Abstract
Background
Preclinical data suggest that oxytocin reduces hypersensitivity by actions in the spinal cord, but whether it produces antinociception to acute stimuli is unclear. Here we examined the safety of intrathecal oxytocin and screen its effects on acute noxious stimuli.
Methods
Following institutional review board and Food and Drug Administration approval, healthy adult volunteers received 5, 15, 50, or 150 µg intrathecal oxytocin in a dose escalating manner in cohorts of 5 subjects. Hemodynamic and neurologic assessments were performed for 4 hour following injections and 24 hr later, at which time serum sodium was also measured. Cerebrospinal fluid was obtained 60 min after injection, and responses to noxious heat stimuli in arm and leg as well as temporal summation to repeated application of a von Frey filament were obtained.
Results
One subject receiving the highest dose experienced transient hypotension and bradycardia as well as subjective numbness in a lumbo-sacral distribution. No other subject experienced subjective or objective neurologic symptoms. Overall, blood pressure and heart rate increased 1–4 hr after injection by < 15%, with no dose dependency. There was no effect on serum sodium and cerebrospinal fluid oxytocin increased in a dose-dependent manner after injection. Pain scores to noxious heat stimuli were unaffected by oxytocin, and the temporal summation protocol failed to show summation before or after drug treatment.
Conclusions
This small study supports further investigation of oxytocin for analgesia for hypersensitivity states, with continued systematic surveillance for possible effects on blood pressure, heart rate, and neurologic function.
TOC statement
Subarachnoid injections of oxytocin did not produce any major adverse events or complications. In a limited protocol for testing analgesia, no analgesic effects were apparent.
Introduction
Several lines of evidence suggest that oxytocin produces analgesia and that the spinal cord is a major site for this action. Unfortunately, oxytocin enters the central nervous system to a very limited extent after systemic administration, and systemic oxytocin does not produce analgesia to acute nociception in humans, although it mildly potentiates placebo analgesia.1 In contrast, direct administration of oxytocin into cerebrospinal fluid produced analgesia in a patient with cancer pain.2 In animals, activation of oxytocin fibers descending from the paraventricular nucleus to the spinal cord participates in stress induced analgesia3 and reduces hypersensitivity after injury.4 Additionally, we recently showed that pain and hypersensitivity following surgery recovers more quickly in animals and humans when the surgery occurs near the time of delivery, and that this is reversed by spinal injection of an oxytocin receptor-preferring antagonist.5,6 Oxytocin receptors are present in the superficial dorsal horn of human spinal cord7 where it engages inhibitory circuits.3,8,9 Thus, there is a clear rationale to test the efficacy of intrathecal oxytocin for analgesia in humans.
The purpose of this study is the first step in clinical introduction of intrathecal oxytocin for analgesia. Oxytocin has a long history of safe use by systemic administration, although it can cause hypotension,10 QT interval prolongation,11 and could result in hyponatremia12 among other known side effects. In a safety assessment in dogs and rats, intrathecal injection of oxytocin produced no evidence of neurotoxicity in the spinal cord.13 QT interval was not measured in those studies, although intrathecal oxytocin did reduce heart rate. In the rats it caused intense scratching and grooming behavior, consistent with work in mice showing an action of oxytocin on arginine vasopressin receptors to cause itching.14
New drugs for intrathecal use are typically first administered in a dose escalating, open label manner, beginning at doses below and extending above the presumed therapeutic range, with a focus on safety. Based on preclinical studies,15 the therapeutic dose for intrathecal oxytocin was anticipated to be ≥ 20 µg, and doses up to 550 µg had been studied in dogs. We therefore studied a dose range of 5 – 150 µg. Based on clinical observations with systemic drug administration and preclinical observations with intrathecal drug administration, we focused safety assessment on blood pressure, heart rate, QT interval, serum sodium, and presence of itching. Although we would anticipate that intrathecal oxytocin would be most effective against hypersensitivity states, inducing abnormal sensations complicates drug effects in Phase 1 safety studies. Animal studies are conflicting whether oxytocin does15,16 or does not17 produce antinociception to acute stimuli in the normal state, but some data show an effect to reduce response to noxious heat.15 For this reason, we screened for analgesic effects to acute noxious thermal stimuli and to acute hypersensitivity to mechanical stimuli, recognizing that this was exploratory in nature.
Materials and Methods
The study was approved by the Wake Forest School of Medicine Institutional Review Board (Winston Salem, North Carolina) and was performed under Investigational New Drug approval and oversight by the Food and Drug Administration and by a local data safety monitoring committee (DSMC), comprised of an anesthesiologist and neurologist. We employed an open-label, dose-escalation Phase 1 safety design similar to that employed for novel drugs for intrathecal administration.18 Written informed consent was obtained from all subjects.
