Abstract
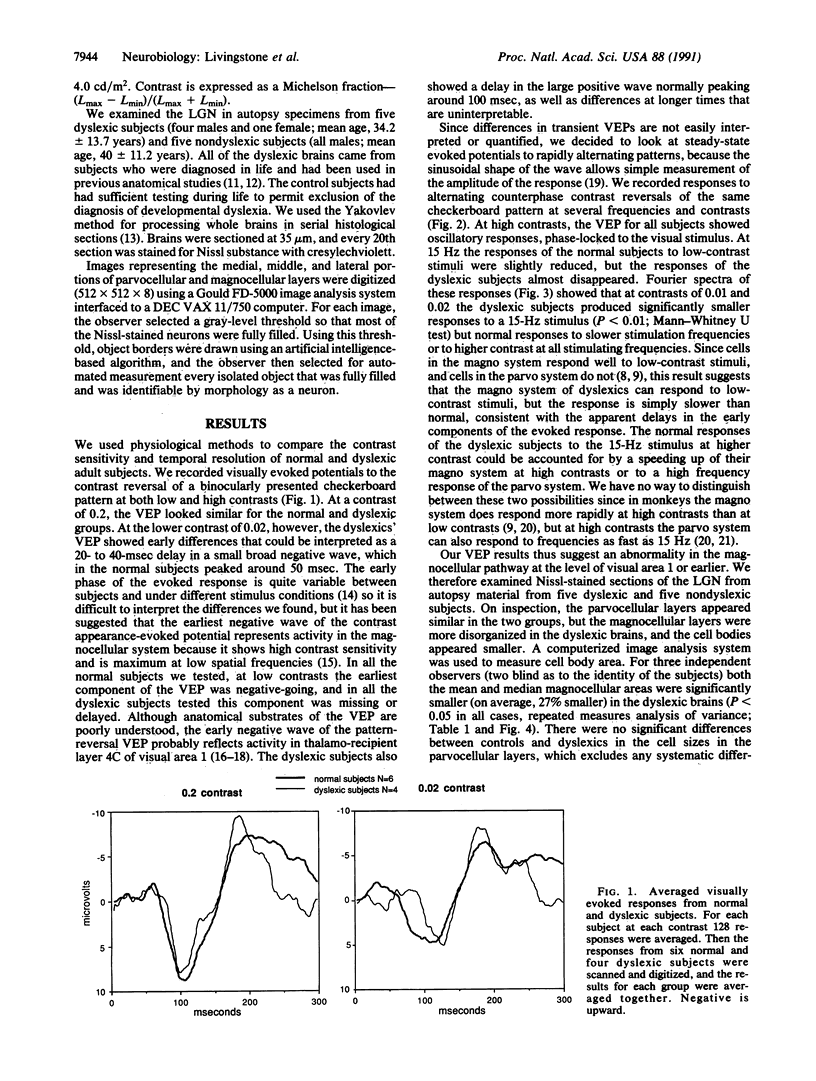
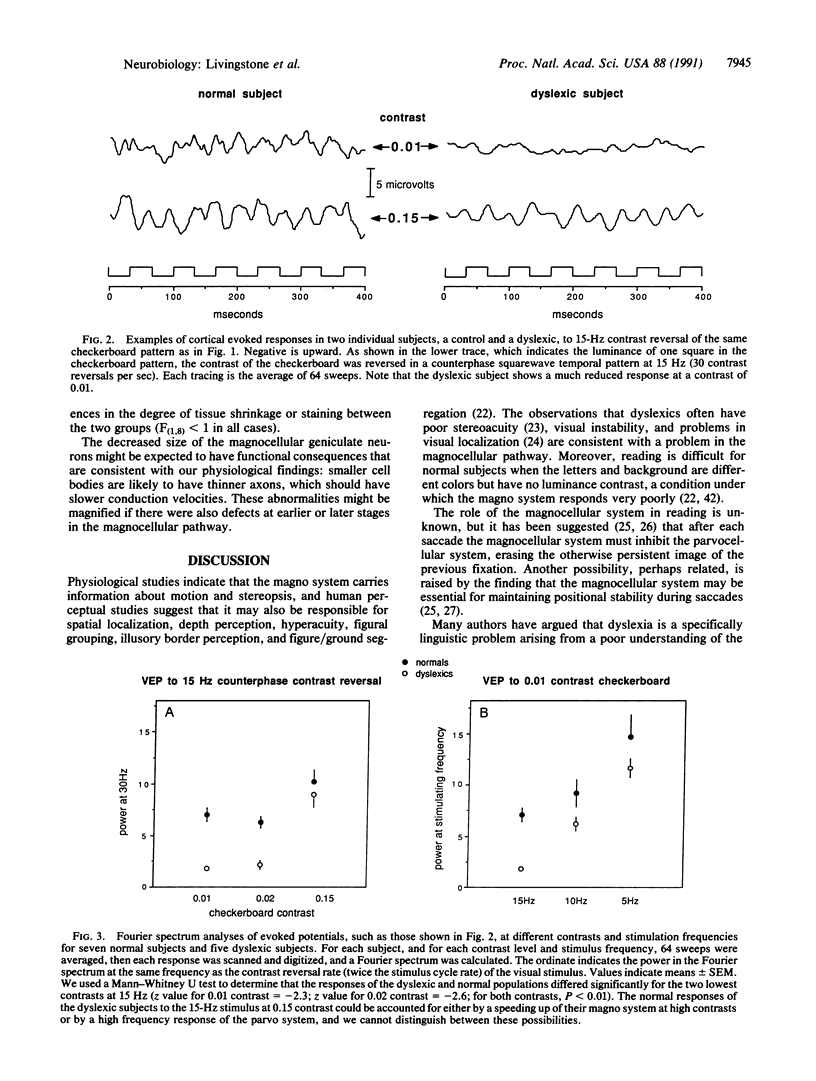
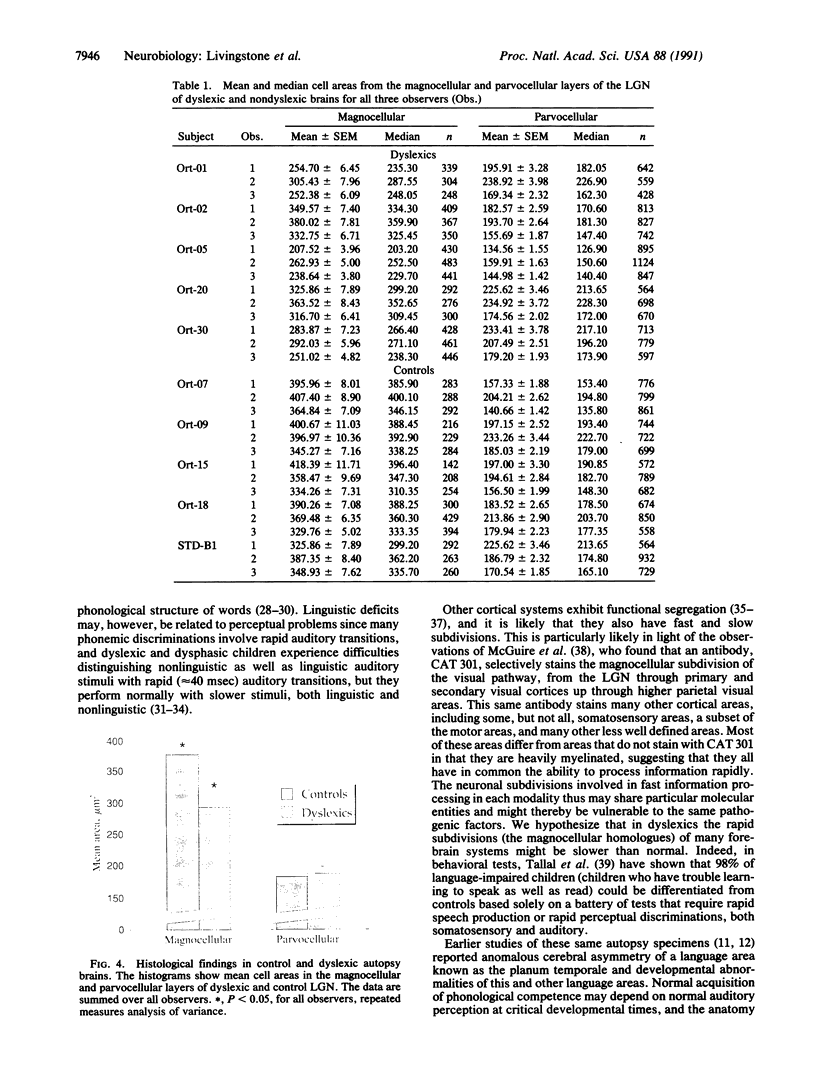
Several behavioral studies have shown that developmental dyslexics do poorly in tests requiring rapid visual processing. In primates fast, low-contrast visual information is carried by the magnocellular subdivision of the visual pathway, and slow, high-contrast information is carried by the parvocellular division. In this study, we found that dyslexic subjects showed diminished visually evoked potentials to rapid, low-contrast stimuli but normal responses to slow or high-contrast stimuli. The abnormalities in the dyslexic subjects' evoked potentials were consistent with a defect in the magnocellular pathway at the level of visual area 1 or earlier. We then compared the lateral geniculate nuclei from five dyslexic brains to five control brains and found abnormalities in the magnocellular, but not the parvocellular, layers. Studies using auditory and somatosensory tests have shown that dyslexics do poorly in these modalities only when the tests require rapid discriminations. We therefore hypothesize that many cortical systems are similarly divided into a fast and a slow subdivision and that dyslexia specifically affects the fast subdivisions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles M., Goldstein M. H., Jr Functional architecture in cat primary auditory cortex: columnar organization and organization according to depth. J Neurophysiol. 1970 Jan;33(1):172–187. doi: 10.1152/jn.1970.33.1.172. [DOI] [PubMed] [Google Scholar]
- Campbell F. W., Maffei L. Electrophysiological evidence for the existence of orientation and size detectors in the human visual system. J Physiol. 1970 May;207(3):635–652. doi: 10.1113/jphysiol.1970.sp009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lollo V., Hanson D., McIntyre J. S. Initial stages of visual information processing in dyslexia. J Exp Psychol Hum Percept Perform. 1983 Dec;9(6):923–935. doi: 10.1037/0096-1523.9.6.923. [DOI] [PubMed] [Google Scholar]
