Abstract
The histopathologic features of adult granulosa cell tumors (AGCTs) are relatively nonspecific, resulting in misdiagnosis of other cancers as AGCT, a problem that has not been well characterized. FOXL2 mutation testing was used to stratify 336 AGCTs from three European centers into three categories: 1) FOXL2 mutant molecularly defined AGCT (MD-AGCT) (n = 256 of 336), 2) FOXL2 wild-type AGCT (n = 17 of 336), 3) misdiagnosed other tumor types (n = 63 of 336). All statistical tests were two-sided. The overall and disease-specific survival of the misdiagnosed cases was lower than in the MD-AGCTs (P < .001). The misdiagnosed cases accounted for 71.9% of disease-specific deaths within five years. In the population-based cohort, overall survival of MD-AGCT patients was not different from age-matched, population-based controls. Even though 35.2% of all the MD-AGCT patients in our study experienced a relapse, AGCT is usually an indolent disease. The historical, premolecular data underpinning our clinical understanding of AGCT was likely skewed by inclusion of misdiagnosed cases, and future management strategies should reflect the potential for surgical cure and long survival even after relapse.
Adult granulosa cell tumor (AGCT) accounts for 3% to 5% of all ovarian cancers (1,2) and is characterized by an unpredictable disease course with reported recurrence rates between 6% to 50% (3–6). AGCTs can show histomorphological patterns similar to a variety of unrelated tumors, and diagnosis can be challenging. In historical series, false-positive diagnosis rates of up to 36% have been recorded (7,8), hampering our ability to understand the clinical behavior of AGCT.
We identified a single somatic point mutation in the forkhead transcription factor FOXL2 (402C>G) C134W in 97% of centrally reviewed AGCTs (9). This mutation is a pathognomonic defining feature of AGCT and is not seen in other tumors, in particular other ovarian cancers (10–15). Analysis of the FOXL2 mutation has proven useful in the differential diagnosis of AGCT, and its incorporation into diagnostic algorithms has been proposed (16–18); the clinical impact of using this diagnostic tool, however, has not been determined. Our objective was to analyze the C134W FOXL2 mutation status and clinical outcomes of three European cohorts of AGCT patients to determine, for the first time, the clinical behavior of true AGCTs when diagnosis is based on a robust molecular marker.
A cohort of 369 ovarian AGCTs were identified in pathology records of three European centers: Helsinki University Hospital, Finland (248 patient cases); the Center for Gynecologic Oncology Amsterdam (CGOA), the Netherlands (79 patient cases); and Tübingen University Hospital, Germany (42 patient cases). The Helsinki and Tübingen University Hospitals both serve populations whereas the CGOA consists of three referral hospitals. Clinical and follow-up data were retrospectively collected, as previously described (19,20). The ethics committees of Helsinki University Central Hospital and the National Supervisory Authority for Welfare and Health approved this study. The study material was strictly handled after anonymization of the data according to national ethical guidelines of ‘Code for Proper Secondary Use of Human Tissue,' developed by the Federation of Medical Societies (FMWV) in the Netherlands. Therefore, the need for obtaining informed consent was waived by the three referral centers. The Independent Ethics Committee (IEC) of the University of Tübingen approved this study.
We were able to perform FOXL2 (C>G) C134W mutation analysis in 336 out of 369 cases with the allelic discrimination assay (9,16). After FOXL2 mutation testing, tumors were stratified as mutation-positive molecularly defined AGCT (MD-AGCT) or negative (FOXL2 wild-type), and all FOXL2 wild-type tumors were subjected to histopathological review. Additional immunohistochemical (IHC) analysis (Supplementary Materials, available online) (11) was performed to further refine diagnoses, and cases assigned to one of three final categories: 1) MD-AGCT (n = 256/336, 76.2%), 2) AGCT FOXL2 wild-type (AGCT-WT) (ie, typical AGCT morphology/immunophenotype but FOXL2 mutation negative) (n = 17/336, 5.1%), or 3) misdiagnosed other tumor types (n = 63/336, 18.8%). The misdiagnosis rate in each of the three cohorts was: Helsinki, 18.8%; Amsterdam, 19.5%; Tübingen 17.1%. The revised diagnoses included other sex cord-stromal tumors (49.2%), epithelial malignancies (44.4%), and miscellaneous tumors (6.3%), many of which have their own distinct molecular features and treatment strategies (Supplementary Table 1, available online). If we disregard the misdiagnosed other tumor types, 256 of 273 (93.8%) AGCTs harbor the FOXL2 mutation.