We recruited adults < 60 years of age, weighing < 100 kg who were healthy (American Society of Anesthesiologists physical status 1) and having normal blood pressure (systolic 90–140 mmHg; diastolic 50–90 mmHg) and resting heart rate of 45–100 beats per minute on screening visit and, on Study Visit 2, prior to spinal injection. Women of child-bearing potential and those < 1 year post-menopausal were included if they had a negative serum pregnancy test at the screening visit and were practicing highly effective methods of birth control. We included both sexes and individuals of different races and ethnicities in accordance with National Institutes of Health guidance. We chose a healthy, young population for this safety study to avoid interactions with other drugs or diseases. We excluded subjects who were breast feeding, had hypersensitivity, allergy, or significant reaction to any ingredient of the study drug or lidocaine and those with any disease, diagnosis, or condition (medical or surgical) that, in the opinion of the Principal Investigator, would place the subject at increased risk (active gynecologic disease in which increased tone would be detrimental e.g., uterine fibroids with ongoing bleeding), or would compromise the subject’s compliance with study procedures. We also excluded those taking prescription medications, with the exception of birth control pills.
Study Visit 1
The participant reported to the general clinical research unit for confirmation of consent and definitive screening. A detailed medical history was obtained and blood was drawn for pregnancy testing as appropriate. Blood pressure, heart rate, and peripheral oxyhemoglobin saturation were recorded and a 12 lead electrocardiogram obtained. Subjects were then trained to estimate pain intensity and unpleasantness from heat stimuli applied to the skin of the arm using a 2 cm2 Peltier-controlled thermode (TSA-II®, Medoc, Ramat Yishai, Israel). Five second periods of fixed temperatures from 39° to 51° C in 2° C increments were presented at 30 sec inter-stimulus intervals for one cycle in an ascending temperature series and then using random presentation as previously described.19 Two-three cycles of random presentation were performed until consistent ratings (within 30% for each temperature) were obtained. The probe remained in contact with the skin for the entire cycle and was moved at the end of each cycle to avoid sensitization. The subject was blinded to temperature being applied and rated pain intensity and unpleasantness separately using a 10 cm mechanical visual analog scale anchored at no pain and the worst pain imagineable or at not unpleasant and as unpleasant as possible.
Study Visit 2
On this visit, at least one day after visit 1, the subject reported to the general clinical research unit in the morning, having had nothing to eat or drink since midnight. An intravenous catheter was inserted into a vein in an upper extremity and lactated Ringers solution infused at 1.5 ml/kg/hr for the duration of the study. After obtaining baseline measures, the volunteer was positioned in the sitting or lateral position and a #27 Whitacre spinal needle (Becton Dickinson, and Company, Franklin Lakes, NJ) was inserted in a lumbar interspace using standard, sterile technique following local infiltration of superficial tissues with 1% lidocaine. After obtaining clear, free flow cerebrospinal fluid (CSF), a 2 ml sample was obtained and rapidly frozen for subsequent measurement of oxytocin. Oxytocin in a 3 ml volume, diluted with sterile normal saline, was then injected over 30 seconds. Oxytocin powder manufactured under Food and Drug Administration oversight for human use was formulated in preservative free solution by Anazao Corporation (Orlando, FL) and used within 48 hr of shipment to Wake Forest School of Medicine. Following spinal injection the volunteer moved to the supine position and the head of the bed was elevated for the remainder of the study. Sixty min following injection the subject was repositioned and a #27 Whitacre tipped spinal needle inserted at the same interspace and 2 ml CSF removed for analysis of oxytocin.
In this dose escalation study, the first 5 volunteers received 5 µg of spinal oxytocin and subsequent groups of 5 volunteers each received 15, 50, and 150 µg of spinal oxytocin. Escalation to the next higher dose occurred only after review by the DSMC of adverse events at the lower dose. Subjects were informed that different doses of oxytocin were being studied, but not which dose they received. We did not assess participant expectations of treatment effects or prior knowledge of oxytocin.
Peripheral oxyhemoglobin saturation, blood pressure and heart rate were measured non invasively before and 15, 30, 60, 120, 180, 240, minutes and approximately 24 hours after administering spinal oxytocin. The electrocardiogram was monitored continuously for 240 min following spinal injection and corrected QT interval (QTc) estimated from manual measurement of printed tracings at 15 min intervals after injection for the first hour, then hourly for 3 more hours.
A screening neurologic exam along with questioning for qualitative symptoms was performed at 45, 90, 150, 210, 240 minutes, and 24 hours after spinal injection. The screening neurologic test consisted assessing upper and lower extremity deep tendon reflexes (biceps, knee, and ankle with standard methods and 0 – 4 standard clinical grading), response to light touch, and extension/withdrawal strength across proximal and distal major joints (plantar-dorsiflexion at the foot, flexion-extension at the knee, palmar-dorsiflexion at the wrist, and flexion-extension at the elbow with standard examiner clinical grading). Based on known adverse effects of oxytocin in clinical studies and on animal data, subjects were specifically questioned whether sedation, anxiety, nausea, dizziness, gastrointestinal or genitourinary symptoms or distress, or weakness was present at the same times as the neurologic examinations. If present, they were asked to rate them on a mild, moderate, severe scale. We also asked for presence of any other symptoms. Subjects were informed through the consent document that oxytocin could cause their blood pressure or heart rate to go up or down and could produce itching, nausea, pelvic or abdominal discomfort, or altered sensations in their legs or arms.
