Summary
A rise in the annual incidence of oropharynx squamous cell cancer, specifically the lingual and palatine tonsils, in white men under the age of 50, non-smokers and non-alcoholics, has been observed over the past decade. This entity is associated with human papilloma virus-16 infection and the risk factors include an increased number of oral and vaginal sex partners at a younger age. The biology of HPV-related oropharynx cancer is distinct with p53 degradation, Rb pathway inactivation, and p16 upregulation. This is in contrast to tobacco related oropharynx cancer which is characterized by p53 mutation and downregulation of p16. The optimal method to detect virus in tumor is controversial and both in situ hybridization and PCR are commonly used; p16 immunohistochemistry may serve as a potential surrogate marker. HPV-related oropharynx cancer appears to be more responsive to chemotherapy and radiation than HPV negative orpharynx cancer. HPV-16 is a prognostic marker for improved overall survival and disease-free survival but has not yet been shown to be a predictive marker. Investigators continue to explore unanswered questions regarding the natural history of oral HPV infection, why the increase dominates in men, the potential of HPV vaccines for primary prevention, developing a commercially available accurate method to detect the virus in tumor, and treatment strategies that reduce toxicity without compromising survival. The goal of this review is to highlight our current understanding of the epidemiology, biology, detection, as well as current management and unresolved issues of HPV-related oropharyngeal HNSCC.
Introduction
Cancers of the head and neck arise from the mucosa lining the oral cavity, oropharynx, hypopharynx, larynx, sinonasal tract and nasophaynx. By far the most common histologic type is squamous cell carcinoma (HNSCC) and grade can vary from well-differentiated, keratinized to undifferentiated, non-keratinizing. Recently, an increase in the incidence of oropharyngeal HNSCC specifically the tonsil and tongue base has been documented in the United States, most notably among individuals ranging in age between 40 and 55 years. Patients with oropharyngeal HNSCC are primarily white men. Unlike most tobacco-related head and neck cancers, patients with oropharyngeal HNSCC often do not have a history of tobacco or alcohol use. Instead, their tumors are positive for oncogenic forms of the human papillomavirus (HPV), particularly the 16 type (Figure One). Approximately 60% of oropharyngeal HNSCC in the United States are HPV16-positive. HPV-associated HNSCC appears to be a distinct clinical entity with a better prognosis than HPV-negative HNSCCs, due in part to increased tumor sensitivity to chemotherapy and radiation therapy. Although HPV is now recognized as an etiologic agent for a subset of oropharyngeal HNSCC, the biology and natural history of oropharyngeal HPV infection and the optimal clinical management of patients with HPV-related HNSCCs is not well understood.
Figure One.
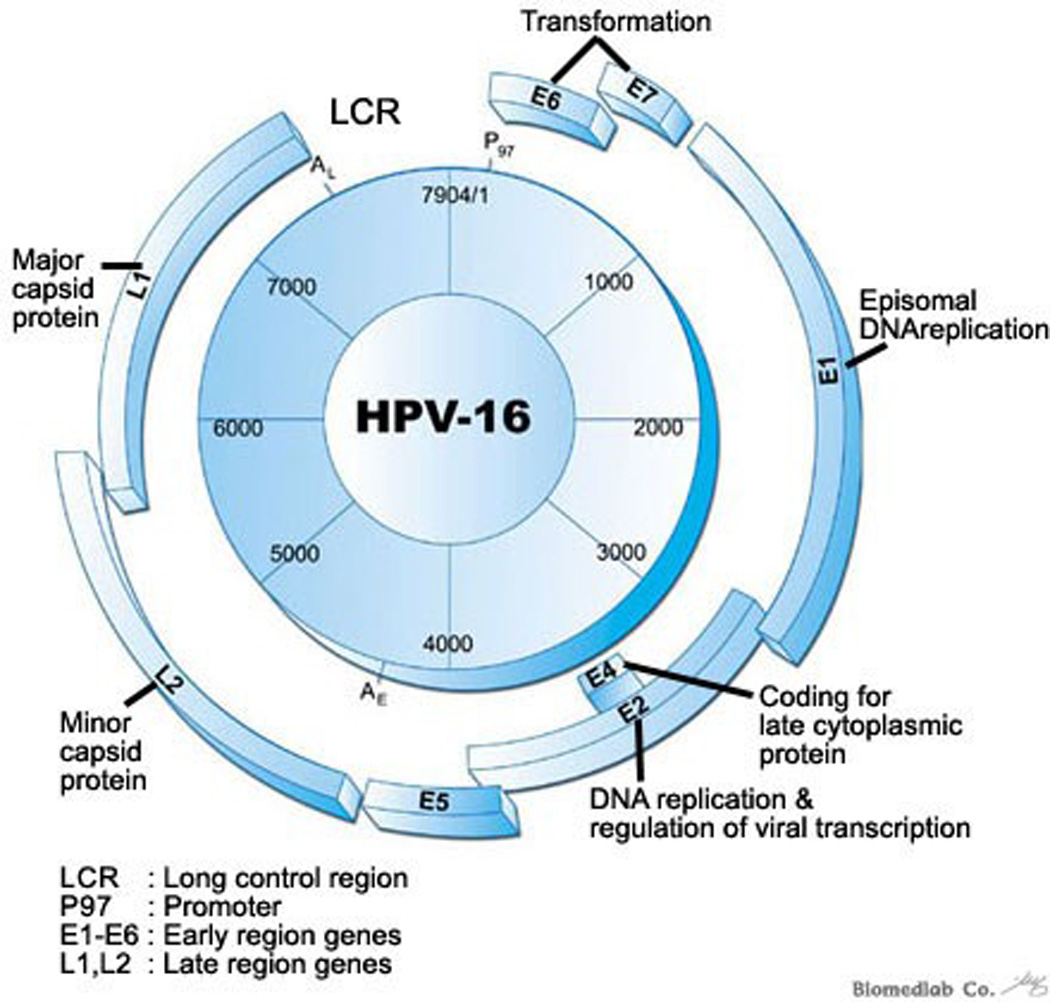
Human Papilloma Virus-16 is the oncogenic form of all HPV types that is frequently associated with oropharyngeal HNSCC. This figure illustrates the genetic composition of HPV-16 DNA virus. E6 and E7 oncoproteins, dysregulation plays a key role in distinct biology of HPV associated oropharyngeal HNSCC. Image obtained from www.dnachip-link.com/Eng/library/HPV.asp. Biomedlab Co.
Distinct Epidemiology and risk factors of HPV-associated head and neck squamous cell carcinoma
Head and neck cancer is the sixth most common cancer worldwide with an estimated annual burden of 563, 826 incident cases (including 274, 850 oral cavity cancers, 159, 363 larynx cancers, and 52, 100 oropharynx cancers) and 301, 408 deaths.1 While human papillomavirus (HPV) has been known to be an important cause of anogenital cancer for some time, only recently has it been recognized that HPV is an etiologic cause of a subset of head and neck squamous cell carcinomas (HNSCC).2 There are over 100 different types of HPV3 and more than 15 types considered to have oncogenic potential.4 However, the majority (>90%) of HPV-associated HNSCC are caused by a single HPV type, HPV16, the same type responsible for the majority of HPV-associated anogenital cancers.
The proportion of HNSCC caused by HPV varies widely (Figure Two), in large part related to the burden of tobacco associated HNSCC in that population. Tobacco, alcohol, poor oral hygiene and genetics remain important risk factors for HNSCC overall, but HPV is now recognized as one of the primary causes of oropharyngeal squamous cell cancers. In the United States 40–80% of oropharyngeal cancers are caused by HPV, while in Europe the proportion varies more widely from >90% in Sweden to <20% in European communities with the highest rates of tobacco use (Figure Two).
Figure Two.
