Abstract
Upper airway muscles subserve many essential for survival orofacial behaviors, including their important role as accessory respiratory muscles. In the face of certain predisposition of craniofacial anatomy, both tonic and phasic inspiratory activation of upper airway muscles is necessary to protect the upper airway against collapse. This protective action is adequate during wakefulness, but fails during sleep which results in recurrent episodes of hypopneas and apneas, a condition known as the obstructive sleep apnea syndrome (OSA). Although OSA is almost exclusively a human disorder, animal models help unveil the basic principles governing the impact of sleep on breathing and upper airway muscle activity. This article discusses the neuroanatomy, neurochemistry, and neurophysiology of the different neuronal systems whose activity changes with sleep-wake states, such as the noradrenergic, serotonergic, cholinergic, orexinergic, histaminergic, GABAergic and glycinergic, and their impact on central respiratory neurons and upper airway motoneurons. Observations of the interactions between sleep-wake states and upper airway muscles in healthy humans and OSA patients are related to findings from animal models with normal upper airway, and various animal models of OSA, including the chronic-intermittent hypoxia model. Using a framework of upper airway motoneurons being under concurrent influence of central respiratory, reflex and state-dependent inputs, different neurotransmitters, and neuropeptides are considered as either causing a sleep-dependent withdrawal of excitation from motoneurons or mediating an active, sleep-related inhibition of motoneurons. Information about the neurochemistry of state-dependent control of upper airway muscles accumulated to date reveals fundamental principles and may help understand and treat OSA.
Introduction
Upper airway muscles play a key role in such important orofacial behaviors as food and fluid ingestion, processing and swallowing, coughing, sneezing, and phonation (39). Under certain physiologic and pathophysiologic conditions, due to their activation having an impact on upper airway patency and stiffness of upper airway walls, upper airway muscles assume very important respiratory function; accordingly, they are classified as accessory respiratory muscles. Accessory, because they do not directly drive movement of air in and out of the lungs, but can importantly facilitate, or impede, this process by changing the resistance of the upper airway.
The effects of sleep on the patency of upper airway have been a subject of major interest due to the high prevalence of respiratory disorders that occur exclusively during sleep; in particular, the obstructive sleep apnea/hypopnea syndrome (OSA). Estimates of the prevalence of OSA in industrialized countries report 5% to 20% of the adult population. The disorder, albeit not acutely lethal, contributes to, or exacerbates, such major cardiovascular disorders as arterial hypertension and stroke. Furthermore, through a chronic disruption of sleep as well as the chronic nocturnal recurrence of hypoxia, OSA increases the probability of driving and workplace accidents and exacerbates all components of the metabolic syndrome (diabetes, obesity, and hypertension). It is through these indirect effects that OSA causes premature mortality (421, 610) and represents a major health problem on par with cancer and degenerative disorders of aging.
OSA patients experience major disruptions of breathing only during sleep, whereas during wakefulness they are fully capable of generating adequate ventilation. From this, it follows that the central neural effects of the state(s) of sleep alter the control of breathing in a manner that leads to OSA. Indeed, OSA is caused by a combination of predisposing anatomical factors and sleep-related decrements of activity in upper airway muscles (435). The anatomical factors predisposing to OSA and the chronic presence of the disorder distinctly modify the magnitude and pattern of activity generated in upper airway muscles across sleep-wake states when compared to the activity observed in humans and animals with a patent upper airway. To fully understand the adaptive and maladaptive processes related to OSA, it is important to first understand how the states of sleep interact with the central control of breathing in healthy subjects in which this fundamental interaction is not complicated by the disorder. With this in mind, a major focus of this overview article is on the neuroanatomy, neurophysiology, and neurochemistry of the interaction between the central neural systems controlling respiratory muscles and those responsible for the generation of sleep-wake states under the normal, physiologic conditions. This knowledge is then used as a reference for considerations of the impact of OSA on the central neural control of the upper airway across sleep-wake states.
The onset of sleep is associated with a reduction in upper airway patency and increase in respiratory resistance, an effect that is not clinically significant, but nevertheless detectable in healthy humans and that is dramatically enhanced in snorers and OSA patients (e.g., 20, 66, 248, 312, 375, 435, 464, 592, 601). The pattern and magnitude of activity in upper airway muscles is but one of several factors that determine the cross-sectional area and resistance of the upper airway. The other major factors are the mechanical properties of the airway walls, head position, pressure, and flow gradients generated during breathing in different compartments of the respiratory tract, and adhesive forces between the inner surfaces of upper airway walls. Within this scheme, electromyographic (EMG) studies of upper airway muscles are important because they provide an insight into the central interaction between the control of sleep and control of breathing. They also allow one to relate observations in humans to data from animal models in which one can experiment with the underlying mechanisms in a manner not possible in human subjects. Therefore, neurophysiological and neuropharmacological studies of activity in respiratory motoneurons that innervate upper airway muscles, central respiratory neurons, and behavioral state-controlling neurons are a major focus of this article. Complementary topics pertaining to the neuromechanical basis of OSA and its clinical and translational aspects have been reviewed in other recent overview articles (100, 206, 207, 586).
Upper Airway Muscles and Their Activity Patterns
Locations, respiratory roles, and sources of motor innervation
The pharynx lacks the rigid supporting structures like those that surround the upper airway in the nasal and laryngeal segments. In the pharynx, three distinct soft tissue elements—the posterior pharyngeal wall, the soft palate, and the base of the tongue—are pulled inward and toward each other by the centripetal force generated by the negative pressure during inspiration (Fig. 1). As the base of the tongue and the posterior pharyngeal walls are pulled toward each other, the soft palate moves to fill the space between them. This reduces the airway cross-sectional area and facilitates airway obstruction. Consequent to this mechanical arrangement, upper airway obstructions occur most commonly in the pharynx (77, 469, 489), although the specific site of highest vulnerability is variable and patient-specific (216).
Figure 1.
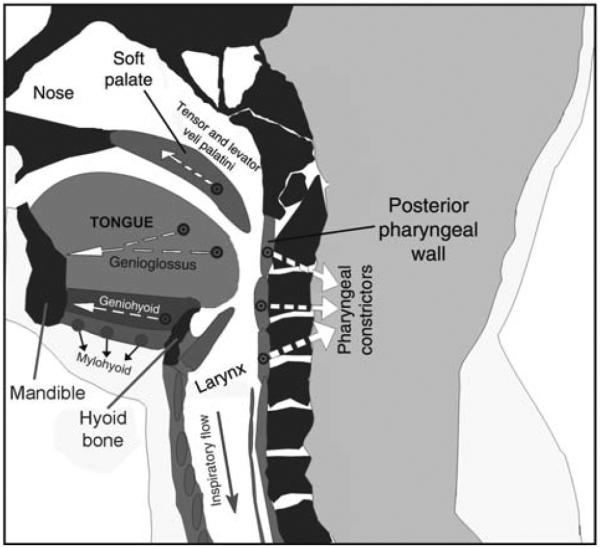
Schematic representation of a sagittal cross-section through the upper airway. During inspiration, negative intraluminar pressure pulls three soft tissue elements, the tongue, posterior pharyngeal walls, and soft palate, toward each, thereby reducing the airway lumen in the pharyngeal region. This airway-collapsing action is opposed by pharyngeal dilator muscles, including the genioglossus, geniohyoid, and tensor and levator veli palatini. Additionally, activation of the pharyngeal constrictors stiffens the airway walls. Arrows show approximate directions of the forces exerted during contraction of these major pharyngeal muscles. Image based on a scan of the upper airway in an OSA patient—courtesy of Dr. Richard J. Schwab at the University of Pennsylvania. (Modified from Fig. 1 in Ref. 270 and republished with permission from Informa Healthcare, a member of the Taylor and Francis Group, obtained via the Copyright Clearance Center, Inc.)
Opposing the centripetal effects of tissue pressure and forces generated during inspiration are the forces generated by those muscles of the upper airway that can stiffen the pharyngeal walls and pull them away from the airway lumen (dilate the airway). Some of the most important upper airway muscles performing this function are shown in Figure 1, and Table 1 lists all major upper airway muscles whose contraction impacts upper airway patency together with the origins of their motor innervation, their predominant action on airway resistance, and the typical patterns of their activity during quiet wakefulness. Due to their importance for the maintenance of upper airway patency, the muscles with airway dilatory function, such as the genioglossus (GG), geniohyoid or tensor and levator veli palatini, have been studied more extensively than airway constrictors. Of those, the GG innervated by hypoglossal (XII) motoneurons has been explored most extensively and in many species, as it is the largest and most accessible of airway dilators. Despite extensive body of research, the impact on the size and stiffness of the upper airway of many lesser muscles has not been studied (Table 1). Nevertheless, it is of note that muscle fibers of the tongue can be functionally classified as causing a tongue-protrusive or tongue-retractive action, and these functional types have been related to predominantly inspiratory or predominantly expiratory patterns of activity (e.g., 109, 333, 453, 556, 597). However, data indicate that protrusor and retractor muscles are simultaneously, rather than reciprocally, activated when they act to protect the airway from collapse, and such an action both stiffens the airway walls and enlarges the airway lumen (159). Data also indicate that, while GG activation correlates well with the maintenance of upper patency and with a reduction of upper airway resistance when the airway is at least partially open during wakefulness and sleep, it is often insufficient to reopen the airway when a full collapse occurs. This indicates that activation of upper airway muscles other than GG is important for the restoration and maintenance of upper airway patency during sleep and that such muscles are not activated during sleep as readily as the GG (e.g., 74, 111). In this context, it is of note that the muscles that pull the airway downward and are innervated by upper cervical motoneurons (e.g., sternohyoid and sternothyroid) also protect the upper airway against obstructions by increasing the distance between the soft palate and the base of the tongue (440, 442, 521).
Table 1.
Major Orofacial Muscles Whose Contraction Affects the Lumen and Patency of the Upper Airway
| Muscle name (cranial nerve providing motor innervation) | Presumed action on upper airway; (bold indicates airway-dilating action) | Typical pattern of activity during wakefulness (I, inspiratory; E, expiratory; t, tonic) | References describing sleep-wake activity in different species (hh, healthy humans; OSAh-, OSA patients; c, cat; d, dog; g, goat; l, lamb; r, rat) |
|---|---|---|---|
| Alae nasi (VII) | Widens nares | I + t | hh: 582,591 |
| E + t | r: 102,491 | ||
| Tensor veli palatini (V) | Moves soft palate up | t (+ I) | hh: 375,541,601 |
| t (+I) | OSAh: 20,74 | ||
| Levator veli palatini (V) | Moves soft palate up | I + t (or silent) | hh: 465,542 |
| Palatopharyngeus (XI) | Moves soft palate down; pharynx up | ||
| Palatoglossus (XII) | Moves tongue up and posterior | I + t | hh: 542 |
| Styloglossus (XII) | Moves tongue up and posterior | t (+ I) | OSAh: 111 |
| Genioglossus (XII) | Moves tongue down and anterior | I + t | hh: 35, 40, 91,280,464,511,541,592,601 |
| I + t | OSAh: 20, 74,111,120,246,247,346,378,465 | ||
| I + t | c: 188,437 | ||
| I + t | d: 225,414,455 | ||
| I + t | g: 400,401 | ||
| I + t or t | r: 82,233,316,340,360,446,520 | ||
| Hyoglossus (XII) | Moves tongue down and posterior | t | OSAh: 111 |
| Stylopharyngeus (IX) | Moves up and widens pharynx | ||
| Pharyngeal constrictors (X) | Stiffen posterior pharyngeal wall; reduce pharyngeal lumen; move hyoid bone posterior | E or silent | hh: 465 |
| E | OSAh: 281 | ||
I 
|
r: 493 | ||
| Digastric anterior (VII) | Moves hyoid bone anterior | t | c: 407 |
| Geniohyoid (XII) | Moves tongue down; hyoid bone up | I + t | hh: 589,590 |
| t | r: 446 | ||
| Mylohyoid (V) | Stiffens floor of the mouth | ||
| Omohyoid (C1-3 via XII) | Moves hyoid bone down | ||
| Thyrohyoid (C1-3 via XII) | Brings closer hyoid bone and thyroid cartilage | ||
| Sternohyoid (C1-3 via XII) | Moves hyoid bone down | I | d: 191 |
| I | r: 341,493 | ||
| Sternothyroid (C1-3 via XII) | Moves thyroid cartilage down | I | r: 341 |
| Posterior cricoarytenoid (X) | Abducts vocal folds | I + post-I +t | hh: 282 |
| I + t | c: 188 | ||
| I + t | l: 253 | ||
| I + t | r: 342,492 | ||
| Lateral cricoarytenoid (X) | Adducts vocal folds | E | r: 342,492 |
| Arytenoid (X) | Adducts vocal folds | E | hh: 278 |
| I/E + t | r: 342 | ||
| Thyroarytenoid (X) | Relaxes vocal folds | E + t | hh: 279 |
| I or silent | l: 253 | ||
| E | r: 342 | ||
| Cricothyroid (X) | Tenses vocal folds | I + t | hh: 284 |
| I + t | l: 253 | ||
| I or I/E | r: 342,492 |
Note. Muscles are listed together with the source of their innervation, possible role in the control of upper airway patency, prevailing pattern of spontaneous activity during quiet wakefulness, and references describing their sleep-wake activity in different species.
While sleep-related upper airway obstructions do not typically occur in the nasal or laryngeal portion of the airway, these compartments determine the magnitude of the negative pressure generated in the pharyngeal region by acting as pressure dividers. Normally, nasal resistance is about half the total airway resistance (419). Since the walls of the nasal passages are relatively noncompliant, neuromuscular effects on nasal resistance are small (582). However, pressure drop along the nasal compartment affects the level of negative pressure within the relatively compliant and collapsible pharyngeal compartment. An increase in nasal resistance (e.g., caused by mucosal swelling, congestion, or reduced local vasoconstrictory activity) will increase the magnitude of negative inspiratory pressure generated in the pharynx. This, in turn, will facilitate a collapse of the pharyngeal walls.
In contrast to the nasal airway, dynamic changes in neuromuscular activity in the larynx may have large effects on upper airway resistance (see 38 for a review). Many laryngeal muscles have been studied relatively extensively, with the studies often being motivated by an important role of the larynx in protecting the airway during ingestive behaviors, or in actively blocking the airway under certain pathophysiologic conditions (e.g., larygospasm). However, active laryngoconstriction is not a part of the clinical picture in OSA. Rather, the larynxes (and the nose) are important sources of afferent information for the reflex control of all upper airway muscles. This is because the afferent information from receptors located in the nose and larynx has more prominent reflex effects on upper airway muscle activity than that from pharyngeal receptors (219,329,449; reviewed in 462,588). Thus, the segment of the upper airway where obstructions are most likely to occur is flanked by more rigid compartments that provide sensory information important for the reflex control of upper airway muscle activity.
Respiratory-modulated and tonic components of upper airway muscle activity
Upper airway muscle activity during sleep is often quantified relative to that observed during the preceding period of quiet wakefulness, but the methodology used to obtain and scale EMG measurements varies among studies. In studies in humans, it is common to scale the integrated EMG activity recorded during sleep and wakefulness relative to the amount of activity generated by the same subject during maximal voluntary activation (e.g., 345). This approach reveals that the total magnitude of combined tonic and respiratory-phasic baseline activity in upper airway muscles recorded during quiet wakefulness from healthy human subjects is very low (e.g., 67,246,278,280,455,541,576,601). In different studies and subjects, it ranged between 1% and 11% of the peak voluntarily generated activity (e.g., 247,346,510,541,576). With the baseline level of activity being low, accurate determination of the level of recorded signal at which recording noise ends and genuine activity starts is of the essence. To date, no commonly accepted procedures of measuring and subtracting baseline noise from EMG signals recorded from upper airway muscles in humans have been established.
Voluntarily generated maximal activation cannot be used to scale EMG recordings in animal models. Therefore, one approach to scale the records has been to measure the average level of activity over extended periods of undisturbed wakefulness. Multiple periods with no EMG activity also were identified, which allowed one to measure the level of integrated signal corresponding to the level of electrical noise only. With these two measurements established, one can quantify EMG across sleep-wake states (e.g., 316). The approach has been validated in experiments in which upper airway EMG activity levels were compared across multiple recording sessions within a subject and across different subjects (445,446). Unfortunately, the methods of EMG acquisition, processing, and quantification currently differ greatly among laboratories which makes comparison of the results is difficult. With high-quality and long-term digital acquisition of EMG signals now being widely available, it should be possible to further standardize the methods of EMG measurements.
In humans, some tonic activity and a degree of inspiratory modulation are frequently present in EMG records from GG and other pharyngeal muscles during both quiet wakefulness and nonrapid eye movement (NREM) sleep (e.g., 192, 452, 464, 541). In different muscles the tonic and phasic components are expressed to variable degrees and with a pattern characteristic of that muscle. For example, during eupneic breathing in wakefulness, tensor veli palatini, and digastricus show only low level tonic, or no, activity but respiratory modulation appears in response to hypercapnia or airway occlusion (346, 374, 541, 576). In healthy humans, the combined phasic inspiratory and tonic components represent less than 2% of maximal spontaneous GG activity (247). Humans who do not meet the clinical criteria for OSA despite having smaller than normal upper airway cross-sectional area produce larger anterior movements of the tongue during inspiration than other control subjects (90). This suggests that, at least in humans studied during wakefulness, a subclinical reduction of upper airway patency elicits a compensatory activation of upper airway dilating muscles during inspiration. In rats, measurable levels of inspiratory modulation of GG activity were reported at normocapnic levels in some studies (e.g., 233, 341, 358), but in other studies inspiratory modulation of lingual EMG occurred intermittently and more often during deep NREM sleep than during any other sleep or wakefulness state (315, 520). GG activity also was minimal or absent during quiet wakefulness in dogs (455).
Several studies suggested that the magnitude of respiratory modulation of the tongue EMG in humans varies with the recording site (119, 464). This has been recently confirmed in healthy humans (576), but was not observed in rats (315). The magnitude of the total, or only the respiratory-modulated component, of lingual muscle activity in awake healthy humans, rats, and cats depends on the head position, being increased with ventral flexion or when the animal assumes a curled position (52, 341, 370, 475). This positional influence is only partially dependent on reflex stimulation of upper airway muscle tone by upper airway mechanoreceptors sensitive to negative upper airway pressure (52). In healthy humans, GG activity is higher in the supine than in the upright position which is, at least in part, due to gravitational effects on the tongue (112, 475, 576).
Origins of respiratory modulation of upper airway muscle activity
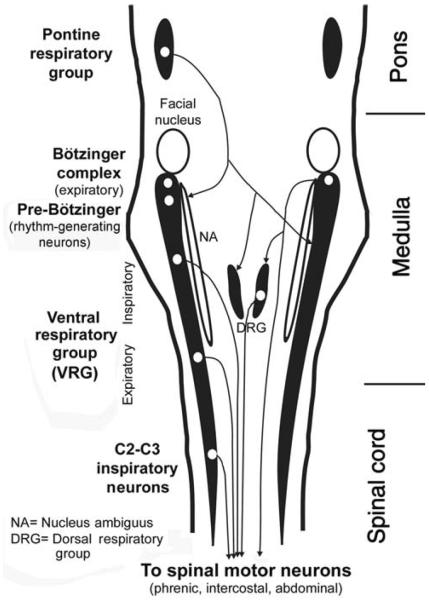
The central pattern generators for all essential rhythmic behaviors, such as suckling, mastication/swallowing and breathing, reside within the medulla and pons. The key site for the respiratory rhythm has been localized bilaterally at a site within the rostral, ventrolateral medulla oblongata, and it is referred to as the pre-Bötzinger complex (Fig. 2). Neurons producing rhythmic respiratory activity are present at this site and their rhythmic activity persists even under in vitro conditions and when synaptic connectivity is blocked by pharmacological means (231,241,383,504). This suggests that, at least in neonate mammals, the basic respiratory rhythm is generated by pacemaker neurons. Many respiratory neurons of the pre-Bötzinger region express neurokinin 1 (NK1) receptors that mediate excitatory effects of substance P (SP). Selective chemical destruction of NK1 receptor-expressing neurons in the pre-Bötzinger region causes irregular breathing with long apneic episodes. Depending on the neurochemical nature of lesioned cells, disruption of the respiratory rhythm occurs mainly during sleep or wakefulness (337, 540).
Figure 2.
Distribution of the major groups of respiratory neurons in the brainstem and upper spinal cord, as seen in a dorsal view. Brainstem respiratory neurons form longitudinal columns, of which the most prominent one is the VRG located in the ventrolateral medullary reticular formation. The rostralmost part of the VRG, the Bötzinger complex, contains mainly late expiratory neurons. Next region caudally, the pre-Bötzinger complex, contains pacemaker neurons that, at least in neonatal animals, are capable of producing basic respiratory rhythm under in vitro conditions. Farther caudal is a large group of mainly inspiratory-modulated neurons, of which many send axons to spinal motoneurons that innervate the diaphragm and external intercostal muscles. Nucleus ambiguus runs parallel to this part of VRG and contains cell bodies of laryngeal motoneurons. Caudal to the inspiratory part of the VRG is an expiratory region whose neurons send axons to spinal expiratory motoneurons that control internal intercostal and abdominal muscles. A spinal extension of the VRG, the C2 and C3 group, again contains inspiratory neurons whose function may be to reinforce the actions of the VRG. The dorsal respiratory group is located in the viscerosensory nucleus of the solitary tract. It contains mostly inspiratory-modulated neurons, of which some have connections with spinal motoneurons and some receive input from pulmonary and laryngeal receptors. The pontine respiratory group located in the dorsolateral pons comprises cells with different patterns of respiratory modulation. These neurons integrate peripheral and central respiratory and nonrespiratory inputs and have descending projections to medullary respiratory neurons. (Modified from Fig. 2 in Ref. 266 and republished with permission from the American Academy of Sleep Medicine.)
In adult mammals, the respiratory rate and the pattern of activity in different respiratory muscles are determined by complex synaptic interactions among different types of respiratory-modulated and nonrespiratory neurons (see 14,114,129,425 for reviews). This activity is also under continuous reflex control from a host of peripheral and central receptors, including the sensors of blood and brain tissue O2, CO2, and pH, and lung and airway mechanoreceptors (reviewed in 128, 133, 176, 268, 297). The central respiratory activity is shaped by reflexes and then differentially distributed to motoneurons that innervate the inspiratory pump muscles (diaphragm and external intercostals), expiratory pump muscles (internal intercostal and abdominal), and accessory respiratory muscles of the upper airway.
Based on single-cell recordings and differential effects of various experimental manipulations, three phases are distinguished within the respiratory cycle: inspiration, postinspiratory period, and late-expiratory period (426). Mechanically, these three phases correspond to the period of active inhalation, passive exhalation whose rate is limited by postinspiratory activity, and active expiration, respectively. Central respiratory neurons are classified on the basis of timing of their activity relative to the three phases of the respiratory cycle. During wakefulness, respiratory-modulated neurons are found in many brain regions (395, 566). However, during NREM sleep or under anesthesia, respiratory modulation is found at relatively few locations, and cells with similar activity patterns aggregate in distinct groups or columns (107,114,129,391,488,496). In a dorsal view of the pons, medulla, and upper spinal cord, respiratory-modulated neurons occur bilaterally in columns located in the ventrolateral medullary reticular formation (Fig. 2). The Bötzinger complex, a site containing late-expiratory and phase-spanning neurons, is located in the rostralmost end, followed by the pre-Bötzinger complex, the ventral respiratory group (VRG), with inspiratory neurons in its rostral part and expiratory neurons in its caudal part, and the upper cervical inspiratory group that extends to the third segment of the spinal cord. The dominant neuronal types in the successive segments of the VRG differ both in regard to their activity patterns and efferent projections. Some are involved in the generation of the respiratory rhythm and/or relaying it to cranial respiratory-modulated motoneurons (trigeminal, facial, XII, and vagal), while others have axons that descend into the spinal cord. Parallel, and adjacent to, the VRG runs a column of vagal motoneurons (nucleus ambiguus) that innervate laryngeal and posterior pharyngeal muscles. Another important group of mainly inspiratory neurons is referred to as the dorsal respiratory group (DRG) and it is located in the ventrolateral portion of the nucleus of the solitary tract of the dorsomedial medulla. The pontine respiratory group is located in the parabrachial region of the dorsolateral pons and it contains neurons with diverse patterns of activity. The pontine group is not necessary for generation of the basic respiratory rhythm but it plays an important role in the development and adaptation of the respiratory system to changes in the external and internal environments (118). A major portion of respiratory drive to spinal respiratory motoneurons that innervate the diaphragm, intercostal and abdominal muscles originates in the VRG and DRG, with additional contributions from the parabrachial region of the pons (see 14, 114, 129, 133 for reviews).
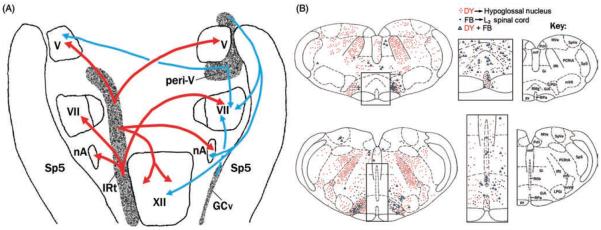
In contrast to extensive studies of bulbospinal respiratory neurons that transmit respiratory drive to spinal motoneurons, the location and characteristics of the neurons that provide respiratory drive to upper airway motoneurons are not well established. Retrograde tracing studies indicate that the XII, trigeminal, and facial motor nuclei all receive their main afferent innervation from just a few distinct pontomedullary sites (109, 132, 304, 305, 322, 557). Figure 3 shows data from two retrograde tracing studies that delineated the main sites of origin of projections to the XII and other orofacial motor nuclei. These sites include the intermediate medullary reticular region (IRt), the ventral and ventromedial medullary reticular areas, the medullary raphé nuclei, and the reticular region surrounding the trigeminal motor nucleus in the pons. According to one study (551), only the IRt region has strong and bilateral projections to all orofacial motor nuclei, whereas the premotor cells of the peritrigeminal region and ventromedial medullary gigantocellular region typically have unilateral projections (Fig. 3A). However, other tracing studies report considerable bilateral projections from the ventromedial medulla to the XII motor nucleus (323,552). Many cells located in the caudal half of the IRt region extending between the ventrolateral border of the XII nucleus and the VRG have phasic-inspiratory activity and provide inspiratory drive to XII motoneurons (262, 309, 384, 408, 600). The rostral extension of the IRt region is important for the transmission of reflexes from upper airway pressure receptors to XII motoneurons and less so for the transmission of central inspiratory drive (80). Strong and bilateral efferent projections from the IRt region to all orofacial motor nuclei (Fig. 3A) have been interpreted as appropriate for coordination of activity in different orofacial muscles during ingestive behaviors (551). Some cells in the ventral and ventromedial medullary reticular formation have divergent projections to the XII nucleus and the lumbar spinal cord (Fig. 3B), which suggests their involvement in state-dependent regulation of muscle tone. Recent recordings from a subpopulation of such cells identified as synthesizing the inhibitory gamma-aminobutyric acid (GABA) revealed that they are activated during both REM sleep and ingestive behaviors occurring during wakefulness (579).
Figure 3.
Major sources of pontomedullary afferent projections to the orofacial motor nuclei. (A) Schematic dorsal view of the most prominent afferent pathways common to all orofacial motor nuclei. The medullary IRt is the main source of bilateral projections to the trigeminal (V), facial (VII), hypoglossal (XII), and ambiguus (nA) motor nuclei (red arrows). The projections to these motor nuclei tend to exhibit a distinct rostrocaudal pattern. The projections to orofacial motor nuclei that originate in the pontine reticular region surrounding the trigeminal motor nucleus (peri-V), and the ventrolateral medullary gigantocellular region (GCv) tend to be unilateral (blue arrows). (Fig. 12 from Ref. 551 colorized and republished with permission from John Wiley and Sons obtained via the Copyright Clearance Center, Inc.) (B) Major medullary sources of afferent projections to the XII nucleus in relation to the descending spinal collaterals of selected XII premotor neurons, as revealed by large retrograde tracer injections into the XII motor nucleus and ventral horns of the lumbar (L2) spinal cord. The two medullary levels illustrated are located rostral to the XII nucleus. Consistent with data in A, the IRt region contains the largest number of neurons retrogradely labeled with Diamidino Yellow (DY) tracer from the XII nucleus (red dots). Notably, no cells retrogradely labeled with Fast Blue (FB) tracer from the spinal cord (black dots) are located in this region. Additional medullary XII premotor neurons are located along the midline (enlarged images in the middle), the gigantocellular region pars α (GiA), and the lateral paragigantocellular (LPGi) region. A small fraction of cells in these medial and ventral locations has divergent projections to the XII nucleus and lumbar spinal cord (blue triangles). The key to anatomical regions is shown on the right. (Modified from Fig. 3B in Ref. 323 and republished with permission from John Wiley and Sons obtained via the Copyright Clearance Center, Inc.) Additional abbreviations in A and B: Gi, gigantocellular reticular region; mlf, medial longitudinal fasiculus; MVe, medial vestibular nucleus; PCRtA, parvicellular reticular area; PrH, nucleus prepositus hypoglossi; py, pyramidal tract; RMg, Rob, Rpa, raphé magnus, pallidus, and obscurus nuclei; Sp5, spinal trigeminal sensory nucleus; SpVe, spinal vestibular nucleus.
Many anatomically identified XII premotor cells located in the IRt region are either glutamatergic or cholinergic (553, 572). Indeed, the inspiratory drive from central respiratory neurons to XII motoneurons is mediated by glutamate through non-NMDA and, to a lesser extent, NMDA receptors (164, 222, 513, 514).
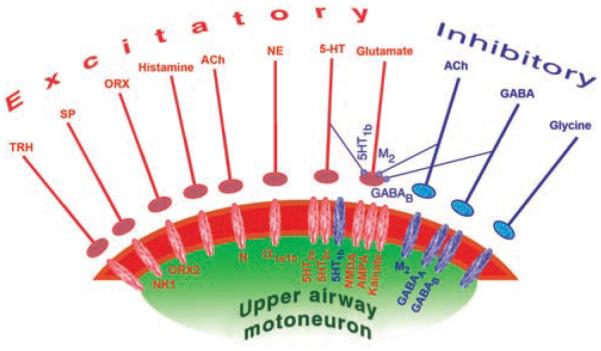
As will be discussed in subsequent sections, brainstem cells that release norepinephrine (NE), serotonin (5-HT), and acetylcholine (ACh) have activity patterns that predictably vary across the different sleep-wake states. This gives them the ability to modulate the activity of central respiratory neurons and respiratory motoneurons in a state-dependent manner. Neuromodulatory actions of NE can be mediated by three major subclasses of receptors (α1-, α2-, and β-adrenoceptors), and 5-HT may use seven different receptors (designated as type 1 through 7). Within these major classes of receptors, further subdivisions have been distinguished based on their pharmacology and genetic identity. The effects of ACh can be mediated by muscarinic (M) and nicotinic (N) receptors. There are five known members of the M receptor family, M1 through M5, all functioning as G protein-coupled receptors (76, 291, 580). N receptors are pentameric ion channels that may be composed of nine different α, and two β, subunits (227, 296). NE, 5-HT, and ACh receptors may function as postsynaptic receptors located on cell bodies of central respiratory neurons and motoneurons and as presynaptic receptors modulating synaptic transmission. When located on synaptic terminals (presynaptically) they can either up- or downregulate the release of other transmitters. Some of these receptors mediate inhibitory (hyperpolarizing) effects on their targets, whereas others mediate excitation (depolarization) (see 196, 213, 619 for an overview of NE, 5-HT, and ACh receptors).
Excitatory and inhibitory effects of 5-HT, NE, and ACh may act in the same or opposite directions at different levels of the respiratory system. For example, the net effect of 5-HT on medullary respiratory neurons is inhibition mediated, at least in part, by 5-HT1A receptors (22, 81, 180, 289, 290, 427). In contrast, 5-HT has predominantly excitatory effect on motoneurons, including those innervating upper airway muscles, with the effect being mediated by 5-HT2A and 5-HT2c receptors (22,56,139,276,293,559). If endogenous inhibitory effects of 5-HT dominate at the level of premotor inspiratory neurons, whereas excitatory effects dominate at the level of motoneurons, the respiratory motor output will be determined by the difference between these two opposing effects. In different pools of respiratory motoneurons, the two opposing effects may have different magnitudes. Similarly, data from medullary slices from neonatal rats indicate that XII premotor neurons of the IRt region can be controlled by both the excitatory α1-adrenoceptors that are often located postsynaptically and the inhibitory α2-adrenoceptors that appear to be mainly located on synaptic terminals contacting these premotor neurons (presynaptically) (368).
