Abstract
Botulinum neurotoxins, causative agents of botulism in humans, are produced by Clostridium botulinum, an anaerobic spore-former Gram-positive bacillus. Botulinum neurotoxin poses a major bioweapon threat because of its extreme potency and lethality; its ease of production, transport, and misuse; and the need for prolonged intensive care among affected persons. This paper aims at discussing botulinum neurotoxin, its structure, mechanism of action, pharmacology, its serotypes and the reasons for wide use of type A, the various indications and contraindications of the use of botulinum neurotoxin and finally the precautions taken when botulinum neurotoxin is used as a treatment approach. We have searched relevant articles on this subject in various medical databases including Google Scholar, PubMed Central, ScienceDirect, Wiley Online Library, Scopus, and Copernicus. The search resulted in more than 2669 articles, out of which a total of 187 were reviewed. However, the review has been further constricted into only 54 articles as has been presented in this manuscript keeping in mind the page limitation and the limitation to the number of references. A single gram of crystalline toxin, evenly dispersed and inhaled, can kill more than one million people. The basis of the phenomenal potency of botulinum toxin (BT) is enzymatic; the toxin is a zinc proteinase that cleaves neuronal vesicle-associated proteins responsible for acetylcholine release into the neuromuscular junction. A fascinating aspect of BT research in recent years has been the development of the most potent toxin into a molecule of significant therapeutic utility. It is the first biological toxin which is licensed for the treatment of human diseases. The present review focuses on both warfare potential as well as medical uses of botulinum neurotoxin.
Keywords: Botulinum toxin, botulism poisoning, facelift, Frey's syndrome, neuromuscular junction
Introduction
The neurotoxin called botulinum toxin (BT) is produced by the bacterium Clostridium botulinum. This toxin is capable of causing muscle paralysis by blocking the release of Ach at the neuromuscular (NM) junction of striated muscle. Among the several types of BT, subtype A (Botox, Allergan, Inc., Irvine, CA, USA) is the most potent toxin produced by the bacterium.[1] The basis of botulinum neurotoxin therapy is the localized inhibition of exocytosis from selected neurons after the toxin has been injected into specific target areas such as muscles. The local route of administration along with the specific features of the neurotoxin formulation results in such properties.[2] The BT Type A (BT-A) is a purified protein which causes partial paralysis when injected into a muscle, thereby reducing spasticity and muscle stiffness.
Structural Aspects of Botulinum Toxin
The seven different serotypes of BT known today are A, B, C1, D, E, F, and G. Till now only serotype A was available for commercial use in the forms of Botox (Allergan, Inc., Irvine, CA, USA) and Dysport (Ipsen Ltd., Slough, Berkshire).[3]
BT is synthesized as a single chain (150 kd) and subsequently cleaved into a di-chain molecule with a disulfide bridge. The light chain acts as a zinc endopeptidase with proteolytic activity located at the N-terminal end [Figure 1]. The heavy chain provides cholinergic specificity and binding of the toxin to presynaptic receptors. This promotes translocation of the toxin across the endosomal membrane. Despite having different antigenic properties, the serotypes of BT are found to exhibit similar structure within themselves.
Figure 1.
