Abstract
Aim:
The purpose of this study was to compare the efficacy of platelet-rich plasma (PRP), platelet-rich fibrin (PRF), and hydroxyapatite (HA) for reduction of pain and swelling, absence of dry socket, soft tissue healing, and bone regeneration after mandibular third molar extraction in human patients.
Materials and Methods:
Forty patients requiring extraction of mandibular third molars were randomly grouped as control, PRP, PRF, and HA-treated. The patients were assessed for postoperative pain, swelling, dry socket, and soft tissue healing on the 3rd, 7th, and 14th day of postoperative periods depending on the standard methods. Radiological assessment of the extraction site was done at 1, 2, and 6 months interval to compare the change in bone density in the sockets in control and treated patients.
Results:
Pain and swelling were less on PRP and PRF site when compared to HA and control site. PRP and PRF site showed better soft tissue healing when compared to HA and control site. Radiographic assessment showed comparatively lesser bone density values in PRP, PRF, and control site at 1, 2, and 6 months than HA site.
Conclusion:
Our study showed that PRP and PRF are better graft materials than HA regarding pain, swelling, dry socket, and soft tissue healing. Bone regeneration is induced promptly by HA as compared to other graft materials. However, a more elaborate study with a larger number of clinical cases is very much essential to be more conclusive regarding the efficacy of the graft materials.
Keywords: Bone density, hydroxyapatite, mandibular third molar extraction, pain, platelet-rich fibrin, platelet-rich plasma, swelling
Introduction
Abundant graft materials are used nowadays for the purpose of reducing the signs and symptoms of postoperative phase after surgical extraction and promote soft tissue healing and bone regeneration. Among the several materials used to repair bone defects, autologous graft is still considered as the gold standard.[1,2,3] However, the use of platelet-rich plasma (PRP) and protein-rich fibrin (PRF) as bone graft material has increased in recent years. The beneficial growth factors from platelets were first described by Ross et al.[4] Platelets trapped within fibrin matrix release growth factors after activation that stimulates the mitogenic response in the periosteum for bone repair.[5] PRP contain important growth promoting factors such as platelet-derived growth factors (PDGF), transforming growth factors-β, vascular endothelial growth factor, and epithelial growth factor that are responsible for increased cell mitosis, collagen production, and recruitment of other cells to the site of injury, thereby initiating vascular in-growth and cell differentiation. PRP also contains cell adhesive such as fibrin, fibronectin, and vitronectin responsible for osteoconduction and used as a matrix for bone, connective tissue, and epithelial migration.[6] Choukroun's PRF is a fibrin matrix in which platelet cytokines and cells are trapped and can serve as a resorbable membrane when released after a certain time.[7] More recently, PRF is shown to be a suitable scaffold for breeding human periosteal cells in vitro, thus also being beneficial for bone tissue engineering applications.[5,8]
At present, several biocompatible materials have emerged as substitutes of autologous bone. Among synthetic biomaterials artificial or synthetic hydroxyapatite (HA), the bioglass and bioceramics are also widely accepted. The unique chemical similarity of HA with the mineralized phase of bone makes it osteoconductive and biocompatible.[9] It is widely used in dental, craniofacial, and orthopedic surgery.[10,11,12]
This clinical study was undertaken to compare the efficacy of PRP, PRF, and HA for reduction of pain and swelling, absence of dry socket, soft tissue healing, and bone regeneration after mandibular third molar extraction, to compare whether any difference exist between postoperative stages of each type of treatment and to determine which one is the best graft material for bone regeneration.
Materials and Methods
The CONSORT guidelines were followed for reporting this study. This was a randomized, controlled, parallel group study.[13] This study was conducted in the Department of Oral and Maxillofacial surgery, Vyas Dental College and Hospital (in Jodhpur, Rajasthan, India), after obtaining Institutional Ethical Clearance. The subjects for the study comprised selected patients (total 40) who were referred to the Department of Oral and Maxillofacial Surgery for the removal of mandibular third molar (ipsilateral third molar was selected due to lack of patients with bilateral), including both genders male and female.
Inclusion criteria
Patient's age between 17 and 36 years
Patients with mandibular third molar indicated for extractions.
Exclusion criteria
Patients with pericoronitis, periapical infection, or lesions with respect to impacted third molars
Opposing traumatic occlusion or impinging upper third molars
Smokers, alcoholics, and any systemic diseases
Female patients on oral contraceptives
Those patients with incomplete follow-up were excluded from the study.
After obtaining and recording the history of each of the case, the patients were clinically examined thoroughly. Then, the procedure for the treatment, its complications, and follow-up period involved in the study was explained to them individually. Willing patients were enrolled for the study after getting their written consent. Preoperative intraoral periapical (IOPA) radiograph was taken instead of computer tomography (CT) scan and cone beam CT because of cost-effectiveness.
