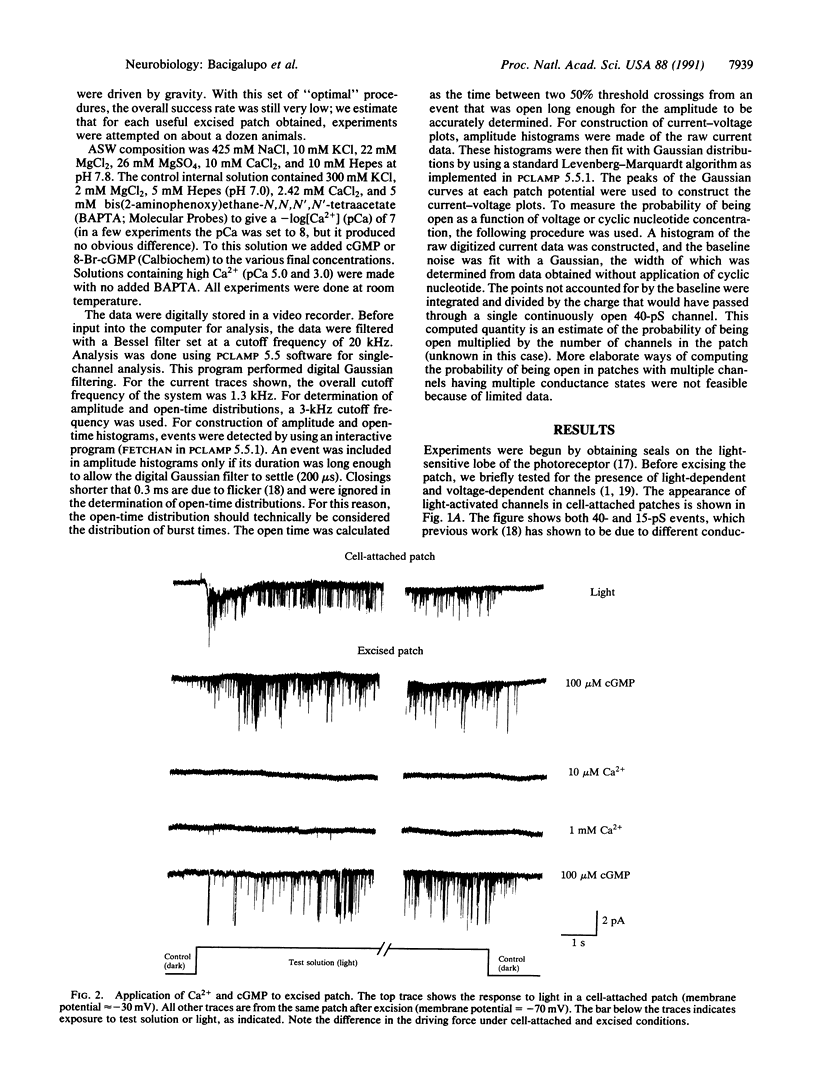
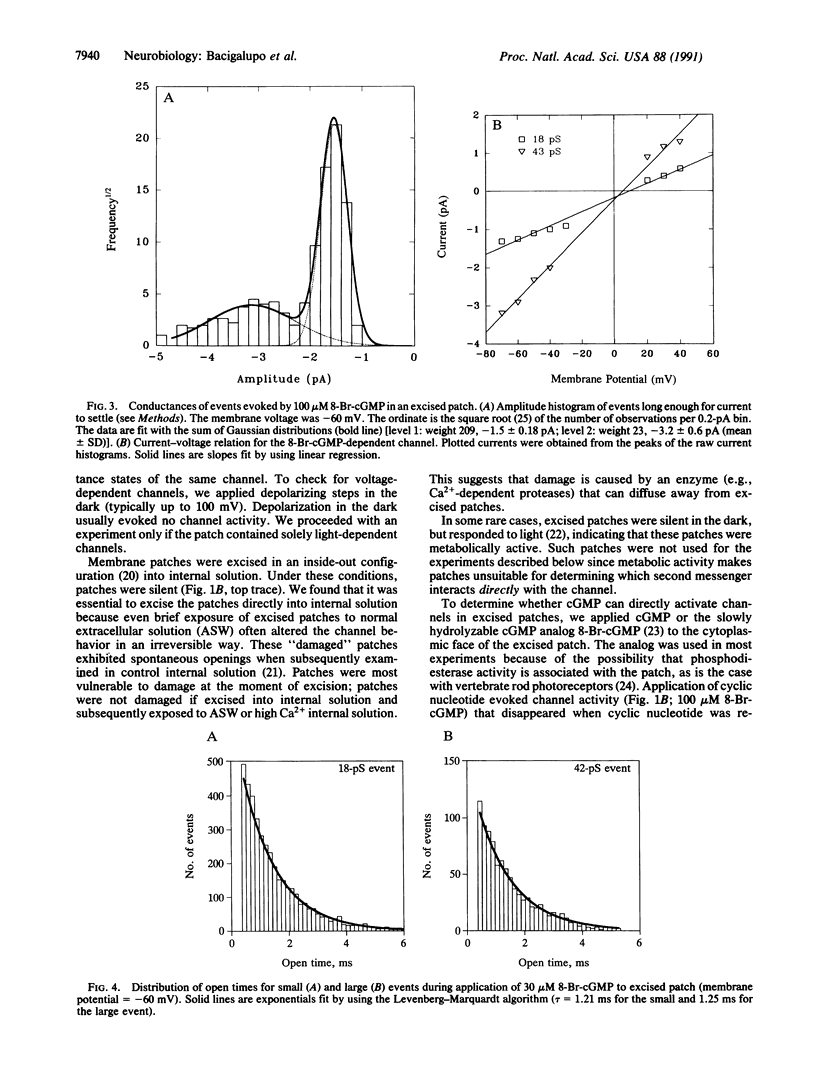
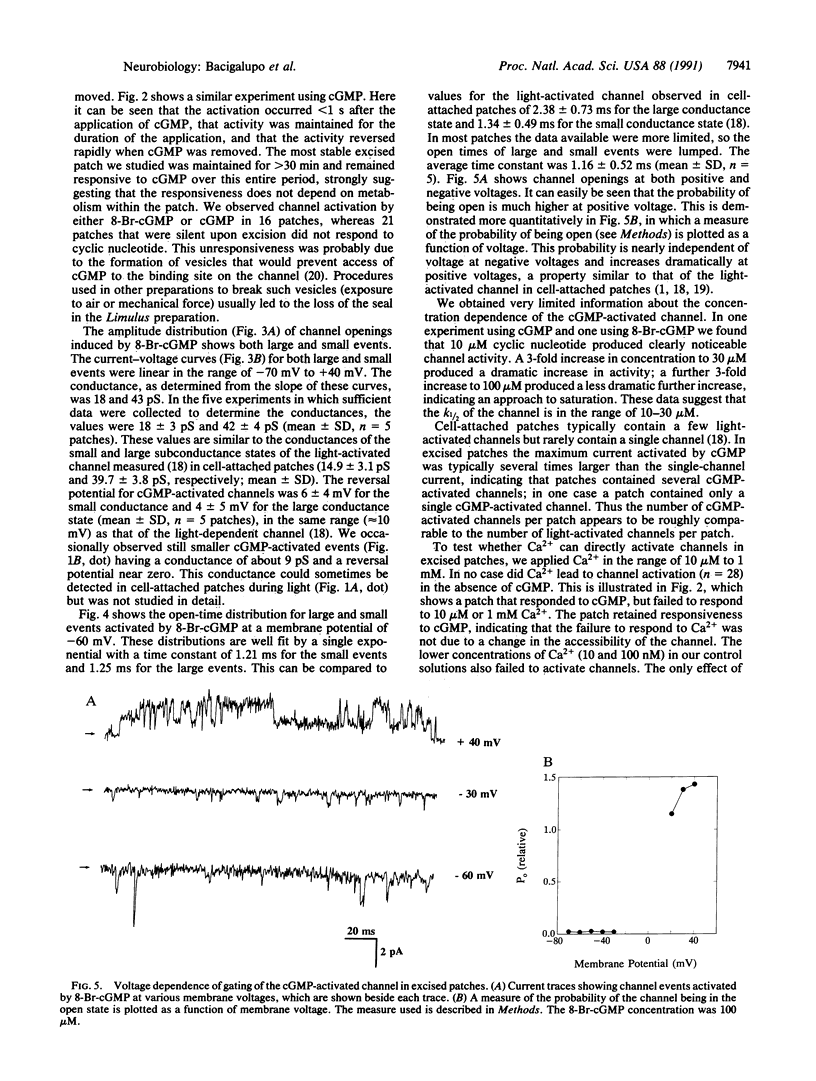
Abstract
The identity of the second messenger that directly activates the light-dependent conductance in invertebrate photoreceptors remains unclear; the available evidence provides some support for cGMP and Ca2+. To resolve this issue we have applied these second messengers to membrane patches excised from the light-sensitive lobe of Limulus ventral photoreceptors. Our results show that these patches contain channels that can be opened by cGMP, but not by Ca2+. These cGMP-activated channels closely resemble the channels activated by light in cell-attached patches. This evidence suggests that cGMP is the messenger that opens the light-dependent channel in invertebrate photoreceptors.
