Abstract
New therapeutic targets for noncognitive reductions in energy intake, absorption, or storage are crucial given the worldwide epidemic of obesity. The gut microbial community (microbiota) is essential for processing dietary polysaccharides. We found that conventionalization of adult germ-free (GF) C57BL/6 mice with a normal microbiota harvested from the distal intestine (cecum) of conventionally raised animals produces a 60% increase in body fat content and insulin resistance within 14 days despite reduced food intake. Studies of GF and conventionalized mice revealed that the microbiota promotes absorption of monosaccharides from the gut lumen, with resulting induction of de novo hepatic lipogenesis. Fasting-induced adipocyte factor (Fiaf), a member of the angiopoietin-like family of proteins, is selectively suppressed in the intestinal epithelium of normal mice by conventionalization. Analysis of GF and conventionalized, normal and Fiaf knockout mice established that Fiaf is a circulating lipoprotein lipase inhibitor and that its suppression is essential for the microbiota-induced deposition of triglycerides in adipocytes. Studies of Rag1-/- animals indicate that these host responses do not require mature lymphocytes. Our findings suggest that the gut microbiota is an important environmental factor that affects energy harvest from the diet and energy storage in the host.
Keywords: symbiosis, nutrient processing, energy storage, adiposity, fasting-induced adipose factor
There are now >500 million adult humans in the world who are overweight [body mass index (BMI) of 25.0-29.9 kg/m2] and 250 million who are obese (BMI ≥ 30 kg/m2) (1). This growing epidemic threatens both industrialized and developing countries and has been accompanied by worldwide increases in obesity-related disorders, including type II diabetes, hypertension, cardiovascular pathology, and nonalcoholic fatty liver disease. In the United States, 64% of adults are overweight or obese (2), prompting the Surgeon General to designate this condition as the most important public health challenge of our time (3). Most people are unable to make willful, lifelong dietary changes needed for weight management (4). Therefore, developing foods or identifying new therapeutic targets that produce noncognitive reductions in total energy intake, absorption, or storage has considerable importance for public health.
The human gut contains an immense number of microorganisms, collectively known as the microbiota. This community consists of at least 1013 citizens, is dominated by anaerobic bacteria, and includes ≈500-1,000 species whose collective genomes are estimated to contain 100 times more genes than our own human genome (5, 6). The microbiota can be viewed as a metabolic “organ” exquisitely tuned to our physiology that performs functions that we have not had to evolve on our own. These functions include the ability to process otherwise indigestible components of our diet, such as plant polysaccharides. Defining host signaling pathways regulated by the microbiota provides an opportunity to identify new therapeutic targets for promoting health. In the current study, we use normal and genetically engineered gnotobiotic mice to address the hypothesis that the microbiota acts through an integrated host signaling pathway to regulate energy storage in the host.
Materials and Methods
Animals. C57BL/6J (B6) WT and Rag1-/- mice were purchased from The Jackson Laboratory. B6 peroxisome proliferator-activator receptor-α (Ppara)-/- mice were kindly provided by F. J. Gonzales (National Institutes of Health, Bethesda) (7). Fasting-induced adipocyte factor (Fiaf) +/- heterozygotes on a mixed B6:129/Sv background were generated as described below, and Fiaf+/+, Fiaf+/-, and Fiaf-/- littermates, obtained from crosses of Fiaf+/- heterozygotes were compared. Animals were genotyped by using PCR protocols outlined in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Conventionally raised (CONV-R) WT and knockout mice were rederived as germ-free (GF) as described (8). GF animals were maintained in gnotobiotic isolators (8), under a strict 12-h light cycle (lights on at 0600 hours), and fed an autoclaved chow diet (B & K Universal, East Yorkshire, U.K.) ad libitum. All manipulations of mice were performed by using protocols approved by the Washington University Animal Studies Committee.
Colonization of GF Mice. The cecal contents of each 8-week-old CONV-R mouse were resuspended in 10 ml of sterile PBS, and 2-ml aliquots were spread on the fur of 7- to 10-week-old GF recipients. The resulting conventionalized (CONV-D) mice were housed in gnotobiotic isolators for 10-28 d under the same conditions and fed the same diet as their GF counterparts.
CONV-R animals were maintained in microisolator cages in a specified pathogen-free state in a barrier facility on the autoclaved B & K diet. They were transferred to gnotobiotic isolators 2 weeks before they were killed at 8-10 weeks of age to mimic the housing conditions of GF and CONV-D mice.
Eight- to 10-week-old GF mice were orally gavaged with 109 Bacteroides thetaiotaomicron strain VPI-5482. Colonization density in the distal intestine, cecum, and colon ranged from 108 to 1011 colony-forming units/ml luminal contents, as defined by culturing samples of luminal contents on BHI blood agar for 2-3 d at 37°C under anaerobic conditions.