Surgical procedure
The surgical procedure in all the groups was done by same experienced oral and maxillofacial surgeon. Extraction of mandibular third molars was done under local anesthesia using the standard technique. A triangular flap using ward-I or ward-II incision or an envelope flap was raised. Buccal guttering and ditching were done using a tungsten carbide bur and micromotor handpiece. After extraction of the tooth and achieving hemostasis, the socket was thoroughly irrigated with 40 ml of normal saline. The patients were randomly distributed into the following four groups, each consisting of 10 patients (sample size = 10/group):
Control: Involved patients having extraction socket closed without any graft material [Figures 1 and 2]
PRP-treated group: Comprised patients having extraction socket filled with PRP before closure of the socket [Figures 3 and 4]
PRF-treated group: Comprised patients having extraction socket filled with PRF before closure of the socket [Figures 5 and 6]
HA-treated group: Comprised patients having extraction socket filled with HA before closure of the socket [Figures 7 and 8].
Figure 1.

Representative pictures of pre-, post- and intra-operative periods of surgical extraction of mandibular third molar in control patient
Figure 2.
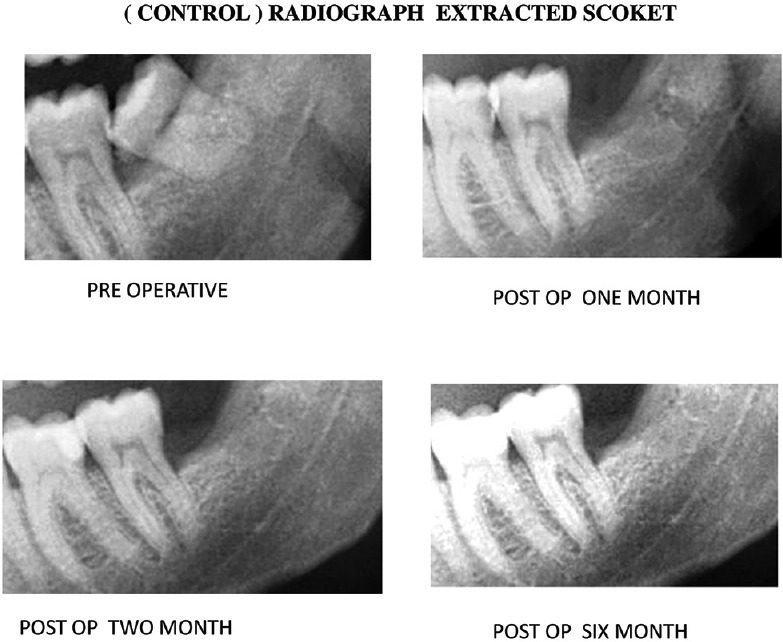
Representative radiographs (intraoral periapical) showing bone healing in control patients
Figure 3.
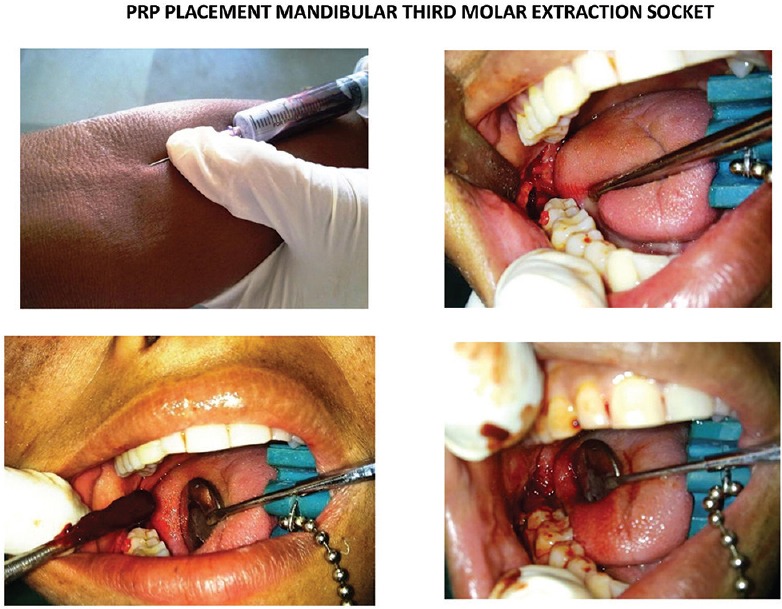
Representative pictures showing pre-, post- and intra-operative periods of surgical extraction of mandibular third molar in platelet-rich plasma-treated patients
Figure 4.
