Abstract
The number of species coexisting in ecological communities must be a consequence of processes operating on both local and regional scales. Although a great deal of experimental work has been devoted to local causes of diversity, little is known about the effects of regional processes on local diversity and how they contribute to global diversity patterns in marine systems. We tested the effects of latitude and the richness of the regional species pool on the species richness of local epifaunal invertebrate communities by sampling the diversity of local sites in 12 independent biogeographic regions from 62°S to 63°N latitude. Both regional and local species richness displayed significant unimodal patterns with latitude, peaking at low latitudes and decreasing toward high latitudes. The latitudinal diversity gradient was represented at the scale of local sites because local species richness was positively and linearly related to regional species richness. The richness of the regional species pool explained 73-76% of local species richness. On a global scale, the extent of regional influence on local species richness was nonrandom—the proportion of regional biota represented in local epifaunal communities increased significantly from low to high latitudes. The strong effect of the regional species pool implies that patterns of local diversity in temperate, tropical, and high-latitude marine benthic communities are influenced by processes operating on larger spatiotemporal scales than previously thought.
Understanding the forces that shape spatiotemporal variation in species diversity remains one of the major issues confronting ecologists (1). Experiments conducted at local sites (spatial scale of meters to hundreds of meters) demonstrate that biological interactions, productivity, habitat complexity, disturbance, and environmental stress interact to produce variability in local species richness (i.e., number of species) (2, 3). But, local diversity must also be affected by regional-scale processes [spatial scale of 200 to thousands of kilometers (4)] because local communities are integral components of larger biogeographic regions. Tests of the importance of regional-scale phenomena typically involve quantifying the relationship between regional and local species richness.
On a global scale, species diversity typically declines with increasing latitude toward the poles, especially on land (5, 6). The linear or modal function describing the relationship between diversity and latitude varies by taxa and sampling resolution, but an emerging generalization from empirical (6) and meta-analyses of latitudinal diversity patterns (7) is that the latitudinal pattern is chiefly based on the decline of regional (gamma) diversity toward the poles and is not due to variation in local (alpha) community richness. In the ocean, however, there are few studies of local diversity across broad latitudinal gradients (7, 8). Because of the remote (e.g., ship-based) methods commonly used to sample the diversity of soft-bottom benthic communities, local samples often represent a mix of species from several habitat types, confounding analyses of regional influences on local diversity with effects of habitat heterogeneity. These limitations make it difficult to evaluate the scaling of latitudinal gradients to local marine communities. A tropical-polar marine diversity decline is supported by regional-scale data on molluscs (9, 10), fish (11), and bryozoans (12) but not by local-scale patterns of intertidal epifauna (13) and macrobenthic infauna (14). The generality of an interhemispheric, tropical-polar diversity gradient has been questioned by recent findings of high biodiversity in Antarctic waters (15, 16). It is apparent from reviews of both the regional-local diversity relationship (17, 18, 28) and latitudinal diversity gradients (7, 8, 19) that most of the analyses have been conducted on species in taxonomically restricted (i.e., single genera, classes, or phyla) subsets of communities, suggesting that our understanding of large-scale drivers of the local diversity of multiphyletic communities is incomplete.
We investigated the relationship between local, regional, and latitudinal patterns of species richness in epifaunal invertebrate communities encrusting subtidal vertical rock walls bordering continents, islands, and ledges across a wide latitudinal range from 62°S (Antarctica) to 63°N (Iceland.) The sessile invertebrates forming these communities interact via interference competition for space, exploitative competition for food, facilitation, and adult-larval interactions (20, 21). The spatial scales of these local biological interactions range from centimeters (22) to tens of square meters (23). Competition for space in marine epifaunal communities is thought to be intense and frequently involves members of different phyla (20). Typical examples include overgrowth competition between sponges and corals, ascidians and sponges, cnidarians and calcareous polychaetes, and bryozoans and ascidians. The diversity is also impacted by predation from consumers (fish, sea urchins, etc.) and various forms of disturbance (24).
Theory predicts that if regional-scale processes are important, local species richness will increase as a positive linear function of the size of the regional species pool [unsaturated type I relation (25)]. In contrast, if local species richness levels off with increased regional richness and is better described by a nonlinear function [saturated type II relation (25, 26)], then factors other than the size of the regional species pool constrain local richness. Local biological interactions (e.g., competition) are usually invoked to explain a nonlinear saturating function, although the evidence is often indirect and controversial (see below). Testing the form of the relationship requires estimates of local richness (alpha diversity) from independent regions varying in species richness and an estimate of the number of species in each region (gamma diversity).