Measurement of Total Body Fat Content and Metabolic Rate (Oxygen Consumption). Total body fat content was determined 5 min after mice were anesthesized with an i.p. injection of ketamine (10 mg/kg body weight) and xylazine (10 mg/kg). The protocol used for dual-energy x-ray absorptiometry (Lunar PIXImus Mouse, GE Medical Systems, Waukesha, WI) has been described (9).
Oxygen consumption was determined in conscious, individually caged mice, in a fed state, by using open-circuit indirect calorimetry (single-chamber small-animal Oxymax system, Columbus Instruments, Columbus, OH). Animals were allowed to adapt to the metabolic chamber for 20 min before VO2 was measured every 30 s for 1 h.
SYBR-Green-Based Real-Time Quantitative RT-PCR (qRT-PCR). RNA was isolated as described in Supporting Materials and Methods and reverse-transcribed by using SuperScript II and dT15 primers (Invitrogen). qRT-PCR assays were performed as described (10), except that each 25-μl reaction contained cDNA corresponding to 1 ng of total RNA and 900 nM gene-specific primers (Table 1, which is published as supporting information on the PNAS web site). All assays were performed in triplicate with an ABI Prism 7700 Sequence Detector (Applied Biosystems). Data were normalized to L32 RNA (ΔΔCT analysis).
Assays of Lipoprotein Lipase (LPL). LPL activity in epididymal fat pads was determined according to ref. 11. For details see Supporting Materials and Methods.
Statistics. Statistically significant differences were determined by using Student's t tests. Comparisons between more than two groups of mice were made by a one-way ANOVA followed by Tukey's post hoc multiple comparison test.
Results and Discussion
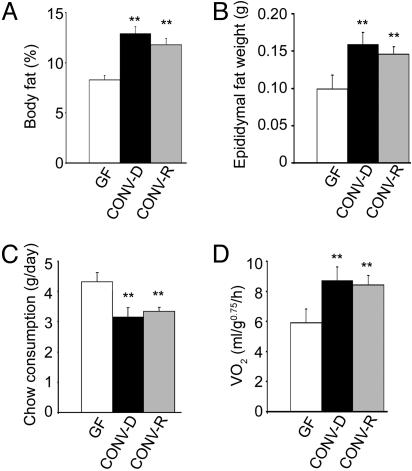
Introduction of a Gut Microbiota into Adult GF Mice Produces a Rapid Increase in Body Fat Content Despite Reduced Chow Consumption. Comparisons of 8- to 10-week-old male B6 mice raised in the absence of any microorganisms (GF) with mice that harbored a microbiota beginning at birth (CONV-R) revealed that CONV-R animals contain 42% more total body fat, as defined by dual energy x-ray absorptiometry (Fig. 1A). Epididymal fat pad weights were also significantly greater (47%; Fig. 1B). The higher levels of body fat observed in CONV-R animals is intriguing given that their daily consumption of a standard rodent chow diet (57% carbohydrates, 5% fat) was 29% less than their GF counterparts (Fig. 1C).
Fig. 1.
Phenotyping WT gnotobiotic mice. Three groups of 8- to 10-week-old adult male B6 mice [those raised in a GF state, those allowed to acquire a microbiota from birth to adulthood (CONV-R), and those raised GF until adulthood and then colonized for 2 weeks with an unfractionated cecal microbiota harvested from CONV-R donors (CONV-D)] were analyzed for total body fat content by dual energy x-ray absorptiometry (n = 21-25 per group) (A), epididymal fat weight (n = 10-20 per group) (B), chow consumption (average daily value over the 3 d before termination of the experiment; n = 10 per group) (C), and oxygen consumption (defined by open circuit calorimetry just before the animals were killed; n = 10 per group) (D). Mean values ± SEM are plotted. **, P < 0.01 compared with GF.
A 14-d colonization of 8- to 10-week-old male GF B6 recipients with an unfractionated microbiota harvested from the distal intestines (cecums) of adult CONV-R donors, a process known as conventionalization, produced a dramatic 57% increase in their total body fat content (Fig. 1 A) and a 61% increase in epididymal fat weight (Fig. 1B). The increase in body fat was associated with a 7% decrease in lean body mass, resulting in no significant differences in total body weight between the two groups [23.5 ± 2.6 g (GF) versus 23.4 ± 2.6 g (CONV-D); n = 21; P > 0.05]. Fasting serum triglyceride values were similar (P > 0.05) in both GF and CONV-D mice (data not shown).
A similar increase in total body fat content was observed after a shorter, 10-d conventionalization (66%; P > 0.05 compared to 14 d). A more prolonged conventionalization (28 d) did not produce further increments in total body fat content or epididymal fat pad weight (data not shown). The increased fat storage produced by a 14-d conventionalization also occurred in the face of decreased chow consumption (27% lower than GF; Fig. 1C).