Neurochemically Distinct Central Neuronal Systems with State-Dependent Activity Patterns
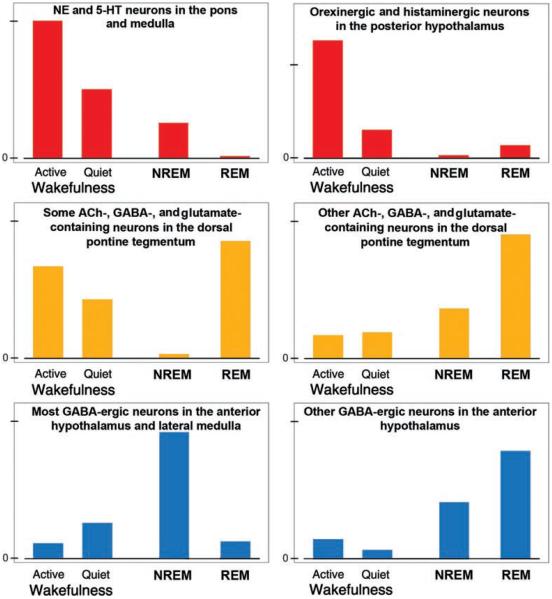
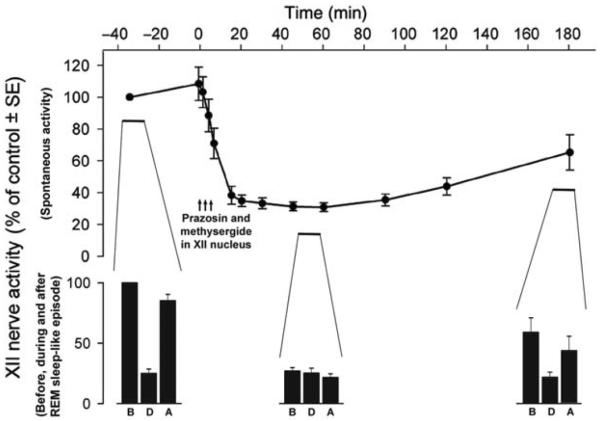
Figure 4 shows the typical patterns of changes in activity of neurochemically distinct groups of brain neurons in relation to the sleep-wake cycle. Neurons containing 5-HT aggregate in the brainstem as distinct groups designated as B1 through B9 (230), and brainstem NE neurons are designated as groups A1 through A7. When compared to wakefulness, both 5-HT and NE neurons have reduced or abolished activity during NREM sleep and are consistently silent during REM sleep (29,230,335,432,532,603). This pattern has been originally ascertained for only two NE groups, the locus coeruleus (LC, or A6 group) and neurons of the pontine subcoeruleus (SubC) region (29,432). More recently, it was determined that ventrolateral pontine A5 cells are silenced during pharmacologically elicited REM sleep-like state (140) and that the dorsal medullary A2/C2 catecholaminergic neurons have activity reduced in proportion to the prior time spent in REM sleep-like state when assessed based on c-Fos expression (444). In contrast, recordings from the ventrolateral medullary adrenergic neurons (C1) revealed that these cells are activated, rather than silenced, during the REM sleep-like episodes (519). Thus, the picture emerging from these studies is that most brainstem 5-HT and NE neurons are silenced during REM sleep, whereas at least one group of adrenergic neurons (C1) is activated.
Figure 4.
Typical activity patterns during wakefulness and sleep states of selected neurochemically distinct groups of central neurons. Some groups have the highest activity during wakefulness (top/red); others have peaks during both wakefulness and REM sleep or during REM sleep only (middle/yellow), and still others during NREM or REM sleep (bottom/blue). These neuronal groups have direct and indirect connections with central respiratory neurons and upper airway motoneurons through which they impart state-dependent changes onto the respiratory system.
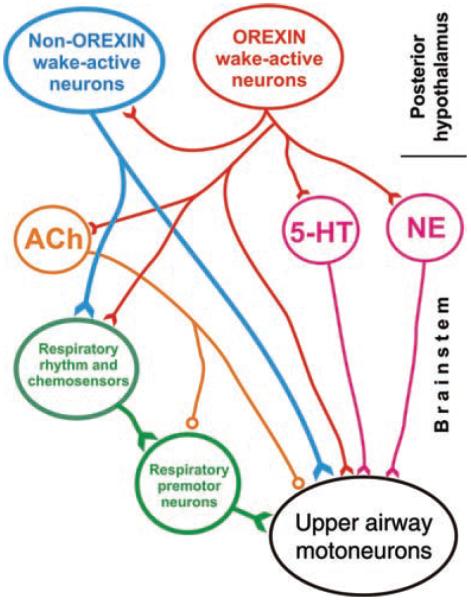
Similar to 5-HT and NE neurons, the cells located in the perifornical region of the posterior hypothalamus and containing the excitatory peptides, orexins, are most active during active wakefulness, have moderate levels of activity during quiet wakefulness, minimal or no activity during NREM sleep, and are silent during REM sleep, with the exception of occasional phasic bursts (299, 347, 531). Interestingly, hypothalamic orexinergic cells are excited by ventrolateral medullary adrenergic C1 neurons (48), but it remains to be determined whether the rostrally projecting C1 neurons are REM sleep-active similarly to the more caudally located and caudally projecting C1 neurons (519). If so, this ascending connectivity may be an important part of the central arousal circuit.
Histaminergic cells located in the posterior hypothalamic tuberomammillary region also have maximal activity during wakefulness, reduced activity during NREM sleep, and are silent during REM sleep (234, 258, 533). Thus, both orexins and histamine can maximally affect their targets, including the respiratory system, during wakefulness, less so during NREM sleep, and minimal during REM sleep.
In contrast, activity of a subpopulation of pontine ACh-containing neurons selectively increases during REM sleep, other ACh cells increase their activity only in association with certain phasic events of REM sleep, and some have increased activity during both REM sleep and wakefulness. Regardless of these differences, the lowest level of ACh cell activity coincides with NREM sleep (53, 127, 249, 260, 456, 516; see 272 for a review). There are also relatively many putative glutamatergic neurons located in the dorsomedial rostral pons that have sleep-wake activity patterns similar to those of the pontine ACh neurons (53, 93, 287, 457). Furthermore, the dorsal pons and the adjacent midbrain contain neurons that synthesize GABA and are distinctly activated during NREM sleep. A possible function of these cells is to suppress the generation of REM sleep (50,53,463). As such, these cells would act parallel to the GABAergic/galaninergic neurons of the anterior hypothalamus that have widespread ascending and descending projections and are maximally activated during NREM sleep (7, 490). NREM sleep-active and putatively inhibitory neurons are also present in the rostral, ventrolateral medullary reticular formation (18).
The presence of cells with REM sleep-related activity in the ventromedial medullary reticular formation has been often described, but the reported locations and properties of such cells varied greatly among the studies (169,242,243,369,499,502). One widely considered but still hypothetical role of these cells has been to relay inhibitory effects of REM sleep from the dorsal pontine tegmentum to spinal motoneurons (e.g., 51, 181, 534; see 498 for a review). However, recent studies suggest considerable diversity of neurochemical phenotypes and functions among the ventral medullary cells with REM sleep-related activity. Some are located in the rostral ventrolateral medulla and have been recently identified as epinephrine-containing neurons of the C1 group (519). Another group of REM sleep-active cells recently investigated in the medullary lateral paragigantocellular region (LPGi) is indeed GABAergic but, similarly to the C1 neurons, these cells appear to have both ascending and descending axonal projections and are also active during wakefulness in relation to ingestive behaviors (579). Such cells may contribute to the generation of the state of REM sleep or some of its characteristic hallmarks (e.g., REM sleep-related twitches of orofacial muscles).
Connectivity between the respiratory and state-dependent neural systems
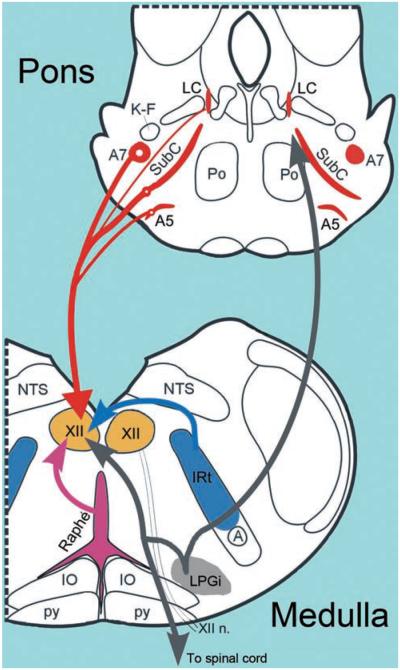
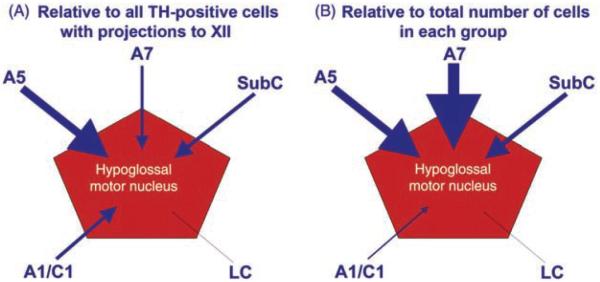
While the dorsal raphé nucleus of the caudal midbrain (B7 group) and the LC (A6 group) of the dorsomedial, rostral pons are the largest and most extensively studied groups representing 5-HT and NE neurons, respectively, these groups do not contribute significant aminergic innervation to upper airway motoneurons. Rather, 5-HT afferents to the XII nucleus and other orofacial motor nuclei mainly originate in 5-HT neurons located along the medullary midline (raphé pallidus and obscurus nuclei) and in the lateral wings of medullary raphé (154, 155, 205, 306, 322, 524) (Fig. 5). The sources of NE afferents to the XII and other orofacial nuclei are distributed more widely and include the pontine A7 group and the SubC region, and possibly also the A5 group (10,11,154,155,447). The contribution, if any, from the ventrolateral medullary A1 group is small (447) (Fig. 5).
Figure 5.
Schematic representation of the four key neuroanatomically and neurochemically distinct inputs to the XII nucleus. NE afferents originate mainly in the pontine A7 and A5 groups and the SubC region, whereas the largest group of NE neurons, the LC, has negligible projections to the XII nucleus. For clarity, NE projections are shown on one side only; they are bilateral with a minor ipsilateral predominance. 5-HT afferents come from the medullary raphé pallidus and obscurus nuclei, as well as the lateral wings of the medullary raphé. The medullary IRt contains glutamatergic and cholinergic XII premotor cells; the former provide inspiratory drive to XII motoneurons, and the latter may mediate pre- or postsynaptic modulatory influences that are either respiratory or state dependent. The LPGi and the adjacent areas contain cells that have been hypothesized to mediate active inhibitory effects of REM sleep to motoneurons. GABAergic, REM sleep-active neurons with divergent projections ascending to the pons and descending to the spinal cord have been located in this area (579). It is not known whether the same cells also have axonal projections to the XII nucleus. Abbreviations: A, nucleus ambiguus; IO, inferior olive, Po, nucleus pontis oralis; K-F, Kölliker-Fuse nucleus; NTS, nucleus of the solitary tract; py, pyramidal tract.
Both NE and 5-HT-containing terminals are present at moderate to high densities in all orofacial motor nuclei (9, 11–13, 23, 263, 454). In the rat, there is a clear topographic organization of NE afferents to the XII nucleus, such that their highest concentration occurs in the ventromedial quadrant of the caudal half of the nucleus (11, 443) (Fig. 6A). The relevance of this is that the XII motoneurons that innervate the GG, the main protrusor of the tongue, are located in this region (109, 333). The density of 5-HT terminals in the rat XII nucleus also has been reported to be nonuniform, with more terminals in the dorsal than the ventral half of the nucleus (11, 12), but this nonuniformity was not as evident as for NE terminals in another study (443) (Fig. 6B). Terminals containing epinephrine or dopamine, two other major derivatives of tyrosine, are not present in the XII nucleus (239, 240) which indicates that adrenergic or dopaminergic neurons do not directly control XII motoneurons. In addition to the orofacial motor nuclei, a variable density of 5-HT- and NE-containing terminals is present throughout the brainstem, including a particularly high density of terminals in the viscerosensory NTS (239, 515). Aminergic synaptic contacts also exist on central respiratory neurons and XII premotor neurons of the IRt region (412, 552, 575). Thus, the sleep-wake cycle variations in the release of 5-HT and NE may affect upper airway motor tone in a state-dependent manner by acting on motoneurons, as well as at different premotor sites. It is, however, of note that the net effects of NE or 5-HT applied onto orofacial motoneurons are uniformly excitatory (6, 244, 276, 285, 293, 331, 436, 473), whereas, as discussed earlier, the effects of these amines at other respiratory sites are less uniform. This justifies particular interest in the magnitude and pattern of state-dependent effects exerted by 5-HT and NE at the motoneuronal level.
Figure 6.
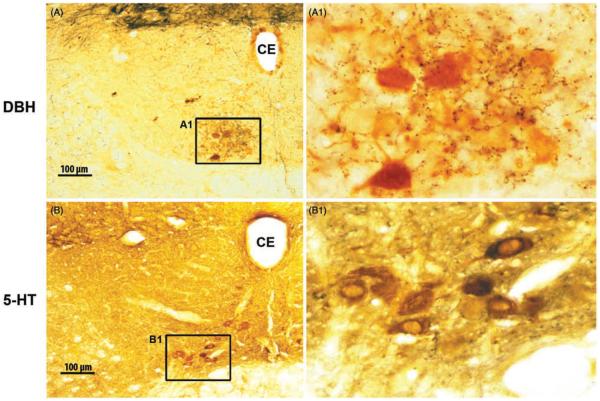
NE- and 5-HT-containing axon terminals are present throughout the XII nucleus. (A and B) Microscopic images of coronal cross-sections through the XII nucleus on one side. The section in A was immunostained for dopamine-ß-hydroxylase (DBH), which in the XII nucleus labels noradrenergic fibers and terminals (black). The section in B was immunostained for 5-HT fibers and terminals. In both panels, selected XII motoneurons that innervate the tongue also were labeled by retrograde transport from the base of the tongue (brown). Typical of motoneurons innervating the genioglossus, most labeled motoneurons are located in the ventromedial quadrant of the XII nucleus. Panels A1 and B1 show enlarged details framed in the corresponding panels on the left. The small dark-brown and black particles in the enlarged images represent fine axonal ramifications and terminals containing NE (in A1) and 5-HT (in B1). NE terminals are especially numerous in the ventromedial portion of the XII nucleus. CE, central canal. (Unpublished data from the study described in Ref. 443.)
The effects of NE on motoneurons may be exerted through multiple receptors located either postsynaptically on motoneurons or presynaptically on interneurons or afferent pathways to motoneurons. Of the seven major classes of 5-HT receptors and their subclasses, only the 5-HT1B, 5-HT2A, 5-HT2C, and possibly 5-HT7 types are expressed in XII motoneurons (380, 569, 614). Pharmacological studies show that excitatory effects in orofacial motoneurons are mainly mediated by 5-HT2 receptors and α1-adrenoceptors (3, 165, 276, 293). mRNA for all α1-adrenoceptor subtypes (α1A, α1B, and α1D) and 5-HT1B, 5-HT2A, and 5-HT2C receptors are present in orofacial motor nuclei (97, 131, 343, 380, 406,424,485–487,569,602,614). Of the three α2-adrenoceptor subtypes (α2A, α2B, and α2C), only the α2C is significantly present in upper airway motor nuclei (441, 467, 537, 595). In immature XII motoneurons, α2-adrenoceptors may mediate postsynaptic inhibition of motoneurons but this effect decreases with development (2, 402, 403, 477). In mature animals, α2-adrenoceptors may exert a more prominent role at various premotor sites, including those that control the respiratory rate (81, 236, 612). α2- and β-Adrenoceptors that are present in orofacial motor nuclei are probably located on astrocytes or neuronal elements other than motoneurons because, when studied at the single cell level, XII motoneurons of juvenile rats expressed mRNA only for the α1 subtypes (424, 486, 571).
5-HT1B and α2-adrenoceptors located within orofacial motor nuclei may also mediate presynaptic effects. Activation of these receptors suppresses the release of various transmitters, both excitatory and inhibitory. As such, the direction of the effect depends on the nature of the prevailing input that is under such a presynaptic control. In medullary slices from juvenile rats, 5-HT1B receptors presynaptically suppressed both the postsynaptic glycinergic inhibitory and glutamatergic excitatory effects on XII motoneurons (44, 56, 501). It is, however, of note that 5-HT1B receptor mRNA is present in cell bodies of many XII motononeurons and many XII motoneurons are immunopositive for 5-HT1B receptor-like protein (44, 569). This would suggest that 5-HT1B receptors may mediate postsynaptic inhibition of XII motoneurons. In anesthetized rats, a 5-HT1B agonist injected into the XII nucleus caused a moderate suppression (about 20%) of XII nerve activity (379), and in vitro studies reveal a small change in XII motoneuron membrane resistance (56). During sleep, when 5-HT neurons have reduced activity or become silent, reduced stimulation of 5-HT1B receptors located in the XII nucleus may slightly increase XII motoneuronal excitability by means of disinhibition (withdrawal of postsynaptic inhibition), but the effect is likely masked by the concurrent effects of reduced postsynaptic excitation mediated by 5-HT2 receptors.
Data from rodents suggest that, in addition to 5-HT1B and 5-HT2 receptors, 5-HT1A and 5-HT3 receptors (or their mRNA) are also present in orofacial motor nuclei (263, 325, 355, 546, 614). However, in adult animals, neither agonists nor antagonists of 5-HT1A receptors have any effects on the activity of orofacial motoneurons when they are applied directly onto motoneurons (3, 276, 293, 379, 538), and neither mRNA nor 5-HT1A receptor-like protein are present in upper airway motor nuclei of adult rats (252,380). For 5-HT3 receptors, microinjections of selective 5-HT3 receptor agonists and antagonists into the XII nucleus had no effect on XII nerve activity (135). mRNA studies suggest that 5-HT4 and 5-HT5 receptors also are present in XII motoneurons (614), but their functions have not been tested.
5-HT and NE neurons may have intrinsic chemosensitivity, such that their excitability increases when extracellular CO2 increases or pH declines (105, 133, 177, 178, 199, 438). Due to the state-dependent nature of activity of 5-HT and NE neurons, the component of central chemosensitivity that they mediate would be reduced during NREM sleep and lost during REM sleep.
The brainstem contains two major groups of ACh-containing neurons with well-documented state-dependence of their activity. They are the pedunculopontine tegmental nucleus (PPN) and the laterodorsal tegmental nucleus (LDN) and are designated as Ch5 and Ch6, respectively (24,237,344,377,478). PPN and LDN are an important component of the reticular activating system. In this role, they may also contribute to state-dependent changes in the respiratory output. In addition to LDN and PPN, less densely packed ACh neurons are present in the intermediate medullary reticular formation (parvocellular region in cats, IRt region in rats), around the superior olivary nucleus, in the medullary paragigantocellular area, and in the dorsomedial, caudal NTS (24, 203, 529). About 50% of PPN/LDN neurons project to the thalamus and about 20% to the medulla (451, 494). ACh cell-specific cytotoxic lesions of medullary reticular neurons destroy a relatively small fraction of medullary cholinergic terminals (202), suggesting that most of medullary ACh afferents originate outside of the medulla.
Respiratory effects of ACh can be mediated by muscarinic (M) and nicotinic (N) receptors. M1 and M3 receptor binding is present in all brainstem respiratory nuclei, with a very high density in the medial NTS, and moderate M3 density in the XII nucleus and the pontine parabrachial area (320). The distribution of M2 protein indicates that these receptors function as both autoreceptors and presynaptic receptors on terminals of ACh and other neurons (300). ACh binding under the conditions favoring the M2 subtype is very high in the pontine nuclei, and slightly lower in the NTS, spinal trigeminal sensory nucleus, and the trigeminal, facial and XII motor nuclei (33,68,422,567). mRNA for all five M receptor subtypes was detected in the retrofacial/pre-Bötzinger region, with M2 and M3 receptor mRNA being the most abundant (286, 567).
In medullary slices from juvenile rats, M2 receptors presynaptically inhibit excitatory glutamatergic transmission to XII motoneurons (43). In contrast, N receptors mediate postsynaptic excitation of motoneurons (79,483,613). Postsynaptic excitation mediated by M3 receptors has been detected in inspiratory neurons of the pre-Bötzinger complex (482), whereas N receptors composed of α4 and β2 subunits mediated presynaptic excitatory effects on the respiratory rhythm in this area (483, 484). In vivo studies with iontophoretic administration of cholinergic agonists and antagonists revealed excitatory (59), mixed inhibitory/excitatory (49, 179, 180, 356, 468), or no consistent effects (264) in inspiratory and expiratory neurons in rabbits, cats, and dogs.
Orexins are excitatory peptides whose actions can be mediated by two types of receptors that are distributed throughout the brain with characteristic patterns (327, 410). For example, NE cells typically express orexin type 1 receptors, motoneurons, and hypothalamic histaminergic neurons have mainly type 2 receptors, and 5-HT cells express both types. In dogs, a genetic defect of just one of these receptors, type 2, results in cataplectic episodes which occur as sudden and disconnected from the normal behavioral context spells of postural atonia and other electrophysiological features of REM sleep while the dog is fully aware of the external environment (308). However, in mice, it takes genetic ablation of both orexin receptors, or of orexin synthesis, to elicit cataplectic attacks (87, 460). Because hypothalamic orexin neurons are active mainly during wakefulness, the loss of their release during NREM sleep may contribute to the relative depression of breathing. Another interesting aspect of the effects of orexin on upper airway motoneurons is related to the phasic bursts of activity that orexin cells generate during REM sleep (347, 531). Specifically, it appears plausible that such bursts contribute to phasic activation of upper airway motoneurons during this state (316, 446, 550). When tested in vitro, orexin neurons have intrinsic pH chemosensitivity similar to that reported for brainstem NE and 5-HT neurons (593). Multiple antagonists of the two known orexin receptors have been developed because of their potential usefulness for treatment of insomnia (46, 62, 466).
Neurons having state-dependent activity patterns often synthesize additional small molecule, as well as peptidergic transmitters. For example, 5-HT neurons commonly contain two excitatory peptides, thyrotropin-releasing hormone (TRH) and SP, and some may produce enkephalin whose effects are inhibitory (27, 195, 200, 235, 238, 373). Orexin neurons synthesize dynorphin whose actions are usually inhibitory (92). Pontine cholinergic cells contain nitric oxide synthase, as well as SP, atrial natriuretic peptide, and corticotrophin releasing factor (261,568; reviewed in 450). Synaptic release of peptides may preferentially occur when cells generate high levels of activity (229). Accordingly, respiratory effects of the peptides colocalized with small molecule transmitters would be most pronounced during active wakefulness.
Interactions between the state-controlling and respiratory networks
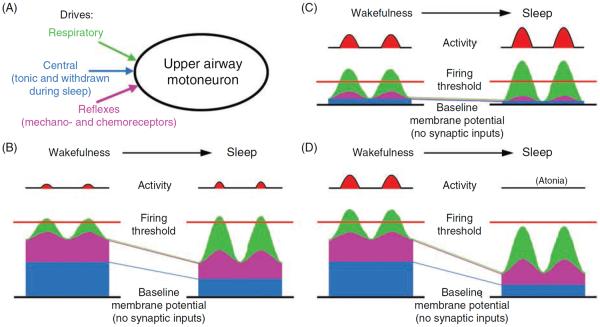
Activity of upper airway muscles is the net result of excitatory and inhibitory inputs (drives) that are synaptically transmitted to motoneurons from various premotor sources. Some upper airway muscles often exhibit both tonic and respiratory phasic components of activity (e.g., the inspiratory-phasic and tonic components of GG activity in human subjects). These two components may occur in different proportions in different muscles and may change independently of each other under different conditions. Hence, it is clear that motoneurons innervating upper airway muscles receive distinct inputs from respiratory-modulated and tonic sources. In addition to the central respiratory and tonic drives, upper airway motoneurons are under reflex control, especially from the central and peripheral chemoreceptors and mechanoreceptors of the lungs and airways (see 34, 176, 223, 268 for reviews). To analyze how these functionally different pathways shape upper airway muscle activity across sleep-wake states, it is practical to examine how sleep affects each one of them separately (cf., 452,541). The reflex drives are principally tonic, but the phasic nature of the mechanical events in the respiratory system and transmission of these inputs through central respiratory neurons lead to respiratory modulation of reflexes. Thus, central phasic respiratory, central tonic, and reflex drives represent three functionally distinct inputs to upper airway motoneurons (Fig. 7A).
Figure 7.
Different firing rate outcomes resulting from different patterns of convergence of three functionally distinct excitatory inputs onto an upper airway motoneuron. The scheme shows three scenarios of how different combinations of inputs from functionally different sources may shape state-dependent changes in motoneuronal activity. (A) The three distinct excitatory inputs considered—respiratory, state-dependent, and reflex—are shown converging on an upper airway motoneuron. In B to D, the depolarizing actions contributed by each of these three inputs summate above the “baseline membrane potential” (black line at the bottom of each panel), which may result in the membrane potential crossing the firing threshold. Of the three distinct drives, the respiratory input is distinctly rhythmic (phasic), the central state-dependent input is tonic, and the reflex input includes both a phasic respiratory and a tonic component. The respiratory input is assumed to increase during sleep (e.g., to make up for reduced ventilation during NREM sleep, or as a result of central activation during REM sleep). In the three scenarios shown in B to D, different relative magnitudes of the three drives during wakefulness and their different changes at sleep onset result in quantitatively and qualitatively different firing rate outcomes at the motoneuronal level. (B) A case with a moderate phasic respiratory, moderate reflex, and strong central tonic input during wakefulness. A large drop of the tonic input at the onset of sleep is compensated for by an increase in the respiratory input and the associated phasic component of the reflex input. As a result, the motoneuron's activity minimally changes on the transition from wakefulness to sleep. (C) A case with a strong phasic respiratory input and weak central and reflex inputs during wakefulness. The increase in respiratory input during sleep more than makes up for the loss of the reflex and tonic drives. As a result, the motoneuron is more active during sleep than during wakefulness. (D) A case with all three inputs having similar magnitudes during wakefulness and the state-dependent input being profoundly reduced during sleep. In spite of a prominent increase in the respiratory input and a moderate increase in the reflex input during sleep, the motoneuron becomes silent. (Modified from Fig. 5 in Ref. 270 and republished with permission from Informa Healthcare, a member of the Taylor and Francis Group, obtained via the Copyright Clearance Center, Inc.)
The relative contribution of each of these three drives to the membrane potential (subthreshold changes) and the level of activity (once the firing threshold is reached) varies among motoneurons innervating different upper airway muscles. Panels B to D of Figure 7 show, schematically, three scenarios where, due to different basal levels of the three drives and differential effects of sleep on these drives, transitions to sleep result in different net changes in motoneuronal activity.
Both the sources and the mechanisms responsible for inspiratory modulation of activity are different in motoneurons that innervate the upper airway and respiratory pump muscles. Whereas the central inspiratory drive to motoneurons innervating the phrenic and external intercostal muscles mainly originates in inspiratory neurons of the VRG (Fig. 2), inspiratory input to XII motoneurons mainly originates in the IRt region located between the XII nucleus and VRG (Figs. 3 and 5). Furthermore, inspiratory modulation of the phrenic and external intercostal motoneurons is achieved through a rhythmic alteration between active excitatory inputs arriving during the inspiratory phase of the respiratory cycle and active inhibition that arrives during the expiratory phase. In contrast, in the inspiratory-modulated laryngeal, facial and XII motoneurons, there is little or no phasic inhibition during expiration (37, 215, 599). The separation of sources of inspiratory drive and the absence of an active, phasic expiratory inhibition in inspiratory-modulated upper airway motoneurons make it possible to engage the latter in a variety of nonrespiratory orofacial functions without a need to interfere with ventilation.
Wakefulness stimulus for breathing
The characteristic slowing down of breathing and its stability during NREM sleep have been referred to as resulting from a withdrawal of “the wakefulness stimulus for breathing” (387, 391). In support of this concept, recordings from medullary respiratory neurons in chronically instrumented, behaving cats revealed that tonic excitatory inputs that maintain central respiratory activity decrease on transition from wakefulness to NREM sleep. The brainstem reticular formation and the midbrain and forebrain structures that exert behavioral control over the respiratory system have been originally seen as the key sources of this wake-related drive.
When the idea of the wakefulness stimulus for breathing was first conceived its underlying neurochemical basis was not known. It is now clear that the effects of NE, 5-HT, histamine, and orexins on breathing and upper airway muscle activity represent important neurochemical substrates of the wake-related drive, as all these systems are more active during wakefulness than during sleep and mediate predominantly activating effects on respiratory motoneurons. Furthermore, since activity of many state-dependent cell groups declines during NREM sleep to reach a nadir during REM sleep, the concept of the wakefulness stimulus can be extended to include both NREM sleep and REM sleep, as both these states are relatively devoid of the excitatory drives mediated by NE, 5-HT, histamine, and orexins. Such an extension of the concept of wakefulness stimulus for breathing should facilitate the analysis of the effects of REM sleep on breathing by helping to operationally separate the suppressant effects caused by a withdrawal of tonic, wake-related excitatory influences (disfacilitation) from phasic effects that may be elicited by transient excitatory and inhibitory inputs mediated by neural systems distinct from those that mediate the tonic effects.
Non-REM sleep
Effects of NREM sleep on ventilation
On the transition from quiet wakefulness to NREM sleep, inspiratory tidal volume may increase while the respiratory rate is reduced and becomes very regular (391). As a net effect, ventilation may be slightly reduced relative to the metabolic need. The resulting small increase in systemic CO2 provides additional stimulation to the respiratory system that compensates for the loss of other wake-related central influences. In human subjects and animals with fully patent upper airway, most tonic and respiratory-modulated activity of accessory respiratory muscles of the pharyngeal region ceases (247,315,455,520). This may slightly reduce the stiffness and cross-sectional area of the upper airway and otherwise healthy humans may exhibit a small degree of flow limitation and occasional snoring. However, as long as the episodes of flow limitation do not reach the threshold of 5 or 10 per hour, the effects are not seen as clinically significant or sufficient to diagnose OSA. In humans, after an initial decline at sleep onset, there is usually a secondary increase of pharyngeal muscle activity which is probably driven by reflexes from upper airway negative pressure receptors and central CO2 receptors (339, 429, 609).
Effects of NREM sleep on respiratory motoneurons
The effect of NREM sleep on motoneurons innervating respiratory pump muscles is different from that on upper airway motoneurons. In particular, motoneurons innervating the diaphragm remain active during NREM sleep with only a small change when compared to quiet wakefulness. The rate of activity increase during inspiration (slope) is reduced, whereas the peak inspiratory activation may be slightly reduced, unchanged or slightly increased due to a concurrent prolongation of inspiratory time (Ti). Overall, the changes in respiratory pump muscle activity closely follow the behavior of central respiratory premotor neurons. In contrast, the pattern of changes in activity of upper airway muscles between quiet wakefulness and NREM sleep varies considerably among the muscles (e.g., 541, 542, 601), and probably also among the species. In healthy humans, tonic and phasic-respiratory components of activity slightly or profoundly decrease, disappear altogether, or increase moderately during NREM sleep when compared to wakefulness (e.g., 35, 247, 283, 541). When considering this variability, it is important to note that the changes observed at NREM sleep onset are often more profound that those observed when deep NREM sleep is fully established. This is particularly clear in human subjects, even in those not exhibiting clinical signs of OSA, because there is usually a small reduction in airway patency at NREM sleep onset which then triggers a compensatory increase in central respiratory drive and the associated, reflexly driven, secondary increase of upper airway muscle activity (e.g., 339, 601, 608). Thus, breath-to-breath monitoring of changes in upper airway muscle activity around the time of sleep onset can more accurately reflect the central effects of state on upper airway muscle output than a discontinuous comparison between the established states of quiet wakefulness and deep NREM sleep because the results from the latter approach may be confounded by reflexes.
When activity of individual motor units is recorded from the base of the tongue in healthy humans during transitions from wakefulness to NREM sleep, changes in activity vary with the type of motor unit, as classified based on its activity pattern during wakefulness in relation to the phases of the respiratory cycle. Thus, the phasic, inspiratory-modulated motor units (i.e., those that cease firing during at least a part of expiration) show the largest decline of firing rate, the inspiratory-tonic motor units (continuously active with increases bound to inspiration) either have no declines or abruptly become silent (Fig. 8A). In contrast to the inspiratory phasic motor units, the expiratory phasic, expiratory tonic, and tonic throughout motor units well maintain their activity during the wake-to-NREM sleep transitions. Based on this finding and similar observations from respiratory-modulated and tonic single motor units recorded from the tensor veli palatini, it has been suggested that, in humans, state-dependent depression of upper airway muscle activity affects the phasic inspiratory modulated, but not the expiratory-tonic or tonic motor units (375, 555, 592). There are, however, a few caveats with the classification of single motor units based on their activity patterns observed under baseline conditions, and also with the conclusions derived from such a classification. First, the motor unit-firing pattern observed during certain experimental conditions is not an invariant property of that unit. Indeed, depending on the level of central inspiratory drive (and other inputs), the same motor unit may have only phasic inspiratory activity, inspiratory modulation superimposed on a level of tonic activity, or just tonic activity (the schemes in Fig. 7 can be used to visualize how these different patterns may result from summation of different subthreshold inputs). Second, it appears that individual upper airway motoneurons often have a relatively narrow range of firing rates that they can attain and relatively steep synaptic input-firing output characteristics. As a result, reports indicate that upper airway motoneurons often alternate between being active and silent by means of recruitment and derecruitment, rather than continuously responding to varying inputs by gradually changing their firing rates (e.g., 35,139,418,453,592). These caveats need to be kept in mind when conclusions are drawn about the central control of motoneurons that exhibit different firing patterns under certain experimental conditions.
Figure 8.