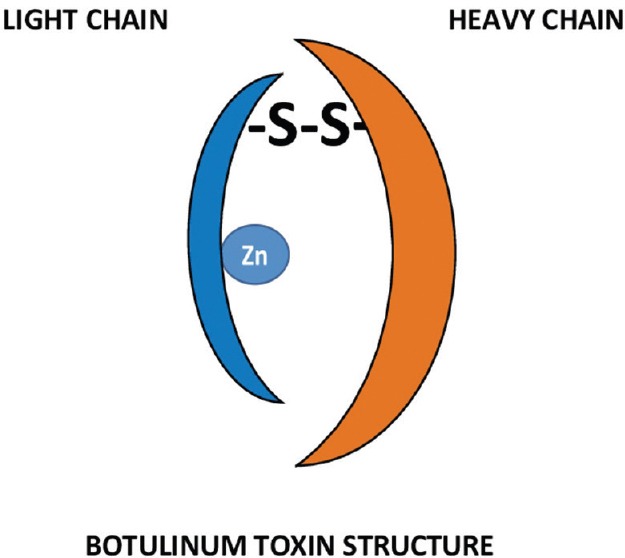
Structure of botulinum toxin
A single-chain inactive 150 kDa protein is formed during BT synthesis which is found to be bound to the nontoxic proteins as a complex. This prevents BT from damage when ingested in oral botulism poisoning.[4]
Mechanism of Action of Botulinum Toxin
Botulinum neurotoxin prevents the release of Ach at the presynaptic NM junction thereby inducing flaccid paralysis. This toxin-mediated paralysis includes three principal steps: binding, internalization, and inhibition of the release of neurotransmitter.[5] BT acts locally in the peripheral nervous system by blocking the release of Ach in the presynaptic NM junction. In contrast, the tetanus toxin migrates retrograde into the central nervous system from the same presynaptic NM junction to affect the interneurons in the spinal cord from releasing glycine and g-amino-butyric acid. At the presynaptic terminal, the cleavage of synaptosome-associated protein (SNAP-25) takes place by serotypes A, C, and E, but only serotype C primarily affects the protein syntaxin also. The other BT serotypes B, D, F, and G affects the cleavage of vesicle-associated membrane protein. BT results in reversible denervation atrophy at the NM junction after two important phases: (1) An initial phase in which sprouting of non-collateral axons occur. (2) Development of a new functional NM junction at the incipient nerve terminals, thus replacing the original motor endplate. It takes about 3 months for the new terminals to regress and the parent terminal to reestablish its functional core.[3]
Pharmacology of Botulinum Neurotoxins
The two different marketed brands for BT-A, namely Botox and Dysport have remarkably different potencies. One unit of Botox is considered to be equivalent to approximately 3–4 units of Dysport[6] though there remains a controversy for the precise conversion. Electromyographic data measurement has been done in human patients to evaluate the duration of Botox and Myobloc on opposite extensor digitorum muscles.[7] The BT-A has been in therapeutic use since the late 1970s[8] and has been recognized for its remarkable safety profile. The mainstay of therapy is the injectable form and it is also significantly highly safer than the oral pharmacologic version of the toxin.[9] It is evident that only a localized effect is produced by the injectable form even in clinical conditions requiring larger injection amounts (e.g., for cervical dystonia).[10] Stability of the toxin is a prominent concern for clinical use. During the manufacturing process of BT-A, lyophilization or vacuum drying is important for direct impact on the activity of the toxin and denaturation of proteins. This leads to formation of antibody without any beneficial clinical efficiency. The reconstitution of BT-A with saline makes the pH of the solution 7.3. At this neutral pH, there is rapid loss of its clinical efficacy within 12 h despite refrigeration. In contrast, BT-B complex undergoes extreme adverse conditions during its production but still manages to remain intact and highly purified.[11]
Indications of Botulinum Toxin
Facial rejuvenation
Forehead rhytids
Transverse rhytids in the forehead region are treated by directly injecting BT in the frontalis muscles. Successful rejuvenation of the rhytids requires relaxation of the frontalis muscles but usually associated with subsequent brow ptosis through the unopposed depressor activity of the procerus, orbicularis oculi, and corrugator muscles.
Glabellar rhytids
The injection of Botox in procerus and corrugators muscles respectively successfully resulted in the relaxation of transverse and vertical rhytids lying between the brows in the glabellar regions. This method helps to minimize the possible diffusion through the orbital septum that causes eyelid ptosis, thus achieving maximum effect. Since procerus and corrugators muscles act as brow depressors, a treatment of the entire glabella appropriately with Botox chemodenervation can achieve a slight medial brow elevation.
Crow's feet
It is generally the action of underlying orbicularis oculi muscle that results into lateral orbital rhytids, or crow's feet. The repeated squinting or blinking cause excessive muscular activity, thus leads to pleating of the overlying skin in a radial fashion from the lateral canthus and orbital rim.[12]
Marionette lines
In the perioral region, the vertical lines found at the oral commissures are called the classic “marionette lines,” or melomental rhytids that give the impression of sadness or disapproval.
Vertical lip rhytids
It is the persistent, repetitive contraction of the orbicularis oris muscle that leads to the development of vertical rhytids of the upper and lower lip. These wrinkles are named as smoker's lines as these are more frequently observed in individuals who smoke. An overaggressive treatment usually results in lip asymmetry and possible speech or drinking impairment. A better treatment for these rhytids is the skin resurfacing technique, such as with chemical peels or a CO2 laser treatment.
