Abstract
Cryoablation for atrial fibrillation (AF) has rapidly become a mainstream treatment for AF. In this report, 163 patients who had undergone a cryoablation procedure at one clinical center were contacted by telephone 33.1 ± 3.3 months after the procedure. All patients had received cryoablation of the pulmonary vein ostia, although concomitant procedures were performed at the same time in over 50% of the patients, including radiofrequency and/or cryoablation of other areas of the left atrium. Freedom from a repeat ablation procedure was 87%, while freedom from recurrent hospitalization for AF was 89%, as compared to previous reports of 65%. Of the 13 patients who had a repeat ablation procedure, only one was found to have a reconnection of pulmonary veins, while 4 were found to have atrial flutter. Cryoablation for AF produces a durable result in most patients out to 3 years with better outcomes than previously reported.
Pulmonary vein isolation (PVI) has emerged as the gold standard of ablative strategies to treat medically refractory paroxysmal and persistent atrial fibrillation (AF) (1). But despite the superiority of catheter ablation based on PVI over antiarrhythmic drug therapy (2–4), recurrence rates of AF remain higher than desired (5). Among large trials, freedom from recurrent AF is about 65% (range 48%–77%), with follow-up limited to 12 to 28 months (6–18). For the cryoablation procedure, the second-generation cryoballoon catheter provides a larger and more uniform cooling zone (8, 9). We sought to determine the overall need for recurrent ablation procedures and hospitalizations for atrial arrhythmias among a group of consecutive patients who underwent ablation with the second-generation cryoballoon catheter.
METHODS
This study was a retrospective assessment of consecutive subjects who underwent cryoballoon-based catheter ablation of AF by 8 electrophysiologists (with 4 physicians performing 95% of the procedures) at the Baylor Heart and Vascular Hospital from May 18, 2012, through June 6, 2013. Follow-up after ablation was performed according to American Heart Association/Heart Rhythm Society guidelines for the first year and was at the discretion of each physician subsequently.
Patients were brought to the electrophysiology lab in a fasted state off all antiarrhythmic drug therapy. Under general anesthesia, a decapolar diagnostic catheter was placed in the coronary sinus via the femoral approach. After a single transseptal puncture, the 28 mm cryoballoon (Artic Front or Artic Front Advance, Medtronic, Inc., Minneapolis, MN) was introduced into the left atrium via a 12F steerable sheath (FlexCath, Medtronic). Pulmonary vein mapping was performed using either a 20-pole circular mapping catheter or an 8-pole transluminal circular mapping guide (Achieve, Medtronic). Catheter positioning was assessed by transluminal contrast injection and/or intracardiac echocardiography with the goal of complete pulmonary vein occlusion prior to lesion delivery. Cryo energy was delivered for 180 to 240 seconds at operator discretion, with a bonus lesion following isolation. Lesion duration was impacted by assessment for phrenic nerve dysfunction, esophageal temperature, balloon nadir temperature, and time to isolation, when measured. Only the 28 mm cryo balloon was used. PVI was reassessed 30 minutes after the final application at each vein.
A total of 218 patients were identified, and 163 patients were successfully contacted and interviewed. Patients were queried about whether any symptoms of AF had recurred, how the recurrent AF was diagnosed, how they perceived symptom resolution, if they had another ablation procedure, and if they had any subsequent hospitalizations for AF (separate from any ablation admissions).
For all patients, a chart review of the ablation procedure was performed. Information collected included the type of AF that had been present (paroxysmal, persistent, or both); if cardioversions for AF had ever been done; the subjective frequency of the AF preablation; the presence of the risk factors of hypertension, coronary artery disease, and cardiomyopathy; the presence and names of anticoagulant and antiarrhythmic medications before or after the procedure; the exact procedure that was done (including information on concomitant ablation sites treated); the number of pulmonary veins present and ablated; and the presence of any complications of the procedure. This study was reviewed and approved by the Baylor Research Institute institutional review board. All patients provided verbal phone consent.
