Abstract
Memory T cells can persist for extended periods in the absence of antigen, and long-term T cell immunity is often seen after acute infections. Paradoxically, there have been observations suggesting that T cell memory may be antigen-dependent during chronic infections. To elucidate the underlying mechanisms we have compared memory CD8 T cell differentiation during an acute versus chronic infection by using the mouse model of infection with lymphocytic choriomeningitis virus. We found that during a chronic infection virus-specific CD8 T cells failed to acquire the cardinal memory T cell property of long-term antigen-independent persistence. These chronically stimulated CD8 T cells were unable to undergo homeostatic proliferation, responded poorly to IL-7 and IL-15, and expressed reduced levels of the IL-7 and IL-15 receptors, thus providing a possible mechanism for the inability of these cells to persist long term in the absence of antigen. In striking contrast, virus-specific memory CD8 T cells that developed after an acute lymphocytic choriomeningitis virus infection could persist without antigen, were capable of self-renewal because of homeostatic proliferation, responded efficiently to IL-7 and IL-15, and expressed high levels of receptors for these two cytokines. Thus, memory CD8 T cells generated after acute infections are likely to have a competitive advantage over CD8 T cells that develop during chronic infections. These findings raise concerns about using vaccines that may persist and also suggest that there may be limitations and challenges in designing effective immunological interventions for the treatment of chronic infections and tumors.
A hallmark of memory T cells is their ability to persist in the absence of antigen (1). Memory CD8 T cells are able to do this because they can survive for extended periods of time because of higher levels of antiapoptotic molecules such as Bcl-2 (2) and, more importantly, because unlike naïve T cells, they can undergo antigen-independent proliferation to maintain their numbers (3–5). The unique ability of memory CD8 T cells to undergo homeostatic proliferation in response to IL-15 and IL-7 allows for their self-renewal and the maintenance of a functional pool of memory T cells that can rapidly respond to reinfection and confer protective immunity. Despite numerous studies in both animal models and humans demonstrating antigen-independent memory T cell persistence, other studies suggest that antigen persistence may be necessary to maintain protective immunity in some chronic infections (6–10). For example, antibiotic treatment in a murine model of Bacille Calmette-Guérin (bacillus Calmette–Guérin) infection results in a decline in antigen-specific CD8 T cell numbers (11). Also, the elimination of Leishmania major parasite burden spontaneously or with drug treatment is associated with loss of protective immunity, whereas in persistently infected mice resistance to reinfection is maintained (9, 10).
Why do memory T cells and protective immunity appear antigen-independent in some settings, but antigen-dependent in others? One striking distinction is that data supporting antigen-independent immunological memory come from studies on acute infections, whereas observations suggesting a role for antigen in T cell maintenance come from models of chronic infections. These studies suggest that the normal memory CD8 T cell differentiation pathway that culminates in the generation of memory T cells that can persist without antigen (12–14) may not occur during chronic infections. To address this question we have examined memory CD8 T cell differentiation in the mouse model of infection with lymphocytic choriomeningitis virus (LCMV) where it is possible to track the same CD8 T cell response after an acute or chronic viral infection. This model allows one to analyze virus-specific CD8 T cell responses after an acute infection that is fully cleared within a week and during two types of chronic infections: (i) during a chronic infection with high viral load in multiple tissues for the life of the mouse; and (ii) during a chronic infection that is characterized by high virus levels in multiple tissues for 2–3 months followed by control of infection in most tissues, but not complete elimination of the virus (15–17). An important distinction between these two conditions of persistence is that in the former the virus-specific CD8 T cells encounter antigen constantly for the life of the mouse, whereas in the latter the CD8 T cells see antigen continuously for the first 2–3 months and then encounter antigen only periodically. For the purpose of this study we chose the latter condition as our model of chronic infection, because many human chronic infections show this pattern, i.e., initial high levels of infection followed by control and then low-grade persistence of the pathogen. Moreover, only this model of chronic infection allows one to analyze the properties of “resting” antigen-specific CD8 T cells because T cells taken several months after infection are not continuously seeing high levels of antigen. Our results demonstrate that T cells that experience prolonged exposure to antigen during chronic LCMV infection do not acquire the cardinal properties of memory CD8 T cells, including antigen-independent persistence via homeostatic proliferation in response to IL-7 and IL-15. Rather, virus-specific CD8 T cells generated during chronic LCMV infection fail to persist when removed from antigen. These results provide a potential explanation for the loss of immunity observed after control of some chronic infections and have implications for our understanding of protective immunity to persisting pathogens and tumors.
Materials and Methods
Animals and Virus. Four- to 6-week-old female C57BL/6 mice were purchased from The Jackson Laboratory. Mice were infected with 2 × 105 plaque-forming units of LCMV Armstrong i.p. or 2 × 106 plaque-forming units of LCMV clone 13 i.v. as described (17). Viral titers were determined by plaque assay as described (15).
Lymphocyte Isolation. Lymphocytes were isolated from tissues as described (17). Liver and lung were perfused with ice-cold PBS before removal and lymphocyte isolation.
Flow Cytometry. MHC class I peptide tetramers were generated and used as described (17). All antibodies were purchased from BD Bioscience (San Diego) except for granzyme B (Caltag, Burlingame, CA), IL-15Rα (R & D Systems), CD127 (eBioscience, San Diego), and pSTAT5 (Cell Signaling Technology, Beverly, MA). All surface and intracellular cytokine staining was performed as described (17). Staining for the intracellular molecules Bcl-2 and pSTAT5 was performed by using the same procedure as for intracellular cytokine staining.