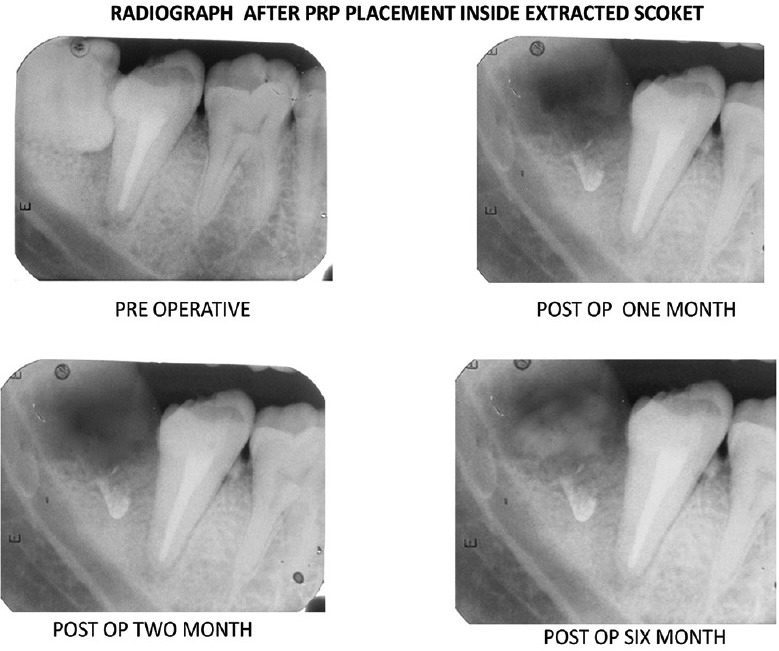
Representative radiographs (intraoral periapical) showing bone healing in platelet-rich plasma treated patients
Figure 5.
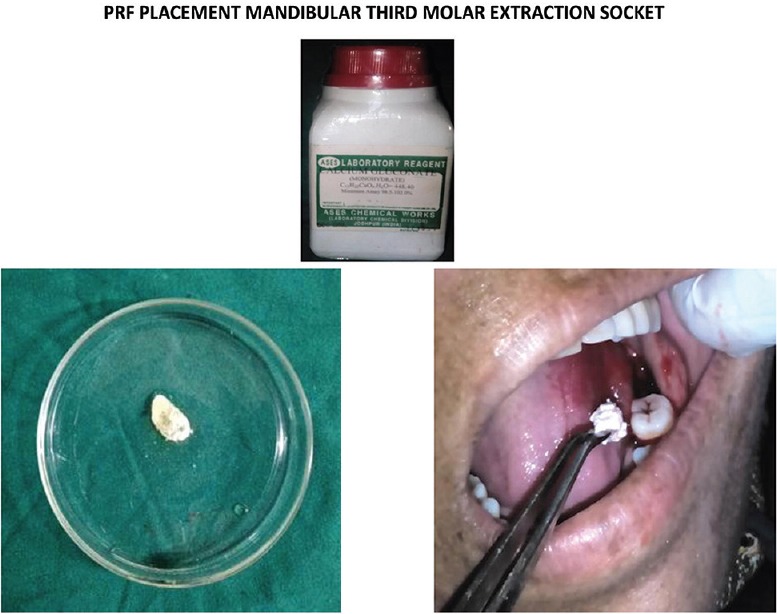
Representative pictures showing pre-, post- and intra-operative periods of surgical extraction of mandibular third molar in protein-rich fibrin-treated patients
Figure 6.
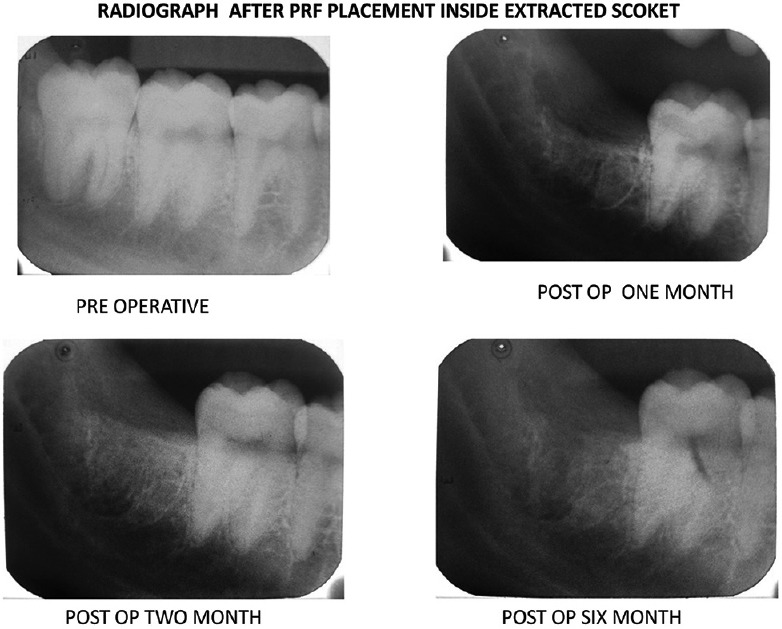
Representative radiographs (intraoral periapical) showing bone healing in protein-rich fibrin-treated patients
Figure 7.