Means, standard deviations, medians, interquartile range, and percentages were calculated to describe the study cohort. Freedom from recurrent atrial ablation or hospitalization for AF was estimated with a Kaplan-Meier analysis. Estimates were used to generate freedom from recurrent atrial ablation and freedom from hospitalization for AF curves in this study cohort.
RESULTS
A total of 163 patients were interviewed by telephone a mean of 33 months after the cryoablation procedure. The mean age of the patients was 64 years, and the group was divided about equally between men and women (Table 1). Most patients were white. Prior to the ablation, patients had had AF for 4½ years, and two-thirds had paroxysmal AF. Interestingly, one-third had a concomitant diagnosis of atrial flutter by history. This index AF ablation procedure was the second procedure for 7% and the third procedure for 3%.
Table 1.
Baseline characteristics of 163 patients who underwent a cryoablation procedure
| Variable | Value |
|---|---|
| Age, years (mean ± SD) | 63.6 ± 12.0 |
| Male | 95 (58.3%) |
| Female | 68 (41.7%) |
| Race/ethnicity | |
| White | 157 (96.3%) |
| Black | 2 (1.2%) |
| Other | 0 (0%) |
| Hispanic or Latino | 4 (2.5%) |
| Duration of atrial fibrillation, months (mean ± SD) | 56.2 ± 63.9 |
| Time between ablation and interview, months (mean ± SD) | 34.0 ± 3.3 |
| Type of atrial fibrillation | |
| Paroxysmal | 108 (66.3%) |
| Persistent | 33 (20.3%) |
| Both | 22 (13.1%) |
| Concomitant atrial flutter, by patient history | 63 (38.7%) |
| Index ablation procedure was the | |
| First ablation procedure | 146 (90.0%) |
| Second ablation procedure | 12 (7.4%) |
| Third ablation procedure | 5 (3.1%) |
| Risk factors | |
| Hypertension | 93 (57.1%) |
| Coronary artery disease | 21 (12.9%) |
| Cardiomyopathy | 25 (15.3%) |
Many patients (68%) were on an anticoagulant prior to the procedure, including dabigatran (37.6%), warfarin (34.8%), rivaroxaban (27.0%), and apixaban (0.9%). Similarly, many (66%) were on an antiarrhythmic medication at the time of evaluation for the ablation procedure (although all antiarrhythmic medication was held prior to the procedure), which included flecainide (32.4%), sotalol (19.4%), amiodarone (16.7%), dronedarone (13.0%), propafenone (9.3%), dofetilide (6.5%), and diltiazem (2.8%).
All patients in this study underwent cryoballoon PVI (Table 2). In addition, 10% to 20% of patients had either radiofrequency or cryoablation in other parts of the left atrium, and 20% specifically had an atrial flutter ablation in addition to the AF ablation. There were 16 complications, all of which resolved within 30 days (Table 3).
Table 2.
Procedures performed among 163 patients who underwent a cryoablation procedure
| Procedure | N (%) |
|---|---|
| Cryoablation pulmonary vein isolation | 163 (100.0%) |
| Cryoablation plus radiofrequency to complete the pulmonary vein isolation | 18 (11.0%) |
| Cryoablation plus balloon lesions to nonpulmonary vein areas | 20 (12.3%) |
| Cryoablation plus radiofrequency to nonpulmonary vein areas | 17 (10.4%) |
| Cryoablation plus typical atrial flutter ablation | 33 (20.3%) |
| Cryoablation plus ablation on nonpulmonary vein atrial tachycardia | 1 (0.6%) |
Some patients had more than one concomitant site treated.
Table 3.