Adoptive Transfers and Carboxyfluorescein Diacetate Succinimidyl Ester (CFSE) Labeling. CFSE labeling was performed as described (13). For all adoptive transfer experiments CD8 T cells were purified from donor spleens by using MACS beads (Miltenyi Biotec, Auburn, CA) as described (ref. 13; ≈95–98% pure). Donor T cell populations were adoptively transferred by i.v. injection. Longitudinal monitoring of T cell persistence was performed by retroorbital bleeding into 4% sodium citrate under isofluorane anesthesia. Peripheral blood mononuclear cells (PBMC) were purified by using Hystopaque (Sigma) and stained.
In Vitro Proliferation. Purified CD8 T cells were labeled with CFSE and mixed with unlabeled naïve spleen as antigen-presenting cells in 96-well flat-bottom plates. Cytokines (IL-7 or IL-15; each 5 ng/ml) or peptide (0.2 μg/ml) were added, and proliferation was assessed by MHC tetramer staining after ≈60 h.
Results
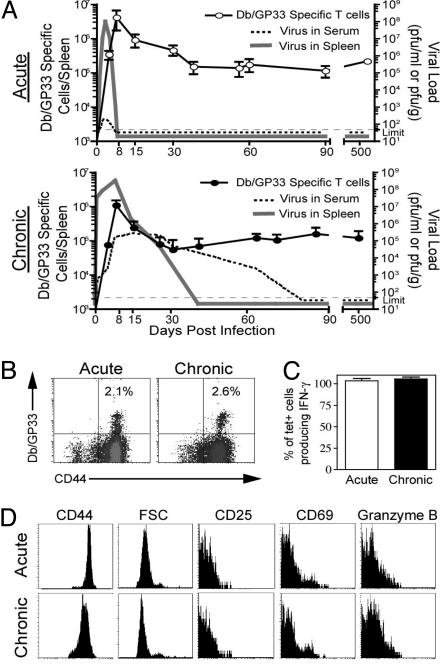
Adult C57BL/6 mice were infected with either the LCMV Armstrong strain that causes an acute infection and is cleared from all tissues within a week or LCMV clone 13 that establishes a chronic infection with 2–3 months of viremia (Fig. 1A) followed by persistence in selected tissues (15, 18). Virus-specific CD8 T cells recognizing the GP33-41 epitope were induced and maintained for >1.5 years after Armstrong (acute) or clone 13 (chronic) infection (Fig. 1 A and B). It should be noted that the Armstrong and clone 13 strains of LCMV differ by only two amino acids and neither of these changes affects any of the defined CD8 T cell epitopes (19). For all experiments performed in this study, Db/GP33-specific CD8 T cell populations were isolated from clone 13-infected mice at time points after viremia had been controlled (after day 100; Fig. 1). At these time points virus-specific CD8 T cells from both acutely and chronically infected mice displayed the phenotype of “resting” memory T cells; they were CD44Hi and small in size, did not express markers of recent activation, such as CD25, CD69, or high levels of granzyme B, and were capable of producing IFN-γ upon peptide restimulation in vitro (Fig. 1 C and D). Hereafter, we will use the terms acute memory and chronic memory to refer to Db/GP33-specific CD8 T cells generated after an acute LCMV Armstrong infection or a chronic infection with LCMV clone 13, respectively.
Fig. 1.
Characterization of acute memory and chronic memory CD8 T cells. (A) The kinetics of viral infection (dashed line, serum; solid line, spleen) and Db/GP33-specific CD8 T cell responses after LCMV Armstrong and LCMV clone 13 infection. (B) Db/GP33 tetramer staining of CD8 T cells from the spleen after acute or chronic infection (days 500 and 825 p.i., respectively; similar results were observed between days 30 and 800). (C) Intracellular cytokine staining for IFN-γ after 5 h of GP33 peptide stimulation at day 180 acute or chronic infection. Data are the percent of Db/GP33+ CD8 T cells that produced IFN-γ. Similar results were observed at other time points after day 100 p.i. (data not shown). (D) The phenotype of LCMV Db/GP33+ CD8 T cells day 140 after acute or chronic LCMV infection. Plots were gated on Db/GP33+ CD8 T cells. Similar results were observed at multiple time points after day 100 of chronic infection. Similar results were observed for Db/GP276+ CD8 T cells (data not shown).