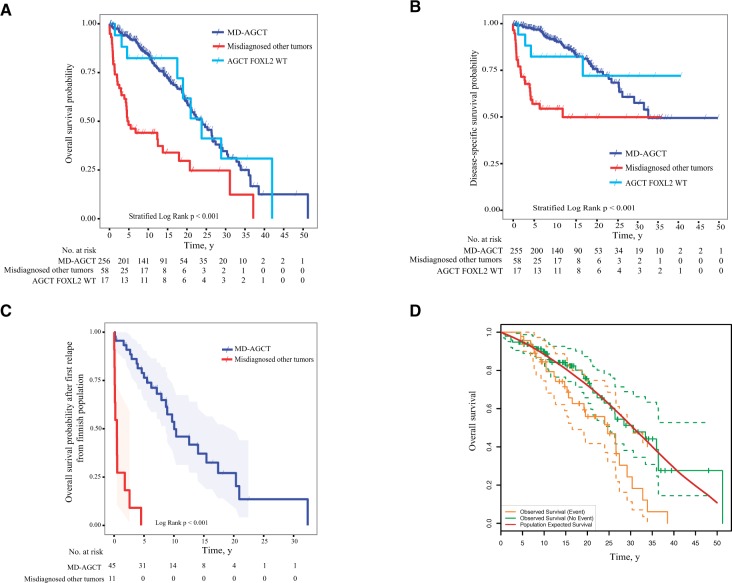
Clinical characteristics of patients with FOXL2 MD-AGCTs, AGCT-WT, and misdiagnosed other tumor types are described in Table 1. All calculated P values are two-sided, and statistical significance was assessed at the .05 level. Univariate associations were examined using Fisher’s exact test for categorical variables and t tests for continuous ones (Supplementary Materials, available online). As this was a multicenter study, we used a stratified log-rank (SLR) test to account for cohort. The overall survival (OS) (Figure 1A) and disease-specific survival (DSS) (Figure 1B) were clearly distinct (P < .001) between MD-AGCTs and the misdiagnosed other tumor types. Molecularly defined diagnoses remain statistically significant when cohort, stage, and age at diagnosis were included as covariates in a Cox proportional hazard model; however, we note that because of the long-term follow-up in one cohort, proportional hazard assumptions are not met (Supplementary Table 2, available online). Although the number of AGCT-WT cases was small (n = 17), these tumors were associated with a similar OS compared with the MD-AGCT cases (Figure 1A). Although 35.2% of all MD-AGCT recurred, we found that 71.9% (23/32) of all disease-specific deaths in the first five years after diagnosis were within the subset of misdiagnosed other types of tumors. We determined the OS of patients after their first disease relapse and found that all patients with recurrent misdiagnosed other tumor types (primarily carcinomas) died within five years of the first relapse (Figure 1C). In contrast, 76.4% of the patients with relapsed MD-ACGT survive to five years, and 53.6% survive to 10 years after their first relapse.
Table 1.