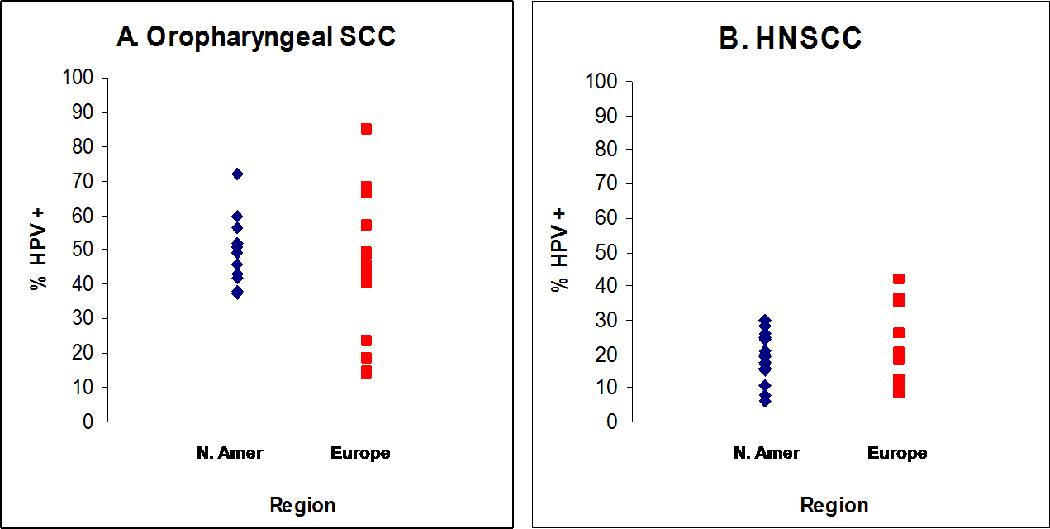
Proportion of oropharyngeal (panel A) and head and neck (panel B) squamous cell carcinoma (HNSCC) that are caused by HPV in North America and Europe. Only studies with more than 25 oropharynx (panel A: 27 studies)2, 5–13 or 50 HNSCC (panel B: 30 studies)5–9, 11, 14–16 cases were included.
The incidence of head and neck cancers overall in the United States has decreased in recent years, consistent with the decrease in tobacco use in the U.S. In contrast, the incidence of HPV-associated oropharyngeal cancer appears to be increasing, highlighting the increasing importance of this etiologic association.17–19 A recent U.S. study using Surveillance, Epidemiology, and End Results (SEER) data found the incidence of oropharyngeal cancers (which are the most likely to be HPV-associated) increased by 1.3% for base of tongue cancers and 0.6% for tonsillar cancers each year between 1973 to 2004. In contrast, the incidence of oral cavity cancers (HPV-unassociated) decreased by 1.9% each year during the same time period.17 The increasing incidence for oropharynx cancers was observed predominantly among white men (but not among women) in this study, and at younger ages (Figure Three). As in the U.S., an increase in the incidence of oropharyngeal cancers has been reported internationally including studies in Sweden,20 the Netherlands,21 the United Kingdom22 and Scotland.23 A study from the Swedish Cancer Registry during the same time period,24 also found increasing oropharyngeal rates, but the increases were substantially larger and occurred in both women and men. In this study, the age-adjusted incidence of tonsillar cancer increased 3.5-fold in women and 2.6-fold in men between 1970–2002.24 The increasing incidence of these HPV-associated oropharyngeal cancers represents an emerging viral epidemic of cancer.
Figure Three.
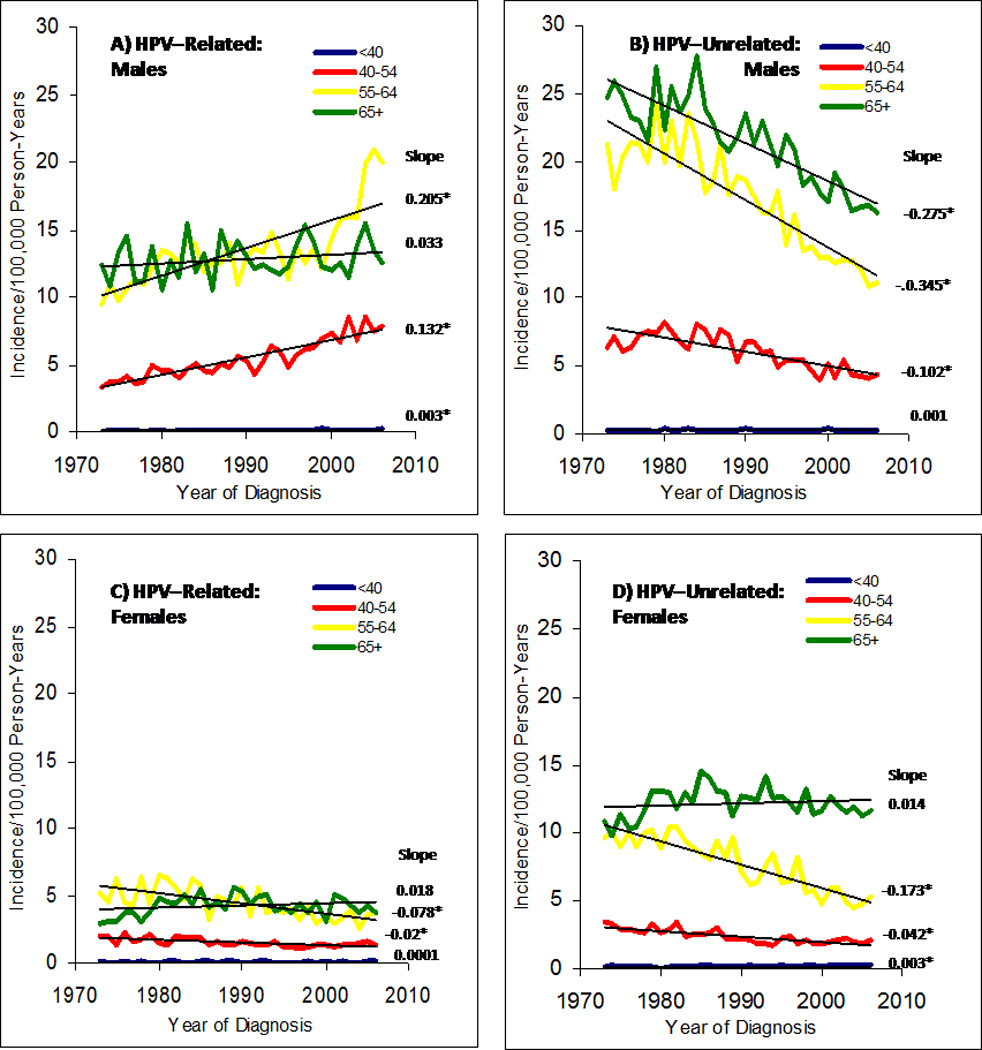
Age-adjusted incidence rates of HPV-related (panels A & C) and HPV-unrelated (panels B & D) head and neck squamous cell cancer between 1973 and 2006, stratified by age at diagnosis. The annual percent change in incidence for each age category is shown next to each line; * indicates slope with p<0.05. As per Chaturvedi et al17, HPV-related sites includes base of tongue, lingual tonsil, tonsil, oropharynx, and Waldeyer ring and HPV-unrelated sites includes other and unspecified parts of tongue, gum, floor of mouth, palate, and other parts of mouth. Graph generated with the assistance of Anil Chaturvedi, modified from similar graph in Chaturvedi et al. 2008 article.17
It is not known why the increased incidence of HPV-associated oropharyngeal cancer appears more pronounced among younger individuals. It may be due to changes in sexual norms (i.e. more oral sex partners or oral sex at an earlier age in recent than past generations) combined with less tobacco associated cancers among younger cohorts making the effects of HPV-associated cancers more visible. It is also unclear whether the higher rates of HPV-associated oropharyngeal cancers in men compared to women are explained solely by differences in sexual behavior, or whether there are biological differences in viral clearance that may contribute to the higher burden of these cancers among men. It is possible the HPV prevalence in cervical than penile tissue can increase the chances of HPVinfection when performing oral sex on a woman, contributing to the higher rate of HPV-associated oropharyngeal cancer among men.