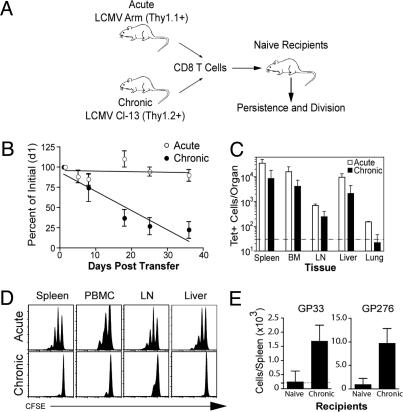
The first question we addressed was whether chronic memory CD8 T cells had acquired the memory T cell quality of antigen-independent persistence. To directly compare the ability of chronic memory CD8 T cells versus acute memory CD8 T cells to persist in the absence of antigen we adoptively transferred both acute memory and chronic memory CD8 T cells together into the same naïve uninfected recipients and used the congenic Thy1 marker (Thy1.1 vs. Thy1.2) to distinguish the two cell populations (Fig. 2A). Care was taken in these experiments to ensure that antigen was not transferred with the chronic memory CD8 T cells. First, at the time of adoptive transfer no virus was detectable in the spleens of donor mice and, second, purified CD8 T cells (>95% pure) were used in all experiments to reduce the chance of antigen carryover because LCMV clone 13 does not infect CD8 T cells (20). As shown in Fig. 2B, the chronic memory CD8 T cells failed to persist in uninfected mice and their frequency declined over time. This finding was in striking contrast to the efficient maintenance of the acute memory CD8 T cells present in the same recipient mice. This decline in the blood (Fig. 2B) was not caused by redistribution of the cells because ≈3- to 10-fold fewer chronic memory CD8 T cells were recovered from both lymphoid and nonlymphoid tissues compared with the cotransferred acute memory CD8 T cells (Fig. 2C).
Fig. 2.
Chronic memory CD8 T cells do not persist in the absence of antigen. (A) Experimental design for adoptive transfers. Acute memory (after day 30 p.i.) and chronic memory (after day 120 p.i.) CD8 T cells were purified, mixed to contain equal numbers of Db/GP33+ CD8 T cells, and transferred to naïve mice. Acute memory (Thy1.1+) and chronic memory CD8 T cells (Thy1.2+) were tracked independently in the same recipient. Similar results were obtained when acute and chronic memory CD8 T cells were transferred into separate groups of naïve mice (data not shown). (B) Recipients were bled longitudinally, and the frequency of Db/GP33+ acute and chronic memory CD8 T cells in the PBMC is expressed as a percentage of their initial frequency ≈36 h posttransfer. Data represent three to eight mice per group in five independent experiments, and the difference between acute and chronic memory CD8 T cells is significant (P < 0.05) on days 18, 25, and 35 posttransfer. We have fit a straight line to the curve, but it is also possible that this T cell loss is biphasic with some of the chronic memory CD8 T cells waning faster than others. (C) The number of transferred Db/GP33+ acute and chronic memory CD8 T cells was quantified on day 35 in the indicated tissues by tetramer staining (n = 2 mice per group and is representative of five independent experiments). Dotted line indicates the limit of detection. (D) Acute memory (day 150) and chronic memory (day 120) CD8 T cells were labeled with CFSE before adoptive transfer, and homeostatic proliferation was assessed 35 days later. Histograms are gated on acute memory (Db/GP33+Thy1.1+) or chronic memory CD8 T cells (Db/GP33+Thy1.2+) isolated from the indicated tissues of naïve recipient mice. Data are representative of five independent experiments with two to three mice each. For B–D similar results were observed for Db/GP276+ CD8 T cells (data not shown). (E) Chronic memory CD8 T cells (day 150) were purified and adoptively transferred into naïve or chronically infected mice (≈130 days p.i.). On day 56 significant differences were observed between the number of donor Db/GP33+ and Db/GP276+ chronic memory CD8 T cells in the spleen determined by tetramer staining (GP33, P = 0.02; GP276, P = 0.01). Data are representative of two independent experiments with two to three mice per group.
We next examined whether the loss of chronic memory CD8 T cells in naive recipients was caused by defects in homeostatic proliferation. Acute memory and chronic memory CD8 T cells were labeled with CFSE and cotransferred into naïve recipients. At 1 month posttransfer, the vast majority of the chronic memory CD8 T cells had failed to divide in any tissue examined (Fig. 2D). In contrast, the acute memory CD8 T cells had undergone efficient homeostatic proliferation, and many of them had divided one, two, and even three times (Fig. 2D). Thus, chronic memory CD8 T cells were dramatically impaired in their ability to persist long term when adoptively transferred to antigen-free mice, which correlated with a failure to undergo efficient homeostatic division.
Although the chronic memory CD8 T cells were maintained efficiently in the original clone 13-infected mice (Fig. 1 A), they declined rapidly after adoptive transfer into naïve mice (Fig. 2B). This observation suggested that transfer of the chronic memory CD8 T cells back into the clone 13-infected mice should result in better maintenance of these cells. This idea was formally tested, and we found that ≈5- to 10-fold more Db/GP33 and Db/GP276 (another LCMV epitope)-specific chronic memory CD8 T cells were recovered (56 days later) after adoptive transfer into clone 13-infected mice [greater than day 130 postinfection (p.i.)] compared with naïve mice (Fig. 2E). Thus, these results show that chronic memory CD8 T cells declined rapidly in an antigen-free environment, but were maintained in the chronically infected mice.
