Abstract
Anticonvulsant hypersensitivity syndrome (AHS) is a potentially fatal multiorgan drug reaction that presents with various cutaneous eruptions. There is a genetic predisposition to such reactions. We present a young woman with AHS due to carbamazepine that presented as an atypical erythema multiforme with elevated liver enzymes.
Anticonvulsant hypersensitivity syndrome (AHS) is a rare condition that can be life-threatening if misdiagnosed. It is characterized by the triad of fever, skin eruptions, and lymphadenopathy, typically with systemic involvement, that occurs 1 to 8 weeks after the initiation of anticonvulsants (1). Cyclical anticonvulsants including pheny-toin, phenobarbital, and carbamazepine are the most common culprits, but AHS has also been reported with lamotrigine and valproic acid (1). The mechanism is believed to be related to genetic variations in the metabolism of these medications. There seems to be a strong genetic component (2). AHS is estimated to occur in 1:1000 to 1:10,000 patients prescribed anticonvulsants. The syndrome is frequently undetected and underreported (1). We present a case of a young woman who presented with a diffuse skin rash and elevated liver enzymes after starting carbamazepine.
CASE DESCRIPTION
A 30-year-old white woman with a history of bipolar disorder, congenital heart disease, and migraines was admitted to the hospital with complaints of a generalized, painful rash, nausea, vomiting, facial edema, and malaise. The lesions started on her ears and arms and progressed to involve her entire body. Approximately 6 days prior to the onset of the rash, she was started on carbamazepine for bipolar disorder. She was seen 2 days later at an outside facility for a migraine and prescribed propranolol and an oral methylprednisolone 6-day taper starting at 24 mg a day. A few days later, she presented to the emergency department for urinary retention and nausea and was found to have a nondescript rash on her chest and upper extremities. The rash produced a burning sensation and was hypersensitive to touch. She was instructed to discontinue carbamazepine and was given a dose of diphenhydramine, ondansetron, and morphine and discharged on propoxyphene and ondansetron. Her rash continued to progress to involve her face, lips, abdomen, back, hands, and feet. She was seen again by her primary care provider, who instructed her to go to the emergency room to be admitted.
On the day of admission, she developed facial edema and worsening xerosis of the lips. Examination revealed multiple, deep red macules that coalesced into patches, predominantly on the upper extremities, chest, upper back, face, and ears. Some of the patches had dusky centers and developed edematous vesicles (Figure 1). There were more dispersed erythematous 3- to 5-mm macules located on her abdomen, lower back, upper thighs, distal fingertips, palms, and soles (Figure 2). There were no mucosal ulcerations or lymphadenopathy. Associated symptoms included nausea, nonbloody emesis, arthralgia, back pain, dyspnea, and nonproductive cough. She denied fevers but reported chills. She was afebrile throughout her hospitalization.
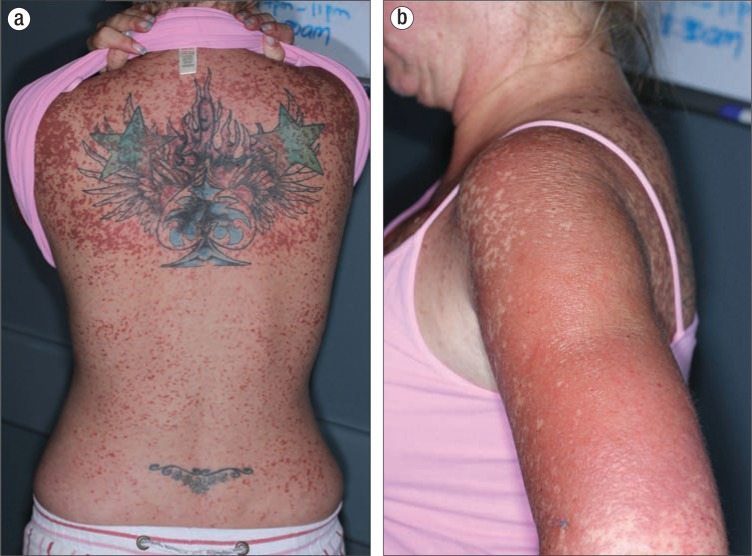
Figure 1.
Painful, dusky, erythematous 3- to 5-mm edematous papules coalescing into plaques on the (a) back and (b) upper arm.
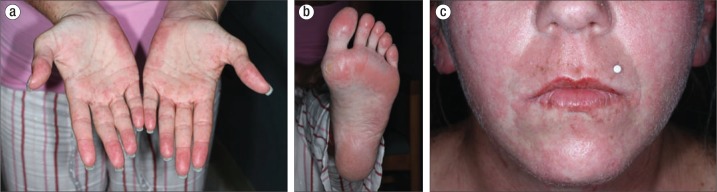
Figure 2.