As tobacco use has decreased in the last decades, the proportion of all head and neck cancers that are oropharyngeal in origin has correspondingly increased dramatically in both the US and internationally. SEER data suggest that ~18% of all U.S. head and neck cancers were located in the oropharynx in 1973, compared to 31% of HNSCC in 2004.19 Similarly, in Sweden, the proportion of oropharyngeal cancers caused by HPV has steadily increased from 23% in the 1970s, 57% in the 1990s, and as high as 93% in 2007.13, 25 This data highlights that HPV is now the primary cause of tonsillar cancers in Western countries.
Despite the recently recognized importance of HPV in many oropharyngeal cancers, the epidemiology of oral HPV infection is not well understood (Table One). Initial studies suggest that oral HPV prevalence increases with age. Prevalent oral HPV infection is detected in 3–5% of adolescents26–28 and 5–10% of adults.14, 29 It is not yet known whether oral HPV natural history or risk factors for persistent HPV infection in the oropharynx differ from that known for anogenital HPV infection (Table One). Initial studies suggest oral HPV prevalence increases with number of sexual partners, is more common among men, HIV-infected individuals, and current tobacco users.26–28, 30, 31
Table One.
Summary of what is known about HPV-related head and neck squamous cell carcinoma (HNSCC) and what questions remain to be clarified.
| KNOWN | UNKNOWN |
|---|---|
| HPV causes a subset of oropharyngeal cancers | Whether HPV causes cancer at any other HNSCC sub-sites |
| Oral HPV is sexually transmitted | Exact sexual behaviors associated with transmission |
|
HPV-positive and HPV-negative HNSCC are distinct cancers |
How common is prevalent and persistent oral HPV infection in the general population |
|
Anogenital HPV infections are common but most clear on their own |
Oral HPV natural history Median time from oral HPV infection to cancer Factors which affect oral HPV persistence and progression to cancer |
|
Incidence of HPV-positive oropharyngeal cancer is increasing in some groups |
Why the increase in OP cancer incidence is: Seen in men but not women. more apparent in younger cohorts |
|
p16 IHC is strongly associated with tumor HPV16 status of oropharyngeal cancers |
Whether HPV-negative/p16-positive and HPV- positive/p16-positive oropharyngeal cancers have similar survival outcomes |
|
HPV-positive HNSCC have better median survival than HPV-negative HNSCC |
Whether the treatment of HPV-positive and HPV- negative HNSCC should be different |
|
Among HPV-positive HNSCC, nonsmokers have better median survival than smokers |
Biologic mechanism for why survival is different among HPV-positive HNSCC tobacco users and non-users. |
|
Oropharyngeal cancers are often detected at a late stage |
Are there pre-cancerous oropharyngeal lesions. (oral Pap)? Can testing for persistent oral HPV infection be a useful screening tool? |
|
There is no known cure for established HPV infection but HPV-vaccines can prevent new cervical HPV infections |
Efficacy of HPV-vaccines against oral HPV infection |
Given the importance of tobacco use in HNSCC, it is not surprising that most cases of HNSCC seen among nonsmokers are HPV-related. However, oral HPV infection is common among both smokers and nonsmokers and is an important cause of oropharyngeal cancer in both groups. For example, in recent case series only 13–16% of individuals with HPV-positive HNSCC were non-smoker non-drinkers (NSND).32, 33 While a higher proportion of individuals with HPV-positive than with HPV-negative cancers are non-smokers or NSND, many with HPV-positive HNSCC have a history of alcohol and tobacco use. In fact, 10–30% of HPV-positive HNSCC in recent studies were heavy tobacco and alcohol users.32, 33 This underscores that HPV-associated HNSCC is not only a disease of nonsmoker and nondrinkers, but also occurs in people with the traditional risk factors of tobacco and alcohol use.
HPV is a sexually transmitted infection and studies suggest that the number of lifetime sexual partners is an important risk factor for developing HPV-associated HNSCC. In case control studies the odds of HPV-positive HNSCC increased two-fold in individuals with 1–5 lifetime oral sexual partners and five-fold in those with six or more lifetime oral sexual partners, compared to those reporting no oral sex.32–34 However, it should be noted that HPV-positive HNSCC occurs among individuals reporting few sexual partners. For example, more than half of HPV-positive HNSCC in studies report five or fewer lifetime oral sexual partners and 8–40% of HPV-positive HNSCC cases report never having oral sex.32, 33 Therefore, while sexual behavior is an important risk factor for HPV-positive HNSCC, the absence of a high number of sexual partners does not exclude the diagnosis.
Distinct Biology and Clinical presentation of HPV associated Head and Neck Squamous Cell Carcinoma (HNSCC)
HPV associated HNSCC arises most commonly from the lingual and palatine tonsils.35 HPV preferentially targets the highly specialized reticulated epithelium lining the tonsillar crypts, but the intrinsic properties of this epithelium that renders it vulnerable to HPV infection are not yet recognized.36 Once the virus integrates its DNA genome within the host cell nucleus, it dysregulates the expression of the oncoproteins E6 and E7.37 The E6 protein induces degradation of p53, through ubiquitin-mediated p53 proteolysis, leading to a significant loss of p53 activity. The normal function of p53 is to arrest cells in G1 or induce apoptosis to allow host DNA to be repaired. The E6-expressing cells are not capable of this p53 mediated response to DNA damage and hence are susceptible to genomic instability. The E7 protein binds and inactivates the retinoblastoma(Rb) tumor suppressor gene product, causing the cell to enter into S-phase leading to cell cycle disruption, proliferation and malignant transformation.37
Morphologically, HPV-positive HNSCs deviate from the moderately differentiated keratinizing morphology that typifies most HNSCCs. HPV-positive HNSCCs consistently: a) arise from the tonsillar crypts; b) are unassociated with dysplasia of the surface epithelium; c) exhibit lobular growth; d) are permeated by infiltrating lymphocytes; e) lack significant keratinization; and f) demonstrate a prominent “basaloid” morphology.38 Clinically, HPV positive tumors tend to present at an early T stage and more advanced nodal stage (Table Two).39 In general, the HPV associated oropharynx cancers at presentation are Stage III or IV. The nodal metastases tend to be cystic and multilevel.40
Table Two.
Summary of the differences between HPV-positive and HPV-negative HNSCC
| HPV-positive | HPV-negative | |
|---|---|---|
| Anatomic site | Tonsil / BOT | All sites |
| Histology | Non-keratinized | Keratinized |
| Age | Younger | Older |
| Gender | 3:1 men | 3:1 men |
| Stage | Tx, T1–2 | Variable |
| Risk factors | Sexual behavior | Alcohol / tobacco |
| Incidence | Increasing | Decreasing |
| Survival | Improved | Unchanging |
BOT: Base of Tongue,
Current trends in pathological diagnosis of HPV-related HNSCC
HPV detection may ultimately serve a more comprehensive role than mere prognostication. HPV detection is emerging as a valid biomarker for discerning the presence and progress of disease encompassing all aspects of patient care from early cancer detection,41 to more accurate tumor staging (e.g. localization of tumor origin),42, 43 to selection of patients most likely to benefit from specific therapies44, to post-treatment tumor surveillance.45, 46 Consequently, there is a pressing need for a method of HPV detection that is highly accurate, reproducible from one diagnostic laboratory to the next, and practical for universal application in the clinical arena.
Despite a growing consensus for routine HPV testing of all oropharyngeal carcinomas, the optimal method for HPV detection is currently unsettled. A variety of methods are in current use ranging from consensus and type-specific PCR methods, real-time PCR assays to quantify viral load, type-specific DNA in-situ hybridization (ISH), detection of serum antibodies directed against HPV epitopes, and immunohistochemical detection of surrogate biomarkers (e.g. p16 protein). While PCR-based detection of HPV E6 oncogene expression in frozen tissue samples is generally regarded as the gold standard for establishing the presence of HPV, selection of HPV assays for clinical use will ultimately be influenced by a variety of concerns relating to sensitivity, specificity, reproducibility, cost, and feasibility.