The nature of the relationship between local and regional diversity has been used extensively to test for the impact of regional-scale processes on natural communities. Recently, a number of criticisms have been raised about the validity of this approach based on methodological or statistical concerns (27, 28), appropriate scales (28-30), and alternative interpretations (18, 31-33). Our study avoids many of the potential pitfalls that have been raised about this theoretical approach. We estimated local and regional richness independently and incorporated replication at local (within region) and at regional scales. Local richness was sampled to near completion, and the range of regional diversity spans the entire spectrum of marine epifaunal communities found globally. Moreover, identical methodology was used to sample a single habitat (subtidal rock walls) that is quite similar worldwide. Entire multiphyletic communities were sampled at small spatial scales characteristic of species interaction, capturing both positive (34) and negative (3, 20) effects of local ecological interactions on species richness.
Although we have dealt with most of the methodological, statistical, and scale issues, the interpretation of linear or nonlinear relationships between local and regional diversity remains controversial. Much of this controversy arises because various models find linear relationships between local and regional diversity even when strong local interactions are incorporated into the models (31, 33, 35). For example, it has been recognized since the early models of Caswell and Cohen (31) that a linear relationship can occur despite strong local interactions. In their study, disturbance alleviated the impact of strong local interactions and thus increased the relative importance of regional-scale (dispersal among patches) processes in controlling the number of species within a patch. Recent models by Mouquet and colleagues (33, 35) also challenge the typical interpretation of local-regional diversity plots. They found that when they altered the importance of dispersal (33) or the time since last disturbance (35) in their models, the local-regional relationship switched between linear and saturating functions despite no change in the strength of the competitive interactions. This result occurs because the relative importance of regional-scale processes (e.g., dispersal) changed. In both cases, dispersal among patches becomes relatively more important because the rate of dispersal increased, or disturbance increased, which alleviated competition and thereby increased the importance of dispersal. The results from these models, therefore, are not inconsistent with the typical interpretation of the relationship between local and regional diversity. A linear relationship suggests that the number of species within local communities is not determined solely by local processes but is influenced by processes operating at regional scales. It cannot be used to argue that local interactions are weak, or unimportant.
The questions addressed in our study were these: (i) Is local diversity in the same subtidal habitat in different areas of the world influenced by the size of the regional species pool? (ii) Do latitudinal patterns in local diversity exist for multiphyletic, epifaunal communities in the ocean? (iii) If so, can the relationship between regional and local species richness provide insight into latitudinal patterns of diversity?
Materials and Methods
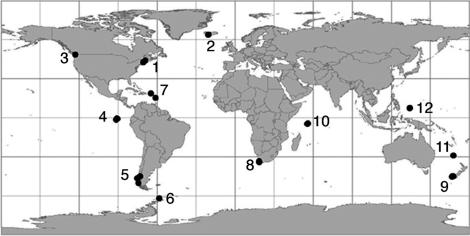
Field Sampling. We sampled the species richness of epifaunal invertebrates in local communities by taking high resolution photographs of quadrats (0.25 m2 area) at random locations along replicate transects stretched horizontally across rock wall habitats at a 10- to 15-m depth. The spatial scale of the local community referred to here is an ≈30- to 120-m-long horizontal span of rock wall habitat. The total area of the photo quadrats randomly sampled within this area was determined by species accumulation curves (below) and ranged from 5.0 to 18.25 m2 per site. Photo quadrats were taken with a “quadrapod” camera framer, mounted with a Nikonos V (Nikon) camera equipped with a 15-mm wide-angle lens and two strobes as in ref. 36. Rock walls are uniquely well suited for studying the effects of regional and local processes on community structure across a broad range of latitudes because they are nearly flat vertical surfaces, minimizing potentially confounding differences in habitat heterogeneity between sites and regions. Furthermore, they are geographically widespread in temperate, tropical, and polar regions varying greatly in species richness, allowing us to estimate the influence of the regional species pool on a global scale. By using identical methodology, three to five sites were sampled per region yielding a total of 49 local sites sampled in 12 biogeographic regions from 1991 to 2003 (Fig. 1). The only exception to this level of site replication occurred in Antarctica, where logistical constraints prevented sampling more than two local sites. Over half of the sites sampled for species richness were located in marine reserves (Galapagos Islands, Seychelles Islands, and Palau Islands), national parks (Eastern Caribbean and Norfolk Islands), or a biosphere reserve (New Zealand Fiords and Antarctica).
Fig. 1.
Equal area projection map showing the location of sites sampled for the species richness of epifaunal communities. At this scale, most of the dots representing individual sites within a region are superimposed on each other. Lines of latitude are in 30° increments. 1, Gulf of Maine; 2, Iceland; 3, northeast Pacific; 4, Galapagos Islands; 5, Chilean Patagonia; 6, Antarctic Peninsula; 7, eastern Caribbean; 8, southwest Africa; 9, southwest New Zealand; 10, Seychelles Islands; 11, Norfolk Islands; 12, Palau Islands.