These effects were not unique to males: CONV-D B6 females exhibited increases in total body fat (85%) and reductions in lean body mass (9%) that were not significantly different from age-matched males (P > 0.05). In addition, the fat storage phenotype was not limited to the B6 inbred strain: a 14-d conventionalization of 8-week-old male mice belonging to the NMRI inbred strain produced a 90% increase in total body fat content (P < 0.01 compared with GF) and a 31% decrease in chow consumption (P < 0.05).
Sequence-based 16S rDNA enumeration studies of the cecal microbiota revealed great similarities in the fractional representation of the predominant species in CONV-R donors and CONV-D B6 recipients (Fig. 6 and Table 2, which are published as supporting information on the PNAS web site). As in many humans, Bacteroides and Clostridium were the most prevalent genera. B. thetaiotaomicron is a prominent member of the human distal gut microbiota with an extraordinary capacity for acquiring and degrading plant polysaccharides (12). For example, its proteome contains 172 glycosylhydrolases that are predicted to cleave most glycosidic linkages encountered in human diets. Studies in GF mice colonized with B. thetaiotaomicron have shown that its polysaccharide processing activity is associated with induction of host monosaccharide transporters (13). Therefore, we colonized 8-week-old male GF B6 mice for 2 weeks with the sequenced strain (VPI-5482) to determine whether a single saccharolytic bacterial species could, by itself, effect host fat storage. Colonization produced a statistically significant increase in total body fat content, although the magnitude of the increase was less than that obtained with an unfractionated cecal microbiota (23% versus 57%, respectively; n = 10 mice per group; P < 0.01).
Metabolic Rate Is Higher in CONV-D Mice Than in Their GF Counterparts. Because the microbiota-mediated increase in body fat content was not caused by increased chow consumption, open-circuit indirect calorimetry was performed to determine whether it reflected decreased energy expenditure. This explanation was excluded when we found that the leaner GF mice had a metabolic rate (VO2) that was 27% lower than age- and gender-matched (male) B6 mice conventionalized for 14 d (P < 0.01; Fig. 1D). CONV-D mice had VO2 values that were not significantly different from age- and gender-matched CONV-R animals (Fig. 1D).
The increase in VO2 observed with conventionalization could reflect increased metabolic rate in the host and/or the metabolic activity of their recently acquired microbial community. There are no available methods for measuring the metabolic activity of the microbiota in vivo. However, microanalytic biochemical assays of freeze-clamped gastrocnemius muscle and liver revealed significant increases in the steady-state levels of tricarboxylic acid cycle intermediates in CONV-D versus GF animals. Despite this evidence of increased cycle activity, there were no significant alterations in tissue high-energy phosphate stores (n = 5 animals per group; Table 3, which is published as supporting information on the PNAS web site). Increasing oxygen consumption without increasing high-energy phosphate stores implies the presence of futile cycles, a biochemical correlate of inefficient metabolism in the host (see below).
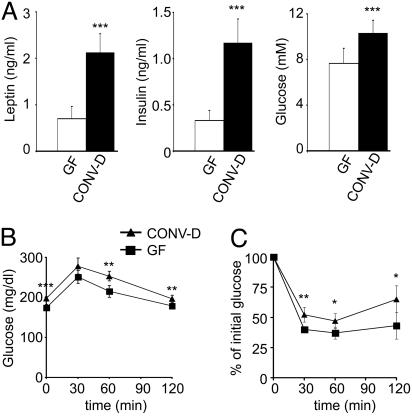
Leptin is an adipocyte-derived hormone whose expression correlates with adipocyte lipid content (14). Moreover, leptin is known to reduce food intake and increase energy expenditure in mice (15). Leptin levels increase upon colonization (Fig. 2A). The increase is proportional to the increase in body fat (r2 = 0.977).
Fig. 2.
A 14-d conventionalization of WT GF B6 mice increases circulating leptin levels and decreases sensitivity to insulin. (A) Sera were obtained after a 4-h fast and analyzed for leptin, insulin, and glucose (n = 8 animals per group). Numbers represent mean values ± SEM. Glucose tolerance (B) and insulin tolerance (C) tests were performed after a 4-h fast (n = 8 mice per group). Mean values ± SEM are plotted. ***, P < 0.001; **, P < 0.01; and *, P < 0.05 compared with GF.
The increase in fat content was accompanied by statistically significant elevations in fasting glucose and insulin levels (Fig. 2 A) and an insulin-resistant state, as defined by glucose and insulin tolerance tests (Fig. 2 B and C).