For the routine assessment of oropharyngeal carcinoma, ISH is in routine use in many diagnostic pathology laboratories. The development of nonfluorescent chromogens now allows visualization of DNA hybridization using conventional light microscope; and adaptation of ISH to formalin-fixed and paraffin-embedded tissues has made this technique compatible with standard tissue-processing procedures and amendable to retrospective analysis of archival tissue blocks. Most PCR-based methods, on the other hand, require a higher level of technical sophistication and are optimized to fresh frozen samples.) ISH permits direct visualization of HPV distribution in tissue samples (Figure 4). Localization of the HPV genome to tumor cell nuclei allows one to distinguish between etiologically revelant HPV detection (clonal presence in all tumor cells) and virus or contamination (low copy detection in only a very few cells). In contrast, mere detection of virus by non-quantitative PCR-based methods does not distinguish transcriptionally active (i.e. clinically relevant) from transcriptionally inactive (i.e. clinically irrelevant) HPV infections. The superior sensitivity of ISH does not compromise its specificity. The introduction of various signal amplification steps has significantly improved the sensitivity of this technique, even to the point of viral detection down to one viral copy per cell.
Figure Four.
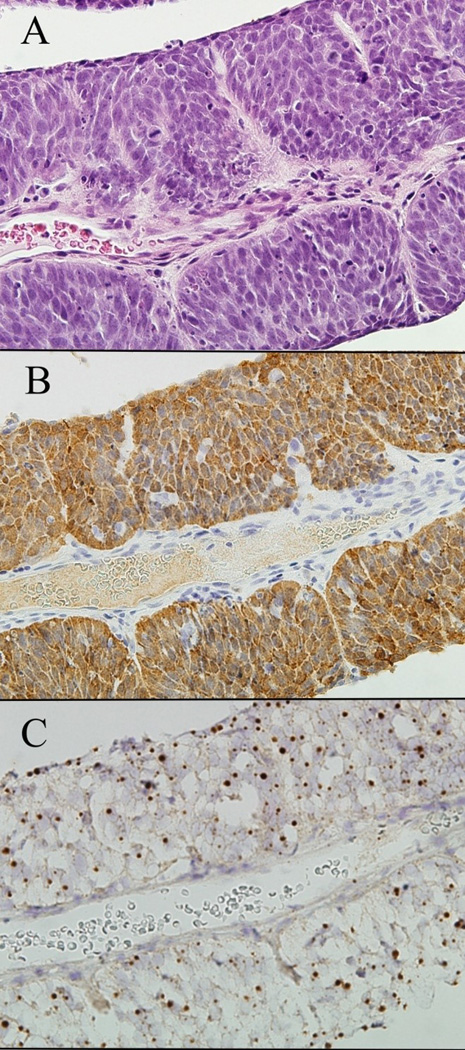
Strips of metastatic non-keratinized squamous cell carcinoma aspirated from a cystic neck mass (A, hematoxylin and eosin). The presence of HPV is visualized as strong cytoplasmic and nuclear staining for p16 by immunohistochemistry (B), and as dot-like hybridization signals within the nuclei of tumor cells by HPV-16 in-situ hybridization (C).
In HPV-positive oropharyngeal carcinomas, as described previously, the transcription of the viral oncoprotein E7 is known to functionally inactive the Retinoblastoma (Rb) gene product, causing a perturbation of other key components of the Rb pathway and known to induce an up regulation of p16 expression, reaching levels that can be readily detected by immunohistochemistry.47, 37 Accordingly, p16 immunohistochemistry is often advocated as a surrogate marker of HPV infection for oropharyngeal cancers.48, 49 In our experience comparing p16 immunohistochemical staining and HPV-16 ISH for large numbers of HNSCCs, the results of p16 immunohistochemistry and HPV-16 ISH are discordant in 7% of cases (unpublished observation). The discrepancies consistently involve cancers that are negative by HPV-16 ISH but positive by p16 immunohistochemistry. As the p16 assay is agnostic for HPV type, the higher positivity rate may reflect detection of non-HPV 16 types that comprise 5–10% of HPV-positive oropharyngeal cancers. Alternatively, p16 overexpression could reflect Rb pathway disturbances unrelated to HPV (e.g. mutational inactivation of Rb). Using E6/E7 mRNA levels as conclusive evidence of HPV involvement, p16 immunostaining of HNSCCs is 100% sensitive but only 79% specific as a surrogate marker of HPV infection.50 Although there are indications that p16 overexpression may predict clinical outcomes independent of HPV status,51, 49 replacement of HPV ISH by p16 immunohistochemistry is premature and awaits further confirmation of similar survival outcomes for patients with HPV-negative/p16-positive and HPV-positive/p16-positive oropharyngeal cancers.
The limitations of any single detection assay may be offset using algorithms that combine the strengths of complementary assays.50 One highly feasible algorithm incorporates p16 immunohistochemistry and HPV ISH. Given a sensitivity that approaches 100%, p16 immunostaining is a good first line assay for eliminating HPV-negative cases from any additional analysis. Given a specificity approaching 100%, a positive HPV-16 ISH reduces the number of false positive cases by p16 staining alone. A p16-positive/HPV-16-negative result singles out a subset of tumors that qualify for rigorous analysis for other (i.e. non-16) oncogenic HPV types. This 3rd-tier analysis could comprise wide-spectrum ISH probes that detect an extended panel of HPV types, or PCR-based methods for the detection of transcriptionally active virus.50 Whatever the method for establishing the presence of non-16 HPV types, the upfront use of p16 immunostaining and HPV-16 ISH accurately establishes the HPV status of the vast majority of oropharyngeal cancers.
HPV ISH and p16 immunostaining as a practical diagnostic approach to discerning HPV status can be readily applied to cytologic preparations including fine needle aspirates from patients with cervical lymph node metastases.41, 52 Further expansion of HPV testing to blood and other body fluids would advance the role of HPV as a clinically relevant biomarker, but these specimens would necessitate other detection platforms. PCR-based detection of HPV DNA in the blood53 and saliva54 of patients following treatment of their HPV-positive cancers suggests a future role in tumor surveillance. Detection of serum antibodies to HPV-related epitopes can predict the HPV status of head and neck cancers, and has been advocated as a way to predict clinical outcomes and guide therapy without the constraints of tissue acquisition.55, 53
Although HPV ISH may serve as a starting point for the routine and universal analysis of oropharyngeal carcinomas, HPV detection alone may not fully exploit its potential as a biomarker. A more advanced understanding of HPV-induced tumorigenesis - including the complex interaction of HPV infection with interconnecting molecular genetic pathways – will inevitably drive the implementation of increasingly sophisticated and comprehensive assays. Disruptive p53 mutations,56 aberrant Bcl-2 expression,57 overexpression of EGFR,58 and other pathway disturbances may act individually or in concert to modulate the prognostic impact of HPV detection, necessitating expanded biomarker profiling in conjunction with HPV analysis. In addition, the finding that therapeutic responses may correlate with HPV copy number suggests a future role for quantitative measurement of viral load.58
Management of HPV associated Head and Neck Squamous Cell Carcinoma (HNSCC)
The standard of care for locally advanced (T3 to T4 or N2 to N3) oropharynx cancer is either surgery and adjuvant radiotherapy with or without concurrent cisplatin, as indicated; or more commonly, concurrent chemoradiation for the purpose of preserving speech and swallowing function, particularly applicable to the management of disease at the base of tongue or tonsil. This became standard of care following a multi-center randomized controlled trial of 226 patients with stage III or IV squamous cell cancer of the oropharynx conducted in France.59 Patients were randomly assigned to receive either radiotherapy alone (70 Gy, 35 fractions) or the same radiotherapy with concomitant carboplatin and 5-FU.59 The 3-year survival) (51% versus 31%; p = 0.02) and disease-free survival (42% versus 20%; p = 0.04) rates were significantly improved with the addition of chemotherapy to radiotherapy. Rates of local-regional recurrence and death from oropharynx cancer were also significantly reduced with combined treatment, however, there was no difference in occurrence of distant metastases. These results were maintained at 5 years.60 It is noteworthy that the concomitant treatment group experienced greater acute toxicity including, mucositis related weight loss, feeding tube dependency, and myelosuppression but a significant difference in late effects was noted only for dentition.61 It should also be noted that the low survival outcomes, relative to current data from the US, reflect the population enrolled and traditional risk factors of tobacco and alcohol.