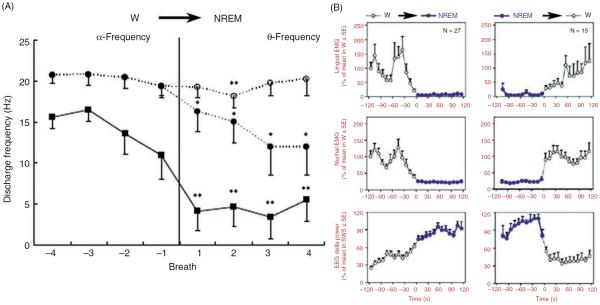
Time course of lingual muscle activity during transition from wakefulness (W) to NREM sleep in healthy humans and rats. (A) In healthy humans, peak firing rate of inspiratory phasic motor units recorded at the base of the tongue significantly declined in association with a transition from quiet wakefulness to NREM sleep (squares). Transitions were defined based on EEG power shift from α-frequency to Θ-frequency. Mean data from 29 inspiratory phasic motor units. In contrast, inspiratory tonic motor units (defined as those firing continuously throughout the respiratory cycle with firing rate increases during inspiration) either had minimal declines of firing rate in association with the onset of NREM sleep or were entirely silenced around the time of the transition. Filled circles show data for all 58 inspiratory tonic motor units studied, including those that became silent at NREM sleep onset. Open circles show data for the subset of inspiratory tonic motor units that were not silenced at the onset of NREM sleep; for this group (n = 29), there was only a very small and transient decrease of firing rate. Asterisks indicate significantly lower firing rate after NREM sleep onset when compared to the mean before the transition. (Fig. 2 in Ref. 592 republished with permission from the American Academy of Sleep Medicine obtained via the Copyright Clearance Center, Inc.) (B) Lingual and nuchal EMGs averaged over multiple transitions from W to NREM sleep and from NREM sleep to W in rats. The top graphs show average levels of lingual EMG determined during successive 10 s intervals over 2-min periods before and after the state transitions, as indicated above the panels. Root mean squares of muscle activity were normalized within each animal and recording session by its average level during W. The middle graphs show the corresponding changes in postural activity recorded from dorsal neck muscles. The bottom panels show the corresponding average changes in cortical delta power which characteristically increases during NREM sleep. Lingual activity is nearly abolished following entry into NREM sleep. Such a low level of activity is maintained throughout the duration of NREM sleep episodes and rapidly returns to the wakefulness level after arousal (right panels). (Modified from Fig. 5 in Ref. 316 and republished with permission from Elsevier.)
Consistent with the limitations associated with classification of individual upper airway motor units (or motoneurons) based on their firing patterns, differences in the behavior of different types of lingual muscle motor units are less evident in studies using multiunit EMG recordings. For example, both the inspiratory phasic and tonic components of activity recorded in healthy humans are similarly depressed at sleep onset (153). The laryngeal cricothyroid muscle activity, which exhibits both tonic and inspiratory-phasic activity, is not depressed during NREM sleep in humans (284), and the inspiratory-modulated and tonic activity of the posterior cricoarytenoid muscle is not depressed in lambs and rats (253, 342, 492).
Multiunit recordings from lingual muscles in rats show suppression of activity during NREM sleep, but considerable different observations are reported regarding the magnitude and pattern of depression. When monitored during transitions from quiet wakefulness to NREM sleep in relation to changes in EEG delta power, lingual EMG is profoundly suppressed, indeed nearly abolished, at NREM sleep onset (Fig. 8B). During most NREM sleep episodes, there is then no trend for a secondary increase and the muscle remains atonic, or nearly atonic (316). Since lingual activity recorded in these studies rarely exhibited any respiratory modulation, the depression evidently affected the tonic activity. In a small number of NREM sleep episodes in which inspiratory modulation of lingual EMG emerged, it appeared with a delay after NREM sleep onset and then gradually increased until the increase was terminated by awakening or transition into REM sleep (520). Such a secondary increase of activity with a distinct phasicinspiratory pattern was similar to the secondary increases of GG muscle activity observed with a delay after the onset of NREM sleep in healthy humans. However, in humans, the effect seems to be driven by reflexes from upper airway mechanoreceptors and central chemoreceptors; whereas in rats, it was correlated with increased power in EEG frequencies in beta range (the relationship to upper airway pressure or systemic CO2 was not determined). This suggests that, in rats, a central component of NREM sleep associated with cortical activity has a small excitatory effect on XII motoneurons.
Other studies in rats report that the GG muscle exhibits both tonic and phasic-inspiratory activity during quiet wakefulness and that both components are similarly suppressed during NREM sleep (e.g., 171, 233, 358, 513). Thus, differences exist among the reports describing the effects of NREM sleep on GG muscle activity. There is an even larger diversity of patterns among different upper airway muscles; this may be related to the different magnitudes of the effects of NREM sleep on different pools of upper airway motoneurons (cf., Fig. 7).
Effect of NREM sleep on central respiratory neurons
Recordings from inspiratory and expiratory neurons located in the region of the VRG in chronically instrumented cats indicate that NREM sleep is associated with a moderate reduction of their peak firing rates (84,156,393,394,420). In most cells with a strong and stable respiratory activity, the average peak firing rate is reduced by 10% to 20%, whereas about 5% to 15% of cells have small increases. The decrease is proportional to the reduction in the tidal volume and slowing of the respiratory rate. Based on the location of typical recording sites, most of the cells with strong respiratory modulation were likely VRG bulbospinal neurons sending axons to spinal respiratory motoneurons (Fig. 2).
In contrast to the strongly respiratory-modulated neurons, cells that had weak and variable respiratory modulation during wakefulness had large sleep-related reductions of their peak firing rates (395). Such cells could be premotor cells for upper airway motoneurons, vagal motoneurons, or neurons involved in mediating respiratory modulation of cardiovascular outputs. Respiratory-modulated neurons located in the pontine parabrachial region increased or decreased their firing rates on transition from wakefulness to NREM sleep, with the average change being a small decrease (187,496). It is of note that the pontine parabrachial region gives origin to projections to orofacial motor nuclei (109, 132, 154, 155, 512, 551, 557).
Overall, the decrease in activity of central respiratory neurons during NREM sleep, and especially those with relatively weak respiratory modulation, may be a part of the wakefulness stimulus for breathing.
REM sleep
Effects of REM sleep on ventilation
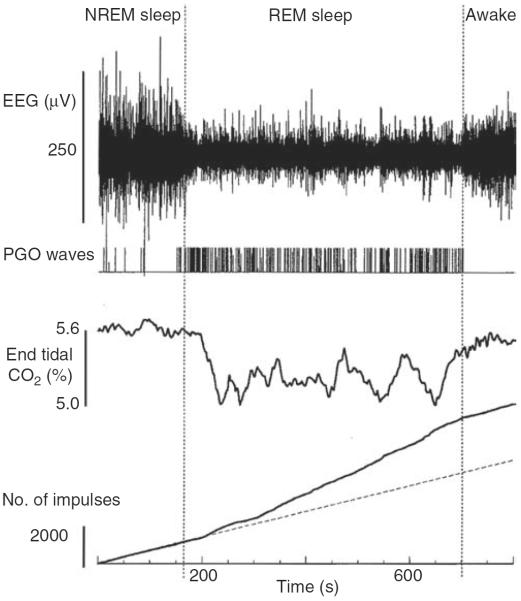
Breathing during REM sleep is characterized by large breath-to-breath variability of both tidal volume and respiratory timing. Particularly short and exceptionally long respiratory cycles and those with high and low tidal volume tend to be clustered together, resulting in a quasi-rhythmic alteration between periods of enhanced and suppressed activity (388). The variability of breathing occurs in parallel to other phasic changes characterizing this state, such as intense REMs, ponto-geniculo-occipital (PGO) waves, surges of heart rate, and arterial blood pressure, and muscle twitches occurring in otherwise atonic postural muscles and distal muscles of the limbs (28). In many regards, breathing during REM sleep is similar to breathing during active wakefulness. Indeed, phasic limb movements observed during normal REM sleep, and even more so during REM sleep with impaired mechanisms of suppression of muscle activity (REM sleep behavior disorder), are at times related to the dream contents (25). The same interpretation has been also proposed to explain the variability of breathing during REM sleep (391).
Effects of REM sleep on respiratory motoneurons
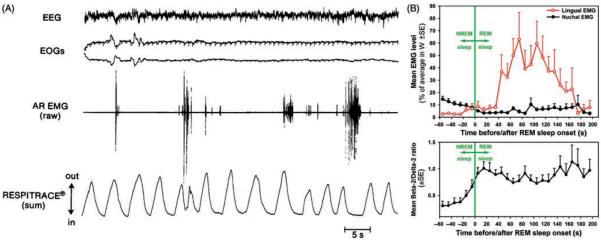
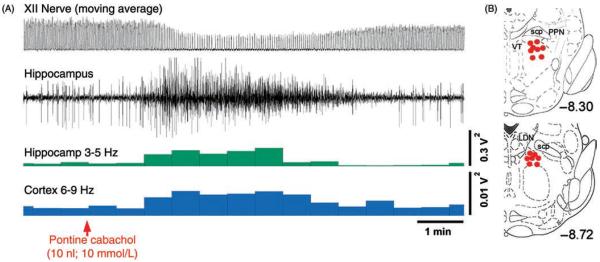
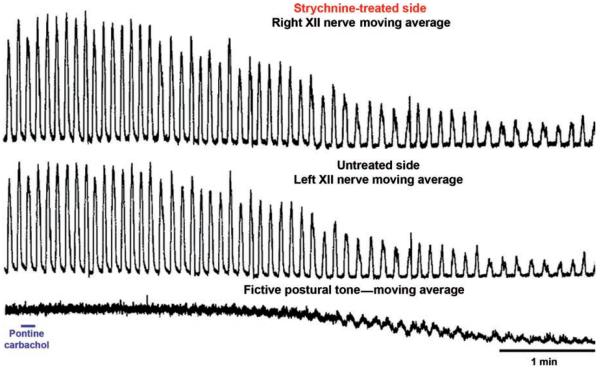
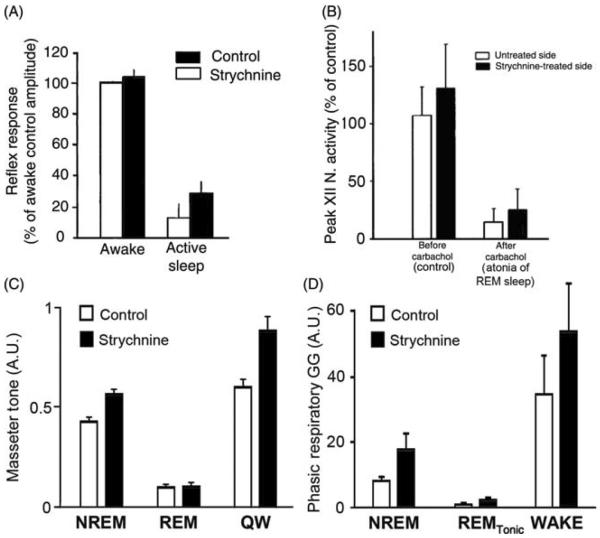
During REM sleep, the rate of inspiratory increase of diaphragmatic activity is variable and often interrupted by transient periods of quiescence lasting ~50 ms (fractionations) (117, 390, 497). Most pharyngeal and some laryngeal upper airway muscles are silent (atonic) with the exception of intermittently occurring phasic twitches (Fig. 9A). Data from rats indicate that the generation of these twitches is governed by a time-dependent process, such that both the magnitude and frequency of twitching gradually increase with the duration of each REM sleep episode and then gradually decline if the episodes extend beyond their average duration without a transition to another state (316, 446). Figure 9B shows the time course of lingual EMG during a 2 min period prior to, and then 2 min after, transition from NREM sleep to REM sleep in rats. Due to averaging across multiple recording segments, the highly phasic nature of activity during REM sleep is smoothed out, revealing a gradual increase and then decline of the mean level of twitching activity with the duration of the episode of REM sleep.
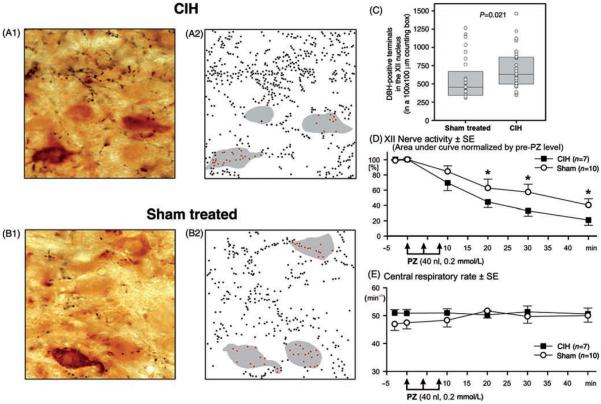
Figure 9.
Upper airway muscle activity during REM sleep in subjects with fully patent upper airway is dominated by nonrespiratory, phasic bursts that emerge from an otherwise atonic state. (A) Activity of the arytenoid (laryngeal) muscle during REM sleep in a healthy human subject. (Fig. 9 in Ref. 278 republished with permission from the American Physiological Society.) (B) Average time course of lingual muscle activity during transitions from NREM to REM sleep in rats. Lingual and nuchal EMG levels were measured during successive 10 s intervals from 60 s before to 200 s after the state transition (time zero) and averaged over multiple transitions in multiple animals. Root mean squares of muscle activity were normalized within each animal by their average levels during wakefulness. The bottom graph shows the corresponding average time course of the ratio of EEG powers in β-2 to Δ-2 bands (the ratio characteristically increases during REM sleep). Lingual and nuchal EMGs follow a different time course during the transition. Whereas nuchal EMG declines prior to the onset of REM sleep and then maintains a low level (atonia), lingual EMG is nearly atonic prior to the onset of REM sleep (see Fig. 8B) and first gradually increases and then declines after REM sleep onset. The relatively smooth time course of the increase and then decline is a result of averaging of many short bursts of activity (twitches) across many NREM to REM sleep transitions. The resulting mean time course indicates that, after the onset of REM sleep, individual twitches first become gradually larger and more frequent and then gradually become smaller and less frequent when the duration of REM sleep episodes extends beyond its mean (90 s in rats). (Modified from Fig. 6 in Ref. 446 and republished with permission from Archives Italiennes de Biologie and the University of Pisa.)
Nonrespiratory bursts of activity superimposed on otherwise quiescent EMG were reported as characteristic of lingual EMG during REM sleep in healthy humans (88), cats (437), dogs (455), and rats (316,340,359), and were also observed in the arytenoideus (laryngeal) muscle in humans (278). Recordings from the diaphragm of cats hyperventilated to apnea during NREM sleep also show that phasic excitatory inputs of nonrespiratory origin reach phrenic motoneurons during REM sleep, and that their intensity gradually increases following the onset of REM sleep (157, 392). The same process seems to determine the pattern of changes in respiratory rate and the occurrence of twitches in postural and orofacial muscles innervated by trigeminal, facial, and vagal motoneurons (19, 64, 157).
Traditionally, the occurrence of phasic events, such as intense REMs, PGO waves, and muscle twitches, has been used to distinguish between the “phasic” REM sleep and “tonic” REM sleep, with the latter characterized by steady suppression of postural muscle tone with little or no phasic events (e.g., 385). The observations that phasic twitches in both accessory respiratory muscles and postural muscles as well as the respiratory rate variability, exhibit an orderly progression within the episodes of REM sleep suggest that the “tonic” and “phasic” periods of REM sleep are not semi-randomly alternating forms of this state but rather result from one continuous neural process that starts with the onset, and ends with the termination, of each REM sleep episode.
Effect of REM sleep on central respiratory neurons
During REM sleep, brainstem respiratory neurons may transiently have a higher or lower activity than during NREM sleep but, on the average, they are more active than during NREM sleep (385, 388, 389, 396) (Fig. 10). The variability at the neuronal level correlates with the breath-to-breath changes in the magnitude of diaphragmatic activity and tracheal pressure (389), as expected given that many of the studied medullary respiratory neurons were bulbo-spinal neurons sending axons to phrenic motoneurons. Similar to medullary respiratory neurons, many pontine parabrachial respiratory and nonrespiratory neurons increase their activity during REM sleep (187).
Figure 10.
REM sleep exerts a powerful excitatory effect on the activity of most brainstem respiratory neurons. In this example, cumulative activity of an augmenting medullary inspiratory neuron was recorded from a chronically instrumented cat over a period covering a transition from NREM to REM sleep and then from REM sleep to awakening. The period of REM sleep is marked by EEG desynchronization, appearance of PGO waves, and a slightly reduced and variable end-expiratory CO2. The bottom panel shows that the neuron has a distinctly increased activity during REM sleep when compared to either the preceding period of NREM sleep or the period after awakening, as indicated by the slope of cumulative activity. (Fig. 1 in Ref. 392 republished with permission from John Wiley and Sons obtained via the Copyright Clearance Center, Inc.)
Significant positive correlation between respiratory cell firing rate and intensity of PGO waves suggests that one excitatory component of the effects of REM sleep on medullary respiratory cells is related to the pontine networks generating these waves. However, an extrapolation of this analysis toward a hypothetical period of REM sleep without PGO waves (i.e., “tonic” REM sleep) indicated that medullary respiratory neurons would still receive both excitatory and suppressant influences during a period of REM sleep without PGO waves (385, 386).
Effects of sleep on respiratory reflexes
Important excitatory reflex effects on upper airway dilator muscles originate in central and peripheral arterial chemoreceptors, pulmonary mechanoreceptors, and multimodal receptors located in the upper airway. The reflexes from chemoreceptors and pulmonary receptors concurrently affect upper airway and respiratory pump muscles, whereas the afferent inputs from the upper airway tend to predominantly affect upper airway muscles. In OSA patients, mechanoreceptors activated by negative pressure in the upper airway provide one level of reflex protection against upper airway from collapse by reflexly enhancing activity of upper airway-dilating muscles (66, 330). Indeed, in humans, a loss of this protective reflex during sleep has been considered an important cause of obstructive apneas and hypopneas during sleep (45, 104, 110, 584). Here, we will relate the findings in human subjects pertinent to state-dependence of upper airway reflexes (e.g., 17, 73, 120, 208, 543, 581, 583) to the extensive information about reflexes from the upper airway in experimental animals (see 34, 223, 269, 462, 588 for reviews).
In the respiratory system, some sleep-related attenuation of various respiratory reflexes has often been reported (e.g., 21,193,209). However, interpretation of the effects of sleep on reflexes is complicated because attenuation of reflex effects may occur as a result of reduced excitability of motoneurons or as a result of an active suppression of reflex transmission at the central level. In this context, it is worth noting that the reflex control of respiratory rate, diaphragmatic activity, heart rate, and arterial blood pressure are well maintained during sleep. For example, negative pressure pulses applied to the airway during expiration produce a similar reflex prolongation of expiration in wakefulness and NREM sleep, and there is only a small attenuation of this response during REM sleep (186). The CO2 threshold for rhythmic respiratory activity stays constant across the states of sleep and wakefulness (210, 224), and ventilatory sensitivity to CO2 is the same during NREM sleep and wakefulness (399, 401). Similarly, reflex control of the heart rate by arterial baroreceptors does not differ between wakefulness and NREM sleep in normal mice (500). Moreover, the apneic and bradycardic responses to instillation of water or inflation of a balloon in the larynx that did not cause arousals were actually more pronounced during REM sleep than NREM sleep (523). The average diaphragmatic response to airway occlusion was reduced during REM sleep when compared to NREM sleep, but further analysis revealed that this occurred only at times when inhibitory effects of REM sleep on diaphragmatic activity predominated, rather than throughout the state (503). In rabbits, reflex activation of the GG muscle was reduced during both NREM sleep and REM sleep to a larger degree than expected from the concomitant suppression of spontaneous GG muscle activity (185). Common to the examples with small, or no, sleep-related suppression of reflex effects is that the set point of the regulated variable was near the middle of the range of regulation, rather than close to the saturation or abolition points.
Data are also conflicting with the regard to the effects of sleep on reflex responses of upper airway muscles to airway negative pressure. Studies show that these reflexes are present, albeit attenuated, in GG and other upper airway muscles during both NREM sleep and REM sleep in chronically instrumented animals (225, 414), healthy humans (193, 209, 318), and OSA patients (194, 361, 362), and that they help prevent upper airway hypotonia during sleep (104,110). In some studies, GG muscle response to negative pressure was nearly abolished during NREM sleep (26, 581, 583). However, in another study in healthy humans, the GG excitatory response to inspiratory resistive load was present during NREM sleep, but absent during wakefulness (541). Recent data suggest that the magnitude of reflex activation of GG by upper airway pressure receptors is proportional to the level of baseline activity (73). This would be consistent with the interpretation that the suppression of reflex excitatory effects during sleep is secondary to the reduced level of baseline activity. Body position may also determine whether GG activation by upper airway negative pressure is, or is not, suppressed during NREM sleep (318).
It has been reported that, in contrast to healthy subjects, sensory input from the upper airway makes a negligible contribution to arousal in severe snorers and OSA patients (78, 98). Reflex activation of upper airway muscles elicited by negative airway pressure or airway occlusion also is reported to be blunted in OSA patients both awake and asleep (334,362). Damage of sensory endings and other morphological abnormalities in upper airway mucosa and muscles described in OSA subjects may be the reason for these impairments (57, 124, 158, 372, 409, 480, 527, 616).
In addition to the sleep-related suppression of reflexes secondary to reduced excitability of motoneurons and the detrimental effect of OSA on sensory endings, there is also distinct neurochemical basis for changes in reflex transmission with sleep-wake states. Neurons with state-dependent changes in activity, such as those containing NE, 5-HT, and ACh, can act presynaptically on axon terminals transmitting information from peripheral receptors. In support of this mechanism, studies in in vitro slices from neonatal and juvenile rats indicate that afferent pathways to XII motoneurons are presynaptically suppressed by 5-HT and other state-dependent transmitters (43, 44, 56, 501, 558). However, in an in vivo study in adult, anesthetized cats, microinjections of 5-HT into the XII nucleus did not change the response of XII motoneurons to negative pressure pulses applied to the upper airway (113), suggesting that transmission in this particular reflex pathway was not modulated by 5-HT at the XII nucleus level.
State-dependent modulation of reflex transmission may occur at the sites where peripheral receptor afferents make their first central synaptic contact. NTS is the main entry point for many relevant viscerosensory afferent pathways, including those mediating reflexes from upper airway receptors. NTS is densely innervated by both 5-HT and NE terminals and contains their receptors (e.g., 97, 324, 325, 441, 447, 537, 546, 547, 595). Stimulation of 5-HT cells and microinjection of 5-HT4 receptor agonists into the NTS suppressed transmission of cardiovascular reflexes (125, 126). SP (often colocalized with 5-HT) modulates transmission in vagal reflexes (364,476). Since most studies indicate that 5-HT and NE have suppressant effects on reflex transmission and the cells that release these amines are most active during wakefulness, their actions would suppress reflex transmission primarily during wakefulness and less so during sleep.
In summary, reflexes from airway mechanoreceptors play an important role in the control of upper airway motoneurons. Their magnitude measured at the motor output levels may vary with the sleep-wake cycle as a result of both state-dependent changes in upper airway motoneuronal excitability and due to state specific, central modulation of transmission in reflex pathways.
Neurotransmitters and Peptides Exerting State-Dependent Control Over Upper Airway Motoneurons
If a neuron makes excitatory connections with motoneurons and its activity is reduced during sleep, the excitation is withdrawn, motoneurons become less active, and the process is called disfacilitation. On the other hand, if a neuron makes inhibitory connections with motoneurons and its activity is increased during sleep, this will cause a reduction in motoneuronal activity and the mechanism will be called sleep-specific, active inhibition. This framework is useful for considering the mechanisms underlying the effects of sleep on upper airway muscle tone from the perspective of motoneurons that innervate upper airway muscles.
Insights from reduced animal models versus studies during natural sleep-wake states
Interpretation of the mechanisms underlying the effects of sleep on muscle tone observed during natural sleep-wake states is complicated by a host of reflexes and behavioral influences. The secondary increase of upper airway muscle tone occurring with a delay after the onset of NREM sleep is but one example of how reflexes can mask the central effects of state on the respiratory output. Data from animal studies show that not only the magnitude of the effects may be altered, but even their direction may be reversed, leading to incorrect interpretations. For example, in studies of the effect of the atonia of REM sleep on different pools of upper airway and respiratory pump muscles, the magnitude of depression was smaller when measured in spontaneously breathing, decerebrate cats than when the same preparation was paralyzed and artificially ventilated at a constant rate and volume (549). In another study in anesthetized rats, the effects of central microinjections of a cholinergic agonist, carbachol, into a ventral region of the pons included a profound depression of XII nerve activity when the experiment was conducted in spontaneously breathing animals. This suggested that the effect represented pharmacological activation of a REM sleep-related network. However, the same injections made in paralyzed and artificially ventilated rats revealed that the depression of XII nerve activity observed in spontaneously breathing rats was due to stimulation of breathing and hyperventilation. It also turned out that the injections caused an increase in activity of LC neurons, which was consistent with brain activation but contrary to the known effects of REM sleep on NE neurons (145). These examples show that interactions between the central effects of sleep-wake states and the reflex controls of breathing can significantly influence the final outcomes and their interpretations. Hence, studies of the effects of sleep-wake states on upper airway muscle activity conducted in reduced preparations in which reflex effects are minimized or eliminated help unravel direct influences of sleep on the respiratory system.
Dissociation between respiratory reflexes and central effects of behavioral states on upper airway activity can be achieved when ventilation and gas exchange are kept constant despite sleep-related changes in respiratory muscle activity. This can be achieved by recording from respiratory motoneurons, rather than muscles, in animals with neuromuscular blockade and artificial ventilation at a constant rate and volume. Such an approach has been used to study the effect REM sleep on upper airway motoneurons in conjunction with pharmacological triggering of a REM sleep-like state. The approach was developed based on the evidence that REM sleep-like neural phenomena can be triggered in both unanesthetized (chronically instrumented or decerebrate) and anesthetized animals by microinjections of a cholinergic agonist, carbachol, into a discrete region of the dorsomedial pontine reticular formation (1, 54, 255, 314, 530; see 32, 265, 272 for reviews). In unanesthetized, acutely decerebrated cats and rats, these REM sleep-like phenomena include the postural atonia, silencing of medullary 5-HT neurons, depression of activity in XII motoneurons, and eye movements (255, 354, 530, 549, 598). The pattern of depression elicited in decerebrate, paralyzed, and artificially ventilated cats in different respiratory motor outputs is typical of the natural REM sleep in that activity of phrenic motoneurons is only slightly affected, expiratory-modulated vagal motoneurons that innervate pharyngeal muscles and XII motoneurons are nearly silenced, whereas laryngeal and intercostal motoneurons are suppressed to an intermediate degree (138,255,274). The strong suppression of XII motoneurons is similar to the changes with sleep in EMG activity in naturally sleeping rats and goats in which baseline GG activity was increased by hypercapnia or hypoxia to ensure that the muscles maintained some activity during NREM sleep prior to the transition into REM sleep (211, 400, 401). The suppression of expiratory-modulated vagal motoneurons is similar to that observed in pharyngeal constrictors (283). Thus, in the carbachol models of REM sleep, there is a centrally determined pattern of the suppressant effects of the state on the activity of different pools of upper airway motoneurons that is analogous to that observed during natural REM sleep.
Changes in cortical EEG and respiratory output analogous to those occurring during natural states of NREM and REM sleep can be observed under general anesthesia, and urethane anesthesia in particular (103,173,218,326,397,398). Furthermore, pontine injections of carbachol in urethane-anesthetized rats can effectively elicit cortical, hippocampal, and central neuronal changes that are similar to those occurring during natural REM sleep (140, 530, 600; see 265, 272 for reviews). Figure 11A shows a typical episode of REM sleep-like state elicited by pontine carbachol injection in a urethane-anesthetized rat. As during natural REM sleep, NE cells of LC and A5 group are silenced (140, 265). Also as with natural REM sleep, pharmacological activation of wake-related neurons in the posterior, lateral hypothalamus blocks the ability of pontine carbachol injections to trigger REM sleep-like episodes (314). In acute experiments under anesthesia, microinjections can be precisely placed and can have very small volumes, thus allowing for precise anatomical localization of the sensitive region. The carbachol-induced REM sleep-like episodes often occur within less than 1 min after the injection, indicating a close proximity of the episode-triggering site to the site of injection. In decerebrate cats, the episodes may last in excess of 30 min. In contrast, in anesthetized or decerebrate rats, they are considerably shorter, 2 to 10 min, end relatively abruptly and, importantly, can be elicited repeatedly by additional carbachol injections placed at the same site. The ability to elicit REM sleep-like episodes repeatedly and in a controllable manner makes feasible experiments at the whole animal and single cell levels that are difficult to conduct and interpret in behaving animals.
Figure 11.
REM sleep-like episodes of depression of XII nerve activity accompanied by the characteristic cortical and hippocampal activation can be elicited by microinjections of a cholinergic agonist, carbachol, into the dorsal mesopontine tegmentum in urethane-anesthetized, paralyzed, and artificially ventilated rats. (A) Typical REM sleep-like episode. The moving average of XII nerve activity (top) shows inspiratory bursts of activity. The amplitude of the bursts is reduced and the central respiratory rate declines within less than 2 min following pontine carbachol injection (at arrow). Parallel to the depression of XII nerve activity, there is cortical and hippocampal activation. The second trace from top shows raw signal recorded from the hippocampus. The third trace from top shows the power of hippocampal activity in the 3 to 5 Hz frequency band, which represents theta-like rhythm under urethane anesthesia. The increase in power of cortical EEG in the 6 to 9 Hz frequency band (bottom) indicates activation relative to the period prior to carbachol injection. The entire episode lasts about 5 min, after which all signals return to their precarbachol levels and patterns. The ability to repeatedly elicit such REM sleep-like episodes allows one to investigate cellular, neurochemical, and network mechanisms of the interaction between REM sleep and the regulation of breathing and upper airway muscle activity. (Unpublished record from the study described in Ref. 142.) B: The sites at which carbachol microinjections elicit REM sleep-like episodes in urethane-anesthetized rats superimposed onto two standard cross sections of the brainstem from levels 8.3 mm and 8.72 mm caudal to bregma. Abbreviations: LDN, laterodorsal tegmental nucleus; PPN, pedunculopontine tegmental nucleus; scp, superior cerebellar peduncle; VT, ventral tegmental nucleus. (Modified from Fig. 2 in Ref. 272 and republished with permission from Elsevier.)
In cats, a discrete site located in the dorsomedial pontine reticular formation ventral to LC has been identified at which carbachol most effectively elicits a REM sleep-like state characterized by activation of the cortical EEG, REMs, postural atonia, and PGO waves (560). In anesthetized rats, REM sleep-like responses are produced from a discrete area in the dorsal pontine tegmentum located lateral to the ventral tegmental nucleus and rostral to the subcoeruleus region (142,143,314) (Fig. 11B). Small carbachol injections made in chronically instrumented, behaving rats into the same region most effectively elicited frequently occurring REM sleep episodes (54).
In the decerebrate cat and anesthetized rat carbachol models of REM sleep, activity of medullary inspiratory neurons is minimally suppressed, or even increased, while inspiratory activity of spinal and cranial respiratory nerves is suppressed to a variable degree (274, 600). The absence of a major suppressant effect of REM sleep-like state on activity of strongly inspiratory modulated central neurons is consistent with the recordings from VRG neurons during natural REM sleep in behaving cats (388, 396). The disparity between no suppression of activity in central respiratory neurons and very large in some respiratory motor outputs suppression of motoneuronal activity indicates that REM sleep-related input, whether disfacilitatory or inhibitory, reaches respiratory motoneurons through pathways that bypass central respiratory premotor neurons.
Another reduced model of the effects of REM sleep on respiratory motor output is based on an in vitro preparation of an arterially perfused brainstem/spinal cord of juvenile rat. In this model, dorsal pontine carbachol injections can also repeatedly elicit episodes of depression of upper airway muscle activity and respiratory rate acceleration (60). Since the extracellular environment can be manipulated in this preparation by intra-arterial perfusion with various drugs, the model should prove useful for analysis of transmitters and modulators essential for the generation of REM sleep and its atonia.
In vivo studies using carbachol models yielded new major findings about the neurochemical basis of REM sleep influences on upper airway motoneurons. In particular, the first studies revealing that disfacilitatory mechanisms cause the depression of activity in XII motoneurons during REM sleep were conducted using carbachol models (137,142,273). Carbachol models also made possible investigation of the effects of REM sleep on activity of pontomedullary catecholaminergic neurons of the A1/C1, A2/C2, and A5 groups that are difficult to access in behaving animals (140,444,519).
One notable phenomenological difference between natural REM sleep and REM sleep-like state elicited in different preparations by pontine carbachol injections is that the latter occurs without muscle twitches or the within-state variabilities of the heart rate, respiratory rate, or tidal volume. To explain this difference, it has been proposed that variabilities and phasic events may be caused by rapid changes in the levels of endogenous transmitters that trigger and maintain REM sleep, and that such a changes do not occur when the network responsible for the generation of REM sleep is activated by an exogenous cholinergic agonist (255).
Inhibitory amino acids
Intracellular recordings from trigeminal motoneurons in freely behaving cats inspired the concept that postsynaptic inhibition is the cause of motoneuronal hyperpolarization during REM sleep (83, 365). The key evidence derived from those experiments was that synaptic activity, both excitatory and inhibitory, declined during REM sleep in association with motoneuronal hyperpolarization. Subsequent evidence for these effects being caused by active inhibition has been strongly influenced by studies of spinal motoneurons. Intracellular recordings obtained from lumbar motoneurons during either natural REM sleep or REM sleep-like atonia elicited in decerebrate cats by pontine carbachol injections revealed that REM sleep was associated with the appearance of inhibitory postsynaptic potentials (IPSPs) of which some had uniquely large amplitudes (168, 351, 353, 354). The potentials were abolished by strychnine, an antagonist of chloride-dependent inhibition mediated by glycine, when the drug was administered by iontophoresis into the vicinity of the recorded from motoneuron (86). In one study, motoneuronal hyperpolarization during REM sleep also was nearly abolished (506). These studies demonstrated that large IPSPs recorded from spinal motoneurons during REM sleep were mediated by glycine. Collectively, the findings were interpreted as evidence that the motor atonia of REM sleep is caused by an active, postsynaptic inhibition of motoneurons (85).