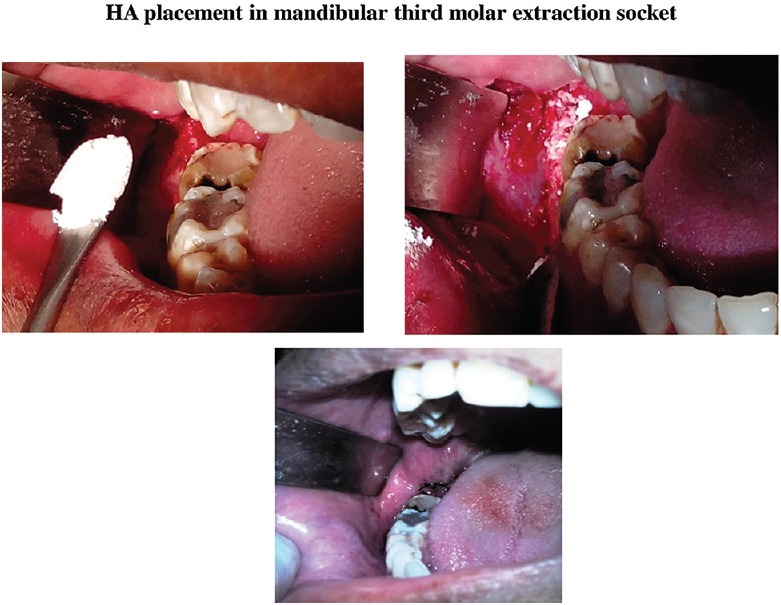
Representative pictures showing pre-, post- and intra-operative periods of surgical extraction of mandibular third molar in hydroxyapatite-treated patients
Figure 8.
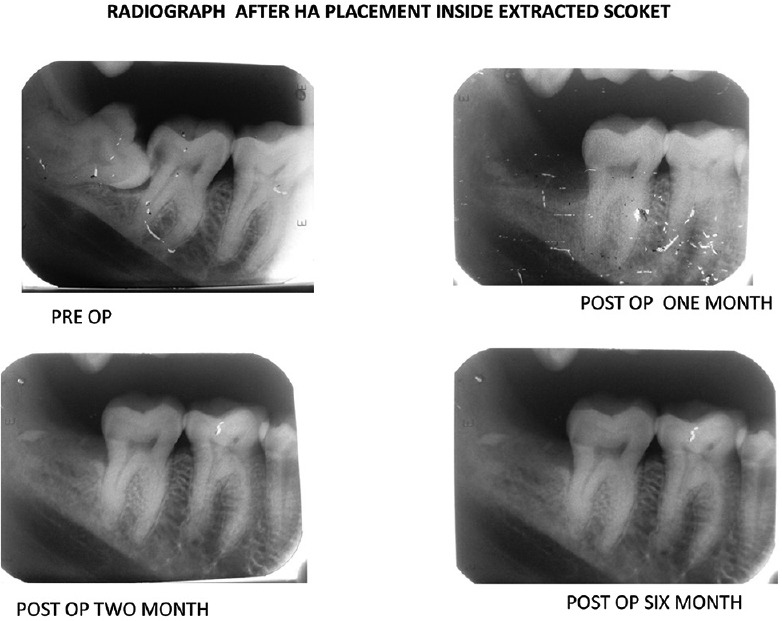
Representative radiographs (intraoral periapical) showing bone healing in hydroxyapatite treated patients
Postoperative monitoring and variables
Patients of all the groups were assessed on the postoperative days 3, 7, and 14 for the assessment of swelling, pain, dry socket, and soft tissue healing, and radiographic (IOPA) assessment for bone healing was conducted after 1, 2, and 6 months. Each patient received identical postoperative antibiotics, analgesics, and instructions. Patients were specifically instructed not to take any other drug without informing the investigator. Data were gathered for statistical analysis:
Assessment of pain using visual analog scale based on six-point facial Wong-Baker Scale[14]
Evaluation of facial edema and swelling was based on the modification of three line measurement using five fixed points on surgical side of the face [Figure 9] before and after 3rd, 7th, and 14th day of surgery. Line 1: Horizontal line joining the outer corner of the mouth to the midline of the tragus of the ear lobe (AC); line 2: Horizontal line joining the pogonium to the midline of the tragus of the ear lobe (AD); line 3: Vertical line joining the outer canthus of the eye and point on mandibular angle (BE). The average data calculated from the difference of postoperative and preoperative values[14]
Evaluation of soft tissue healing was also based on the standard method[15]
Criteria for dry socket assessment were based on Blum's method[16]
Mean radiographic score (using IOPA) for assessment of bony healing at various time lags between groups. The criteria for bone healing (including lamina dura, overall density, and trabecular pattern) and scoring system were based on the Kelley's method with some modification.[17]
Figure 9.
