Dear Editor,
Inherited muscular disorder is a heterogeneous group of genetic disorders that primarily affect skeletal muscle fibers. We have recently reported compound heterozygous mutations in ADSSL1 in two Korean families with adolescent-onset distal myopathy.1 However, ultrastructural changes associated with adenylosuccinate synthetase-like 1 (ADSSL1) myopathy have not been demonstrated previously. Here we report a third Korean family with ADSSL1 mutations and describe the ultrastructural features of ADSSL1 myopathy.
A 15-year-old girl (Supplementary Fig. 1A and B in the online-only Data Supplement, II-2) presented with gait disturbance. She was the second child of healthy, nonconsanguineous parents. She had first noticed difficulty running when aged 8 years, and the muscle weakness had progressed very slowly. When we examined her at an age of 15 years, she could still ambulate independently. A neurological examination revealed diffuse muscle weakness, predominantly in the leg muscles. She did not exhibit facial weakness, sensory deficits, joint contractures, or a high-arch palate. Her serum creatine kinase (CK) level was 281 IU/l (reference value <185 IU/l). The findings of needle electromyography were compatible with generalized myopathy. Her electrocardiography findings were normal. Lower-limb MRI showed subtle fatty replacement of the tibialis anterior and gastrocnemius muscles (Supplementary Fig. 1C-G in the online-only Data Supplement).
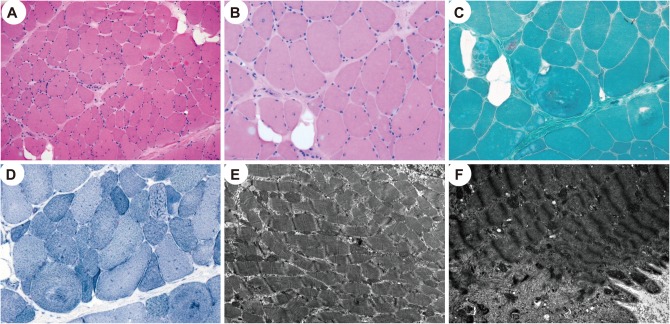
The vastus lateralis muscle was biopsied when she was 15 years old. The myofibers showed marked size and shape variations along with frequent internalization of the sarcolemmal nuclei and splitting; vacuolization of the sarcoplasm, degenerating myofibers, and abnormal (whorled) arrangement of the myofibrils were also noted (Fig. 1A and B). In addition, focal endomysial fibrosis and fatty infiltration were present. Periodic acid-Schiff and Oil Red O staining did not produce abnormal positive reactions. Modified Gomori trichrome and nicotinamide adenine dinucleotide-tetrazolium reductase staining revealed whorled fibers and occasional rimmed vacuoles without ragged red fibers, cytoplasmic bodies, or nemaline rods (Fig. 1C and D). The ATPase reaction with preincubations at different pH values and immunostaining with myosin heavy chain (fast), myosin heavy chain (slow), and myosin IIa revealed a predominance of type I fibers. An electron microscopy examination showed myofibers with varying degrees of disorganization, and disorientation of the myofibrils with loss of their normal striation pattern (Fig. 1E). The myofibers showing diffuse or localized myofibrillar destruction contained remnants of the myofibrils, irregular electron-dense material of Z-line origin, and rounded and swollen mitochondria (Fig. 1F). In addition, various abnormalities of the Z-disc (including thickening) were noted, as well as displaced and replicated triads.
Fig. 1. Histopathological findings of vastus lateralis muscle biopsy. A and B: Hematoxylin and eosin staining revealed marked variations of the fiber size and shape with frequent internalization of sarcolemmal nuclei and splitting; vacuolization of the sarcoplasm, focal endomysial fibrosis, and fatty infiltration were also occasionally noted. C: Modified Gomori trichrome staining showed whorled myofibers and occasional rimmed vacuoles. D: Nicotinamide adenine dinucleotide-tetrazolium reductase staining revealed a disorganized myofibrillar arrangement. E and F: An electron microscopy examination showed varying degrees of disorganization of the myofibrils with loss of their normal striation pattern (E). Myofibers showing localized myofibrillar destruction contained remnants of the myofibrils, irregular electron-dense material of Z-line origin, and swollen mitochondria (F) (A, ×100; B, C, and D: ×200; E: ×10,000; F: ×15,000).
