Abstract
A recent meta-analysis suggested that circulating fatty acids do not play an important role in prostate carcinogenesis. We hypothesized that the relation between circulating fatty acids and prostate cancer (PCa) risk is modified by time between blood draw and diagnosis. We tested this hypothesis in a prospective case-control study of 476 PCa cases and matched control subjects nested in the Physicians’ Health Study. The previously reported associations between fatty acids and PCa in this cohort were dramatically stronger among men diagnosed 10 or more years after blood collection. Statistically significant effect modification by time since blood collection was identified for mono-unsaturated and poly-unsaturated fatty acids and was more pronounced for aggressive tumors. Among men diagnosed fewer than 10 years since blood collection, the relative risks per interquartile range were 1.03 (95% confidence interval [CI] = 0.86 to 1.25) for total mono-unsaturated fatty acids (MUFA) and 0.95 (95% CI = 0.78 to 1.15) for total poly-unsaturated fatty acids (PUFA) whereas among men diagnosed 10 or more years after blood draw the relative risks per interquartile range were 1.69 (95% CI = 1.21 to 2.34) for MUFA (Pheterogeneity = .01) and 0.59 (95% CI = 0.42 to 0.83) for PUFA (Pheterogeneity = .02). These data suggest that the results of the meta-analysis may be partly explained by insufficient follow-up time. Furthermore, they suggest that some environmental and metabolic factors may play a role in prostate carcinogenesis decades before clinical identification of this disease.
The literature on the relation between circulating fatty acid (FA) levels and prostate cancer (PCa) risk has been inconsistent (1–12). For example, mono-unsaturated fatty acid levels have been related to higher risk of PCa in some studies (1,11) but not in others (3,6,7). A recent meta-analysis that pooled individual-level data from seven prospective biomarker studies, including the Physicians’ Health Study (PHS) (4,5,11), did not find evidence for strong associations between circulating FAs and PCa risk (13). While compelling at face value, some of the limitations of this pooled analysis may explain its results. Chief among these are the relatively short follow-up time and the underrepresentation of clinically aggressive disease, possibly resulting from most studies having enrolled patients after PSA screening became widespread. The average time between blood draw and PCa diagnosis was five years in the pooled analysis, compared with nine years in the PHS. Also, the proportion of aggressive PCa cases (defined in terms of stage and lethality) in the pooled analysis was 9%, as compared with 20% in PHS. This reflects in part the proportion of cases diagnosed after 1995, which was 86% in the pooled analysis vs 15% in PHS. Given that increasing evidence suggests that exposures taking place decades before disease identification may be more relevant in prostate carcinogenesis than more proximal exposures (14), we hypothesized that the previously reported associations between blood fatty acids and PCa in the PHS would become stronger with increasing follow-up time. We also evaluated whether this hypothesized interaction would differ according to tumor characteristics.
The study design has been previously described (4,5,11). Briefly, PHS was a randomized trial of aspirin and β-carotene among 22 071 US men age 40 to 84 years in 1982. Written informed consent was obtained, and the institutional review boards of Partners HealthCare and the Harvard T. H. Chan School of Public Health approved this study. When PCa was reported in annual follow-up questionnaires, medical records and pathology reports were obtained and reviewed by study physicians to confirm diagnosis and obtain clinical information, including tumor stage, grade, and prostate-specific antigen at diagnosis. Baseline blood specimens were obtained before random assignment and stored at -82 °C (15).Whole blood FA levels were determined for 476 men diagnosed with PCa over 13 years of follow-up and matched to 476 control subjects by age and smoking status at baseline (4,5,11). The FA levels in each sample were measured by gas-liquid chromatography and expressed as the percentages of total FAs. Long-term storage does not have a major impact on measurement of FA levels (16). Logistic regression models conditioned on the matching variables were used to estimate the relative risks (RRs) and 95% confidence intervals (CIs) of PCa per one interquartile range (IQR) increase and across quintiles of FA levels stratified by time between blood draw and PCa diagnosis (<5 years vs ≥ 5 years, <10 years vs ≥10 years, and continuous yearly exclusion, ie, ≥1, ≥2…). We also fitted these models in subgroups defined by tumor characteristics (stage, grade, lethality—metastasis or death vs not—and aggressiveness, defined as clinical stage T3/T4/M1 or Gleason score ≥ 8 at diagnosis or subsequent metastasis or death). Heterogeneity tests to compare risks of two time intervals were performed by including a product term of time intervals (dichotomous) and FA levels (continuous). All statistical analyses were carried out by SAS, version 9.3 (SAS Institute, Cary, NC). Results were considered to be statistically significant when P values were less than .05 (two-sided).