Species Richness. Species in a total of 1,503 photo quadrats of epifaunal communities were identified to the lowest taxonomic level possible and counted. Species identifications were made in consultation with taxonomic experts, by referring to voucher specimens collected from the quadrats and to photo libraries constructed from species in the photo quadrats. Where identifications could not be made to the species level, a descriptive name was assigned to the organism and referenced to the photo library for consistency. Because of the difficulty of sampling thinly encrusting species of sessile invertebrates by destructive methods, these photographic estimates of species richness can be equivalent to or higher than estimates obtained by destructive sampling. For instance, comparisons of destructive (airlift) vs. photo quadrat methods to estimate mean species richness (SA and SP, respectively) of epifauna at three rock wall sites in the Gulf of Maine indicated either no difference in mean richness per 0.25 m2 between the two methods [Ammen Rock, SA = 10.3 (1.04 SE), SP = 10.4 (0.73SE), ANOVA, F = 0.08, P ≤ 0.777, nonsignificant] or that mean species richness estimated by the photographic method was significantly higher than that estimated by destructive sampling [Columbia Ledge, SA = 8.6 (0.77) vs. SP = 12.9 (0.65), F = 18.4, P ≤ 0.005; Machias Seal Island, SA = 10.0 (0.64) vs. SP = 14.1 (0.84), F = 15.7, P ≤ 0.01]. The statistical comparisons were all based on n = 10 quadrats per method, resulting in 1, 18 df for each ANOVA.
To fully understand the relationships between local, regional, and latitudinal patterns of species richness, we used two estimators of local species richness and three types of regional species pools. The local species richness estimates were based on species accumulation curves (richness as a function of number of quadrats) constructed from 100 randomized poolings of the quadrats at each site by using estimates software (Fig. 2) (37). Local species richness at each site was estimated as the asymptote of the species accumulation curve, where increased sampling does not add an appreciable number of new species. We used both the species observed (Sobs) and Chao2 as estimates of local species richness. Chao2 estimates the asymptote of the species accumulation curve by taking into account the effect of rare species on total richness and may provide a better estimate of true species richness for small numbers of samples (38).
 |
where Sobs is the number of species observed in the pooled quadrat samples, Q1 is the number of species occurring in one quadrat, and Q2 is the number of species occurring in two quadrats.
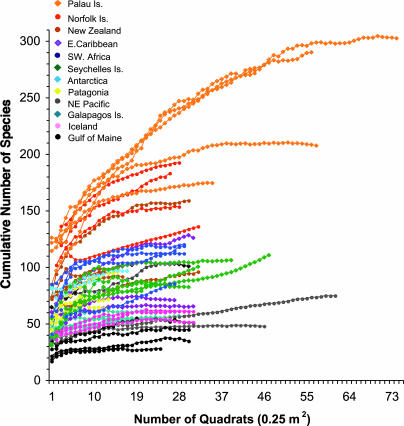
Fig. 2.
Species accumulation curves for 49 sites plotted as a function of number of quadrats censused, by using the Chao2 estimate of species richness. Curves are color-coded by region, representing the same regions shown in Fig. 1. The endpoint of each curve was used as local species richness in regressions of regional vs. local species richness.
The regional species pool was estimated as the number of epifaunal invertebrate species capable of living on hard substrata at shallow depths (<20 m). The standard regional pool was assembled from published species lists and by consulting taxonomic experts in each region. The boundaries of the regions were defined by biogeographic limits to the fauna, usually delineated by oceanographic currents and physical geography. Biogeographic regions were initially selected to represent at least three replicates of low, intermediate, and high regional species richness, based on a map of global shallow-water diversity (39). Because the minimum body size of organisms identifiable to species in the photo quadrats was 1.0 mm, the small invertebrate component (hydroids, bryozoans) of the standard regional pool was filtered by removing congeneric species of these groups, assuming that we may not have been able to recognize small individuals of congeners as different species. Using this reduced regional pool in the regressions of local vs. regional species richness guarded against the possibility of biasing the regional-local richness relationship toward saturation, due to potential underestimation of local richness. Summing the species lists of all sites within a region assembled the third regional species pool. This summed regional pool was used to avoid any unknown, potentially confounding effects of underestimating the regional pool in regressions of latitude vs. the local-regional richness ratio, or in tests of latitudinal variation in the extent of regional influence on local species richness.