The increasing incidence of oropharyngeal cancer in younger populations and substantially higher survival rates with current treatment approaches stands in contrast to survival achieved in older individuals with co-morbid conditions associated with tobacco and alcohol history. Several head and neck patient characteristics have been associated with a favorable prognosis including nonsmoker, minimal exposure to alcohol, excellent performance status, and no comorbid conditions, all of which are associated with HPV-positive tumor status. Retrospective analyses suggest higher response rates to chemotherapy and radiation and improved survival62–64, 66 for HPV positive oropharynx cancer patients compared to HPV negative oropharynx cancers. An increased sensitivity to chemotherapy and radiotherapy has been attributed to absence of exposure to tobacco and the presence of functional, unmutated p53.63, 64, 65 Improved survival of HPV positive cancer is also likely due in part to the absence of field cancerization related to tobacco and alcohol exposure.67 The HPV positive tumors are more sensitive to cytotoxic chemotherapy and DNA damage-induced apoptosis secondary to the incorporation of viral oncoproteins E6 and E7.68, 69
In 2008, the first prospective HNSCC clinical trial to correlate tumor HPV status with outcome was published.70 The U.S. NCI-funded Eastern Cooperative Oncology Group (ECOG) conducted a prospective Phase II trial (ECOG 2399) testing non-surgical management of patients with clinical stage III or IV squamous cell carcinoma of the oropharynx or larynx. All tumors were evaluated for HPV16 by in-situ hybridization and p16 status by immunohistochemistry. Treatment consisted of induction chemotherapy with two cycles of carboplatin/paclitaxel followed by weekly paclitaxel concurrent with standard-fractionation radiation therapy, total dose 70 Gy in 35 fractions over 7 weeks. HPV16 was detected by in-situ hybridization in 63% (38/60) of oropharynx tumor specimens and all demonstrated high expression of p16.70 These patients were predominantly white men with less than 20 pack-years of cigarette use and the histology was poorly differentiated SCC with basaloid features. HPV status was correlated with treatment response, progression-free survival, and overall survival and all outcomes were better in the HPV positive compared to the HPV negative patient groups: response rates to induction chemotherapy were 82% and 55%, p=0.01; response at the completion of chemoradiation - 84% and 57%, p= 0.06; progression-free survival rate at 2 years - 86% and 53%, p = 0.02 respectively; and overall survival at 2 years - 95% vs. 62%, p = 0.0050 respectively.70
When the analysis was restricted to patients with oropharyngeal cancer,70 patients with HPV-positive tumors had a significantly better outcome compared to HPV negative oropharynx patients: overall survival rate at 2 years was 94% and 58%, p=0.004 respectively; and progression-free survival at 2 years 85% vs. 50%, p=0.05 respectively. In the same study, it was noted that acute toxicity was acceptable with this regimen, 49% of oropharyngeal cancer patients had moderate-to-severe swallowing impairment 3 months following treatment, and only 3% were still feeding tube dependent after 12 months. The excellent survival results suggesting increased sensitivity to chemotherapy and radiotherapy in HPV-positive patients have generated interest in evaluating the association between HPV 16, p16 and tobacco exposure and designing clinical trials with less toxic regimens for HPV-positive patients.
The association between HPV status, p16, tobacco exposure and survival was explored in a retrospective analysis of a large phase III trial (RTOG 0129) comparing standard fractionation radiotherapy and cisplatin to accelerated fraction radiotherapy and cisplatin.71 This study enrolled over 400 oropharynx cancers of which ; 61% (198/323) were HPV 16 positive by in situ hybridization. P16 was positive in 96% of HPV positive patients and 22% of HPV negative patients. The results of the analysis were consistent with the findings of the ECOG prospective trial. At a median follow-up of 4.4 years, patients with HPV-positive cancer had significantly better 2-year overall survival of 87.9% vs. 65.8% (p=<0.001) and 2 year progression-free survival of 71.8% vs. 50.4% (p=<0.001) compared to HPV negative patients. The survival outcomes for HPV 16 positive and p16 positive patients were similar. The pattern of failure results revealed significantly lower rates of locoregional failure, and second primary in HPV positive patients compared to HPV negative patients and no difference in the distant metastases between the two groups. When survival was assessed after adjusting for tobacco exposure the HPV positive <20 pack year smokers had a 2 year overall survival of 95% compared to 80% in HPV positive > 20 pack year smokers and 63% in HPV negative ≥20 pack year smokers. This data clearly indicated tobacco exposure altered the biology in HPV positive tumors and is an important prognostic factor as well.
A second retrospective analysis of a large Phase III trial of chemoradiation in over 800 patients enrolled from international sites recently reported the association between HPV positive, p16 positive oropharynx tumors and survival outcomes.72 This was a retrospective sub-study analysis of 195 available tumor samples in patients with an oropharynx primary of which 28% were HPV-positive and 58% were p16 positive. It was noted the HPV-positive tumors had an 2-year overall survival of 94% and 2 year failure-free survival of 86% compared to 77% and 75% respectively, in HPV-negative patients. When the coexpression of HPV and p16 was correlated with survival outcomes it was noted the HPV positive/p16 positive patient had a 2 year overall survival of 95 % compared to 88%.in HPV negative/p16 positive and 71% (p=0.003)in HPV negative/p16 negative patients. Similar results were noted with 2 year failure free survival (89%, 86%, vs 69% respectively p=0.002) and time to locoregional failure (93%, 95%, vs 84% respectively, p=0.051). A multivariable analysis identified the HPV16 and p16 as independent prognostic factors. After a median follow-up of 27 months it was noted that the locoregional failure rates were significantly lower in HPV positive or p16 positive patients, and no difference in distant failure compared to HPV negative /p16 negative patients. This study concluded that the HPV 16 positive and p16 positive patients had a better prognosis compared to HPV negative, p16 negative patients.
Investigators from the University of Michigan analyzed oropharynx tumor specimens from two sequential Phase 2 chemoradiation trials for presence of HPV 16.73 The first trial had a median follow-up of 76 months and the second study had a median follow-up of 36 months. In this study HPV DNA was detected by PCR analysis that could detect 15 high-risk subtypes. Approximately 81% (102/124) of patients were HPV-positive. HPV status and tobacco use were correlated with local, regional or distant failure, development of second primary tumors and survival outcomes. Among the HPV-positive patients 32% were never smokers, 45% were former smokers and 23% were current smokers. The investigators reported that HPV positive never smokers were a more favorable group with improved survival outcomes and time to recurrence compared to the HPV positive current or former smokers. Of the never smokers, 88% remained alive with no evidence of disease recurrence at median follow-up of 76 months from the first trial and 36 months from the second trial. This study highlights the need to further study the impact of tobacco exposure on the biology of the HPV positive patients.