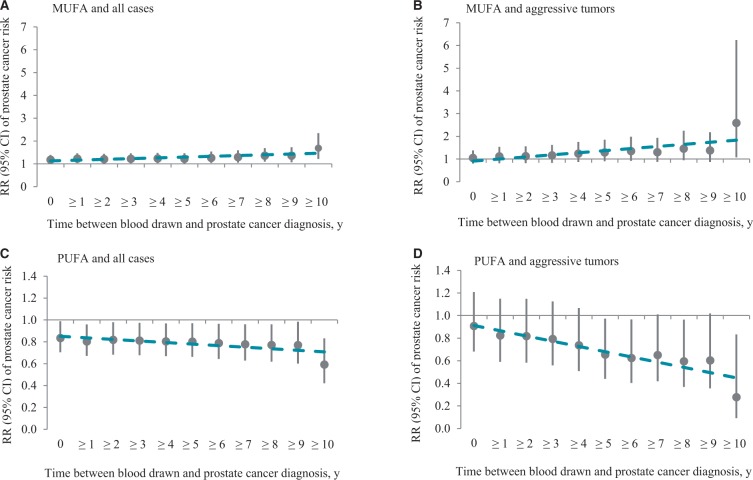
The median follow-up time was nine years (25th–75th percentile = 7–11 years). The previously reported associations of mono-unsaturated (MUFA) (11) and poly-unsaturated (PUFA) (4) FAs with PCa risk were stronger among men diagnosed 10 or more years after blood draw than among men diagnosed earlier (Table 1). Among men diagnosed fewer than 10 years since blood collection, the relative risks per IQR were 1.03 (95% CI = 0.86 to 1.25) for total MUFA and 0.95 (95% CI = 0.78 to 1.15) for total PUFA; among men diagnosed 10 or more years after blood draw, the relative risks per IQR were 1.69 (95% CI = 1.21 to 2.34) for MUFA (Pheterogeneity = .01) and 0.59 (95% CI = 0.42 to 0.83) for PUFA (Pheterogeneity = .02). A similar pattern was observed when follow-up time was dichotomized at five years (data not shown). Finer stratification of follow-up time showed progressively stronger associations, particularly for aggressive tumors (Figure 1). Among men diagnosed with aggressive PCa, as the time between blood draw and diagnosis increased by year, the relative risks per IQR increased for MUFA from 1.05 (95% CI = 0.80 to 1.38) of all aggressive cases to 2.59 (95% CI = 1.08 to 6.24) of cases diagnosed 10 or more years since blood draw and decreased for PUFA from 0.91 (95% CI = 0.68 to 1.21) of all aggressive cases to 0.28 (95% CI = 0.09 to 0.83) of cases diagnosed 10 years after blood collection. This pattern was more evident when fatty acids were modeled as quintiles (Supplementary Figure 1, available online).