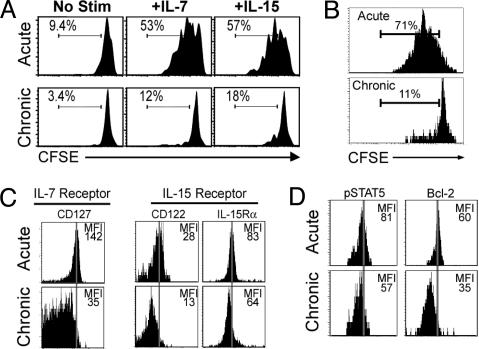
The γ chain cytokines IL-7 and IL-15 are key signals for homeostatic proliferation (4, 5, 21–24), and responsiveness to these cytokines is crucial for the persistence of memory CD8 T cells in the absence of antigen. The failure of chronic memory CD8 T cells to undergo homeostatic proliferation, therefore, could reflect a lack of responsiveness to these cytokines. Indeed, we found that chronic memory CD8 T cells responded poorly to both IL-7 and IL-15 in vitro, whereas acute memory CD8 T cells proliferated efficiently to both cytokines (Fig. 3A). IL-15 is “transpresented” to CD8 T cells in vivo through a yet unidentified cell type (25, 26), and it was possible that this cell type was defective in chronically infected mice. To control for this possibility we tested the responsiveness of acute memory and chronic memory CD8 T cells to IL-15 in the same environment in vivo after coadoptive transfer into naïve mice followed by IL-15 injections. Even when present in the same in vivo setting, chronic memory CD8 T cells responded poorly to IL-15 compared with the efficient proliferation of acute memory CD8 T cells after IL-15 injection (Fig. 3B), indicating that the poor responsiveness to IL-15 was an inherent defect in the chronic memory CD8 T cells.
Fig. 3.
Chronic memory CD8 T cells respond poorly to IL-7 and IL-15. (A) Acute memory (day 180) or chronic memory (day 240) CD8 T cells were CFSE-labeled and tested for responsiveness to IL-7 and IL-15 in vitro (5 ng/ml each). Division was assessed after 60 h. Numbers indicate the percent of D/bGP33+ CD8 T cells that had divided at least once. (B) Acute memory (day 140) and chronic memory (day 115) CD8 T cells were purified and labeled with CFSE, and equal numbers of Db/GP33+ cells were cotransferred into naïve recipients. IL-15 was injected i.p. daily (5 μg per injection), and division of Db/GP33+ CD8 T cells was analyzed on day 5. (C) IL-7 and IL-15 receptors on acute memory (day 150) and chronic memory (day 120) CD8 T cells. Histograms are gated on Db/GP33+ CD8 T cells. (D) Staining for intracellular pSTAT5 and Bcl-2 expression in the same populations as in C. For all panels similar results were observed for the LCMV Db/GP276+ CD8 T cell population (data not shown). The lower expression of cytokine receptors (except CD132 and IL-15Ra) and intracellular molecules by chronic memory Db/GP33+ CD8 T cells was statistically significant (Fig. 6).
The IL-7 receptor is composed of the common γ chain, CD132, and a high-affinity α chain, CD127 (27). The IL-15 receptor also uses the common γ chain, CD132, as well as the IL-2/IL-15 β chain, CD122, and a unique IL-15R α chain (27). When the expression of these cytokine receptors was examined directly ex vivo, we found that the levels of CD127 (IL-7Rα) were dramatically reduced on virus-specific CD8 T cells from chronically infected mice (Fig. 3C and Fig. 6, which is published as supporting information on the PNAS web site). CD122 (IL-2/15Rβ) expression was also substantially reduced on chronic memory CD8 T cells compared with acute memory CD8 T cells, whereas there was a slight reduction in expression of IL-15Rα, but not CD132 (common γ chain) (Figs. 3C and 6). An important signaling molecule downstream of the IL-7 and IL-15 receptors, phosphorylated STAT5 (pSTAT5) (27), was also reduced in the chronic memory CD8 T cells (Fig. 3D). Bcl-2 expression can be up-regulated or maintained by IL-7 and IL-15 signaling (27). The expression of this antiapoptotic molecule was also lower in chronic memory CD8 T cells compared with acute memory cells (Fig. 3D), suggesting that not only homeostatic turnover, but also survival of these cells may be compromised. Previous studies have shown that IL-15 is necessary for homeostatic proliferation of memory CD8 T cells, and in the absence of this self-renewal, memory T cell numbers gradually decline (4, 5, 24). It has also been shown that memory CD8 T cell numbers decrease in the absence of IL-7, although homeostatic proliferation is not as dramatically affected as in the absence of IL-15 (5). If both IL-7 and IL-15 signals are absent, loss of memory CD8 T cells is even more severe, suggesting an important role for IL-7 in survival, as well as homeostatic turnover, of memory CD8 T cells (5). Thus, based on the results presented in Figs. 2 and 3 it appears that the underlying defect in chronic memory CD8 T cells is the lack of responsiveness to both the proliferative and survival signals that normally allow acute memory CD8 T cells to persist long term in the absence of antigen.