Confluent dusky erythematous edematous papules on the (a) palms, (b) foot, and (c) face without mucosal involvement.
Laboratory abnormalities included a mild thrombocytopenia (platelets of 133,000/mcL) and a mild increase in liver enzymes (aspartate transaminase, 57 U/L [normal, 0–40 U/L]; alanine transaminase, 75 U/L [normal, 0–69 U/L]). Dermatology performed a 4-mm punch biopsy from the left upper extremity that showed interface dermatitis with areas of full-thickness epidermal necrosis. Given the lack of mucosal involvement and typical targetoid lesions on exam, she was diagnosed with an atypical erythema multiforme, likely secondary to carbamazepine. She was treated with methylprednisolone 60 mg intravenously every 6 hours for 2 days, then changed to oral prednisone 60 mg daily. Her prednisone was tapered over 6 days and discontinued upon discharge. She was also prescribed topical triamcinolone 0.1% ointment twice a day from the neck down and daily to her face for a total of 14 days. Her rash significantly improved over the next 2 weeks. Desquamation, specifically of the ears, palms, and soles, occurred with resolution of the rash.
Her half-sister and her father also had bipolar disorder. When her sister was visiting, she reported a similar reaction to carbamazepine, and her father noted the same reaction when asked. This incidentally acquired family history led to the diagnosis of AHS. Using the World Health Organization's classification for adverse drug reactions, this case would be categorized as a “probable” causal relationship, given the family history of similar responses to the same medication and her response to withdrawal of the medication. The patient and her family were instructed to avoid all cross-reactive medications in the future.
DISCUSSION
AHS was first described in 1950. Recently, there has been a movement to reclassify AHS under the broad term “drug-induced hypersensitivity syndrome,” which would also include drug reaction with eosinophilia and systemic symptoms, hypersensitivity syndrome, and drug-induced delayed multiorgan hypersensitivity syndrome. Eosinophilia is not a requirement for any of these conditions. Fever and malaise frequently precede the skin lesions. The skin eruption of AHS is nonspecific and can range from a morbilliform eruption to toxic epidermal necrolysis (1).
Hypersensitivity reactions are common with anticonvulsants. The mechanism of these reactions is thought to be secondary to toxic drug metabolites. Aromatic anticonvulsants are metabolized by cytochrome P-450 to intermediate metabolites such as arene oxides, which are detoxified by epoxide hydroxylase. If there are defects in metabolism, these toxic metabolites may cause cell necrosis or apoptosis and induce secondary immuno-logical responses (2). Patients should be advised to avoid all aromatic anticonvulsants and tricyclic antidepressants, as cross-reactivity may be as high as 75%.
Patients may be predisposed to hypersensitivity reactions through various genetic factors. The genetic allele HLA-A∗3101 has been associated with carbamazepine-induced cutaneous drug reactions in many different populations (3). For example, >15% of Japanese, Native American, and Southern Indian patients are thought to carry this allele (3). Unfortunately, testing for HLA-A∗3101 yields a high false-positive rate (2).
Treatment for hypersensitivity syndromes is primarily supportive after discontinuation of the offending agent. Systemic corticosteroids are frequently used, although there have been no clinical trials to prove efficacy. Steroid-sparing agents have also been used with success in other hypersensitivity reactions. Relapse is common given the high cross-reactivity among anticonvulsants.
While hypersensitivity drug reactions are well known, the hereditary component of AHS remains unfamiliar to physicians who prescribe anticonvulsants, as demonstrated by our case. Given the high rate of HLA-A∗3101 carriers worldwide, improving physician awareness of AHS is essential in decreasing the preventable serious morbidity in family members of affected patients.
References
- 1.Mehta M, Shah J, Khakhkhar T, Shah R, Hemavathi KG. Anticonvulsant hypersensitivity syndrome associated with carbamazepine administration: case series. J Pharmacol Pharmacother. 2014;5(1):59–62. doi: 10.4103/0976-500X.124428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knowles SR, Dewhurst N, Shear NH. Anticonvulsant hypersensitivity syndrome: an update. Expert Opin Drug Saf. 2012;11(5):767–778. doi: 10.1517/14740338.2012.705828. [DOI] [PubMed] [Google Scholar]
- 3.Tegretol [package insert] East Hanover, NJ: Novartis Pharmaceuticals Corporation, 2014; pp. 4–6. [Google Scholar]