Table 1. RRs and 95% CIs for prostate cancer per IQR increase in blood fatty acids stratified by follow-up time (10 years)*†
| Circulating fatty acids | All cases |
Tumor aggressiveness‡ |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <10 y |
≥10 y |
<10 y |
≥10 y |
|||||||
| RR (95% CI) | P§ | RR (95% CI) | P§ | Pheter‖ | RR (95% CI) | P§ | RR (95% CI) | P§ | Pheter‖ | |
| No., case/control | 304/304 | 172/172 | 115/115 | 36/36 | ||||||
| Median follow-up (25th–75th percentile), y | 7.5 (5.1 to 9.0) | 11.8 (10.7 to 12.4) | 7.0 (4.4 to 8.8) | 11.9 (10.8 to 12.6) | ||||||
| Saturated fatty acids | 1.01 (0.81 to 1.25) | .96 | 1.21 (0.88 to 1.66) | .24 | .34 | 1.02 (0.73 to 1.43) | .91 | 2.86 (1.03 to 7.93) | .04 | .06 |
| Myristic acid (14:0) | 1.08 (0.90 to 1.30) | .42 | 1.32 (0.96 to 1.80) | .09 | .29 | 1.00 (0.77 to 1.31) | .98 | 1.28 (0.61 to 2.68) | .52 | .55 |
| Palmitic acid (16:0) | 1.01 (0.81 to 1.28) | .90 | 1.49 (1.02 to 2.17) | .04 | .09 | 0.94 (0.65 to 1.34) | .72 | 2.8 (1.06 to 7.45) | .04 | .04 |
| Stearic acid (18:0) | 0.97 (0.78 to 1.20) | .75 | 0.74 (0.55 to 1.01) | .06 | .17 | 1.12 (0.80 to 1.56) | .51 | 0.76 (0.38 to 1.54) | .45 | .33 |
| Mono-unsaturated fatty acids | 1.03 (0.86 to 1.25) | .73 | 1.69 (1.21 to 2.34) | .002 | .01 | 0.91 (0.68 to 1.23) | .54 | 2.59 (1.08 to 6.24) | .03 | .03 |
| Myristoleic acid (14:1n-5) | 1.05 (0.90 to 1.23) | .51 | 1.35 (1.04 to 1.76) | .03 | .11 | 0.97 (0.80 to 1.19) | .78 | 1.60 (0.86 to 2.99) | .14 | .13 |
| Palmitoleic acid (16:1n-7) | 1.08 (0.91 to 1.27) | .39 | 1.58 (1.15 to 2.17) | .01 | .04 | 0.95 (0.76 to 1.20) | .67 | 1.81 (0.87 to 3.80) | .12 | .10 |
| Oleic acid (18:1n-9) | 1.02 (0.84 to 1.24) | .82 | 1.63 (1.18 to 2.26) | .003 | .02 | 0.92 (0.68 to 1.26) | .61 | 2.48 (1.04 to 5.94) | .04 | .04 |
| Poly-unsaturated fatty acids | 0.95 (0.78 to 1.15) | .59 | 0.59 (0.42 to 0.83) | .003 | .02 | 1.06 (0.78 to 1.44) | .71 | 0.28 (0.09 to 0.83) | .02 | .02 |
| n-3 poly-unsaturated fatty acid | 0.89 (0.73 to 1.08) | .25 | 0.67 (0.48 to 0.92) | .01 | .14 | 0.97 (0.71 to 1.33) | .85 | 0.56 (0.24 to 1.34) | .19 | .25 |
| α-linolenic acid (18:3n-3) | 1.04 (0.89 to 1.21) | .65 | 0.97 (0.76 to 1.24) | .81 | .66 | 1.01 (0.79 to 1.31) | .91 | 0.83 (0.43 to 1.58) | .57 | .57 |
| EPA + DPA + DHA | 0.89 (0.73 to 1.08) | .23 | 0.66 (0.48 to 0.93) | .02 | .15 | 0.97 (0.70 to 1.34) | .85 | 0.58 (0.24 to 1.38) | .22 | .28 |
| n-6 poly-unsaturated fatty acid | 0.98 (0.81 to 1.19) | .83 | 0.68 (0.50 to 0.92) | .01 | .05 | 1.07 (0.80 to 1.45) | .64 | 0.38 (0.15 to 0.95) | .04 | .03 |
| Linoleic acid (18:2n-6) | 0.89 (0.72 to 1.10) | .27 | 0.77 (0.56 to 1.04) | .09 | .44 | 0.98 (0.70 to 1.37) | .91 | 0.36 (0.14 to 0.91) | .03 | .05 |
| Gamma-linolenic acid (18:3n-6) | 1.13 (0.92 to 1.39) | .25 | 1.15 (0.90 to 1.47) | .26 | .91 | 0.95 (0.68 to 1.31) | .74 | 1.18 (0.66 to 2.09) | .58 | .52 |
| Dihomo-γ-linolenic acid (20:3n-6) | 1.22 (1.01 to 1.48) | .04 | 1.28 (0.99 to 1.65) | .06 | .78 | 1.32 (0.95 to 1.83) | .10 | 1.10 (0.69 to 1.74) | .70 | .53 |
| Arachidonic acid (20:4n-6) | 1.10 (0.90 to 1.36) | .35 | 0.76 (0.56 to 1.04) | .08 | .05 | 1.13 (0.82 to 1.57) | .45 | 1.04 (0.51 to 2.11) | .91 | .83 |
*Circulating fatty acids per interquartile range was calculated and used in the conditional logistic regression. The relative risks were conditioned on the matching variables of age at baseline, smoking status at baseline, and length of follow-up. CI = confidence interval; DHA = docosahexaenoic acid; DPA = docosapentaenoic acid; EPA = eicosapentaenoic acid; IQR = interquartile range; RR = relative risk.