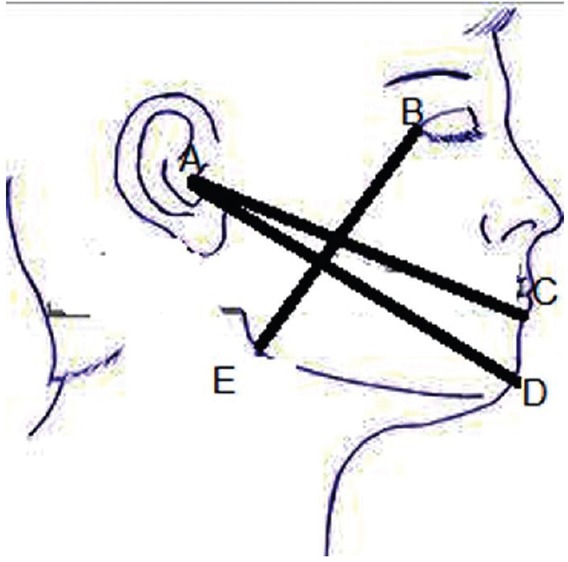
Representative picture showing facial swelling measurement by joining the three lines AC, AD, and BE
Preparation of platelet-rich plasma gel
Collection of blood: Under all aseptic techniques, 5 ml of venous blood was collected from the antecubital fossa. A volume of 3.6 ml of this blood was placed in a vial containing 0.4 ml citrate-phosphate-dextrose-adenine anticoagulant solution and gently mixed.
Preparation of platelet-rich plasma: The tube was placed in a centrifuge machine and counterbalanced. Then, it was centrifuged at 2000 rpm for 15 min. This separated the whole blood into a lower red blood cell region and upper straw-colored plasma. The uppermost region of this plasma contains less platelet (platelet poor plasma, [PPP]) and the central region contains high concentration of platelets and white blood cell (buffy coat). With a micropipette, the PPP and the buffy coat layer including 1 mm below the central region were collected in a sterile test tube and centrifuged at 3000 rpm for 10 min. After the second centrifuge, the upper half was removed, and the lower half was used as PRP. The PRP was activated with calcium chloride (CaCl2) to form a PRP gel.[18]
Preparation of protein-rich fibrin
After the first spin of the blood top layer consisting of PPP was discarded. The second layer was transferred to a neatly incubated test tube. Calcium gluconate (Ases chemical works, Jodhpur, India) was added to this solution (PRP). For 2 ml of PRP, 0.5 ml of calcium gluconate is added and allowed to stand for 10 min for conversion into the gel. The resultant gel obtained is PRF.[19]
Graft material
The graft material used was G-Bone (SHAG-3, G. Surgiwear Limited, Shahjahanpur, Uttar Pradesh, India), a bioceramic composite material which contains 90% HA and 10% beta-tricalcium phosphate. These grafts are white and granular measuring 1–2 mm in size and with porosity of 500–1200/mm. The material is dispensed in a sterile disposable 2 cc syringe in a blister pack.[20]
Statistical analysis
The results obtained are expressed as mean ± standard error of the mean. The data were statistically analyzed with the SPSS 16.0 for Windows (SPSS Inc., Chicago, USA). A multivariate analysis was done for all variables. Results with P < 0.05 were considered statistically significant for the study.
Results
Forty patients were included in the study and allocated randomly divided into four groups - control, PRP, PRF, and HA, each group having at least 10 patients. Of these 40 patients included in the study, 27 were males, and 13 were females. The median age of patients in our study is 27 years, with a minimum of 17 years and maximum of 36 years (mean: 27; standard deviation [SD] 5). The mean age of patients in years (± SD) in control, PRP, PRF, and HA groups were 28 ± 6, 27 ± 6, 26 ± 4, 26 ± 5, respectively. There was no significant difference in average age of patients among different groups.