Full text
PDF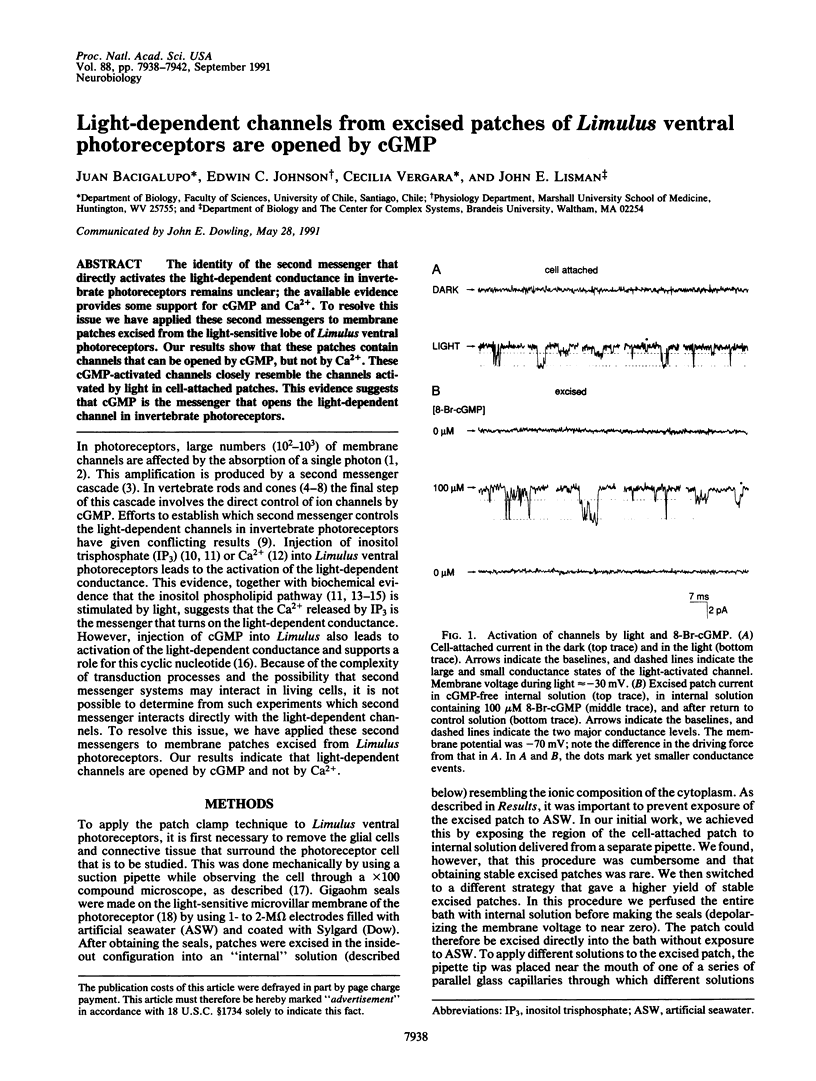

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacigalupo J., Chinn K., Lisman J. E. Ion channels activated by light in Limulus ventral photoreceptors. J Gen Physiol. 1986 Jan;87(1):73–89. doi: 10.1085/jgp.87.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacigalupo J., Lisman J. E. Single-channel currents activated by light in Limulus ventral photoreceptors. Nature. 1983 Jul 21;304(5923):268–270. doi: 10.1038/304268a0. [DOI] [PubMed] [Google Scholar]
- Bloomquist B. T., Shortridge R. D., Schneuwly S., Perdew M., Montell C., Steller H., Rubin G., Pak W. L. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988 Aug 26;54(5):723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Rubin L. J., Ghalayini A. J., Tarver A. P., Irvine R. F., Berridge M. J., Anderson R. E. myo-Inositol polyphosphate may be a messenger for visual excitation in Limulus photoreceptors. Nature. 1984 Sep 13;311(5982):160–163. doi: 10.1038/311160a0. [DOI] [PubMed] [Google Scholar]
- Devary O., Heichal O., Blumenfeld A., Cassel D., Suss E., Barash S., Rubinstein C. T., Minke B., Selinger Z. Coupling of photoexcited rhodopsin to inositol phospholipid hydrolysis in fly photoreceptors. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6939–6943. doi: 10.1073/pnas.84.19.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel E. A. Excised patches of plasma membrane from vertebrate rod outer segments retain a functional phototransduction enzymatic cascade. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4226–4230. doi: 10.1073/pnas.87.11.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein A., Payne R., Corson D. W., Berridge M. J., Irvine R. F. Photoreceptor excitation and adaptation by inositol 1,4,5-trisphosphate. Nature. 1984 Sep 13;311(5982):157–160. doi: 10.1038/311157a0. [DOI] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hanke W., Cook N. J., Kaupp U. B. cGMP-dependent channel protein from photoreceptor membranes: single-channel activity of the purified and reconstituted protein. Proc Natl Acad Sci U S A. 1988 Jan;85(1):94–98. doi: 10.1073/pnas.85.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L. W., Kay A. R., Yau K. W. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986 May 1;321(6065):66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- Haynes L., Yau K. W. Cyclic GMP-sensitive conductance in outer segment membrane of catfish cones. Nature. 1985 Sep 5;317(6032):61–64. doi: 10.1038/317061a0. [DOI] [PubMed] [Google Scholar]
