Abstract
Bone marrow (BM) hematopoietic stem cells differentiate to common lymphoid progenitors (CLP) that emigrate to the thymus to form T cells or differentiate into immature B cells that then migrate to the spleen for maturation. Rapid in vivo suppression of BM progenitor cells by a single oral or intraperitoneal dose of 7,12‐dimethylbenz(a)anthracene (DMBA) subsequently decreased mature lymphoid populations in BM, spleen, and thymus. These suppressions depended on BM CYP1B1, but not on aryl hydrocarbon receptor (AhR) activity. Suppression of pre‐B colony formation at 6 h, correlated with subsequent decreases in mature BM, spleen, and thymus populations (48–168 h). Thymus T‐cell ratios were unaffected, suggesting low local toxicity. DMBA treatment suppressed progenitor cells 24‐h post treatment in wild type (WT), AhRb mice, but not in Cyp1b1‐ko mice. The stem cell populations were sustained. Benzo(a)pyrene (BP) mediated a similar progenitor suppression up to 6 h, but reversal rapidly ensued. This recovery was absent in mice with a polycyclic aromatic hydrocarbon (PAH)‐resistant, AhRd genotype. This AhR‐dependent progenitor recovery with BP induction accounts for the absence of suppression of B220+ BM and spleen populations at 48–168 h. However, DMBA and BP produced similar profiles for thymus cell suppression, independent of AhR genotype. Thus, lymphoid progenitors may exit the BM to the thymus prior to the BP reversal. This progenitor recovery is associated with elevated chemokines and cytokines that depend on AhR‐mediated induction of CYP1A1. This response increased constitutively in Cyp1b1‐ko BM, demonstrating that CYP1B1 metabolizes local stimulants that impact a basal progenitor protection process.
Keywords: 7, 12‐dimethylbenz(a)anthracene; Ah receptor; benzo(a)pyrene; cytochrome P450 1b1; lymphoid progenitors; polycyclic aromatic hydrocarbons; spleen; thymus
Abbreviations
- AhR
aryl hydrocarbon receptor
- BM
bone marrow
- BP
benzo(a)pyrene
- CFU
colony‐forming unit assay
- CLP
common lymphoid progenitor
- CMP
common myeloid progenitor
- CYP1A1
cytochrome P450 1A1
- CYP1B1
cytochrome P450 1B1
- DMBA
12dimethylbenz(a)anthracene
- GMP
granulocyte‐macrophage progenitor
- HSC
hematopoietic stem cells
- MEP
megakaryocyte‐erythroid progenitor
- MMP
multipotential progenitor
- PAH
polycyclic aromatic hydrocarbon
- TCDD
2,3,7,8‐tetrachlorodibenzo‐pdioxin
Introduction
Polycyclic aromatic hydrocarbons (PAHs) cause immune suppression in mice via effects on lymphocyte populations in the bone marrow (BM), spleen, and thymus. Benzo(a)pyrene (BP) and 7,12‐dimethylbenz(a)anthracene (DMBA) are PAHs that are both carcinogenic and immunosuppressive (Burchiel and Luster 2001). Intraperitoneal (IP) or oral administration of DMBA caused substantial diminution of BM cellularity in mice 48‐h post treatment, which was not seen with BP (Galvan et al. 2003, 2006; N'Jai et al. 2010). Daily DMBA treatments decreased the activity of isolated spleen cells, in parallel with increased phosphorylation of p53 and ATM (Gao et al. 2008). PAH suppression of BM cellularity required p53 (Page et al. 2003). Similarly, DMBA decreased thymus weight and T‐cell function, even though DMBA dihydrodiol formation was undetectable in isolated thymus cells (Miyata et al. 2001).
The BM and spleen cell suppression effects are mediated by cytochrome P450 1B1 (CYP1B1), as shown by the minimal responses in Cyp1b1‐ko mice (Heidel et al. 2000; Galvan et al. 2005; Gao et al. 2007). CYP1B1 is constitutively expressed in many extrahepatic tissues, including the BM, spleen and thymus, and is induced by PAHs via Ah Receptor (AhR) activation (Galvan et al. 2003, 2005 and Choudhary et al. 2005; Uno et al. 2006). CYP1B1‐dependent metabolism of PAHs to PAH dihydrodiol epoxides commonly initiates carcinogenesis through formation of DNA adducts (Buters et al. 1999), and has been linked to BM lymphocyte and myeloid suppression (Galvan et al. 2006). CYP1B1 also mediates physiological processes related to oxidative stress and fatty acid homeostasis (Larsen et al. 2015).
In the BM, hematopoietic stem cells (HSCs) differentiate into progenitors that produce both myeloid and lymphoid lineages (Eaves 2015). The common lymphoid progenitors (CLP) emigrate from the BM to the thymus or differentiate into B cells that either populate the BM or are exported to the spleen for maturation. In the thymus, CLPs differentiate into immature thymocytes that provide the repertoire of immunocompetent T cells (Park and Pan 2015). Naive T cells also migrate from the thymus to the spleen, where an alternate tissue‐specific expansion occurs (Temchura et al. 2005). Additionally, T‐cell subpopulations in the thymus are selectively sensitive to AhR activation (Laiosa et al. 2010; Rohlman et al. 2012). The various lymphocyte subpopulations in the spleen and thymus may thus provide a measure of the local effects of the PAHs, determined by AhR activity or metabolic activation. Here, we show that CYP1B1, but not AhR, mediates these PAH‐directed suppressions.
DMBA treatment (6 h) extensively suppressed BM progenitors that form lymphoid and myeloid lineages (CFU‐preB and CFU‐GM, respectively) (N'Jai et al. 2010, 2011). Recovery is dependent on the mode of delivery, dose, and type of progenitor. Flow cytometry and microarray analyses showed that the majority of BM populations are unaffected 24‐h post treatment, despite their extensive depletion at 48 h.
We hypothesize that PAHs first suppress the mature BM cell populations by preventing the expansion of HSC‐derived progenitor cells. The mature cells become depleted when their exit from the BM is not replenished from the progenitor pool. Similar depletion occurs in the spleen and thymus, as the supply of precursor cells from the BM declines.
Here, we use single low and high intraperitoneal (IP) or oral PAH doses to vary the kinetic profile as a means to test whether their effects on BM progenitor expansion are predictive of later effects on the total cell populations of the BM, thymus, and spleen. Progenitor expansion is evaluated by changes in the CFU‐preB assay. The participation of AhR induction in these PAH‐mediated responses is assessed through use of congenic mice that express the low affinity, AhRd allele, which impairs PAH binding (Okey et al. 1989). Previous reports show that AhR activation plays an important role in the regulation of BM HSC maturation (Gasiewicz et al. 2014). Deletions of Cyp1b1 and Cyp1a1 are used to assess their contributions to PAH activation. Cyp1b1 is appreciably expressed in the BM, but not the liver, whereas Cyp1a1 shows the reversed selectivity.
Novel features of PAHs, compared to 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin (TCDD) (AhR participation), arise from metabolism of the former. Thus, AhR mediates protection rather than suppression because of CYP1A1‐mediated metabolism of BP. CYP1B1 metabolism of endogenous substrates targets similar inflammatory genes as TCDD, by enhancing noncanonical AhR signaling. The appreciable AhR induction of total CYP1B1 in BM does not contribute to PAH suppression, which is minimal in multipotential mesenchymal cells (Heidel et al. 2000). The mechanism of AhR‐mediated BP protection does not extend to the thymus. We discuss the involvement of BM progenitors in thymus development.
Materials and Methods
Reagents and antibodies
DMBA and BP were purchased from AccuStandard, Inc (New Haven, CT). RPMI 1640 was purchased from Sigma Chemical (St Louis, MO). Collagenase (Type 1) was purchased from the Worthington Biochemical Corp. (Lakewood, NJ). The CFU‐preB (M3630) media were obtained from StemCell Technologies (Vancouver, BC, Canada). Fetal bovine serum was obtained from Atlanta Biologicals (Flowery Branch, GA). The following monoclonal antibodies (mAbs) for FACS analysis of splenocyte and thymocyte cell populations were purchased from BD Pharmingen (San Jose, CA): CD45/B220‐Percp–, Gr‐1‐fluorescein isothiocyanate (FITC), CD4‐APC, CD8‐Percp, CD8‐PeCy7, CD62L‐FITC, and CD44‐PE, whereas F4/80 (FITC), Sca‐1(PE) and c‐kit (FITC) were obtained from eBioscience (San Diego, CA).
Animals and treatments
C57BL/6j (wild type (WT), AhRb) and C57BL/6.D2N‐AhRd (AhRd) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Cyp1b1‐ko mice (C57BL/6j background) were bred, as described previously (Buters et al. 1999), and back‐crossed for at least 10 generations. Mice were housed in the AAALAC‐certified University of Wisconsin Madison School of Veterinary Medicine and School of Medicine and Public Health animal care facilities, and used in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Groups of female mice (6 weeks old) were randomly selected and injected IP or treated by oral gavage with DMBA or BP (10 or 50 mg/kg) in olive oil (0.2 mL). The maximal dose of DMBA (50 mg/kg) is similar to that used to initiate tumors in rodent carcinogenicity studies (Buters et al. 1999), which is consistent or lower than those used in numerous previously published investigations (Heidel et al. 2000; Galvan et al. 2003, 2005, 2006; Uno et al. 2006; Gao et al. 2008; N'Jai et al. 2010, 2011). Time‐matched vehicle control animals received an equivalent volume of olive oil. Mouse body, spleen and thymus weights were recorded following CO2 euthanasia, at indicated time points (6‐, 48‐, and 168‐h post treatment).
Measurement of PAH in blood
PAH levels were determined, as previously described (N'Jai et al. 2011). Briefly, blood was collected via cardiac puncture from PAH‐ or vehicle‐treated mice, at the indicated times. Residual PAH was extracted (2:1, acetone:acetonitrile) and separated by reverse‐phase HPLC (Beckman C18 Ultrasphere column), using a Waters 2695 Separation module interfaced to a Waters 470 scanning Fluorescence Detector, incorporating a 50–100% methanol gradient over a 30 min period. Total pmol PAH were quantified relative to a standard curve (0.5–50 pmol).
BM cell isolation
DMBA‐, BP‐ and vehicle control‐treated WT, AhRd, Cyp1a1‐ko and Cyp1b1‐ko mice were euthanized by CO2 asphyxiation. Femurs were dissected free of muscle tissue and the ends of the bones removed with a surgical blade. BM cells were either flushed from the femurs with 2 mL of RPMI culture medium supplemented with 2% FBS, using a syringe equipped with a 25‐gauge needle or crushed and incubated for 60 min with 3 mg/mL Type 1 collagenase, as described. A 1:50 dilution of cells in 3% acetic acid was used to lyse red blood cells and enumerate the hematopoietic cells in a hemocytometer.