Results and Discussion
The local species richness of epifaunal invertebrates (Fig. 2) varied greatly between biogeographic regions, with a 10.7-fold difference between the lowest richness in the Gulf of Maine (28.2 species) and the highest species richness in Palau (303.7 species, both Chao2 estimates). Rock wall sites in the Norfolk Islands, Australia contained the second highest levels of local species richness, with sites in New Zealand fiords ranked third (Fig. 2). The species accumulation curves separate into two groups roughly demarcated at 136 species per local site (Fig. 2). The nine sites with greater numbers of species than this (three sites in the Norfolk Islands, Bauza Island in New Zealand, and all five sites in Palau) support epifaunal communities that are exceptionally diverse. Worldwide, the epifaunal communities were composed of species belonging to 10 invertebrate phyla, with sponges, cnidarians, and urochordates (ascidians) accounting for the three highest percentages of the fauna (unpublished data).
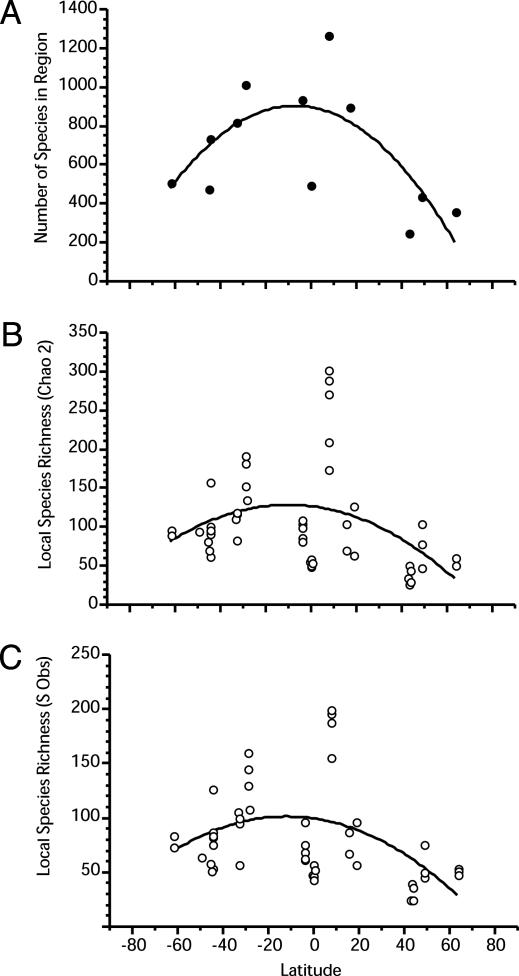
Latitudinal patterns of species richness were evident at both regional and local spatial scales (Fig. 3) in the form of a unimodal (concave down parabola) relation where species richness increased from high latitudes toward peak values at low latitudes (≈7.0°N). The effect of latitude on species richness was stronger at regional (r2 = 0.39) than at local spatial scales (r2 = 0.136, Chao2; r2 = 0.155, Sobs; Table 1). The lower percent variance explained by the effect of latitude on species richness at the local scale was due to high variation in local S at sites from 4.4°S to 7.3°N latitude.
Fig. 3.
Species richness as a function of latitude. (A) Regional species richness (standard regional pool). (B) Local species richness based on the Chao2 estimate. (C) Local species richness as Sobs. Lines represent significant, best fits to second-order polynomial equations (Table 1).
Table 1. Second-order polynomial (quadratic) regressions of latitude vs. species richness.
| Equation | r2 | P |
|---|---|---|
| S vs. latitude | ||
| Regional S = 896.353 - 2.034 latitude - 0.141 latitude2 | 0.391 | 0.043 |
| Local S (Chao) = 126.702 - 0.359 latitude - 0.018 latitude2 | 0.136 | 0.013 |
| Local S (Sobs) = 99.532 - 0.304 latitude - 0.013 latitude2 | 0.155 | 0.007 |
| Local/regional S vs. latitude | ||
| Chao/regional S = β0 - β1 latitude - β2 latitude2 | 0.071 | NS |
| Sobs/regional S = 0.354 + 0.00014 latitude + 0.000012 latitude2 | 0.129 | 0.041 |
| Chao/sum regional S = 0.765 + 0.001 latitude + 0.0000724 latitude2 | 0.261 | 0.0004 |
| Sobs/sum regional S = 0.670 + 0.000469 latitude + 0.00000595 latitude2 | 0.280 | 0.0002 |
The first equation has 2, 9 df; all others have 2, 46 df. Local-regional S ratios were arcsin square-root-transformed prior to regression analysis. NS, nonsignificant.
Local species richness often varied strongly among sites within regions (Fig. 3 B and C). For example, estimates of local richness at the Norfolk Islands, the Palau Islands, and South Africa varied by a factor of 1.5-2 among sites within these regions (Fig. 3 B and C). Although we presently lack sufficient information to elucidate the key physical and biological processes causing high intersite differences in species richness, the unusually low species richness at some of the sites was related to the effects of competitive dominance and predation. For instance, the lowest species richness values in South Africa (Sobs = 58, -33.5°S latitude midpoint; Fig. 3C) occurred at the Pannekoek site, where an encrusting sponge, Spirastrella sp., dominated space on the walls. Rock walls with low cover of this species were 2 times more diverse. The lowest species richness in the Norfolk Islands occurred on Kingston Reef walls (Sobs = 109, -29.5°S latitude midpoint), where sea urchin grazing apparently reduced invertebrate diversity and a competitively dominant encrusting coral, Montipora tuberculosa, was common (J.D.W., unpublished observations).