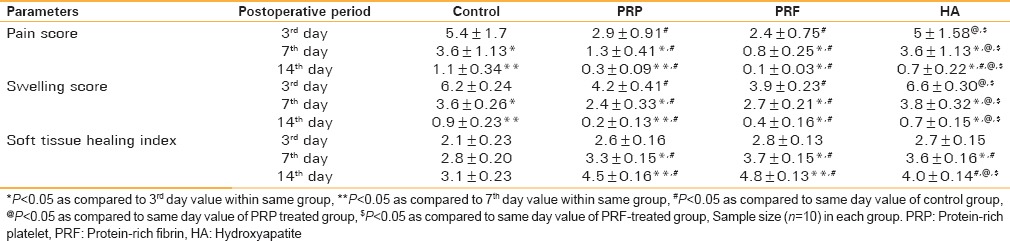
The intensity of pain and swelling in these different groups was scored on the 3rd day, 7th day, and 14th day. The results represented in Table 1 shows a reduction in the intensity of pain along with reduced swelling in all the groups from day 1, though the maximum reduction of pain, and swelling being in PRF- and PRP-treated patients (P < 0.05), respectively. Only a few patients were reported to have the presence of dry sockets only on the 3rd day of the postoperative period (2 control cases, 2 PRP-treated cases and 1 PRF-treated case). The soft tissue healing scores were also significantly higher in PRP- and PRF-treated groups [Table 1]; however, there was no significant difference in between these two groups.
Table 1.
Comparison of pain and swelling scores and soft tissue healing index of patients (results are mean±standard error of mean)
Radiographic assessment
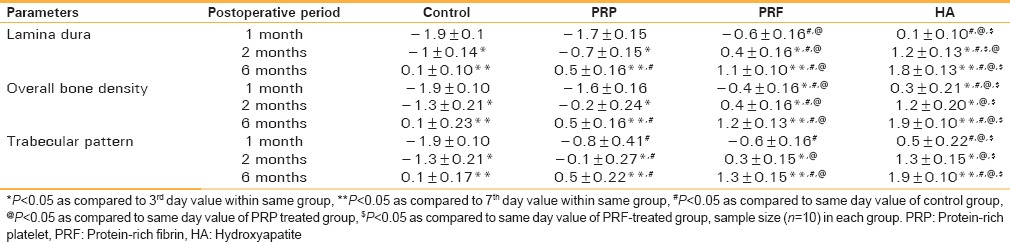
Radiographic assessment of bone healing lamina dura revealed an increased score in all the groups over the 6-month duration of the healing period [Table 2], significantly higher score (P < 0.05) being in the HA-treated group. Table 2 summarizes the mean bone healing scores for overall density and trabecular pattern at different periods in all the groups. Assessment of bone healing overall density demonstrated significantly higher scores (P < 0.05) in HA-treated group.
Table 2.
Comparison of bone healing index of patients (results are mean±standard error of mean)
Discussion
Several studies including in vivo animal studies suggest that biological mediators such as growth factors can be used to accelerate the healing of soft tissue and bone. Our study demonstrated that the use of PRP and PRF in extraction socket was more beneficial in reducing pain, swelling, and in accelerating the healing of soft tissue than HA. PDGF and epidermal growth factor are mainly involved in the migration of fibroblast, their proliferation, and collagen synthesis, thereby accelerating soft tissue wound healing.[21] Thus, the use of platelets in bone defects to improve, fasten healing, and stimulate new bone formation is quite justifiable.[22] It has also been shown in our previous study that healing of surgical sites enhanced with PRP is 2–3 times greater than that of normal surgical sites without PRP.[23] PRP accelerates wound maturity and epithelialization and hence decreases scar formation.[24] The activation of PRP to form PRP gel causes degranulation of α-granules present in the platelets, thus releasing the growth factors. In this study, we have mixed CaCl2 along with PRP to form an autologous platelet gel. This gel was prepared from the patient's own blood, thus preventing any antigen-antibody reaction.
PRF is mostly preferred over other concentrates because it releases the growth factors at a sustained rate over a longer period, thereby optimizing wound healing.[25] PRF is also beneficial for the regeneration of periodontal defects as it stimulates the growth of osteoblasts and periodontal ligament cells.[25,26]
The presence of dry socket (alveolar osteitis) is a common complication after tooth extraction, occurring due to disruption of blood clot prematurely in the extraction socket leaving bone unprotected and exposed to the oral environment.[16] The result from the assessment of dry socket supports previous report indicating that HA can be an effective preventive factor for dry socket (though results are not statistically significant).[27]
However, our radiographic results revealed better bone healing at HA site than other sites, suggesting that HA has an excellent bone conductive property. Porous HA permits the growth of osteogenic cells from existing bone surfaces into adjacent bone graft material.[28] Studies have shown that HA is well tolerated by the surrounding uninflamed tissues and is suitable for application in humans.[29,30]
In our study, all the procedures were done comfortably under local anesthesia on an outpatient basis without any complication. All the materials used in this study were found to be biocompatible with no detectable evidence of foreign body reaction or rejection of the material. All the materials not only fill and obliterate the extraction socket defect but also maintain the existing height of the alveolar bone. One of the disadvantages of PRP and PRF over HA is that former preparation is cumbersome procedure. Both preparations need patient's blood collection and taking to laboratory which requires some time while HA is readily available and can be placed immediately. The use of HA granules (G Bone) is cost-effective for the patients compared to other bone graft materials. The limitation of this study was that 6 months postoperative follow-up period was short to comment on the efficacy of various graft materials in complete bone regeneration process but adequate to evaluate the effects of initiating and enhancing the role in pain, swelling, dry socket prevention also in soft tissue healing. However, long-term follow-up along with histological study of the bone is required for assessment of the efficacy of various graft materials.