Spleen and thymus cell preparation
Single cell suspensions were prepared from spleen and thymus tissues of individual mice (three to four per treatment group). The organs were dissected, weighed and placed in 3 mL of RPMI containing 1% FBS. Each organ was dispersed, using the rubber plug of a 3 mL syringe to macerate the tissue on a metal screen placed in a 60 mm petri dish. Cell suspensions were transferred to a 15 mL conical centrifuge tube via the addition of 10 mL RPMI media. Cells were pelleted by centrifugation at 2000 rpm (~900g) for 9 min at 4°C, the supernatant discarded, and the cells resuspended in fresh media. Spleen erythrocytes were lysed by adding 1 mL of 0.83% NH4Cl for 1 min, with gentle rotation, followed by the immediate addition of 12 mL RPMI containing 1% FBS. Cell debris was allowed to settle, and the supernatant transferred to a new tube. Cells were pelleted by centrifugation at 2000 rpm (~900g) for 9 min at 4°C and resuspended in 3 mL of RPMI containing 10% FBS. Viable cells were enumerated in a hemocytometer, based on their exclusion of trypan blue and used for flow cytometry.
Flow cytometry of spleen and thymus cells
Freshly isolated BM, spleen, and thymus cells were suspended at 1 × 106 cells per 0.1 mL media. Aliquots of cells (1 × 106) were maintained on ice for 20 min with 0.5 μg purified rat anti‐mouse CD16/CD32 (Fcγ III/II Receptor) (Mouse BD Fc Block, Caltag; BD Biosciences; San Jose, CA) to block Fc receptors. The spleen and thymus cells were incubated with 1 μg of one of the following anti‐mouse antibodies cocktails; 1) CD45/B220‐Percp, Gr‐1‐FITC, CD4‐APC and CD62L‐PE; 2) CD8‐Percp, CD4‐APC, CD44‐FITC and CD62L‐PE; 3) CD45/B220‐Percp, Sca‐1‐PE, C‐kit‐FITC and CD4‐APC; 4) F4/80‐FITC, CD8‐Percp, CD4‐APC, and CD62L‐PE. Cell suspensions were incubated at 4° C for 30 min and washed twice with 0.2 mL of 1% PBS. Cells were resuspended in 0.2 mL of 1% PBS and fixed with 2% paraformaldehyde. Fixed cells were stored in the dark at 4°C, until data were acquired and analyzed within 3 days of staining. Two hundred thousand events were acquired for each sample using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). Populations of B lymphocytes, granulocytes, T lymphocytes, macrophage, and lineage negative stem cell markers were gated as described previously (Thurmond and Gasiewicz 2000). Data were analyzed using Flow Jo 6.4 software.
Flow cytometry of hematopoietic progenitor cell populations
Hematopoietic stem and progenitor cell populations were isolated from the BM of mouse femurs (as described above) and analyzed on either a FACS ARIA III or an LSRII flow cytometer (BD Biosciences). Cells were stained with antibodies recognizing lineage markers (CD3, CD4, CD5, CD8, B220, GR1, MAC1, and TER119), c‐KIT, SCA1, CD34, IL7R, FLK2, and/or FcgrII‐PE and defined by the gating strategies indicated in Figure 5A (Boyer et al. 2011).
Colony‐forming unit assays
Freshly isolated BM cells were resuspended at concentrations of 1.0 × 106 cells per mL in RPMI1640 media supplemented with 2% FBS and penicillin/streptomycin. Colony‐forming unit (CFU) assays were completed according with the manufacturer's protocol. Briefly, an aliquot (0.3 mL) of resuspended BM cells was added to 3 mL of CFU‐preB Methocult media, vortexed and allowed to stand for 5 min for bubbles to dissipate. The media were then dispensed into duplicate pretested culture dishes using a syringe and blunt‐end needle. The BM cells were incubated for 7 days in a humidified incubator at 37°C and 5% CO2. CFU‐preB progenitor colonies were evaluated and counted using an inverted microscope and gridded scoring dishes.
mRNA expression analysis
RNA was purified from freshly isolated BM cells using the RNAeasy isolation kit (Qiagen, Hilden,Germany), as per manufacturer's instructions. RNA quality was confirmed using 260/280 and 260/230 ratios on a Nanodrop DU800 (Beckman Coulter, Brea, CA) and visualized on a 1% agarose/formaldehyde gel. Microarray analyses were completed using the Agilent dual‐color 44 K platform. Data were analyzed using the EDGE3 software package (Vollrath et al. 2009; N'Jai et al. 2011). The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE82242 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE82242).
Data analysis
Data from PAH‐treated animals are expressed as the percent of the value of vehicle (olive oil)‐treated mice, where the vehicle controls are set at 100 percent. Results presented are the mean ± standard error of the mean (SEM) in each experiment. Anova statistical evaluation, followed by Tukey's post hoc test, was completed unless otherwise specified (Prism GraphPad 5 software, San Diego, CA). Student's t‐tests (unpaired, two‐tailed), where indicated, were completed using Prims GraphPad 6 software.
Results
Experimental design to test the effect of PAH treatment on BM progenitors
This study tests the hypothesis that suppression of lymphoid progenitors in the BM, by a single dose of PAH, mediates a decrease in mature lymphocyte populations in BM, spleen, and thymus, which is proportional to the BM CLP population. The export of lymphocytes from the spleen and thymus requires their replacement from the rapidly replicating BM progenitor pool. The mature cells in each tissue are much less susceptible to PAH treatment and require repeated dosing to realize tissue‐selective changes. Each tissue has very different characteristics for AhR and metabolite‐mediated effects of PAH.
BM hematopoietic stem cells (HSC) differentiate through multipotential progenitors: CLP, common myeloid progenitor (CMP) and megakaryocyte‐erythroid progenitor (MEP) to form the respective BM lineages (Fig. 1A). BM differentiation is controlled by cytokines released from the fibroblastic and vascular cells that comprise the diverse BM niches. Splenic B lymphocytes arise from further maturation of B220+ BM progenitor cells. CLPs also undergo an alternative modification, whereby they migrate to the thymus (CLPt) and initiate the complex differentiation of T lymphocytes.
Figure 1.
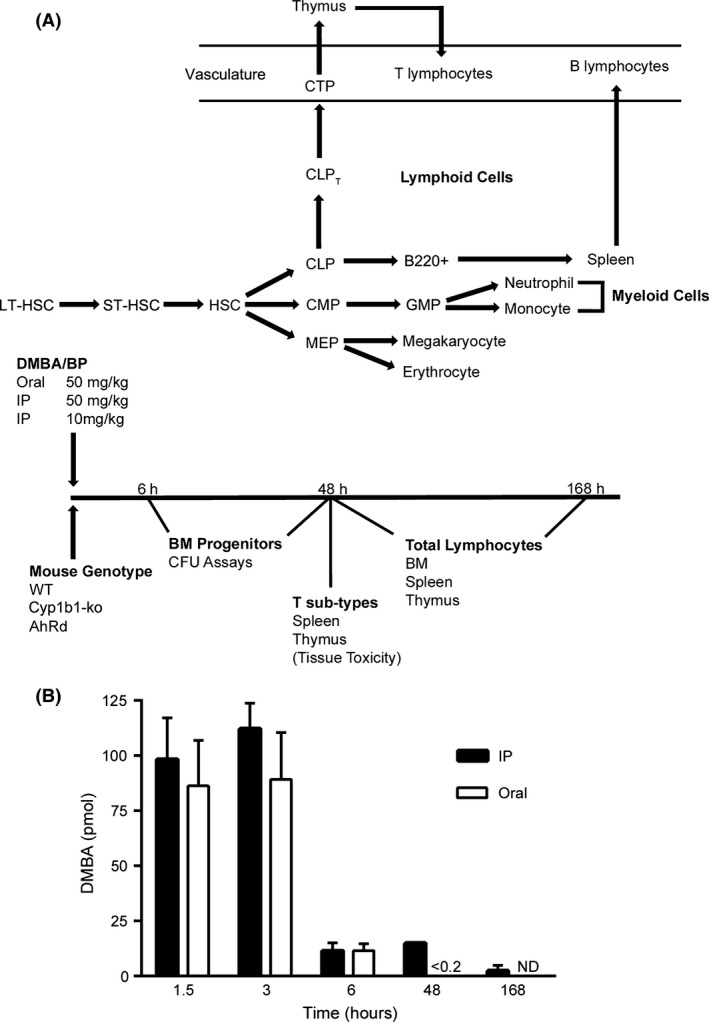
Experimental design to evaluate polycyclic aromatic hydrocarbon (PAH) effects on cell populations in bone marrow (BM), spleen, and thymus. (A) The scheme for development of cell lineages from BM hematopoietic stem cells (HSC). Standard abbreviations are used for progenitors (long‐term HSC, LT‐HSC; short‐term HSC, ST‐HSC; common lymphoid progenitor, CLP; common myeloid progenitor, CMP; megakaryocyte‐erythroid progenitor, MEP; granulocyte‐macrophage progenitor, GMP) as defined in the Introduction. B220+ cells are B‐lymphocytes that migrate to the spleen for further maturation. CLPT are lymphoid progenitors modified for export to the thymus as circulating thymic progenitors (CTP). (B) Time‐dependent clearance of circulating dimethylbenz(a)anthracene (DMBA), administered to wild type (WT) mice by Intraperitoneal (IP) injection or oral gavage (50 mg/kg in olive oil). Blood was collected by cardiac puncture and DMBA concentration measured by HPLC. The results represent the average of 2–3 experiments with n = 3–4 mice per assay.
Spleen and thymus mass are substantially determined by expansion of the BM‐derived lymphocyte progenitors. In vivo PAH treatment causes extensive loss of mass in these tissues, due to lymphocyte depletion (Thurmond et al. 1987; Burchiel and Luster 2001). Here, we measure tissue weight and cell population ratios, determined by flow cytometry, to assess the PAH effects on each organ relative to early depletion of BM progenitor cell numbers, as assessed by colony‐forming activities (as CFU assays).
Different treatment methods and doses are employed to distinguish rapid effects of PAH on BM progenitors. We relied on the large pharmacokinetic differences between oral and IP PAH administrations, or a fivefold difference in IP dose. Clearance of orally administered PAHs is substantially dependent on first pass metabolism in the liver by AhR‐induced CYP1A1 (Uno et al. 2004; Nebert et al. 2013). DMBA and BP each generate circulating PAH dihydrodiols and phenols, whereas BP generates a distinct set of quinones through CYP1A1 metabolism (Legraverend et al. 1984; N'Jai et al. 2010). Residual PAHs and their metabolites transfer through the blood to the BM, where further metabolism is directed by CYP1B1. Orally administered DMBA or BP is completely cleared within 24 h (Uno et al. 2004, 2006 and N'Jai et al. 2010), whereas IP administration is biphasic, with an additional sustained release from abdominal adipose tissue that maintains elevated blood levels for 6–48 h post treatment (Fig. 1B).
Figures 2, 3, 4, 5 show that time‐dependent responses of mature lymphocytes to DMBA treatment in the BM, spleen and thymus are temporally related to the suppression and recovery of BM pre‐B progenitor activity. These occur under conditions that appreciably and selectively change the PAH pharmacokinetic profile and local metabolism in these tissues. In these experiments, we compared oral versus IP administration at low and high doses, AhR status (AhRb vs. AhRd) and CYP1B1 participation.