- Ducati A., Fava E., Motti E. D. Neuronal generators of the visual evoked potentials: intracerebral recording in awake humans. Electroencephalogr Clin Neurophysiol. 1988 Mar-Apr;71(2):89–99. doi: 10.1016/0168-5597(88)90010-x. [DOI] [PubMed] [Google Scholar]
- Galaburda A. M., Sherman G. F., Rosen G. D., Aboitiz F., Geschwind N. Developmental dyslexia: four consecutive patients with cortical anomalies. Ann Neurol. 1985 Aug;18(2):222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- Goldman P. S., Nauta W. J. Columnar distribution of cortico-cortical fibers in the frontal association, limbic, and motor cortex of the developing rhesus monkey. Brain Res. 1977 Feb 25;122(3):393–413. doi: 10.1016/0006-8993(77)90453-x. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Livingstone M. S. Color and contrast sensitivity in the lateral geniculate body and primary visual cortex of the macaque monkey. J Neurosci. 1990 Jul;10(7):2223–2237. doi: 10.1523/JNEUROSCI.10-07-02223.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys P., Kaufmann W. E., Galaburda A. M. Developmental dyslexia in women: neuropathological findings in three patients. Ann Neurol. 1990 Dec;28(6):727–738. doi: 10.1002/ana.410280602. [DOI] [PubMed] [Google Scholar]
- Jones R., Keck M. J. Visual evoked response as a function of grating spatial frequency. Invest Ophthalmol Vis Sci. 1978 Jul;17(7):652–659. [PubMed] [Google Scholar]
