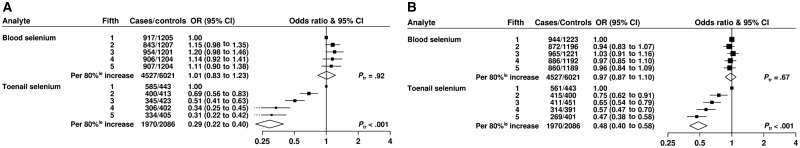
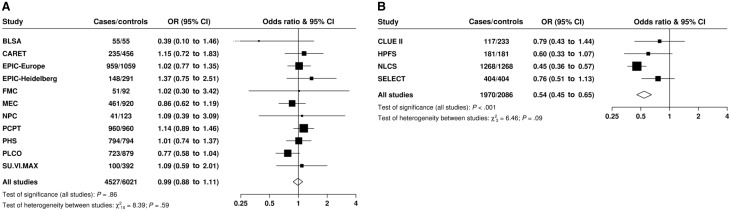
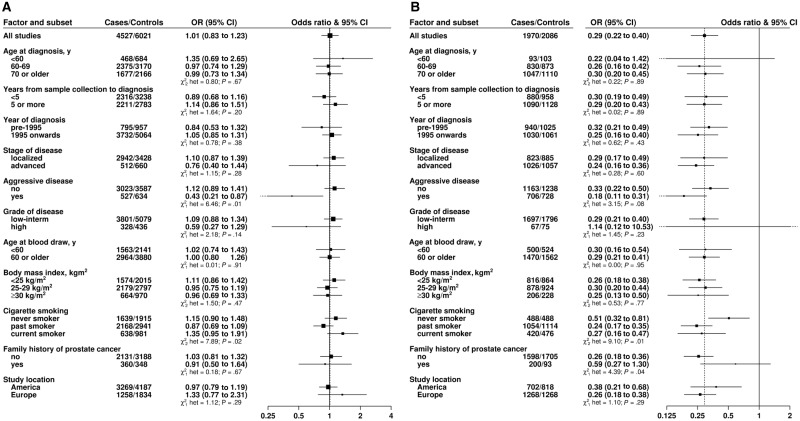
Naomi E Allen
Naomi E Allen
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,✉,
Ruth C Travis
Ruth C Travis
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Paul N Appleby
Paul N Appleby
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Demetrius Albanes
Demetrius Albanes
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Matt J Barnett
Matt J Barnett
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Amanda Black
Amanda Black
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
H Bas Bueno-de-Mesquita
H Bas Bueno-de-Mesquita
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Mélanie Deschasaux
Mélanie Deschasaux
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Pilar Galan
Pilar Galan
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Gary E Goodman
Gary E Goodman
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Phyllis J Goodman
Phyllis J Goodman
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Marc J Gunter
Marc J Gunter
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Markku Heliövaara
Markku Heliövaara
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Kathy J Helzlsouer
Kathy J Helzlsouer
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Brian E Henderson
Brian E Henderson
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,*,
Serge Hercberg
Serge Hercberg
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Paul Knekt
Paul Knekt
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Laurence N Kolonel
Laurence N Kolonel
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Christina Lasheras
Christina Lasheras
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Jakob Linseisen
Jakob Linseisen
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
E Jeffrey Metter
E Jeffrey Metter
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Marian L Neuhouser
Marian L Neuhouser
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Anja Olsen
Anja Olsen
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Valeria Pala
Valeria Pala
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Elizabeth A Platz
Elizabeth A Platz
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Harri Rissanen
Harri Rissanen
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Mary E Reid
Mary E Reid
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Jeannette M Schenk
Jeannette M Schenk
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Meir J Stampfer
Meir J Stampfer
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Pär Stattin
Pär Stattin
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Catherine M Tangen
Catherine M Tangen
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Mathilde Touvier
Mathilde Touvier
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Antonia Trichopoulou
Antonia Trichopoulou
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Piet A van den Brandt
Piet A van den Brandt
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1,
Timothy J Key
Timothy J Key
1Affiliations of authors: Clinical Trial Service Unit and Epidemiological Studies Unit (NEA) and Cancer Epidemiology Unit (RCT, PNA, TJK), Nuffield Department of Population Health, University of Oxford, Oxford, UK; Division of Cancer Epidemiology and Genetics, US National Cancer Institute, Bethesda, MD (DA, AB); Division of Public Health Science (MJB, GEG, MLN), SWOG (formerly the Southwest Oncology Group) Statistical Center (PJG, CMT), and Cancer Prevention Program (JMS), Fred Hutchinson Cancer Research Center, Seattle, WA; Department of Epidemiology (JMS) and Department of Biostatistics (CMT), University of Washington, Seattle, WA; Department for Determinants of Chronic Diseases, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (HBBdM); Department of Gastroenterology and Hepatology, University Medical Centre, Utrecht, the Netherlands (HBBdM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (HBBdM, MJG); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (HBBdM); Sorbonne Paris Cité Epidemiology and Biostatistics Research Center, Nutritional Epidemiology Research Team, Inserm U1153, Inra U1125, Cnam, University Paris 13, University Paris 5, University Paris 7, Bobigny, France (MD, PG, SH, MT); National Institute for Health and Welfare, Helsinki, Finland (MH, PK, HR); The Prevention and Research Center Mercy Medical Center, Baltimore, MD (KJH); Department of Preventive Medicine, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA (BEH); University of Hawaii Cancer Center, Honolulu, HI (LNK); Department of Functional Biology, Faculty of Medicine, University of Oviedo, Asturias, Spain (CL); Institute of Epidemiology II, Helmholtz-Zentrum München, Neuherberg, Germany (formerly of Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany) (JL); Intramural Research Program, National Institute on Aging, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN (EJM); Danish Cancer Society Research Center, Copenhagen, Denmark (AO); Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy (VP); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (EAP); Roswell Park Cancer Institute, New York, NY (MER); Department of Epidemiology, Harvard School of Public Health, Boston, MA (MJS); Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (MJS); Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (PS); Hellenic Health Foundation, Athens, Greece (AT); Department of Epidemiology, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands (PAvdB)
1;
on behalf of the Endogenous Hormones, Nutritional Biomarkers and Prostate Cancer Collaborative Group1