Figure 2.
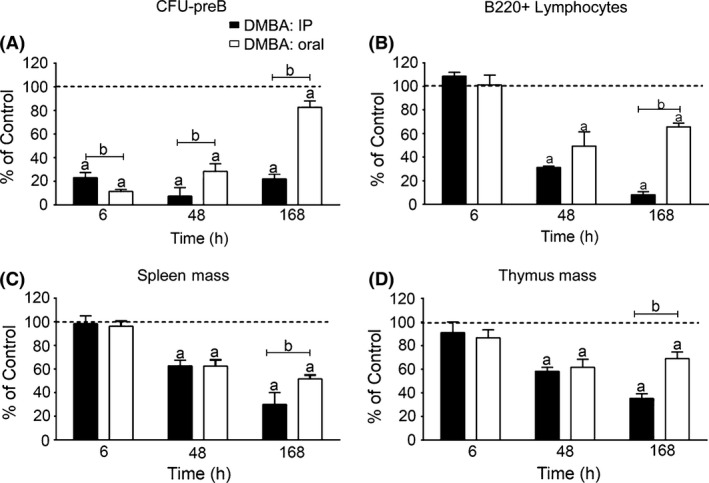
Reduction in pre‐B colony‐forming activity (CFU‐preB) and lymphoid progenitor (B220+) cellularity in BM, and tissue mass loss in spleen and thymus after Intraperitoneal (IP) or oral dimethylbenz(a)anthracene (DMBA) treatment in WT mice. (A) BM CFU‐pre‐B formed after oral and IP DMBA (50 mg/kg) treatment. (B) FACS quantification of BM B220+ lymphocytes after oral and IP DMBA (50 mg/kg) treatment. (C) Spleen mass after oral and IP DMBA (50 mg/kg) treatment. (D) Thymus mass after oral and IP DMBA (50 mg/kg) treatment. The results in each panel are expressed as percent of values in vehicle‐treated (olive oil) control mice (set at 100%), and are the mean ± SEM of 3–4 mice per group. Significant differences between control and DMBA treatment is denoted by the letter “a”, whereas “b” denotes significant differences between the two routes of administration (P < 0.05). WT, wild type.
Figure 3.
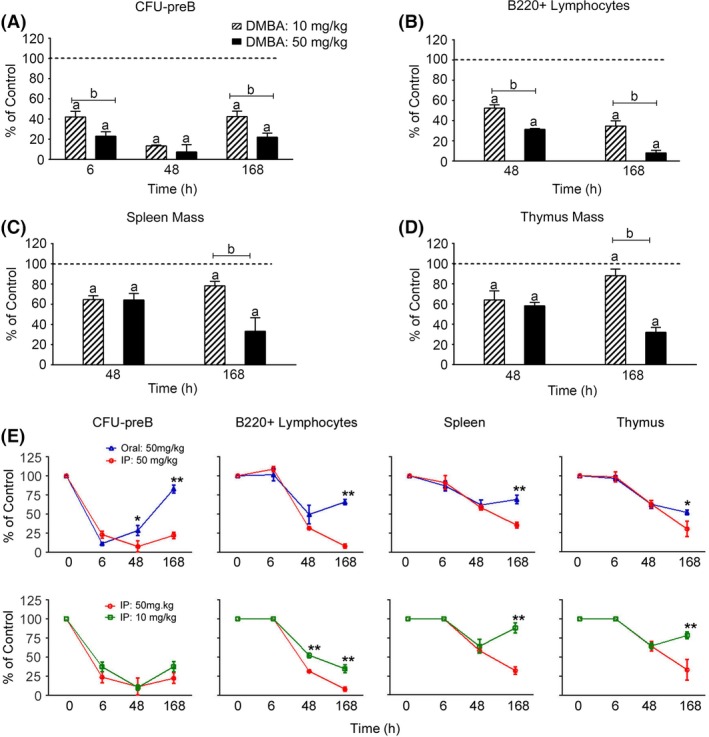
The effectiveness of a fivefold lower Intraperitoneal (IP) dose of dimethylbenz(a)anthracene (DMBA) in the reduction in lymphoid colony‐forming activity (CFU‐preB), lymphoid progenitor (B220+) cellularity, and spleen and thymus mass in WT mice. (A) BM CFU‐preB formation after IP DMBA (10 and 50 mg/kg) treatment. (B) FACS quantification of BM B220+ lymphocytes after IP DMBA (10 and 50 mg/kg) treatment. (C) Spleen mass after IP DMBA (10 and 50 mg/kg) treatment. (D) Thymus mass after IP DMBA (10 and 50 mg/kg) treatment. (E) Line graph representation of the time course data presented in panels A–D shows the parallel recovery that is observed in BM B220+ lymphocytes, spleen and thymus tissues 48–168 h post DMBA treatment (*P < 0.05, **P < 0.01). The results in each panel (A–D) are expressed as percent of values in vehicle‐treated (olive oil) control mice (set at 100%), and are the mean ± SEM of 3–4 mice per group. Significant differences between control and DMBA treatment is denoted by the letter “a”, whereas “b” denotes significant differences between the two DMBA concentrations (P < 0.05). WT, wild type.
Figure 4.
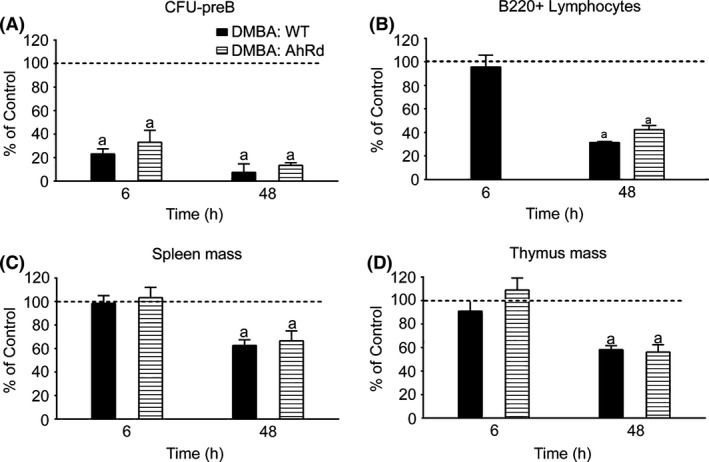
Dimethylbenz(a)anthracene (DMBA)‐mediated suppression of CFU‐preB activity, B220+ cellularity and organ weights in AhRd mice. (A) CFU‐preB activity in wild type (WT) and AhRd BM following Intraperitoneal (IP) DMBA (50 mg/kg) treatment. (B) FACS quantification of B220+ lymphocytes in WT and AhRd BM following IP DMBA (50 mg/kg) treatment. (C) Spleen mass in DMBA‐treated (IP, 50 mg/kg) WT and AhRd mice (D) Thymus mass in DMBA‐treated (IP, 50 mg/kg) WT and AhRd mice. The results in each panel are expressed as percent of values in vehicle‐treated (olive oil) WT control mice (set at 100%), and are the mean ± SEM of 3–4 mice per group. Significant differences between control and DMBA treatment is denoted by the letter “a”, whereas “b” denotes significant differences between the two respective strains (P < 0.05).
Figure 5.
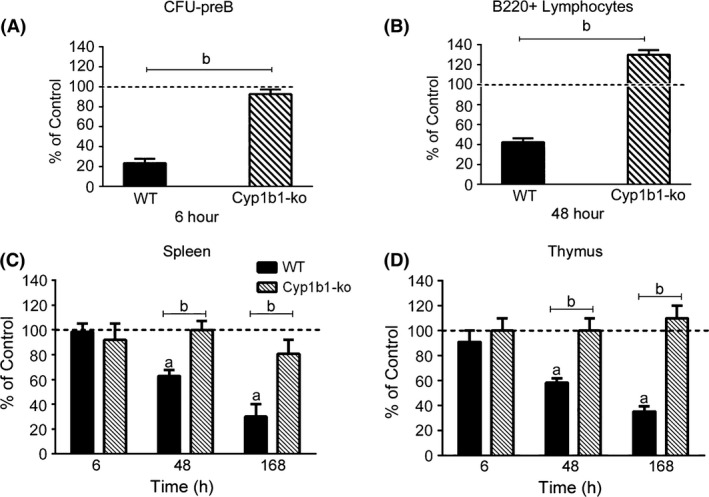
Dimethylbenz(a)anthracene (DMBA)‐mediated suppression of CFU‐preB activity, B220+ cellularity and organ weights is dependent upon Cyp1b1 expression. (A) BM CFU‐preB activity 6 h after DMBA treatment (Intraperitoneal [IP], 50 mg/kg) of wild type (WT) and Cyp1b1‐ko mice. (B) FACS quantification of BM B220+ lymphocytes following DMBA (IP, 50 mg/kg) treatment of WT and Cyp1b1‐ko mice. (C) Spleen mass in DMBA‐treated (IP, 50 mg/kg) WT and Cyp1b1‐ko mice. (D) Thymus mass in DMBA‐treated (IP, 50 mg/kg) WT and Cyp1b1‐ko mice. The results in each panel are expressed as percent of values in vehicle‐treated (olive oil) WT control mice (set at 100%), and are the mean ± SEM of 3–4 mice per group. Significant differences between control and DMBA treatment is denoted by the letter “a”, whereas “b” denotes significant differences between the two respective strains (P < 0.05).
DMBA effects on BM progenitor CFU activity predict later changes in mature lymphoid cells in the BM, spleen, and thymus
Figure 2A compares the effects of oral and IP DMBA treatment on CFU‐preB activity, normalized to time‐matched vehicle controls. CFU suppression is extensive within 6 h for both routes of administration (oral > IP treatment). The CFU‐preB activities are partially restored 48 h following oral treatment, but decline further after IP treatment. This difference corresponds to the early clearance of DMBA following oral treatment compared to the persistence of DMBA after IP administration. B220+ lymphocytes and spleen and thymus mass show divergences between oral and IP DMBA after 48 and 168 h, respectively, which corresponds to the divergence of preB progenitors after 6 h (Fig. 2B–D).
IP administration of a fivefold lower dose of DMBA (10 vs. 50 mg/kg) attenuated the CFU‐preB suppression by twofold (Fig. 3A). This difference was maintained for BM lymphocytes at 48 and 168 h, and for spleen and thymus masses at 168 h (Fig. 3B–D). CFU‐preB activities exhibit similar threefold recoveries between 48 and 168 h post treatment, following either low or high IP doses of DMBA. Overall, the 48 and 168 h decreases mediated by the lower dose are similar for mature cells in each tissue. They are also comparable to the differences obtained between oral and IP treatments (Fig. 3E). The lower IP dose matches the oral profile because the slow release component of DMBA is very dose sensitive. The various time profiles suggest that recoveries can sustain or restore mature populations in each tissue once the progenitor levels recover to 20–30 percent of normal.