- Kaplan E., Shapley R. M. X and Y cells in the lateral geniculate nucleus of macaque monkeys. J Physiol. 1982 Sep;330:125–143. doi: 10.1113/jphysiol.1982.sp014333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. B., Martin P. R., Valberg A. Sensitivity of macaque retinal ganglion cells to chromatic and luminance flicker. J Physiol. 1989 Jul;414:223–243. doi: 10.1113/jphysiol.1989.sp017685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J Neurosci. 1987 Nov;7(11):3416–3468. doi: 10.1523/JNEUROSCI.07-11-03416.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M., Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988 May 6;240(4853):740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Lovegrove W. J., Garzia R. P., Nicholson S. B. Experimental evidence for a transient system deficit in specific reading disability. J Am Optom Assoc. 1990 Feb;61(2):137–146. [PubMed] [Google Scholar]
- Lovegrove W. J., Garzia R. P., Nicholson S. B. Experimental evidence for a transient system deficit in specific reading disability. J Am Optom Assoc. 1990 Feb;61(2):137–146. [PubMed] [Google Scholar]
- MOUNTCASTLE V. B. Modality and topographic properties of single neurons of cat's somatic sensory cortex. J Neurophysiol. 1957 Jul;20(4):408–434. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- Maier J., Dagnelie G., Spekreijse H., van Dijk B. W. Principal components analysis for source localization of VEPs in man. Vision Res. 1987;27(2):165–177. doi: 10.1016/0042-6989(87)90179-9. [DOI] [PubMed] [Google Scholar]
- Martin F., Lovegrove W. Flicker contrast sensitivity in normal and specifically disabled readers. Perception. 1987;16(2):215–221. doi: 10.1068/p160215. [DOI] [PubMed] [Google Scholar]
- May J. G., Williams M. C., Dunlap W. P. Temporal order judgements in good and poor readers. Neuropsychologia. 1988;26(6):917–924. doi: 10.1016/0028-3932(88)90059-0. [DOI] [PubMed] [Google Scholar]