Summary and Conclusion
In summary, the results obtained from our study clearly indicate that in cases treated with PRP and PRF there was obvious improvement in the pain, swelling, and healing of soft tissue. Regeneration of bone after third molar surgery in HA is much better as compared to the control, PRP- and PRF-treated patients postoperatively. Moreover, PRP used in this study formed a biological gel which functioned as an adhesive and formed stable clot. In addition, the procedure for the preparation of PRP and PRF in this study is simple, economical, and exhibited fruitful results. However, since this study was done with a follow-up of 6 months only, further clinical trials with follow-up of longer duration with larger sample size needs to be done to get more affirmative and conclusive result.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The work has been done in Vyas Dental College, Jodhpur, Rasjasthan, India. All the authors are thankful to the Chairman, Principal and HOD of the Department of OMFS for providing the infrastructural support for conducting the research.
References
- 1.Aghazadeh A, Rutger Persson G, Renvert S. A single-centre randomized controlled clinical trial on the adjunct treatment of intra-bony defects with autogenous bone or a xenograft: Results after 12 months. J Clin Periodontol. 2012;39:666–73. doi: 10.1111/j.1600-051X.2012.01880.x. [DOI] [PubMed] [Google Scholar]
- 2.Meyle J, Hoffmann T, Topoll H, Heinz B, Al-Machot E, Jervøe-Storm PM, et al. A multi-centre randomized controlled clinical trial on the treatment of intra-bony defects with enamel matrix derivatives/synthetic bone graft or enamel matrix derivatives alone: Results after 12 months. J Clin Periodontol. 2011;38:652–60. doi: 10.1111/j.1600-051X.2011.01726.x. [DOI] [PubMed] [Google Scholar]
- 3.Misch CM. Autogenous bone: Is it still the gold standard? Implant Dent. 2010;19:361. doi: 10.1097/ID.0b013e3181f8115b. [DOI] [PubMed] [Google Scholar]
- 4.Ross R, Glomset J, Kariya B, Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro . Proc Natl Acad Sci U S A. 1974;71:1207–10. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gassling V, Douglas T, Warnke PH, Açil Y, Wiltfang J, Becker ST. Platelet-rich fibrin membranes as scaffolds for periosteal tissue engineering. Clin Oral Implants Res. 2010;21:543–9. doi: 10.1111/j.1600-0501.2009.01900.x. [DOI] [PubMed] [Google Scholar]
- 6.Marx RE. Platelet-rich plasma: Evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–96. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 8.Anitua E, Sánchez M, Nurden AT, Nurden P, Orive G, Andía I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006;24:227–34. doi: 10.1016/j.tibtech.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Nandi SK, Kundu B, Ghosh SK, De DK, Basu D. Efficacy of nano-hydroxyapatite prepared by an aqueous solution combustion technique in healing bone defects of goat. J Vet Sci. 2008;9:183–91. doi: 10.4142/jvs.2008.9.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minami M, Takechi M, Ohta K, Ohta A, Ninomiya Y, Takamoto M, et al. Bone formation and osseointegration with titanium implant using granular- and block-type porous hydroxyapatite ceramics (IP-CHA) Dent Mater J. 2013;32:753–60. doi: 10.4012/dmj.2012-169. [DOI] [PubMed] [Google Scholar]
- 11.Moreira-Gonzalez A, Jackson IT, Miyawaki T, DiNick V, Yavuzer R. Augmentation of the craniomaxillofacial region using porous hydroxyapatite granules. Plast Reconstr Surg. 2003;111:1808–17. doi: 10.1097/01.PRS.0000055432.20074.93. [DOI] [PubMed] [Google Scholar]
- 12.Itokazu M, Matsunaga T, Ishii M, Kusakabe H, Wyni Y. Use of arthroscopy and interporous hydroxyapatite as a bone graft substitute in tibial plateau fractures. Arch Orthop Trauma Surg. 1996;115:45–8. doi: 10.1007/BF00453217. [DOI] [PubMed] [Google Scholar]