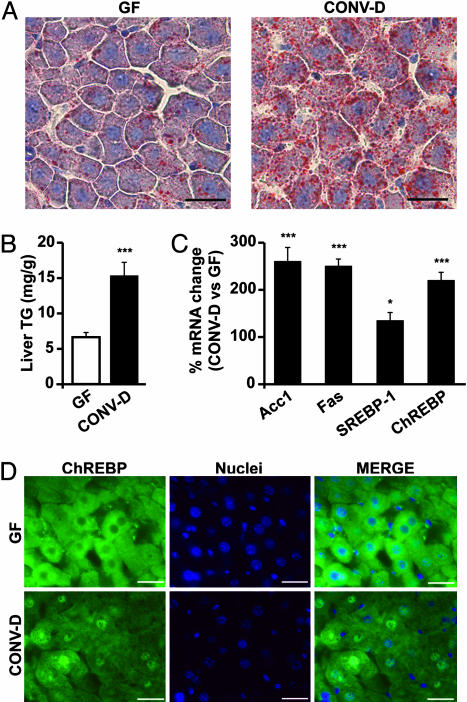
The Microbiota Directs the Host to Increase Hepatic Production of Triglycerides. Glucose and insulin are known to induce expression of lipogenic enzymes in the liver (16). A 14-d conventionalization of GF mice produced a 2.3-fold increase in liver triglyceride content (Fig. 3 A and B), but no appreciable changes in total liver free fatty acids or cholesterol (P > 0.05; data not shown). qRT-PCR assays confirmed that conventionalization was accompanied by statistically significant elevations in liver mRNAs encoding two key enzymes in the de novo fatty acid biosynthetic pathway, acetyl-CoA carboxylase (Acc1), and fatty acid synthase (Fas) (Fig. 3C).
Fig. 3.
Conventionalization induces hepatic lipogenesis and nuclear import of the basic helix-loop-helix transcription factor ChREBP. (A) Oil-red O stains of paraformaldehyde-fixed liver sections prepared from 8-week-old WT male GF and CONV-D B6 mice. (B) Liver triglyceride (TG) levels (n = 5 per group; mean values ± SEM; ***, P < 0.001 compared to GF). (C) qRT-PCR assays of liver RNAs from GF and CONV-D mice [n = 15 per group; mean values ± SEM are expressed relative to levels in GF animals (GF set at 100%); *, P < 0.05; ***, P < 0.001 compared with GF]. (D) Immunohistochemical study of paraformaldehyde-fixed sections of livers from GF or CONV-D mice. Sections were stained with rabbit polyclonal antibodies to mouse ChREBP (green). Nuclei are labeled dark blue with 4′,6-diamidino-2-phenylindole. (Bars: 25 μm.)
Sterol response element binding protein 1 (SREBP-1) and carbohydrate response element binding protein (ChREBP), two basic helix-loop-helix/leucine zipper transcription factors, mediate hepatocyte lipogenic responses to insulin and glucose and appear to act synergistically (17). Both Acc1 and Fas are known targets of ChREBP and SREBP-1 (16). qRT-PCR assays of liver RNAs revealed that conventionalization increases liver ChREBP mRNA, and to a lesser extent, SREBP-1 mRNA levels (Fig. 3C).
ChREBP is translocated from the cytoplasm to the nucleus after it is dephosphorylated by the serine/threonine phosphatase PP2A (18, 19). PP2A, in turn, is activated by xylulose-5-phosphate (Xu5P) (20), an intermediate in the hexose monophosphate shunt. Mice colonized with a microbiota had elevated levels of liver Xu5P compared with their GF counterparts (1.6 ± 0.4 versus 2.6 ± 0.3 μmol/g wet weight of liver; P < 0.01; see Scheme 1, which is published as supporting information on the PNAS web site for assay) and more nuclear-localized ChREBP (Fig. 3D).
We obtained direct biochemical evidence that the presence of the microbiota promotes increased monosaccharide uptake from the gut. GF mice and their CONV-D counterparts (n = 4 per group) were given a single gavage of 100 μl of a mixture of 5 mM glucose and 0.2 mM 2-deoxyglucose and killed 15 min later, and 2-deoxyglucose 6-phosphate levels were measured in their distal intestines. Levels were 2-fold higher in CONV-D mice (1.15 ± 0.013 versus 0.55 ± 0.04 pmol/μg protein; P < 0.001). Once taken up into the intestine, transfer of monosaccharides to the portal circulation is facilitated through an additional effect of the microbiota. We have shown previously that conventionalization results in a doubling of the density of capillaries that underlie the small intestinal villus epithelium to levels equivalent to that of age-matched CONV-R animals (21).
Together, these findings are consistent with an increase in processing of dietary polysaccharides by microbial glycosylhydrolases in CONV-D mice, increased delivery of monosaccharides to their livers, and increased transactivation of lipogenic enzymes by ChREBP and perhaps SREBP-1. The liver has at least two ways of responding to this augmented delivery of calories: increasing inefficient metabolism (futile cycles) and exporting these calories in the form of fat for deposition in peripheral tissues.