Mentalis crease
A long-term hyperfunctioning mentalis muscle develops a deep transverse crease in the mentum. The origin of mentalis muscle lies in the mentum inferior to the incisor fossa and extends vertically medially to fuse with the orbicularis oris.[13]
Neck bands
During aging, the platysma separates in the midline anteriorly causing the overlying skin loses its elasticity and forming vertical bands in the neck. These diverging bands are apparent in repose but become more noticeable during animated conservation.
Botox and brow lift
The Botox-assisted brow lift has been shown to provide stable, long-lasting, esthetic results with minimal morbidity.[14] Brow lifts may have an unpredictable cosmetic outcome based on postsurgical healing. It is important to stabilize brow musculature for a predictable final brow position.
Botox and laser resurfacing
In general, lasers address static wrinkles and cause stimulation of new collagen, whereas Botox cause prevention of the recurrence of dynamic wrinkles.[15,16,17] Better results can be produced by giving Botox injection 2–3 weeks before laser resurfacing.[16]
Botox and peels
Botox should be done 2–3 days before chemical peels, otherwise if done immediately before or after peels it can cause soft tissue swelling and increased migration of the toxin.[18]
Botox and fillers
Botox may decrease the amount of fillers required for lines. Botox treatment 2–4 weeks prior to fillers softens the muscle contractions and gives a more accurate idea of how much filler is required.[18]
Hyperhidrosis
It is most common to have hyperhidrosis in the axilla, palms, or soles. For all these sites, Botox has effectively decreased sweating.[19,20,21,22,23,24]
Management of Frey's syndrome and hypersialorrhea
Recent research interest has led to the use of BT-A to block another important autonomic dysfunction. It has been shown recently that intraglandular injection of BT-A reduces salivation in neurologically-impaired patients who have hypersialorrhea.[25,26]
Treatment of primary axillary hyperhidrosis
The use of BT-A in the treatment of primary axillary hyperhidrosis is relatively recent. Similar levels of efficacy and safety are provided by both Botox and Dysport in the treatment of primary axillary hyperhidrosis in a condition in which when a conversion factor of 1:3 is used.[27]
Neuromuscular correction of gummy smile
The temporary improvement of gummy smiles caused due to hyperfunctional upper lip elevator muscles by injecting BT-A at preselected sites is a novel, cosmetically effective, minimally invasive alternative.[28,29]
Treatment of hemimasticatory spasm
Hemimasticatory spasm is a rare movement disorder caused due to trigeminal motor nerve dysfunction of unknown origin. Most often it is misdiagnosed as hemifacial spasm caused due to dysfunction of the facial nerve. A case of hemimasticatory spasm has been reported to be associated with localized scleroderma and facial hemiatrophy; and excellent results have been obtained with the use of BT-A injections.[30]
Control of pain of trigeminal neuralgia
Neurological improvement seen who have received BT-A injections in the trigger zone of trigeminal neuralgia.[31]
Treatment for reduction of plantar flexors spasticity after stroke
BT-A is a potent neurotoxin that blocks local synaptic transmission at cholinergic terminals. It has become a useful therapeutic alternative for spasticity management as it is safe and also effective option in the treatment of poststroke spasticity. Its principal advantage is that it has a focal, selective, and reversible effect in the injected muscles without having much adverse effects.[32] BT is significantly associated with reduced spasticity, increased range of motion, and improved upper extremity function. The use of electrical stimulation in combination with BT injection improves upper extremity function. The Reduction of shoulder pain and improvement of passive range of motion is more with denervation of the subscapularis muscle using BT than denervation of the pectoralis major muscle. Denervation of lower extremity muscles by injecting BT reduces spasticity but does not improve function. A combination of BT with electrical stimulation may improve spasticity and function.[33]
Treatment of cerebral palsy in children and adolescents
Reasons for considering botulinum toxin Type A as a treatment approach
The following reasons justify the use of BT-A as treatment approach in children and adolescents suffering from cerebral palsy:[34]
Reduces spasticity to free up movement in the arm and hand
Prevents shortening of muscles
Improves the appearance of the hand and arm
Reduces pain or discomfort
Improves the use of arm and hand
Reduces the need to wear splints
The outcome of possible surgery can be predicted
The need for surgery can be delayed.
Steps to be taken before treatment with botulinum toxin Type A
The child is usually admitted as a day patient at hospital
Injections are given under a general anesthesia for easy and accurate injection of BT into the relevant muscles
A muscle stimulator is used to make sure that BT-A is injected into the correct muscle as most of the muscles in the forearm and hand are very small and lie adjacent to each other.