Over 90% of head and neck cancers express EGFR and high expression of EGFR and EGFR gene copy number is associated with poor prognosis.74 Correlation between EGFR expression, p-16, bcl-xL, p53, HPV titer and response to treatment (induction chemotherapy, chemoradiation) in 50 patients with HPV-16 positive oropharynx tumors was explored by Kumar and colleagues.58 There was a high correlation between the combination of low EGFR and high p16 expression and a better clinical outcome in contrast to patients who had high EGFR expression and low HPV titer or high EGFR and low p16 expression after adjustment for age, sex, smoking status, TN stage and primary site. This study emphasizes the need to include EGFR status in addition to HPV status in future clinical trials of oropharynx cancers. This would be an additional prognostic factor that would help identify the high risk HPV positive patients.
Future direction of treatment of HPV associated HNSCC
Based on these prospective and retrospective data analyses from clinical trials, HPV-positive oropharyngeal cancer is now recognized as a distinct subset of HNSCC with a favorable outcome. Future clinical trials will at the very least need to stratify for HPV status. There is now an opportunity to explore less intense treatment strategies that do not compromise survival outcomes but lower the risk of potentially debilitating late effects. For the most part, patients with HPV positive oropharyngeal cancer are young and in excellent health. Thus, providing a high level of quality of life and a minimum of treatment complications are important considerations. The potential long term side effects of concurrent chemoradiation include dysphagia, xerostomia, feeding tube dependency from fibrosis and scarring of the pharyngeal muscles, chronic aspiration, and chronic fatigue.
Recently, the National Cancer Institute (NCI) Head and Neck Steering Committee and Task Forces, met in Washington, D.C. to consolidate the data available on the epidemiology, natural history, diagnosis of HPV-associated HNSCC, and reviewed all completed and ongoing clinical trials that have evaluated the HPV status.75 One of the major issues discussed in this review is the statistical and design issues and their impact in the development of future clinical trials based on HPV-status.
The ECOG and Radiation Therapy Oncology Group (RTOG) are currently planning Phase II clinical trials in patients with HPV positive tumors. The ECOG proposes induction chemotherapy with a triple drug regimen to reduce the tumor burden to subclinical disease (clinical complete response at primary site) followed by lower dose radiation, total dose 54 Gy and concurrent cetuximab. Overall survival and progression-free survival outcomes will be assessed and compared to the results of ECOG 2399. The main purpose of this study is to evaluate the potential for a lower dose of radiation to control disease and to explore toxicity and quality of life parameters.
Conclusion
In summary tumor HPV status is a prognostic factor for overall survival and progression free survival and may also be a predictive marker of response to treatment. The method of in situ hybridization provides a feasible tool for implementation in most diagnostic pathology laboratories; and immunohistochemical staining for p16 may be useful as a surrogate marker for HPV status. It appears that locoregional recurrence but not the rate of distant disease is lower in the HPV positive patients. Smoking and tobacco exposure seems to modify the survival and recurrence of HPV positive tumors and should be considered in future trials for risk stratification of HPV positive patients. HPV associated oropharynx cancer represents a distinct clinical and biologic entity with many unresolved issues that will be investigated in future translational, clinical research. We need to further explore and understand why the disease occurs predominantly in males, and whether the natural history of oral HPV infection differences in men and women. Optimal diagnostic tests are needed for HPV diagnosis and the utility of HPV DNA copy for outcome and early relapse needs to be explored. Opportunities for primary and secondary prevention need to be evaluated including the use of HPV vaccines for prevention of infection and of therapeutic vaccines in the adjuvant setting for prevention of locoregional recurrence and distant disease. Finally we face the challenge of designing clinical trials with appropriate risk stratification that will lead to the identification of the least morbid treatment that can cure patients with this disease. Longer follow-up of this recently recognized entity is essential to better understand the natural history and failure patterns.
Footnotes
Search strategy and selection criteria
Data for this Review were identified by searches of MEDLINE, Current Contents, PubMed, and references from relevant articles using the search terms “head and neck squamous cell cancer”, “oropharynx”, “HPV16” and “p16”. Abstracts and reports from meetings were included only when they related directly to previously published work. Only papers published in English between January 1980 and November 2009 were included.
Contributors
SM, GD, WHW and AAF equally contributed to the planning, writing, editing and figures.
Conflicts of Interest
The authors declared not conflicts of interest.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 3.WHO. IARC Monographs on the Evaluation of carcinogenic risks to humans. Lyon, France: International Agency for Research on Cancer; 2007. [PMC free article] [PubMed] [Google Scholar]
- 4.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 5.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 6.Applebaum KM, Furniss CS, Zeka A, Posner MR, Smith JF, Bryan J, et al. Lack of association of alcohol and tobacco with HPV16-associated head and neck cancer. J Natl Cancer Inst. 2007;99(23):1801–1810. doi: 10.1093/jnci/djm233. [DOI] [PubMed] [Google Scholar]
- 7.Pintos J, Black MJ, Sadeghi N, Ghadirian P, Zeitouni AG, Viscidi RP, et al. Human papillomavirus infection and oral cancer: a case-control study in Montreal, Canada. Oral Oncol. 2008;44(3):242–250. doi: 10.1016/j.oraloncology.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Smith EM, Ritchie JM, Summersgill KF, Hoffman HT, Wang DH, Haugen TH, et al. Human papillomavirus in oral exfoliated cells and risk of head and neck cancer. J Natl Cancer Inst. 2004;96(6):449–455. doi: 10.1093/jnci/djh074. [DOI] [PubMed] [Google Scholar]
- 9.Koch WM, Lango M, Sewell D, Zahurak M, Sidransky D. Head and neck cancer in nonsmokers: a distinct clinical and molecular entity. Laryngoscope. 1999;109(10):1544–1551. doi: 10.1097/00005537-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Tachezy R, Klozar J, Rubenstein L, Smith E, Salakova M, Smahelova J, et al. Demographic and risk factors in patients with head and neck tumors. J Med Virol. 2009;81(5):878–887. doi: 10.1002/jmv.21470. [DOI] [PubMed] [Google Scholar]
- 11.Termine N, Panzarella V, Falaschini S, Russo A, Matranga D, Lo Muzio L, et al. HPV in oral squamous cell carcinoma vs. head and neck squamous cell carcinoma biopsies: a meta-analysis (1988–2007) Ann Oncol. 2008;19(10):1681–1690. doi: 10.1093/annonc/mdn372. [DOI] [PubMed] [Google Scholar]
- 12.Romanitan M, Nasman A, Ramqvist T, Dahlstrand H, Polykretis L, Vogiatzis P, et al. Human papillomavirus frequency in oral and oropharyngeal cancer in Greece. Anticancer Res. 2008;28(4B):2077–2080. [PubMed] [Google Scholar]
