Abstract
Injectable hydrogels have been widely used for a number of biomedical applications. Here, we report a new strategy to form an injectable and glucose-responsive hydrogel using the boronic acid–glucose complexation. The ratio of boronic acid and glucose functional groups is critical for hydrogel formation. In our system, polymers with 10–60% boronic acid, with the balance being glucose-modified, are favorable to form hydrogels. These hydrogels are shear-thinning and self-healing, recovering from shear-induced flow to a gel state within seconds. More importantly, these polymers displayed glucose-responsive release of an encapsulated model drug. The hydrogel reported here is an injectable and glucose-responsive hydrogel constructed from the complexation of boronic acid and glucose within a single component polymeric material.
Graphical abstract

Hydrogels have been broadly used for biomedical applications, including drug delivery and tissue engineering.1–3 Injectable hydrogels that flow under modest pressure and exhibit self-healing recovery following cessation of pressure offer many advantages for medical applications. Specifically, injectable implantation can be self-administered and is minimally invasive, leading to improved patient compliance.1 Injectable hydrogels have been used in medical practice, with examples such as hylaform gel, a chemically modified hylauronic acid for facial rejuvenation, that have received FDA approval.4 In addition, a number of injectable hydrogels have been evaluated preclinically or in early stage clinical trials, including hydrogels for cancer therapy and bone repair.5 In order to prepare injectable hydrogels, a variety of cross-linking mechanisms have been leveraged, including in situ covalent cross-linking as well as physical cross-linking that include salt bridges, peptide interactions, molecular recognition motifs, and/or van der Waals forces.2,5 Preparing hydrogels with cross-links that can respond to a specific biologic stimulus, such as elevation of blood glucose levels in diabetes, could further expand the utility of this class of material in preparing new therapies.
Early efforts to prepare glucose-responsive materials for insulin delivery evaluated the complexation of a glycosylated insulin derivative with the lectin concanavalin A (Con A), a natural carbohydrate binding protein.6–8 The competitive binding to Con A of glucose and glycosylated insulin regulates the breakdown of the complex, leading to glucose-responsive insulin release.9–11 However, possible immunogenicity of Con A, as well as a requirement for a special modified insulin derivative, would prove limiting to the translation of this approach.12 Another method to prepare glucose-responsive materials utilizes the enzymatic actuation, leveraging the catalytic conversion of glucose into gluconic acid by glucose oxidase. The drop in pH that arises through this conversion can be used to trigger hydrogel swelling, leading to the release of encapsulated insulin.13,14 This method has been widely used in a number of insulin delivery systems, including injectable networks.8,15–18 However, this strategy also has risks associated with enzyme immunogenicity along with toxicity of the hydrogen peroxide byproduct produced in the conversion.
Phenylboronic acids (PBAs) are Lewis acids that can bind reversibly to cis-1,2 or cis-1,3 diols, including glucose, to form a stable five-membered ring complex.19 In 1959, Lorand and Edwards reported the first quantitative study describing the complexation of boronic acids and polyols.20 Extensive studies since this time have investigated the binding affinity of boronic acids with different diols including fructose, glucose, and other sugars.19,21,22 Kataoka and co-workers have reported on the synthesis of numerous PBA-containing polymers and evaluated these for glucose responsiveness.23,24 When these polymers are combined with poly(vinyl alcohol), a hydrogel is formed through PBA–diol complexation that can sense changes in glucose levels with the release of encapsulated insulin.24 These studies explored polymers prepared from various acrylamide and modified PBA monomers, several of which are responsive to changing glucose concentrations.25–27 However, little is known about their mechanical properties including injectability.
On the basis of previous work in the preparation of glucose-responsive polymeric hydrogels from PBA–diol complexation,28 we endeavored to design a hydrogel leveraging cross-linking between PBA and glucose-like diols that could be injectable (i.e., self-healing) (Figure 1). In this system, polymers containing multiple PBA groups would cross-link through interaction with multiple glucose units installed within the same polymer to form a stable hydrogel network. This PBA–glucose complexation is reversible, enabling the preparation of an injectable self-healing hydrogel as verified through rheological measurements. Herein, we report a hydrogel cross-linked via the complexation of PBA and glucose that exhibits injectable self-healing properties, and this is accomplished through a single component polymeric material.