Steps to be taken after treatment with botulinum toxin Type A
Usually, the effect of the injection is within 7–10 days, some reported within 24–36 h
The occupational therapist provides an intervention or group program and keeps in touch with the child and family to monitor progress. A home activity program is also often provided. Evidence support that BT-A injections in combination with a therapy and home program help the clients to make greater gains in movement and function
The occupational therapist reviews the child approximately 3 months following BT-A injection to determine the outcomes of the intervention.
Treatment of depression
Major depression is a very serious and common condition that may be resistant to routine pharmacologic and psychotherapeutic treatment approaches. BT-A is used to treat glabellar frown lines in treating patients with major depression.[35]
Treatment of masseteric hypertrophy
The diagnosis of head and neck masses reveal a rare condition called masseter muscle hypertrophy in the cheek with unknown cause. Various treatment options ranging from simple pharmacotherapy to more invasive surgical reduction have been reported. BT-A, a powerful neurotoxin produced by the anaerobic organism C. botulinum causes interference with the neurotransmitter mechanism when injected into a muscle and produces selective paralysis, subsequently causing muscular atrophy. Injection of BT-A into the masseter muscle is considerably a less invasive modality and often used for cosmetic sculpting of the lower face. BT-A injection is considered as a beneficial treatment modality in patients with masseter muscle hypertrophy.[36]
Use in migraine surgery
The patients who claimed to have migraine headaches were examined by the neurologists' team to confirm its diagnosis. After answering an extensive questionnaire, Botox (25 U) was injected in the corrugators supercilii muscle of those patients. The treatment procedure using Botox and surgery in the elimination of migraine headaches is colossal, elimination of migraine headaches following forehead rejuvenation.[37,38]
Use in esthetic oculoplastic surgery
The facial augmentation and rejuvenation in the periocular region require medical and surgical management. Laser resurfacing such as fractional lasers, use of dermal fillers, dermabrasion, chemical peeling, and BT injections are considered as medical measures. Tissue augmentation also involves increased use of hyaluronic acid injections. Commonly practiced surgical procedures are mid face lift, browplasty, and blepharoplasty. It is safe and effective to use BT-A (Botox; Allergan, Inc.; Irvine, CA, USA) as a muscle paralytic agent for both short- and long-term durations. BT can be injected by esthetic oculoplastic clinicians either in only one area or many areas to provide maximum eyelid elevation. It should be noted that the use of BT for any area other than the glabellar frown line is off label.[1]
Ocular uses
Strabismus
When used for the treatment of strabismus, it is hypothesized that BT-A causes lengthening of the muscle in which it is injected and a corresponding shortening of the antagonist muscle.
Entropion
BT has been used safely for the treatment of spastic and congenital entropion[39] by injecting in the pretarsal portion of the orbicularis oculi muscle (5 U). Reports also suggest its use in the treatment of senile entropion[40] though it is temporary and surgery remains the only choice for permanent treatment.
Treatment of dysphagia
Many investigators have used BT to chemically denervate the muscle to allow for relaxation and passage of the food bolus. It is easy to identify the cricopharyngeus muscle lateral and posterior to the cricoid cartilage using electromyography. There is constant firing of this muscle at rest which abates at the onset of a voluntary swallow. This allows for injection even in office or in the operating room under direct laryngoscopy. Response to chemodenervation with BT can predictably be a successful surgical outcome for patients not wanting repeated injections for recurrent dysphagia.[41]
Treatment of puberophonia
Adolescent boys and men suffering from puperophonia or mutational dysphonia can also be treated by BT injection into the cricothyroid muscles. Usually puperophonia patients speak with the higher fundamental frequency of their prepubescent years. As this disorder is primarily mediated by inappropriate psychological controls and behavior, hence the primary treatment involves a combination of speech and behavioral therapy.[42]
Treatment of bilateral vocal fold paralysis
Patients suffering from bilateral abductor vocal fold paralysis/paresis are injected with BT into the thyroarytenoid and interarytenoid muscles adductor muscles of the larynx are actually weakened by those muscles. BT injection increases the patency of airway at rest and with activity. Less dyspnea was reported by such patients after BT injections.