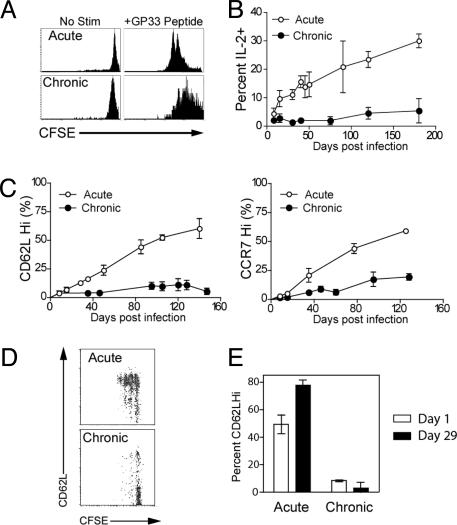
Memory CD8 T cell differentiation after acute infection results in the progressive acquisition of memory T cell qualities (12, 13). These qualities include not only homeostatic proliferation and responsiveness to IL-15 and IL-7, but also high proliferative potential upon reexposure to antigen, IL-2 production, and several phenotypic changes, including reexpression of the lymph node homing receptors CD62L and CCR7 (12, 13). We next asked whether the development of these other memory CD8 T cell traits was also impaired during chronic infection. We first tested the recall of chronic memory cells CD8 T cells compared with acute memory CD8 T cells. Chronic memory CD8 T cells did proliferate to GP33 peptide stimulation, but had a reduced proliferative capacity compared with acute memory CD8 T cells (Fig. 4A) in agreement with recent studies in HIV patients (28, 29). Chronic memory CD8 T cells also failed to progressively acquire the ability to produce IL-2 in response to antigen stimulation, whereas this property is characteristic of acute memory CD8 T cell differentiation (Fig. 4B and ref. 13). In addition, some of the phenotypic changes that accompany memory CD8 T cell differentiation showed a different pattern during chronic infection. As shown in Fig. 4C, chronic memory CD8 T cells failed to undergo substantial conversion to CD62LHi or CCR7Hi over ≈120–150 days, whereas acute memory CD8 T cells showed the typical pattern of phenotypic conversion from CD62LLoCCR7Lo to predominantly CD62LHiCCR7Hi over this time period (Fig. 4C and ref. 13). We next wanted to determine whether this phenotypic conversion could be restored if chronic memory CD8 T cells were removed from antigen. Acute memory CD8 T cells (≈50% CD62LHi) were transferred to naïve recipients, and the change in CD62L expression on these cells was monitored over time along with cell division. Many of the CD62LLo acute memory CD8 T cells converted to CD62LHi and underwent homeostatic proliferation; 1 month later, this acute memory CD8 T cell population was nearly 80% CD62LHi (Fig. 4 D and E) in agreement with previous observations (13). In contrast, adoptive transfer of chronic memory CD8 T cells to an antigen-free environment did not restore this differentiation pattern, and no increase in CD62L expression on this population was observed after ≈1 month in naïve uninfected recipients (Fig. 4 D and E). Similar results were also seen with CCR7 and CD127 (data not shown). Thus, our results indicate that not only is normal memory CD8 T cell differentiation altered during chronic infection, but also that the normal memory T cell differentiation program cannot be restored by simply removing the chronic memory CD8 T cells from antigen.
Fig. 4.
Impaired memory CD8 T cell differentiation during chronic infection. (A) CFSE-labeled acute memory (day 240) or chronic memory (day 240) CD8 T cells were stimulated in vitro with GP33 peptide, and division of Db/GP33+ CD8 T cells was assessed after 60 h. (B) The ability to produce IL-2 after in vitro stimulation with GP33 peptide was assessed at the indicated times by intracellular cytokine staining. Percentage indicates the percent of Db/GP33+ CD8 T cells from the spleen that produced IL-2; n = 2–7 for all points. The difference between acute and chronic infection is significant (P < 0.05) after day 8. (C) CD62L and CCR7 expression by Db/GP33+ CD8 T cells after acute versus chronic infection; n = 2–7 for all points. The difference between acute and chronic infection is significant (P < 0.05) after day 8 for CD62L and day 15 for CCR7. (D) Acute memory (day 100) and chronic memory (days 140–160) CD8 T cells were purified and transferred to naïve mice. Thirty-four days later, division and phenotype of CFSE-labeled, Db/GP33+ CD8 T cells and acute or chronic memory CD8 T cells were analyzed by costaining for CD62L. Results shown are for the spleen. Similar results were observed for lymph nodes, PBMC, and liver (data not shown). (E) CD62L expression on Db/GP33+ acute and chronic memory CD8 T cells in the PBMC after adoptive transfer to naïve recipients. There was a significant increase in the percentage of CD62LHi acute memory CD8 T cells (P < 0.01) after 34 days in naïve adoptive hosts, but no significant change in the percentage of CD62LHi chronic memory. Similar results were observed in the spleen (data not shown). n = two to three per group, and data are representative of four independent experiments. Similar results were observed for CCR7 and CD127 expression (data not shown). For all panels, similar results were observed for the Db/GP276+ CD8 T cells (data not shown).
Discussion
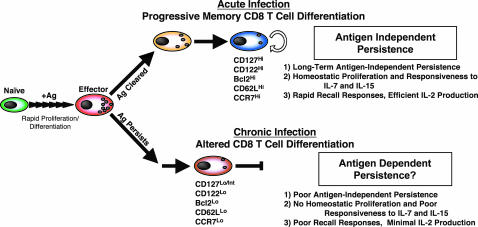
The normal memory CD8 T cell differentiation program that occurs after acute infection is linear and progressive and culminates in the formation of memory CD8 T cells that are capable of long-term antigen-independent persistence as a result of slow homeostatic proliferation in response to IL-7 and IL-15 (Fig. 5 Upper and refs. 12–14). The data presented in this study demonstrate that during chronic LCMV infection this normal memory CD8 T cell differentiation pathway does not proceed efficiently and virus-specific CD8 T cells fail to acquire the cardinal memory T cell property of antigen-independent persistence (Fig. 5 Lower). These cells also fail to acquire two antigen-driven memory T cell properties that gradually develop after acute infection: high proliferative potential and IL-2 production. T cell antigen receptor stimulation during acute infection occurs for a finite period and then, after antigen elimination, differentiation into memory T cells occurs in the absence of antigen. Our results suggest that prolonged exposure to antigen without rest results in CD8 T cells that have developed an antigen addiction and that cannot persist in an antigen-independent manner. Thus, our study raises concerns about vaccine strategies that use persisting antigen because antigen-independent memory T cells may not develop.