Figure 1.
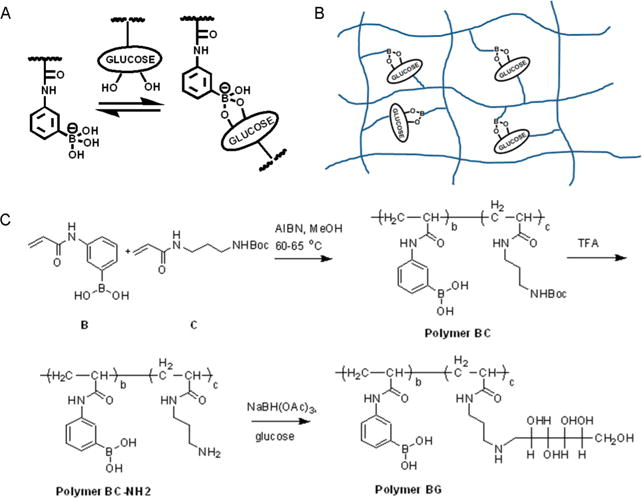
Design and synthesis of injectable hydrogel based on complexation between phenylboronic acid (PBA) and glucose. (A) PBA forms a five-membered ring in complex with diols such as glucose. (B) Illustration of PBA–glucose complexation on polymer chains. (C) Synthetic routes to polymer BG, which can self-assemble into an injectable and glucose-responsive hydrogel.
To incorporate boronic acid and glucose functional groups within the same polymer, we designed a synthetic route that used radical polymerization (Figure 1C). The PBA-containing monomer (monomer B) was copolymerized with a Boc-protected monomer (monomer C) using azobis-(isobutyronitrile) (AIBN) as an initiator in methanol, affording polymer BC. This polymer was subsequently treated with trifluoroacetic acid (TFA) to remove the Boc protecting group, yielding polymer BC-NH2. Finally, reductive amination of polymer BC-NH2 in combination with glucose at room temperature gave the final product, polymer (3-propionamidophenyl)boronic acid (N-(3-((2,3,4,5,6-pentahydroxyhexyl)amino)propyl)propionamide) (polymer BG).29 Eleven different polymers (BG 1–11) were obtained by varying the ratio of monomers B and C in the initial polymerization, and structures of all polymers, including BC and BC-NH2 precursors, were confirmed with 1H NMR (Supporting Information).
Next, we explored hydrogel formation in water and quantified mechanical properties. Polymers BG 5–10 formed self-supporting hydrogels at 5% by weight in water (Figure 2A), while polymers BG 1–4 and 11 were in either solid or liquid state in water (Supporting Information Table 1). Addition of glucose to the deprotected primary amines is essential for hydrogel formation, as a control polymer of BC-NH2 does not form a hydrogel. This supports PBA–glucose complexation as the mechanism for hydrogel formation. Using a standard syringe and needle, hydrogel BG-5 could be loaded and easily extruded through the needle, reforming a hydrogel after extrusion (Figure 2B). Cryogenic SEM imaging revealed the hydrogel to have a homogeneously porous structure, with a pore size around 1 um (Figure 2C).
Figure 2.
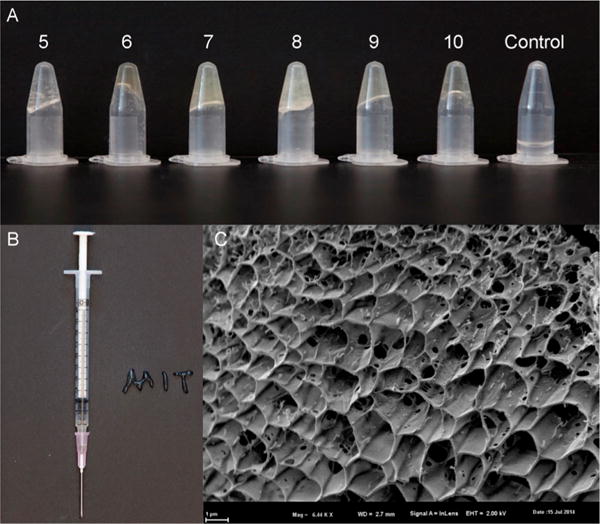
Hydrogels and their properties. (A) 5% polymer in water. Polymers BG 5–10 formed hydrogel in 5% water, while polymers BG 1–4 and 11 were in solid or liquid state in water. The control polymer BC-NH2 5 does not form a hydrogel. (B) Polymer BG 6 (50% PBA/50% glucose) is a soft and injectable hydrogel. (C) A cryo-SEM image of BG 6. Scale bar: 1 um.