†Follow-up time was time interval from blood collection to diagnosis.
‡Tumor aggressiveness was defined if ultimately died, had metastasis, had clinical stage T3/T4/M1, or Gleason score ≥ 8 at diagnosis.
§P value was calculated by conditional logistic regression modeling fatty acid per IQR as the independent variable. All statistical tests were two-sided.
‖Pheterogeneity was calculated by including a product term of fatty acid per IQR and dichotomous time interval of blood collection since diagnosis. All statistical tests were two-sided.
Figure 1.
Relative risks (RRs) and 95% confidence intervals of prostate cancer per interquartile range (IQR) increase in total mono-unsaturated fatty acids (A and B) and total poly-unsaturated fatty acids (C and D) among all cases (A and C) and aggressive tumors (B and D) by increasing follow-up time since blood collection. The relative risks (RRs) were conditioned on the matching variables (smoking status at baseline and length of follow-up). Regression line (dashed line) was obtained by linearly plotting RR of prostate cancer by circulating fatty acids per IQR vs years. CI = confidence interval; MUFA = mono-unsaturated fatty acids; PUFA = poly-unsaturated fatty acids; RR = relative risk.
These findings are in agreement with our hypothesis that nutritional and metabolic factors captured by circulating FA levels play a role in prostate carcinogenesis in its early stages and with the more general hypothesis that exposures taking place decades before any clinical evidence of disease may be particularly important for the development of PCa (14). If our hypothesis is true, it could explain why studies with longer follow-up time (time between blood draw and PCa diagnosis), a greater proportion of advanced/high-grade cases, and fewer cases identified after PSA screening became widespread tend to report strong associations between circulating FAs and PCa risk (1,11) whereas studies with shorter follow-up and more localized/low-grade cases tend to report either no association and associations with PCa in the opposite direction (6,8,10).
Our findings also raise important considerations regarding the design of epidemiologic studies aimed at identifying risk factors for PCa. If, as suggested by our data, exposures predating PCa diagnosis by decades are more relevant to the development of this disease than more proximal exposures, epidemiologic studies focused on older men, as well as epidemiologic studies with short follow-up, may have limited value in identifying modifiable risk factors for PCa, particularly the factors playing a role in the initiation of the carcinogenic process and for factors subject to important within-subject variability over extended periods of time such as circulating fatty acids (17). Moreover, this type of study may be more likely to identify spurious associations (eg, associations because of reverse causality) or to fail to identify associations at all by missing relevant susceptibility windows.
The most important limitation of the study is the possibility of residual confounding. Nevertheless, we have previously shown (4,5,11) that adjustment for a large number of potential confounders has no to minimal impact on the relation between fatty acids and prostate cancer risk in this cohort. The most salient strength of the study is the large number of cases and long follow-up period since blood draw, which allowed for testing our hypothesis with adequate statistical power.
In summary, we found that previously reported associations between circulating FAs and PCa risk in the PHS varied by follow-up time. Specifically, these associations became stronger with increasing follow-up time, particularly for aggressive tumors. Our results offer a possible explanation for the inconsistencies across studies addressing these relations and for the findings of a recent pooled analysis of prospective studies. Furthermore, they highlight the importance of long follow-up (>10 years) in epidemiologic studies aimed at identifying risk factors for PCa, including biomarker-based studies.