Outcomes of 163 patients who underwent a cryoablation procedure
| Outcome | Value |
|---|---|
| Months between ablation and phone call | 34.1 ± 3.3 |
| Postprocedure vascular complications | 8 (4.9%) |
| Postprocedure phrenic nerve dysfunction | 8 (4.9%) |
| Postprocedure pericardiocentesis | 0 (0.0%) |
| Repeat hospitalization | 19 (11.7%) |
| Repeat ablation procedure | 21 (12.9%) |
| Patient-reported symptom resolution (N = 143) | |
| 50% fewer spells | 20 (13.9%) |
| 75% fewer spells | 14 (9.7%) |
| 90% fewer spells | 23 (16.0%) |
| No further spells | 86 (60.1%) |
Nineteen patients had another ablation procedure, and for 13 of these 19, the next procedure was performed at our institution. In one patient with a repeat ablation procedure, there was a reconnection of the pulmonary veins (19). Seven patients were found to have residual potential in a pulmonary vein antrum. Four patients had atrial flutter, including atypical atrial flutter. Various other locations of an electrical focus were found on the left atrial roof, posterior wall, superior vena cava, left atrial appendage, and right atrial appendage.
DISCUSSION
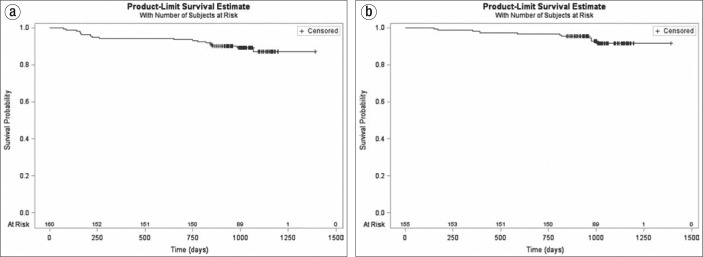
The main findings of our study were that, with long-term follow-up of an ablation procedure using the second-generation cryoballoon, 1) freedom from recurrent ablation was observed in 87% of subjects (Figure 1a); 2) freedom from hospitalization for atrial arrhythmias was seen in 88% of subjects (Figure 1b); and 3) most were either free of AF symptoms or had a reduced burden after a follow-up of nearly 3 years. Complication rates were acceptably low, and no procedural-related issues emerged late in follow-up. When AF recurred, there was a finding in the pulmonary veins, consistent with either a reconnection or incomplete ablation at the index procedure. All patients with recurrence underwent a successful second ablation procedure.
Figure 1.
Freedom from (a) repeat ablation procedure and (b) any hospitalization for atrial fibrillation among 163 patients who underwent a cryoablation procedure.
The majority of publications on AF and cryoablation have focused on freedom from 30 seconds of AF during the time period of 3 to 12 months postablation. More recent reports have focused on cryoablation for somewhat longer times and have shown a 65% rate of freedom from AF for 12 to 28 months postablation (Table 4) (6–18). This study thus demonstrated a higher success rate over a longer time of observation.
Table 4.
Freedom from atrial fibrillation with radiofrequency ablation and cryoablation∗
| First author, year of publication | N | Radiofrequency ablation | Cryoablation | Follow-up (months) |
|---|---|---|---|---|
| Knecht, 2014 | 208 | 56% | 48% | 28 |
| Mugnai, 2014 | 396 | 73% | 63% | 23 |
| Cheng, 2015 | 1216 | 65% | 67% | 16.5 |
| Wasserlauf, 2015 | 201 | 61% | 60% | 12 |
| Squara, 2015 | 376 | 76% | 73% | 18 |
| Aryana, 2015 | 1196 | 60% | 77% | 12 |
| Luik, 2015 | 315 | 63% | 64% | 6 |
| Straube, 2016 | 373 | 60% | 70% | 17 |
From references 11 to 18.
While freedom from AF represents a standard and important endpoint, it does not directly reflect the need for recurrent, expensive resource utilization. The cost of a typical AF ablation has been estimated at $80,000 and a hospitalization related to AF at $35,000. Prevention of repeat ablation procedures can also mitigate the risk of additional complications.
This was a retrospective, observational study, and 55 patients in the consecutive series could not be reached. This was a single-center study involving multiple operators. In this study, the presence of recurrent AF was assessed historically, and a routine systematic approach to AF monitoring was not performed. We were not able to correlate symptoms of palpitations in these patients to the presence or absence of any specific arrhythmia. Finally, we did not have data on resource utilization prior to patients' index ablation.