Polymers prepared with 10–60% of the PBA-containing monomer B are required for hydrogel formation. Characterization of these hydrogels by rheology showed minimal differences in mechanical properties for hydrogels prepared from BG 5–10 (Figure 3 and Supporting Information Figure 1). The frequency dependence of the storage and loss moduli (G′ and G″, respectively) is characteristic of a typical hydrogel, as the G′ and G″ curves are linear and parallel and G′ is dominant across the range of frequencies measured (Figure 3A). Step–strain measurements were also performed in order to investigate the recovery time of the hydrogel BG 6 following deformation at high strains. The hydrogel structure is broken using high-magnitude stain (ε = 500%), and then reforms with complete restoration of properties following a shift to a low magnitude strain (ε = 0.5%). This recovery occurs rapidly, within seconds, indicative of shear-thinning and self-healing behaviors consistent with an injectable hydrogel.
Figure 3.
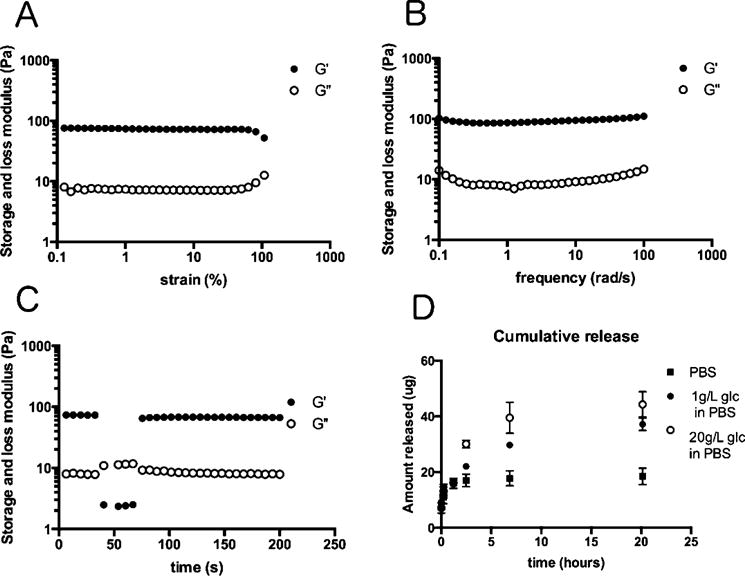
Glucose responsiveness of BG6. (A) Strain dependence (ω = 10 rad/s) of the storage and loss moduli of BG6. (B) Frequency dependence (ε = 2%) of the storage and loss moduli of BG6. (C) Step–strain measurements of BG 6 to investigate the ability of the hydrogel to recover following deformation at high strains. This recovery occurs completely and rapidly, within seconds, indicative of shear-thinning and self-healing behaviors consistent with an injectable hydrogel. (D) Glucose responsiveness in vitro. BG 6 released a higher amount of rhodamine B in a 20 g/L solution than that in 1 g/L glucose and PBS only solutions. BG 6 (50% PBA/50% glucose).
We further studied glucose-responsive release of hydrogels BG 5–10 using rhodamine B as a model drug compound (Figure 3D). Rhodamine B was incorporated into hydrogels by using a solution of this small molecule in water to hydrate BG 5–10, which then spontaneously forms hydrogels. Hydrogels were then incubated in different concentrations of glucose (1 and 20 g/L). BG 6 released a higher amount of rhodamine B in a 20 g/L solution than that in 1 g/L glucose and PBS only solutions (Figure 3D). These results suggest glucose-responsive release for these hydrogels.
In summary, injectable hydrogels have broad potential for biomedical applications. Using complexation between phenylboronic acid and glucose, hydrogels were formed that are both injectable and glucose responsive. These polymers may have potential application for sensors to monitor blood glucose levels.