Clinical characteristics of patients with molecularly defined AGCT
| Characteristic | Total | Finland | Netherlands | Germany | P† |
|---|---|---|---|---|---|
| Age at last birthday, y | |||||
| Median (IQR) | 54 (45–61) | 54 (44–62) | 52 (45–58) | 57 (47–70) | .28 |
| Stage (FIGO 2009), No. (%) | |||||
| Stage I | 218 (85.2) | 148 (90.2) | 46 (76.7) | 24 (75.0) | .03 |
| I | 11 (4.3) | 6 (3.7) | 5 (8.3) | 0 (0) | .002 |
| Ia | 130 (50.8) | 88 (53.7) | 21 (35.0) | 21 (65.6) | |
| Ib | 4 (1.6) | 1 (0.6) | 2 (3.3) | 1 (3.1) | |
| Ic | 73 (28.5) | 53 (32.3) | 18 (30.0) | 2 (6.2) | |
| Stage II | 23 (9.0) | 10 (6.1) | 8 (13.3) | 5 (15.6) | |
| Stage III | 10 (3.9) | 3 (1.8) | 4 (6.7) | 3 (9.4) | |
| Unknown | 5 (2.0) | 3 (1.8) | 2 (3.3) | 0 (0) | |
| Type of surgery, No. (%) | <.001 | ||||
| HYS+BSO | 157 (61.3) | 112 (68.3) | 26 (43.3) | 19 (59.4) | |
| USO | 66 (25.8) | 31 (18.9) | 28 (46.7) | 7 (21.9) | |
| BSO | 26 (10.2) | 14 (8.5) | 6 (10.0) | 6 (18.6) | |
| Other/unknown | 5 (2.0) | 5 (3.0) | 0 (0.0) | 0 (0) | |
| Any adjuvant treatment*, No. (%) | .15 | ||||
| Yes | 52 (20.3) | 40 (24.4) | 8 (13.3) | 4 (12.5) | |
| No | 197 (77.0) | 123 (75.0) | 52 (86.7) | 22 (68.8) | |
| Unknown | 7 (2.7) | 1 (0.6) | 0 (0.0) | 6 (18.8) | |
| Follow-up time, y | |||||
| Reverse KM | 10.49 | 14.32 | 8.96 | 5.34 | |
| OS (95% CI), % | |||||
| 5-y | 93.3 (91.5 to 98.4) | 94.9 (91.5 to 98.4) | 92.6 (85.8 to 99.9) | 85.2 (72.7 to 99.8) | |
| 10-y | 84.4 (79.5 to 89.5) | 89.8 (81.4 to 92.5) | 80.5 (69.7 to 91.3) | 79.5 (64.6 to 98.0) | |
| 15-y | 72.1 (65.6 to 79.2) | 77.0 (70.0 to 84.7) | 59.4 (44.7 to 79.1) | 39.8 (9.8 to 100) | |
| Deaths, No. (%) | .07 | ||||
| Yes | 92 (35.9) | 65 (39.6) | 6 (18.8) | 21 (35.0) | |
| No | 164 (64.1) | 99 (60.4) | 26 (81.2) | 39 (65.0) | |
| DSS (95% CI), % | |||||
| 5-y | 97.4 (95.3 to 99.5) | 98.6 (96.8 to 100) | 94.6 (88.9 to 100) | 95.5 (87.1 to 100) | |
| 10-y | 91.8 (88.0 to 95.8) | 93.2 (88.9 to 97.6) | 87.4 (78.3 to 97.5) | 95.5 (87.1 to 100) | |
| 15-y | 85.4 (80.0 to 91.2) | 89.0 (83.5 to 95.0) | 71.9 (57.6 to 89.7) | 95.5 (87.1 to 100) | |
| Deaths of disease, No. (%) | .01 | ||||
| Yes | 42 (16.4) | 25 (15.2) | 1 (3.1) | 16 (26.7) | |
| No | 213 (83.2) | 138 (84.1) | 31 (96.9) | 44 (73.3) | |
| Unknown | 1 (0.4) | 1 (0.6) | 0 (0.0) | 0 (0.0) | |
| Number of patients with recurrent disease, No. (%) | <.001 | ||||
| Yes | 90 (35.2) | 46 (28.0) | 38 (63.3) | 6 (18.8) | |
| No | 143 (55.9) | 110 (67.1) | 20 (33.3) | 13 (40.6) | |
| Unknown | 23 (9.0) | 8 (4.9) | 2 (3.3) | 13 (40.6) | |
| Time to event for patients who recurred, median, y | |||||
| OS | 13.4 | 16.5 | 3.2 | 11.6 | |
| DSS | 12.7 | 14.7 | 3.3 | 12.5 | |
| RFS | 13.9 | 7.2 | 4.2 | 5.3 | |
*Includes chemotherapy and radiation. The percentages shown are reflected in the column groupings. BSO = bilateral salpingo-oophorectomy; CI = confidence interval; DSS = disease-specific survival; HYS = hysterectomy; IQR = interquartile range; OS = overall survival; RFS = relapse-free survival; USO = unilateral salpingo-oophorectomy.