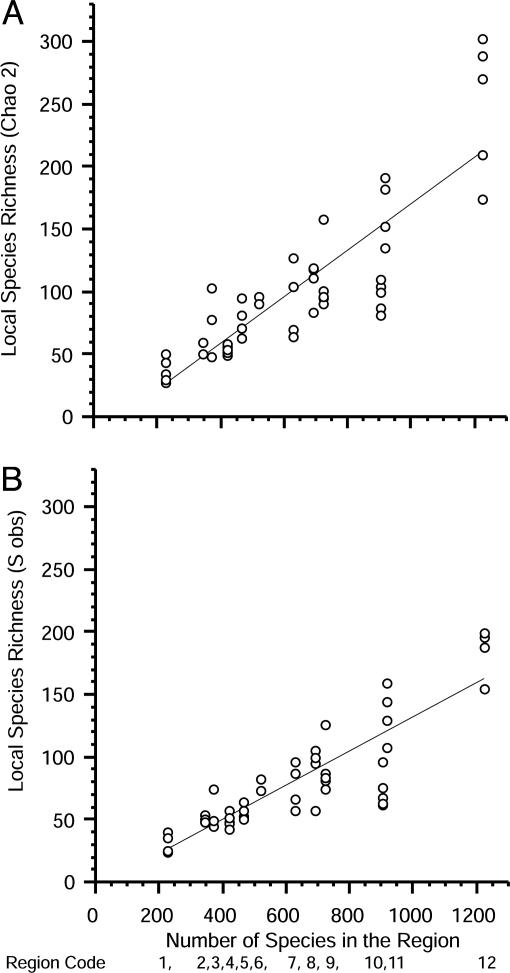
Linear and nonlinear least-squares techniques were used to test the nature of the relationship between local and regional diversity. The nonlinear models included a power function and an exponential function (Table 2). All models estimate two parameters so that the variance explained can be used to distinguish between saturation and regional enrichment. The best model in each case was linear, which explained 73% and 76% of the variance in Chao2 and Sobs, respectively (Table 2 and Fig. 4). For both dependent variables, the power function accounts for slightly more variance (Table 2), but the exponent is > indicating that the curve is concave up, rather than concave down as would be predicted by saturation. Moreover, plots of both nonlinear curves are strongly linear over the range of diversity values in our data. For a variety of statistical reasons and because the exact form of a saturating function has not been specified theoretically, others have suggested using the slope of a log-log plot as a test for saturation (40). A slope significantly less than 1 is indicative of saturation, whereas a slope that does not differ from 1 implies regional enrichment. For both Sobs and Chao2, the slopes in a log-log analysis do not differ from 1 [Sobs slope = 0.931 ± 0.146, 95% confidence interval (C.I.); Chao2 slope = 0.978 ± 0.156, 95% C.I.], also implying a strong influence of regional enrichment. Similar findings resulted from a model II regression analysis: Sobs major axis slope = 1.06 ± 0.152, 95% C.I.; Chao2 major axis slope = 1.13 ± 0.166, 95% C.I. If the mean of local site richness within a region [instead of individual site values (27)] is used in linear regression analysis, the percent variation of local richness explained by the richness of the regional species pool increases to 82.3-82.4% (Chao2 and Sobs, P < 0.0001).
Table 2. Results of regression analyses of regional species richness (y) on local species richness (x) for linear and nonlinear models.
| Equation | a | b | r2 |
|---|---|---|---|
| Chao2 | |||
| y = a + bx | -11.8 | 0.185 | 0.733 |
| y = a(1 - e-bx) | 880 | 2.1 × 10-4 | 0.71 |
| y = axb | 0.04 | 1.21 | 0.744 |
| Sobs | |||
| y = a + bx | -2.242 | 0.137 | 0.761 |
| y = a(1 - e-bx) | 558 | 2.7 × 10-4 | 0.742 |
| y = axb | 0.077 | 1.08 | 0.763 |
All were significant at P < 0.001.
Fig. 4.
Plots of regional vs. local species richness based on the Chao2 (A) and Sobs (B) estimates of species richness. Lines represent significant, best fits by least-squares linear regression (Table 2). Region codes below the x axis are the same as in Fig. 1 and here identify the order of regional species richness (reduced regional pool).
These findings imply that even in the most diverse regions of the world, the number of species coexisting in local communities of epifaunal invertebrates is influenced by the size of the regional species pool (type I). No saturation is evident.