Fig. 5.
Model of CD8 T cell differentiation during acute versus chronic infections. During the first week of infection naïve antigen-specific CD8 T cells undergo antigen-driven proliferation and differentiation into effector CD8 T cells. If antigen is cleared (i.e., acute infection) 5–10% of CD127Hi effector CD8 T cells (14) survive and undergo further antigen-independent differentiation, resulting in the generation of memory CD8 T cells that have acquired three defining memory T cell properties: (i) long-term antigen-independent persistence, (ii) homeostatic proliferation in response to IL-7 and IL-15, and (iii) rapid recall responses including vigorous antigen-driven proliferation, secretion of cytokines (IFN-γ, tumor necrosis factor α, and IL-2), and acquisition of cytotoxicity (2, 12–14) (Upper). In contrast, if antigen persists past the effector stage and a chronic infection ensues (Lower) the antigen-independent phase of memory CD8 T cell differentiation does not occur and the resulting CD8 T cells do not optimally develop the three main memory T cell properties.
It will be important to determine whether the defect in IL-7 and IL-15 responsiveness by chronic memory CD8 T cells can be overcome by increasing the expression of IL-7 and IL-15 receptors alone or whether other downstream deficiencies also exist. Moreover, the failure to restore normal memory T cell differentiation of chronic memory CD8 T cells after ≈1 month in a naïve antigen-free recipient suggests that this differentiation state may not be readily reversible in the absence of antigen, perhaps because of permanent changes in gene expression or epigenetic silencing of memory T cell properties. The defect in antigen-driven proliferation demonstrated here and also observed during other chronic infections (28, 29) suggests that there may be global changes in the proliferative machinery of T cells that have experienced prolonged exposure to antigen. Memory CD8 T cells are normally poised for vigorous proliferation because of an altered G0/G1 state that favors rapid entry into cell cycle (30, 31). It will be of interest to compare the cell cycle regulation in antigen-specific T cells from chronic infection with memory T cells generated after acute infection.
During many chronic infections, including chronic LCMV (Fig. 4), virus-specific CD8 T cells often retain a CD62LLoCCR7Lo phenotype (32–34) that favors homing to nonlymphoid tissues. Therefore, it was striking that in our adoptive transfer experiments (Fig. 2C) we found ≈3- to 10-fold fewer LCMV-specific chronic memory CD8 T cells in the liver and lung compared with acute memory CD8 T cells 1 month after adoptive transfer to naïve mice (Fig. 2C). These results suggest that even in nonlymphoid organs maintenance of chronic memory T cells is inefficient compared with acute memory T cells.
The lymphoid compartment contains only a finite amount of “space” for any given lymphocyte subset, including memory CD8 T cells (35). This constraint results in a competition between memory CD8 T cells for the limiting resources involved in homeostatic maintenance of the memory T cell pool. Chief among these resources for memory CD8 T cells are IL-7 and IL-15 (4, 5, 21–24). The inefficient use of the IL-7 and IL-15 pathways by chronic memory CD8 T cells suggests that these cells will be at a competitive disadvantage with true memory T cells for antigen-independent homeostatic maintenance. Indeed, our analysis indicates that, when placed in such a competitive situation, chronic memory CD8 T cells are outcompeted by true memory CD8 T cells and the number of chronic memory CD8 T cells declines (see Fig. 2).
This study may provide a possible mechanism for the loss of T cell immunity observed in other chronic infections and cancer. A key aspect of concomitant immunity originally described for tumors was that protection from a secondary tumor challenge was maintained if the original tumor persisted, but not if it was removed (36). Similar protective immunity associated with persistent parasitic or bacterial infections has also been described. For example, the loss of protective immunity to L. major when persisting infection is eliminated (9, 10), or loss of specific T cells when antibiotics are given to bacillus Calmette–Guérin-infected mice (11), may indicate that T cells generated during these chronic infections do not acquire the memory T cell property of antigen-independent persistence. A similar situation may also occur during HIV infection. When viremia is controlled with effective antiretroviral therapy, or when antigen stimulation declines because of epitope mutation, the number of circulating HIV-specific CD8 T cells can substantially decline in chronically infected individuals (37–40). One prediction of our study is that if antigen exposure is terminated during the early stages of infection (the acute phase), T cells may experience the necessary rest from antigen stimulation to differentiate into long-lived antigen-independent memory T cells. Indeed, recent studies have shown that if antiretroviral therapy is initiated during the early phase of infection, HIV-specific CD8 T cells are more efficiently maintained (38, 39). Also, it has been reported that during latent gammaherpesvirus infection of mice virus-specific CD8 T cells appear to be maintained in an IL-15-independent manner (41). Together, these observations support the idea that prolonged antigen exposure in the context of chronic infections has a negative effect on the development of memory T cell properties, whereas a shorter duration of antigenic stimulation followed by antigen removal allows for more efficient formation of memory CD8 T cells. It remains possible, however, that aspects of chronic infections other than prolonged antigen exposure can also regulate CD8 T cell differentiation. For example, it is known that the two strains of LCMV have different tropism (19), which could impact the quality of CD8 T cell stimulation in vivo. Future studies are necessary to address these issues and will be important to evaluate how chronic antigen stimulation during different types of persisting infections and cancer can impact the acquisition of key antigen-independent memory T cell properties.