As in spinal motoneurons, large IPSPs also occur in XII and trigeminal motoneurons during the atonia of REM sleep (160, 162, 407, 605), and increased levels of both glycine and GABA were detected in the XII nucleus region in association with the REM sleep-like motor atonia produced by electrical stimulation within the dorsal pontine tegmentum (259). However, none of the studies in which attempts were made to directly address the hypothesis that active inhibition mediated by glycine is the cause of the atonia of REM sleep yielded results supportive of this hypothesis. The first study questioning the role of active inhibition as the cause of REM sleep-related suppression of activity in XII motoneurons was conducted using a decerebrate cat carbachol model of REM sleep (273). In that study, the REM sleep-like depression of spontaneous activity of XII motoneurons was not diminished by microinjections into the XII motor nucleus of antagonists of either glycine or GABAA receptors, and the authors concluded that REM sleep-related depression of XII motoneuronal activity was not caused by active inhibition (Fig. 12). Subsequent similarly designed studies using the anesthetized rat carbachol model of REM sleep also indicated that inhibitory amino acids contributed minimally, or not at all, to the depression of activity in XII motoneurons (141, 142). Observations pointing to a small role of glycine and GABA in causing the REM sleep-related suppression of XII and trigeminal motoneuronal activity were also derived from experiments with chronically instrumented and naturally sleeping rats (64, 358). Additionally, based on an earlier evidence that inhibition of orofacial motoneurons may be mediated by GABAB receptors (44, 381), attempts were made to simultaneously antagonize glycine, GABAA and GABAB receptors in the trigeminal motor nucleus to determine whether this would abolish the atonia of REM sleep (65). As a matter of conclusion from these experiments, it was proposed that a combined antagonism of all three inhibitory receptors released trigeminal motoneurons from a portion of REM sleep-related inhibition. However, the study also indicated that the effects of the three antagonists combined were more powerful during wakefulness and NREM sleep than during REM sleep. Consequently, when expressed relative to the mean level of activity during wakefulness, the depression of activity observed during REM sleep was more profound with than without the antagonists. Finally, it is of note that the first study that attempted to directly assess the role of inhibitory amino acids, glycine or GABA, in the REM sleep-related suppression of trigeminal motoneurons indicated that neurotransmitters other than glycine or GABA must significantly contribute to the depression of excitability of these motoneurons during REM sleep (505). In that study, large injections of bicuculline or picrotoxin (to antagonize GABAA receptors) or strychnine (to block glycine receptors) were made into the trigeminal motor nucleus of chronically instrumented cats and reflexly evoked motoneuronal activity was measured across different sleep-wake states with and without the antagonists. The study revealed that the antagonists reduced the suppressant effect of REM sleep on reflexly evoked motoneuronal response by less than 20%. Unfortunately, in the interpretation of this result, the authors emphasized the importance of the postulated active inhibitory mechanisms at the expense of the evidence for mechanisms other than active inhibition that their study clearly uncovered. Figure 13 shows the results from the experiments designed to test the causality of amino acid-mediated inhibition for the generation of the atonia of REM sleep in a format conductive for quantitative comparison of measurements collected in different labs and model systems. Despite many technical differences, all studies have consistently pointed to the inability to abolish, or even reduce in a major way, the atonia of REM sleep in either XII or trigeminal motoneurons by administration of inhibitory amino acid receptor antagonists into the corresponding motor nuclei.
Figure 12.
Antagonism of glycinergic inhibition at the level of the XII motor nucleus did not prevent the REM sleep-like depression of XII nerve activity elicited in decerebrate, paralyzed, vagotomized, and artificially ventilated cats by pontine microinjections of a cholinergic agonist, carbachol. Strychnine, a blocker of glycinergic receptors, was repeatedly microinjected into the right XII nucleus 3 to 16 min prior to the beginning of the record. The traces show moving averages of activities recorded from both XII nerves (upward deflections represent inspiratory bursts) and a cervical nerve branch that innervates dorsal neck muscles (a marker of postural activity). Pontine carbachol injection made at the marker initiated the REM sleep-like suppression of activity in both XII nerves and the postural nerve. The activities of both XII nerves were similarly depressed despite the prior injection of strychnine into the right XII nucleus. (Modified from Fig. 2B in Ref. 273 and republished with permission from Elsevier.)
Figure 13.
Results from different laboratories and different animal models consistently indicate that antagonism of glycinergic inhibition at the motoneuronal level by strychnine does not abolish the REM sleep-related depression of activity in orofacial motoneurons. (A) In chronically instrumented, behaving cats, microinjections of strychnine into the trigeminal motor nucleus only marginally diminished the REM sleep-related depression of reflexly elicited activation of the masseter muscle. (Modified from Fig. 3 in Ref. 505 and republished with permission from Elsevier.) (B) In unanesthetized, decerebrate cats, the REM sleep-like depression of spontaneous activity of the XII nerve elicited by microinjection of a cholinergic agonist, carbachol, into the dorsomedal pontine tegmentum was well maintained despite microinjections of strychnine into the XII nucleus. (Modified from Fig. 5 in Ref. 273 and republished with permission from Elsevier.) (C) REM sleep atonia of the masseter muscle was not reduced in naturally sleeping rats by continuous microperfusion of strychnine into the trigeminal motor nucleus. QW, quiet wakefulness. (Data extracted and replotted from Fig. 4D in Ref. 64 with the authors' permission.) (C) In naturally sleeping rats, depression of activity of the genioglossus muscle (GG) measured in arbitrary units (A.U.) during the period of REM sleep without muscle twitches (“TONIC”) occurred with a similar magnitude during continuous microperfusion of the XII nucleus region with strychnine and when the nucleus was perfused with a vehicle (Control). (Data extracted and re-plotted from Fig. 4 in Ref. 358 with the authors' permission.)
Thus, while there is evidence that all orofacial motor nuclei receive dense glycinergic innervation (75, 428), this neurotransmitter may have an important role in mediation of inhibitory reflex effects and coordination of complex motor tasks required for ingestive behaviors and phonation (e.g., 313, 535, 604), whereas its role in the atonia of REM sleep is negligible.
Norepinephrine and serotonin
The negative evidence for active postsynaptic inhibition as the main cause of depression of upper airway muscle tone during REM sleep prompted a search for alternative mechanisms. A withdrawal from upper airway motoneurons of postsynaptic excitation mediated by 5-HT and NE (disfacilitation) has been proposed as the main cause of XII motoneuronal depression during REM sleep (271, 276). Subsequently, a withdrawal of aminergic excitation was found to be both necessary and sufficient to explain the REM sleep-related suppression of XII motoneuronal activity in the anesthetized rat carbachol model of REM sleep (142).
Aminergic disfacilitation was initially proposed based on studies using the decerebrate cat carbachol model of REM sleep. In decerebrate, vagotomized, paralyzed, and artificially ventilated cats, microinjections of 5-HT2 receptor antagonists into the XII nucleus caused about a 50% decline of spontaneous activity of the XII nerve. This suggested that about 50% of spontaneous XII nerve activity was dependent on endogenous serotonergic drive (276). The neural nature of this drive was confirmed in microdialysis experiments in which, in decerebrate cats, extracellular level of 5-HT in the XII nucleus region decreased during the REM sleep-like atonia and when medullary 5-HT neurons were silenced by pharmacological means (275). An estimate of the magnitude of serotonergic effect on upper airway muscle tone in English bulldogs (a natural model of OSA) conducted by means of a systemic administration of a 5-HT2 receptor antagonist and recording of sternohyoid muscle activity also revealed that about 50% of spontaneous activity of this muscle during wakefulness depended on endogenous 5-HT (191, 564). In two studies in anesthetized and vagotomized rats, microinjections of various antagonists of 5-HT2 receptors reduced XII nerve activity by about 60% (136,508). However, in a third study in anesthetized and vagotomized rats, multiple microinjections of a broad-spectrum 5-HT receptor antagonist, methysergide, placed along the entire rostro-caudal extent of the XII nucleus caused a reduction of only 15% to 30% (142). In a study in chronically instrumented, unanesthetized rats with vagi intact and microperfusion of a portion of the XII nucleus with a 5-HT2 receptor antagonist, mianserin, spontaneous GG EMG, was not significantly reduced (509). To explain the absence of significant effect in unanesthetized, behaving rats, it was proposed that the endogenous 5-HT drive was lower in these studies than in the other ones due to the absence of vagotomy. However, another plausible explanation is that local microperfusion with an antagonist was not sufficient to reveal endogenous 5-HT effects of a statistically significant magnitude because the antagonist was delivered to only a limited portion of the XII nucleus and under the conditions when the level of spontaneous activity in XII motoneurons was low. Nevertheless, collectively, these results suggested that the endogenous excitatory effect of 5-HT on XII motoneurons is weaker in rats than in other species.
In unanesthetized, decerebrate, vagotomized, paralyzed, and artificially ventilated cats, microinjections of 5-HT into the XII nucleus attenuated, but did not abolish, the REM sleep-like depression of XII nerve activity elicited by pontine carbachol (277). Similar findings were obtained from chronically instrumented, naturally sleeping rats in which administration of 5-HT into the XII nucleus region by reverse microdialysis only modestly attenuated the REM sleep-related depression of GG muscle activity (233). In further support of these findings, it was noted that even the most extensive microinjections of 5-HT receptor antagonist, methysergide into the XII nucleus made in either decerebrate cats or anesthetized rats were not able to reduce XII nerve activity by the same amount as that seen during the experimentally elicited state of the atonia of REM sleep (142, 276). Thus, the withdrawal of serotonergic excitatory effects mediated by 5-HT2 receptors may make a measurable, but only a partial, contribution to the REM sleep-related decrements of XII motoneuronal activity.
As with 5-HT, the levels of NE were also reduced in the XII nucleus region when motoneuronal activity was depressed in decerebrate cats by electrical stimulation within the dorsomedial pontine REM sleep-triggering region (288). Also as with 5-HT, measurements of the magnitude of the excitatory effect of NE on XII motoneurons suggested species differences. In urethane-anesthetized, paralyzed and artificially ventilated rats, microinjection into the XII nucleus of prazosin, a selective antagonist of α1-adrenoceptors, caused about an 80% decrease of respiratory-modulated XII nerve activity, indicating that, under these experimental conditions, XII motoneurons of rats receive a strong endogenous NE excitatory drive (142). Similarly, in unanesthetized, chronically instrumented rats, local antagonism of NE excitation in the XII nucleus region caused a profound suppression of GG EMG during wakefulness (82). In contrast, in cats, serotonergic (and also histaminergic) excitation was considerably stronger than that mediated by NE (371). Nevertheless, exogenous stimulation of α1-adrenoceptors in the XII nucleus region in chronically instrumented rats could not prevent the depression of GG muscle activity during natural REM sleep (82).
Based on the findings that NE and 5-HT partially contribute portions of endogenous excitatory drive to XII motoneurons and that both NE and 5-HT cells are silenced during REM sleep, experiments were designed to test the hypothesis that a combined pharmacological antagonism of 5-HT and NE receptors in the XII nucleus is functionally equivalent to the depression of XII motoneuronal activity during REM sleep (137,141,142). The premise of these experiments was that, if the depression of activity of XII motoneurons during REM sleep is caused by a concurrent withdrawal of excitations mediated by NE and 5-HT, then no additional REM sleep-like depression should occur following pharmacological antagonism of the relevant aminergic receptors at the level of the XII nucleus. Based on studies of the major types of 5-HT and NE receptors that may mediate excitatory effects in the XII nucleus, the α1-adrenergic receptor blocker, prazosin, and a broad-spectrum 5-HT receptor antagonist, methysergide, were used to eliminate aminergic excitatory effects. A control REM sleep-like episode was elicited by pontine carbachol injection, after which the XII nucleus was injected with a mix of NE and 5-HT receptor antagonists, and then additional REM sleep-like episodes were elicited at approximately 30 min intervals as the aminergic antagonists first attained their full effect and then started to diffuse away (Fig. 14).
Figure 14.
Evidence that the REM sleep-related depression of activity in XII motoneurons is caused by a concurrent withdrawal of endogenous activation mediated by NE and 5-HT. When a cocktail containing the α1-adrenergic receptor antagonist, prazosin, and a broad-spectrum 5-HT receptor antagonist, methysergide, is injected into the XII nucleus at multiple antero-posterior levels, spontaneous XII nerve activity is depressed to 20% to 30% of its control level and remains depressed for several hours (line graph at the top). This indicates that XII motoneurons are under a strong endogenous excitatory drive mediated by NE and 5-HT. The three sets of bar graphs at the bottom show XII nerve activity levels measured just before (B), during (D), and just after (A) REM sleep-like episodes elicited by pontine microinjections of carbachol. The first set of bars characterizes typical REM sleep-like depression elicited under the control conditions (before prazosin and methysergide injection into the XII nucleus; see Fig. 11A for a detailed time course and pattern of a typical REM sleep-like episode). Under these control conditions, XII nerve activity is depressed to 20% to 30% of the control. The second set of bars represents XII nerve activity during REM sleep-like episodes elicited 40 to 50 min after prazosin and methysergide microinjections, that is, when the antagonists exert maximal effect (cf. top graph). Under these conditions, there is no additional depression of XII nerve activity during the REM sleep-like episode beyond that already caused by the antagonists. The third set of bars shows XII nerve activity during the REM sleep-like episodes elicited when XII nerve activity partially recovered from the effect of the antagonists. At this time after the antagonist injections (about 170 min), XII nerve activity is again depressed during the REM sleep-like episodes. Thus, when endogenous activation of XII motoneurons mediated by NE and 5-HT is fully blocked (middle set of bars), no depression of XII nerve activity occurs during the REM sleep-like episodes. This indicates an occlusion between the depression caused by the antagonists and the effect of REM sleep-like state that takes place at the level of XII motoneurons. (Data from Ref. 142 visualized as Fig. 3 in Ref. 267 and republished with permission from Elsevier.)
The experiments revealed that the two antagonists injected together reduced XII nerve activity to approximately the same level as that attained during the REM sleep-like episodes elicited by pontine carbachol injections not preceded by the antagonists. Conversely, when REM sleep-like episodes were elicited while the antagonists were fully active, there was no further reduction of XII nerve activity, whereas other changes characteristic of the REM sleep-like state did occur (Fig. 14). This finding was consistent with an occlusion occurring between the depressant effect of REM sleep-like state and the combined NE and 5-HT receptor antagonism. It is also of note in Figure 14A that the depression of XII nerve activity elicited by the combined prazosin and methysergide injections developed over a period of about 10 to 20 min. This is a relatively long period during which, according to relevant estimates conducted in another study (381), the antagonist could start diffusing beyond the boundaries of the XII nucleus. Based on this observation, it has been suggested that a part of the occlusion between the effects of REM sleep-like state and pharmacological antagonism of NE and 5-HT receptors could take place outside the XII nucleus (134). While this is possible, one needs also to note that many dendrites of XII motoneurons extend considerably far outside the XII nucleus (16) and that both NE and 5-HT terminals also occur in the reticular formation lateral, ventral and ventrolateral to the XII nucleus (albeit at a lower density than within the XII nucleus). Therefore, if any relevant for these experiments antagonism of NE and 5-HT receptors occurred outside the XII nucleus, its targets could be distal dendrites of XII motoneurons and possibly cell bodies and terminals of XII premotor neurons located in the IRt region lateral and ventrolateral to the XII nucleus (Fig. 3) because these cells may also have NE and 5-HT receptors (368).
Additional experiments revealed that, when REM sleep-like atonia was elicited after injections of prazosin only or methysergide only, XII nerve activity still exhibited a significant decline. Thus, combined microinjections of prazosin and methysergide were both necessary and sufficient to fully mimic the REM sleep-like suppression of XII nerve activity (142). As such, the experiments demonstrated that, at least in the anesthetized rat model, the REM sleep-related depression of XII motoneuronal activity can be fully explained as caused by a combined withdrawal of only two excitatory inputs, noradrenergic and serotonergic. A broader implication of this finding pertains also to the role of NE and 5-HT in the control of upper airway muscle activity during NREM sleep. As discussed earlier, activity of 5-HT and NE neurons declines during NREM sleep and reaches a nadir during REM sleep. Thus, aminergic disfacilitation may contribute to a partial reduction of muscle tone during NREM sleep and then to its loss during REM sleep.
The finding that, at least in urethane-anesthetized rats, the REM sleep-like depression of XII motoneuronal activity is caused by withdrawal of motoneuronal excitation mediated by only two modulators, NE and 5-HT, and that, at least in rats, the endogenous drive mediated by NE has a dominant role in setting the baseline level of activity in XII motoneurons prompted questions about the relative role of different sources of NE projections to the XII nucleus in the REM sleep-related disfacilitation of XII motoneurons. One earlier study found that NE axonal projections to the XII nucleus originate mainly in the pontine A5, SubC, and A7 groups (10). Specifically, when quantified relative to all pontine NE neurons retrogradely labeled from the XII nucleus, the cells located in the SubC region represented 69% of the total, those in the A7 represented 21%, and those in the A5 group, 10%. In another similar study which also included medullary catecholaminergic neurons, the distribution was 43.5% for the A5 group, 21.0% for the SubC, 15% for A7 group, 18.5% for the combined medullary A1/C1 group, and only 1.7% for LC (Fig. 15A) (447). Since there are no adrenergic fibers in the XII nucleus (239), the projections from the ventrolateral medulla must have originated from the NE neurons of the A1 group. Importantly, when data from the latter study were expressed relative to the total numbers of NA cells in each group, 7.5% of all A7 cells, 5.1% of A5 cells, 3.1% of SubC cells, 0.9% of A1/C1 cells, and 0.1% of LC cells were retrogradely labeled from the XII nucleus (Fig. 15B). This suggests that, compared to other NE groups, a particularly large proportion of neurons of the A7 group provides endogenous NE drive to XII motoneurons.
Figure 15.
Semiquantitative representation of afferent projections to the XII nucleus from distinct groups of pontomedulary NE neurons based on retrograde tracing studies. (A) When expressed relative to the total number of retrogradely labeled NE neurons found throughout the brainstem following tracer injections into the XII nucleus, projections from the A5 group are most prominent, followed by SubC region, and then A1/C1 and A7 groups. (B) When the numbers of NE cells retrogradely labeled from the XII nucleus found in each group are expressed relative to the total numbers of neurons present in this cell group, the A7 group contains the highest percentage of cells sending axons to the XII nucleus, followed by the A5 group and SubC. Regardless of the quantification method, projections from the LC are negligible. In both schemes, arrow thickness is proportional to the corresponding percentage of cells retrogradely labeled from the XII nucleus. (Data from Ref. 447 modified from Fig. 4 in Ref. 267 and republished with permission from Elsevier.)
To obtain a functional estimate of the relative strength of the endogenous NE excitatory drive to XII motoneurons from different NE cell groups, experiments were conducted in urethane-anesthetized, paralyzed, and artificially ventilated rats. Since NE cells can be silenced by stimulation of their α2-adrenergic autoreceptors (214, 431), local microinjections of the α2-adrenergic receptor agonist, clonidine, were made targeting different sites of origin of NE projections to the XII nucleus with the goal to eliminate NE input from these sites and measure the impact of the injections on spontaneous XII nerve activity. In separate experiments, the A5, SubC, or A7 groups were targeted (140, 144, 146). Of all sites tested, only the injections placed in the A7 group significantly reduced spontaneous XII nerve activity by 31% following unilateral injections (146). Thus, unilateral pharmacological inhibition of A7 cells resulted in significant decrements of XII nerve activity, whereas silencing of A5 or SubC cells did not. The absence of significant effects from the A5 or SubC groups is not fully consistent with anatomical evidence for NE projections from these groups to the XII nucleus. One possible explanation is that efferent projections exist but they make relatively weak excitatory connections.
For 5-HT, neuroanatomical and neurophysiological data show that substantial differences exist in the density of innervation and magnitude of effects among different groups of orofacial motoneurons. These differences are of interest because they may be related to the differences in the effect of sleep on different upper airway muscles. For example, laryngeal (vagal) motoneurons have a higher density of 5-HT terminals than XII motoneurons (23, 525), suggesting that 5-HT may exert stronger effects on laryngeal than other orofacial motoneurons. However, the sensitivity of XII motoneurons to iontophoretically applied 5-HT was higher than that of laryngeal motoneurons when measured relative to the baseline firing rate (139). One reason for this discrepancy may be that many 5-HT terminals do not make identifiable synaptic contacts and, instead, contribute to what is called “volume transmission” (4). In this type of transmission, neurotransmitters are released into the extracellular space and then diffuse to their target receptors. Such “free” 5-HT terminals may account for about 75% of 5-HT terminals in the XII nucleus (13), whereas in the trigeminal motor nucleus they represent less than 25% of all 5-HT terminals (454). Volume transmission may be a particularly suitable mechanism for the regulation of cell excitability in relation to behavioral states of wakefulness and sleep. Therefore, the high incidence of “free” 5-HT terminals in the XII nucleus is consistent with 5-HT playing a role of a global, state-dependent modulator of excitability of XII motoneurons. In the XII nucleus, 5-HT terminals are frequently closely apposed to the distal dendrites and only rarely to the proximal dendrites and cell bodies of XII motoneurons (12, 13, 525). This is also consistent with 5-HT playing a role of a state-dependent modulator of XII motoneuronal excitability.
In addition to the differences among motoneuronal groups innervating different upper airway muscles, data discussed in this section suggest that species differences exist in the effects of 5-HT and NE at least in the case of XII motoneurons. In rats, the relative contribution of endogenous activation mediated by NE is larger than that of 5-HT, whereas in cats it is the opposite. Data also suggest that the excitatory effects mediated by 5-HT (but not by NE) vary with circadian time because mRNA and protein levels for the excitatory 5-HT2A receptors are higher in the XII nucleus during the active period (night in rats) than during the rest period (574). Accordingly, one would expect to find that endogenous activation of XII motoneurons is stronger during the active period than during the rest period of the circadian cycle. These regional, circadian and species differences uncovered in animal studies point to the need to better quantify the relative roles of 5-HT and NE in the control of upper airway muscles in humans in whom aminergic effects on upper airway motoneurons are especially relevant for the pathophysiology of OSA.
Excitatory peptides—orexins, thyrotropin-releasing hormone, and substance P
Orexin cells are localized exclusively in the perifornical region of the posterior hypothalamus (410, 461). They have the highest level of activity during active wakefulness, minimal or no activity during NREM sleep and low-level, bursty activity during REM sleep (Fig. 4). They send axons to many sites within the forebrain, brainstem, and spinal cord, and in particular to those regions that are active during wakefulness (Fig. 16). Among their major targets are motoneurons, including those innervating orofacial muscles, brainstem NE, 5-HT and ACh neurons, and sympathetic preganglionic neurons (115, 161, 212, 573, 615). Given the excitatory nature of orexins and strong connections of orexin neurons with other wake-related targets, orexins are able to amplify other excitatory effects in the CNS in relation to wakefulness. Conversely, a loss of orexinergic excitation results in reduced alertness and reduced motor activation.
Figure 16.
Pathways transmitting activation from the hypothalamic wake-active orexin neurons to orofacial motoneurons. Anatomical studies indicate that ACh, 5-HT, and NE brainstem neurons as well as motoneurons, are among the major targets of axons descending from hypothalamic orexin-containing neurons. However, physiological and pharmacological studies reveal that nonorexin cells located in the posterior hypothalamic orexinergic region also can significantly influence the respiratory output, including upper airway motoneurons. In particular, neither the combined antagonism of 5-HT and NE-mediated excitation at the XII nucleus level (147), nor microinjections of a dual orexin receptor antagonist into the XII nucleus (518), could significantly attenuate activation of XII motoneurons from the hypothalamic region containing orexin neurons. On the other hand, microinjections of a dual orexin receptor antagonist into the hypothalamic orexin cell field significantly attenuated activation of XII motoneurons from this hypothalamic region (518). These results support the presence of an excitatory pathway that descends from the posterior hypothalamus parallel to the orexinergic pathway and targets different components of the respiratory system, including upper airway motoneurons. Additional connections may exist, such as nonorexinergic excitatory hypothalamo-brainstem pathways to ACh, 5-HT and NE neurons, and there may be additional orexinergic connections to central respiratory neurons, although specific details are not yet available. Cholinergic effects within the respiratory network may be excitatory or inhibitory (marked with yellow circles).
Orexin-containing cells are under strong endogenous inhibition mediated by GABAA receptors in both chronically instrumented, behaving and anesthetized animals (8, 314). This allows one to experimentally increase orexin cell activity by locally blocking GABAergic inhibition with antagonists such as bicuculline or gabazine. In unanesthetized rats, disinhibition of orexin and other cells located in the hypothalamic perifornical region increases wakefulness and suppresses sleep (8,570). In anesthetized rats, bicuculline microinjections into the perifornical region activate upper airway muscle tone, increase the respiratory rate and arterial blood pressure, and cause cortical and hippocampal activation (314).
Although anatomical data show that NE and 5-HT neurons are among the major targets of orexinergic projections and exogenous orexin strongly activates these neurons (55, 212, 228), experimental activation of orexin and other cells located in the perifornical hypothalamus can increase activity of XII motoneurons even when 5-HT2 and α1-adrenergic receptors are blocked at the level of the XII nucleus (147). This could happen if activation of XII motoneurons were mediated by direct descending projections of hypothalamic orexin neurons to the XII motor nucleus (Fig. 16). However, activation of XII motoneurons elicited by activation of posterior hypothalamic perifornical neurons was not attenuated when the orexin type 2 receptor antagonist, almorexant (15 mg/kg) (62) was injected into the XII nucleus (518).
In the same model, a systemic administrations of almorexant attenuated, but did not abolish, the tachypneic and sympathoexcitatory effects of hypothalamic microinjections of bicuculline (220). Thus, as proposed in Figure 16, a part of the response elicited by disinhibition of hypothalamic perifornical neurons may be due to activation of nonorexinergic neurons located in this region. One role of endogenous orexin in the control of upper airway motoneurons that has been revealed to date is exerted at the very site where orexin-containing cells are located. Specifically, when almorexant was injected into the posterior hypothalamus prior to activation of posterior hypothalamic cells with the GABAA receptor antagonist, gabazine, the resulting activation of XII motoneurons was delayed and attenuated and the increases in respiratory rate and heart rate were diminished when compared to the effects of hypothalamic gabazine without almorexant pretreatment (518). Collectively, these findings suggest that the effects elicited by disinhibition of cells in the perifornical hypothalamic region originate in both orexin-containing and other neurons with overlapping projections and redundant functions. It also appears that orexin cells excite other adjacent neurons that, in turn, distribute this excitation to different cardiorespiratory outputs.
The loss of orexinergic activation is associated with reduced respiratory chemosensitivity (101). Stimulation of breathing by elevated inspiratory CO2 increases expression of the early gene c-Fos in orexin and other adjacent hypothalamic neurons (526). Increased c-Fos expression in these neurons suggests that their firing rate is increased. This may be related to anxiety triggered by the feeling of dyspnea. Indeed, activation of orexin neurons during hypercapnia may lead to an increased arousal and anxiety (593). Regardless of the underlying mechanisms, orexin cell activation secondary to hypercapnia can facilitate ventilatory effort. It is also of note that even before information about the location and neurochemical nature of orexin neurons was established, the perifornical region of the posterior hypothalamus was recognized as an important site for the maintenance of wakefulness and for the coordination of the behavioral “fight or flight” response (e.g., 36,108,121). There is now compelling support for orexinergic excitation being one of the neurochemical substrates of both the “fight or flight” response and the wakefulness stimulus for breathing.
Baseline ventilation in orexin knockout (KO) mice does not differ from that in the wild-type strains but the KO animals exhibit reduced ventilatory response when breathing is stimulated by increased levels of inspired CO2. Other evidence from rodents further supports a role of orexins in ventilatory activation elicited by hypercapnia and central acidification. In arterially perfused brainstem-spinal cord juvenile rat preparation, respiratory rate response to hypercapnia is attenuated by bath application of an orexin-1 receptor antagonist (94). This suggests that any endogenous orexin that might be present in the brainstem of this preparation acts at the brainstem level to facilitate transmission of CO2 stimulus to the central respiratory rhythm generating neurons. Such an action may be exerted in the ventrolateral medullary retrotrapezoid nucleus neurons which are established central sensors of brain acidification (295). However, an alternative explanation for these findings would be that relevant orexin receptors are constitutively active and pH-sensitive in the conditions of these experiments.
Investigation of the sleep-wake state-dependent role of orexins in modulation of CO2 sensitivity in orexin KO and wild-type mice suggests that orexins do not exert any effects on the hypoxic ventilatory response and facilitate ventilatory response to hypercapnia only during wakefulness (366). In another study, facilitatory effects of orexins on hypercapnic ventilatory response were strongest during wakefulness bouts occurring during the dark (active) phase of the circadian cycle, and the ventrolateral medullary retrotrapezoid region was found to be an important site where such a state- and circadian time-dependent facilitation could take place (106). The state dependence of the effect of orexin on ventilatory chemosensitivity is consistent with the well-established state dependence of orexin cell activity. Orexin KO mice also have more frequent sleep-related central apneas (350, 366). The increased expression of central sleep apneas in orexin KO mice is puzzling because orexin cells are nearly silent during NREM sleep. Nevertheless, the increased propensity for central apneas in orexin KO mice may be taken to suggest that withdrawal of orexinergic excitation elevates the hypocapnic apneic threshold. Appropriate experiments directly addressing this possibility remain to be conducted.
TRH and SP are colocalized in medullary 5-HT neurons and may be coreleased from their axon terminals (167, 195, 197, 204, 525, 536). Both are excitatory to upper airway motoneurons (41,165,434,587), with both neurokinin receptors (for SP) and TRH receptors expressed in orofacial motor nuclei (165, 189, 321, 367; see 163 for a review). The effects of SP on XII motoneurons are similar to those of NE, with both sharing the same intracellular pathways (606). It is not known under what conditions TRH and SP exert endogenous excitatory effects on upper airway motoneurons, but it has been proposed that the release of peptides from axon terminals of raphé neurons requires higher levels of activity than those needed to release 5-HT (229). During strong motor and respiratory efforts, the firing rate of caudal raphé cells increases by 50% to 100% above the 1 to 4 Hz typical for quiet wakefulness and may occur in bursts (563). This raises the possibility that TRH and/or SP are released at times of enhanced respiratory effort; e.g., during exercise or arousals triggered by airway occlusion. In addition to the role of firing rate, complex pre- and postsynaptic interactions between 5-HT and associated peptides were proposed as important determinants of the conditions under which peptides are likely to be released (201,348). The powerful effects observed when exogenous SP or TRH are applied onto upper airway motoneurons warrant more research, as there is a potential for their therapeutic use in OSA, but progress in this area is hampered by limited availability of suitable receptor agonists and antagonists.
Acetylcholine
Cholinergic cells of the IRt region are probably the main source of ACh afferents to the orofacial motor nuclei (553, 572). This region extends throughout the length of the medulla and also contains inspiratory-modulated neurons that mediate inspiratory drive to XII motoneurons, cells that mediate reflex effects to XII motoneurons (80, 512, 551) and cells important for the generation of twitches that occur during REM sleep in the masseter muscles (19). Most of these cells are probably glutamatergic (69,164,513), whereas the role of ACh cells of the IRt region remains to be defined. Activity of many inspiratory-modulated IRt neurons increases during carbachol-induced REM sleep-like state (600), whereas the effect of REM sleep-like state on activity of identified ACh neurons of the IRt region is unknown. Additional ACh projections to the XII nucleus originate in the dorsal pontine tegmentum. These projections appear to be relatively scant and primarily originate in the pars-compacta of the PPN and α part of the LDN (448). These cells may have state dependent activity typical of pontine ACh neurons (Fig. 4); some may be maximally active during both wakefulness and REM sleep, others may have relatively selective activity increases during REM sleep, and still others may generate phasic bursts in association with phasic events of REM sleep.
ACh exerts both pre- and postsynaptic effects on orofacial motoneurons (43,79,423,613). Postsynaptic excitatory effects are mediated by nicotinic (N) receptors (79,613), but mRNAs for both N and M receptors are present in XII premotor neurons of the IRt region and neurons of the VRG (484, 572; see 272 for a review). Accordingly, these medullary inspiratory cells may express both N and M receptors as postsynaptic receptors on their cell bodies or as presynaptic receptors on their axon terminals. M2 receptors most commonly occur as presynaptic receptors that negatively control ACh release (291). The presence of M2 receptor mRNA in glutamatergic XII premotor neurons of the medullary IRt region additionally suggests an arrangement where ACh may presynaptically inhibit glutamate release, thereby suppressing the magnitude of inspiratory drive to XII motoneurons. Presynaptic inhibition of glutamatergic activation of XII motoneurons mediated by M2 receptors has been demonstrated in in vitro medullary slices from juvenile rats (43). In these experiments, the effect occurred without changes in postsynaptic properties of motoneurons and was associated with a reduction in the frequency, but not amplitude, of spontaneous postsynaptic currents, as would be expected from inhibition of glutamate release from presynaptic terminals. Both N excitation and M suppression of GG EMG was also observed in in vivo studies in urethane-anesthetized rats in which various cholinergic agonists and antagonists were microperfused into the XII nucleus region (310). Under these conditions, the suppressant effects mediated by M receptors were more powerful than the excitation mediated by N receptors. M receptor-mediated activation was also reported in neonatal XII motoneurons (221). These excitatory effects could be mediated by M1 receptors (291).