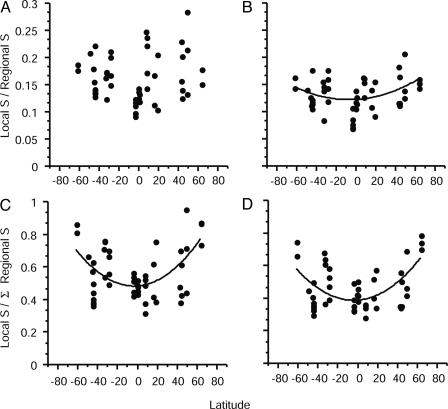
To place these results in a global perspective, we investigated whether the strong regional enrichment of local diversity varies with latitude. The null hypothesis tested was that the influence of the regional pool on local richness is invariant with latitude. This was addressed by testing for latitudinal variation in the ratio of local (site) richness to regional richness (i.e., alpha/gamma diversity), using both the standard regional pool and the summed regional pool. Using the summed pool as the denominator in the second ratio enabled us to measure the extent of regional pool influence without any unknown bias stemming from inaccurately estimating the regional species pool in different latitudinal areas. Three of the four analyses of latitudinal variation in the local-regional richness ratio [Sobs/standard regional pool (Fig. 5B), Chao/summed regional pool (Fig. 5C), and Sobs/summed regional pool (Fig. 5D)] display significant parabolic, concave-up relationships to latitude (Table 1), indicating that the proportion of the regional species pool present in local communities increases from low to high latitudes. Because significant quadratic fits to latitude were obtained by using ratios based on the summed regional pool as well as the standard pool, latitudinal patterns in the local:regional ratio were not affected by unknown biases in estimating the size of the regional pool. Another potential source of bias in the characterization of latitudinal diversity gradients occurs when sampling regimes or diversity measures are spatially autocorrelated (19). In this study, it was possible that the ratios of local to regional species richness calculated with the summed regional species pool might be higher among proximate local sites than among more distant ones, because proximate sites might have more species in common. Consequently, average intersite distances were calculated for local sites within regions by using the geodistances program in the r software package (41). Regression analyses yielded no significant linear (P > 0.63) or nonlinear (second order polynomial, P > 0.75) relationships of intersite distance to latitude, leading us to conclude that latitudinal variation in the extent of regional influence on local species richness is an emergent property of the data.
Fig. 5.
Plots of the ratio of local to regional species richness as a function of latitude. Untransformed data are plotted here for ease of interpretation. (A) Chao2 local S/standard regional pool. (B) Sobs local S/standard regional pool. (C) Chao2 local S/summed regional pool. (D) Sobs local S/summed regional pool. All ratios but that in A are significant functions of latitude (polynomial equations on arcsin square-root-transformed data in Table 1).
To our knowledge, this is the first shallow-water (non-deep sea) diversity study to report significant latitudinal variation in species richness at both regional and local spatial scales. Because the latitudinal effect was stronger at regional than at local spatial scales, we envision that the main effects of latitudinal mechanisms on local diversity are expressed through variation in regional pool size and thus are likely to be evolutionary in nature. The variation in species richness among sites within regions probably reflects local-scale processes such as intersite variability in abiotic conditions, biological interactions, habitat heterogeneity, and stochastic variation in recruitment (42). However, much of the variation in local richness among regions at global scales reflects differences in the size of the regional species pool, implicating historical and evolutionary processes. Understanding to what extent the variance in local diversity reflects ecological or evolutionary mechanisms will be essential for identifying the forces that ultimately structure these communities.
The finding that regional enrichment of local marine diversity increased from low to high latitudes contrasts with the result that fig wasp communities are equally influenced by the regional species pool across a tropical-temperate (6.0°N to 34.0°S latitude) gradient (43). An important field study of coral species richness (44) corroborated earlier results from literature-based studies (45) that the richness of coral communities is strongly influenced by regional pool richness across a longitudinal diversity gradient and showed that the regional effect is similarly strong in three reefal habitats. At this point, we can only frame hypotheses for future investigation to explain potential causes of a greater regional species pool effect in local marine communities at high latitudes than at low latitudes. The pattern may be related to (i) latitudinal differences in the process of colonization from the regional pool at low and high latitudes, such as greater dispersal of larvae or propagules at high latitudes or greater predation on competitive dominants (2) at high latitudes, facilitating colonization; (ii) latitudinal differences in disturbance, resulting in greater amounts of open substrate at high latitudes; (iii) latitudinal differences in enhanced competitive resource partitioning in the tropics resulting in fewer vacant niches, greater saturation, and less regional pool influence (5); (iv) latitudinal differences in productivity, because the regional pool effect might be greater at intermediate levels of productivity (latitudes 40-60°) where other factors (competition, disturbance) are not limiting diversity as described in ref. 29; and (v) a reduced regional pool size at high latitudes that permits local communities to support a greater complement of the regional diversity.