Augmenting T cell responses to persisting pathogens and tumors through cytokine therapy and therapeutic vaccination is an important goal, but the results of this study suggest that there may be limitations to these approaches. The poor responsiveness of T cells during chronic infection to γ chain cytokines, including IL-7 and IL-15, suggests that therapies based solely on these cytokines could be difficult. Similarly, therapeutic vaccination approaches that aim to provide antigen restimulation to T cells during persisting infections may have to overcome defects in proliferative potential. Adoptive immunotherapy is another approach that is proving useful for treatment of some tumors and persisting infections (42, 43). In this context, when T cells are highly stimulated and expanded in vitro it may be important to determine the capacity of the transferred cells to acquire antigen-independent memory T cell properties. Assessing the expression levels of IL-7 and/or IL-15 receptors or the responsiveness to IL-7 and IL-15 may be one approach to monitor formation of memory T cells in this setting. Rest from antigenic stimulation is a critical requirement for efficient completion of normal memory T cell differentiation. Antiviral therapy or chemotherapy for cancer may provide rest from T cell antigen receptor stimulation and allow partial recovery of some memory T cell functions. Perhaps by combining drug treatment with appropriate therapeutic vaccination or cytokine therapies loss of T cell memory can be prevented and more effective long-term protective immunity from reencounter or reemergence of a pathogen or tumor can be established.
Supplementary Material
Acknowledgments
We thank members of the Ahmed laboratory for technical support and scientific input. This work was supported by grants from the National Institutes of Health (Grant AI30048 to R.A.), the Damon Runyon–Walter Winchell Cancer Research Fund and the Burroughs Wellcome Fund (to S.M.K.), and the Cancer Research Institute (to E.J.W).
Author contributions: E.J.W. and R.A. designed research; E.J.W., D.L.B., S.M.K., and J.N.B. performed research; E.J.W. and R.A. analyzed data; and E.J.W. and R.A. wrote the paper.
Abbreviations: LCMV, lymphocytic choriomeningitis virus; CFSE, carboxyfluorescein diacetate succinimidyl ester; PBMC, peripheral blood mononuclear cells; p.i., postinfection.
References
- 1.Kaech, S. M., Wherry, E. J. & Ahmed, R. (2002) Nat. Rev. Immunol. 2, 251–262. [DOI] [PubMed] [Google Scholar]
- 2.Grayson, J. M., Zajac, A. J., Altman, J. D. & Ahmed, R. (2000) J. Immunol. 164, 3950–3954. [DOI] [PubMed] [Google Scholar]
- 3.Jameson, S. C. (2002) Nat. Rev. Immunol. 2, 547–556. [DOI] [PubMed] [Google Scholar]
- 4.Becker, T. C., Wherry, E. J., Boone, D., Murali-Krishna, K., Antia, R., Ma, A. & Ahmed, R. (2002) J. Exp. Med. 195, 1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldrath, A. W., Sivakumar, P. V., Glaccum, M., Kennedy, M. K., Bevan, M. J., Benoist, C., Mathis, D. & Butz, E. A. (2002) J. Exp. Med. 195, 1515–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tawfik, A. F. & Colley, D. G. (1986) Am. J. Trop. Med. Hyg. 35, 110–117. [DOI] [PubMed] [Google Scholar]
- 7.Moloney, N. A., Hinchcliffe, P. & Webbe, G. (1987) Trans. R. Soc. Trop. Med. Hyg. 81, 247–254. [DOI] [PubMed] [Google Scholar]
- 8.van Rie, A., Warren, R., Richardson, M., Victor, T. C., Gie, R. P., Enarson, D. A., Beyers, N. & van Helden, P. D. (1999) N. Engl. J. Med. 341, 1174–1179. [DOI] [PubMed] [Google Scholar]
- 9.Uzonna, J. E., Wei, G., Yurkowski, D. & Bretscher, P. (2001) J. Immunol. 167, 6967–6974. [DOI] [PubMed] [Google Scholar]
- 10.Belkaid, Y., Piccirillo, C. A., Mendez, S., Shevach, E. M. & Sacks, D. L. (2002) Nature 420, 502–507. [DOI] [PubMed] [Google Scholar]
- 11.Dudani, R., Chapdelaine, Y., van Faassen, H., Smith, D. K., Shen, H., Krishnan, L. & Sad, S. (2002) J. Immunol. 168, 5737–5745. [DOI] [PubMed] [Google Scholar]
- 12.Kaech, S. M., Hemby, S., Kersh, E. & Ahmed, R. (2002) Cell 111, 837–851. [DOI] [PubMed] [Google Scholar]
- 13.Wherry, E. J., Teichgraber, V., Becker, T. C., Masopust, D., Kaech, S. M., Antia, R., von Andrian, U. H. & Ahmed, R. (2003) Nat. Immunol. 4, 225–234. [DOI] [PubMed] [Google Scholar]