To screen for potential analgesic effects of oxytocin to acute pain processing, we examined response to both thermal and mechanical stimuli. For the former, subjects rated pain and unpleasantness to 5 sec heat stimuli between 39°C and 51°C presented in random order separately on the arm and leg, using the same scales and device used on Study visit 1. A stimulus response was obtained prior to spinal injection and 15, 30, 60, 120, 180, 240, minutes and approximately 24 hours after administering spinal oxytocin. At these same times we also determined temporal summation to 10 applications of a 255 mN von Frey filament application to the arm and leg using a method previously described.20 For this, the filament was manually applied briefly to the bending point once per second for a total of 10 applications and pain scores obtained with the first and last application. Temporal summation was defined as the pain score on the last application minus the first.
Subjects were discharged in the care of an accompanying adult from the clinical trials unit 240 min after oxytocin on the condition that blood pressure and heart rate were within normal limits (140/50− 90/50 mm Hg and 45–100 beats per minute, respectively), the subject could ambulate without difficulty, and there were no adverse events which had not resolved at this time.
Study Visit 3
Approximately 24 hours after the spinal oxytocin injection the volunteer returned to the general clinical research unit for screening neurologic examination, querying of any adverse events, and measurement blood pressure, heart rate, and pain ratings in the arm and leg to thermal stimuli and repeated von Frey filament application as described in Study Visit 2. Because of concerns of potential hyponatremia following exposure to large doses of oxytocin, a blood sample was obtained at this time for subjects who received 50 and 150 µg for serum sodium.
Long term follow up
Subjects were contacted by telephone daily for 5 days following spinal injection, then weekly for one month, then at 6 months. They were specifically queried at these times regarding side effects from the procedure (headache, backache), for any focal or general neurologic symptoms, and for any adverse events since the time of study.
Volunteers were paid according to a graduated schedule with a total of $400 for completion of all phases of study.
CSF oxytocin prior to injection was measured using an ultra-sensitive, proprietary radioimmunoassay (RIAgnosis, Regensburg, Germany, limit of sensitivity 0.5 pg/sample) and CSF oxytocin after injection was measured with a commercially available radioimmunoassay (Phoenix Pharmaceuticals, Inc, Burlingame, CA, limit of sensitivity 10 pg/sample). Serum sodium was measured in a commercial laboratory (Laboratory Corporation of America, Winston Salem, NC).
Statistical analysis
The number of subjects at each dose level was chosen by tradition for phase 1 safety trials with intrathecal drug administration, balancing the risks associated with the invasive drug administration and the assessment of adverse event rates. A formal power calculation was not performed.
Data are presented as mean ± SD or median [25th,75th percentile] as appropriate. For blood pressure, heart rate, and QTc interval, a two factor, repeated measures ANOVA was performed with time and dose defined as the factors. For pain scores, two approaches were used. The primary analysis was a two factor, repeated measures ANOVA with time and dose defined as the factors and average visual analog pain score at the two highest stimuli (49 and 51° C) as the outcome measure. We chose these two since these were the only temperature stimuli to result in non-zero pain scores from all individuals at all times. We secondarily explored analgesic effects and potential sex differences in two separate generalized estimating equation models to predict pain or unpleasantness by (1) site (arm or leg), (2) dose (5, 15, 50, 150 mcg), (3) time (5 –1440 min) and (4) stimulus temperature (39–51°C). Data were first explored to determine distributions and linear associations between predictors and outcomes.
Measurements were viewed as nested within subject with repeated measures for dose, time and stimulus temperatures. A normal distribution with identity link was specified and the robust estimator was used to provide the covariance estimation along with an independent correlation matrix. Site, dose, time and stimulus temperature were entered into the model as fixed factors. As there were no missing values in either model, all cases were included in the analysis. The model residuals were examined following prediction. Two-tailed p < 0.05 was considered statistically significant, with no attempts made to adjust for the two models. All analyses were conducted using the Statistical Package for the Social Sciences (SPSS for Windows, version 22.0, Chicago, IL).
Results
Twenty subjects were studied between Feb, 2012 and Aug, 2013. They were 37 ± 12 years old, 172 ± 10 cm tall, and 76 ± 18 kg in weight. Twelve of the subjects were women, one was Hispanic, four were African-American, and fifteen were Caucasian. All subjects completed the study and none were lost to follow up for the 6 month period of study.
Safety measures
Sensory and motor examination, as well as deep tendon reflexes in the upper and lower extremities were normal in all subjects prior to intrathecal injection and unchanged following injection. In response to pre-specified, systematic questioning, one subject reported anxiety prior to and 45 min after intrathecal injection (50 µg dose) and no subject reported weakness, sedation, nausea, itching, or gastrointestinal or bladder discomfort. Three subjects reported mild headache at the 24 hr visit (2 at the 5 µg dose and 1 at the 150 µg dose) which resolved spontaneously over the next few days.