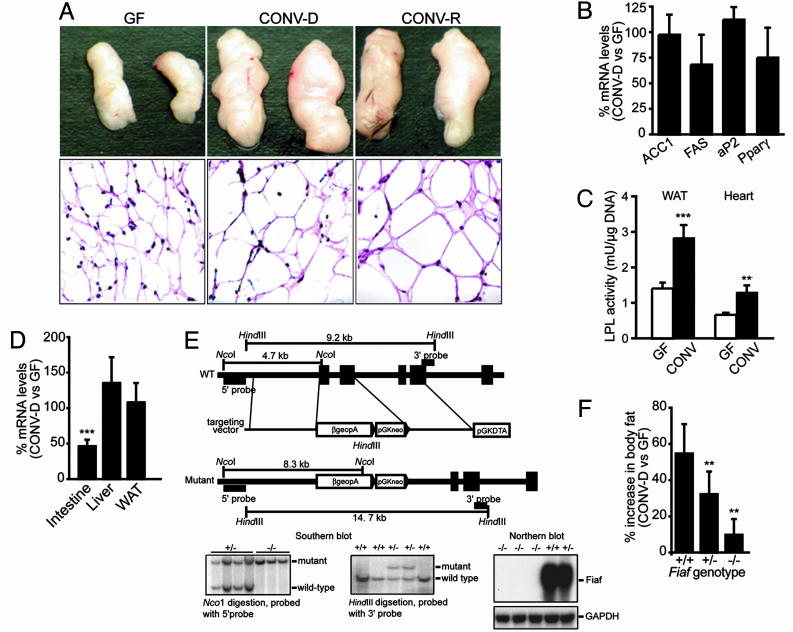
The Microbiota Promotes Storage of Triglycerides in Adipocytes Through Suppression of Intestinal Expression of a Circulating LPL Inhibitor. The DNA content of epididymal fat pads recovered from GF and CONV-D mice were not significantly different. This observation, together with histochemical studies, allowed us to conclude that the microbiota-induced increase in epididymal fat pad weight reflected adipocyte hypertrophy (Fig. 4A). Moreover, qRT-PCR analyses of fat pad RNA revealed that neither biomarkers of adipogenesis (aP2 and PPAR-γ) or lipogenesis (Acc1 and Fas) were significantly changed after conventionalization (Fig. 4B).
Fig. 4.
Conventionalization promotes adipocyte hypertrophy by suppressing Fiaf expression in the intestine. (A Upper) Epididymal fat pads from 8-week-old WT male GF, CONV-D, and CONV-R B6 mice. (A Lower) The corresponding hematoxylin- and eosin-stained sections are shown. (B) qRT-PCR assays of epididymal fat pad RNAs harvested from WT mice reveal that conventionalization does not produce significant changes in expression of mediators or biomarkers of lipogenesis and adipogenesis (mean values ± SEM are plotted; P > 0.05; n = 15 per group). (C) LPL activity is increased upon conventionalization in both epididymal fat pads and heart of WT mice (**, P < 0.01 and ***, P < 0.001 compared to GF; n = 5 per group). (D) qRT-PCR assays of Fiaf expression in WT animals [***, P < 0.001 compared to GF (set at 100%); n = 13]. (E) Generation of Fiaf knockout mice. Structures are shown for the WT Fiaf locus, the targeting vector, and the mutated locus with exons 1-3 replaced by a βgeopA cassette. The desired disruption was verified by Southern blot analysis. Northern blots of adipocyte RNA establish the absence of detectable Fiaf mRNA in Fiaf-/- animals. (F) The absence of Fiaf markedly attenuates the increase in total body fat content after a 14-d conventionalization (**, P < 0.01 compared with WT; n = 7-8 per group).
LPL is a key regulator of fatty acid release from triglyceride-rich lipoproteins in muscle, heart, and fat (22). Increased adipocyte LPL activity leads to increased cellular uptake of fatty acids and adipocyte triglyceride accumulation. In white fat, LPL is regulated posttranscriptionally by nutritional status: fasting reduces and refeeding increases enzyme activity (23). Intriguingly, we found that a 14-d conventionalization increased LPL activity 122% in epididymal fat pads (Fig. 4C). Moreover, the effect was not confined to fat: enzymatic assays of heart revealed a 99% increase with conventionalization (Fig. 4C). Increased insulin levels produce reductions in muscle LPL activity (24). Therefore, our findings indicated that the microbiota induces the observed general increase in LPL through another mechanism.
Fiaf, also known as angiopoietin-like protein 4, is produced by brown and white fat, liver, and intestine (13, 25, 26). This secreted protein is an inhibitor of LPL in vitro [IC50 = 200 nM (27)]. qRT-PCR analysis of intestinal Fiaf expression during the postnatal period disclosed that the gene is induced in GF mice during the suckling-weaning transition. Induction does not occur in CONV-R animals, resulting in significantly lower levels of Fiaf mRNA in adult CONV-R versus GF intestine (Fig. 7, which is published as supporting information on the PNAS web site). During the suckling-weaning transition, the diet switches from lipid/lactose-rich mother's milk to low-fat/polysaccharide-rich chow, with coincident expansion of the microbiota and a shift from facultative to obligate anaerobes (e.g., Bacteroides). These observations suggested that Fiaf could provide a signal that links conventionalization with a change in host energy partitioning.
qRT-PCR assays disclosed that conventionalization of adult GF mice suppressed Fiaf expression in their small intestines (ileum), but not in their livers or white fat (Fig. 4D). Follow-up qRT-PCR studies of laser capture microdissected intestinal crypt and villus epithelium, and the villus mesenchyme, established that microbial suppression of Fiaf occurs in differentiated villus epithelial cells (data not shown).