In chronically instrumented, behaving rats, microdialysis perfusion of the XII nucleus region with a broad spectrum M receptor antagonist, scopolamine, resulted in a significant increase of GG muscle activity during wakefulness, NREM sleep and the REM sleep periods without phasic muscle twitches (170). Because the absolute magnitude of this effect was similar in all three states, the increases expressed relative to the control activity level in each state were more prominent during NREM sleep than during wakefulness, and even more so during REM sleep without muscle twitches. Remarkably, the activity-enhancing effect of scopolamine was most powerful during the periods of REM sleep with muscle twitches. The increases during this period were significantly larger than those observed during either wakefulness or NREM sleep regardless of whether the absolute or relative magnitude was considered as a reference. This suggests that scopolamine preferentially facilitated transmission to XII motoneurons of phasic excitatory synaptic inputs that cause muscle twitches.
Scopolamine is a broad-spectrum M receptor antagonist but the effects of its delivery to the XII nucleus region across sleep-wake states combined with the known distributions of different M receptors in the brainstem have led to the suggestion that scopolamine enhanced GG muscle activity preferentially during REM sleep with twitches due to its antagonistic action on M2 receptors (170). This hypothesis needs to be further explored using more selective antagonists of M2 receptors applied over a range of doses and in combination with selective agonists. It will also be important to determine whether the XII nucleus is the main site where the state-dependent effect of scopolamine is exerted because the microdialysis probes used were sufficiently long to extend considerably beyond the boundaries of the XII nucleus. The sites located dorsal and ventrolateral to the XII nucleus include the viscerosensory NTS and the dorsomedial part of the IRt region, both of which contain various M receptors (33, 68, 320, 422, 567). Nevertheless, the findings with scopolamine prompted Grace et al. (170) to focus on the possibility that M2 receptors are the most likely target of scopolamine in their experiments. This, in turn, directed their attention to certain class of potassium (K+) channels that are implicated in mediation of M2 receptor effects, as discussed in the next section.
Potassium channels
Pharmacological and genetic studies have led to the identification of diverse of K+ channels. These channels occur both in the CNS and peripheral organs, including the heart, kidney and lungs; they play major roles in normal physiologic processes and contribute to pathologies as diverse as cancer and diabetes (see 88,95,317,319,481,528,548,554 for reviews of K+ channels in the brain). Under most conditions, opening of these channels results in an increased flow of K+ ions out of the cell and, consequently, a shift toward a more hyperpolarized membrane potential. The channels differ in their kinetics and second messenger pathways, have different activating and modulating factors, and some are voltage dependent. Among the latter, some are activated only when membrane potential is close to the equilibrium potential for K+ ions, which effectively makes them act as stabilizers of membrane potential near a relatively hyperpolarized level. K+ channels of different types occur with different densities in different brain regions.
The diversity of K+ channels is highly relevant to the discussion of state-dependent control of the motor output to upper airway muscles across sleep-wake states because many of the transmitters and modulators that act in a state-dependent manner exert their effects by acting on these channels. For example, neuronal depolarization elicited by 5-HT, NE, orexins, TRH, and SP is achieved through a closure of constitutively open K+ channels (3, 163, 165, 228, 293, 554, 606). Conversely, when release of these modulators is reduced, K+ channels partially reopen, leading to hyperpolarization. K+ channels also mediate different components of afterhyperpolarization that follows each action potential; this, in turn, stabilizes the neuronal firing rate. Other K+ channels are positively coupled to inhibitory receptors, such as GABAB or μ-opioid receptors, resulting in their opening and cell hyperpolarization when the receptors are stimulated by their ligands. In a subset of K+ channels, their opening varies with pH; such channels were found in brainstem cells responsible for central respiratory chemosensitivity, and their elimination resulted in impaired ventilatory response to CO2 (577, 578). The availability of specific pharmacological tools for identification of different K+ channels in relation to their different roles is limited. Nevertheless, the large diversity and powerful effects of K+ channel manipulations on neuronal excitability make these channels an attractive target for experimental and therapeutic interventions.
The potential to target K+ channels with a goal to increase upper airway muscle tone during sleep as the means for a pharmacological treatment of OSA has been considered in a recent review (172). Based on their own data and evidence published by others, the authors proposed that M2 receptors located within the XII nucleus and coupled to K+ channels mediate an inhibitory effect on XII motoneurons that is preferentially exerted during REM sleep. The basis for this was the finding that perfusion of the XII nucleus region with Tertiapin-Q, a compound acting mainly on a subset of K+ channels (the inwardly rectifying channels: GIRK1/Kir 3.1, GIRK4/Kir 3.4 and ROMK1/Kir 1.1; as well as the calcium-activated (KCa) large conductance (BK) channel), had quantitatively similar effects on GG muscle activity as the perfusion of the XII nucleus with the M receptor antagonist, scopolamine (170). Furthermore, since M2 receptors are typically coupled to a subset of Kir channels, Grace et al. (172) proposed that the K+ channels involved in the state-dependent control of XII motoneurons may include the Kir2.4 subunit because this subunit is prominently expressed in the orofacial motor nuclei. The relative role of this one versus other K+ channels that may also regulate other aspects of orofacial motoneuronal activity remains to be investigated.
While certain K+ channels may be actively involved in mediation of the NREM or REM sleep-related depression of activity in upper airway muscles, the well-established role of voltage-dependent (Kv), and tandem pore domain (K2P/TASK) K+ channels in mediation of the excitatory effects of NE, 5-HT, orexins, TRH and SP makes them worth of interest as potential targets for enhancement of upper airway muscle activity during sleep in OSA. With this in mind, Grace et al. (171) investigated the effects of microdialysis perfusion of the XII nucleus region with various K+ channel blockers in chronically instrumented, behaving rats. The key findings from that study are illustrated in Figure 17. When barium ions (Ba+2) were included in the perfusate to preferentially block Kir channels, GG muscle activity was elevated in all sleep-wake states (Fig. 17A), with the effect being most prominent during wakefulness and during REM sleep with muscle twitches (REM(+) in the figure). XII nucleus perfusion with an experimental antagonist of TASK channels, methanandamide, yielded effects different from the effects of Ba+2 in that excitation was limited to the period of wakefulness (Fig. 17B). This could be explained by assuming that the main action of this antagonist was on the same K+ channels that are being closed by activation of 5-HT, NE, and other receptors that are mainly active during wakefulness and only minimally so during NREM or REM sleep. In contrast, perfusion with 4-aminopyridine (4-AP) or tetraethylammonium (TEA) which, respectively, block the voltage-dependent Kv4 and Kv2 channels, increased GG activity across all sleep-wake states with no preference for wakefulness (Fig. 17C). Interestingly, both 4-AP and TEA were especially effective during REM sleep periods with twitches, suggesting a suppressant effect of certain K+ channel opening on the transmission of phasic twitches to XII motoneurons. Collectively, these experiments demonstrated that closure of certain K+ channels can elevate GG muscle activity to, or above, the levels of activity during wakefulness. By extension, a sufficiently strong activation may also be achievable in OSA subjects whose upper airway muscle tone needs to be higher than in subjects with fully patent upper airway to protect the airway from collapse. The questions of which K+ channels can be targeted most effectively and selectively, and how to deliver the drugs without prohibitive side effects, remain to be resolved through additional experiments.
Figure 17.
Effects of microdialysis perfusion of the XII nucleus region with different K+ channel blockers in chronically instrumented, behaving rats. (A) Perfusion with a solution containing barium ions, which primarily block the inwardly rectifying (Kir) channels, resulted in GG muscle activity being elevated in all sleep-wake states and attaining the highest levels during wakefulness and REM sleep with muscle twitches (REM(+)). (B) During perfusion with an antagonist of the tandem pore domain (TASK) channels, methanandamide, activation was limited to wakefulness. (C) Perfusion with 4-aminopyridine (4-AP, a blocker of voltage-dependent Kv4 channels) or TEA (a blocker of voltage-dependent Kv2 channels) increased GG activity across all sleep-wake states, with the strongest activation attained during REM sleep with twitches. Collectively, these experiments demonstrate that closure of certain K+ channels can elevate GG muscle activity during sleep to, or above, the levels seen during wakefulness. In all panels, integrated GG activity was measured during inspiration within different sleep-wake states using arbitrary units while the XII nucleus region was perfused with a vehicle [artificial cerebrospinal fluid (ACSF) with or without an emulsificant] or with one of the blockers. Symbols in the graphs indicate statistically significant elevation of activity with the blocker when compared to vehicle. (Panels A–C show Figs. 1E, 2B and 3C, respectively, from Ref. 171 and are republished with permission from the American Academy of Sleep Medicine obtained via the Copyright Clearance Center, Inc.)
Convergence of state-dependent pathways on upper airway motoneurons
As a result of many years of research motivated by clinical problems posed by OSA, a large body of knowledge has been accumulated about the basic neurochemistry and neurophysiology of the different pathways that converge on upper airway motoneurons and control their respiratory behaviors or changes with the states of wakefulness and sleep. The scheme in Figure 18 shows how such inputs converge on an upper airway motoneuron. Considerably more is currently known about the state-dependent effects mediated by 5-HT, NE and ACh than about histamine or peptidergic effects. The role of glutamate as the main transmitter mediating respiratory drive to motoneurons and the different modulatory pathways that regulate this input mainly at the presynaptic level and in a state-dependent manner have been explored extensively. There is a consensus that active postsynaptic inhibition mediated by amino acids, albeit detectable in upper airway motoneurons, plays a minor role in sleep-related decrements of their activity. However, there is multifaceted evidence that such postsynaptic inhibition is very important for coordination of reflexly and centrally controlled use of upper airway motoneurons for their nonrespiratory function. For more information on excitation and active inhibition of orofacial motoneurons during nonrespiratory behaviors the reader is referred to other sources (e.g., 39, 313, 535).
Figure 18.
Summary of neurochemically distinct pathways that converge on upper airway motoneurons and determine the respiratory and sleep-wake state-dependent patterns of motoneuronal activity. Among the many excitatory pathways (shown on the left) the glutamatergic one mediates the respiratory drive, whereas the effects of all the remaining ones are likely to be wakefulness (or wakefulness and REM sleep) related. Among the inhibitory pathways (shown on the right), GABA, and glycine are especially important for shaping the reflex responses and rhythmic activities of orofacial motoneurons, whereas ACh may mediate pre- and postsynaptic, state-dependent inhibitory effects through muscarinic (M) receptors [in addition to the excitatory effects of ACh mediated by nicotinic (N) receptors]. Additional state-dependent and state-independent presynaptic effects are exerted on the respiratory (glutamatergic) input to motoneurons by 5-HT and GABA.
With a lot already known, it is worthwhile to acknowledge a few important gaps in the present knowledge and consider possible future research directions. First, it is now apparent that there is a disparity between our understanding of the mechanisms exerting state-dependent control over the motor output to the upper airway relative to the corresponding control of the axial and limb muscles. This is somewhat ironic because the original inspiration for the studies of state dependence of upper airway motoneurons was derived from research on the effects of sleep on postural muscles (e.g., 168, 352–354, 417, 506). The current picture is that, qualitatively, the sets of neurochemically distinct, state-dependent inputs converging on upper airway and spinal motoneurons are similar. Beyond this, however, there are probably significant quantitative differences that have not been addressed because the progress with studies of state-dependent control of postural muscles of the spinal cord has largely stalled in recent years while significant advances were being made in the corresponding research on the control of upper airway muscles. In particular, experiments with direct administration of inhibitory amino acid antagonists into the orofacial motor nuclei revealed a very limited role of active inhibition during REM sleep (Fig. 13). This, in turn, resulted in intense exploration of alternative mechanisms and led to the discovery of important roles of 5-HT, NE, and ACh and their interactions with glutamate (82, 142, 170, 233, 275, 276, 474). In contrast, no such experiments have been undertaken to explore spinal motoneurons. This is, in part, due to technical limitations; spinal motor columns are less amenable to experimental approaches based on local and selective infiltration of targeted motor nuclei with neurotransmitter receptor agonists and antagonists than the cranial motor nuclei. Nevertheless, this line of research on spinal motoneurons is needed to determine, both qualitatively and quantitatively, whether the mechanisms of state-dependent control differ between different pools of spinal motoneurons (e.g., those innervating axial vs. distal muscles, or upper spinal vs. lumbar motoneurons), and between spinal and orofacial pools. It is of note that there has been progress with quantification and comparative studies of the pattern and intensity of muscle twitches occurring in different orofacial, axial and distal muscles innervated by spinal nerves (e.g., 47, 157, 245, 332, 439, 446). This should help with cellular studies of the neurochemical, neurophysiological, and neuroanatomical basis of differences between state-dependent control of spinal and orofacial motoneurons.
Another question that needs a reconciliation emerged as a result of findings from rat XII motoneurons that appear to propose alternative mechanisms of depression of activity in XII motoneurons during REM sleep. On the one hand, Fenik et al. (142) proposed that the REM sleep-related depression of XII motoneuronal activity can be nearly completely explained as caused by a combined withdrawal of excitation mediated by just two transmitters, 5-HT and NE. In a related study, they also demonstrated that any additional active inhibitory process mediated by GABA or glycine was negligible (137). On the other hand, Grace et al. (170) provided evidence suggesting that a major part of the REM sleep-related suppression of activity in XII motoneurons is dependent on an active inhibitory process that resides within or adjacent to the XII nucleus and is mediated by M2 receptors. Furthermore, based on the findings that a direct administration of 5-HT and/or NE receptor agonists into the XII nucleus region could not counter the REM sleep-related depression of activity in XII motoneurons, the same group concluded that mechanisms other than disfacilitation resulting from a withdrawal of excitatory effects of 5-HT and NE may play an important role (82, 233). Since all these results were obtained from rats, species differences can be excluded as a reason for different conclusions. However, several other important differences and technical issues need to be pointed out. First, while the conclusion that a combined withdrawal of 5-HT and NE is sufficient was derived from studies with microinjections of the relevant receptor antagonists into the XII nucleus, the opposite conclusion that withdrawal of 5-HT and NE is not sufficient was obtained by attempts to infiltrate the XII nucleus with receptor agonists. Because it is technically more difficult, if possible at all, to fully saturate the relevant receptors with agonists without a concomitant side effect from exogenous stimulation of relevant receptors located in the regions adjacent to the target site, it is not unexpected that agonist treatment could not eliminate the REM sleep-related depression. Indeed, the same limitation was also considered in earlier studies in cats (277). Second, it is important to note that the data pointing to a major and possibly sufficient role of withdrawal of 5-HT- and NE-mediated excitation was obtained from a rat carbachol model of the atonia of REM sleep, a model in which non-respiratory phasic bursts of motor activation do not occur. In contrast, the most prominent antisuppressant effect of the M receptor antagonist, scopolamine, was observed during the periods of natural REM sleep with phasic muscle twitches. Thus, it is possible that the effects of scopolamine, interpreted as reflective of its M2 receptor antagonistic properties, are exerted on the pathway(s) that mediate phasic muscle twitches during REM sleep. As such, the roles of 5-HT, NE, and ACh would be complementary, with little overlap between their distinct roles in the control of upper airway motoneurons during REM sleep.
Lastly, it is worth noting in Figure 18 that, among the different wakefulness-related excitatory effects on motoneurons innervating upper airway muscles, there are those mediated by TRH, SP, orexins and histamine. Common to all these sources is that there is so far no evidence for the presence of endogenous activation of motoneurons mediated by these pathways. It is possible that such endogenous effects are exerted only under certain emergency conditions. Studies with appropriate antagonists conducted over a range of physiologic and pathophysiologic states are needed to explore this question. The long-term relevance of this is that peptidergic activation may modulate endogenous effects mediated by transmitters such as 5-HT, NE, and ACh by convergence on the same second messenger systems. Furthermore, if some of these peptidergic effects are rarely activated under the physiologic conditions, the intracellular pathways activated by these peptides may prove to be an attractive target for pharmacological interventions to treat OSA in a manner that does not interfere with the basic endogenous mechanisms of state-dependent control of motoneurons.
Neural Control of the Upper Airway in Sleep-Disordered Breathing
The focus of this section is on the neurophysiological and neurochemical aspects of upper airway control specific to OSA in adult humans. Several other distinct disorders of breathing also occur in a state-dependent manner. These include the Pickwickian syndrome/obesity hypoventilation syndrome, central sleep apnea, Cheyne-Stokes, and other forms of periodic breathing, sudden infant death syndrome (SIDS), Rett syndrome, and congenital central hypoventilation syndrome (Hirschsprung disease or Ondine's curse). Their pathophysiology has been reviewed in other articles of this series (166, 232, 413, 545). The reader is also referred to several recent reviews for a discussion of similarities and differences between OSA in adults and children (175, 251, 470).
Upper airway muscle activity in OSA patients
Most mechanistic studies discussed so far used various animal models to uncover the mechanisms underlying the sleep-related decrements of upper airway muscle activity. Although the fundamental mechanisms that regulate sleep-wake behavior and the control of breathing are similar in humans and other mammals, OSA is an almost uniquely human disorder. The likely reasons for the special vulnerability of the human upper airway to collapse may be the elongation of the upper airway and high mobility of the hyoid bone that evolved to enable the production of speech. Be it as it may, data derived from animal models that do not chronically experience recurrent periods of hypoxia and repeated sleep disruptions, such as rats or cats, may miss important aspects of the central neural control of breathing during sleep that are activated in association with the pathophysiological conditions of OSA. By the same token, upper airway control in OSA patients may differ from that in healthy humans due to the chronic presence of the disorder and in association with anatomical predispositions to OSA.
OSA patients commonly have structural features of the upper airway that result in a reduced upper airway lumen and collapsible pharyngeal walls. These features may include excessive accumulation of fat in upper airway soft tissue, large pharyngeal organs (tongue, soft palate, and tonsils), or altered bony structures (71,357,405,470). Also, recurrent nocturnal episodic hypoxia as well as the loss and fragmentation of sleep, have multiple adverse cardiorespiratory, metabolic, and other consequences. Although obstructive episodes of OSA are not acutely life-threatening, the incidence of arterial hypertension, insulin resistance, hyperlipidemia, stroke, and the overall mortality are all increased in OSA patients (30, 301, 421, 544, 610).
OSA patients adapt to the structural vulnerability of their upper airway by increasing activity of upper airway dilator muscles during wakefulness when compared to the level of activity seen in healthy individuals (247, 281, 311, 345). However, this adaptation is partially lost during NREM sleep and even more so during REM sleep. Simultaneous recordings of upper airway muscle activity and airflow consistently show that flow limitations and occlusions occur at the time when upper airway muscle activity reaches its nadir (e.g., 66, 378, 435). In OSA, the airway cross-sectional area decreases to such an extent that the patients experience episodes of clinically significant hypoventilation or asphyxia. Also as a consequence of the compensatory increase of upper airway muscle tone during wakefulness, the magnitude of sleep-related decrease of upper airway muscle tone at sleep onset is higher in sleep apneics than in normal subjects (246,346). The initial precipitous decline caused by the transition from wakefulness to NREM sleep, or from NREM sleep to REM sleep, may then be partially corrected by a reflexly elicited increase of upper airway muscle activity which, in a subpopulation of OSA patients, may effectively protect the airway from collapse. Such patients may experience periods of hypoventilation during sleep, but their sleep is not disrupted by arousals that are otherwise needed to increase upper airway muscle tone, reopen the airway, and re-establish ventilation (429,609). More typically, however, reflex stimulation of respiratory effort of the diaphragm, intercostal, and abdominal muscles secondary to the initial hypoventilation leads to an upper airway closure and the obstruction elicits arousal. A delayed timing and insufficient magnitude of inspiratory activation of upper airway muscles relative to the respiratory pump muscles also may facilitate upper airway closure due to inadequate compensation for the airway-collapsing effect of negative inspiratory pressure (217). In a subset of OSA patients whose critical closing pressure (Pcrit) is positive (111, 226, 256), the airway may collapse in association with the cessation of expiratory flow near the end of expiration even prior to the onset of inspiratory effort. In severe OSA, obstructive episodes may occur as frequently as 50 times per hour, resulting in a dramatic sleep loss and fragmentation, episodic hypoxia, and profound daytime sleepiness.
According to one hypothesis, reflex activation of upper airway dilator muscles from upper airway negative pressure mechanoreceptors is the cause of the increased upper airway muscle tone during wakefulness in OSA subjects (345,346,585). The observation that positive pressure applied to the upper airway elicits a larger drop of upper airway muscle activity in OSA patients than in normal subjects has been interpreted as evidence that the reflex drive is enhanced in OSA subjects (345, 346). Along the same line, hyperventilation also caused a larger increase in upper airway resistance in OSA patients than in normal subjects (31, 479). However, direct comparisons of the gain of upper airway reflex responses to negative airway pressure either did not reveal significant differences between OSA patients and control subjects (152,198) or provided evidence that the reflex control of upper airway muscle tone in OSA patients is impaired (334). Another explanation for the increased upper airway muscle activity during wakefulness in OSA patients would be that the perception of upper airway resistance is responsible for the increase. This mechanism would, by definition, no longer function during sleep, thus contributing to the larger sleep-related decreases in upper airway motor tone in OSA patients than in normal subjects (294). The increased upper airway muscle tone in OSA may also be driven centrally as one of the long-term consequences of OSA. The result would be an increase of the baseline level of activity in upper airway motoneurons. As a secondary effect, this could also facilitate transmission of reflex effects to upper airway motoneurons. There is evidence from studies in rats subjected to intermittently occurring hypoxia that such a central upregulation does occur (discussed in subsequent sections).
In addition to the elevated level of upper airway muscle tone in OSA patients during wakefulness, a comparison of the sleep-wake patterns of activity in upper airway muscles in animals with no upper airway vulnerability to the pattern in humans, and OSA patients in particular, reveals other differences. With upper airway muscle tone being very low during quiet wakefulness in animals and humans with non-collapsible upper airway, upper airway muscle activity often entirely disappears at the onset of NREM sleep. Consequently, no further decline can be measured at the onset of REM sleep (247, 316, 360), and an increase then occurs in association with muscle twitches (191, 315, 316, 437, 446, 455). In the same subjects or species, when the baseline tone is experimentally increased, be it by an increased chemical drive for breathing (211,400,401), application of stimulants onto upper airway motoneurons (233), vagotomy (255), or in the context of OSA (338), a measurable level of upper airway muscle tone is usually maintained throughout NREM sleep and a distinct decline occurs at the entry into REM sleep. Interestingly, nonrespiratory bursts of activity (twitches) reported in some recordings during REM sleep in healthy humans (91, 278) are absent (or excluded from analysis) in the few published recordings of upper airway muscle activity in OSA subjects. The absence of such bursts would suggest that the need to maintain a steady airway-dilating tone in OSA is associated with a suppression of the central sources responsible for the phasic bursts of activity during REM sleep.
Thus, the baseline levels of upper airway muscle activity in wakefulness and the pattern of changes in upper airway muscle tone across the sleep-wake cycle differ between healthy subjects and OSA patients. Whereas in the former activity is often abolished at the onset of NREM sleep, in the latter, activity is characteristically increased during wakefulness as a matter of a compensatory response to the mechanical vulnerability of the upper airway, the increase is at least partially carried over into NREM sleep, and a nadir occurs during REM sleep. These differences are exemplified in Figure 19 based on a comparison of measurements from control dogs and English bulldogs who have anatomically compromised upper airway and experience sleep-related hypotonia (190, 191).
Figure 19.
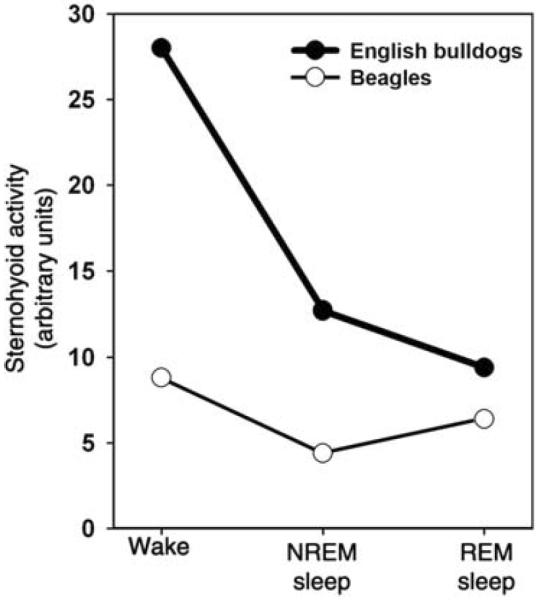
Upper airway muscle activity changes across sleep-wake states have different patterns in healthy subjects and OSA subjects with anatomically compromised upper airway. The graph compares measurements obtained from recordings of sternohyoid muscle activity in English bulldogs, who present with OSA (especially during REM sleep), and in normal dogs (beagles). During wakefulness, sternohyoid EMG is higher in English bulldogs than in beagles. Furthermore, whereas in bulldogs sternohyoid EMG steadily declines from wakefulness to NREM sleep and then REM sleep, in beagles, there is a decline between wakefulness and NREM sleep and then an increase during REM sleep. The increase during REM sleep is similar to that in rats (315, 316, 340), other healthy dogs (455), cats (437), and healthy humans (91, 278). (Graphical representation of numerical data in Ref. 191 published as Fig. 3B in Ref. 270 and republished with permission from Informa Healthcare, a member of the Taylor and Francis Group, obtained via the Copyright Clearance Center, Inc.)
Several distinct neurochemical and genetic changes have been associated with OSA. A polymorphism in 5-HT2A and 5-HT2C receptor genes has been reported in different populations (292,458), and serotonergic deficiency was identified in the brains of infant victims of the SIDS (116,545). These findings appear to well correspond to the data from experimental animals showing an important role of excitation of upper airway motoneurons mediated by 5-HT in the maintenance of upper airway patency, as discussed in the preceding sections. However, the 5-HT2A receptor link did not reach statistical significance in a follow up association study (404), and no association was observed between either 5-HT2A or 5-HT2C receptor gene polymorphism and OSA in another study in Japanese population (458).
OSA patients have reduced plasma levels of orexin (70, 376) which are then increased following treatment with continuous positive airway pressure (CPAP) (459). A plausible explanation of these findings is that the excessive sleepiness typically present in OSA patients results in lower levels of orexin cell activity and, consequently, reduced plasma orexin levels. Once sleep is normalized, sleepiness is at least partially alleviated, and orexin cell activity rebounds. However, gene association studies also reveal a significant relationship between the genetic predisposition for narcolepsy-cataplexy and OSA. Specifically, a distinct polymorphism of the preproorexin gene has been associated with OSA (5, 89). It is possible that subtle metabolic consequences of orexin deficiency contribute to the link between OSA and narcolepsy.
Interestingly, in contrast to the findings in rats, patients who have the human leukocyte antigen allele that predisposes to narcolepsy-cataplexy (DQB1*0602) do not exhibit reduced ventilatory sensitivity to hypercapnia. Instead, they have a depressed ventilatory response to hypoxia (184). The discrepancy between the impairment of the hypoxic ventilatory response in humans versus hypercapnic ventilatory response in orexin-deficient mice (discussed earlier) suggests a complex and only indirect, rather than a strictly deterministic, impact of orexins on the control of breathing or the predisposition to OSA.
In humans, attempts to treat sleep-disordered breathing by enhancing the central levels of 5-HT with reuptake blockers or L-tryptophan have yielded mixed results (307,561). However, data from rats point to a stronger excitatory effect of NE than 5-HT on upper airway motoneurons (142) as well as a reduced availability of the excitatory 5-HT receptors in XII motoneurons during the normal sleep period (574). If the same is the case in humans, components of the NE system may prove to be a more appropriate target for attempts to treat OSA than the 5-HT system.
Sensitization of upper airway mechanoreceptor reflexes has been proposed as the means of increasing upper airway muscle tone to treat OSA (596), but this approach is at early stages of experimentation. In contrast, increasing the upper airway motor tone by electrical stimulation of the XII nerve, or lingual muscles, has now advanced from being an experimental approach for improving upper airway patency (42,382,471,607) to a recognized treatment modality (250, 472, 522).
Animal models of sleep-disordered breathing
Attempts to assess how the physiologic mechanisms of respiratory control across sleep-wake states function in the context of sleep-disordered breathing and to investigate the impact of OSA on cardiorespiratory regulation are hampered by a limited availability of suitable animal models. In particular, there is a paucity of natural animal models, which exhibit at least some features typical of OSA. Among those, only in two models, the English bulldogs and extremely obese Yucatan miniature pigs, can one observe naturally occurring sleep-related hypopneas and upper airway obstructions (190, 191, 311). Unfortunately, the limited availability of these animals and the limited ability to conduct fundamental neuropharmacological and genetic studies using these species make their use in OSA research impractical.
Obese and extremely obese rodent strains that were originally developed to explore the regulation of metabolism and energy balance have been adopted for use in OSA research with an expectation that their obesity would be associated with physiologically significant and sleep-dependent respiratory flow limitations. In a partial support of this, studies with the leptin- or leptin receptor-deficient obese mice, obese Zucker rats, and New Zealand obese mice revealed that these rodents have smaller and more collapsible upper airway when subjected to negative pressure than their wild-type counterparts (e.g., 61, 415, 430). However, no evidence is available to date that upper airway obstructions occur spontaneously during sleep in any rodent model. Furthermore, in contrast to data from OSA patients, GG muscle tone is not elevated during wakefulness in obese Zucker rats when compared to control rats (507). Thus, rodents with genetically induced obesity and altered metabolism are used in OSA research to explore the potential role of metabolic derangements as a comorbidity in OSA (e.g., 302,416). Such rodents are also used as models for induced OSA-like conditions achieved through exposure to chronic intermittent hypoxia (CIH), as discussed later. More common, however, is the use of natural rodent models in research on sleep-related central apneic events in which the respiratory rhythm transiently stops without a concurrent flow limitation. This is beyond the scope of this article and has been reviewed elsewhere (96).
In the absence of satisfactory natural models, various induced models have been developed and tested with the goal to explore selected aspects of OSA and/or its consequences (see 99 for an overview). Such models include: (1) induction of repeated upper airway obstructions or intermittent periods of hypoxia in human subjects with the goal to explore the consequences of these interventions on cardiovascular variables and the level of sleepiness (256, 539); (2) induction of repeated periods of asphyxia associated with closure of the airway in instrumented dogs or rats, again with the main goal to study various cardiovascular and/or inflammatory sequellae (15, 63, 130, 254); (3) exposure of various strains of rodents, mice, or rats, for prolonged periods to CIH that, in a few studies, has been synchronized with the sleep-wake cycle (182, 183), and in most studies is being applied at a fixed rate during the rest period of the circadian cycle (see 100, 150, 328, 617 for reviews); and (4) mechanical interference with upper airway anatomy in otherwise healthy animals with the goal to obtain sleep related flow limitations and/or airway obstructions; experiments of this kind have been typically conducted with larger animals, such as dogs, monkeys, pigs, cats, or rabbits (298, 370, 411, 455, 594, 611), but were also attempted in rats (445). Of the different induced models, only the latter ones and a small fraction of the studies using CIH in rodents have been designed to study the impact of OSA on the control of upper airway muscles or the central interactions between sleep and breathing. The relevant CIH studies are discussed in the next section.
In several animal species, attempts were made to induce sleep-related flow limitations and obstructions by imposing a mechanical load on the soft tissue of the upper airway in a manner similar to the increased accumulation of fat in OSA patients. In Yorkshire pigs and Yucatan mini pigs, balloons were implanted in the lateral walls of the pharyngeal airway adjacent to the location of the lateral fat pads. Inflation of the balloons resulted in increased upper airway resistance, but no attempts were made to apply balloon inflations periodically and in synchrony with different sleep-wake states (594). In small monkeys, Macaca fasciculataris, collagen injections were made at 2-week intervals into the uvula, tongue, and lateral pharyngeal walls with concurrent monitoring of sleep-wake amounts and respiratory parameters (411). The injections increased the number of respiratory events (apneas and hypopneas) during sleep from 4.8 to 27.9 per hour, reduced sleep time, and totally eliminated stage III/IV NREM sleep and REM sleep. These results suggested that the model successfully replicated some of the most salient features of OSA. It is also of interest that the injected collagen was gradually absorbed and degraded, which offered the opportunity to study the impact of induced OSA-like conditions followed by a gradual recovery from the “disorder.” In another conceptually similar study, liquid silicone was injected into the base of the tongue in rabbits (611). This caused sleep-related hypopneas (~29 per hour) and apneas (~10 per hour) that persisted at the same level during a 3-month follow up. Concurrently, sleep was curtailed with only a partial recovery between the first and the third month after the intervention. Unfortunately, upper airway muscle activity was not monitored in any of these studies, but the outcomes suggest that the same models could be used to investigate the impact of mechanically compromised upper airway on upper airway muscle activity.
Activity of the GG muscle was monitored in chronically instrumented dogs (455) and cats (370) in which head position was varied or compression was acutely applied to the pharyngeal region to elicited compensatory increases of GG activity. In the cat model, CPAP was applied through a nose mask and, as expected, this enlarged the airway and normalized breathing (370). However, multiple technical limitations of these models did not allow for systematic manipulations of upper airway patency in synchrony with the sleep-wake cycle. In another study in rats, the tendons through which the geniohyoid and hyoglossus muscles are attached to the hyoid apparatus were surgically severed to compromise the neuromuscular support of the upper airway (445). The hypothesis behind these experiments was that, if these two muscles contributed to the stiffness of the upper airway and acted synergistically with the GG, then their surgically induced impairment should elicit a compensatory increase of GG activity and/or increase the propensity for sleep-related respiratory disorders. Sleep-wake behavior, diaphragmatic, and GG activity were concurrently monitored in sham-operated rats and rats subjected to mechanical disconnection of the geniohyoid and hyoglossus muscles from the hyoid bone for 22 days after the intervention. Ultimately, while the effects were consistent with the original hypothesis, their magnitude was too small to regard the model as sufficiently robust. Sleep was not significantly disrupted, and the rats with injured upper airway muscles had only marginally increased GG muscle activity during NREM sleep when compared to the sham-operated animals, an effect that was significant only when measured within subjects relative to the mean level of activity during wakefulness. Also, there was no evidence for sleep-related breathing irregularities, and the occurrence of inspiratory activation of GG was as rare in the rats with injured upper airway muscles as in the sham-operated animals. Thus, these results were consistent with the overall notion that the upper airway of rodents is not vulnerable to collapse even in the face of a surgically implemented weakening of neuromuscular support.