References
- 1.Marine JE. Catheter ablation therapy for supraventricular arrhythmias. JAMA. 2007;298(23):2768–2778. doi: 10.1001/jama.298.23.2768. [DOI] [PubMed] [Google Scholar]
- 2.Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A, Macle L, Daoud EG, Calkins H, Hall B, Reddy V, Augello G, Reynolds MR, Vinekar C, Liu CY, Berry SM, Berry DA ThermoCool AF Trial Investigators. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303(4):333–340. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 3.Packer DL, Kowal RC, Wheelan KR, Irwin JM, Champagne J, Guerra PG, Dubuc M, Reddy V, Nelson L, Holcomb RG, Lehmann JW, Ruskin JN STOP AF Cryoablation Investigators. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61(16):1713–1723. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 4.Morillo CA, Verma A, Connolly SJ, Kuck KH, Nair GM, Champagne J, Sterns LD, Beresh H, Healey JS, Natale A RAAFT-2 Investigators. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA. 2014;311(7):692–700. doi: 10.1001/jama.2014.467. [DOI] [PubMed] [Google Scholar]
- 5.Andrade JG, Khairy P, Macle L, Packer DL, Lehmann JW, Holcomb RG, Ruskin JN, Dubuc M. Incidence and significance of early recurrences of atrial fibrillation after cryoballoon ablation: insights from the Multicenter Sustained Treatment of Paroxysmal Atrial Fibrillation (STOP AF) trial. Circ Arrhythm Electrophysiol. 2014;7(1):69–75. doi: 10.1161/CIRCEP.113.000586. [DOI] [PubMed] [Google Scholar]
- 6.Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS, Sullivan T, Roberts-Thomson KC, Sanders P. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2(2):e004549. doi: 10.1161/JAHA.112.004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrade JG, Khairy P, Guerra PG, Deyell MW, Rivard L, Macle L, Thibault B, Talajic M, Roy D, Dubuc M. Efficacy and safety of cryoballoon ablation for atrial fibrillation: a systematic review of published studies. Heart Rhythm. 2011;8(9):1444–1451. doi: 10.1016/j.hrthm.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 8.Aryana A, Morkoch S, Bailey S, Lim HW, Sara R, d'Avila A, O'Neill PG. Acute procedural and cryoballoon characteristics from cryoablation of atrial fibrillation using the first- and second-generation cryoballoon: a retrospective comparative study with follow-up outcomes. J Interv Card Electrophysiol. 2014;41(2):177–186. doi: 10.1007/s10840-014-9942-7. [DOI] [PubMed] [Google Scholar]
- 9.Pandya B, Sheikh A, Spagnola J, Bekheit S, Lafferty J, Kowalski M. Safety and efficacy of second-generation versus first-generation cryoballoons for treatment of atrial fibrillation: a meta-analysis of current evidence. J Interv Card Electrophysiol. 2016;45(1):49–56. doi: 10.1007/s10840-015-0075-4. [DOI] [PubMed] [Google Scholar]
- 10.Reddy VY, Sediva L, Petru J, Skoda J, Chovanec M, Chitovova Z, Di Stefano P, Rubin E, Dukkipati S, Neuzel P. Durability of pulmonary vein isolation with cryoballoon ablation: results from the Sustained PV Isolation with Arctic Front Advance (SUPIR) study. J Cardiovasc Electrophysiol. 2015;26(5):493–500. doi: 10.1111/jce.12626. [DOI] [PubMed] [Google Scholar]