Together these findings suggest that the microbiota acts to stimulate hepatic triglyceride production through effects mediated by transcription factors such as ChREBP and to promote LPL-directed incorporation of these triglycerides into adipocytes through transcriptional suppression of an intestinal epithelial gene encoding a circulating LPL inhibitor. We tested this hypothesis by generating mice with a null Fiaf allele (Fig. 4E) and rederiving them as GF.
Eight-week-old male GF Fiaf-/- mice have 67% higher epididymal fat pad LPL activity than GF littermates containing the WT Fiaf allele (P < 0.01), confirming that Fiaf is an important inhibitor of this lipase in vivo. Conventionalization of GF knockout mice did not produce significant changes in LPL activity in fat pads (or heart) (P > 0.05; n = 10 animals).
GF Fiaf-/- animals have the same amount of total body fat as their age- and gender-matched CONV-D (Fiaf-suppressed) WT littermates (12.8 ± 1.1% of body weight versus 14.2 ± 1.9, P > 0.05). Moreover, a 14-d conventionalization of already Fiaf-deficient GF knockout animals produced only minor increases in total body fat (10 ± 8% increase versus 55 ± 16% increase in WT littermates; Fig. 4F). Fiaf+/- heterozygotes had an intermediate increase (33 ± 12%). These results establish the importance of Fiaf as a prominent mediator of microbial regulation of peripheral fat storage.
The Effect of the Microbiota on Fiaf Expression and Fat Storage Does Not Depend on Mature Lymphocytes or PPAR-α. We found that the zebrafish homolog of mouse Fiaf is suppressed by the microbiota when GF fish are conventionalized, indicating that this response has been highly conserved over the course of vertebrate evolution (28). We applied two methods to identify conserved regulatory elements in the 10 kb of DNA sequence 5′ to the transcriptional start site of human, mouse, rat, zebrafish, and fugu Fiaf orthologs. First, we searched for novel motifs by using phylocon (29). Two statistically significant motifs were identified: one overlaps with the PPAR binding site; the other is similar to the Heb binding site, which contains an E-box (Fig. 8A, which is published as supporting information on the PNAS web site). Second, we searched the transfac database (30) of 466 vertebrate-specific transcription factor scoring matrices with patser (G. Hertz and G. Stormo, personal communication, http://ural.wustl.edu) for high-scoring binding sites that appear in all five Fiaf orthologs and in conserved sequence blocks between the human and mouse genes. More than 40 matrices satisfied these two selection criteria (Table 4, which is published as supporting information on the PNAS web site), including sites recognized by several fork head domain-containing factors (e.g., HNF3, HNF4α, and FKH8) and an IFN-stimulated response element (Fig. 8).
Fiaf was identified during a screen for PPAR-α targets in liver (25). PPAR-α is an important regulator of energy metabolism in a variety of tissues including intestine, liver, heart, and kidney (31). We found that PPAR-α mRNA levels decrease modestly (1.7 ± 0.2-fold) in the small intestines of CONV-D compared with GF animals, but remain unchanged in their livers and fat pads (P < 0.05; see Fig. 9, which is published as supporting information on the PNAS web site). To directly test the role of PPAR-α in regulating the microbiota-directed change in body fat content and suppression of Fiaf, B6 Ppara knockout mice were rederived as GF. Eight- to 10-week-old male GF Ppara-/- mice had the same amount of total body fat as their age- and gender-matched GF WT littermates (Fig. 9). Moreover, Ppara-/- animals had no impairment in their microbiota-induced increase in body fat content (Fig. 9). Finally, qRT-PCR assays of intestinal RNAs isolated from GF and CONV-D WT and Ppara-/- mice indicated that the absence of PPAR-α did not prevent transcriptional suppression of Fiaf upon conventionalization (Fig. 9). We concluded that the host fat storage response to the microbiota does not require PPAR-α. A comparable analysis of the role of PPAR-γ could not be performed because Pparg-/- mice die at embryonic day 10 (29).
Finding a conserved IFN-stimulated response element in the orthologous Fiaf genes was intriguing in light of our previous genechip analyses of intestinal RNAs that revealed that conventionalization of B6 GF mice regulates expression of a number of genes involved in B and T cell responses (28). Therefore, we rederived B6 Rag1-/--deficient mice as GF to determine whether the presence or absence of mature T and B cells had an effect on the capacity of the microbiota to increase body fat content or modulate Fiaf. Rag1+/+ and Rag1-/- littermates had equivalent increases in body fat content after a 14-d conventionalization (59 ± 16% versus 67 ± 16%; P > 0.05) and similar degrees of Fiaf suppression (2.8 ± 0.3- and 3.8 ± 0.3-fold, respectively; P < 0.05 compared with GF). Thus, it appears that these cellular components of the adaptive immune system are not required to process signals or metabolic products emanating from the gut microbiota that promote fat storage.