†All P values are two-sided and calculated using the Fisher’s exact test to assess associations with categorical variables, and t tests were used for continuous ones.
Figure 1.
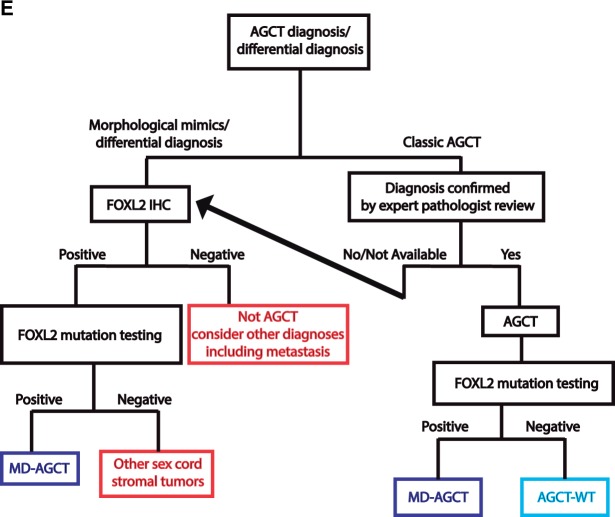
Survival curves of molecularly defined adult granulosa cell tumor patients (MD-AGCT), AGCT FOXL2 wild-type (WT), and misdiagnosed other tumor types. A) Overall survival (OS) of the combined three AGCT (adult granulosa cell tumor) cohorts from three European centers. All outcome analyses were performed using Kaplan-Meier methods with log-rank (LR) testing and stratified log-rank (SLR) testing where cohorts were combined. All statistical tests were two-tailed. B) Disease-specific survival (DSS) of the combined three AGCT cohorts from three European centers. C) Overall survival after the first recurrence of MD-AGCT patients compared with patients diagnosed with misdiagnosed other types of cancers. Shown is only the patient cohort from Helsinki, Finland. Survival data is only after a patient’s first recurrence in the MD-AGCT group and the misdiagnosed other cancer types group. D) Overall survival for MD-AGCT from the Finnish population for patients who recurred and did not recur compared with an age-, sex-, and calendar year–matched Finnish population acquired as one-year intervals from the human mortality database (www.mortality.org). The green solid lines indicate MD-ACGTs with no observed survival event, the orange solid lines indicate MD-AGCTs with an observed survival event, and the red solid line indicates the expected survival of the general population. Ninety-five percent confidence bands are indicated by broken lines. Overlapping confidence bands indicates that there is no statistically significant difference in survival between those patients who recurred or did not recur compared with the survival of the matched population. E) Recommended diagnostic algorithm for accurate diagnosis of AGCT. Morphological assessment, FOXL2 immunohistochemistry, and FOXL2 mutation testing are applied in a step-wise fashion, as indicated, to identify MD-AGCTs, morphologically typical AGCT-WT (FOXL2 mutation negative), and other tumors (eg, sex cord stromal tumors, carcinomas, metastasis). AGCT = adult granulosa cell tumor; IHC = immunohistochemistry; MD-AGCT = molecularly defined adult granulosa cell tumor; WT = wild-type.