DMBA suppression of BM lymphoid progenitors depends on CYP1B1, but not the AhR
DMBA responses in WT (AhRb) mice were compared with those in congenic AhRd mice, which are completely resistant to AhR induction by PAHs. This reveals the AhR suppression contribution (Galvan et al. 2006). There is no effect of AhR genotype on DMBA‐mediated suppression of BM CFU‐preB activity, reduction in B220+ lymphocytes or loss of spleen and thymus tissue mass (Fig. 4A–D). We concluded that the suppression of these responses, therefore, is not mediated by AhR. This contrasts with the appreciable AhR‐mediated effects of TCDD on CFU activities, BM progenitors and thymic T cells (Laiosa et al. 2003, 2010 and Singh et al. 2009). Importantly, the direct AhR‐mediated suppression of CFU‐preB activity and B220+ cellularity does not reach levels that were comparable to our study until 7‐days post treatment (Singh et al. 2009).
As the loss of BM cellularity is prevented in Cyp1b1‐ko mice (Galvan et al. 2006), we measured the effects of DMBA on BM progenitors from these mice. Cyp1b1‐ko mice were completely resistant to the effects of DMBA on CFU‐preB activity and the BM lymphocyte populations (Fig. 5A and B). The spleens of Cyp1b1‐ko mice were also resistant to DMBA‐mediated toxicity, as reported previously (Gao et al. 2008). Likewise, there was no effect on thymus mass (Fig. 5C and D).
Identification of BM progenitor populations and their suppression by DMBA
The progenitor cells that produce the lymphoid, myeloid, and erythroid lineages represent only approximately 0.1% of the total BM cells. Colonies formed in the CFU assays each derive from a single progenitor and recapitulate the proportional distribution of total BM populations. To determine whether the DMBA‐mediated decrease in CFU activity represents a decrease in the respective progenitor numbers or their proliferative response to CFU stimuli, we examined progenitor numbers in BM preparations by flow cytometry. Figure 6A shows the gating strategy and flow resolution of HSC, multipotential progenitor (MPP), CMP, CLP, MEP, granulocyte macrophage progenitor (GMP), and mature B‐cell and T‐cell populations.
Figure 6.
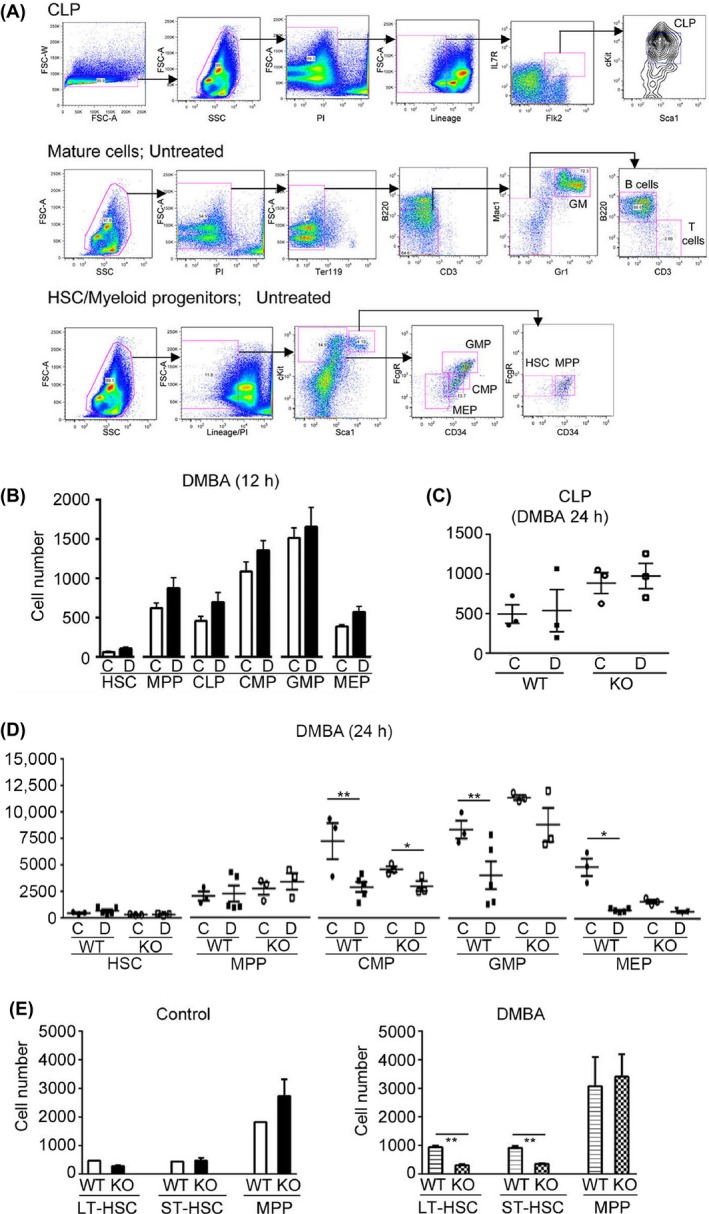
Dimethylbenz(a)anthracene (DMBA) treatment suppressed BM progenitor populations 24 h post treatment, but failed to affect the HSC population. (A) Gating strategy for FACS analysis of BM hematopoietic stem and progenitor cell populations. (B) Quantification of FACS analysis of BM isolated from wild type (WT) mice 12 h post DMBA (Intraperitoneal [IP], 50 mg/kg) treatment. BM was isolated with collagenase digestion. (C) FACS quantification of BM CLP isolated from WT and Cyp1b1‐ko (KO) mice 24 h post DMBA (IP, 50 mg/kg) treatment. BM was isolated with collagenase digestion. These data represent the average of two independent experiments. (D) FACS quantification of BM isolated from WT and Cyp1b1‐ko (KO) mice 24 h post DMBA (IP, 50 mg/kg) treatment. BM was isolated with collagenase digestion. These data represent the average of two independent experiments. (E) Quantification of FACS analysis of the effect of DMBA (IP, 50 mg/kg) on BM stem cell populations in WT and KO mice. BM was isolated 24 h post treatment, with collagenase digestion. The data presented in B and E represents the average 3–5 mice, of mixed gender. Statistical significance was assessed using student t‐test analyses. *P < 0.05, **P < 0.01.
An improved BM cell isolation procedure (collagenase digestion of the crushed femur) (Fig. S1) increased cell yields and progenitor subpopulations allowing FACS analysis from single mice. A 12 h DMBA exposure failed to significantly affect the progenitor populations, as shown in Figure 6B. However, a 24 h DMBA exposure depleted the CMP population 2.5‐fold (Fig. 6D). The MEP population, which derives from the CMP, was further suppressed by 6.5‐fold. The GMP population was decreased by twofold. Suppression was also observed in the much smaller CLP cell population, although the low numbers of cells were more difficult to quantify (Fig. 6C).
DMBA suppression of the committed progenitor populations was less prominent in Cyp1b1‐ko mice than in their WT counterparts, although still detectable (Fig. 6C and D). Collectively, the HSC populations were of similar magnitude in WT and Cyp1b1–ko mice (Fig. 6E). DMBA treatment, however, mediated a 2.5‐fold increase in LT‐HSC and ST‐HSC populations in WT mice (n = 3, P < 0.01), whereas there were no significant differences in the MPP or committed progenitor populations (Fig. 6E). These increases in LT‐HSC and ST‐HSC may arise because of a sustained block by DMBA in their progression to MPP, which results from slower conversion to the three committed progenitors. In contrast, DMBA treatment decisively decreased these early progenitors in Cyp1b1‐ko mice.
Early benzo(a)pyrene (BP) suppression of BM progenitor cells parallels thymus suppression; progenitor recovery parallels BM and spleen resistance
BP treatment, like DMBA, significantly decreased CFU‐preB activity within 6 h following oral or IP administration (Fig. 7A and 2A). However, unlike DMBA, CFU‐preB activities recovered completely within 48 h. As previously shown, BP had no effect on total BM cellularity, B220+ lymphocytes (Galvan et al. 2006) or total spleen cells (Fig. 7C).
Figure 7.
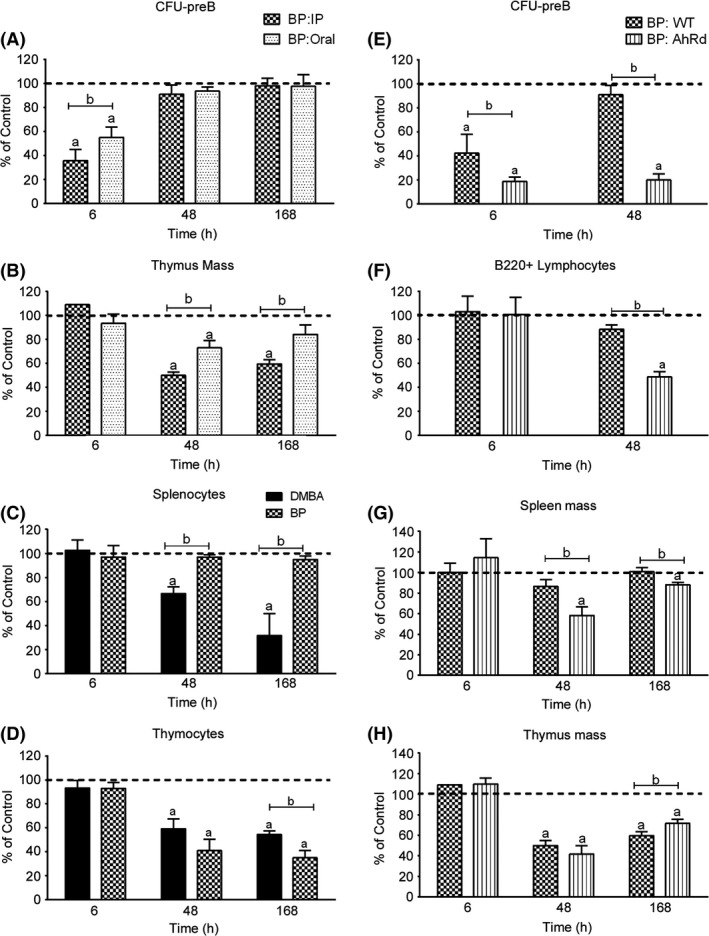
Effects of BP administration on CFU‐preB activity, B220 lymphocytes, spleen mass and thymus mass. The response in AhRd mice demonstrates that BP‐mediated recovery of CFU‐preB activity and B220+ lymphocyte cellularity is dependent on the AhR. (A) BM CFU‐preB activity following oral or Intraperitoneal (IP) BP (50 mg/kg) administration. (B) Thymus mass following oral or IP BP (50 mg/kg) administration. (C) Comparison of the effect of IP BP and dimethylbenz(a)anthracene (DMBA) (50 mg/kg) administration on splenocytes, as measured by FACS analysis. (D) Comparison of the effect of IP BP and DMBA (50 mg/kg) administration on thymocytes, as measured by FACS analysis. (E) BM CFU‐preB activity in wild type (WT) and AhRd mice following IP BP (50 mg/kg) administration. (F) FACS quantification of BM B220+ lymphocytes in WT and AhRd mice following IP BP (50 mg/kg) administration. (G) Spleen mass in WT and AhRd mice following IP BP (50 mg/kg) administration. (H) Thymus mass in WT and AhRd mice following IP BP (50 mg/kg) administration. The results in each panel are expressed as percent of values in vehicle‐treated (olive oil) WT control mice (set at 100%), and are the mean ± SEM of 3–4 mice per group. Significant differences (P < 0.05) between control and BP treatment is denoted by the letter “a”, whereas “b” denotes significant differences (P < 0.05) between PAHs, routes of administration or mouse strains.