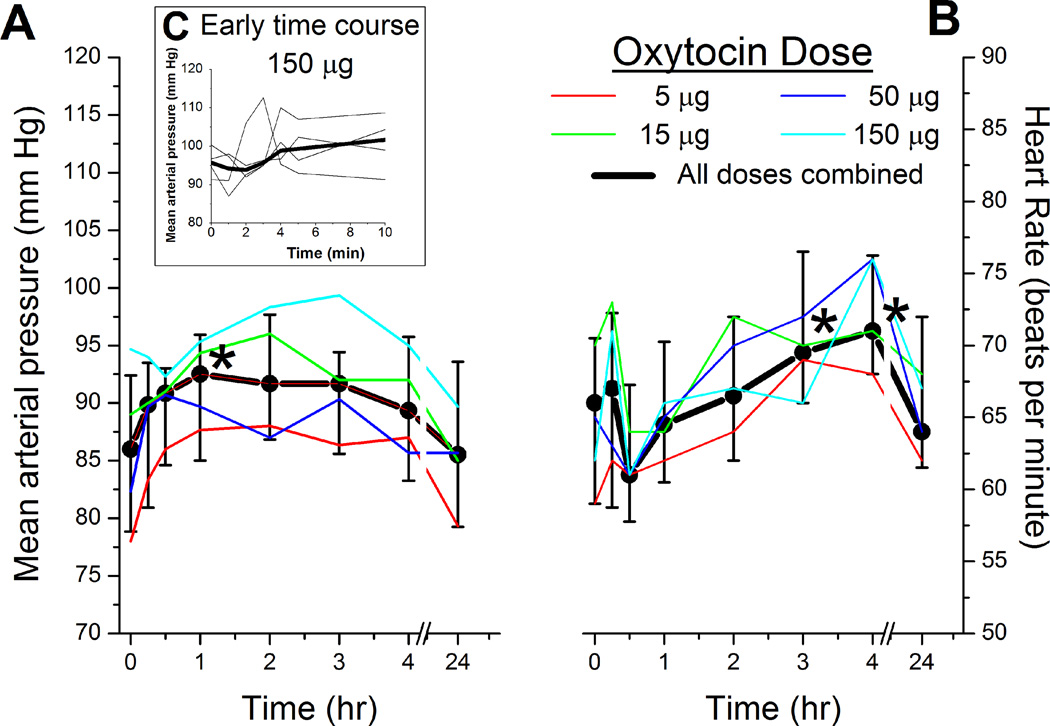
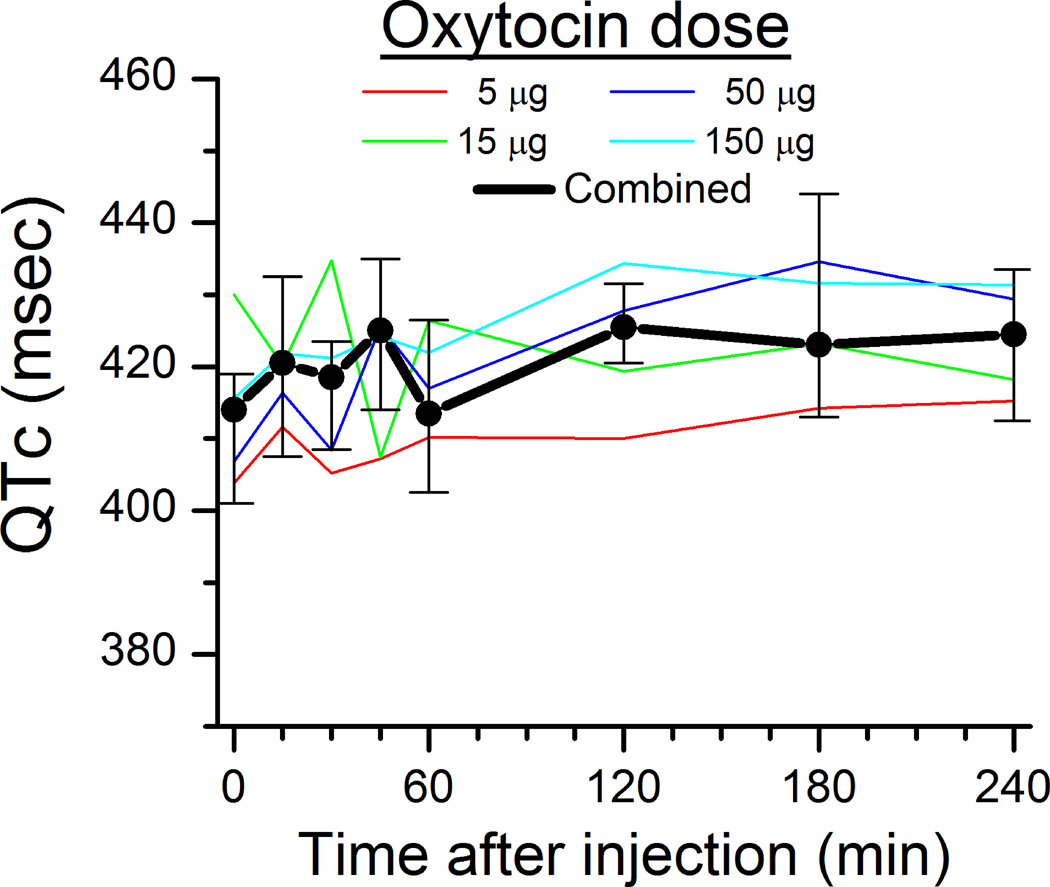
There was a dose independent effect of intrathecal oxytocin on blood pressure and heart rate, with a small (<15%) increase in both parameters which was transient and occurred earlier for blood pressure than heart rate (Figure 1A and 1B). QTc interval did not change after oxytocin injection (Figure 2), and no subject met criteria for QTc prolongation. The median serum sodium value 24 hr after spinal injection was 137 mmol/L (range 137–142 mmol/L) in subjects who received 50 µg oxytocin and 139 mmol/L (range 137–146 mmol/L) in subjects who received 150 µg oxytocin. One subject who received 150 µg oxytocin and a serum sodium of 146 mmol/L was outside the normal range (134–144 mmo/L).