As MD-AGCTs generally show an indolent disease course, we assessed whether the life expectancies of Finnish MD-AGCT patients differ from an age- and sex-matched Finnish population. The OS of MD-AGCT patients with and without recurrence were similar to the population for the first 10 years (Figure 1D). The expected survival curves were estimated using the Hakulinen method (21) from life tables. Data with diagnoses prior to 2010 were used to compare with expected population data in order to match to available survival dates.
This is the first large retrospective clinical outcomes study of AGCTs where unrelated tumors that mimic the histomorphological features of AGCT have been excluded. We confirmed the presence of the FOXL2 mutation in 94.1% of AGCTs from three European centers, with all showing a similar misdiagnosis rate (17%–20%). AGCT-WT cases are morphologically typical, are rare but do exist, and merit further study; they appear to have similar clinical features to MD-AGCTs. The role of other mutations in modifying the behavior of MD-AGCT is unknown. The conflicting data from previous studies on AGCT prognosis (22,23) is attributable to the inclusion of non-AGCT cases. The majority (71.8%) of disease-specific deaths were caused by misdiagnosed tumors. Conversely, only six (2.2%) patients with MD-AGCTs died of disease within the first five years, and 56.8% of all MD-AGCT patients who experienced a recurrence were alive five years thereafter. Most recurrences and deaths in historical, molecularly unconfirmed cohorts of AGCT likely occur in patients with misdiagnosed other tumor types. We propose that in all cases with diagnostic uncertainty where the differential diagnosis includes AGCT, FOXL2 mutation testing should be performed to allow for accurate diagnosis and appropriate treatment and clinical follow-up (Figure 1E) (24,25). Incorporating FOXL2 mutation testing into routine pathological assessment will aid in moving toward reproducible diagnosis of AGCT, allowing for future studies to accurately study the clinical course of true ovarian AGCTs and creating uncontaminated cohorts for clinical trials. The FOXL2 mutation frequency in any future AGCT series should be over 90%, or the accuracy of primary diagnoses is questionable.
The median time to recurrence of MD-AGCT, for those who recurred in the Finnish population–based cohort, was 7.2 years, suggesting that both the clinical standard follow-up time of five years, as recommended by the NCCN (26), SGO (27), and ESMO (28), is illogical and of unproven benefit.
Our study cohort originates from three different European centers, which results in limitations typical of multicenter studies. The Helsinki and Tübingen centers provide service to a general population; however, the Amsterdam center consists of three tertiary hospitals, making the Dutch cohort a selected population. This has likely resulted in a selection bias, resulting in an overestimation of disease recurrences in the Amsterdam cohort. The cohort from Germany is also limited by the shorter follow-up time. Differences in stage distributions between the cohorts may be explained by differences in staging methods and procedures over the 60-year ascertainment period of this study (19). Because of these potential limitations, we have presented analyses for both the individual and combined cohorts. It was not possible to properly generalize conclusions to the combined cohorts for some specific clinicopathological features and outcome analysis, such as stage and relapse-free survival. However, the distribution and prognosis of MD-AGCTs were comparable within each cohort, and we therefore have presented survival curves based on the combined cohorts. Moreover, because cancer survival is known to vary greatly between countries (29), these data from the three combined cohorts will putatively more accurately depict the clinical behavior of MD-AGCT.
This study provides an explicit view of the clinical behavior of MD-AGCT, in particular the long latency to relapse and overall favorable outcomes, highlighting the critical importance of accurate diagnosis.