- 13.Nasman A, Attner P, Hammarstedt L, Du J, Eriksson M, Giraud G, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: An epidemic of viral-induced carcinoma? Int J Cancer. 2009;125(2):362–366. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 14.Hansson BG, Rosenquist K, Antonsson A, Wennerberg J, Schildt EB, Bladstrom A, et al. Strong association between infection with human papillomavirus and oral and oropharyngeal squamous cell carcinoma: a population-based case-control study in southern Sweden. Acta Otolaryngol. 2005;125(12):1337–1344. doi: 10.1080/00016480510043945. [DOI] [PubMed] [Google Scholar]
- 15.Brandsma JL, Abramson AL. Association of papillomavirus with cancers of the head and neck. Arch Otolaryngol Head Neck Surg. 1989;115(5):621–625. doi: 10.1001/archotol.1989.01860290079018. [DOI] [PubMed] [Google Scholar]
- 16.Smith EM, Hoffman HT, Summersgill KS, Kirchner HL, Turek LP, Haugen TH. Human papillomavirus and risk of oral cancer. Laryngoscope. 1998;108(7):1098–1093. doi: 10.1097/00005537-199807000-00027. [DOI] [PubMed] [Google Scholar]
- 17.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 18.Ryerson AB, Peters ES, Coughlin SS, Chen VW, Gillison ML, Reichman ME, et al. Burden of potentially human papillomavirus-associated cancers of the oropharynx and oral cavity in the US, 1998–2003. Cancer. 2008;113(10 Suppl):2901–2909. doi: 10.1002/cncr.23745. [DOI] [PubMed] [Google Scholar]
- 19.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer. 2005;103(9):1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 20.Hammarstedt L, Dahlstrand H, Lindquist D, Onelov L, Ryott M, Luo J, et al. The incidence of tonsillar cancer in Sweden is increasing. Acta Otolaryngol. 2007;127(9):988–992. doi: 10.1080/00016480601110170. [DOI] [PubMed] [Google Scholar]
- 21.Braakhuis BJ, Visser O, Rene Leemans C. Oral and oropharyngeal cancer in The Netherlands between 1989 and 2006: Increasing incidence, but not in young adults. Oral Oncol. 2009;45(9):e85–e89. doi: 10.1016/j.oraloncology.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Conway DI, Stockton DL, Warnakulasuriya KA, Ogden G, Macpherson LM. Incidence of oral and oropharyngeal cancer in United Kingdom (1990–1999) – recent trends and regional variation. Oral Oncol. 2006;42(6):586–592. doi: 10.1016/j.oraloncology.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Robinson KL, Macfarlane GJ. Oropharyngeal cancer incidence and mortality in Scotland: are rates still increasing? Oral Oncol. 2003;39(1):31–36. doi: 10.1016/s1368-8375(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 24.Hammarstedt L, Lindquist D, Dahlstrand H, Romanitan M, Dahlgren LO, Joneberg J. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–2623. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 25.Dalianis T, Nasman A, Attner P, Hammarstedt L, Du J, Eriksson M, et al. International Papillomavirus Conference. Malom, Sweden: 2009. Human Papillomavirus in tonsillar cancer, an epidemic of viral carcinoma. [Google Scholar]
- 26.Summersgill KF, Smith EM, Levy BT, Allen JM, Haugen TH, Turek LP. Human papillomavirus in the oral cavities of children and adolescents. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91(1):62–69. doi: 10.1067/moe.2001.108797. [DOI] [PubMed] [Google Scholar]
- 27.Smith EM, Swarnavel S, Ritchie JM, Wang D, Haugen TH, Turek LP. Prevalence of human papillomavirus in the oral cavity/oropharynx in a large population of children and adolescents. Pediatr Infect Dis J. 2007;26(9):836–840. doi: 10.1097/INF.0b013e318124a4ae. [DOI] [PubMed] [Google Scholar]
- 28.D'Souza G, Agrawal Y, J H, Bodison D, Gillison ML. Oral sexual behaviors associated with prevalent oral human papillomavirus (HPV) infection. Journal of Infectious Disease. 2009;199:1–7. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz SM, Daling JR, Doody DR, Wipf GC, Carter JJ, Madeleine MM, et al. Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection. J Natl Cancer Inst. 1998;90(21):1626–1636. doi: 10.1093/jnci/90.21.1626. [DOI] [PubMed] [Google Scholar]
- 30.Kreimer AR, Alberg AJ, Daniel R, Gravitt PE, Viscidi R, Garrett ES, et al. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis. 2004;189(4):686–698. doi: 10.1086/381504. [DOI] [PubMed] [Google Scholar]
- 31.D'Souza G, Fakhry C, Sugar EA, Seaberg EC, Weber K, Minkoff HL, et al. Six-month natural history of oral versus cervical human papillomavirus infection. Int J Cancer. 2007;121(1):143–150. doi: 10.1002/ijc.22667. [DOI] [PubMed] [Google Scholar]
- 32.Smith EM, Ritchie JM, Summersgill KF, Klussmann JP, Lee JH, Wang D, et al. Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancers. Int J Cancer. 2004;108(5):766–772. doi: 10.1002/ijc.11633. [DOI] [PubMed] [Google Scholar]
- 33.Gillison ML, D'Souza G, Westra W, Sugar E, Xiao W, Begum S, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 34.Anaya-Saavedra G, Ramirez-Amador V, Irigoyen-Camacho ME, Garcia-Cuellar CM, Guido-Jimenez M, Mendez-Martinez R, et al. High association of human papillomavirus infection with oral cancer: a case-control study. Arch Med Res. 2008;39(2):189–197. doi: 10.1016/j.arcmed.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Klussmann J, Weissenborn S, Wieland U, et al. Prevalence, distribution, and viral load of human papillomavirus 16 DNA in tonsillar carcinomas. Cancer. 2001;92:2875–2884. doi: 10.1002/1097-0142(20011201)92:11<2875::aid-cncr10130>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 36.Pai SI, Westra WH. Molecular pathology of head and neck cancer: implications for diagnosis, prognosis, and treatment. Annu Rev Pathol. 2009;4:49–70. doi: 10.1146/annurev.pathol.4.110807.092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiest T, Schwarz E, Enders C, Flechtenmacher C, Bosch FX. Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene. 2002;21(10):1510–1517. doi: 10.1038/sj.onc.1205214. [DOI] [PubMed] [Google Scholar]
- 38.Begum S, Westra WH. Basaloid squamous cell carcinoma of the head and neck is a mixed variant that can be further resolved by HPV status. Am J Surg Pathol. 2008;32(7):1044–1050. doi: 10.1097/PAS.0b013e31816380ec. [DOI] [PubMed] [Google Scholar]
- 39.Hafkamp HC, Manni J, Haesevoets A, et al. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int J Cancer. 2008;122:2656–2664. doi: 10.1002/ijc.23458. [DOI] [PubMed] [Google Scholar]
- 40.Goldenberg D, Begum S, Westra WH. Cystic lymph node metastasis in patients with head and neck cancer: an HPV-associated phenomenon. Head Neck. 2008;30:898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 41.Zhao M, Rosenbaum E, Carvalho AL, Koch W, Jiang W, Sidransky D, Califano J. Feasibility of quantitative PCR-based saliva rinse screening of HPV for head and neck cancer. Int J Cancer. 2005;117(4):605–610. doi: 10.1002/ijc.21216. [DOI] [PubMed] [Google Scholar]
- 42.Begum S, Gillison ML, Nicol TL, Westra WH. Detection of human papillomavirus-16 in fine-needle aspirates to determine tumor origin in patients with metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13(4):1186–1191. doi: 10.1158/1078-0432.CCR-06-1690. [DOI] [PubMed] [Google Scholar]
- 43.Begum S, Gillison ML, Ansari-Lari MA, Shah K, Westra WH. Detection of human papillomavirus in cervical lymph nodes: a highly effective strategy for localizing site of tumor origin. Clin Cancer Res. 2003;9(17):6469–6475. [PubMed] [Google Scholar]