Figure 1.
A) Blood pressure and B) heart rate before and after spinal injection of different doses of oxytocin. There was an effect of time on blood pressure and heart rate across all groups, with no dose dependency. C) Insert shows blood pressure for the first 10 min after injection of 150 µg oxytocin in 4 volunteers (thin lines) and the median value (thick line). There was not an effect of time on blood pressure during this time period.
For the A) and B) thin lines represent the median of 5 individuals, and the thick line represents the median ± interquartile range of the population.
* < 0.05 versus Time 0 by Bonferroni test, which followed a significant two way ANOVA for repeated measures.
Figure 2.
Corrected QT interval (QTc) before and after spinal injection of different doses of oxytocin. Thin lines represent the median of 5 individuals, and the thick line represents the median ± interquartile range of the population. No significant effect of time or dose.
Serious adverse event
One subject had a symptomatic decrease in heart rate and blood pressure. This subject, the first to receive the largest dose (150 µg) was noted to appear pale and felt clammy just prior to intrathecal injection. She denied light headedness. At the end of the injection the subject was positioned from the sitting to the right lateral position, when blood pressure following repositioning (73/58 mm Hg) was considerably below her baseline just prior to positioning for spinal injection (114/67 mm Hg), with a heart rate of 58, no apparent change to the narrow QRS complex on electrocardiographic monitoring, but with loss of P waves. She felt better over the next 5 min, and by 15 min after intrathecal oxytocin injection, felt normal. Although P waves were present and bradycardia resolved shortly after she was placed in the lateral position, she remained hypotensive (86/49 mg Hg) 15 min after intrathecal injection. This met protocol criteria for treatment (more than 20% reduction in mean arterial pressure from baseline), and, given the known hypotensive effect of oxytocin, it was considered that this was possibly due to study drug. Ephedrine, 5 mg was administered intravenously, and blood pressure increased 5 min thereafter to 96/47 mm Hg and 15 min thereafter to 111/71, with no recurrent episodes of hypotension. On further questioning, she noted that she had experienced similar feelings of clamminess associated with times of anxiety.
Approximately 20 min after injection, the same volunteer who experienced hypotension following oxytocin injection noted numbness in buttocks and left 3rd–5th toes. She was still in the right lateral decubitus position, and she moved to the supine position. On physical examination there were no motor deficits including foot plantar flexion, deep tendon reflexes were normal and unchanged from baseline, and there were no sensory deficits to light touch in lower extremities or on buttocks. No sensory abnormalities were noted on testing of the foot (sensory threshold to von Frey filament application or light stroking with a cotton swab). After repositioning, over the next 10 min she noted expansion of a tingling / numbness sensation extending from the buttocks to the perineum. She was asked to perform a pelvic floor exercise (Kegel) and she reported this as normal. This altered sensation began to resolve 60 min after injection and was completely resolved 90 min after injection. She urinated before leaving the General Clinical Research Center and reported no difficulties with urination or ambulation. Follow up at 12 and 24 hr as well as pre-specified follow up intervals to 6 months after study were normal. Her demographic measures, cerebrospinal fluid oxytocin concentrations, pain scores, and resting hemodynamic values were within the 25th to 75th percentile range of the subject population.
This episode was reported within 24 hr to the Institutional Review Board, the Food and Drug Administration, and the DSMC. The DSMC considered that the timing and characteristics of the initial hypotension following spinal needle insertion were consistent with a vaso-vagal episode, although it was possible that the lingering and less severe hypotension 15 min after injection was due to systemic absorption of oxytocin and its action on peripheral blood vessels. This hypotension clearly and rapidly responded to ephedrine. The cause of the subjective, negative sensory symptoms was unclear. The study was placed on hold for 30 days and the protocol revised for more frequent blood pressure monitoring in the first 10 min following spinal injection. None of the subsequent 4 individuals who received 150 µg oxytocin experienced hypotension (Figure 1C), bradycardia, or altered sensation.
CSF oxytocin concentrations were 9.5 ± 3.1 pg/ml prior to injection, and increased in a dose dependent manner 60 min after 5, 15, 50, and 150 µg oxytocin injection to 26 [22,28], 154 [126,170]. 313 [266,379], and 1200 [1187,1448] ng/ml respectively. The subject with the serious adverse event had a CSF oxytocin concentration of 1448 ng/ml.