- Johnson E. C., Bacigalupo J., Vergara C., Lisman J. E. Multiple conductance states of the light-activated channel of Limulus ventral photoreceptors. Alteration of conductance state during light. J Gen Physiol. 1991 Jun;97(6):1187–1205. doi: 10.1085/jgp.97.6.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. C., Robinson P. R., Lisman J. E. Cyclic GMP is involved in the excitation of invertebrate photoreceptors. Nature. 1986 Dec 4;324(6096):468–470. doi: 10.1038/324468a0. [DOI] [PubMed] [Google Scholar]
- Karpen J. W., Zimmerman A. L., Stryer L., Baylor D. A. Gating kinetics of the cyclic-GMP-activated channel of retinal rods: flash photolysis and voltage-jump studies. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1287–1291. doi: 10.1073/pnas.85.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G. Single-channel recordings demonstrate that cGMP opens the light-sensitive ion channel of the rod photoreceptor. Proc Natl Acad Sci U S A. 1987 Jan;84(1):299–302. doi: 10.1073/pnas.84.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne R., Corson D. W., Fein A., Berridge M. J. Excitation and adaptation of Limulus ventral photoreceptors by inositol 1,4,5 triphosphate result from a rise in intracellular calcium. J Gen Physiol. 1986 Jul;88(1):127–142. doi: 10.1085/jgp.88.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne R., Corson D. W., Fein A. Pressure injection of calcium both excites and adapts Limulus ventral photoreceptors. J Gen Physiol. 1986 Jul;88(1):107–126. doi: 10.1085/jgp.88.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P. R., Cote R. H. Characterization of guanylate cyclase in squid photoreceptors. Vis Neurosci. 1989 Jul;3(1):1–7. doi: 10.1017/s0952523800012451. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern J., Chinn K., Bacigalupo J., Lisman J. Distinct lobes of Limulus ventral photoreceptors. I. Functional and anatomical properties of lobes revealed by removal of glial cells. J Gen Physiol. 1982 Dec;80(6):825–837. doi: 10.1085/jgp.80.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Vandenberg C. A., Montal M. Light-regulated biochemical events in invertebrate photoreceptors. 2. Light-regulated phosphorylation of rhodopsin and phosphoinositides in squid photoreceptor membranes. Biochemistry. 1984 May 22;23(11):2347–2352. doi: 10.1021/bi00306a004. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986 May 1;321(6065):70–72. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Yamanaka G., Eckstein F., Baylor D. A., Stryer L. Interaction of hydrolysis-resistant analogs of cyclic GMP with the phosphodiesterase and light-sensitive channel of retinal rod outer segments. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8813–8817. doi: 10.1073/pnas.82.24.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]