Prospectus: The Microbiota as an Environmental Factor That Affects Predisposition Toward Adiposity. Adult humans are composed of an estimated 10 times more resident microbial than human cells (5). Our microbial partners have coevolved with us to forge mutually beneficial (symbiotic) relationships. These relationships are typically founded on nutrient sharing. The studies described in this paper indicate that one manifestation of this symbiotic relationship is microbial processing of components of the diet and deposition the extracted energy in host fat depots. The ability to store energy would be a beneficial attribute for ancient humans who had variable access to food. However, in modern, developed societies, where there is ready access to large-portion, high-calorie diets, this “benefit” becomes a detriment.
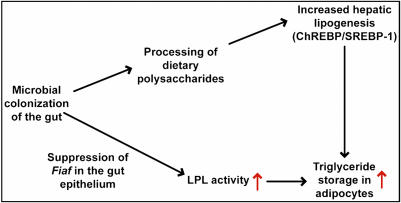
Our finding that microbial suppression of intestinal Fiaf promotes adiposity, through the mechanism summarized in Fig. 5, suggests that increasing Fiaf expression and/or activity may promote leanness. We also speculate that changes in microbial ecology prompted by Western diets, and/or differences in microbial ecology between individuals living in these societies, may function as an “environmental” factor that affects predisposition toward energy storage and obesity.
Fig. 5.
Schematic view of how the gut microbiota effects host fat storage. The microbiota acts through Fiaf to coordinate increased hepatic lipogenesis with increased LPL activity in adipocytes, thereby promoting storage of calories harvested from the diet into fat. See text for further details.
Supplementary Material
Acknowledgments
We thank David O'Donnell, Maria Karlsson, Jill Manchester, Sabrina Wagoner, Trey Coleman, and Xiaoli Wu for technical assistance and John Rawls, Peter Kang, Peter Crawford, and Justin Sonnenburg for helpful advice. This work was supported in part by National Institutes of Health Grants DK30292, DK56341, and HL58427 and the Canadian Institute of Health Research. F.B. is the recipient of a postdoctoral fellowship from the Wenner-Gren Foundation.
Author contributions: F.B., C.F.S., and J.I.G. designed research; F.B., H.D., and L.V.H. performed research; F.B., H.D., G.Y.K., and A.N. contributed new reagents/analytic tools; F.B., T.W., A.N., C.F.S., and J.I.G. analyzed data; F.B. and J.I.G. wrote the paper.
Abbreviations: GF, germ-free; Fiaf, fasting-induced adipocyte factor; B6, C57BL/6J; PPAR, peroxisome proliferator-activator receptor; CONV-R, conventionally raised; CONV-D, conventionalized; qRT-PCR, quantitative RT-PCR; LPL, lipoprotein lipase; Acc1, acetyl-CoA carboxylase; Fas, fatty acid synthase; SREBP-1, sterol response element binding protein 1; ChREBP, carbohydrate response element binding protein.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AY667702-AY668946).
References
- 1.Bouchard, C. (2000) N. Engl. J. Med. 343, 1888-1889. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad, A. H., Marks, J. S., Stroup, D. F. & Gerberding, J. L. (2004) J. Am. Med. Assoc. 291, 1238-1245. [DOI] [PubMed] [Google Scholar]
- 3.Department of Health and Human Services (2001) The Surgeon General's Call to Action to Prevent and Decrease Overweight and Obesity (Public Health Service, Washington, DC).