Oral and IP BP treatment, however, each reduced thymus mass at 48 h, with differences similar to those observed for DMBA (Fig. 2D and 7B). Figure 7D shows that the suppression of thymocytes was similar for IP administrations of BP or DMBA. Thus, the BP‐initiated recovery mechanism does not extend to the thymus. CLP that emigrate to the thymus to maintain the thymocyte population, therefore, are not subject to the recovery. This exit may occur in the 3‐ to 6‐h period that precedes the CFU recovery.
BP suppression is followed by an AhR‐mediated recovery that by‐passes progenitor emigration to the thymus
The suppression of BM CFU‐preB (6 h) by BP was significantly enhanced in AhRd mice (Fig. 7E), to comparable levels as observed for DMBA treatment (Fig. 4A). This lymphoid progenitor activity remained suppressed at 48 h, paralleling the changes seen in DMBA‐treated WT mice (Fig. 4A). In the absence of the AhR activity, the three tissues each respond similar to BP and DMBA, further supporting the concept that each response derives from the shared BM progenitor suppression.
The involvement of CYP1A1 in this recovery is shown by the enhanced suppression of BM cellularity in BP‐treated Cyp1a1‐ko mice (Uno et al. 2004). BP clearance after oral treatment is principally determined by AhR induction of CYP1A1 in the liver (Uno et al. 2004, 2006 and Nebert et al. 2013). After IP administration, however, serum BP levels are sustained between 6 and 24 h in both WT and AhRd mice (Fig. S2), much as shown for DMBA (Fig. 1B). The serum levels were somewhat lower in AhRd mice, despite diminished liver CYP1A1 expression. The serum level of BP is, therefore, not the discriminatory factor in the recovery phase following IP‐administered BP in WT mice. In addition, BP and DMBA remain equally effective in the induction of AhR‐responsive genes (Table 1). Even at a fivefold lower dose of DMBA, significant BM suppression is sustained for at least 48 h (Fig. 3). We have previously shown that BP differs from DMBA by stimulating high levels of multiple inflammatory gene responses in the recovery period (N'Jai et al. 2011).
Table 1.
BM microarray expression levels of classical AhR‐responsive genes and genes associated with inflammation following in vivo polycyclic aromatic hydrocarbon exposure
| Gene | WT | Cyp1b1‐ko | Cyp1a1‐ko | Double‐ko | |||||
|---|---|---|---|---|---|---|---|---|---|
| BPa (6 h) | DMBAa (12 h) | TCDDa (12 h) | Controla (12 h) | DMBAb/ KO (12 h) | Controla (12 h) | BPc/ KO (12 h) | Controla (12 h) | BPd/ KO (12 h) | |
| Classical | |||||||||
| Cyp1b1 | 3.3* | 2.5* | −1.6 | NA | NA | −1.5 | 3.5 | NA | NA |
| Cyp1a1 | 11.4 | 15.0* | 2.1 | −6.7 | 10.6 | NA | NA | NA | NA |
| Ahrr | 8.3* | 4.1* | 3.8* | −5.0* | 10.0 | −2.2 | 10.4** | −1.4 | 12.4* |
| Spint1 | 5.2* | 4.1* | 5.0** | −5.4* | 11.6** | −2.2 | 12.5** | −3.8 | 27.9* |
| Acpp | 4.0** | 2.3** | 3.5** | −1.6* | 2.4 | NC | 3.2** | 1.4 | 3.5 |
| Cxcl3 | 10.4** | 2.7* | 3.7* | −3.9* | 9.5** | −1.5 | 5.0 | 1.4 | 3.0 |
| Dnahc2 | 4.6** | 2.3** | 2.3** | 1.6 | 1.8 | NC | 3.6** | 1.7 | 3.2** |
| Hic1 | 8.3** | 1.8* | 4.2** | −1.5* | 3.8** | 2.0 | 2.4 | NC | 4.7* |
| Inflammation | |||||||||
| Cxcl12 | NC | 1.5 | −2.4 | 2.5* | −1.4 | 1.8 | NC | 2.0 | −2.4 |
| Cxcl10 | 7.7** | NC | 2.0* | 1.5 | 2.5** | 1.7* | NC | 2.3* | NC |
| Ccl2 | 2.0* | 1.6** | NC | 1.7* | NC | NC | 1.7* | 1.6* | −1.3 |
| Ccl3 | 2.8* | NC | 1.5 | 1.7 | 1.6 | NC | NC | 1.7 | NC |
| Ccl4 | 3.9* | NC | NC | 1.3 | 1.7* | NC | NC | 1.9* | NC |
| Ccl5 | 2.1** | 1.3* | 1.5* | 2.4** | NC | NC | NC | 1.7* | NC |
| Il1a | 4.5* | 1.9 | 3.0* | 1.5 | NC | NC | 1.4 | 2.2 | 1.8 |
| Il1b | 4.5** | NC | 1.3 | 1.4 | 2.4 | NC | 2.0 | NC | NC |
| Il6 | 4.5** | NC | 3.0** | 2.7** | 1.8 | 2.4 | NC | 7.7** | −1.8 |
| Tnf | 5.6** | NC | 2.0** | 1.3 | 1.8* | 2.8** | ‐1.3 | 2.4** | NC |
| Ifnb1 | 13.7* | 2.4 | 7.8** | 2.1 | NC | 1.9 | NC | 3.4 | 1.5 |
| Ifng | 3.4** | NC | 2.6** | 3.1** | NC | 1.5 | NC | 7.0** | ‐1.6 |
| Ptgs2 | 11.2** | NC | NC | −1.7 | 9.3* | −1.4 | 1.9 | −1.9 | −1.3 |
| Egr1 | 5.4** | NC | NC | 2.0* | 1.4 | NC | 1.7 | NC | −1.3 |
| Egr2 | 5.9** | NC | 2.9** | 1.5* | 2.3* | 1.6 | 1.7 | 1.5 | 1.3 |
| Socs2 | 2.4** | NC | 2.0** | −1.3 | 2.0 | NC | 1.3 | 1.3 | NC |
| Socs3 | 2.6** | 1.3* | 1.7* | 1.9** | 1.5* | 1.6** | NC | 1.6* | 1.4 |
| Nfkbiz | 8.6** | NC | 2.6** | 1.5 | 1.5 | 1.4 | 1.3 | 2.5* | NC |
| Tnfaip3 | 5.2** | NC | 2.1* | NC | 1.9* | NC | 1.4 | 1.5 | NC |
Female mice were treated with vehicle or polycyclic aromatic hydrocarbon for 6 or 12 h prior to BM isolation, as indicated. Microarrays were completed on BM isolated from individual mice (n = 2–3 mice/microarrays per treatment group). WT, wild type; DMBA, dimethylbenz(a)anthracene; NA, not applicable; NC, not change.
*P < 0.05,** P < 0.01.
Fold change relative to WT control.
Fold change relative to Cyp1b1‐ko control.
Fold change relative to Cyp1a1‐ko control.
Fold change relative to Double‐ko control.
In AhRd mice, BP suppressed B220+ lymphocytes and both spleen and thymus tissue masses at 48 h post treatment (Fig. 7F–H), to the same extent as observed for DMBA (Fig. 4B–D). In the thymus, the time course for the effect of BP was very similar for WT and AhRd mice (Fig. 7H). Thus, without AhR‐mediated recovery, BP‐ (AhRd mice) and DMBA‐ (WT mice) treated mice exhibit similar relationships between 6‐h BM progenitor suppression and subsequent losses of mature lymphocytes at the three sites. The residual level of progenitors following BP treatment (20%) correspond to similar residual levels of B220+ lymphocytes (50%), spleen mass (60%), and thymus mass (40%) at 48 h (Figs. 2, 7). The similarity of these levels also suggests dependence on shared progenitor mechanisms.
Effects of PAH treatment on FACS analyses of spleen lymphocyte subpopulations
We used FACS analysis of the relative populations of splenocytes to test for local toxicity 48 h after a single IP dose of DMBA or BP. In the spleen, B220+ cells from the BM mature through two transitional steps into mature B cells that reside in the marginal zone and follicular B‐cell compartments (Pieper et al. 2013). Naive T lymphocytes migrate from the thymus to the spleen, wherein they differentiate into multiple subpopulations. B and T lymphocytes normally are present at a 2:1 ratio. The number of lymphoid progenitors are much lower in the spleen than in the BM (data no shown), consistent with the emigration of immature cells from the BM (Temchura et al. 2005). Despite the extensive DMBA‐mediated suppression of B220+ cells in the spleen, the proportions of thymic B220+ lymphocytes and CD4+ and CD8+ T lymphocytes were largely unaffected by either DMBA or BP treatment (Fig. S3A). The expansion and differentiation of CD4+ and CD8+ T lymphocytes are established by the spleen environment, which is, therefore, relatively unaffected by DMBA or BP. Opposing effects of DMBA and BP were observed for the small population of spleen granulocytes (Gr‐1+) and, to a lesser extent, macrophages (F4/80+).
Effects of DMBA and BP on thymocyte subpopulations
To further test for local toxic effects of the PAHs, we examined thymocyte T‐cell subpopulations by FACS analysis 48 h post IP treatment, which corresponds to approximately 50 percent suppression of thymocytes. Mature T lymphocytes of the thymus are characterized by the presence of CD4 or CD8 receptors on the cell surface (Schwarz and Bhandoola 2006). The CLP initially expand through four stages that lack CD4 or CD8 (DN 1‐4), before differentiating to CD4+CD8+ double‐positive cells. The transition to DN3 and DN4 is suppressed when AhR is activated by TCDD (Laiosa et al. 2003, 2010; Rohlman et al. 2012). Thymus mass is comparably suppressed by BP in the WT and AhRd mice, indicating that this loss is not affected at this early stage by PAH activation of AhR activity. CD4+CD8+ cells, which comprise 75% of the T cells, transfer to the thymic cortex where they mature under the control of histocompatibility antigen‐directed processes that promote either loss of CD8 to generate CD4+ cells (T helper cells) or loss of CD4 to provide CD8+ cells (cytotoxic T cells), in a ratio of approximately 5–1 (Li et al. 2012). Despite the extensive suppression of thymocytes by DMBA and BP (Fig. 7D), the relative proportions of these three populations were scarcely affected by either PAH. We used two additional markers, CD44 and CD62L, to characterize these populations (Fig. S3B). The proportions of high, intermediate and low CD44 provide a measure of IL‐17 and IFN‐γ secretion in the CD4 population (Schumann et al. 2015), whereas CD44 and CD62L positive expression provides a measure of memory status in the T8+ population. Apart from modest decreases in CD44 expression, the proportions of these populations were also largely unaffected by the PAHs.