Thus, BT injection effectively caused the rebalancing of the position of paralyzed vocal folds to a more abducted position by weakening of the adductor muscles.[43]
Treatment of parotid fistula after face lifting
BT-A injection causes inhibition of Ach release from the preganglionic sympathetic and cholinergic autonomic parasympathetic neurons at the secretary end of the salivary gland, in addition to inhibition of NM junction. The use of BT in the treatment of sialorrhea was first reported in 1997. The injuries to parotid duct or gland with parotid fistula are not very common are the result due to traumatic surgical conditions. Such patients reported constant leakage of serous fluid and swelling cheek after facelift surgery. Those patients had to undergo an amylase test, starch-iodine test, and sialography. About 50 units of BT was injected into the parotid gland of the patients after diagnosis of parotid fistula and were advised for facial bandage, scopolamine, and instructions were given to them to reduce the movement of temporomandibular joint. Within 2 weeks, there was a decrease in leakage volume, and symptoms subsided. There were no functional problems or other complications. Thus, it can be concluded that BT provides an effective treatment for a parotid fistula after facial surgery and should be preferred considered before invasive surgical treatment.[44]
Treatment of persistent parotid sialocele drooling, salivary fistulas, and sialadenitis
BT-A acts by blocking Ach release, thereby inhibiting transmission of a nerve impulse at the secretomotor parasympathetic autonomic NM junction responsible for the secretion of saliva. The treatment of parotid sialocele is with BT-A is considered to be effective when conventional therapy fails.[45] BT dependably reduces salivary secretion. In general, the effect lasts for 3–4 months. During extended follow-up intervals, side effects were not observed. Thus, local injection of BT-A into salivary glands is a dependable and side-effect-free therapeutic option in patients with drooling, salivary fistulas, and chronic sialadenitis.[46]
Use on cleft lip revision surgery scar
When BT-A (Botox) is injected immediately after suturing, it causes local paralysis of the musculature which lies below the surgical wound. This helps in reducing the repetitive tensile forces on the skin wound edges. This results in a decreased fibroblastic response and subsequent hypertrophic scar formation.[47]
Treatment of disorders of autonomic nervous system
BT-A has become an important method of treatment of neurological disorders and other disorders associated with Ach such as excessive muscular contraction and glandular hypersecretion. It is one of the most potent neurotoxin which has been effective in many fields for the past 20 years. Thus, autonomic nervous system disorders such as Frey's syndrome, sialorrhea, hyperhidrosis, and hyperlacrimation involving excessive activity can be treated by BT injections in cholinergic autonomic parasympathetic and postganglionic nerve synapses.[48]
Contraindications of Botulinum Toxin
The following conditions should be avoided for treatments with BT:[49]
Patients who have known NM disorders or sensitivity to the ingredients in the formulation
During pregnancy or nursing
Drugs such as calcium channel blockers, quinine, or aminoglycoside antibiotics increase the potency of BT; patients already taking these medications should be treated cautiously
Patients having previous surgical procedures at the same site as BT injection site which was repositioned or weakened the muscle of interest should be considered as suboptimal candidates
Allergic to BT and its components (i.e., BT, human albumin, saline, lactose, and sodium succinate)
Dependent on intact and active movements of facial muscles and expressions on the faces for their living (e.g., musicians, singers, actors, and other media personalities).
General Precautions
When treatment with BT-A is selected as a therapeutic approach following precautions should be taken into consideration:[50]
The use of aminoglycoside antibiotics or drugs interfering with NM transmission (e.g., tubocurarine-type muscle relaxants) can usually potentiate the effect of BT. Therefore, a detailed drug history should be elicited before injection of the toxin
Precautions should be taken when BT-A is utilized for the treatment of disorders such as myasthenia gravis, Eaton-Lambert Syndrome, and others which produce a depletion of Ach
There is the possibility of anaphylactic reaction as occurs with all biologic products. Necessary precautions should be taken and epinephrine should be available
No data is available on the use of BT-A in pregnant women or of its excretion in human milk. Therefore, one should be cautious about the use of BT in pregnant and lactating women
It is not established to be safe for use in children under 12 years of age
Patients with BT administration should resume activity gradually.