In one additional study, breathing was monitored during drug-induced sleep in rabbits whose tongue muscles were paralyzed by injections of botulinum toxin (611). This intervention resulted in a widely ranging severity of respiratory events that appear to have been preferentially bound to the periods of induced sleep. About one third of the animals did not exhibit any significant sleep-related respiratory events, another one third died of what might have been unresolved upper airway obstruction during sleep, and in the remaining third, the rate of apneic and hypopneic events varied from 2 to 242 per hour. Thus, it appears that, at least at this stage, the severity of respiratory events was difficult to manage in these experiments, and it is not clear whether flow limitations and obstructions also would occur in this model during natural sleep.
From this summary, it is apparent that considerable attempts were made to explore various induced models of OSA. The success of these attempts has been limited because of the high effort required to maintain and manage the models in larger animals and due to difficulties with obtaining a rodent model with spontaneously occurring sleep-related flow limitations and obstructions. One may hope that the experience accumulated to date will guide future research toward improved animal models of upper airway control in OSA.
Effects of chronic intermittent hypoxia on the control of upper airway
Most studies of the basic mechanisms controlling the motor output to the upper airway across sleep-wake states discussed so far used animal models with fully patent upper airway. However, it is well documented that the chronic sleep and respiratory disruption characteristic of OSA is associated with a host of adaptive and maladaptive changes occurring at multiple levels, both centrally and peripherally. For example, as discussed earlier, OSA patients exhibit an adaptive increase of activity in upper airway dilator muscles during wakefulness (cf. Fig. 19). There is now also evidence that a chronic need to cope with recurring nocturnal hypoxemia leads to adaptive changes in upper airway motor control through mechanisms involving the central NE and 5-HT systems. Other studies demonstrate that severe chronic recurrent hypoxemia leads to a damage of central neurons responsible for the maintenance of vigilance. These novel results have been obtained using rodents exposed to CIH as a simple to implement model of one major pathophysiologic aspect of OSA.
Our group employed a rat CIH model to assess the neuroanatomic and neurophysiologic changes in the control exerted by NE and 5-HT over XII motoneurons. Rats were subjected to CIH or sham treatment for 35 days, with O2 oscillating with 180 s period between a mild hyperoxia (24%) and hypoxic nadirs of 6.9% for 10 h daily. Brains were harvested about 17 h after the last period of exposure to hypoxia to minimize any acute effects of hypoxic exposure on the measured outcomes. After immunohistochemical staining, the density of NE and 5-HT terminals was quantified in the ventromedial quadrant of the XII nucleus (cf. Fig. 6), and immunostaining for α1-adrenoceptor-like and 5-HT2A receptor-like proteins was quantified throughout the XII nucleus. When compared to sham-treated rats, CIH rats had about 40% higher density of NE terminals (Fig. 20A–C), and about 20% higher density of 5-HT terminals. Concurrently, XII motoneurons expressing α1-adrenoceptors were about 10% more numerous in CIH rats, whereas 5-HT2A receptor density was not significantly altered (443). In a parallel study, CIH-exposed rats also had elevated NE terminal density in the spinal trigeminal sensory nucleus and a similar trend was present in the trigeminal motor nucleus (349), but the effects were not as prominent as in the XII nucleus.
Figure 20.
Rats exposed to CIH have increased density of NE terminals in the ventromedial quadrant of the XII nucleus (top section) and increased endogenous excitatory drive to XII motoneurons mediated by α1-adrenergic receptors (bottom section). (A1 and B1) Comparison of the appearance of NE-containing terminals in 100 × 100 μm images of the ventromedial quadrant of the XII nucleus in a rat exposed to CIH for 35 days (A1) and a sham-treated animal (B1). Also visible are several XII motoneurons retrogradely labeled from the base of the tongue (dark brown). (A2 and B2) Graphic renditions of all NE-containing terminals found in the images shown in A1 and B1. Red dots indicate terminals that were closely apposed to the somatic membrane of retrogradely labeled XII motoneurons (gray), whereas black dots represent the remaining NE terminals. (C) Average numbers of NE terminals (immunostained for dopamine-β-hydroxylase) counted in 24 matched for the anteroposterior level pairs of brain sections from eight pairs of CIH/sham-treated rats. (Panels A–C modified from Fig. 2 and 3 in Ref. 443 and republished with permission from the American Thoracic Society.) (D) Microinjections of the α1-adrenergic receptor antagonist, prazosin (PZ), into the XII nucleus caused larger decrements of spontaneous XII nerve activity in anesthetized, paralyzed, and artificially ventilated rats that were earlier exposed to CIH for 35 days than in sham-treated animals. (E) Prazosin injections did not cause any changes of the central respiratory rate. (Panels D and E modified from Fig. 3 in Ref. 517 and republished with permission from the American Physiological Society.)
The combination of NE terminal sprouting and increased expression of α1-adrenergic receptors following the exposure to CIH could enhance endogenous activation of XII motoneurons mediated by NE. To test this, rats were subjected to 35 days of CIH or sham treatment and then the effects of α1-adrenergic receptor agonists and antagonists injected into the XII nucleus on XII nerve activity were measured under urethane anesthesia (517). While the magnitude of the excitatory effect of the agonist (phenylephrine) did not differ between the CIH- and sham-treated rats, microinjections of the antagonist (prazosin) caused larger decrements of spontaneous XII nerve activity in CIH animals (Fig. 20D and E). These findings suggested that CIH exposure enhanced the endogenous excitatory drive to XII motoneurons mediated by NE. This could be due to a combination of NE terminal sprouting and increased expression of α1-adrenoceptors on XII motoneurons. Such a CIH-elicited enhancement of central excitatory drive to XII motoneurons offers a possible mechanistic explanation for the clinical observation that OSA patients have elevated upper airway muscle tone during wakefulness.
In addition to an increased NE terminal density and increased α1-adrenoceptor expression, CIH could also increase the baseline levels of catecholamines in the brain because, in other studies, CIH exposure resulted in increased plasma levels of catecholamines and increased baseline activity of the sympathetic system (72, 148, 149, 151, 495). However, in Wistar-Kyoto rats, only dopamine levels were increased, whereas NE or 5-HT levels were not, following hypocapnic CIH when measured in tissue homogenates collected from different brain regions (303). Similarly, when measured using c-Fos accumulation in neuronal nuclei as an indirect marker of the level of NE cell activity under standardized conditions, c-Fos expression was not increased in CIH rats in any of the pontomedullary catecholaminergic neuronal groups when compared to sham-treated animals. Indeed, for the medullary groups (A1/C1, A2/C2, and A5), the percentage of catecholaminergic cells with nuclear c-Fos expression tended to be lower in CIH than in sham-treated animals (519). Thus, spontaneous activity of central catecholaminergic cells does not appear to be persistently increased following CIH. In several other studies, increases of c-Fos and other early genes were reported in various brain regions and cells when tested immediately after exposure to CIH; this could be due to acute effects of episodic hypoxia, rather than being indicative of a reconfiguration leading to persistent hyperactivity (e.g., 174, 257, 363). Interestingly, locomotor activity of Wistar-Kyoto rats was increased for at least 3 days after 35 days of exposure to CIH despite the absence of evidence for an increased NE or 5-HT metabolism in the brain (303). This is similar to the increased activation of XII motoneurons by endogenous NE despite the absence of evidence for increased baseline activity of brainstem NE neurons (517, 519).
Other studies revealed that severe CIH combined with hypercapnia may adversely affect contractile properties of upper airway muscles (58, 122). However, follow-up experiments did not detect impaired reflex activation of GG muscles in rats exposed to CIH when tested under anesthesia (123). These experiments used relatively low nadirs of hypoxia (5%), but the duration of exposures might have been too short (7 days) for a full expression of any adaptive or maladaptive changes. Indeed, available data suggest that the direction and magnitude of the effects of CIH on the control of upper airway muscles varies with the duration and severity of CIH (see later).
It is relatively mundane and laborious to conduct systematic exploration over a wide range of CIH conditions, such as the hypoxic nadirs, durations of the hypoxic bouts and periods of exposure, with the goal to generate satisfactory dose-response curves for different outcome variables. Indeed, few such studies have been performed (e.g., 148, 336, 433). However, in the absence of such data, the unknown relationship between the emergence and magnitude of different outcomes and the severity of CIH is one possible source of variable results generated in different laboratories. It is possible that qualitatively different effects of CIH have different thresholds, but there is currently no common scale with which one can compare the levels of CIH applied in different experimental settings. As an example, data from what seems to be a moderate level of CIH yielded results indicative of an enhancement of endogenous excitatory drive to XII motoneurons mediated by NE (443, 517). However, in other CIH studies in mice, a more prolonged and possibly more severe CIH elicited evidence of injury in wake-active brain neurons, including the LC but not other NE groups (618), sleepiness that persisted after termination of hypoxia (562), and reduced ability of glutamate and 5-HT to activate XII motoneurons (565). Thus, the findings from mice were not compatible with those from rats, but it is not clear whether the differences were related to different severity of hypoxia, different species, or other factors. With an unquestionable utility of CIH models for exploration of the various physiologic and pathophysiologic mechanisms relevant to OSA, it will be important to establish common standards for comparison of CIH exposure protocols. Such standards may be based on an agreed upon set of biochemical markers or on a limited set of simple to collect physiologic parameters, such as the impact of CIH on body weight or tissue oxygen saturation measured in a standardized manner.
Conclusion
There is currently extensive evidence that multiple neurotransmitter systems control upper airway muscle activity in a state-dependent manner. Of those, NE, 5-HT, and ACh have been particularly well explored. Furthermore, there is now evidence that, among these systems, at least the endogenous excitatory drive to upper airway motoneurons mediated by NE is enhanced following exposure to CIH administered in a manner intended to mimic episodic hypoxia of OSA.
One challenge for future studies will be to determine to what extent the findings derived from animal models can be applied to the control of upper airway muscles in humans, including OSA patients. To achieve this, the relative roles on NE, 5-HT, ACh and other transmitters will need to be compared among different species, models, and experimental conditions. Significant progress has been made toward delineating which neurotransmitter receptor classes mediate changes in upper airway muscle tone across sleep-wake states or with a circadian pattern, which act in a state-independent manner, and which are silent under most physiologic conditions. A pharmacotherapy for OSA may take advantage of any one of these systems. Studies to date have provided an important neurochemical background, but dose-response characteristics need to be determined more precisely and a broader range of pharmacologically active compounds needs to be tested across different model systems. Importantly, while OSA may be alleviated by increasing upper airway muscle activity during sleep, neural changes, such as the decline of aminergic activity during NREM and REM sleep and cholinergic activation during REM sleep play physiologically important roles in the regulation of the occurrence of normal sleep per se. Therefore, it is unlikely that a systemic interference with these mechanisms with the goal to enhance upper airway muscle tone can be achieved without significant adverse effects on sleep and other vital functions. With this caveat in mind, there is a need to explore novel methods of drug delivery to achieve a selective action on the motor output to upper airway motoneurons in a manner devoid of undesirable side effects.
Acknowledgements
Our research discussed in this review was supported by grants HL-042236, HL-047600, HL-060287, HL-071097, and HL-116508 from the National Heart, Lung, and Blood Institute of the National Institutes of Health. The author thanks Ms. Kate B. Herr for editorial assistance.
References
- 1.Adachi M, Nonaka S, Katada A, Arakawa T, Ota R, Harada H, Takakusaki K, Harabuchi Y. Carbachol injection into the pontine reticular formation depresses laryngeal muscle activities and airway reflexes in decerebrate cats. Neurosci Res. 2010;67:40–50. doi: 10.1016/j.neures.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Adachi T, Robinson DM, Miles GB, Funk GD. Noradrenergic modulation of XII motoneuron inspiratory activity does not involve α2-receptor inhibition of the Ih current or presynaptic glutamate release. J Appl Physiol. 2005;98:1297–1308. doi: 10.1152/japplphysiol.00977.2004. [DOI] [PubMed] [Google Scholar]
- 3.Aghajanian GK, Sprouse JS, Sheldon P, Rasmussen K. Electrophysiology of the central serotonin system: Receptor subtypes and transducer mechanisms. Ann N Y Acad Sci. 1990;600:93–103. doi: 10.1111/j.1749-6632.1990.tb16875.x. [DOI] [PubMed] [Google Scholar]
- 4.Agnati LF, Zoli M, Strömberg I, Fuxe K. Intercellular communication in the brain wiring versus volume transmission. Neuroscience. 1995;69:711–726. doi: 10.1016/0306-4522(95)00308-6. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed WA, Tsutsumi M, Nakata S, Mori T, Nishimura Y, Fujisawa T, Kato I, Nakashima M, Kurahashi H, Suzuki K. A functional variation in the hypocretin neuropeptide precursor gene may be associated with obstructive sleep apnea syndrome in Japan. Laryngoscope. 2012;122:925–929. doi: 10.1002/lary.23179. [DOI] [PubMed] [Google Scholar]
- 6.Al-Zubaidy ZA, Erickson RL, Greer JJ. Serotonergic and noradrenergic effects on respiratory neural discharge in the medullary slice preparation of neonatal rats. Pfl ü gers Arch. 1996;431:942–949. doi: 10.1007/s004240050089. [DOI] [PubMed] [Google Scholar]
- 7.Alam MN, McGinty D, Szymusiak R. Neuronal discharge of preoptic/anterior hypothalamic thermosensitive neurons: Relation to NREM sleep. Am J Physiol. 1995;269:R1240–R1249. doi: 10.1152/ajpregu.1995.269.5.R1240. [DOI] [PubMed] [Google Scholar]
- 8.Alam MN, Kumar S, Bashir T, Suntsova N, Methippara MM, Szymusiak R, McGinty D. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. J Physiol (Lond) 2005;563:569–582. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldes LD. Topographically organized projections from the nucleus subceruleus to the hypoglossal nucleus in the rat: A light and electron microscopic study with complementary axonal transport techniques. J Comp Neurol. 1990;302:643–656. doi: 10.1002/cne.903020318. [DOI] [PubMed] [Google Scholar]
- 10.Aldes LD, Chapman ME, Chronister RB, Haycock JW. Sources of noradrenergic afferents to the hypoglossal nucleus in the rat. Brain Res Bull. 1992;29:931–942. doi: 10.1016/0361-9230(92)90168-w. [DOI] [PubMed] [Google Scholar]
- 11.Aldes LD, Chronister RB, Shelton CI, Haycock JW, Marco LA, Wong DL. Catecholamine innervation of the rat hypoglossal nucleus. Brain Res Bull. 1988;21:305–312. doi: 10.1016/0361-9230(88)90245-6. [DOI] [PubMed] [Google Scholar]
- 12.Aldes LD, Chronister RC, Marco LA, Haycock JW, Thibault J. Differential distribution of biogenic amines in the hypoglossal nucleus of the rat. Exp Brain Res. 1988;73:305–314. doi: 10.1007/BF00248222. [DOI] [PubMed] [Google Scholar]
- 13.Aldes LD, Marco LA, Chronister RB. Serotonin-containing axon terminals in the hypoglossal nucleus of the rat. An immunoelectronmicroscopic study. Brain Res Bull. 1989;23:249–256. doi: 10.1016/0361-9230(89)90154-8. [DOI] [PubMed] [Google Scholar]
- 14.Alheid GF, McCrimmon DR. The chemical neuroanatomy of breathing. Respir Physiol Neurobiol. 2008;164:3–11. doi: 10.1016/j.resp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almendros I, Farre R, Planas AM, Torres M, Bonsignore MR, Navajas D, Montserrat JM. Tissue oxygenation in brain, muscle, and fat in a rat model of sleep apnea: Differential effect of obstructive apneas and intermittent hypoxia. Sleep. 2011;34:1127–1133. doi: 10.5665/SLEEP.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altschuler SM, Bao X, Miselis RR. Dendritic architecture of hypoglossal motoneurons projecting to extrinsic tongue musculature in the rat. J Comp Neurol. 1994;342:538–550. doi: 10.1002/cne.903420404. [DOI] [PubMed] [Google Scholar]
- 17.Amis TC, O'Neill N, Wheatley JR, Van der Touw T, Di Somma E, Brancatisano A. Soft palate muscle response to negative upper airway pressure. J Appl Physiol. 1999;86:523–530. doi: 10.1152/jappl.1999.86.2.523. [DOI] [PubMed] [Google Scholar]
- 18.Anaclet C, Lin JS, Vetrivelan R, Krenzer M, Vong L, Fuller PM, Lu J. Identification and characterization of a sleep-active cell group in the rostral medullary brainstem. J Neurosci. 2012;32:17970–17976. doi: 10.1523/JNEUROSCI.0620-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anaclet C, Pedersen NP, Fuller PM, Lu J. Brainstem circuitry regulating phasic activation of trigeminal motoneurons during REM sleep. PLoS ONE. 2010;5:e8788. doi: 10.1371/journal.pone.0008788. doi: 10.1371/journal.pone.0008788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anch AM, Remmers JE, Sauerland EK, DeGroot WJ. Oropharyngeal patency during waking and sleep in the Pickwickian syndrome: Electromyographic activity of the tensor veli palatini. EMG Clin Neurophysiol. 1981;21:317–330. [PubMed] [Google Scholar]
- 21.Anderson CA, Dick TE, Orem J. Respiratory responses to tracheobronchial stimulation during sleep and wakefulness in the adult cat. Sleep. 1996;19:472–478. doi: 10.1093/sleep/19.6.472. [DOI] [PubMed] [Google Scholar]
- 22.Arita H, Ochiishi M. Opposing effects of 5-hydroxytryptamine on two types of medullary inspiratory neurons with distinct firing patterns. J Neurophysiol. 1991;66:285–292. doi: 10.1152/jn.1991.66.1.285. [DOI] [PubMed] [Google Scholar]
- 23.Arita H, Sakamoto M, Hirokawa Y, Okado N. Serotonin innervation patterns differ among the various medullary motoneuronal groups involved in upper airway control. Exp Brain Res. 1993;95:100–110. doi: 10.1007/BF00229659. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong DM, Saper CB, Levey AI, Wainer BH, Terry RD. Distribution of cholinergic neurons in rat brain: Demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol. 1983;216:53–68. doi: 10.1002/cne.902160106. [DOI] [PubMed] [Google Scholar]
- 25.Arnulf I. The `scanning hypothesis' of rapid eye movements during REM sleep: A review of the evidence. Arch Ital Biol. 2011;149:367–382. doi: 10.4449/aib.v149i4.1246. [DOI] [PubMed] [Google Scholar]
- 26.Aronson RM, Onal E, Carley DW, Lopata M. Upper airway and respiratory muscle responses to continuous negative airway pressure. J Appl Physiol. 1989;66:1373–1382. doi: 10.1152/jappl.1989.66.3.1373. [DOI] [PubMed] [Google Scholar]
- 27.Arvidsson J, Cullheim S, Ulfhake B, Bennett GW, Fone KCF, Cuello AC, Verhofstad AAJ, Visser TJ, Hökfelt T. 5-hydroxytryptamine, substance P, and thyrotropin-releasing hormone in the adult cat spinal cord segment L7: Immunohistochemical and chemical studies. Synapse. 1990;6:237–270. doi: 10.1002/syn.890060305. [DOI] [PubMed] [Google Scholar]
- 28.Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–274. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- 29.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayas NT, Hirsch AA, Laher I, Bradley TD, Malhotra A, Polotsky VY, Tasali E. New frontiers in obstructive sleep apnoea. Clin Sci. 2014;127:209–216. doi: 10.1042/CS20140070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badr MS, Kawak A, Skatrud JB, Morrell MJ, Zahn BR, Babcock MA. Effect of induced hypocapnic hypopnea on upper airway patency in humans during NREM sleep. Respir Physiol. 1997;110:33–45. doi: 10.1016/s0034-5687(97)00072-8. [DOI] [PubMed] [Google Scholar]
- 32.Baghdoyan HA. Cholinergic mechanisms regulating REM sleep. In: Schwartz WJ, editor. Sleep Science: Integrating Basic Research and Clinical Practice. Karger; Basel: 1997. [Google Scholar]
- 33.Baghdoyan HA. Location and quantification of muscarinic receptor subtypes in rat pons: Implications for REM sleep generation. Am J Physiol. 1997;273:R896–R904. doi: 10.1152/ajpregu.1997.273.3.R896. [DOI] [PubMed] [Google Scholar]
- 34.Bailey EF, Fregosi RF. Modulation of upper airway muscle activities by bronchopulmonary afferents. J Appl Physiol. 2006;101:609–617. doi: 10.1152/japplphysiol.00204.2006. [DOI] [PubMed] [Google Scholar]
- 35.Bailey EF, Fridel KW, Rice AD. Sleep/wake firing patterns of human genioglossus motor units. J Neurophysiol. 2007;98:3284–3291. doi: 10.1152/jn.00865.2007. [DOI] [PubMed] [Google Scholar]
- 36.Bailey TW, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus elevates plasma ACTH in conscious rats. Am J Physiol. 2001;280:R8–R15. doi: 10.1152/ajpregu.2001.280.1.R8. [DOI] [PubMed] [Google Scholar]
- 37.Barillot JC, Grèlot L, Reddad S, Bianchi AL. Discharge patterns of laryngeal motoneurones in the cat: An intracellular study. Brain Res. 1990;509:99–103. doi: 10.1016/0006-8993(90)90314-2. [DOI] [PubMed] [Google Scholar]
- 38.Bartlett D., Jr Respiratory functions of the larynx. Physiol Rev. 1989;69:33–57. doi: 10.1152/physrev.1989.69.1.33. [DOI] [PubMed] [Google Scholar]
- 39.Bartlett D, Jr, Leiter JC. Coordination of breathing with nonrespiratory activities. Compr Physiol. 2012;2:1387–1415. doi: 10.1002/cphy.c110004. doi: 10.1002/cphy.c110004. [DOI] [PubMed] [Google Scholar]
- 40.Basner RC, Ringler J, Schwartzstein RM, Weinberger SE, Weiss JW. Phasic electromyographic activity of the genioglossus increases in normals during slow-wave sleep. Respir Physiol. 1991;83:189–200. doi: 10.1016/0034-5687(91)90028-h. [DOI] [PubMed] [Google Scholar]
- 41.Bayliss DA, Viana F, Berger AJ. Mechanisms underlying excitatory effects of thyrotropin-releasing hormone on rat hypoglossal motoneurons in vitro. J Neurophysiol. 1992;68:1733–1745. doi: 10.1152/jn.1992.68.5.1733. [DOI] [PubMed] [Google Scholar]
- 42.Bellemare F, Pecchiari M, Bandini M, Sawan M, D'Angelo E. Reversibility of airflow obstruction by hypoglossus nerve stimulation in anesthetized rabbits. Am J Respir Crit Care Med. 2005;172:606–612. doi: 10.1164/rccm.200502-190OC. [DOI] [PubMed] [Google Scholar]
- 43.Bellingham MC, Berger AJ. Presynaptic depression of excitatory synaptic inputs to rat hypoglossal motoneurons by muscarinic M2 receptors. J Neurophysiol. 1996;76:3758–3770. doi: 10.1152/jn.1996.76.6.3758. [DOI] [PubMed] [Google Scholar]
- 44.Berger AJ, Huynh P. Activation of 5HT1B receptors inhibits glycinergic synaptic inputs to mammalian motoneurons during postnatal development. Brain Res. 2002;956:380–384. doi: 10.1016/s0006-8993(02)03464-9. [DOI] [PubMed] [Google Scholar]
- 45.Berry RB, Kouchi KG, Bower JL, Light RW. Effect of upper airway anesthesia on obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:1857–1861. doi: 10.1164/ajrccm.151.6.7767531. [DOI] [PubMed] [Google Scholar]
- 46.Betschart C, Hintermann S, Behnke D, Cotesta S, Fendt M, Gee CE, Jacobson LH, Laue G, Ofner S, Chaudhari V, Badiger S, Pandit C, Wagner J, Hoyer D. Identification of a novel series of orexin receptor antagonists with a distinct effect on sleep architecture for the treatment of insomnia. J Med Chem. 2013;56:7590–7607. doi: 10.1021/jm4007627. [DOI] [PubMed] [Google Scholar]
- 47.Bliwise DL, He L, Ansari FP, Rye DB. Quantification of electromyographic activity during sleep: A phasic electromyographic metric. J Clin Neurophysiol. 2006;23:59–67. doi: 10.1097/01.wnp.0000192303.14946.fc. [DOI] [PubMed] [Google Scholar]
- 48.Bochorishvili G, Nguyen T, Coates MB, Viar KE, Stornetta RL, Guyenet PG. The orexinergic neurons receive synaptic input from C1 cells in rats. J Comp Neurol. 2014;522:3834–38346. doi: 10.1002/cne.23643. doi: 10.1002/cne.23643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Böhmer G, Dinse HR, Fallert M, Sommer TJ. Microelectrophoretic application of antagonists of putative neurotransmitters onto various types of bulbar respiratory neurons. Arch Ital Biol. 1979;117:13–22. [PubMed] [Google Scholar]
- 50.Boissard R, Fort P, Gervasoni D, Barbagli B, Luppi P-H. Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep onset. Eur J Neurosci. 2003;18:1627–1639. doi: 10.1046/j.1460-9568.2003.02861.x. [DOI] [PubMed] [Google Scholar]
- 51.Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi P-H. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: A combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16:1959–1973. doi: 10.1046/j.1460-9568.2002.02257.x. [DOI] [PubMed] [Google Scholar]
- 52.Bonora M, Bartlett D, Jr, Knuth SL. Changes in upper airway muscle activity related to head position in awake cats. Respir Physiol. 1985;60:181–192. doi: 10.1016/0034-5687(85)90102-1. [DOI] [PubMed] [Google Scholar]
- 53.Boucetta S, Cisse Y, Mainville L, Morales M, Jones BE. Discharge profiles across the sleep-waking cycle of identified cholinergic, GABAergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J Neurosci. 2014;34:4708–4727. doi: 10.1523/JNEUROSCI.2617-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bourgin P, Escourrou P, Gaultier C, Adrien J. Induction of rapid eye movement sleep by carbachol infusion into the pontine reticular formation in the rat. NeuroReport. 1995;6:532–536. doi: 10.1097/00001756-199502000-00031. [DOI] [PubMed] [Google Scholar]
- 55.Bourgin P, Huitrón-Reséndiz S, Spier AD, Fabre V, Morte B, Criado JR, Sutcliffe JG, Henriksen SJ, de Lecea L. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bouryi VA, Lewis DI. The modulation by 5-HT of glutamatergic inputs from the raphe pallidus to rat hypoglossal motoneurons in vitro. J Physiol (Lond) 2003;553:1019–1031. doi: 10.1113/jphysiol.2003.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boyd JH, Petrof BJ, Hamid Q, Fraser R, Kimoff RJ. Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:541–546. doi: 10.1164/rccm.200308-1100OC. [DOI] [PubMed] [Google Scholar]
- 58.Bradford A, McGuire M, O'Halloran KD. Does episodic hypoxia affect upper airway dilator muscle function? Implications for the pathophysiology of obstructive sleep apnoea. Respir Physiol Neurobiol. 2005;147:223–234. doi: 10.1016/j.resp.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 59.Bradley PB, Lucy AP. Cholinoceptive properties of respiratory neurones in the rat medulla. Neuropharmacology. 1983;22:853–858. doi: 10.1016/0028-3908(83)90131-4. [DOI] [PubMed] [Google Scholar]
- 60.Brandes IF, Stettner GM, Morschel M, Kubin L, Dutschmann M. REM sleep-like episodes of motoneuronal depression and respiratory rate increase are triggered by pontine carbachol microinjections in in situ perfused rat brainstem preparation. Exp Physiol. 2011;96:548–555. doi: 10.1113/expphysiol.2010.056242. doi: 10.1113/expphysiol.2010.056242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brennick MJ, Pack AI, Ko K, Kim E, Pickup S, Maislin G, Schwab RJ. Altered upper airway and soft tissue structures in the New Zealand obese mouse. Am J Respir Crit Care Med. 2009;179:158–169. doi: 10.1164/rccm.200809-1435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, Mueller C, Nayler O, van GJ, de Haas SL, Hess P, Qiu C, Buchmann S, Scherz M, Weller T, Fischli W, Clozel M, Jenck F. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13:150–155. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- 63.Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension. J Clin Invest. 1997;99:106–109. doi: 10.1172/JCI119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brooks PL, Peever JH. Glycinergic and GABAA-mediated inhibition of somatic motoneurons does not mediate rapid eye movement sleep motor atonia. J Neurosci. 2008;28:3535–3545. doi: 10.1523/JNEUROSCI.5023-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brooks PL, Peever JH. Identification of the transmitter and receptor mechanisms responsible for REM sleep paralysis. J Neurosci. 2012;32:9785–9795. doi: 10.1523/JNEUROSCI.0482-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brouillette RT, Thach BT. A neuromuscular mechanism maintaining extrathoracic airway patency. J Appl Physiol. 1979;46:772–779. doi: 10.1152/jappl.1979.46.4.772. [DOI] [PubMed] [Google Scholar]
- 67.Brown EC, Hudson AL, Butler JE, McKenzie DK, Bilston LE, Gandevia SC. Single motor unit recordings in human geniohyoid reveal minimal respiratory activity during quiet breathing. J Appl Physiol. 2011;110:1054–1059. doi: 10.1152/japplphysiol.00454.2010. [DOI] [PubMed] [Google Scholar]
- 68.Buckley NJ, Bonner TI, Brann MR. Localization of a family of muscarinic receptor mRNAs in rat brain. J Neurosci. 1988;8:4646–4652. doi: 10.1523/JNEUROSCI.08-12-04646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burgess C, Lai D, Siegel J, Peever J. An endogenous glutamatergic drive onto somatic motoneurons contributes to the stereotypical pattern of muscle tone across the sleep-wake cycle. J Neurosci. 2008;28:4649–4660. doi: 10.1523/JNEUROSCI.0334-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Busquets X, Barbé F, Barceló A, de la Peña M, Sigritz N, Mayoralas LR, Ladaria A, Agustí A. Decreased plasma levels of orexin-A in sleep apnea. Respiration. 2004;71:575–579. doi: 10.1159/000081757. [DOI] [PubMed] [Google Scholar]
- 71.Cakirer B, Hans MG, Graham G, Aylor J, Tishler PV, Redline S. The relationship between craniofacial morphology and obstructive sleep apnea in whites and in African-Americans. Am J Respir Crit Care Med. 2001;163:947–950. doi: 10.1164/ajrccm.163.4.2005136. [DOI] [PubMed] [Google Scholar]
- 72.Campen MJ, Shimoda LA, O'Donnell CP. Acute and chronic cardiovascular effects of intermittent hypoxia in C57BL/6J mice. J Appl Physiol. 2005;99:2028–2035. doi: 10.1152/japplphysiol.00411.2005. [DOI] [PubMed] [Google Scholar]