Funding
This work was supported by the Terry Fox Research Institute Program Project grant #1021 and the Canadian Institutes of Health Research (CIHR; 200903MOP-200281-CPT-CAAA-106737). MKM was supported by a CIHR Doctoral Fellowship, and HMH is supported by a translational research fellowship from the Dutch Cancer Society (KWF 2013-5869). AF, SB, LUK, MH, and MA were supported by Helsinki University Hospital Research Funds, The Paulo Foundation (MA), and the Sladjana M. Crosley Funds for GCT Research. MH was supported by the Academy of Finland and Sigrid Jusélius Foundation. We would also like to thank the Granulosa Cell Tumor of the Ovary Foundation and the Granulosa Cell Tumor Research Foundation for their continued support.
Note
The funders did not have a role in design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
References
- 1.van Meurs HS, Bleeker MC, van der Velden J, et al. The incidence of endometrial hyperplasia and cancer in 1031 patients with a granulosa cell tumor of the ovary: long-term follow-up in a population-based cohort study. Int J Gynecol Cancer. 2013;23(8):1417–1422. [DOI] [PubMed] [Google Scholar]
- 2.Unkila-Kallio L, Leminen A, Tiitinen A, et al. Nationwide data on falling incidence of ovarian granulosa cell tumours concomitant with increasing use of ovulation inducers. Hum Reprod. 1998;13(10):2828–2830. [DOI] [PubMed] [Google Scholar]
- 3.Malmstrom H, Hogberg T, Risberg B, et al. Granulosa cell tumors of the ovary: prognostic factors and outcome. Gynecol Oncol. 1994;52(1):50–55. [DOI] [PubMed] [Google Scholar]
- 4.Haroon S, Zia A, Idrees R, et al. Clinicopathological spectrum of ovarian sex cord-stromal tumors; 20 years' retrospective study in a developing country. J Ovarian Res. 2013;6(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu-Rustum NR, Restivo A, Ivy J, et al. Retroperitoneal nodal metastasis in primary and recurrent granulosa cell tumors of the ovary. Gynecol Oncol. 2006;103(1):31–34. [DOI] [PubMed] [Google Scholar]
- 6.Rosario R, Wilson M, Cheng WT, et al. Adult granulosa cell tumours (GCT): clinicopathological outcomes including FOXL2 mutational status and expression. Gynecol Oncol. 2013;131(2):325–329. [DOI] [PubMed] [Google Scholar]
- 7.Cronje HS, Niemand I, Bam RH, et al. Review of the granulosa-theca cell tumors from the emil Novak ovarian tumor registry. Am J Obstet Gynecol. 1999;180(2 Pt 1):323–327. [DOI] [PubMed] [Google Scholar]
- 8.Miller BE, Barron BA, Wan JY, et al. Prognostic factors in adult granulosa cell tumor of the ovary. Cancer. 1997;79(10):1951–1955. [DOI] [PubMed] [Google Scholar]
- 9.Shah SP, Kobel M, Senz J, et al. Mutation of FOXL2 in granulosa-cell tumors of the ovary. N Engl J Med. 2009;360(26):2719–2729. [DOI] [PubMed] [Google Scholar]
- 10.Schrader KA, Gorbatcheva B, Senz J, et al. The specificity of the FOXL2 c.402C>G somatic mutation: a survey of solid tumors. PLoS One. 2009;4(11):e7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Agha OM, Huwait HF, Chow C, et al. FOXL2 is a sensitive and specific marker for sex cord-stromal tumors of the ovary. Am J Surg Pathol. 2011;35(4):484–494. [DOI] [PubMed] [Google Scholar]
- 12.Gershon R, Aviel-Ronen S, Korach J, et al. FOXL2 C402G mutation detection using MALDI-TOF-MS in DNA extracted from Israeli granulosa cell tumors. Gynecol Oncol. 2011;122(3):580–584. [DOI] [PubMed] [Google Scholar]