Conclusion
Scientific research into the established serotype of BT-A as well as others has continued to provide important clinical information about the proper use of this neurotoxin. Nowadays, while BT-A remains the mainstay for clinical therapy, BT Type B has gained importance in the treatment of patients having acquired antibody resistance to BT-A. However, further research is required to define morphological alterations in the BT-injected injected tissues, its duration of action, the formation of neutralizing antibody, and the development of possible long-term effects. All the authors of this manuscript hope that this article has provided interesting background information for the clinician and an informative literature for understanding the clinical role of BT to improve delivery of patient care.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Stupak HD, Maas CS. New procedures in facial plastic surgery using botulinum toxin A. Facial Plast Surg Clin North Am. 2003;11:515–20. doi: 10.1016/S1064-7406(03)00092-0. [DOI] [PubMed] [Google Scholar]
- 2.Aoki KR. Pharmacology of botulinum neurotoxins. Operative techniques in otolaryngology. Head Neck Surg. 2004;15:81–5. [Google Scholar]
- 3.Lam SM. The basic science of botulinum toxin. Facial Plast Surg Clin North Am. 2003;11:431–8. doi: 10.1016/S1064-7406(03)00073-7. [DOI] [PubMed] [Google Scholar]
- 4.Hanson MA, Stevens RC. In: Structural view of botulinum neurotoxin in numerous functional states. Scientific and Therapeutic Aspects of Botulinum Toxin. Brin MF, Hallet M, Jankovic J, editors. Philadelphia: Lippincott Williams and Wilkins; 2002. pp. 11–28. [Google Scholar]
- 5.Brin MF. In: Botulinum toxin therapy: Basic science and overview of other therapeutic applications. Management of Facial Lines and Wrinkles. Blitzer A, Binder WJ, Boyd JB, Carruthers A, editors. Philadelphia: Lippincott Williams and Wilkins; 2000. pp. 279–302. [Google Scholar]
- 6.Brin MF, Blitzer A. Botulinum toxin: Dangerous terminology errors. J R Soc Med. 1993;86:493–4. [PMC free article] [PubMed] [Google Scholar]
- 7.Sloop RR, Cole BA, Escutin RO. Human response to botulinum toxin injection: Type B compared with type A. Neurology. 1997;49:189–94. doi: 10.1212/wnl.49.1.189. [DOI] [PubMed] [Google Scholar]
- 8.Schantz EJ, Johnson EA. In: Preparation and characterization of botulinum toxin type A for human treatment. Therapy with Botulinum Toxin. Jankovic J, Hallett M, editors. Vol. 109. New York: Marcel Dekker; 1994. pp. 10–24. [Google Scholar]
- 9.Brans JW, Lindeboom R, Snoek JW, Zwarts MJ, van Weerden TW, Brunt ER, et al. Botulinum toxin versus trihexyphenidyl in cervical dystonia: A prospective, randomized, double-blind controlled trial. Neurology. 1996;46:1066–72. doi: 10.1212/wnl.46.4.1066. [DOI] [PubMed] [Google Scholar]
- 10.Tsui JK, Eisen A, Stoessl AJ, Calne S, Calne DB. Double-blind study of botulinum toxin in spasmodic torticollis. Lancet. 1986;2:245–7. doi: 10.1016/s0140-6736(86)92070-2. [DOI] [PubMed] [Google Scholar]
- 11.Callaway JE, Grethlein AJ. In: Production, quality and stability of botulinum toxin type B (Myobloc) for clinical use. Scientific and Therapeutic Aspects of Botulinum Toxin. Brin MF, Jankovic J, Hallett M, editors. Philadelphia: Lippincott Williams and Wilkins; 2002. pp. 115–22. [Google Scholar]
- 12.Blitzer A, Binder WJ. Current practices in the use of botulinum toxin A in the management of facial lines and wrinkles. Facial Plast Surg Clin North Am. 2001;9:395–404. [PubMed] [Google Scholar]
- 13.Janfaza P, Cheney ML. In: Superficial structures of the face, head, and parotid region. Surgical Anatomy of the Head and Neck. Janfaza P, Nadol JB, Galla RJ, Fabian RL, Montgomery WW, editors. Philadelphia: Lippincott Williams and Wilkins; 2000. pp. 1–48. [Google Scholar]