- 73.Carberry JC, Hensen H, Fisher LP, Saboisky JP, Butler JE, Gandevia SC, Eckert DJ. Mechanisms contributing to the response of upper-airway muscles to changes in airway pressure. J Appl Physiol. 2015;118:1221–1228. doi: 10.1152/japplphysiol.01103.2014. [DOI] [PubMed] [Google Scholar]
- 74.Carberry JC, Jordan AS, White DP, Wellman A, Eckert DJ. Upper airway collapsibility (Pcrit) and pharyngeal dilator muscle activity are sleep stage dependent. Sleep. 2016;39:511–521. doi: 10.5665/sleep.5516. doi: 10.5665/sleep.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cassell MD, Roberts L, Talman WT. Glycine-containing terminals in the rat dorsal vagal complex. Neuroscience. 1992;50:907–920. doi: 10.1016/0306-4522(92)90214-m. [DOI] [PubMed] [Google Scholar]
- 76.Caulfield MP. Muscarinic receptors–characterization, coupling and function. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 77.Chaban R, Cole P, Hoffstein V. Site of upper airway obstruction in patients with idiopathic obstructive sleep apnea. Laryngoscope. 1988;98:641–647. doi: 10.1288/00005537-198806000-00013. [DOI] [PubMed] [Google Scholar]
- 78.Chadwick GA, Crowley P, Fitzgerald MX, O'Regan RG, McNicholas WT. Obstructive sleep apnea following topical oropharyngeal anesthesia in loud snorers. Am Rev Respir Dis. 1991;143:810–813. doi: 10.1164/ajrccm/143.4_Pt_1.810. [DOI] [PubMed] [Google Scholar]
- 79.Chamberlin NL, Bocchiaro CM, Greene RW, Feldman JL. Nicotinic excitation of rat hypoglossal motoneurons. Neuroscience. 2002;115:861–870. doi: 10.1016/s0306-4522(02)00454-2. [DOI] [PubMed] [Google Scholar]
- 80.Chamberlin NL, Eikermann M, Fassbender P, White DP, Malhotra A. Genioglossus premotoneurons and the negative pressure reflex in rats. J Physiol (Lond) 2007;579:515–526. doi: 10.1113/jphysiol.2006.121889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Champagnat J, Denavit-Saubié M, Henry JL, Leviel V. Catecholaminergic depressant effects on bulbar respiratory mechanisms. Brain Res. 1979;160:57–68. doi: 10.1016/0006-8993(79)90600-0. [DOI] [PubMed] [Google Scholar]
- 82.Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am J Respir Crit Care Med. 2006;174:1264–1273. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- 83.Chandler SH, Chase MH, Nakamura Y. Intracellular analysis of synaptic mechanisms controlling trigeminal motoneuron activity during sleep and wakefulness. J Neurophysiol. 1980;44:359–371. doi: 10.1152/jn.1980.44.2.359. [DOI] [PubMed] [Google Scholar]
- 84.Chang F-CT. Modification of medullary respiratory-related discharge patterns by behaviors and states of arousal. Brain Res. 1992;571:281–292. doi: 10.1016/0006-8993(92)90666-w. [DOI] [PubMed] [Google Scholar]
- 85.Chase MH, Morales F. The atonia and myoclonia of active (REM) sleep. Annu Rev Psychol. 1990;41:557–584. doi: 10.1146/annurev.ps.41.020190.003013. [DOI] [PubMed] [Google Scholar]
- 86.Chase MH, Soja PJ, Morales FR. Evidence that glycine mediates the postsynaptic potentials that inhibit lumbar motoneurons during the atonia of active sleep. J Neurosci. 1989;9:743–751. doi: 10.1523/JNEUROSCI.09-03-00743.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 88.Chen H, Chatelain FC, Lesage F. Altered and dynamic ion selectivity of K+ channels in cell development and excitability. Trends Pharmacol Sci. 2014;35:461–469. doi: 10.1016/j.tips.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen W, Ye J, Han D, Yin G, Wang B, Zhang Y. Association of prepro-orexin polymorphism with obstructive sleep apnea/hypopnea syndrome. Am J Otolaryngol. 2012;33:31–36. doi: 10.1016/j.amjoto.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 90.Cheng S, Brown EC, Hatt A, Butler JE, Gandevia SC, Bilston LE. Healthy humans with a narrow upper airway maintain patency during quiet breathing by dilating the airway during inspiration. J Physiol (Lond) 2014;592:4763–4774. doi: 10.1113/jphysiol.2014.279240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chokroverty S. Phasic tongue movements in human rapid-eye-movement sleep. Neurology. 1980;30:665–668. doi: 10.1212/wnl.30.6.665. [DOI] [PubMed] [Google Scholar]
- 92.Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21:RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clement O, Sapin E, Berod A, Fort P, Luppi P-H. Evidence that neurons of the sublaterodorsal tegmental nucleus triggering paradoxical (REM) sleep are glutamatergic. Sleep. 2011;34:419–423. doi: 10.1093/sleep/34.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Corcoran A, Richerson G, Harris M. Modulation of respiratory activity by hypocretin-1 (orexin A) in situ and in vitro. Adv Exp Med Biol. 2010;669:109–113. doi: 10.1007/978-1-4419-5692-7_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.D'Adamo MC, Servettini I, Guglielmi L, Di MV, Di MR, Di GG, Pessia M. 5-HT2 receptors-mediated modulation of voltage-gated K+ channels and neurophysiopathological correlates. Exp Brain Res. 2013;230:453–462. doi: 10.1007/s00221-013-3555-8. [DOI] [PubMed] [Google Scholar]
- 96.Davis EM, O'Donnell CP. Rodent models of sleep apnea. Respir Physiol Neurobiol. 2013;188:355–361. doi: 10.1016/j.resp.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Day HEW, Campeau S, Watson SJ, Jr, Akil H. Distribution of α1a-α1b- and α1d-adrenergic receptor mRNA in the rat brain and spinal cord. J Chem Neuroanat. 1997;13:115–139. doi: 10.1016/s0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- 98.Deegan PC, Mulloy E, McNicholas WT. Topical oropharyngeal anesthesia in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:1108–1112. doi: 10.1164/ajrccm/151.4.1108. [DOI] [PubMed] [Google Scholar]
- 99.Dematteis M, Godin-Ribuot D, Arnaud C, Ribuot C, Stanke-Labesque F, Pepin JL, Levy P. Cardiovascular consequences of sleep-disordered breathing: Contribution of animal models to understanding the human disease. ILAR J. 2009;50:262–281. doi: 10.1093/ilar.50.3.262. [DOI] [PubMed] [Google Scholar]
- 100.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Deng BS, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Contribution of orexin in hypercapnic chemoreflex: Evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol. 2007;103:1772–1779. doi: 10.1152/japplphysiol.00075.2007. [DOI] [PubMed] [Google Scholar]
- 102.Deschenes M, Haidarliu S, Demers M, Moore J, Kleinfeld D, Ahissar E. Muscles involved in naris dilation and nose motion in rat. Anat Rec (Hoboken, N J) 2015;298:546–553. doi: 10.1002/ar.23053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Detari L, Rasmusson DD, Semba K. Phasic relationship between the activity of basal forebrain neurons and cortical EEG in urethane-anesthetized rat. Brain Res. 1997;759:112–121. doi: 10.1016/s0006-8993(97)00252-7. [DOI] [PubMed] [Google Scholar]
- 104.DeWeese EL, Sullivan TY. Effects of upper airway anesthesia on pharyngeal patency during sleep. J Appl Physiol. 1988;64:1346–1353. doi: 10.1152/jappl.1988.64.4.1346. [DOI] [PubMed] [Google Scholar]
- 105.Dias MB, Li A, Nattie E. Focal CO2 dialysis in raphe obscurus does not stimulate ventilation but enhances the response to focal CO2 dialysis in the retrotrapezoid nucleus. J Appl Physiol. 2008;105:83–90. doi: 10.1152/japplphysiol.00120.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dias MB, Li A, Nattie EE. Antagonism of orexin receptor-1 in the retrotrapezoid nucleus inhibits the ventilatory response to hypercapnia predominantly in wakefulness. J Physiol (Lond) 2009;587:2059–2067. doi: 10.1113/jphysiol.2008.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dick TE, Bellingham MC, Richter DW. Pontine respiratory neurons in anesthetized cats. Brain Res. 1994;636:259–269. doi: 10.1016/0006-8993(94)91025-1. [DOI] [PubMed] [Google Scholar]
- 108.DiMicco JA, Abshire VM, Hankins KD, Sample RH, Wible JH., Jr Microinjection of GABA antagonists into posterior hypothalamus elevates heart rate in anesthetized rats. Neuropharmacology. 1986;25:1063–1066. doi: 10.1016/0028-3908(86)90203-0. [DOI] [PubMed] [Google Scholar]
- 109.Dobbins EG, Feldman JL. Differential innervation of protruder and retractor muscles of the tongue in rat. J Comp Neurol. 1995;357:376–394. doi: 10.1002/cne.903570305. [DOI] [PubMed] [Google Scholar]
- 110.Doherty LS, Nolan P, McNicholas WT. Effects of topical anesthesia on upper airway resistance during wake-sleep transitions. J Appl Physiol. 2005;99:549–555. doi: 10.1152/japplphysiol.01221.2004. [DOI] [PubMed] [Google Scholar]
- 111.Dotan Y, Pillar G, Schwartz AR, Oliven A. Asynchrony of lingual muscle recruitment during sleep in obstructive sleep apnea. J Appl Physiol. 2015;118:1516–1524. doi: 10.1152/japplphysiol.00937.2014. [DOI] [PubMed] [Google Scholar]
- 112.Douglas NJ, Jan MA, Yildirim N, Warren PM, Drummond GB. Effect of posture and breathing route on genioglossal electromyogram activity in normal subjects and in patients with the sleep apnea/hypopnea syndrome. Am Rev Respir Dis. 1993;148:1341–1345. doi: 10.1164/ajrccm/148.5.1341. [DOI] [PubMed] [Google Scholar]
- 113.Douse MA, White DP. Serotonergic effects on hypoglossal neural activity and reflex responses. Brain Res. 1996;726:213–222. [PubMed] [Google Scholar]
- 114.Duffin J. Functional organization of respiratory neurones: A brief review of current questions and speculations. Exp Physiol. 2004;89:517–529. doi: 10.1113/expphysiol.2004.028027. [DOI] [PubMed] [Google Scholar]
- 115.Dun NJ, Dun SL, Chen CT, Hwang LL, Kwok EH, Chang JK. Orexins: A role in medullary sympathetic outflow. Reg Pept. 2000;96:65–70. doi: 10.1016/s0167-0115(00)00202-0. [DOI] [PubMed] [Google Scholar]
- 116.Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL, Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dunin-Barkowski WL, Orem JM. Suppression of diaphragmatic activity during spontaneous ponto-geniculo-occipital waves in cat. Sleep. 1998;21:671–675. [PubMed] [Google Scholar]
- 118.Dutschmann M, Dick TE. Pontine mechanisms of respiratory control. Compr Physiol. 2012;2:2443–2469. doi: 10.1002/cphy.c100015. doi: 10.1002/cphy.c100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eastwood PR, Allison GT, Shepherd KL, Szollosi I, Hillman DR. Heterogeneous activity of the human genioglossus muscle assessed by multiple bipolar fine-wire electrodes. J Appl Physiol. 2003;94:1849–1858. doi: 10.1152/japplphysiol.01017.2002. [DOI] [PubMed] [Google Scholar]
- 120.Eckert DJ, Malhotra A, Lo YL, White DP, Jordan AS. The influence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleep. Chest. 2009;135:957–964. doi: 10.1378/chest.08-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Economo C von. Sleep as a problem of localization. J Nerv Ment Dis. 1930;71:249–259. [Google Scholar]
- 122.Edge D, Bradford A, Jones JF, O'Halloran KD. Chronic intermittent hypoxia alters genioglossus motor unit discharge patterns in the anaesthetized rat. Adv Exp Med Biol. 2012;758:295–300. doi: 10.1007/978-94-007-4584-1_40. [DOI] [PubMed] [Google Scholar]
- 123.Edge D, McDonald FB, Jones JF, Bradford A, O'Halloran KD. Effect of chronic intermittent hypoxia on the reflex recruitment of the genioglossus during airway obstruction in the anesthetized rat. Progr Brain Res. 2014;209:147–168. doi: 10.1016/B978-0-444-63274-6.00008-4. [DOI] [PubMed] [Google Scholar]
- 124.Edstrom L, Larsson H, Larsson L. Neurogenic effects on the palatopharyngeal muscle in patients with obstructive sleep apnoea: A muscle biopsy study. J Neurol Neurosur Psych. 1992;55:916–920. doi: 10.1136/jnnp.55.10.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Edwards E, Paton JFR. 5-HT4 receptors in nucleus tractus solitarii attenuate cardiopulmonary reflex in anesthetized rats. Am J Physiol. 1999;277:H1914–H1923. doi: 10.1152/ajpheart.1999.277.5.H1914. [DOI] [PubMed] [Google Scholar]
- 126.Edwards E, Paton JFR. Glutamate stimulation of raphe pallidus attenuates the cardiopulmonary reflex in anaesthetised rats. Auton Neurosci Bas Clin. 2000;82:87–96. doi: 10.1016/S0165-1838(00)00072-2. [DOI] [PubMed] [Google Scholar]
- 127.El Mansari M, Sakai K, Jouvet M. Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats. Exp Brain Res. 1989;76:519–529. doi: 10.1007/BF00248908. [DOI] [PubMed] [Google Scholar]
- 128.Euler C von. Brain stem mechanisms for generation and control of breathing pattern. Compr Physiol. 2011(Suppl. 11):1–67. [Google Scholar]
- 129.Ezure K. Reflections on respiratory rhythm generation. Progr Brain Res. 2004;143:67–74. doi: 10.1016/S0079-6123(03)43007-0. [DOI] [PubMed] [Google Scholar]
- 130.Farre R, Nacher M, Serrano-Mollar A, Galdiz JB, Alvarez FJ, Navajas D, Montserrat JM. Rat model of chronic recurrent airway obstructions to study the sleep apnea syndrome. Sleep. 2007;30:930–933. doi: 10.1093/sleep/30.7.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fay R, Kubin L. Pontomedullary distribution of 5-HT2A receptor-like protein in the rat. J Comp Neurol. 2000;418:323–345. [PubMed] [Google Scholar]
- 132.Fay RA, Norgren R. Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus III. Lingual muscle motor systems. Brain Res Rev. 1997;25:291–311. doi: 10.1016/s0165-0173(97)00028-3. [DOI] [PubMed] [Google Scholar]
- 133.Feldman JL, Mitchell GS, Nattie EE. Breathing: Rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fenik VB. Revisiting antagonist effects in hypoglossal nuleus: Brainstem circuit for the state-dependent control of hypoglossal motoneurons: A hypothesis. Front Neurol. 2015;6:254. doi: 10.3389/fneur.2015.00254. doi: 10.3389/fneur.2015.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fenik P, Ogawa H, Veasey SC. Hypoglossal nerve response to 5-HT3 drugs injected into the XII nucleus and vena cava in the rat. Sleep. 2001;24:871–878. doi: 10.1093/sleep/24.8.871. [DOI] [PubMed] [Google Scholar]
- 136.Fenik P, Veasey SC. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. Am J Respir Crit Care Med. 2003;167:563–569. doi: 10.1164/rccm.200202-107OC. [DOI] [PubMed] [Google Scholar]
- 137.Fenik V, Davies RO, Kubin L. Combined antagonism of aminergic excitatory and amino acid inhibitory receptors in the XII nucleus abolishes REM sleep-like depression of hypoglossal motoneuronal activity. Arch Ital Biol. 2004;142:237–249. [PubMed] [Google Scholar]
- 138.Fenik V, Davies RO, Pack AI, Kubin L. Differential suppression of upper airway motor activity during carbachol-induced, REM sleep-like atonia. J Appl Physiol. 1998;275:R1013–R1024. doi: 10.1152/ajpregu.1998.275.4.R1013. [DOI] [PubMed] [Google Scholar]
- 139.Fenik V, Kubin L, Okabe S, Pack AI, Davies RO. Differential sensitivity of laryngeal and pharyngeal motoneurons to iontophoretic application of serotonin. Neuroscience. 1997;81:873–885. doi: 10.1016/s0306-4522(97)00215-7. [DOI] [PubMed] [Google Scholar]
- 140.Fenik V, Marchenko V, Janssen P, Davies RO, Kubin L. A5 cells are silenced when REM sleep-like signs are elicited by pontine carbachol. J Appl Physiol. 2002;93:1448–1456. doi: 10.1152/japplphysiol.00225.2002. [DOI] [PubMed] [Google Scholar]
- 141.Fenik VB, Davies RO, Kubin L. Noradrenergic, serotonergic and GABAergic antagonists injected together into the XII nucleus abolish the REM sleep-like depression of hypoglossal motoneuronal activity. J Sleep Res. 2005;14:419–429. doi: 10.1111/j.1365-2869.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- 142.Fenik VB, Davies RO, Kubin L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med. 2005;172:1322–1330. doi: 10.1164/rccm.200412-1750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fenik VB, Kubin L. Differential localization of carbachol- and bicuculline-sensitive pontine sites for eliciting REM sleep-like effects in anesthetized rats. J Sleep Res. 2009;18:99–112. doi: 10.1111/j.1365-2869.2008.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fenik VB, Marchenko V, Davies RO, Kubin L. Inhibition of A5 neurons facilitates the occurrence of REM sleep-like episodes in urethane-anesthetized rats: A new role for noradrenergic A5 neurons? Front Neurol. 2012;3:119. doi: 10.3389/fneur.2012.00119. doi: 10.3389/fneur.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fenik VB, Ogawa H, Davies RO, Kubin L. Carbachol injections into the ventral pontine reticular formation activate locus coeruleus cells in urethane-anesthetized rats. Sleep. 2005;28:551–559. doi: 10.1093/sleep/28.5.551. [DOI] [PubMed] [Google Scholar]
- 146.Fenik VB, Rukhadze I, Kubin L. Inhibition of pontine noradrenergic A7 cells reduces hypoglossal nerve activity in rats. Neuroscience. 2008;157:473–482. doi: 10.1016/j.neuroscience.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fenik VB, Rukhadze I, Kubin L. Antagonism of α1-adrenergic and serotonergic receptors in the hypoglossal motor nucleus does not prevent motoneuronal activation elicited from the posterior hypothalamus. Neurosci Lett. 2009;462:80–84. doi: 10.1016/j.neulet.2009.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fenik VB, Singletary T, Branconi JL, Davies RO, Kubin L. Glucoregulatory consequences and cardiorespiratory parameters in rats exposed to chronic-intermittent hypoxia: Effects of the duration of exposure and losartan. Front Neurol. 2012;3:51. doi: 10.3389/fneur.2012.00051. doi: 10.3389/fneur.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fletcher EC. Sympathetic activity and blood pressure in the sleep apnea syndrome. Respiration. 1997;64(Suppl 1):22–28. doi: 10.1159/000196732. [DOI] [PubMed] [Google Scholar]
- 150.Fletcher EC. Invited review. Physiological consequences of intermittent hypoxia: Systemic blood pressure. J Appl Physiol. 2001;90:1600–1605. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- 151.Fletcher EC, Bao G, Li R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension. 1999;34:309–314. doi: 10.1161/01.hyp.34.2.309. [DOI] [PubMed] [Google Scholar]
- 152.Fogel RB, Malhotra A, Pillar G, Edwards JK, Beauregard J, Shea SA, White DP. Genioglossal activation in patients with obstructive sleep apnea versus control subjects. Mechanisms of muscle control. Am J Respir Crit Care Med. 2001;164:2025–2030. doi: 10.1164/ajrccm.164.11.2102048. [DOI] [PubMed] [Google Scholar]
- 153.Fogel RB, Trinder J, Malhotra A, Stanchina M, Edwards JK, Schory KE, White DP. Within-breath control of genioglossal muscle activation in humans: Effect of sleep-wake state. J Physiol (Lond) 2003;550:899–910. doi: 10.1113/jphysiol.2003.038810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fort P, Luppi P-H, Sakai K, Salvert D, Jouvet M. Nuclei of origin of monoaminergic, peptidergic, and cholinergic afferents to the cat trigeminal motor nucleus: A double-labeling study with cholera-toxin as a retrograde tracer. J Comp Neurol. 1990;301:262–275. doi: 10.1002/cne.903010209. [DOI] [PubMed] [Google Scholar]
- 155.Fort P, Sakai K, Luppi P-H, Salvert D, Jouvet M. Monoaminergic, peptidergic, and cholinergic afferents to the cat facial nucleus as evidenced by a double immunostaining method with unconjugated cholera toxin as a retrograde tracer. J Comp Neurol. 1989;283:285–302. doi: 10.1002/cne.902830209. [DOI] [PubMed] [Google Scholar]
- 156.Foutz AS, Boudinot E, Morin-Surun M-P, Champagnat J, Gonsalves SF, Denavit-Saubié M. Excitability of' “silent” respiratory neurons during sleep-waking states: An iontophoretic study in undrugged chronic cats. Brain Res. 1987;404:10–20. doi: 10.1016/0006-8993(87)91350-3. [DOI] [PubMed] [Google Scholar]
- 157.Fraigne JJ, Orem JM. Phasic motor activity of respiratory and non-respiratory muscles in REM sleep. Sleep. 2011;34:425–434. doi: 10.1093/sleep/34.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Friberg D, Ansved T, Borg K, Carlsson-Norlander B, Larsson H, Svan-borg E. Histological indications of a progressive snorers disease in the upper airway muscles. Am J Respir Crit Care Med. 1998;157:586–593. doi: 10.1164/ajrccm.157.2.96-06049. [DOI] [PubMed] [Google Scholar]
- 159.Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol (Lond) 1999;519:601–613. doi: 10.1111/j.1469-7793.1999.0601m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fung SJ, Chase MH. Postsynaptic inhibition of hypoglossal motoneurons produces atonia of the genioglossal muscle during rapid eye movement sleep. Sleep. 2015;38:139–146. doi: 10.5665/sleep.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fung SJ, Yamuy J, Sampogna S, Morales FR, Chase MH. Hypocretin (orexin) input to trigeminal and hypoglossal motoneurons in the cat: A double-labeling immunohistochemical study. Brain Res. 2001;903:257–262. doi: 10.1016/s0006-8993(01)02318-6. [DOI] [PubMed] [Google Scholar]
- 162.Fung SJ, Yamuy J, Xi MC, Engelhardt JK, Morales FR, Chase MH. Changes in electrophysiological properties of cat hypoglossal motoneurons during carbachol-induced motor inhibition. Brain Res. 2000;885:262–272. doi: 10.1016/s0006-8993(00)02955-3. [DOI] [PubMed] [Google Scholar]
- 163.Funk GD. Neuromodulation: Purinergic signaling in respiratory control. Compr Physiol. 2013;3:331–363. doi: 10.1002/cphy.c120004. doi: 10.1002/cphy.c120004. [DOI] [PubMed] [Google Scholar]
- 164.Funk GD, Smith JC, Feldman JL. Generation and transmission of respiratory oscillations in medullary slices: Role of excitatory amino acids. J Neurophysiol. 1993;70:1497–1515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- 165.Funk GD, Smith JC, Feldman JL. Development of thyrotropin-releasing hormone and norepinephrine potentiation of inspiratory-related hypoglossal motoneuron discharge in neonatal and juvenile mice in vitro. J Neurophysiol. 1994;72:2538–2541. doi: 10.1152/jn.1994.72.5.2538. [DOI] [PubMed] [Google Scholar]
- 166.Gallego J. Genetic diseases: Congenital central hypoventilation, Rett, and Prader-Willi syndromes. Compr Physiol. 2012;2:2255–2279. doi: 10.1002/cphy.c100037. doi: 10.1002/cphy.c100037. [DOI] [PubMed] [Google Scholar]
- 167.Gatti PJ, Llewellyn-Smith IJ, Sun QJ, Chalmers D, Pilowsky P. Substance P-immunoreactive boutons closely appose inspiratory protruder hypoglossal motoneurons in the cat. Brain Res. 1999;834:155–159. doi: 10.1016/s0006-8993(99)01515-2. [DOI] [PubMed] [Google Scholar]
- 168.Glenn LL, Dement WC. Membrane potential, synaptic activity, and excitability of hindlimb motoneurons during wakefulness and sleep. J Neurophysiol. 1981;46:839–854. doi: 10.1152/jn.1981.46.4.839. [DOI] [PubMed] [Google Scholar]
- 169.Goutagny R, Luppi P-H, Salvert D, Lapray D, Gervasoni D, Fort P. Role of the dorsal paragigantocellular reticular nucleus in paradoxical (rapid eye movement) sleep generation: A combined electrophysiological and anatomical study in the rat. Neuroscience. 2008;152:849–857. doi: 10.1016/j.neuroscience.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 170.Grace KP, Hughes SW, Horner RL. Identification of the mechanism mediating genioglossus muscle suppression in REM sleep. Am J Respir Crit Care Med. 2013;187:311–319. doi: 10.1164/rccm.201209-1654OC. [DOI] [PubMed] [Google Scholar]
- 171.Grace KP, Hughes SW, Horner RL. Identification of a pharmacological target for genioglossus reactivation throughout sleep. Sleep. 2014;37:41–50. doi: 10.5665/sleep.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Grace KP, Hughes SW, Shahabi S, Horner RL. K+ channel modulation causes genioglossus inhibition in REM sleep and is a strategy for reactivation. Respir Physiol Neurobiol. 2013;188:277–288. doi: 10.1016/j.resp.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 173.Grahn DA, Radeke CM, Heller HC. Arousal state vs. temperature effects on neuronal activity in subcoeruleus area. Am J Physiol. 1989;256:R840–R849. doi: 10.1152/ajpregu.1989.256.4.R840. [DOI] [PubMed] [Google Scholar]
- 174.Greenberg HE, Sica AL, Scharf SM, Ruggiero DA. Expression of c-fos in the rat brainstem after chronic intermittent hypoxia. Brain Res. 1999;816:638–645. doi: 10.1016/s0006-8993(98)01222-0. [DOI] [PubMed] [Google Scholar]
- 175.Guilleminault C, Akhtar F. Pediatric sleep-disordered breathing: New evidence on its development. Sleep Med Rev. 2015;24:46–56. doi: 10.1016/j.smrv.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 176.Guyenet PG. Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol. 2014;4:1511–1562. doi: 10.1002/cphy.c140004. doi: 10.1002/cphy.c140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Guyenet PG, Abbott SB, Stornetta RL. The respiratory chemoreception conundrum: Light at the end of the tunnel? Brain Res. 2013;1511:126–137. doi: 10.1016/j.brainres.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol (Lond) 2008;586:2043–2048. doi: 10.1113/jphysiol.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Haji A, Furuichi S, Takeda R. Effects on iontophoretically applied acetylcholine on membrane potential and synaptic activity of bulbar respiratory neurones in decerebrate cats. Neuropharmacology. 1996;35:195–203. doi: 10.1016/0028-3908(95)00159-x. [DOI] [PubMed] [Google Scholar]
- 180.Haji A, Takeda R, Okazaki M. Neuropharmacology of control of respiratory rhythm and pattern in mature mammals. Pharmacol Ther. 2000;86:277–304. doi: 10.1016/s0163-7258(00)00059-0. [DOI] [PubMed] [Google Scholar]
- 181.Hajnik T, Lai YY, Siegel JM. Atonia-related regions in the rodent pons and medulla. J Neurophysiol. 2000;84:1942–1948. doi: 10.1152/jn.2000.84.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hamrahi H, Chan B, Horner RL. On-line detection of sleep-wake states and application to produce intermittent hypoxia only in sleep in rats. J Appl Physiol. 2001;90:2130–2140. doi: 10.1152/jappl.2001.90.6.2130. [DOI] [PubMed] [Google Scholar]
- 183.Hamrahi H, Stephenson R, Mahamed S, Liao KS, Horner RL. Regulation of sleep-wake states in response to intermittent hypoxic stimuli applied only in sleep. J Appl Physiol. 2001;90:2490–2501. doi: 10.1152/jappl.2001.90.6.2490. [DOI] [PubMed] [Google Scholar]
- 184.Han F, Mignot E, Wei YC, Dong SX, Li J, Lin L, An P, Wang LH, Wang JS, He MZ, Gao HY, Li M, Gao ZC, Strohl KP. Ventilatory chemoresponsiveness, narcolepsy-cataplexy and human leukocyte antigen DQB1*0602 status. Eur Respir J. 2010;36:577–583. doi: 10.1183/09031936.00174609. [DOI] [PubMed] [Google Scholar]
- 185.Harasawa Y, Inoue M, Ariyasinghe S, Yamamura K, Yamada Y. Changes in reflex responses of the genioglossus muscle during sleep in rabbits. Brain Res. 2005;1065:79–85. doi: 10.1016/j.brainres.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 186.Harms CA, Zeng Y-J, Smith CA, Vidruk EH, Dempsey JA. Negative pressure-induced deformation of the upper airway causes central apnea in awake and sleeping dogs. J Appl Physiol. 1996;80:1528–1539. doi: 10.1152/jappl.1996.80.5.1528. [DOI] [PubMed] [Google Scholar]
- 187.Harper RM, Sieck GC. Discharge correlations between neurons in the nucleus parabrachialis medialis during sleep-waking states. Brain Res. 1980;199:343–358. doi: 10.1016/0006-8993(80)90694-0. [DOI] [PubMed] [Google Scholar]
- 188.Haxhiu MA, van Lunteren E, Mitra J, Cherniack NS. Comparison of the response of diaphragm and upper airway dilating muscle activity in sleeping cats. Respir Physiol. 1987;70:183–193. doi: 10.1016/0034-5687(87)90049-1. [DOI] [PubMed] [Google Scholar]
- 189.Helke CJ, Shults CW, Chase TN, O'Donohue TL. Autoradiographic localization of substance P receptors in rat medulla: Effect of vagotomy and nodose ganglionectomy. Neuroscience. 1984;12:215–223. doi: 10.1016/0306-4522(84)90148-9. [DOI] [PubMed] [Google Scholar]
- 190.Hendricks JC, Kline LR, Kovalski RJ, O'Brien JA, Morrison AR, Pack AI. The English bulldog: A natural model of sleep-disordered breathing. J Appl Physiol. 1987;63:1344–1350. doi: 10.1152/jappl.1987.63.4.1344. [DOI] [PubMed] [Google Scholar]
- 191.Hendricks JC, Petrof BJ, Panckeri K, Pack AI. Upper airway dilating muscle hyperactivity during non-rapid eye movement sleep in English bulldogs. Am Rev Respir Dis. 1993;148:185–194. doi: 10.1164/ajrccm/148.1.185. [DOI] [PubMed] [Google Scholar]
- 192.Henke KG. Upper airway muscle activity and upper airway resistance in young adults during sleep. J Appl Physiol. 1998;84:486–491. doi: 10.1152/jappl.1998.84.2.486. [DOI] [PubMed] [Google Scholar]
- 193.Henke KG, Badr MS, Skatrud JB, Dempsey JA. Load compensation and respiratory muscle function during sleep. J Appl Physiol. 1992;72:1221–1234. doi: 10.1152/jappl.1992.72.4.1221. [DOI] [PubMed] [Google Scholar]
- 194.Henke KG, Sullivan CE. Effects of high-frequency oscillating pressures on upper airway muscles in humans. J Appl Physiol. 1993;75:856–862. doi: 10.1152/jappl.1993.75.2.856. [DOI] [PubMed] [Google Scholar]
- 195.Henry JN, Manaker S. Colocalization of substance P or enkephalin in serotonergic neuronal afferents to the hypoglossal nucleus in the rat. J Comp Neurol. 1998;391:491–505. [PubMed] [Google Scholar]
- 196.Hieble JP, Bylund DB, Clarke DE, Eikenburg DC, Langer SZ, Lefkowitz RJ, Minneman KP, Ruffolo RR., Jr International union of pharmacology. X. Recommendation for nomenclature of a1-adrenoceptors: Consensus update. Pharmacol Rev. 1995;47:267–270. [PubMed] [Google Scholar]
- 197.Hinrichsen CFL, Weston S. Substance P in the hypoglossal nucleus of the rat. Arch Oral Biol. 1999;44:683–691. doi: 10.1016/s0003-9969(99)00040-0. [DOI] [PubMed] [Google Scholar]
- 198.Hlavac MC, Catcheside PG, Adams A, Eckert DJ, McEvoy RD. The effects of hypoxia on load compensation during sustained incremental resistive loading in patients with obstructive sleep apnea. J Appl Physiol. 2007;103:234–239. doi: 10.1152/japplphysiol.01618.2005. [DOI] [PubMed] [Google Scholar]
- 199.Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hökfelt T, Fuxe K, Johansson O, Jeffcoate S, White N. Distribution of thyrotropin-releasing hormone (TRH) in the central nervous system as revealed with immunohistochemistry. Eur J Pharmacol. 1975;34:389–392. doi: 10.1016/0014-2999(75)90269-1. [DOI] [PubMed] [Google Scholar]
- 201.Hökfelt T, Johansson O, Goldstein M. Chemical anatomy of the brain. Science. 1984;225:1326–1334. doi: 10.1126/science.6147896. [DOI] [PubMed] [Google Scholar]
- 202.Holmes CJ, Jones BE. Importance of cholinergic, GABAergic, serotonergic and other neurons in the medial medullary reticular formation for sleep-wake states studied by cytotoxic lesions in the cat. Neuroscience. 1994;62:1179–1200. doi: 10.1016/0306-4522(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 203.Holmes CJ, Mainville LS, Jones BE. Distribution of cholinergic, GABAergic and serotonergic neurons in the medial medullary reticular formation and their projections studied by cytotoxic lesions in the cat. Neuroscience. 1994;62:1155–1178. doi: 10.1016/0306-4522(94)90351-4. [DOI] [PubMed] [Google Scholar]
- 204.Holtman JR., Jr Immunohistochemical localization of serotonin- and substance P-containing fibers around respiratory muscle motoneurons in the nucleus ambiguus of the cat. Neuroscience. 1988;26:169–178. doi: 10.1016/0306-4522(88)90135-2. [DOI] [PubMed] [Google Scholar]
- 205.Holtman JR, Jr., Marion LJ, Speck DF. Origin of serotonin-containing projections to the ventral respiratory group in the rat. Neuroscience. 1990;37:541–552. doi: 10.1016/0306-4522(90)90422-z. [DOI] [PubMed] [Google Scholar]
- 206.Horner RL. Neural control of the upper airway: Integrative physiological mechanisms and relevance for sleep disordered breathing. Compr Physiol. 2012;2:2012. doi: 10.1002/cphy.c110023. doi: 10.1002/cphy.c110023. [DOI] [PubMed] [Google Scholar]