In conclusion, our results suggest that the number of species coexisting in local marine communities is influenced by the diversity of the regional species pool and is not determined solely by small-scale ecological processes operating within communities. The richness of the regional species pool is determined by rates of speciation, immigration, and extinction and by geologic events (25), all of which operate on larger scales of space and time than typically considered in community ecology. Regional influences on local richness assume dispersal from the regional pool to local communities, a phenomenon that is both historical and contemporary. Because these epifaunal communities are not saturated, they also may be particularly susceptible to the influence of invasive species as they immigrate to new biogeographic regions (42). Regional-level effects such as larval dispersal, metapopulation dynamics, landscape ecology, and the evolutionary buildup of the regional species pool will interact with small-scale biotic and abiotic processes to regulate the number and composition of locally coexisting species. This result does not imply that local ecological processes are unimportant, but rather that broadening the perspective to incorporate the effects of regional and latitudinal scale processes is needed to fully understand the origin and maintenance of diversity in local marine communities.
Shallow-water marine communities are being severely impacted worldwide by pollution, habitat destruction, and over-fishing (46). Our findings have important implications for understanding the forces that shape spatial and temporal variation in diversity as well as for conserving and managing marine biodiversity. To preserve and manage these communities, conservation strategies will need to incorporate much larger spatial and temporal scales not only to maintain local diversity, but also to foster evolution to ensure adequate dispersal from a diverse regional species pool.
Acknowledgments
Earlier versions of the manuscript were improved by critical comments from M. Rex, J. Hughes, and J. H. Brown. Statistical advice was kindly provided by A. Solow. This study could not have been accomplished without help from G. Branch, J. Byrnes, R. Bustamante, J. Bruno, T. Brito, L. Candisani, P. Edmunds, J. Ellis, K. Grange, S. Genovese, P. Colin, L. Colin, J. Collie, P. Jonssson, M. Hardwick-Witman, J. Leichter, S. Mayfield, R. Miller, P. Meredith, P. Pelletier, C. Rogers, and C. Siddon. Invaluable assistance with invertebrate taxonomy (samples and/or literature) was kindly provided by C. Battershill, D. Calder, L. Colin, M. Dethier, D. Gordon, C. Griffiths, P. Heywood, W. Hartman, C. Hickman, E. Kozloff, G. Lambert, D. Lee, C. Monniot, A. Olaffson, M. Page, T. Samaii, R.Van Soest, and J.Winston. We thank the National Science Foundation (NSF) (Biological Oceanography) for the funding that enabled us to complete this project. Additional funding was provided by the National Undersea Research Program (Avery Point, CT), the Andrew Mellon Foundation, the NSF CORONA project under the direction of C. Cunningham for travel to Iceland, and the Helen and Merrill Bank Foundation.
Author contributions: J.D.W. and R.J.E. designed research; J.D.W., R.J.E., and F.S. performed research; J.D.W., R.J.E., and F.S. analyzed data; and J.D.W. and R.J.E. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Gaston, K. J. (2000) Nature 405, 220-227. [DOI] [PubMed] [Google Scholar]
- 2.Paine, R. T. (1966) Am. Nat. 100, 65-75. [Google Scholar]
- 3.Huston, M. A. (1994) Biological Diversity (Cambridge Univ. Press, Cambridge, U.K.).
- 4.Mittlebach, G. G., Steiner, C. F., Scheiner, S. M., Gross, K. L. Reynolds, H. L. Waide, R. B., Willig, M. R., Dodson, S. L. & Gough, L. (2001) Ecology 82, 2381-2396. [Google Scholar]
- 5.Rosensweig, M. L. (1995) Species Diversity in Space and Time (Cambridge Univ. Press, Cambridge, U.K.).
- 6.Stevens, R. D. & Willig, M. R. (2002) Ecology 83, 545-560. [Google Scholar]
- 7.Hillebrand, H. (2004) Am. Nat. 163, 192-211. [DOI] [PubMed] [Google Scholar]
- 8.Clarke, A. & Crame, A. (1997) in Marine Diversity: Patterns and Processes, eds. Ormond, R. F., Gage, J. D. & Angel, M. D. (Cambridge Univ. Press, Cambridge, U.K.), pp. 122-147.