EXPERIMENTS
General Methods
1H NMR spectra were measured on a 300 and 500 MHz Varian spectrometer using TMS as the internal standard. Gel permeation chromatography (GPC) was carried out in H2O on glucose-modified divinylbenzene columns, both utilizing a Malvern Viscotek TDA 305 triple detection system.
General Procedures of Polymer Synthesis
A heating block which can hold 20 mL vials was preheated to 65 °C. Twenty mL of glass vials with septa were used in a multireaction setup. 3-(Acrylamino)phenylboronic acid (A) and tert-butyl (3-acrylamidopropyl)carbamate (B) in a desired ratio were dissolved in MeOH (3.5 mL) at rt with stirring. Nitrogen was purged in solution for 30 min. 12.5% of 2,2′-azobis(2-methylpropionitrile) (it was recrystallized before using) was added. The solution was continuously degassed for an additional 30 min, heated at 65 °C with stirring for 1 day, and then cooled to rt. It was dropwise added to 200 mL of Et2O. The precipitate was filtered by suction, washed with Et2O (3 × 50 mL), and dried to get a white solid. Yield: 100% of B, 86%; 90:10% of B/C, 82%; 80:20% of B/C, 92%; 70:30% of B/C, 91%; 60:40% of B/C, 90%; 50:50% of B/C, 84%; 40:60% of B/C, 80%; 30:70% of B/C, 87%; 20:80% of B/C, 68%; 10:90% of B/C, 62%; 100% of C, 78%.
General Procedures of Deprotection
To a polymer (~200 mg) were added dichloromethane (6 mL) followed by TFA (3 mL). The suspension was stirred at rt for 1 day. The solvents were evaporated on a rotavap. Methanol was added to dissolve oily residue and evaporated subsequently. This procedure was repeated three times to get rid of excess TFA as much as possible to leave a white solid, which was dried further under high vacuum pump overnight.
General Procedures of Reductive Amination
A mixture of deprotected polymer, glucose, and sodium triacetoxyborohydride in DMF (3 mL) and THF (6 mL) was stirred at rt for 1 day. The amount of glucose (1 equiv) and sodium triacetoxyborohydride (1.2 equiv) depended on the amount of B in polymer synthesis. The majority of the solvents were evaporated. The residue was dissolved in ultrapure water (~20 mL), dialyzed (MWCO 1000), and lyophilized to have a white solid.
Rheological Characterization
Rheological characterization was performed using a TA Instruments AR-G2 stress controlled rheometer fitted with a Peltier stage. Dynamic oscillatory strain amplitude sweep measurements were conducted at a frequency of 10 rad/s (unless otherwise noted). Dynamic oscillatory frequency sweep measurements were conducted at a 2% strain amplitude (unless otherwise noted). All measurements were performed using a 20 mm, four-cone geometry and analyzed using TA Instruments TRIOS software.
Cryogenic Scanning Electron Microscopy (Cryo-SEM)
Cryo-SEM images of gels where acquired using a Zeiss NVision 40 (Carl Zeiss SMT, Inc.) field emission scanning electron microscope at acceleration voltages of 1–2 kV. To prepare samples for imaging approximately 100 μL of microcapsules where transferred to a sample stub and then plunged into a slushy liquid and solid nitrogen bath. The samples were next transferred to an EM VCT100 vacuum cryo transfer system (Leica Microsystems, Inc.) to selectively remove surface water (ice) by controlled specimen sublimation. The frozen samples were then further fractured with a sharp blade and sputter coated with a thin layer of platinum and palladium metals prior to imaging.