To identify the genetic cause, exome sequencing was performed on the proband (Supplementary Table 1 in the online-only Data Supplement). Screening of myopathy-related genes revealed 27 functionally significant variants (Supplementary Table 2 in the online-only Data Supplement). We subsequently identified compound heterozygous c.910G>A (p.D340N) and c.1048delA (p.I350fs) mutations in ADSSL1, which we recently reported to be the underlying cause of distal myopathy.1 Subsequent capillary analysis showed that the ADSSL1 mutations cosegregated completely with the affected status (Supplementary Fig. 1A and B in the online-only Data Supplement).
This study identified compound heterozygous ADSSL1 mutations in a Korean patient with congenital myopathy. The ADSSL1 protein encoded by ADSSL1 is a muscle-specific enzyme that has a role in purine nucleotide interconversion by catalyzing the initial reaction in the conversion of inosine monophosphate to adenosine monophosphate.2 Although a previous study using cell and zebra-fish models demonstrated the damaging effect on ADSSL1, the pathogenesis of ADSSL1 myopathy has remained unclear.1 The mutational, clinical, and pathological manifestations of the present patient showed both similarities with and differences from those in previously reported cases of ADSSL1 myopathy (Supplementary Table 3 in the online-only Data Supplement). Identical ADSSL1 mutations have been identified in three Korean families, which suggests that the c.910G>A and c.1048delA mutations are founder ADSSL1 mutations in the Korean population. Clinically the patients show normal early motor milestones, very slow disease progression, and mild CK elevation. However, the patient in the present study showed diffuse muscle weakness without facial involvement. The muscle biopsy similarly demonstrated myopathic changes with rare rimmed vacuoles. The present study is the first to reveal pathology findings at the electron microscopy level, including irregular electron-dense material of Z-line origin, and rounded and swollen mitochondria. However, these findings are nonspecific for inherited muscular disorders.
In conclusion, this is the first report on the ultrastructural pathology of ADSSL1 myopathy and it extends the known clinical and pathological features.
Acknowledgements
The authors would like to thank the patients and their families for their essential help with this work.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
Supplementary Materials
The online-only Data Supplement is available with this article.
Pedigree, sequencing chromatograms, and limb MR images in the patient with ADSSL1 mutations. A: Pedigree of the present patient with compound heterozygous ADSSL1 mutations. Arrow indicates the proband (square: male; circle: female; filled: affected; and non-filled: unaffected). B: Sequencing chromatograms of c.910G>A (p.D340N) and c.1048delA (p.I350fs) mutations in the ADSSL1 gene. Arrows indicate mutation sites. C-G: MR images of the present patient with compound heterozygous ADSSL1 mutations. C: T1-weighted coronal images of the hip and thigh levels showed normal findings. At the calf level, subtle fatty replacement was observed. D and E: In the T1-weighted axial images of the hip and thigh, the muscles appeared nearly normal. F and G: At the calf level, the tibialis anterior and gastrocnemius muscles showed mild fatty hyperintense signal changes.
Exome sequencing analysis of patient
Functionally significant variants in myopathy-related genes
Comparison of phenotypes between the present patient and previously reported patients with ADSSL1 mutations
References
- 1.Park HJ, Hong YB, Choi YC, Lee J, Kim EJ, Lee J, et al. ADSSL1 mutation relevant to autosomal recessive adolescent onset distal myopathy. Ann Neurol. 2016;79:231–243. doi: 10.1002/ana.24550. [DOI] [PubMed] [Google Scholar]
- 2.Lipps G, Krauss G. Adenylosuccinate synthase from Saccharomyces cerevisiae: homologous overexpression, purification and characterization of the recombinant protein. Biochem J. 1999;341(Pt 3):537–543. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pedigree, sequencing chromatograms, and limb MR images in the patient with ADSSL1 mutations. A: Pedigree of the present patient with compound heterozygous ADSSL1 mutations. Arrow indicates the proband (square: male; circle: female; filled: affected; and non-filled: unaffected). B: Sequencing chromatograms of c.910G>A (p.D340N) and c.1048delA (p.I350fs) mutations in the ADSSL1 gene. Arrows indicate mutation sites. C-G: MR images of the present patient with compound heterozygous ADSSL1 mutations. C: T1-weighted coronal images of the hip and thigh levels showed normal findings. At the calf level, subtle fatty replacement was observed. D and E: In the T1-weighted axial images of the hip and thigh, the muscles appeared nearly normal. F and G: At the calf level, the tibialis anterior and gastrocnemius muscles showed mild fatty hyperintense signal changes.
Exome sequencing analysis of patient
Functionally significant variants in myopathy-related genes
Comparison of phenotypes between the present patient and previously reported patients with ADSSL1 mutations