- 14.Dyer WK, Yung RT. Botulinum toxin-assisted brow lift. Facial Plast Surg. 2000;8:343–54. [Google Scholar]
- 15.Edelstein C, Shorr N, Jacobs J, Balch K, Goldberg R. Oculoplastic experience with the cosmetic use of botulinum A exotoxin. Dermatol Surg. 1998;24:1208–12. doi: 10.1111/j.1524-4725.1998.tb04099.x. [DOI] [PubMed] [Google Scholar]
- 16.Carruthers J, Carruthers A. The adjunctive usage of botulinum toxin. Dermatol Surg. 1998;24:1244–7. doi: 10.1111/j.1524-4725.1998.tb04105.x. [DOI] [PubMed] [Google Scholar]
- 17.Fulton JE. Botulinum toxin. The Newport Beach experience. Dermatol Surg. 1998;24:1219–24. [PubMed] [Google Scholar]
- 18.Werschler P, Baumann L. Everything you need to know about Botox injection. Skin Aging. 2001;9:36–42. [Google Scholar]
- 19.Glogau RG. Botulinum A neurotoxin for axillary hyperhidrosis. No sweat Botox. Dermatol Surg. 1998;24:817–9. doi: 10.1111/j.1524-4725.1998.tb04257.x. [DOI] [PubMed] [Google Scholar]
- 20.Naumann M, Hofmann U, Bergmann I, Hamm H, Toyka KV, Reiners K. Focal hyperhidrosis: Effective treatment with intracutaneous botulinum toxin. Arch Dermatol. 1998;134:301–4. doi: 10.1001/archderm.134.3.301. [DOI] [PubMed] [Google Scholar]
- 21.Odderson IR. Axillary hyperhidrosis: Treatment with botulinum toxin A. Arch Phys Med Rehabil. 1998;79:350–2. doi: 10.1016/s0003-9993(98)90020-x. [DOI] [PubMed] [Google Scholar]
- 22.Odderson IR. Hyperhidrosis treated by botulinum A exotoxin. Dermatol Surg. 1998;24:1237–41. doi: 10.1111/j.1524-4725.1998.tb04104.x. [DOI] [PubMed] [Google Scholar]
- 23.Schnider P, Moraru E, Kittler H, Voller B, Auff E. High-dose botulinum toxin type A for axillary hyperhidrosis. Arch Dermatol. 2000;136:1567. doi: 10.1001/archderm.136.12.1567. [DOI] [PubMed] [Google Scholar]
- 24.Shelley WB, Talanin NY, Shelley ED. Botulinum toxin therapy for palmar hyperhidrosis. J Am Acad Dermatol. 1998;38(2 Pt 1):227–9. doi: 10.1016/s0190-9622(98)70242-7. [DOI] [PubMed] [Google Scholar]
- 25.Jost WH. Treatment of drooling in Parkinson's disease with botulinum toxin. Mov Disord. 1999;14:1057. doi: 10.1002/1531-8257(199911)14:6<1057::aid-mds1033>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Porta M, Gamba M, Bertacchi G, Vaj P. Treatment of sialorrhoea with ultrasound guided botulinum toxin type A injection in patients with neurological disorders. J Neurol Neurosurg Psychiatry. 2001;70:538–40. doi: 10.1136/jnnp.70.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talarico-Filho S, Mendonça DO Nascimento M, Sperandeo DE Macedo F, DE Sanctis Pecora C. A double-blind, randomized, comparative study of two type A botulinum toxins in the treatment of primary axillary hyperhidrosis. Dermatol Surg. 2007;33:S44–50. doi: 10.1111/j.1524-4725.2006.32331.x. [DOI] [PubMed] [Google Scholar]
- 28.Patel DP, Thakkar SA, Suthar JR. Botulinum toxin: An aid for the neuromuscular correction of gummy smile – Review. Indian J Basic Appl Med Res. 2013;2:764–72. [Google Scholar]
- 29.Rubin LR, Mishriki Y, Lee G. Anatomy of the nasolabial fold: The keystone of the smiling mechanism. Plast Reconstr Surg. 1989;83:1–10. doi: 10.1097/00006534-198901000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Kim HJ, Jeon BS, Lee KW. Hemimasticatory spasm associated with localized scleroderma and facial hemiatrophy. Arch Neurol. 2000;57:576–80. doi: 10.1001/archneur.57.4.576. [DOI] [PubMed] [Google Scholar]
- 31.Ngeow WC, Nair R. Injection of botulinum toxin type A (BOTOX) into trigger zone of trigeminal neuralgia as a means to control pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e47–50. doi: 10.1016/j.tripleo.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 32.Baricich A, Carda S, Bertoni M, Maderna L, Cisari C. A single-blinded, randomized pilot study of botulinum toxin type A combined with non-pharmacological treatment for spastic foot. J Rehabil Med. 2008;40:870–2. doi: 10.2340/16501977-0251. [DOI] [PubMed] [Google Scholar]