- 4.Friedman, J. M. (2004) Nat. Med. 10, 563-569. [DOI] [PubMed] [Google Scholar]
- 5.Savage, D. C. (1977) Annu. Rev. Microbiol. 31, 107-133. [DOI] [PubMed] [Google Scholar]
- 6.Xu, J. & Gordon, J. I. (2003) Proc. Natl. Acad. Sci. USA 100, 10452-10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee, S. S., Pineau, T., Drago, J., Lee, E. J., Owens, J. W., Kroetz, D. L., Fernandez-Salguero, P. M., Westphal, H. & Gonzalez, F. J. (1995) Mol. Cell. Biol. 15, 3012-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooper, L. V., Mills, J. C., Roth, K. A., Stappenbeck, T. S., Wong, M. H. & Gordon, J. I. (2002) in Molecular Cellular Microbiology, eds. Sansonetti, P. & Zychlinsky, A. (Academic, San Diego), Vol. 31, pp. 559-589. [Google Scholar]
- 9.Bernal-Mizrachi, C., Weng, S., Li, B., Nolte, L. A., Feng, C., Coleman, T., Holloszy, J. O. & Semenkovich, C. F. (2002) Arterioscler. Thromb. Vasc. Biol. 22, 961-968. [DOI] [PubMed] [Google Scholar]
- 10.Stappenbeck, T. S., Hooper, L. V., Manchester, J. K., Wong, M. H. & Gordon, J. I. (2002) Methods Enzymol. 356, 167-196. [DOI] [PubMed] [Google Scholar]
- 11.Iverius, P. H. & Ostlund-Lindqvist, A. M. (1986) Methods Enzymol. 129, 691-704. [DOI] [PubMed] [Google Scholar]
- 12.Xu, J., Bjursell, M. K., Himrod, J., Deng, S., Carmichael, L. K., Chiang, H. C., Hooper, L. V. & Gordon, J. I. (2003) Science 299, 2074-2076. [DOI] [PubMed] [Google Scholar]
- 13.Hooper, L. V., Wong, M. H., Thelin, A., Hansson, L., Falk, P. G. & Gordon, J. I. (2001) Science 291, 881-884. [DOI] [PubMed] [Google Scholar]
- 14.Maffei, M., Fei, H., Lee, G. H., Dani, C., Leroy, P., Zhang, Y., Proenca, R., Negrel, R., Ailhaud, G. & Friedman, J. M. (1995) Proc. Natl. Acad. Sci. USA 92, 6957-6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelleymounter, M. A., Cullen, M. J., Baker, M. B., Hecht, R., Winters, D., Boone, T. & Collins, F. (1995) Science 269, 540-543. [DOI] [PubMed] [Google Scholar]
- 16.Towle, H. C. (2001) Proc. Natl. Acad. Sci. USA 98, 13476-13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dentin, R., Pegorier, J. P., Benhamed, F., Foufelle, F., Ferre, P., Fauveau, V., Magnuson, M. A., Girard, J., Postic, C., Kabashima, T., et al. (2004) J. Biol. Chem. 279, 20314-20326. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita, H., Takenoshita, M., Sakurai, M., Bruick, R. K., Henzel, W. J., Shillinglaw, W., Arnot, D. & Uyeda, K. (2001) Proc. Natl. Acad. Sci. USA 98, 9116-9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawaguchi, T., Takenoshita, M., Kabashima, T., Uyeda, K., Yamashita, H., Sakurai, M., Bruick, R. K., Henzel, W. J., Shillinglaw, W. & Arnot, D. (2001) Proc. Natl. Acad. Sci. USA 98, 13710-13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabashima, T., Kawaguchi, T., Wadzinski, B. E., Uyeda, K., Takenoshita, M., Yamashita, H., Sakurai, M., Bruick, R. K., Henzel, W. J., Shillinglaw, W., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 5107-5112.12684532 [Google Scholar]
- 21.Stappenbeck, T. S., Hooper, L. V. & Gordon, J. I. (2002) Proc. Natl. Acad. Sci. USA 99, 15451-15455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preiss-Landl, K., Zimmermann, R., Hammerle, G. & Zechner, R. (2002) Curr. Opin. Lipidol. 13, 471-481. [DOI] [PubMed] [Google Scholar]
- 23.Bergo, M., Olivecrona, G. & Olivecrona, T. (1996) Biochem. J. 313, 893-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lithell, H., Boberg, J., Hellsing, K., Lundqvist, G. & Vessby, B. (1978) Atherosclerosis 30, 89-94. [DOI] [PubMed] [Google Scholar]
- 25.Kersten, S., Mandard, S., Tan, N. S., Escher, P., Metzger, D., Chambon, P., Gonzalez, F. J., Desvergne, B. & Wahli, W. (2000) J. Biol. Chem. 275, 28488-28493. [DOI] [PubMed] [Google Scholar]
- 26.Yoon, J. C., Chickering, T. W., Rosen, E. D., Dussault, B., Qin, Y., Soukas, A., Friedman, J. M., Holmes, W. E. & Spiegelman, B. M. (2000) Mol. Cell. Biol. 20, 5343-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida, K., Shimizugawa, T., Ono, M. & Furukawa, H. (2002) J. Lipid Res. 43, 1770-1772. [DOI] [PubMed] [Google Scholar]
- 28.Rawls, J. F., Samuel, B. S. & Gordon, J. I. (2004) Proc. Natl. Acad. Sci. USA 101, 4596-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, T. & Stormo, G. D. (2003) Bioinformatics 19, 2369-2380. [DOI] [PubMed] [Google Scholar]
- 30.Matys, V., Fricke, E., Geffers, R., Gossling, E., Haubrock, M., Hehl, R., Hornischer, K., Karas, D., Kel, A. E., Kel-Margoulis, O. V., et al. (2003) Nucleic Acids Res. 31, 374-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braissant, O., Foufelle, F., Scotto, C., Dauca, M. & Wahli, W. (1996) Endocrinology 137, 354-366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.