- 44.Worden FP, Kumar B, Lee JS, Wolf GT, Cordell KG, Taylor JM, et al. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: response and survival positively associated with HPV16 copy number. J Clin Oncol. 2008;26(19):3138–3146. doi: 10.1200/JCO.2007.12.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agrawal Y, Koch WM, Xiao W, Westra WH, Trivett AL, Symer DE, Gillison ML. Oral human papillomavirus infection before and after treatment for human papillomavirus 16-positive and human papillomavirus 16-negative head and neck squamous cell carcinoma. Clin Cancer Res. 2008;14(21):7143. doi: 10.1158/1078-0432.CCR-08-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chuang AY, Chuang TC, Chang S, Zhou S, Begum S, Westra WH, Ha PK, Koch WM, Califano JA. Presence of HPV DNA in convalescent salivary rinses is an adverse prognostic marker in head and neck squamous cell carcinoma. Oral Oncol. 2008;44(10):915–919. doi: 10.1016/j.oraloncology.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andl T, Kahn T, Pfuhl A, Nicola T, Erber R, Conradt C, et al. Etiological involvement of oncogenic human papillomavirus in tonsillar squamous cell carcinomas lacking retinoblastoma cell cycle control. Cancer Res. 1998;58(1):5–13. [PubMed] [Google Scholar]
- 48.Begum S, Cao D, Gillison M, Zahurak M, Westra WH. Tissue distribution of human papillomavirus 16 DNA integration in patients with tonsillar carcinoma. Clin Cancer Res. 2005;11(16):5694–5699. doi: 10.1158/1078-0432.CCR-05-0587. [DOI] [PubMed] [Google Scholar]
- 49.Kuo KT, Hsiao CH, Lin CH, Kuo LT, Huang SH, Lin MC. The biomarkers of human papillomavirus infection in tonsillar squamous cell carcinoma-molecular basis and predicting favorable outcome. Mod Pathol. 2008;21(4):376–386. doi: 10.1038/modpathol.3800979. [DOI] [PubMed] [Google Scholar]
- 50.Smeets SJ, Hesselink AT, Speel EJ, Haesevoets A, Snijders PJ, Pawlita M, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121(11):2465–2472. doi: 10.1002/ijc.22980. [DOI] [PubMed] [Google Scholar]
- 51.Mellin Dahlstrand H, Lindquist D, Björnestål L, Ohlsson A, Dalianis T, Munck-Wikland E, et al. P16(INK4a) correlates to human papillomavirus presence, response to radiotherapy and clinical outcome in tonsillar carcinoma. Anticancer Res. 2005 Nov-Dec;25(6C):4375–4383. [PubMed] [Google Scholar]
- 52.Zhang MQ, El-Mofty SK, Dávila RM. Detection of human papillomavirus-related squamous cell carcinoma cytologically and by in situ hybridization in fine-needle aspiration biopsies of cervical metastasis: a tool for identifying the site of an occult head and neck primary. Cancer. 2008;114(2):118–123. doi: 10.1002/cncr.23348. [DOI] [PubMed] [Google Scholar]
- 53.Smith EM, Rubenstein LM, Ritchie JM, Lee JH, Haugen TH, Hamsikova E, Turek LP. Does pretreatment seropositivity to human papillomavirus have prognostic significance for head and neck cancers? Cancer Epidemiol Biomarkers Prev. 2008;17(8):2087–2096. doi: 10.1158/1055-9965.EPI-08-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Capone RB, Pai SI, Koch WM, Gillison ML, Danish HN, Westra WH, et al. Detection and quantitation of human papillomavirus (HPV) DNA in the sera of patients with HPV-associated head and neck squamous cell carcinoma. Clin Cancer Res. 2000;6(11):4171–4175. [PubMed] [Google Scholar]
- 55.Smith EM, Ritchie JM, Pawlita M, Rubenstein LM, Haugen TH, Turek LP, et al. Human papillomavirus seropositivity and risks of head and neck cancer. Int J Cancer. 2007;120(4):825–832. doi: 10.1002/ijc.22330. [DOI] [PubMed] [Google Scholar]
- 56.Westra WH, Taube JM, Poeta ML, Begum S, Sidransky D, Koch WM. Inverse relationship between human papillomavirus-16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2008;14(2):366–369. doi: 10.1158/1078-0432.CCR-07-1402. [DOI] [PubMed] [Google Scholar]
- 57.Michaud WA, Nichols AC, Mroz EA, Faquin WC, Clark JR, Begum S, et al. Bcl-2 blocks cisplatin-induced apoptosis and predicts poor outcome following chemoradiation treatment in advanced oropharyngeal squamous cell carcinoma. Clin Cancer Res. 2009;15(5):1645–1654. doi: 10.1158/1078-0432.CCR-08-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26(19):3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calais G, Alfonsi M, Bardet E, Sire C, Germain T, Bergerot P, et al. Stage III and IV cancers of the oropharynx: results of a randomized study of Gortec comparing radiotherapy alone with concomitant chemotherapy. Bull Cancer. 2000;87(Spec No):48–53. [PubMed] [Google Scholar]
- 60.Denis F, Garaud P, Bardet E, Alfonsi M, Sire C, Germain T, et al. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol. 2004;22(1):69–76. doi: 10.1200/JCO.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 61.Denis F, Garaud P, Bardet E, Alfonsi M, Sire C, Germain T, et al. Late toxicity results of the GORTEC 94-01 randomized trial comparing radiotherapy with concomitant radiochemotherapy for advanced-stage oropharynx carcinoma:comparison of LENT/SOMA, RTOG/EORTC, and NCI-CTC scoring systems. Int J Radiat Oncol Biol Phys. 2003;55(1):93–98. doi: 10.1016/s0360-3016(02)03819-1. [DOI] [PubMed] [Google Scholar]
- 62.Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer. 2001;92:805–813. doi: 10.1002/1097-0142(20010815)92:4<805::aid-cncr1386>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 63.Butz K, Geisen C, Ullmann A, Spitkovsky D, Hoppe-Seyler F. Cellular responses of HPV-positive cancer cells to genotoxic anti-cancer agents: repression of E6/E7-oncogene expression and induction of apoptosis. Int J Cancer. 1996;68:506–513. doi: 10.1002/(SICI)1097-0215(19961115)68:4<506::AID-IJC17>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 64.Bristow RG, Benchimol S, Hill RP. The p53 gene as a modifier of intrinsic radiosensitivity: implications for radiotherapy. Radiother Oncol. 1996;40:197–223. doi: 10.1016/0167-8140(96)01806-3. [DOI] [PubMed] [Google Scholar]
- 65.Dahm-Daphi J. p53:Biology and role for cellular radiosensitivity. Strahlenther Onkol. 2000;176:278–285. doi: 10.1007/s000660050010. [DOI] [PubMed] [Google Scholar]
- 66.Lindquist D, Romanitan M, Hammarstedt L, Nasman A, Dahlstrand H, Lindholm J, et al. Human papillomavirus is a favourable prognostic factor in tonsillar cancer and its oncogenic role is supported by the expression of E6 and E7. Mol Oncol. 2007;1(3):350–355. doi: 10.1016/j.molonc.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 68.Liu Y, McKalip A, Herman B. Human papillomavirus type 16 E6 and HPV-16 E6/E7 sensitize human keratinocytes to apoptosis induced by chemotherapeutic agents: roles of p53 and caspase activation. J Cell Biochem. 2000;78:334–349. [PubMed] [Google Scholar]
- 69.Liu Y, Xing H, Han X, Shi X, Liang F, Cheng G, Lu Y, Ma D. Apoptosis of HeLa cells induced by cisplatin and its mechanism. J Huazhong Univ Sci Technolog Med Sci. 2008;28(2):197–199. doi: 10.1007/s11596-008-0221-7. [DOI] [PubMed] [Google Scholar]
- 70.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 71.Gillison ML, Harris J, Westra W, Chung C, Ang K, et al. Survival outcomes by tumor human papillomavirus (HPV) status in stage III-IV oropharyngeal cancer in RTOG 0129. ASCO. 2009 (Abstract 6003) [Google Scholar]
- 72.Rischin D, Young R, Fisher R, Fox S, Le Q, Peters L, et al. Prognostic significance of HPV an dp16 status in patients with oropharyngeal cancer treated on a large international phase III trial. ASCO. 2009 (Abstract 6003) [Google Scholar]
- 73.Worden FP, Hooton J, Lee J, Eisbruch A, Wolf GT, Prince M, et al. Association of tobacco (T) use with risk of distant metastases (DM), tumor r4ecurrence, and death in patients (pts) with HPV-positive (+) squamous cell cancer of the oropharynx (SCCOP) ASCO. 2009 (Abstract 6001) [Google Scholar]
- 7.Chung CH, Ely K, McGavran L, Varella-Garcia M, Parker J, Parker N, et al. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol. 2006;24(25):4170–4176. doi: 10.1200/JCO.2006.07.2587. [DOI] [PubMed] [Google Scholar]
- 75.Adelstein DJ, Ridge JH, Gillison ML, Chaturvedi AK, D’Souza G, Chaturvedi AK, et al. Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting; November 9–10, 2008; Washington, DC. [DOI] [PubMed] [Google Scholar]