Efficacy measures
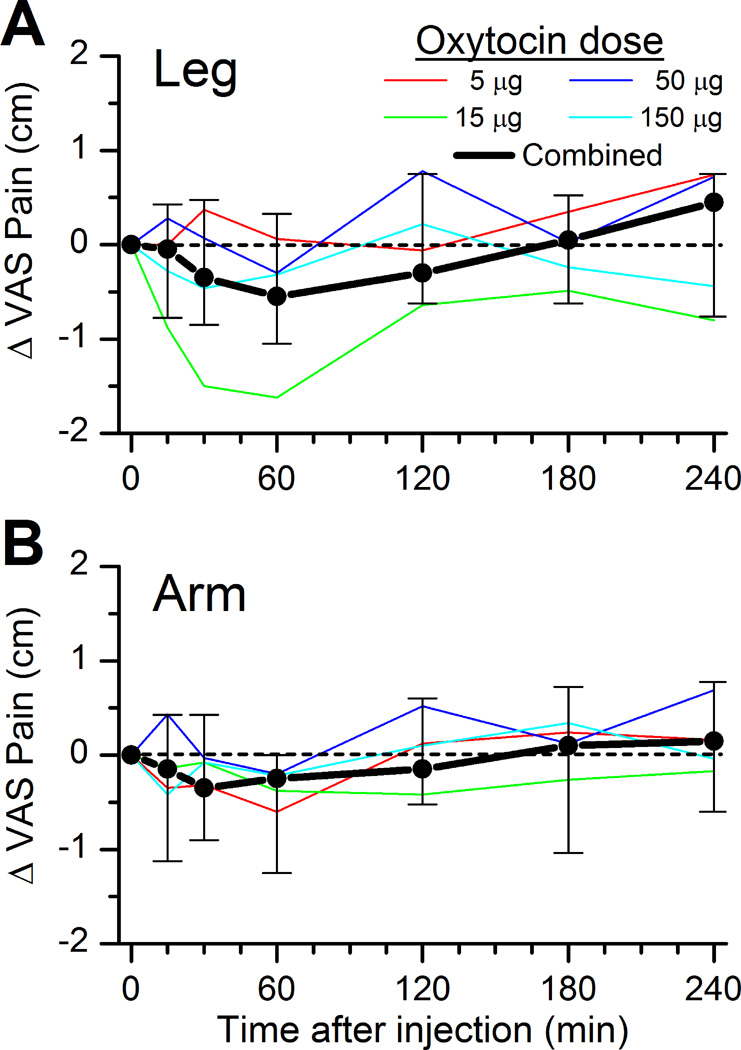
Average pain intensity report for the 49° and 51°C combined prior to spinal injection was 3.7 ± 2.2 cm in the leg and 3.6 ± 2.3 cm in the arm. In the primary analysis, oxytocin did not affect this measure of pain intensity reporting in the leg or arm (Figure 3A and 3B, p=0.11 for leg and p=0.22 for arm). Similarly, oxytocin did not affect pain unpleasantness reporting to the two highest temperature stimuli (data not shown).
Figure 3.
Change in average of the pain intensity report on a 10 cm visual analog scale (VAS) to 5 sec stimuli at 49° and 51°C on the A) leg or B) arm before and after spinal injection of different doses of oxytocin. Thin lines represent the median of 5 individuals, and the thick line represents the median ± interquartile range of the population. No significant effect of time or dose.
Full factorial modeling of time, dose, site, and temperature revealed highly significant 4-way interactions and for each of the 3-way combinations for both pain and unpleasantness (P < 0.0001). Collapsing across time, there was a highly significant 3-way combination for site, dose, and temperature (P < 0.0001), and separate analysis for site revealed a significant interaction between temperature and dose (P < 0.0001). The thermal stimulus response for pain differed by dose in both the arm and the leg, and there was a non-linear relationship between dose and pain report. There was not a sex difference in pain report when this variable was added to the full factorial analysis.
As regards temporal summation, only 5 subjects rated the first mechanical stimulus as painful, making it infeasible to examine the ratio of the 10th to the 1st stimulus as previously described.20 Overall, only 103 of 320 pain ratings were > 0, with an average collapsed across time of 0.4 ± 0.8 on the 1st stimulus and 0.8 ± 1.4 on the 10th stimulus. There was no effect of time or dose on the difference between the 10th and the 1st stimulus.
Discussion
By their nature, first-in-human safety trials expose only a small number of subjects to drug and utilize a wide dose range, further limiting the ability to observe serious safety signals. With only 5 subjects per dose, there is only a 50% chance of observing an adverse event with a true incidence as great as 60%, for example. As such, the conclusion from these trials is either that a drug is unsafe or that it is reasonable to proceed cautiously with continued investigation, rather than that the drug is safe. These data agree with preclinical observations of safety and suggest it is reasonable to proceed cautiously with continued investigation of intrathecal oxytocin.
Interpretation of the adverse event in the subject receiving 150 µg oxytocin is complicated by the onset of symptoms just prior to injection and the presentation of symptoms (clamminess, pallor) and signs (hypotension, bradycardia, narrow complex rhythm in the absence of P waves) consistent with vaso-vagal near syncope. It is conceivable that accidental intravenous injection of oxytocin could cause acute hypotension, but this is highly unlikely because this hypotension is typically associated with tachycardia rather than bradycardia and because CSF concentrations of oxytocin 60 min after injection were consistent with intrathecal injection. Although none of the other 4 volunteers receiving this dose of oxytocin exhibited hypotension, the presence of these symptoms in one individual suggests that future studies should include monitoring of blood pressure and heart rate following injection.