In Vitro Release
Rhodamine B was dissolved in pure ethanol to form a stock solution at 20 mg/mL, and diluted down to a working drug solution of 0.5 mg/mL in Millicule water. A 10 mg portion of dried polymer was reconstituted in 200 ul of drug solution (0.5 mg/mL rhodamine B in water) and mixed thoroughly. Hydrogels formed were incubated in the solutions for 2 days before being used for release experiments. Hydrogels were carefully transferred to a permeable insert in a 24-well plate (HTS Transwell-24 well, Corning). The hydrogels were washed twice with 1 mL of PBS, and immersed in a buffer solution of either 1 g/L glucose solution or 20 g/L glucose solution in PBS. The hydrogels were then incubated at 37 °C without shaking. At specified time points, hydrogel was centrifuged momentarily to remove the buffer, and then replaced with fresh buffer solutions. Samples collected were kept at room temperature. Rhodamine B release was quantified using absorbance at 540 nm relative to a standard curve, and data points represent the mean and standard deviation of two gels for each setup.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Leona M. and Harry B. Helmsley Charitable trust (Award 2014PG-T1D002) along with a generous gift from the Tayebati Family Foundation. Y.D. and B.C.T. acknowledge support from Juvenile Diabetes Research Foundation Postdoctoral Fellowships 3-2011-310 and 3-2013-144, respectively. M.J.W. acknowledges support from the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases) through Ruth L. Kirschstein National Research Service Award F32DK101335. K.X. acknowledges support from the A*STAR Predoctoral National Science Scholarship.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.langmuir.5b04755.
General synthetic procedures and mechanical properties (PDF)
Notes
The authors declare no competing financial interest.
References
- 1.Olsen BD, Kornfield JA, Tirrell DA. Yielding Behavior in Injectable Hydrogels from Telechelic Proteins. Macromolecules. 2010;43(21):9094–9099. doi: 10.1021/ma101434a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appel EA, del Barrio J, Loh XJ, Scherman OA. Supramolecular polymeric hydrogels. Chem Soc Rev. 2012;41(18):6195–214. doi: 10.1039/c2cs35264h. [DOI] [PubMed] [Google Scholar]
- 3.Appel EA, Loh XJ, Jones ST, Dreiss CA, Scherman OA. Sustained release of proteins from high water content supra-molecular polymer hydrogels. Biomaterials. 2012;33(18):4646–52. doi: 10.1016/j.biomaterials.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 4.Kanchwala SK, Holloway L, Bucky LP. Reliable soft tissue augmentation: a clinical comparison of injectable soft-tissue fillers for facial-volume augmentation. Ann Plast Surg. 2005;55(1):30–5. doi: 10.1097/01.sap.0000168292.69753.73. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Rodrigues J, Tomas H. Injectable and biodegradable hydrogels: gelation, biodegradation and biomedical applications. Chem Soc Rev. 2012;41(6):2193–221. doi: 10.1039/c1cs15203c. [DOI] [PubMed] [Google Scholar]
- 6.Sharon N, Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972;177(53):949–59. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- 7.Brownlee M, Cerami A. A glucose-controlled insulin-delivery system: semisynthetic insulin bound to lectin. Science. 1979;206(4423):1190–1. doi: 10.1126/science.505005. [DOI] [PubMed] [Google Scholar]
- 8.Veiseh O, Tang BC, Whitehead KA, Anderson DG, Langer R. Managing diabetes with nanomedicine: challenges and opportunities. Nat Rev Drug Discovery. 2015;14(1):45–57. doi: 10.1038/nrd4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seminoff LA, Gleeson JM, Zheng J, Olsen GB, Holmberg D, Mohammad SF, Wilson D, Kim SW. A self-regulating insulin delivery system. II. In vivo characteristics of a synthetic glycosylated insulin. Int J Pharm. 1989;54(3):251–7. [Google Scholar]
- 10.Makino K, Mack EJ, Okano T, Kim SW. A microcapsule self-regulating delivery system for insulin. J Controlled Release. 1990;12(3):235–9. [Google Scholar]
- 11.Kim SW, Paii CM, Kimiko M, Seminoff LA, Holmberg DL, Gleeson JM, Wilson DE, Mack EJ. Self-regulated glycosylated insulin delivery. J Controlled Release. 1990;11(1–3):193–201. [Google Scholar]