Each PAH produced a twofold loss of B220+ lymphocytes in the thymus (Fig. S3B), where this corresponds to 0.3 percent of the thymocytes (Yamano et al. 2015). Thymus B220+ cells derive either from BM progenitors or via peripheral emigration (Yamano et al. 2015). This suppression occurs in addition to the suppression of the total thymus population, and contrasts with the selectivity of PAH‐mediated suppression of BM B220+ cells. This is compatible with an origin from thymus progenitors rather than BM‐derived peripheral lymphocytes.
Further resolution of CYP1A1 and CYP1B1 contributions to BM gene expression
FACS analyses of BM progenitor populations indicate that some effects observed in DMBA‐treated WT mice are sustained in Cyp1b1‐ko mice (Fig. 6). Previous microarray analyses showed that DMBA induces remarkably few BM changes in WT mice at 12 h, whereas BP extensively and rapidly induces multiple chemokines and cytokines prior to the progenitor recovery phase (N'Jai et al. 2011). CYP1 family members metabolize activators of AhR and endogenous inflammatory agents (Choudhary et al. 2005; Chiaro et al. 2007; Bock 2014). Differences between WT and the Cyp‐ko‐mice derive from increases in endogenous substrates or an absence of active products. We have searched for participation of endogenous substrates of CYP1A1 and CYP1B1 by comparing basal BM gene expression changes in, respectively, Cyp1b1‐ko, Cyp1a1‐ko, or the Cyp1b1/Cyp1a1‐double ko (DKO) mice, with respect to similarly treated WT animals. These Cyp1 substrates may derive from physiological synthesis, including from polyunsaturated fatty acids, or from dietary components.
Two types of response to PAH‐ and TCDD‐mediated activation of the AhR have previously been characterized in the BM (N'Jai et al. 2011). These differences are shown in Table 1. Classical AhR/Arnt responses are equally stimulated by BP, DMBA, and TCDD within 12 h and are sustained for at least 24 h. A second set of gene stimulations, typical of an inflammatory response, is produced by BP within 6 h. For most of these inflammatory genes, the BP responses exceeded the TCDD responses and were minimal for DMBA. We have compared these responses to changes produced by the Cyp1 deletions.
Table 1 shows a set of 19 inflammatory genes, comprising chemokines, cytokines, prostaglandin synthase, and early response genes, which show this signature (BP (6 h) > TCDD >> DMBA). Many of these responses are associated with NF‐κB activation, possibly derived from a noncanonical association of AhR with Rel B (Vogel and Matsumura 2009).
We now report that Cyp1b1 and Cyp1a1 gene deletions alone selectively increase the expression of many of these inflammatory genes, suggesting a response to accumulating endogenous CYP1 substrates (Table 1). These deletions do not elevate endogenous AhR, since neither Cyp1a1 or any other direct AhR targets are stimulated. Notable differences are apparent between Cyp1b1ko, Cyp1a1‐ko, and DKO mice. For Cyp1b1‐ko, 13/19 acute BP target genes were elevated by 50 percent or more (eight with P < 0.05), with fewer in Cyp1a1‐ko mice (8/19; three with P < 0.05). Certain chemokines (Ccl forms 2, 3, 4, 5), Ifng, and the transcription factor, Egr1, each responded selectively to Cyp1b1 deletion. Tnf responded selectively to Cyp1a1 deletion, whereas others were equally responsive (Il6, Ifnb1, Socs3, Cxcl10). The DKO mice shared these responses (15/19; 9 with P < 0.05). Seven showed the highest stimulation, suggesting potentially additive stimulatory effects of the two deletions. The key stem cell regulator, Cxcl12, responded to each Cyp deletion, but not to AhR activation.
BP, in combination with the Cyp1a1‐ko or DKO, increased canonical AhR targets, but did not further stimulate the inflammatory gene expression (Table 1). This supports our hypothesis that BP stimulation of these genes indeed depends on Cyp1a1‐mediated BP metabolism, possibly to BP quinones (N'Jai et al. 2011). It is important to note that CYP1A1, unlike CYP1B1, is not expressed constitutively in the BM and, therefore, any effects derive from distal metabolism.
Surprisingly, DMBA, which is largely inactive in WT mice for these inflammatory responses, enhanced their endogenous gene expression in Cyp1b1‐ko mice (13/19; 7 with P < 0.05), often exceeding stimulation by TCDD. The classic AhR responders were suppressed by Cyp1b1 deletion, but were fully stimulated by DMBA in these mice. Remarkably, Ptgs2, the most selective acute responder to BP in WT animals, only responded to DMBA in Cyp1b1‐ko mice. Cyp1b1 deletion substantially enhances NF‐κB signaling in endothelia and pericytes (Palenski et al. 2013), both of which are present in these BM populations. The simplest explanation for this DMBA enhancement is an interaction with the AhR, which increases the noncanonical stimulation of these inflammatory genes by the accumulated endogenous CYP1B1 substrates.
Discussion
Previous work on PAH‐mediated immune suppression has addressed selective effects on the BM, spleen, or thymus, with respect to the generation of reactive metabolites in each tissue (Ward et al. 1984, 1986; Ladics et al. 1991; Miyata et al. 2001; Galvan et al. 2005; Gao et al. 2005, 2007). A single administration of DMBA, which extensively suppressed lymphoid progenitors in the BM within 6 h, depleted the mature lymphocytes between 24 and 48 h, with minimal impact on gene expression in the depleted population (N'Jai et al. 2010, 2011). We concluded that the primary mechanism of loss was the failure of progenitor cells to replenish exported mature B220+ lymphocytes.
The data presented here shows that this model extends to PAH‐initiated depletion of lymphocytes in spleen and thymus, wherein BM lymphoid progenitors, CLP and B220+ lymphocytes, are unable to migrate to and sustain the populations of the thymus and spleen, respectively.
This rapid loss of BM progenitor activity mediates the cellular tissue depletion, before toxicity is established, via locally generated reactive PAH metabolites, which are dependent on the level of Cyp1 expression. These processes are highly cell type‐dependent and are appreciably different between thymus and spleen (Choudhary et al. 2005; Harper et al. 2015). The maturation of lymphocytes in these tissues is dependent on local cell morphology, gene expression and signaling that controls these processes. We have shown that 48‐h post treatment, at the time of 50 percent cell depletion, the ratio of these different cell types remains largely unchanged. This supports a model in which the availability of BM progenitors is much more affected than the subsequent partitioning between alternative pathways.
Effects of DMBA on lymphoid progenitors determine later changes in mature populations in BM, spleen, and thymus
The relationship between progenitors and the mature cell responses has been pursued using oral and IP administrations of DMBA that distinguish the initial 6‐h post treatment, when BM progenitor suppression occurs, from the subsequent 48 h, when depletion of mature cells is apparent in the tissues. The tissue depletion kinetics reflect turnover rates that have been measured for lymphocytes in these tissues (Scollay et al. 1980).
Initial peak blood levels are similar between both modes of administration, but the rapid clearance of oral PAHs (approximately 6 h) allows a more rapid recovery of progenitor activity. Accordingly, the time profile for recovery of mature cells in each tissue in the 48‐ to 168‐h period matches the 6‐ to 48‐h profile for the BM progenitor cells.
Dependence of progenitor suppression on CYP1B1
Previous work has established the role of Cyp1b1 in DMBA‐mediated progenitor suppression in the BM (Galvan et al. 2003) and spleen (Gao et al. 2007). Here, we show that this extends to the thymus, despite tissue‐specific differences in Cyp1b1‐mediated activation of reactive PAH metabolites (Harper et al. 2015), suggesting that this suppression is initiated in the BM progenitor cells.
BP and DMBA produce similar BM suppression profiles in PAH‐resistant AhRd mice, which are each dependent on basal CYP1B1 expression (Galvan et al. 2003). In this respect, the CYP1B1 dependence matches the expression in the stromal compartment of the BM, which has appreciable basal CYP1B1, but is largely resistant to PAH induction (Galvan et al. 2003). This also marks a distinction from the majority of BM populations, wherein induction is appreciable (N'Jai et al. 2011).
AhR‐mediated recovery of BM progenitors, specifically after BP treatment
BP suppression of CFU‐preB activity in WT mice is completely reversed by an AhR‐mediated protection mechanism within 48 h. Although AhR activation enhances CYP1A1‐mediated clearance of BP in the liver (Uno et al. 2006), this recovery is paralleled by the generation of multiple chemokines, cytokines, and prostanoids that also depend on AhR‐mediated induction of CYP1A1 (N'Jai et al. 2011) (Table 1). Low levels of BP quinones formed in the liver by CYP1A1, which have no equivalent in DMBA metabolism, are likely mediators of this recovery through generation of oxygen radicals that activate NF‐κB. This can directly signal antiapoptotic effects to the progenitors (Mann et al. 2001) that can block the mediation by p53 (Page et al. 2003) or stimulate inflammatory gene products that protect stem cells (Page et al. 2004; Siebenlist et al. 2005).
BP by‐passes AhR‐mediated recovery to suppress the thymus T‐lymphocyte expansion
The thymus is further distinguished by showing very similar response patterns for DMBA and BP in WT mice, irrespective of AhR genotype. This parity is observed despite well‐established effects of AhR activation on the differentiation processes in each tissue (Rohlman et al. 2012; Gasiewicz et al. 2014). The generation of T cells, which predominantly contribute to the thymus weight, is dependent on the export of CLP from the BM and subsequent uptake and propagation in specialized niches of the gland, which is a much more important in this mechanism than thymus‐initiated metabolism (Boehm 2012). CCR7, CCR9, and PSGL‐1 are key mediators of this process (Wurbel et al. 2006; Zlotoff et al. 2010; Sultana et al. 2012). The insensitivity of this expansion to PAH‐mediated AhR activation is remarkable because the expansion of CLP in the thymus (DN1‐DN4 stages) is sensitive to TCDD‐mediated AhR activation (Laiosa et al. 2010), as is the differentiation of CD4+ T cells to form Treg and TH17 cells (Rohlman et al. 2012). The transient window of PAH activity, in this single dose protocol, may be too short compared to the persistent effects of TCDD. We propose that CLP may exit the BM stromal niche in the 3–6 h before AhR‐induced recovery factors can engage the progenitors.
DMBA and CYP1B1 each affect inflammatory signaling to BM progenitor populations
This suppression of CFU‐preB proliferative activity precedes changes in CLP populations, as determined by flow cytometry (6 vs. 24 h post treatment). This 18‐h time difference may reflect the requisite time for the depletion of the cell populations through differentiation to mature cells. The CFU assays indicate that progenitors correspond to 1/10,000 cells. FACS analyses resolve the larger CLP, CMP, GMP, and MEP populations, which each decline between 12 and 24 h post PAH treatment. The HSC population, which is increased by DMBA in WT mice (Galvan et al. 2006), includes both LT‐HSC and ST‐HSC. The reduced HSC levels in DMBA‐treated Cyp1b1‐ko mice arise because of enhanced conversion of HSC to the multiple lineages.