The presence of subjective tingling and numbness in the same volunteer who experienced hypotension is consistent with a drug effect, as it was restricted to sacral dermatomes (perhaps extending to L5), predominantly on the left side while the subject was in the right lateral decubitus position for several minutes after injection. We did not measure baricity of the oxytocin solution, but the diluent, normal saline, would be expected to be hypobaric compared to CSF and drug exposure should be greater to left nerve roots and spinal cord when the subject is in the right lateral decubitus position. The lack of objective signs of altered sensation or objective or subjective weakness, including pelvic floor muscle contraction, lack of alteration of deep tendon reflexes, and rapid resolution of the symptoms are reassuring, as is the lack of similar subjective sensations in other volunteers receiving this dose. Nonetheless, we believe that future investigations of intrathecal oxytocin should include systematic questioning and physical examination for focal neurologic signs and symptoms and extended follow up of all subjects exposed to this treatment.
Aside from this one individual, intrathecal oxytocin produced mild or no side effects. We note that only gross neurologic effects were examined in the current study, and that oxytocin-induced subtle effects on modality-specific sensory thresholds could have occurred without detection. Blood pressure increased by a small amount 60 min after injection. Although we did not measure oxytocin in the systemic circulation in the current study, time of peak absorption after intrathecal injection is approximately 60 min for bupivacaine and morphine,21,22 and it is conceivable that this increase could reflect actions of oxytocin on vascular arginine vasopressin V1a receptors. Arguing against this is the lack of dose dependency in this effect in dogs13 or humans in the current study. Bradycardia was noted in dogs receiving 550 µg intrathecal oxytocin, but, aside from the one episode of symptomatic bradycardia and hypotension, we did not note bradycardia in this clinical study. The increase in heart rate observed 3 and 4 hr after injection was small (<10%) and dose independent. These data are consistent with a review of 1529 subjects receiving 18–72 µg intranasal oxytocin without evidence of toxicity.23
Three special concerns with oxytocin in the non-pregnant patient are effects on QT interval and serum sodium and, after intrathecal administration, itching. We failed to observe a change in QTc in this small safety study, nor did any of the 20 individuals show prolonged QTc after drug administration. Similarly, the only abnormal serum sodium in this study was very mild hypernatremia in one individual, and water intoxication from oxytocin has only been observed in cases with prolonged administration.12 Scratching behavior in rodents following intrathecal oxytocin is directed to the dermatomes near the injection site, consistent with a pruritic effect or, potentially hypesthesia.14 This does not occur in dogs13 and no subject in the current study reported itching or hypesthesia, so it is likely that there is a species dependent effect with rodents being susceptible.
Studies in animals conflict on whether intrathecal oxytocin produces antinociception to acute stimuli in normal individuals. Some studies demonstrate antinociception to thermal,15 chemical,16 and mechanical15 stimuli, although it is unclear whether scratching behaviors elicited by oxytocin in these rodent studies interfered with measurements. Other studies demonstrate an effect only in hypersensitivity states.17 Our exploratory results do not support for an analgesic effect of intrathecal oxytocin to acute noxious heat stimuli in normal humans, although the study was not placebo controlled or adequately powered to observe a subtle effect, and further studies are necessary to definitively test this hypothesis. We were unable to replicate the temporal summation protocol previously described,20 perhaps due to the use of the von Frey filament, which has a larger surface area of point of contact than the custom designed metal filaments used in that study, so could not examine the effects of intrathecal oxytocin on experimentally induced hypersensitivity. A study in progress will examine this effect in a controlled manner.
In summary, these data suggest that intrathecal oxytocin after a single bolus over a dose range of 15–150 µg does not cause a high incidence of effects on blood pressure, heart rate, QT interval, serum sodium, or neurologic symptoms, and does not produce analgesia to acute noxious heat stimuli in normal individuals. One subject did experience transient cardiovascular depression and focal neurologic symptoms, so future studies should carefully assess these systems.
Acknowledgments
Supported in part by grant R37 GM48805 from the National Institute of Health, Bethesda, MD and the Translational Science Institute Clinical Research Unit at the Wake Forest Baptist Medical Center.
Dr. Eisenach has received fees for consultation to Aerial Biopharma (Morrisville, NC) and Adynxx (San Francisco, CA) on topics unrelated to this article.
Footnotes
Competing interests. The other authors have no competing interests.