- 14.Kaech, S. M., Tan, J. T., Wherry, E. J., Konieczny, B. T., Surh, C. D. & Ahmed, R. (2003) Nat. Immunol. 4, 1191–1198. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed, R., Salmi, A., Butler, L. D., Chiller, J. M. & Oldstone, M. B. (1984) J. Exp. Med. 160, 521–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zajac, A. J., Blattman, J. N., Murali-Krishna, K., Sourdive, D. J., Suresh, M., Altman, J. D. & Ahmed, R. (1998) J. Exp. Med. 188, 2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wherry, E. J., Blattman, J. N., Murali-Krishna, K., van der Most, R. & Ahmed, R. (2003) J. Virol. 77, 4911–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matloubian, M., Concepcion, R. J. & Ahmed, R. (1994) J. Virol. 68, 8056–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matloubian, M., Kolhekar, S. R., Somasundaram, T. & Ahmed, R. (1993) J. Virol. 67, 7340–7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed, R., King, C. C. & Oldstone, M. B. (1987) J. Virol. 61, 1571–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schluns, K. S., Kieper, W. C., Jameson, S. C. & Lefrancois, L. (2000) Nat. Immunol. 1, 426–432. [DOI] [PubMed] [Google Scholar]
- 22.Kieper, W. C., Tan, J. T., Bondi-Boyd, B., Gapin, L., Sprent, J., Ceredig, R. & Surh, C. D. (2002) J. Exp. Med. 195, 1533–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan, J. T., Ernst, B., Kieper, W. C., LeRoy, E., Sprent, J. & Surh, C. D. (2002) J. Exp. Med. 195, 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schluns, K. S., Williams, K., Ma, A., Zheng, X. X. & Lefrancois, L. (2002) J. Immunol. 168, 4827–4831. [DOI] [PubMed] [Google Scholar]
- 25.Dubois, S., Mariner, J., Waldmann, T. A. & Tagaya, Y. (2002) Immunity 17, 537–547. [DOI] [PubMed] [Google Scholar]
- 26.Lodolce, J. P., Burkett, P. R., Boone, D. L., Chien, M. & Ma, A. (2001) J. Exp. Med. 194, 1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schluns, K. S. & Lefrancois, L. (2003) Nat. Rev. Immunol. 3, 269–279. [DOI] [PubMed] [Google Scholar]
- 28.Migueles, S. A., Laborico, A. C., Shupert, W. L., Sabbaghian, M. S., Rabin, R., Hallahan, C. W., Van Baarle, D., Kostense, S., Miedema, F., McLaughlin, M., et al. (2002) Nat. Immunol. 3, 1061–1068. [DOI] [PubMed] [Google Scholar]
- 29.Brenchley, J. M., Karandikar, N. J., Betts, M. R., Ambrozak, D. R., Hill, B. J., Crotty, L. E., Casazza, J. P., Kuruppu, J., Migueles, S. A., Connors, M., et al. (2003) Blood 101, 2711–2720. [DOI] [PubMed] [Google Scholar]
- 30.Veiga-Fernandes, H. & Rocha, B. (2004) Nat. Immunol. 5, 31–37. [DOI] [PubMed] [Google Scholar]
- 31.Latner, D. R., Kaech, S. M. & Ahmed, R. (2004) J. Virol. 78, 10953–10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Champagne, P., Ogg, G. S., King, A. S., Knabenhans, C., Ellefsen, K., Nobile, M., Appay, V., Rizzardi, G. P., Fleury, S., Lipp, M., et al. (2001) Nature 410, 106–111. [DOI] [PubMed] [Google Scholar]
- 33.Papagno, L., Spina, C. A., Marchant, A., Salio, M., Rufer, N., Little, S., Dong, T., Chesney, G., Waters, A., Easterbrook, P., et al. (2004) PLoS Biol. 2, 173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urbani, S., Boni, C., Missale, G., Elia, G., Cavallo, C., Massari, M., Raimondo, G. & Ferrari, C. (2002) J. Virol. 76, 12423–12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freitas, A. A. & Rocha, B. (2000) Annu. Rev. Immunol. 18, 83–111. [DOI] [PubMed] [Google Scholar]
- 36.Gershon, R. K., Carter, R. L. & Kondo, K. (1967) Nature 213, 674–676. [DOI] [PubMed] [Google Scholar]
- 37.Casazza, J. P., Betts, M. R., Picker, L. J. & Koup, R. A. (2001) J. Virol. 75, 6508–6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oxenius, A., Price, D. A., Easterbrook, P. J., O'Callaghan, C. A., Kelleher, A. D., Whelan, J. A., Sontag, G., Sewell, A. K. & Phillips, R. E. (2000) Proc. Natl. Acad. Sci. USA 97, 3382–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alter, G., Hatzakis, G., Tsoukas, C. M., Pelley, K., Rouleau, D., LeBlanc, R., Baril, J. G., Dion, H., Lefebvre, E., Thomas, R., et al. (2003) J. Immunol. 171, 477–488. [DOI] [PubMed] [Google Scholar]
- 40.Draenert, R., Verrill, C. L., Tang, Y., Allen, T. M., Wurcel, A. G., Boczanowski, M., Lechner, A., Kim, A. Y., Suscovich, T., Brown, N. V., et al. (2004) J. Virol. 78, 630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Obar, J. J., Crist, S. G., Leung, E. K. & Usherwood, E. J. (2004) J. Immunol. 173, 2705–2714. [DOI] [PubMed] [Google Scholar]
- 42.Dudley, M. E. & Rosenberg, S. A. (2003) Nat. Rev. Cancer 3, 666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho, W. Y., Blattman, J. N., Dossett, M. L., Yee, C. & Greenberg, P. D. (2003) Cancer Cell 3, 431–437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.