CYP1B1 metabolism of endogenous substrates also affects inflammatory signaling
Analysis of the BM microarray data shows that many of the same group of inflammatory chemokines and cytokines that are stimulated by BP metabolites are also elevated in Cyp1b1‐ko mice. BM gene expression changes in Cyp1a1‐ko, Cyp1b1‐ko, and DKO mice indicate that endogenous substrates that are metabolized by both P450 cytochromes function in this respect. Cyp1b1, however, has more impact. The absence of basal Cyp1a1 expression in the BM means that increased expression in Cyp1a1‐ko mice cannot derive from direct metabolism in the BM. The attenuation of metabolism at distal sites where CYP1A1 is constitutively expressed, such as the lung, must therefore contribute this endogenous stimulation in the Cyp1a1‐ko mice.
Endogenous CYP1B1 substrates, which exhibit AhR activation, have been observed in vivo (Chiaro et al. 2007). CYP1B1 is expressed in BM in sufficient amounts to substantially deplete DMBA in this tissue (N'Jai et al. 2011). Cyp1b1 deletion elevated BM Cxcl10, IL6, Ccl3, and Ifng gene expression, which was also observed in the DKO mice. Surprisingly, DMBA treatment doubled many of the responses to Cyp1b1 deletion, without any effect in WT mice. None of the Cyp1 deletions stimulated canonical AhR/Arnt regulation.
Unidentified endogenous CYP1B1 substrates stimulate oxidative stress and NF‐κB activity in endothelia and pericytes (Palenski et al. 2013), suggesting that similar signaling may direct gene expression in the BM. The overlapping effects of BP metabolites, TCDD and Cyp1b1 deletion in the presence of DMBA, indicate an alternative AhR activation mechanism; possibly by Rel partners released from NF‐κB activation (Vogel and Matsumura 2009). Although DMBA quinones are not generated via direct CYP1B1 metabolism, DMBA can potentially participate in this mechanism in Cyp1b1‐ko mice through the conversion of dihydrodiols to ortho‐quinones by Akr1c aldehyde dehydrogenases (Park et al. 2008).
A unified model for systemic PAH effects on lymphocyte distribution
Figure 8 summarizes our model for systemic PAH effects on lymphocytes that are released from BM, spleen and thymus into the blood and lymphatic system. The depleted BM, spleen and thymus cells are selectively restored by maturation of pre‐B progenitors, released from specialized BM niches (Panaroni and Wu 2013). A diverse set of pharmacological manipulations (PAH, dose, route of administration, activities of CYP1B1 and AhR) that rapidly affect the BM progenitors (6 h) produce parallel effects on the lymphocyte populations in all three tissues 48 h post treatment. Local toxicity, which becomes important with repeated dosing, plays a surprisingly small part in the single dose model. The rapid clearance of oral PAH doses and the dramatic suppression of progenitors offers a new approach to studying the recovery processes. The highly effective AhR‐mediated recovery of progenitors, the identification of mediators and the basis for the by‐pass by thymus progenitors provide additional major research opportunities.
Figure 8.
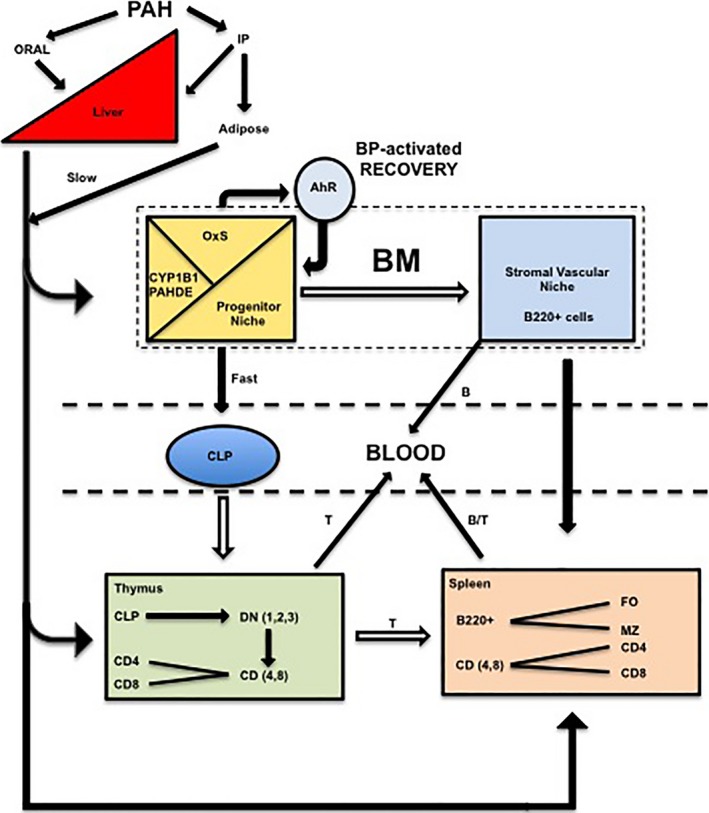
Model for systemic polycyclic aromatic hydrocarbon (PAH) effects on lymphocytes that are released from BM, spleen, and thymus into the blood and lymphatic system. Approximately 50 percent of PAH, administered via oral gavage, is transferred from the gut to the liver via the portal vein. The remainder is not taken up. Oral PAHs are largely cleared from circulation within 12 h by first‐pass metabolism in the liver. Approximately 50 percent of the Intraperitoneal (IP) PAH is transferred to the circulation to be metabolized in the liver, achieving serum levels comparable to oral administration for the first 6‐h post treatment. The remaining IP PAH is sequestered in the adipose tissue with sustained release into the circulation over 168 h. Circulating PAHs are metabolized by stromal CYP1B1 to PAH dihydrodiol epoxides (PAHDE) within the BM progenitor niche. PAHDE mediate the depletion of CLP progenitors, which either differentiate to the BM lymphoid (B220+) populations that transfer to the spleen for maturation and residence within the marginal zone (MZ) or follicular (FO) compartment, or transfer to the thymus where they mature into T lymphocytes (via 4 stages of development, DN1‐4), which also transfer to the spleen. BP mediates a similar suppression of progenitor B‐cell activity, which is rapidly reversed via an AhR‐dependent protection mechanism. This protection mechanism does not extend to the thymus due to rapid transfer of CLP from the BM prior to the onset of recovery. The mature T‐ and B‐cell populations in the thymus and spleen are replaced from BM progenitors after emigration to the blood and to the lymphatic system.
Disclosure
None declared.
Supporting information
Figure S1. The effect of collagenase treatment in the isolation of progenitor and mature BM cell populations.
Figure S2. Circulating BP is threefold lower in AhRd mice than in WT mice 24 h post treatment.
Figure S3. The differential suppression and recovery of spleen and thymus cells following DMBA and BP treatment.
Acknowledgements
We thank Anna Jatzek and Dr. M. Suresh for help with flow cytometry analysis, and Gerald Mikel for assistance in figure preparation.
This work was supported by US Public Health Service grant R01 DK072749 (C.R.J), and the Walter and Martha Renk Endowed Laboratory for Food Safety (C.J.C). This work was also supported by NIH/NIAID (R21AI103656) and NIH/NIDDK (R01DK100917) to ECF; and by CIRM Shared Stem Cell Facilities (CL1‐00506) and CIRM Major Facilities (FA1‐00617‐1) awards to UCSC. ECF is the recipient of a California Institute for Regenerative Medicine (CIRM) New Faculty Award (RN1‐00540) and an American Cancer Society Research Scholar Award (RSG13‐193‐01‐DDC).
Larsen M. C., N'Jai A. U., Alexander D. L., Rondelli C. M., Forsberg E. C., Czuprynski C. J., Jefcoate C. R.. Cyp1b1‐mediated suppression of lymphoid progenitors in bone marrow by polycyclic aromatic hydrocarbons coordinately impacts spleen and thymus: a selective role for the Ah Receptor, Pharma Res Per, 4(4), 2016, e00245, doi: 10.1002/prp2.245
References
- Bock KW (2014). Homeostatic control of xeno‐ and endobiotics in drug‐metabolizing system. Biochem Pharmacol 90: 1–6. [DOI] [PubMed] [Google Scholar]
- Boehm T (2012). Self‐renewal of thymocytes in the absence of competitive precursor replenishment. J Exp Med 209: 1397–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer SW, Schroeder AV, Smith‐Berday S, Forsberg EC (2011). All hematopoietic cells develop from hematopoietic stem cells through Flk2/Flt3‐positive progenitor cells. Cell Stem Cell 9: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchiel SW, Luster MI (2001). Signaling by environmental polycyclic aromatic hydrocarbons in human lymphocytes. Clin Immunol 98: 2–10. [DOI] [PubMed] [Google Scholar]
- Buters JT, Sakai S, Richter T, Pineau T, Alexander DL, Savas U, et al. (1999). Cytochrome P450 CYP1B1 determines susceptibility to 7, 12dimethylbenz[a]anthracene‐induced lymphomas. Proc Nat Acad Sci USA 96: 1977–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaro CR, Patel RD, Marcus CB, Perdew GH (2007). Evidence for an aryl hydrocarbon receptor‐mediated cytochrome P450 autoregulatory pathway. Mol Pharmacol 72: 1369–1379. [DOI] [PubMed] [Google Scholar]
- Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB (2005). Expression patterns of mouse and human CYP orthologs (families 1‐4) during development and in different adult tissues. Arch Biochem Biophys 436: 50–61. [DOI] [PubMed] [Google Scholar]
- Eaves CJ (2015). Hematopoietic stem cells: concepts, definitions, and the new reality. Blood 125: 2605–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan N, Jaskula‐Sztul R, MacWilliams PS, Czuprynski CJ, Jefcoate CR (2003). Bone marrow cytotoxicity of benzo[a]pyrene is dependent on CYP1B1 but is diminished by Ah receptor‐mediated induction of CYP1A1 in liver. Toxicol Appl Pharmacol 193: 84–96. [DOI] [PubMed] [Google Scholar]
- Galvan N, Teske DE, Zhou G, Moorthy B, MacWilliams PS, Czuprynski CJ, et al. (2005). Induction of CYP1A1 and CYP1B1 in liver and lung by benzo(a)pyrene and 7,12dimethylbenz(a)anthracene do not affect distribution of polycyclic hydrocarbons to target tissue: role of AhR and CYP1B1 in bone marrow cytotoxicity. Toxicol Appl Pharmacol 202: 244257. [DOI] [PubMed] [Google Scholar]
- Galvan N, Page TJ, Czuprynski CJ, Jefcoate CR (2006). Benzo(a)pyrene and 7,12dimethylbenz(a)anthrecene differentially affect bone marrow cells of the lymphoid and myeloid lineages. Toxicol Appl Pharmacol 213: 105–116. [DOI] [PubMed] [Google Scholar]
- Gao J, Lauer FT, Dunaway S, Burchiel SW (2005). Cytochrome P450 1B1 is required for 7,12dimethylbenz(a)‐anthracene (DMBA) induced spleen cell immunotoxicity. Toxicol Sci 86: 68–74. [DOI] [PubMed] [Google Scholar]
- Gao J, Lauer FT, Mitchell LA, Burchiel SW (2007). Microsomal expoxide hydrolase is required for 7,12‐dimethylbenz[a]anthracene (DMBA)‐induced immunotoxicity in mice. Toxicol Sci 98: 137–144. [DOI] [PubMed] [Google Scholar]
- Gao J, Mitchell LA, Lauer FT, Burchiel SW (2008). p53 and ATM/ATR regulate 7,12dimethylbenz[a]anthracene‐induced immunosuppression. Mol Pharmacol 73: 137–146. [DOI] [PubMed] [Google Scholar]