- 9.Rex, M. A., Stuart, C. T., Hessler, R. R., Allen, J. A., Sanders, H. L. & Wilson, G. D. F. (1993) Nature 365, 636-639. [Google Scholar]
- 10.Roy, K., Jablonski, D., Valentine, J. W. & Rosenberg, G. (1998) Proc. Natl. Acad. Sci. USA 95, 3699-3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhode, K. (1992) Oikos 65, 514-527. [Google Scholar]
- 12.Clarke, A. & Lidgard, S. (2000) J. Anim. Ecol. 69, 799-814. [DOI] [PubMed] [Google Scholar]
- 13.Coates, M. (1998) Global Ecol. Biogeogr. Lett. 7, 115-124. [Google Scholar]
- 14.Kendall, M. A. & Aschan, M. (1993) J. Exp. Mar. Biol. Ecol. 172, 157-169. [Google Scholar]
- 15.Gray, J. S. (2001) Polar Biol. 24, 633-641. [Google Scholar]
- 16.Gray, J. S. (2002) Mar. Ecol. Prog. Ser. 244, 285-297. [Google Scholar]
- 17.Ricklefs, R. E. (2004) Ecol. Lett. 7, 1-15. [Google Scholar]
- 18.Shurin, J. B. & Srivastava, D. S., Metacommunities, eds. Holyoak, M., Holt, R. & Leibold, M., in press.
- 19.Willig, M. R., Kaufman, D. M. & Stevens, R. D. (2003) Annu. Rev. Ecol. Evol. Syst. 34, 273-309. [Google Scholar]
- 20.Jackson, J. B. C. (1977) Am. Nat. 111, 743-767. [Google Scholar]
- 21.Grosberg, R. K. (1981) Nature 290, 700-702. [Google Scholar]
- 22.Sebens, K. P. (1986) Ecol. Monogr. 56, 73-96. [Google Scholar]
- 23.Smith, F. & Witman, J. D. (1999) Ecology 80, 51-69. [Google Scholar]
- 24.Ayling, A. L. (1981) Ecology 62, 830-847. [Google Scholar]
- 25.Ricklefs, R. E. (1987) Science 235, 167-171. [DOI] [PubMed] [Google Scholar]
- 26.Cornell, H. V. & Lawton, J. H. (1992) J. Anim. Ecol. 61, 1-12. [Google Scholar]
- 27.Srivastava, D. S. (1999) J. Anim. Ecol. 68, 1-16. [Google Scholar]
- 28.Hillebrand, H. & Bleckner, T. (2002) Oecologia 132, 479-491. [DOI] [PubMed] [Google Scholar]
- 29.Huston, M. A. (1999) Oikos 86, 393-401. [Google Scholar]
- 30.Loreau, M. (2000) Ecol. Lett. 3, 73-76. [Google Scholar]
- 31.Caswell, H. & Cohen, J. E. (1993) in Species Diversity in Ecological Communities: Historical and Geographical Perspectives, eds. Ricklefs, R. E. & Schluter, D. (Univ. of Chicago Press, Chicago), pp. 99-107.
- 32.Shurin, J. B. & Allen, E. G. (2001) Am. Nat. 158, 624-637. [DOI] [PubMed] [Google Scholar]
- 33.Mouquet, N. & Loreau, M. (2003) Am. Nat. 162, 544-577. [DOI] [PubMed] [Google Scholar]
- 34.Bruno, J. F., Stachowicz, J. J. & Bertness, M. D. (2003) Trends Ecol. Evol. 18, 119-125. [Google Scholar]
- 35.Mouquet, N., Munguia, P., Kneitel, J. M. & Miller, T. E. (2003) Oikos 103, 618-626. [Google Scholar]
- 36.Witman, J. D. (1985) Ecol. Monogr. 55, 421-445. [Google Scholar]
- 37.Colwell, R. K. (1997) estimates (Univ. of Connecticut, Storrs), Version 5.
- 38.Colwell, R. K. & Coddington, J. A. (1994) Philos. Trans. R. Soc. London B 345, 101-118. [DOI] [PubMed] [Google Scholar]
- 39.Valentine, J. & Moores, E. (1974) Sci. Am. 230, 80-89. [Google Scholar]
- 40.Griffiths, D. (1999) J. Anim. Ecol. 68, 1051-1055. [Google Scholar]
- 41.Casgrain, P. & Legendre, P. (2001) r (Univ. of Montreal, Montreal), Version 4.0d5.
- 42.Lawton, J. H. (2000) Community Ecology in a Changing World (Ecology Institute, Oldendorf/Luhe, Germany).
- 43.Hawkins, B. A. & Compton, S. G. (1992) J. Anim. Ecol. 61, 361-372. [Google Scholar]
- 44.Karlson, R., Cornell, H. V. & Hughes, T. P. (2004) Nature 429, 867-870. [DOI] [PubMed] [Google Scholar]
- 45.Karlson, R. & Cornell, H. V. (2002) Ecology 83, 452-463. [Google Scholar]
- 46.Norse, E. A. (1993) Global Marine Biological Diversity (Island, Washington, DC).