- 33.Kanovský P, Slawek J, Denes Z, Platz T, Comes G, Grafe S, et al. Efficacy and safety of treatment with incobotulinum toxin A (botulinum neurotoxin type A free from complexing proteins; NT 201) in post-stroke upper limb spasticity. J Rehabil Med. 2011;43:486–92. doi: 10.2340/16501977-0796. [DOI] [PubMed] [Google Scholar]
- 34.Galvin J, Sakzewski L. Botulinum toxin A in conjunction with occupational therapy reduces spasticity and improves upper limb function and goal attainment in children with cerebral palsy. Aust Occup Ther J. 2011;58:132–3. doi: 10.1111/j.1440-1630.2011.00925.x. [DOI] [PubMed] [Google Scholar]
- 35.Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: A case series. Dermatol Surg. 2006;32:645–9. doi: 10.1111/j.1524-4725.2006.32136.x. [DOI] [PubMed] [Google Scholar]
- 36.Bas B, Ozan B, Muglali M, Celebi N. Med Oral Patol Oral Cir Bucal. 2010. Treatment of masseteric hypertrophy with botulinum toxin: A report of two cases; pp. e649–52. [PubMed] [Google Scholar]
- 37.Guyuron B, Varghai A, Michelow BJ, Thomas T, Davis J. Corrugator supercilii muscle resection and migraine headaches. Plast Reconstr Surg. 2000;106:429–34. doi: 10.1097/00006534-200008000-00030. [DOI] [PubMed] [Google Scholar]
- 38.Binder WJ, Brin MF, Blitzer A, Schenrock L, Diamond B. Botulinum toxin type A (BTX-A) for migraine: An open label assessment. Mov Disord. 1998;13(Suppl 2):241. [Google Scholar]
- 39.Christiansen G, Mohney BG, Baratz KH, Bradley EA. Botulinum toxin for the treatment of congenital entropion. Am J Ophthalmol. 2004;138:153–5. doi: 10.1016/j.ajo.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 40.Clarke JR, Spalton DJ. Treatment of senile entropion with botulinum toxin. Br J Ophthalmol. 1988;72:361–2. doi: 10.1136/bjo.72.5.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahsan SF, Meleca RJ, Dworkin JP. Botulinum toxin injection of the cricopharyngeus muscle for the treatment of dysphagia. Otolaryngol Head Neck Surg. 2000;122:691–5. doi: 10.1016/S0194-5998(00)70198-7. [DOI] [PubMed] [Google Scholar]
- 42.Woodson GE, Murry T. Botulinum toxin in the treatment of recalcitrant mutational dysphonia. J Voice. 1994;8:347–51. doi: 10.1016/s0892-1997(05)80283-8. [DOI] [PubMed] [Google Scholar]
- 43.Ptok M, Schönweiler R. Botulinum toxin type A-induced rebalancing in bilateral vocal cord paralysis? HNO. 2001;49:548–52. doi: 10.1007/s001060170080. [DOI] [PubMed] [Google Scholar]
- 44.Jung MS, Lee BH, Kim JH, Park SH, Ahn DK, Jeong HS, et al. Treatment of botulinum toxin type A in parotid fistula after face lifting. Arch Aesthetic Plast Surg. 2014;20:120–3. [Google Scholar]
- 45.Chow TL, Kwok SP. Use of botulinum toxin type A in a case of persistent parotid sialocele. Hong Kong Med J. 2003;9:293–4. [PubMed] [Google Scholar]
- 46.Ellies M, Gottstein U, Rohrbach-Volland S, Arglebe C, Laskawi R. Reduction of salivary flow with botulinum toxin: Extended report on 33 patients with drooling, salivary fistulas, and sialadenitis. Laryngoscope. 2004;114:1856–60. doi: 10.1097/00005537-200410000-00033. [DOI] [PubMed] [Google Scholar]
- 47.Galárraga IM. Use of botulinum toxin in cheiloplasty: A new method to decrease tension. Can J Plast Surg. 2009;17:e1–2. doi: 10.1177/229255030901700313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arad A, Blitzer A. Botulinum toxin in the treatment of Autonomic nervous system disorders. Operative techniques in otolaryngology. Head Neck Surg. 2004;15:118–21. [Google Scholar]
- 49.Cather JC, Cather JC, Menter A. Update on botulinum toxin for facial aesthetics. Dermatol Clin. 2002;20:749–61. doi: 10.1016/s0733-8635(02)00043-8. [DOI] [PubMed] [Google Scholar]
- 50.Malhotra C, Waris A, Nagpal RC. Ocular uses of botulinum toxin: An overview. J Med Educ Res. 2008;10:55–7. [Google Scholar]