- Gasiewicz TA, Singh KP, Bennett JA (2014). The Ah receptor in stem cell cycling, regulation, and quiescence. Ann NY Acad Sci 1310: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper TA, Morre J, Lauer FT, McQuistan TJ, Hummel JM, Burchiel SW, et al. (2015). Analysis of dibenzo[def, p]chrysene‐deoxyadenosine adducts in wild‐type and cytochrome P450 1b1 knockout mice using stable‐isotope dilution UHPLC‐MS/MS. Mutation Res 782: 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidel SM, MacWilliams PS, Baird WM, Dashwood WM, Buters JT, Gonzalez FJ, et al. (2000). Cytochrome P4501B1 mediates induction of bone marrow cytotoxicity and preleukemia cells in mice treated with 7,12‐ dimethylbenz[a]anthracene. Cancer Res 60: 3454–3460. [PubMed] [Google Scholar]
- Ladics GS, Kawabata TT, White KL Jr (1991). Suppression of the in vitro humoral immune response of mouse splenocytes by 7,12‐dimethylbenz[a]anthracene metabolites and inhibition of immunosuppression by alpha‐naphthoflavone. Toxicol Appl Pharmacol 110: 31–44. [DOI] [PubMed] [Google Scholar]
- Laiosa MD, Wyman A, Murante FG, Fiore NC, Staples JE, Gasiewicz TA, et al. (2003). Cell proliferation arrest within intrathymic lymphocyte progenitor cells causes thymic atrophy mediated by the aryl hydrocarbon receptor. J Immunol 171: 4582–4591. [DOI] [PubMed] [Google Scholar]
- Laiosa MD, Mills JH, Lai ZW, Singh KP, Middleton FA, Gasiewicz TA, et al. (2010). Identification of stage‐specific gene modulation during early thymocyte development by wholegenome profiling analysis after aryl hydrocarbon receptor activation. Mol Pharmacol 77: 773783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen MC, Bushkofsky JR, Gorman T, Adhami V, Mukhtar H, Wang S, et al. (2015). Cytochrome P450 1B1: an unexpected modulator of liver fatty acid homeostasis. Arch Biochem Biophys 571: 21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legraverend C, Guenthner TM, Nebert DW (1984). Importance of the route of administration for genetic differences in benzo[a]pyrene‐induced in utero toxicity and teratogenicity. Teratol 29: 34–47. [DOI] [PubMed] [Google Scholar]
- Li J, Cai H, Jin J, Wang Q, Miao D (2012). X‐ray irradiation selectively kills thymocytes of different stages and impairs the maturation of donor‐derived CD4+CD8+ thymocytes in recipient thymus. J Biomed Res 26: 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann KK, Doerre S, Schlezinger JJ, Sherr DH, Quadri S (2001). The role of NF‐kB as a survival factor in environmental chemical‐induced pre‐B cell apoptosis. Mol Pharmacol 59: 302309. [DOI] [PubMed] [Google Scholar]
- Miyata M, Furukawa M, Takahashi K, Gonzalez FJ, Yamazoe Y (2001). Mechanism of 7,12dimethylbenz[a]anthracene‐induced immunotoxicity: role of metabolic activation at the target organ. Jap J Pharmacol 86: 302–309. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Shi Z, Galvez‐Peralta M, Uno S (2013). Oral benzo[a]pyrene: understanding pharmacokinetics, detoxication, and consequences‐Cyp1 knockout mouse lines as a paradigm. Mol Pharmacol 84: 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N'Jai AU, Larsen M, Shi L, Jefcoate CR, Czuprynski CJ (2010). Bone marrow lymphoid and myeloid progenitor cells are suppressed in 7,12‐dimethylbenz(a)anthracene (DMBA) treated mice. Toxicol 271: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N'Jai AU, Larsen MC, Bushkofsky JR, Czuprynski CJ, Jefcoate CR (2011). Acute disruption of bone marrow hematopoiesis by benzo(a)pyrene is selectively reversed by aryl hydrocarbon receptor‐mediated processes. Mol Pharmacol 79: 724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okey AB, Vella LM, Harper PA (1989). Detection and Characterization of a low affinity form of cytosolic Ah receptor in livers of mice nonresponsive to induction of cytochrome P1‐450 by 3‐methylcholanthrene. Mol Pharmacol 35: 823–830. [PubMed] [Google Scholar]
- Page TJ, O'Brien S, Holston K, MacWilliams PS, Jefcoate CR, Czuprynski CJ (2003). 7,12dimethylbenz[a]anthracene‐induced bone marrow toxicity is p53‐dependent. Toxicol Sci 74: 85–92. [DOI] [PubMed] [Google Scholar]
- Page TJ, MacWilliams PS, Suresh M, Jefcoate CR, Czuprynski CJ (2004). 7‐12 Dimethylbenz[a]anthracene‐induced bone marrow hypocellularity is dependent on signaling through both TNFR and PKR. Toxicol Appl Pharmacol 198: 21–28. [DOI] [PubMed] [Google Scholar]
- Palenski TL, Gurel Z, Sorenson CM, Hankenson KD, Sheibani, N (2013). Cyp1b1 expression promotes angiogenesis by suppressing NF‐κB activity. Am J Physiol Cell Physiol 305: C1170C1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaroni C, Wu JY (2013). Interactions between B lymphocytes and the osteoblast lineage in bone marrow. Calcif Tissue Int 93: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BV, Pan F (2015). Metabolic regulation of T cell differentiation and function. Mol Immunol 68: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Gelhaus S, Vedantam S, Oliva AL, Batra A, Blair IA, et al. (2008). The pattern of p53 mutations caused by PAH o‐quinones is driven by 8‐oxo‐dGuo formation while the spectrum of mutations is determined by biological selection for dominance. Chem Res Toxicol 21: 1039–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper K, Grimbacher B, Eibel H (2013). Mechanisms of allergic diseases. J Allergy Clin Immunol 131: 959–971. [DOI] [PubMed] [Google Scholar]
- Rohlman D, Pham D, Yu Z, Steppan LB, Kerkvliet NI (2012). Aryl hydrocarbon receptormediated pertubations in gene expression during early stage of CD4+ T‐cell differentiation. Frontiers Immunol. 3: article 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann J, Stanko K, Schliesser U, Appelt C (2015). Differences in CD44 surface expression levels and function discriminates IL‐17 and IFN‐γ producing helper T cells. PLoS ONE 10: e0132479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz BA, Bhandoola A (2006). Trafficking from the bone marrow to the thymus: a prerequisite for thymopoiesis. Immunol Rev 209: 47–57. [DOI] [PubMed] [Google Scholar]
- Scollay RG, Butcher EC, Weissman IL (1980). Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol 10: 210–218. [DOI] [PubMed] [Google Scholar]
- Siebenlist U, Brown K, Claudio E (2005). Control of lymphocyte development by nuclear factor‐κB. Nature Rev Immunol 5: 435–444. [DOI] [PubMed] [Google Scholar]
- Singh KP, Wyman A, Casado FL, Garrett RW, Gasiewicz TA (2009). Treatment of mice with the Ah receptor agonist and human carcinogen dioxin results in altered numbers and function of hematopoietic stem cells. Carcinogenesis 30: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana DA, Zhang SL, Todd SP, Bhandoola A (2012). Expression of functional p‐selectin glycoprotein ligand 1 on hematopoietic progenitors is developmentally regulated. J Immunol 188: 4385–4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temchura VV, Frericks M, Nacken W, Esser C (2005). Role of the aryl hydrocarbon receptor in thymocyte emigration in vivo. Eur J Immunol 35: 2738–2747. [DOI] [PubMed] [Google Scholar]
- Thurmond TS, Gasiewicz TA (2000). A single dose of 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin produces a time‐ and dose‐dependent alteration in the murine bone marrow B‐lymphocyte maturation profile. Toxicol Sci 58: 88–95. [DOI] [PubMed] [Google Scholar]
- Thurmond LM, Lauer LD, House RV, Cook JC, Dean JH (1987). Immunosuppression following exposure to 7,12‐dimethylbenz[a]anthracene (DMBA) in Ah‐responsive and Ah‐nonresponsive mice. Toxicol Appl Pharmacol 91: 450–460. [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Derkenne S, Curran CP, Miller ML, Shertzer HG, et al. (2004). Oral exposure to benzo[a]pyrene in the mouse: detoxication by inducible cytochrome P450 is more important than metabolic activation. Mol Pharmacol 65: 1225–1237. [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Dragin N, Curran CP, Derkenne S, Miller ML, et al. (2006). Oral benzo[a]pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxication, CYP1B1 metabolism required for immune damage independent of total‐body burden and clearance rate. Mol Pharmacol 69: 1103–1114. [DOI] [PubMed] [Google Scholar]
- Vogel CFA, Matsumura F (2009). A new cross‐talk between the aryl hydrocarbon receptor and RelB, a member of the NF‐κB family. Biochem Pharmacol 77: 734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath AL, Smith AA, Craven M, Bradfield CA (2009). EDGE3: a web‐based solution for management and analysis of Agilent two color microarray experiments. BMC Bioinformatics 10: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward EC, Murray MJ, Lauer LD, House RV, Irons R, Dean JH (1984). Immunosuppression following 7,12‐dimethylbenz[a]anthracene exposure in B6C3F1 mice. I. Effects on humoral immunity and host resistance. Toxicol Appl Pharmacol 75: 299–308. [DOI] [PubMed] [Google Scholar]
- Ward EC, Murray MJ, Lauer LD, House RV, Dean JH (1986). Persistent suppression of humoral and cell‐mediated immunity in mice following exposure to the polycyclic aromatic hydrocarbon 7,12‐dimethylbenz[a]anthracene. Int J Immunopharmacol 8: 13–22. [DOI] [PubMed] [Google Scholar]
- Wurbel MA, Malissen B, Campbell JJ (2006). Complex regulation of CCR9 at multiple discrete stages of T cell development. Eur J Immunol 36: 73–81. [DOI] [PubMed] [Google Scholar]
- Yamano T, Steinert M, Klein L (2015). Thymic B cells and central T cell tolerance. Front Immunol 6: 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotoff DA, Sambandam A, Logan TD, Bell JJ, Schwarz BA, Bhandoola A (2010). CCR7 and CCR9 together recruit hematopoietic progenitors to the adult thymus. Blood 115: 1897–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The effect of collagenase treatment in the isolation of progenitor and mature BM cell populations.
Figure S2. Circulating BP is threefold lower in AhRd mice than in WT mice 24 h post treatment.
Figure S3. The differential suppression and recovery of spleen and thymus cells following DMBA and BP treatment.