- 11.Knecht S, Sticherling C, von Felton S, Conen D, Schaer B, Ammann P, Altmann D, Osswald S, Kühne M. Long-term comparison of cryoballoon and radiofrequency ablation of paroxysmal atrial fibrillation: a propensity score matched analysis. Int J Cardiol. 2014;176(3):645–650. doi: 10.1016/j.ijcard.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 12.Mugnai G, Chierchia GB, de Asmundis C, Sieira-Moret J, Conte G, Capulzini L, Wauters K, Rodriguez-Mañero M, Di Giovanni G, Baltogiannis G, Ciconte G, Saitoh Y, Juliá J, Brugada P. Comparison of pulmonary vein isolation using cryoballoon versus conventional radiofrequency for paroxysmal atrial fibrillation. Am J Cardiol. 2014;113(9):1509–1513. doi: 10.1016/j.amjcard.2014.01.425. [DOI] [PubMed] [Google Scholar]
- 13.Cheng X, Hu Q, Zhou C, Liu LQ, Chen T, Liu Z, Tang X. The long-term efficacy of cryoballoon vs irrigated radiofrequency ablation for the treatment of atrial fibrillation: A meta-analysis. Int J Cardiol. 2015;181:297–302. doi: 10.1016/j.ijcard.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Wasserlauf J, Pelchovitz DJ, Rhyner J, Verma N, Bohn M, Li Z, Arora R, Chicos AB, Goldberger JJ, Kim SS, Lin AC, Knight BP, Passman RS. Cryoballoon versus radiofrequency catheter ablation for paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2015;38(4):483–489. doi: 10.1111/pace.12582. [DOI] [PubMed] [Google Scholar]
- 15.Squara F, Zhao A, Marijon E, Latcu DG, Providencia R, Di Giovanni G, Jauvert G, Jourda F, Chierchia GB, De Asmundis C, Ciconte G, Alonso C, Grimard C, Boveda S, Cauchemez B, Saoudi N, Brugada P, Albenque JP, Thomas O. Comparison between radiofrequency with contact force-sensing and second-generation cryoballoon for paroxysmal atrial fibrillation catheter ablation: a multicentre European evaluation. Europace. 2015;17(5):718–724. doi: 10.1093/europace/euv060. [DOI] [PubMed] [Google Scholar]
- 16.Aryana A, Singh SM, Kowalski M, Pujara DK, Cohen AI, Singh SK, Aleong RG, Banker RS, Fuenzalida CE, Prager NA, Bowers MR, D'Avila A, O'Neill PG. Acute and long-term outcomes of catheter ablation of atrial fibrillation using the second-generation cryoballoon versus open-irrigated radiofrequency: a multicenter experience. J Cardiovasc Electrophysiol. 2015;26(8):832–839. doi: 10.1111/jce.12695. [DOI] [PubMed] [Google Scholar]
- 17.Luik A, Radzewitz A, Kieser M, Walter M, Bramlage P, Hörmann P, Schmidt K, Horn N, Brinkmeier-Theofanopoulou M, Kunzmann K, Riexinger T, Schymik G, Merkel M, Schmitt C. Cryoballoon versus open irrigated radiofrequency ablation in patients with paroxysmal atrial fibrillation: the prospective, randomized, controlled, noninferiority FreezeAF study. Circulation. 2015;132(14):1311–1319. doi: 10.1161/CIRCULATIONAHA.115.016871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Straube F, Dorwarth U, Ammar-Busch S, Peter T, Noelker G, Massa T, Kuniss M, Ewertsen NC, Chun KR, Tebbenjohanns J, Tilz R, Kuck KH, Ouarrak T, Senges J, Hoffmann E. FREEZE Cohort Investigators First-line catheter ablation of paroxysmal atrial fibrillation: outcome of radiofrequency vs. cryoballoon pulmonary vein isolation. Europace. 2016;18(3):368–375. doi: 10.1093/europace/euv271. [DOI] [PubMed] [Google Scholar]
- 19.Heeger C-H, Wissner E, Mathew S, Deiss S, Lemes C, Rilling A, Wohlmuth P, Reissmann B, Tilz RR, Ouyang F, Kuck KH, Metzner A. Once isolated, always isolated? Incidence and characteristics of pulmonary vein reconstruction after second-generation cryoballoon-based pulmonary vein isolation. Circ Arrhythm Electrophysiol. 2015;8(5):1088–1094. doi: 10.1161/CIRCEP.115.003007. [DOI] [PubMed] [Google Scholar]