References
- 1.Kessner S, Sprenger C, Wrobel N, Wiech K, Bingel U. Effect of oxytocin on placebo analgesia: A randomized study. JAMA. 2013;310:1733–1735. doi: 10.1001/jama.2013.277446. [DOI] [PubMed] [Google Scholar]
- 2.Madrazo I, Bourland-Franco R, Mena I. Analgesic effect of intracerebroventricular (icv) somatostatin-14 (som-14) arginine vasopressin (avp) and oxytocin (ot) in a patient with terminal cancer pain. Pain. 1987;(Suppl.4):S142. [Google Scholar]
- 3.Robinson DA, Wei F, Wang GD, Li P, Kim SJ, Vogt SK, Muglia LJ, Zhuo M. Oxytocin mediates stress-induced analgesia in adult mice. J Physiol. 2002;540:593–606. doi: 10.1113/jphysiol.2001.013492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miranda-Cardenas Y, Rojas-Piloni G, Martínez-Lorenzana G, Rodríguez-Jiménez J, López-Hidalgo M, Freund-Mercier MJ, Condés-Lara M. Oxytocin and electrical stimulation of the paraventricular hypothalamic nucleus produce antinociceptive effects that are reversed by an oxytocin antagonist. Pain. 2006;122:182–189. doi: 10.1016/j.pain.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez S, Liu B, Hayashida K, Houle TT, Eisenach JC. Reversal of Peripheral Nerve Injury-induced Hypersensitivity in the Postpartum Period: Role of Spinal Oxytocin. Anesthesiology. 2013;118:152–159. doi: 10.1097/ALN.0b013e318278cd21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutierrez S, Hayashida K, Eisenach JC. The puerperium alters spinal cord plasticity following peripheral nerve injury. Neuroscience. 2013;228:301–308. doi: 10.1016/j.neuroscience.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boccia ML, Petrusz P, Suzuki K, Marson L, Pedersen CA. Immunohistochemical Localization of Oxytocin Receptors in Human Brain. Neuroscience. 2013;253:155–164. doi: 10.1016/j.neuroscience.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 8.Rojas-Piloni G, Lopez-Hidalgo M, Martinez-Lorenzana G, Rodriguez-Jimenez J, Condes-Lara M. GABA-mediated oxytocinergic inhibition in dorsal horn neurons by hypothalamic paraventricular nucleus stimulation. Brain Res. 2007;1137:69–77. doi: 10.1016/j.brainres.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 9.Breton JD, Veinante P, Uhl-Bronner S, Vergnano AM, Freund-Mercier MJ, Schlichter R, Poisbeau P. Oxytocin-induced antinociception in the spinal cord is mediated by a subpopulation of glutamatergic neurons in laminas I–II which amplify GABAergic inhibition. Mol Pain. 2008;4:19. doi: 10.1186/1744-8069-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosseland LA, Hauge TH, Grindheim G, Stubhaug A, Langesaeter E. Changes in Blood Pressure and Cardiac Output during Cesarean Delivery: The Effects of Oxytocin and Carbetocin Compared with Placebo. Anesthesiology. 2013;119:541–551. doi: 10.1097/ALN.0b013e31829416dd. [DOI] [PubMed] [Google Scholar]
- 11.Charbit B, Funck-Brentano C, Samain E, Jannier-Guillou V, Albaladejo P, Marty J. QT interval prolongation after oxytocin bolus during surgical induced abortion. Clin Pharmacol Ther. 2004;76:359–364. doi: 10.1016/j.clpt.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Seifer DB, Sandberg EC, Ueland K, Sladen RN. Water intoxication and hyponatremic encephalopathy from the use of an oxytocin nasal spray. A case report. J Reprod Med. 1985;30:225–228. [PubMed] [Google Scholar]
- 13.Yaksh TL, Hobo S, Peters C, Osborn KG, Richter PJ, Jr, Rossi SS, Grafe MR, Eisenach JC. Preclinical Toxicity Screening of Intrathecal Oxytocin in Rats and Dogs. Anesthesiology. 2014;120:951–961. doi: 10.1097/ALN.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, Crawley JN, Hu SB, Nishimori K, Young LJ, Tribollet E, Quirion R, Mogil JS. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci. 2010;30:8274–8284. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu SQ, Lundeberg T, Yu LC. Involvement of oxytocin in spinal antinociception in rats with inflammation. Brain Res. 2003;983:13–22. doi: 10.1016/s0006-8993(03)03019-1. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, Yang Y, Chen JM, Liu WY, Wang CH, Lin BC. Central oxytocin enhances antinociception in the rat. Peptides. 2007;28:1113–1119. doi: 10.1016/j.peptides.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Condes-Lara M, Maie IA, Dickenson AH. Oxytocin actions on afferent evoked spinal cord neuronal activities in neuropathic but not in normal rats. Brain Res. 2005;1045:124–133. doi: 10.1016/j.brainres.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Hood DD, Eisenach JC, Tuttle R. Phase I safety assessment of intrathecal neostigmine in humans. Anesthesiology. 1995;82:331–343. doi: 10.1097/00000542-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Eisenach JC, Hood DD, Curry R. Intrathecal, but not intravenous, clonidine reduces experimental thermal or capsaicin-induced pain and hyperalgesia in normal volunteers. Anesth Analg. 1998;87:591–596. doi: 10.1097/00000539-199809000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain. 2006;123:231–243. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 21.Boico O, Bonnet F, Mazoit JX. Effects of epinephrine and clonidine on plasma concentrations of spinal bupivacaine. Acta Anaesthesiol Scand. 1992;36:684–688. doi: 10.1111/j.1399-6576.1992.tb03544.x. [DOI] [PubMed] [Google Scholar]
- 22.Nordberg G, Hedner T, Mellstrand T, Dahlstrom B. Pharmacokinetic aspects of intrathecal morphine analgesia. Anesthesiology. 1984;60:448–454. doi: 10.1097/00000542-198405000-00010. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011;36:1114–1126. doi: 10.1016/j.psyneuen.2011.02.015. [DOI] [PubMed] [Google Scholar]