- 13.Hes O, Vanecek T, Petersson F, et al. Mutational analysis (c.402C>G) of the FOXL2 gene and immunohistochemical expression of the FOXL2 protein in testicular adult type granulosa cell tumors and incompletely differentiated sex cord stromal tumors. Appl Immunohistochem Mol Morphol. 2011;19(4):347–351. [DOI] [PubMed] [Google Scholar]
- 14.Jamieson S, Butzow R, Andersson N, et al. The FOXL2 C134W mutation is characteristic of adult granulosa cell tumors of the ovary. Mod Pathol. 2010;23(11):1477–1485. [DOI] [PubMed] [Google Scholar]
- 15.Kim MS, Hur SY, Yoo NJ, et al. Mutational analysis of FOXL2 codon 134 in granulosa cell tumour of ovary and other human cancers. J Pathol. 2010;221(2):147–152. [DOI] [PubMed] [Google Scholar]
- 16.Kommoss S, Anglesio MS, Mackenzie R, et al. FOXL2 molecular testing in ovarian neoplasms: diagnostic approach and procedural guidelines. Mod Pathol. 2013;26(6):860–867. [DOI] [PubMed] [Google Scholar]
- 17.McCluggage WG, Singh N, Kommoss S, et al. Ovarian Cellular Fibromas Lack FOXL2 Mutations: A Useful Diagnostic Adjunct in the Distinction From Diffuse Adult Granulosa Cell Tumor. Am J Surg Pathol. 2013;37(9):1450–1455. [DOI] [PubMed] [Google Scholar]
- 18.Stewart CJ, Alexiadis M, Crook ML, et al. An immunohistochemical and molecular analysis of problematic and unclassified ovarian sex cord-stromal tumors. Hum Pathol. 2013;44(12):2774–2781. [DOI] [PubMed] [Google Scholar]
- 19.Bryk S, Farkkila A, Butzow R, et al. Clinical characteristics and survival of patients with an adult-type ovarian granulosa cell tumor: a 56-year single-center experience. Int J Gynecol Cancer. 2015;25(1):33–41. [DOI] [PubMed] [Google Scholar]
- 20.van Meurs HS, Schuit E, Horlings HM, et al. Development and internal validation of a prognostic model to predict recurrence free survival in patients with adult granulosa cell tumors of the ovary. Gynecol Oncol. 2014;134(3):498–504. [DOI] [PubMed] [Google Scholar]
- 21.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. New York: Springer; 2000. [Google Scholar]
- 22.D'Angelo E, Mozos A, Nakayama D, et al. Prognostic significance of FOXL2 mutation and mRNA expression in adult and juvenile granulosa cell tumors of the ovary. Mod Pathol. 2011;24(10):1360–1367. [DOI] [PubMed] [Google Scholar]
- 23.Rosario R, Wilson M, Cheng W-T, et al. Adult granulosa cell tumours (GCT): Clinicopathological outcomes including FOXL2 mutational status and expression. Gynecol Oncol. 2013;131(2):325–329. [DOI] [PubMed] [Google Scholar]
- 24.Kommoss S, Gilks CB, Penzel R, et al. A current perspective on the pathological assessment of FOXL2 in adult-type granulosa cell tumours of the ovary. Histopathology. 2014;64(3):380–388. [DOI] [PubMed] [Google Scholar]
- 25.Maillet D, Goulvent T, Rimokh R, et al. Impact of a second opinion using expression and molecular analysis of FOXL2 for sex cord-stromal tumors. A study of the GINECO group & the TMRO network. Gynecol Oncol. 2014;132(1):181–187. [DOI] [PubMed] [Google Scholar]
- 26.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines) Version 3.2014; Malignant Sex Cord-Stromal tumors, 2014. http://www.nccn.org. Accessed May 2014.
- 27.Salani R, Backes FJ, Fung MF, et al. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am J Obstet Gynecol. 2011;204(6):466–478. [DOI] [PubMed] [Google Scholar]
- 28.Colombo N, Peiretti M, Garbi A, et al. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii20–vii26. [DOI] [PubMed] [Google Scholar]
- 29.De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE--5-a population-based study. Lancet Oncol. 2014;15(1):23–34. [DOI] [PubMed] [Google Scholar]