- 13.Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: Explanation and elaboration. Ann Intern Med. 2001;134:663–94. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 14.Mansuri S, Mujeeb A, Hussain SA, Hussain MA. Mandibular third molar impactions in male adults: Relationship of operative time and types of impaction on inflammatory complications. J Int Oral Health. 2014;6:9–15. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Landry RG, Turnbull RS, Howley T. Effectiveness of benzydamyne HCl in the treatment of periodontal post surgical patients. Res Clin Forums. 1988;10:105–18. [Google Scholar]
- 16.Blum IR. Contemporary views on dry socket (alveolar osteitis): A clinical appraisal of standardization, aetiopathogenesis and management: A critical review. Int J Oral Maxillofac Surg. 2002;31:309–17. doi: 10.1054/ijom.2002.0263. [DOI] [PubMed] [Google Scholar]
- 17.Ogundipe OK, Ugboko VI, Owotade FJ. Can autologous platelet rich plasma gel enhance healing after surgical extraction of mandibular third molars? J Oral Maxillofac Surg. 2011;69:2305–10. doi: 10.1016/j.joms.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Shanaman R, Filstein MR, Danesh-Meyer MJ. Localized ridge augmentation using GBR and platelet-rich plasma: Case reports. Int J Periodontics Restorative Dent. 2001;21:345–55. [PubMed] [Google Scholar]
- 19.Sunitha Raja V, Munirathnam Naidu E. Platelet-rich fibrin: Evolution of a second.generation platelet concentrate. Indian J Dent Res. 2008;19:42–6. doi: 10.4103/0970-9290.38931. [DOI] [PubMed] [Google Scholar]
- 20.Kaur P, Maria A. Efficacy of platelet rich plasma and hydroxyapatite crystals in bone regeneration after surgical removal of mandibular third molars. J Maxillofac Oral Surg. 2013;12:51–9. doi: 10.1007/s12663-012-0382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang HL, Avila G. Platelet rich plasma: Myth or reality? Eur J Dent. 2007;1:192–4. [PMC free article] [PubMed] [Google Scholar]
- 22.Simon D, Manuel S, Geetha V, Naik BR. Potential for osseous regeneration of platelet-rich plasma – A comparative study in mandibular third molar sockets. Indian J Dent Res. 2004;15:133–6. [PubMed] [Google Scholar]
- 23.Dutta SR, Singh P, Passi D, Patter P. Mandibular third molar extraction wound healing with and without platelet rich plasma: A comparative prospective study. J Maxillofac Oral Surg. 2015;14:808–15. doi: 10.1007/s12663-014-0738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SG, Kim WK, Park JC, Kim HJ. A comparative study of osseointegration of Avana implants in a demineralized freeze-dried bone alone or with platelet-rich plasma. J Oral Maxillofac Surg. 2002;60:1018–25. doi: 10.1053/joms.2002.34413. [DOI] [PubMed] [Google Scholar]
- 25.Blair P, Flaumenhaft R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009;23:177–89. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma A, Pradeep AR. Autologous platelet-rich fibrin in the treatment of mandibular degree II furcation defects: A randomized clinical trial. J Periodontol. 2011;82:1396–403. doi: 10.1902/jop.2011.100731. [DOI] [PubMed] [Google Scholar]
- 27.Novaes AB, Jr, Novaes AB. IMZ implants placed into extraction sockets in association with membrane therapy (Gengiflex) and porous hydroxyapatite: A case report. Int J Oral Maxillofac Implants. 1992;7:536–40. [PubMed] [Google Scholar]
- 28.Holmes RE, Wardrop RW, Wolford LM. Hydroxylapatite as a bone graft substitute in orthognathic surgery: Histologic and histometric findings. J Oral Maxillofac Surg. 1988;46:661–71. doi: 10.1016/0278-2391(88)90109-7. [DOI] [PubMed] [Google Scholar]
- 29.Okuda K, Tai H, Tanabe K, Suzuki H, Sato T, Kawase T, et al. Platelet-rich plasma combined with a porous hydroxyapatite graft for the treatment of intrabony periodontal defects in humans: A comparative controlled clinical study. J Periodontol. 2005;76:890–8. doi: 10.1902/jop.2005.76.6.890. [DOI] [PubMed] [Google Scholar]
- 30.Lykins CL, Friedman CD, Costantino PD, Horioglu R. Hydroxyapatite cement in craniofacial skeletal reconstruction and its effects on the developing craniofacial skeleton. Arch Otolaryngol Head Neck Surg. 1998;124:153–9. doi: 10.1001/archotol.124.2.153. [DOI] [PubMed] [Google Scholar]