Funding
This study was supported by grant W81XWH-11-1-0529 from the the US Department of Defense and grants CA42182, CA58684, CA90598, CA141298 CA97193, CA34944, CA40360, CA131945, P50CA90381, 1U54CA155626-01, U54CA155496, P30DK046200, HL26490, and HL34595 from the National Institutes of Health.
Notes
The study funders had no role in design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
References
- 1.Harvei S, Bjerve KS, Tretli S, et al. Prediagnostic level of fatty acids in serum phospholipids: omega-3 and omega-6 fatty acids and the risk of prostate cancer. Int J Cancer. 1997;71(4):545–551. [DOI] [PubMed] [Google Scholar]
- 2.Laaksonen DE, Laukkanen JA, Niskanen L, et al. Serum linoleic and total polyunsaturated fatty acids in relation to prostate and other cancers: a population-based cohort study. Int J Cancer. 2004;111(3):444–450. [DOI] [PubMed] [Google Scholar]
- 3.Mannisto S, Pietinen P, Virtanen MJ, et al. Fatty acids and risk of prostate cancer in a nested case-control study in male smokers. Cancer Epidemiol Biomarkers Prev. 2003;12(12):1422–1428. [PubMed] [Google Scholar]
- 4.Chavarro JE, Stampfer MJ, Li H, et al. A prospective study of polyunsaturated fatty acid levels in blood and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1364–1370. [DOI] [PubMed] [Google Scholar]
- 5.Chavarro JE, Stampfer MJ, Campos H, et al. A prospective study of trans-fatty acid levels in blood and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(1):95–101. [DOI] [PubMed] [Google Scholar]
- 6.Crowe FL, Allen NE, Appleby PN, et al. Fatty acid composition of plasma phospholipids and risk of prostate cancer in a case-control analysis nested within the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008;88(5):1353–1363. [DOI] [PubMed] [Google Scholar]
- 7.Park SY, Wilkens LR, Henning SM, et al. Circulating fatty acids and prostate cancer risk in a nested case-control study: the Multiethnic Cohort. Cancer Causes Control. 2009;20(2):211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brasky TM, Till C, White E, et al. Serum phospholipid fatty acids and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol. 2011;173(12):1429–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassett JK, Severi G, Hodge AM, et al. Plasma phospholipid fatty acids, dietary fatty acids and prostate cancer risk. Int J Cancer. 2013;133(8):1882–1891. [DOI] [PubMed] [Google Scholar]
- 10.Brasky TM, Darke AK, Song X, et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J Natl Cancer Inst. 2013;105(15):1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavarro JE, Kenfield SA, Stampfer MJ, et al. Blood levels of saturated and monounsaturated fatty acids as markers of de novo lipogenesis and risk of prostate cancer. Am J Epidemiol. 2013;178(8):1246–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng TY, King IB, Barnett MJ, et al. Serum phospholipid fatty acids, genetic variation in myeloperoxidase, and prostate cancer risk in heavy smokers: a gene-nutrient interaction in the carotene and retinol efficacy trial. Am J Epidemiol. 2013;177(10):1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowe FL, Appleby PN, Travis RC, et al. Circulating fatty acids and prostate cancer risk: individual participant meta-analysis of prospective studies. J Natl Cancer Inst. 2014;106(9):dju240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutcliffe S, Colditz GA. Prostate cancer: is it time to expand the research focus to early-life exposures? Nat Rev Cancer. 2013;13(3):208–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gann PH, Hennekens CH, Sacks FM, et al. Prospective study of plasma fatty acids and risk of prostate cancer. J Natl Cancer Inst. 1994;86(4):281–286. [DOI] [PubMed] [Google Scholar]
- 16.Matthan NR, Ip B, Resteghini N, et al. Long-term fatty acid stability in human serum cholesteryl ester, triglyceride, and phospholipid fractions. J Lipid Res. 2010;51(9):2826–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeleniuch-Jacquotte A, Chajes V, Van Kappel AL, et al. Reliability of fatty acid composition in human serum phospholipids. Eur J Clin Nutr. 2000;54(5):367–372. [DOI] [PubMed] [Google Scholar]