- 12.Ravaine V, Ancla C, Catargi B. Chemically controlled closed-loop insulin delivery. J Controlled Release. 2008;132(1):2–11. doi: 10.1016/j.jconrel.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Albin GW, Horbett TA, Miller SR, Ricker NL. Theoretical and experimental studies of glucose sensitive membranes. J Controlled Release. 1987;6:267–91. [Google Scholar]
- 14.Traitel T, Cohen Y, Kost J. Characterization of glucose-sensitive insulin release systems in simulated in vivo conditions. Biomaterials. 2000;21(16):1679–87. doi: 10.1016/s0142-9612(00)00050-8. [DOI] [PubMed] [Google Scholar]
- 15.Gu Z, Dang TT, Ma M, Tang BC, Cheng H, Jiang S, Dong Y, Zhang Y, Anderson DG. Glucose-responsive microgels integrated with enzyme nanocapsules for closed-loop insulin delivery. ACS Nano. 2013;7(8):6758–6766. doi: 10.1021/nn401617u. [DOI] [PubMed] [Google Scholar]
- 16.Gu Z, Aimetti AA, Wang Q, Dang TT, Zhang Y, Veiseh O, Cheng H, Langer RS, Anderson DG. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano. 2013;7(5):4194–201. doi: 10.1021/nn400630x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mo R, Jiang T, Di J, Tai W, Gu Z. Emerging micro- and nanotechnology based synthetic approaches for insulin delivery. Chem Soc Rev. 2014;43(10):3595–629. doi: 10.1039/c3cs60436e. [DOI] [PubMed] [Google Scholar]
- 18.Di J, Price J, Gu X, Jiang X, Jing Y, Gu Z. Ultrasound-triggered regulation of blood glucose levels using injectable nano-network. Adv Healthcare Mater. 2014;3(6):811–6. doi: 10.1002/adhm.201300490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Springsteen G, Wang B. A detailed examination of boronic acid-diol complexation. Tetrahedron. 2002;58(26):5291–5300. [Google Scholar]
- 20.Lorand JP, Edwards JO. Polyol Complexes and Structure of the Benzeneboronate Ion. J Org Chem. 1959;24(6):769–774. [Google Scholar]
- 21.Bosch LI, Fyles TM, James TD. Binary and ternary phenylboronic acid complexes with saccharides and Lewis bases. Tetrahedron. 2004;60(49):11175–11190. [Google Scholar]
- 22.Zhu L, Shabbir SH, Gray M, Lynch VM, Sorey S, Anslyn EV. A structural investigation of the N-B interaction in an o-(N,N-dialkylaminomethyl)arylboronate system. J Am Chem Soc. 2006;128(4):1222–1232. doi: 10.1021/ja055817c. [DOI] [PubMed] [Google Scholar]
- 23.Shiino D, Murata Y, Kataoka K, Koyama Y, Yokoyama M, Okano T, Sakurai Y. Preparation and characterization of a glucose-responsive insulin-releasing polymer device. Biomaterials. 1994;15(2):121–8. doi: 10.1016/0142-9612(94)90261-5. [DOI] [PubMed] [Google Scholar]
- 24.Kikuchi A, Suzuki K, Okabayashi O, Hoshino H, Kataoka K, Sakurai Y, Okano T. Glucose-Sensing Electrode Coated with Polymer Complex Gel Containing Phenylboronic Acid. Anal Chem. 1996;68(5):823–8. doi: 10.1021/ac950748d. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto A, Yamamoto K, Yoshida R, Kataoka K, Aoyagi T, Miyahara Y. A totally synthetic glucose responsive gel operating in physiological aqueous conditions. Chem Commun (Cambridge, U K) 2010;46(13):2203–2205. doi: 10.1039/b920319b. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto A, Yoshida R, Kataoka K. Glucose-responsive polymer gel bearing phenylborate derivative as a glucose-sensing moiety operating at the physiological pH. Biomacromolecules. 2004;5(3):1038–1045. doi: 10.1021/bm0345413. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto A, Ishii T, Nishida J, Matsumoto H, Kataoka K, Miyahara Y. A synthetic approach toward a self-regulated insulin delivery system. Angew Chem, Int Ed. 2012;51(9):2124–8. doi: 10.1002/anie.201106252. [DOI] [PubMed] [Google Scholar]
- 28.Yesilyurt V, Webber MJ, Appel EA, Godwin C, Langer R, Anderson DG. Injectable Self-Healing Glucose-Responsive Hydrogels with pH-Regulated Mechanical Properties. Adv Mater. 2016;28:86. doi: 10.1002/adma.201502902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dangerfield EM, Plunkett CH, Win-Mason AL, Stocker BL, Timmer MSM. Protecting-group-free synthesis of amines: synthesis of primary amines from aldehydes via reductive amination. J Org Chem. 2010;75(16):5470–5477. doi: 10.1021/jo100004c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