- McGuire P. K., Hockfield S., Goldman-Rakic P. S. Distribution of cat-301 immunoreactivity in the frontal and parietal lobes of the macaque monkey. J Comp Neurol. 1989 Oct 8;288(2):280–296. doi: 10.1002/cne.902880207. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988 Jul 8;241(4862):170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Schroeder C. E., Tenke C. E., Givre S. J., Arezzo J. C., Vaughan H. G., Jr Striate cortical contribution to the surface-recorded pattern-reversal VEP in the alert monkey. Vision Res. 1991;31(7-8):1143–1157. doi: 10.1016/0042-6989(91)90040-c. [DOI] [PubMed] [Google Scholar]
- Shapley R. Visual sensitivity and parallel retinocortical channels. Annu Rev Psychol. 1990;41:635–658. doi: 10.1146/annurev.ps.41.020190.003223. [DOI] [PubMed] [Google Scholar]
- Spekreijse H., van der Twell L. H., Zuidema T. Contrast evoked responses in man. Vision Res. 1973 Aug;13(8):1577–1601. doi: 10.1016/0042-6989(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Stanley G., Hall R. Short-term visual information processing in dyslexics. Child Dev. 1973 Dec;44(4):841–844. doi: 10.1111/j.1467-8624.1973.tb01163.x. [DOI] [PubMed] [Google Scholar]
- Tallal P. Auditory temporal perception, phonics, and reading disabilities in children. Brain Lang. 1980 Mar;9(2):182–198. doi: 10.1016/0093-934x(80)90139-x. [DOI] [PubMed] [Google Scholar]
- Tallal P., Piercy M. Developmental aphasia: the perception of brief vowels and extended stop consonants. Neuropsychologia. 1975 Jan;13(1):69–74. doi: 10.1016/0028-3932(75)90049-4. [DOI] [PubMed] [Google Scholar]
- Tallal P., Stark R. E., Mellits E. D. Identification of language-impaired children on the basis of rapid perception and production skills. Brain Lang. 1985 Jul;25(2):314–322. doi: 10.1016/0093-934x(85)90087-2. [DOI] [PubMed] [Google Scholar]
- Williams R. W., Rakic P. Elimination of neurons from the rhesus monkey's lateral geniculate nucleus during development. J Comp Neurol. 1988 Jun 15;272(3):424–436. doi: 10.1002/cne.902720310. [DOI] [PubMed] [Google Scholar]