- 207.Horner RL, Hughes SW, Malhotra A. State-dependent and reflex drives to the upper airway: Basic physiology with clinical implications. J Appl Physiol. 2014;116:325–336. doi: 10.1152/japplphysiol.00531.2013. doi: 10.1152/japplphysiol.00531.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Horner RL, Innes JA, Holden HB, Guz A. Afferent pathwyas for pharynyeal dilator reflex to negative pressure in man: A study using upper airway anaesthesia. J Physiol (Lond) 1991;436:31–44. doi: 10.1113/jphysiol.1991.sp018537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Horner RL, Innes JA, Morrell MJ, Shea SA, Guz A. The effect of sleep on reflex genioglossus muscle activation by stimuli of negative airway pressure in humans. J Physiol (Lond) 1994;476:141–151. [PMC free article] [PubMed] [Google Scholar]
- 210.Horner RL, Kozar LF, Kimoff RJ, Phillipson EA. Effects of sleep on the tonic drive to respiratory muscle and the threshold for rhythm generation in the dog. J Physiol (Lond) 1994;474:525–537. doi: 10.1113/jphysiol.1994.sp020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Horner RL, Liu X, Gill H, Nolan P, Liu H, Sood S. Effects of sleep-wake state on the genioglossus vs. diaphragm muscle response to CO2 in rats. J Appl Physiol. 2002;92:878–887. doi: 10.1152/japplphysiol.00855.2001. [DOI] [PubMed] [Google Scholar]
- 212.Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, van den Pol AN. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- 213.Hoyer D, Martin G. 5-HT receptor classification and nomenclature: Towards a harmonization with the human genome. Neuropharmacology. 1997;36:419–428. doi: 10.1016/s0028-3908(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 214.Huangfu D, Guyenet PG. Alpha 2-adrenergic autoreceptors in A5 and A6 neurons of neonate rats. Am J Physiol. 1997;273:H2290–H2295. doi: 10.1152/ajpheart.1997.273.5.H2290. [DOI] [PubMed] [Google Scholar]
- 215.Huangfu D, Koshiya N, Guyenet PG. Central respiratory modulation of facial motoneurons in rats. Neurosci Lett. 1993;151:224–228. doi: 10.1016/0304-3940(93)90025-g. [DOI] [PubMed] [Google Scholar]
- 216.Hudgel DW. Variable site of airway narrowing among obstructive sleep apnea patients. J Appl Physiol. 1986;61:1403–1409. doi: 10.1152/jappl.1986.61.4.1403. [DOI] [PubMed] [Google Scholar]
- 217.Hudgel DW, Harasick T. Fluctuation in timing of upper airway and chest wall inspiratory muscle activity in obstructive sleep apnea. J Appl Physiol. 1990;69:443–450. doi: 10.1152/jappl.1990.69.2.443. [DOI] [PubMed] [Google Scholar]
- 218.Hunter JD, Milsom WK. Cortical activation states in sleep and anesthesia. I. Cardio-respiratory effects. Respir Physiol. 1998;112:71–81. doi: 10.1016/s0034-5687(98)00018-8. [DOI] [PubMed] [Google Scholar]
- 219.Hwang J-C, St. John WM, Bartlett D., Jr Afferent pathways for hypoglossal and phrenic responses to changes in upper airway pressure. Respir Physiol. 1984;55:341–354. doi: 10.1016/0034-5687(84)90056-2. [DOI] [PubMed] [Google Scholar]
- 220.Iigaya K, Horiuchi J, McDowall LM, Lam AC, Sediqi Y, Polson JW, Carrive P, Dampney RA. Blockade of orexin receptors with Almorexant reduces cardiorespiratory responses evoked from the hypothalamus but not baro- or chemoreceptor reflex responses. Am J Physiol. 2012;303:R1011–R1022. doi: 10.1152/ajpregu.00263.2012. [DOI] [PubMed] [Google Scholar]
- 221.Ireland MF, Funk GD, Bellingham MC. Muscarinic acetylcholine receptors enhance neonatal mouse hypoglossal motoneuron excitability in vitro. J Appl Physiol. 2012;113:1024–1039. doi: 10.1152/japplphysiol.00699.2011. [DOI] [PubMed] [Google Scholar]
- 222.Ireland MF, Lenal FC, Lorier AR, Loomes DE, Adachi T, Alvares TS, Greer JJ, Funk GD. Distinct receptors underlie glutamatergic signalling in inspiratory rhythm-generating networks and motor output pathways in neonatal rat. J Physiol (Lond) 2008;586:2357–2370. doi: 10.1113/jphysiol.2007.150532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Iscoe SD. Central control of the upper airway. In: Mathew OP, Sant'Ambrogio G, editors. Respiratory Function of the Upper Airway. Marcel Dekker; New York: 1988. [Google Scholar]
- 224.Issa FG, Bitner S. Effect of route of breathing on the ventilatory and arousal responses to hypercapnia in awake and sleeping dogs. J Physiol (Lond) 1993;465:615–628. doi: 10.1113/jphysiol.1993.sp019696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Issa FG, Edwards P, Szeto E, Lauff D, Sullivan C. Genioglossus and breathing responses to airway occlusion: Effect of sleep and route of occlusion. J Appl Physiol. 1988;64:543–549. doi: 10.1152/jappl.1988.64.2.543. [DOI] [PubMed] [Google Scholar]
- 226.Issa FG, Sullivan CE. Upper airway closing pressures in obstructive sleep apnea. J Appl Physiol. 1984;57:520–527. doi: 10.1152/jappl.1984.57.2.520. [DOI] [PubMed] [Google Scholar]
- 227.Itier V, Bertrand D. Neuronal nicotinic receptors: From protein structure to function. FEBS Lett. 2001;504:118–125. doi: 10.1016/s0014-5793(01)02702-8. [DOI] [PubMed] [Google Scholar]
- 228.Ivanov A, Aston-Jones G. Hypocretin/orexin depolarizes and decreases potassium conductance in locus coeruleus neurons. NeuroReport. 2000;11:1755–1758. doi: 10.1097/00001756-200006050-00031. [DOI] [PubMed] [Google Scholar]
- 229.Iverfeldt K, Serfözö P, Diaz Arnesto L, Bartfai T. Differential release of coexisting neurotransmitters: Frequency dependence of the efflux of substance P, thyrotropin releasing hormone and [3H]serotonin from tissue slices of rat ventral spinal cord. Acta Physiol Scand. 1989;137:63–71. doi: 10.1111/j.1748-1716.1989.tb08721.x. [DOI] [PubMed] [Google Scholar]
- 230.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 231.Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol (Lond) 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol. 2013;3:141–163. doi: 10.1002/cphy.c110057. doi: 10.1002/cphy.c110057. [DOI] [PubMed] [Google Scholar]
- 233.Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J Physiol (Lond) 2001;532:467–481. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.John J, Wu MF, Boehmer LN, Siegel JM. Cataplexy-active neurons in the hypothalamus: Implications for the role of histamine in sleep and waking behavior. Neuron. 2004;42:619–634. doi: 10.1016/s0896-6273(04)00247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Johnson H, Ulfhake B, Dagerlind A, Bennett GW, Fone KC. The serotoninergic bulbospinal system and brainstem-spinal cord content of serotonin-, TRH, and substance P-like immunoreactivity in the aged rat with special reference to the spinal cord motor nucleus. Synapse. 1993;15:63–89. doi: 10.1002/syn.890150108. [DOI] [PubMed] [Google Scholar]
- 236.Johnson SM, Smith JC, Feldman JL. Modulation of respiratory rhythm in vitro: Role of Gi/o protein-mediated mechanisms. J Appl Physiol. 1996;80:2120–2133. doi: 10.1152/jappl.1996.80.6.2120. [DOI] [PubMed] [Google Scholar]
- 237.Jones BE, Beaudet A. Distribution of acetylcholine and catecholamine neurons in the cat brainstem: A choline acetyltransferase and tyrosine hydroxylase immunohistochemical study. J Comp Neurol. 1987;261:15–32. doi: 10.1002/cne.902610103. [DOI] [PubMed] [Google Scholar]
- 238.Kachidian P, Poulat P, Marlier L, Privat A. Immunohistochemical evidence for the coexistence of substance P, thyrotropin-releasing hormone, GABA, methionin-enkephalin, and leucin-enkephalin in the serotonergic neurons of the caudal raphe nuclei: A dual labeling in the rat. J Neurosci Res. 1991;30:521–530. doi: 10.1002/jnr.490300309. [DOI] [PubMed] [Google Scholar]
- 239.Kalia M, Fuxe K, Goldstein M. Rat medulla oblongata. II. Dopaminergic, noradrenergic (A1 and A2) and adrenergic neurons, nerve fibers, and presumptive terminal processes. J Comp Neurol. 1985;233:308–332. doi: 10.1002/cne.902330303. [DOI] [PubMed] [Google Scholar]
- 240.Kalia M, Fuxe K, Goldstein M. Rat medulla oblongata. III. Adrenergic (C1 and C2) neurons, nerve fibers and presumptive terminal processes. J Comp Neurol. 1985;233:333–349. doi: 10.1002/cne.902330304. [DOI] [PubMed] [Google Scholar]
- 241.Kam K, Worrell JW, Janczewski WA, Cui Y, Feldman JL. Distinct inspiratory rhythm and pattern generating mechanisms in the pre-Bötzinger complex. J Neurosci. 2013;33:9235–9245. doi: 10.1523/JNEUROSCI.4143-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kanamori N, Sakai K, Jouvet M. Neuronal activity specific to paradoxical sleep in the ventromedial medullary reticular formation of unrestrained cats. Brain Res. 1980;189:251–255. doi: 10.1016/0006-8993(80)90024-4. [DOI] [PubMed] [Google Scholar]
- 243.Karlsson KA, Blumberg MS. Active medullary control of atonia in week-old rats. Neuroscience. 2005;130:275–283. doi: 10.1016/j.neuroscience.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Katakura N, Chandler SH. An iontophoretic analysis of the pharmacologic mechanisms responsible for trigeminal motoneuronal discharge during masticatory-like activity in the guinea pig. J Neurophysiol. 1990;63:356–369. doi: 10.1152/jn.1990.63.2.356. [DOI] [PubMed] [Google Scholar]
- 245.Kato T, Masuda Y, Morimoto T. Patterns of masseter muscle activities during sleep in guinea pigs. Arch Oral Biol. 2007;52:385–386. doi: 10.1016/j.archoralbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 246.Katz ES, White DP. Genioglossus activity in children with obstructive sleep apnea during wakefulness and sleep onset. Am J Respir Crit Care Med. 2003;168:664–670. doi: 10.1164/rccm.200301-092OC. [DOI] [PubMed] [Google Scholar]
- 247.Katz ES, White DP. Genioglossus activity during sleep in normal control subjects and children with obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:553–560. doi: 10.1164/rccm.200403-262OC. [DOI] [PubMed] [Google Scholar]
- 248.Kay A, Trinder J, Bowes G, Kim Y. Changes in airway resistance during sleep onset. J Appl Physiol. 1994;76:1600–1607. doi: 10.1152/jappl.1994.76.4.1600. [DOI] [PubMed] [Google Scholar]
- 249.Kayama Y, Ohta M, Jodo E. Firing of `possibly' cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res. 1992;569:210–220. doi: 10.1016/0006-8993(92)90632-j. [DOI] [PubMed] [Google Scholar]
- 250.Kezirian EJ, Goding GS, Jr., Malhotra A, O'Donoghue FJ, Zammit G, Wheatley JR, Catcheside PG, Smith PL, Schwartz AR, Walsh JH, Maddison KJ, Claman DM, Huntley T, Park SY, Campbell MC, Palme CE, Iber C, Eastwood PR, Hillman DR, Barnes M. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. J Sleep Res. 2014;23:77–83. doi: 10.1111/jsr.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kheirandish-Gozal L, Gozal D. Genotype-phenotype interactions in pediatric obstructive sleep apnea. Respir Physiol Neurobiol. 2013;189:338–343. doi: 10.1016/j.resp.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kia HK, Miquel M-C, Brisorgueil M-J, Davel G, Riad M, Mestikawy SE, Hamon M, Verge D. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J Comp Neurol. 1996;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 253.Kianicka I, Praud J-P. Influence of sleep states on laryngeal and abdominal muscle response to upper airway occlusion in lambs. Ped Res. 1997;41:862–871. doi: 10.1203/00006450-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 254.Kimoff JR, Makino H, Horner RL, Kozar LF, Lue F, Slutsky AS, Phillipson EA. Canine model of obstructive sleep apnea: Model description and preliminary application. J Appl Physiol. 1994;76:1810–1816. doi: 10.1152/jappl.1994.76.4.1810. [DOI] [PubMed] [Google Scholar]
- 255.Kimura H, Kubin L, Davies RO, Pack AI. Cholinergic stimulation of the pons depresses respiration in decerebrate cats. J Appl Physiol. 1990;69:2280–2289. doi: 10.1152/jappl.1990.69.6.2280. [DOI] [PubMed] [Google Scholar]
- 256.King ED, O'Donnell CP, Smith PL, Schwartz AR. A model of obstructive sleep apnea in normal humans. Role of the upper airway. Am J Respir Crit Care Med. 2000;161:1979–1984. doi: 10.1164/ajrccm.161.6.9904096. [DOI] [PubMed] [Google Scholar]
- 257.Knight WD, Little JT, Carreno FR, Toney GM, Mifflin SW, Cunningham JT. Chronic intermittent hypoxia increases blood pressure and expression of FosB/DeltaFosB in central autonomic regions. Am J Physiol. 2011;301:R131–R139. doi: 10.1152/ajpregu.00830.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ko EM, Estabrooke IV, McCarthy M, Scammell TE. Wake-related activity of tuberomammillary neurons in rats. Brain Res. 2003;992:220–226. doi: 10.1016/j.brainres.2003.08.044. [DOI] [PubMed] [Google Scholar]
- 259.Kodama T, Lai YY, Siegel JM. Changes in inhibitory amino acid release linked to pontine-induced atonia: An in vivo microdialysis study. J Neurosci. 2003;23:1548–1554. doi: 10.1523/JNEUROSCI.23-04-01548.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kodama T, Takahashi Y, Honda Y. Enhancement of acetylcholine release during paradoxical sleep in the dorsal tegmental field of the cat brain stem. Neurosci Lett. 1990;114:277–282. doi: 10.1016/0304-3940(90)90576-u. [DOI] [PubMed] [Google Scholar]
- 261.Kohlmeier KA, Burns J, Reiner PB, Semba K. Substance P in the descending cholinergic projection to REM sleep-induction regions of the rat pontine reticular formation: Anatomical and electrophysiological analyses. Eur J Neurosci. 2002;15:176–196. doi: 10.1046/j.0953-816x.2001.01829.x. [DOI] [PubMed] [Google Scholar]
- 262.Koizumi H, Wilson CG, Wong S, Yamanishi T, Koshiya N, Smith JC. Functional imaging, spatial reconstruction, and biophysical analysis of a respiratory motor circuit isolated in vitro. J Neurosci. 2008;28:2353–2365. doi: 10.1523/JNEUROSCI.3553-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kolta A, Dubuct R, Lund JP. An immunocytochemical and autoradiographic investigation of the serotoninergic innervation of trigeminal mesencephalic and motor nuclei in the rabbit. Neuroscience. 1993;53:1113–1126. doi: 10.1016/0306-4522(93)90494-z. [DOI] [PubMed] [Google Scholar]
- 264.Krolo M, Stuth EA, Tonkovic-Capin M, Hopp FA, McCrimmon DR, Zuperku EJ. Relative magnitude of tonic and phasic synaptic excitation of medullary inspiratory neurons in dogs. Am J Physiol. 2000;279:R639–R649. doi: 10.1152/ajpregu.2000.279.2.R639. [DOI] [PubMed] [Google Scholar]
- 265.Kubin L. Carbachol models of REM sleep: Recent developments and new directions. Arch Ital Biol. 2001;139:147–168. [PubMed] [Google Scholar]
- 266.Kubin L. Respiratory physiology: CNS ventilatory control. In: Amlaner CJ, Fuller PM, editors. Basics of Sleep Guide. 2nd ed Sleep Research Society; Westchester, Il: 2009. [Google Scholar]
- 267.Kubin L. Sleep-wake control of the upper airway by noradrenergic neurons, with and without intermittent hypoxia. In: Holstege G, Beers CM, Subramanian HH, editors. The Central Nervous System Control of Respiration. Elsevier; Amsterdam: 2014. doi: 10.1016/B978-0-444-63274-6.00013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kubin L, Davies RO. Central pathways of pulmonary and airway vagal afferents. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. Marcel Dekker; New York: 1995. [Google Scholar]
- 270.Kubin L, Davies RO. Mechanisms of upper airway hypotonia. In: Pack AI, editor. Sleep Apnea: Pathogenesis, Diagnosis and Treatment. 2nd ed Informa Healthcare; St. Heliers: 2011. [Google Scholar]
- 271.Kubin L, Davies RO, Pack AI. Control of upper airway motoneurons during REM sleep. News Physiol Sci. 1998;13:91–97. doi: 10.1152/physiologyonline.1998.13.2.91. [DOI] [PubMed] [Google Scholar]
- 272.Kubin L, Fenik V. Pontine cholinergic mechanisms and their impact on respiratory regulation. Respir Physiol Neurobiol. 2004;143:235–249. doi: 10.1016/j.resp.2004.04.017. doi: 10.1016/j.resp.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 273.Kubin L, Kimura H, Tojima H, Davies RO, Pack AI. Suppression of hypoglossal motoneurons during the carbachol-induced atonia of REM sleep is not caused by fast synaptic inhibition. Brain Res. 1993;611:300–312. doi: 10.1016/0006-8993(93)90517-q. [DOI] [PubMed] [Google Scholar]
- 274.Kubin L, Kimura H, Tojima H, Pack AI, Davies RO. Behavior of VRG neurons during the atonia of REM sleep induced by pontine carbachol in decerebrate cats. Brain Res. 1992;592:91–100. doi: 10.1016/0006-8993(92)91662-x. [DOI] [PubMed] [Google Scholar]
- 275.Kubin L, Reignier C, Tojima H, Taguchi O, Pack AI, Davies RO. Changes in serotonin level in the hypoglossal nucleus region during the carbachol-induced atonia. Brain Res. 1994;645:291–302. doi: 10.1016/0006-8993(94)91663-2. [DOI] [PubMed] [Google Scholar]
- 276.Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett. 1992;139:243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- 277.Kubin L, Tojima H, Reignier C, Pack AI, Davies RO. Interaction of serotonergic excitatory drive to hypoglossal motoneurons with carbachol-induced, REM sleep-like atonia. Sleep. 1996;19:187–195. [PubMed] [Google Scholar]
- 278.Kuna ST, Insalaco G, Villeponteaux RD. Arytenoideus muscle activity in normal adult humans during wakefulness and sleep. J Appl Physiol. 1991;70:1655–1664. doi: 10.1152/jappl.1991.70.4.1655. [DOI] [PubMed] [Google Scholar]
- 279.Kuna ST, Insalaco G, Woodson GE. Thyroarytenoid muscle activity during wakefulness and sleep in normal adults. J Appl Physiol. 1988;65:1332–1339. doi: 10.1152/jappl.1988.65.3.1332. [DOI] [PubMed] [Google Scholar]
- 280.Kuna ST, Smickley J. Response of genioglosus muscle activity to nasal airway occlusion in normal sleeping adults. J Appl Physiol. 1988;64:347–353. doi: 10.1152/jappl.1988.64.1.347. [DOI] [PubMed] [Google Scholar]
- 281.Kuna ST, Smickley JS. Superior pharyngeal constrictor activation in obstructive sleep apnea. Am J Respir Crit Care Med. 1997;156:874–880. doi: 10.1164/ajrccm.156.3.9702053. [DOI] [PubMed] [Google Scholar]
- 282.Kuna ST, Smickley JS, Insalaco G. Posterior cricoarytenoid muscle activity during wakefulness and sleep in normal adults. J Appl Physiol. 1990;68:1746–1754. doi: 10.1152/jappl.1990.68.4.1746. [DOI] [PubMed] [Google Scholar]
- 283.Kuna ST, Smickley JS, Vanoye CR. Respiratory-related pharyngeal constrictor muscle activity in normal human adults. Am J Respir Crit Care Med. 1997;155:1991–1999. doi: 10.1164/ajrccm.155.6.9196107. [DOI] [PubMed] [Google Scholar]
- 284.Kuna ST, Smickley JS, Vanoye CR, McMillan TH. Cricothyroid muscle activity during sleep in normal adult humans. J Appl Physiol. 1994;76:2326–2332. doi: 10.1152/jappl.1994.76.6.2326. [DOI] [PubMed] [Google Scholar]
- 285.Kurasawa I, Toda K, Nakamura Y. Non-reciprocal facilitation of trigeminal motoneurons innervating jaw-closing and jaw-opening muscles induced by iontophoretic application of serotonin in the guinea pig. Brain Res. 1990;515:126–134. doi: 10.1016/0006-8993(90)90586-z. [DOI] [PubMed] [Google Scholar]
- 286.Lai J, Shao XM, Pan RW, Dy E, Huang CH, Feldman JL. RT-PCR reveals muscarinic acetylcholine receptor mRNA in the pre-Bötzinger complex. Am J Physiol. 2001;281:L1420–L1424. doi: 10.1152/ajplung.2001.281.6.L1420. [DOI] [PubMed] [Google Scholar]
- 287.Lai YY, Clements JR, Wu XY, Shalita T, Wu J-P, Kuo JS, Siegel JM. Brainstem projections to the ventromedial medulla in cat: Retrograde transport horseradish peroxidase and immunohistochemical studies. J Comp Neurol. 1999;408:419–436. doi: 10.1002/(sici)1096-9861(19990607)408:3<419::aid-cne8>3.0.co;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Lai YY, Kodama T, Siegel J. Changes in monoamine release in the ventral horn and hypoglossal nucleus linked to pontine inhibition of muscle tone: An in vivo microdialysis study. J Neurosci. 2001;21:7384–7391. doi: 10.1523/JNEUROSCI.21-18-07384.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Lalley PM. The excitability and rhythm of medullary respiratory neurons in the cat are altered by the serotonin receptor agonist 5-methoxy-N, N, dimethyltryptamine. Brain Res. 1994;648:87–98. doi: 10.1016/0006-8993(94)91909-7. [DOI] [PubMed] [Google Scholar]
- 290.Lalley PM, Bischoff AM, Richter DW. 5-HT1A receptor-mediated modulation of medullary expiratory neurons in the cat. J Physiol (Lond) 1994;476:117–130. [PMC free article] [PubMed] [Google Scholar]
- 291.Langmead CJ, Watson J, Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther. 2008;117:232–243. doi: 10.1016/j.pharmthera.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 292.Larkin EK, Patel SR, Goodloe RJ, Li Y, Zhu X, Gray-McGuire C, Adams MD, Redline S. A candidate gene study of obstructive sleep apnea in European Americans and African Americans. Am J Respir Crit Care Med. 2010;182:947–953. doi: 10.1164/rccm.201002-0192OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Larkman PM, Kelly JS. Ionic mechanisms mediating 5-hydroxytryptamine- and noradrenaline-evoked depolarization of adult rat facial motoneurones. J Physiol (Lond) 1992;456:473–490. doi: 10.1113/jphysiol.1992.sp019347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Launois C, Attali V, Georges M, Raux M, Morawiec E, Rivals I, Arnulf I, Similowski T. Cortical drive to breathe during wakefulness in patients with obstructive sleep apnea syndrome. Sleep. 2015;38:1743–1749. doi: 10.5665/sleep.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lazarenko RM, Stornetta RL, Bayliss DA, Guyenet PG. Orexin A activates retrotrapezoid neurons in mice. Respir Physiol Neurobiol. 2011;175:283–287. doi: 10.1016/j.resp.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Le Novere N, Corringer PJ, Changeux JP. The diversity of subunit composition in nAChRs: Evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol. 2002;53:447–456. doi: 10.1002/neu.10153. [DOI] [PubMed] [Google Scholar]
- 297.Lee LY, Yu J. Sensory nerves in lung and airways. Compr Physiol. 2014;4:287–324. doi: 10.1002/cphy.c130020. doi: 10.1002/cphy.c130020. [DOI] [PubMed] [Google Scholar]
- 298.Lee MC, Lee CH, Hong SL, Kim SW, Lee WH, Lim JY, Joe S, Yoon IY, Kim JW. Establishment of a rabbit model of obstructive sleep apnea by paralyzing the genioglossus. JAMA Otolaryngol Head Neck Surg. 2013;139:834–840. doi: 10.1001/jamaoto.2013.4001. [DOI] [PubMed] [Google Scholar]
- 299.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Levy P, Tamisier R, Arnaud C, Monneret D, Baguet JP, Stanke-Labesque F, Dematteis M, Godin-Ribuot D, Ribuot C, Pepin JL. Sleep deprivation, sleep apnea and cardiovascular diseases. Front Biosci. 2012;4:2007–2021. doi: 10.2741/521. [DOI] [PubMed] [Google Scholar]
- 302.Li J, Grigoryev DN, Ye SQ, Thorne L, Schwartz AR, Smith PL, O'Donnell CP, Polotsky VY. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J Appl Physiol. 2005;99:1643–1648. doi: 10.1152/japplphysiol.00522.2005. [DOI] [PubMed] [Google Scholar]
- 303.Li R, Bao G, El-Mallakh RS, Fletcher EC. Effects of chronic episodic hypoxia on monoamine metabolism and motor activity. Physiol Behav. 1996;60:1071–1076. doi: 10.1016/0031-9384(96)00149-7. [DOI] [PubMed] [Google Scholar]
- 304.Li Y-Q, Takada M, Kaneko T, Mizuno M. Distribution of GABAergic and glycinergic premotor neurons projecting to facial and hypoglossal nuclei in the rat. J Comp Neurol. 1997;378:283–294. doi: 10.1002/(sici)1096-9861(19970210)378:2<283::aid-cne10>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 305.Li YQ, Takada M, Mizuno N. Premotor neurons projecting simultaneously to two orofacial motor nuclei by sending their branched axons. A study with a fluorescent retrograde double-labeling technique in the rat. Neurosci Lett. 1993;152:29–32. doi: 10.1016/0304-3940(93)90475-z. [DOI] [PubMed] [Google Scholar]
- 306.Li Y-Q, Takada M, Mizuno N. The sites of origin of serotoninergic afferent fibers in the trigeminal motor, facial, and hypoglossal nuclei in the rat. Neurosci Res. 1993;17:307–313. doi: 10.1016/0168-0102(93)90114-6. [DOI] [PubMed] [Google Scholar]
- 307.Lin CM, Huang YS, Guilleminault C. Pharmacotherapy of obstructive sleep apnea. Exp Opin Pharmacother. 2012;13:841–857. doi: 10.1517/14656566.2012.666525. [DOI] [PubMed] [Google Scholar]
- 308.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 309.Lipski J, Zhang X, Kruszewska B, Kanjhan R. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res. 1994;640:171–184. doi: 10.1016/0006-8993(94)91871-6. [DOI] [PubMed] [Google Scholar]
- 310.Liu X, Sood S, Liu H, Horner RL. Opposing muscarinic and nicotinic modulation of hypoglossal motor output to genioglossus muscle in rats in vivo. J Physiol (Lond) 2005;565:965–980. doi: 10.1113/jphysiol.2005.084657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Lonergan RP, III, Ware JC, Atkinson RL, Winter WC, Suratt PM. Sleep apnea in obese miniature pigs. J Appl Physiol. 1998;84:531–536. doi: 10.1152/jappl.1998.84.2.531. [DOI] [PubMed] [Google Scholar]
- 312.Lopes JM, Tabachnik E, Muller NL, Levison H, Bryan AC. Total airway resistance and respiratory muscle activity during sleep. J Appl Physiol. 1983;54:773–777. doi: 10.1152/jappl.1983.54.3.773. [DOI] [PubMed] [Google Scholar]
- 313.Lowe AA. The neural regulation of tongue-movements. Progr Neurobiol. 1981;15:295–344. doi: 10.1016/0301-0082(80)90008-8. [DOI] [PubMed] [Google Scholar]
- 314.Lu JW, Fenik VB, Branconi JL, Mann GL, Rukhadze I, Kubin L. Disinhibition of perifornical hypothalamic neurones activates noradrenergic neurones and blocks pontine carbachol-induced REM sleep-like episodes in rats. J Physiol (Lond) 2007;582:52–67. doi: 10.1113/jphysiol.2007.127613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Lu JW, Kubin L. Electromyographic activity at the base and tip of the tongue across sleep-wake states in rats. Respir Physiol Neurobiol. 2009;167:307–315. doi: 10.1016/j.resp.2009.06.004. doi: 10.1016/j.resp.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Lu JW, Mann GL, Ross RJ, Morrison AR, Kubin L. Differential effect of sleep-wake states on lingual and dorsal neck muscle activity in rats. Respir Physiol Neurobiol. 2005;147:191–203. doi: 10.1016/j.resp.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 317.Lujan R, Marron FV, Aguado C, Wickman K. New insights into the therapeutic potential of Girk channels. Trends Neurosci. 2014;37:20–29. doi: 10.1016/j.tins.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Malhotra A, Trinder J, Fogel R, Stanchina M, Patel SR, Schory K, Kleverlaan D, White DP. Postural effects on pharyngeal protective reflex mechanisms. Sleep. 2004;27:1105–1112. doi: 10.1093/sleep/27.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Maljevic S, Lerche H. Potassium channels: A review of broadening therapeutic possibilities for neurological diseases. J Neurol. 2013;260:2201–2211. doi: 10.1007/s00415-012-6727-8. [DOI] [PubMed] [Google Scholar]
- 320.Mallios VJ, Lydic R, Baghdoyan HA. Muscarinic receptor subtypes are differentially distributed across brain stem respiratory nuclei. Am J Physiol. 1995;268:L941–L949. doi: 10.1152/ajplung.1995.268.6.L941. [DOI] [PubMed] [Google Scholar]
- 321.Manaker S, Rizio G. Autoradiographic localization of thyrotropin-releasing hormone and substance P receptors in the rat dorsal vagal complex. J Comp Neurol. 1989;290:516–526. doi: 10.1002/cne.902900406. [DOI] [PubMed] [Google Scholar]
- 322.Manaker S, Tischler LJ. Origin of serotonergic afferents to the hypoglossal nucleus in the rat. J Comp Neurol. 1993;334:466–476. doi: 10.1002/cne.903340310. [DOI] [PubMed] [Google Scholar]
- 323.Manaker S, Tischler LJ, Morrison AR. Raphespinal and reticulospinal axon collaterals to the hypoglossal nucleus in the rat. J Comp Neurol. 1992;322:68–78. doi: 10.1002/cne.903220106. [DOI] [PubMed] [Google Scholar]
- 324.Manaker S, Verderame HM. Organization of serotonin 1A and 1B receptors in the nucleus of the solitary tract. J Comp Neurol. 1990;301:535–553. doi: 10.1002/cne.903010405. [DOI] [PubMed] [Google Scholar]
- 325.Manaker S, Zucchi PC. Autoradiographic localization of neurotransmitter binding sites in the hypoglossal and motor trigeminal nuclei of the rat. Synapse. 1998;28:44–59. doi: 10.1002/(SICI)1098-2396(199801)28:1<44::AID-SYN6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 326.Manns ID, Alonso A, Jones BE. Discharge properties of juxtacellularly labeled and immunohistochemically identified cholinergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. J Neurosci. 2000;20:1505–1518. doi: 10.1523/JNEUROSCI.20-04-01505.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 328.Mateika JH, Syed Z. Intermittent hypoxia, respiratory plasticity and sleep apnea in humans: Present knowledge and future investigations. Respir Physiol Neurobiol. 2013;188:289–300. doi: 10.1016/j.resp.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Mathew OP, Abu-Osba YK, Thach BT. Genioglossus muscle responses to upper airway pressure changes: Afferent pathways. J Appl Physiol. 1982;52:445–450. doi: 10.1152/jappl.1982.52.2.445. [DOI] [PubMed] [Google Scholar]
- 330.Mathew OP, Abu-Osba YK, Thach BT. Influence of upper airway pressure changes on genioglossus muscle respiratory activity. J Appl Physiol. 1982;52:438–444. doi: 10.1152/jappl.1982.52.2.438. [DOI] [PubMed] [Google Scholar]
- 331.McCall RB, Aghajanian GK. Serotonergic facilitation of facial motoneuron excitation. Brain Res. 1979;169:11–27. doi: 10.1016/0006-8993(79)90370-6. [DOI] [PubMed] [Google Scholar]
- 332.McCarter SJ, St Louis EK, Duwell EJ, Timm PC, Sandness DJ, Boeve BF, Silber MH. Diagnostic thresholds for quantitative REM sleep phasic burst duration, phasic and tonic muscle activity, and REM atonia index in REM sleep behavior disorder with and without comorbid obstructive sleep apnea. Sleep. 2014;37:1649–1662. doi: 10.5665/sleep.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.McClung JR, Goldberg SJ. Functional anatomy of the hypoglossal innervated muscles of the rat tongue: A model for elongation and protrusion of the mammalian tongue. Anat Rec. 2000;260:378–386. doi: 10.1002/1097-0185(20001201)260:4<378::AID-AR70>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 334.McGinley BM, Schwartz AR, Schneider H, Kirkness JP, Smith PL, Patil SP. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol. 2008;105:197–205. doi: 10.1152/japplphysiol.01214.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.McGinty DJ, Harper RM. Dorsal raphe neurons: Depression of firing during sleep in cats. Brain Res. 1976;101:569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- 336.McGuire M, Zhang Y, White DP, Ling L. Effect of hypoxic episode number and severity on ventilatory long-term facilitation in awake rats. J Appl Physiol. 2002;93:2155–2161. doi: 10.1152/japplphysiol.00405.2002. [DOI] [PubMed] [Google Scholar]
- 337.McKay LC, Janczewski WA, Feldman JL. Sleep-disordered breathing after targeted ablation of preBotzinger complex neurons. Nat Neurosci. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.McSharry DG, Saboisky JP, Deyoung P, Jordan AS, Trinder J, Smales E, Hess L, Chamberlin NL, Malhotra A. Physiological mechanisms of upper airway hypotonia during REM sleep. Sleep. 2014;37:561–569. doi: 10.5665/sleep.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.McSharry DG, Saboisky JP, DeYoung P, Matteis P, Jordan AS, Trinder J, Smales E, Hess L, Guo M, Malhotra A. A mechanism for upper airway stability during slow wave sleep. Sleep. 2013;36:555–563. doi: 10.5665/sleep.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Megirian D, Cespuglio R, Jouvet M. Rhythmical activity of the rats's tongue in sleep and wakefulness. EEG Clin Neurophysiol. 1978;44:8–13. doi: 10.1016/0013-4694(78)90100-1. [DOI] [PubMed] [Google Scholar]
- 341.Megirian D, Hinrichsen CFL, Sherrey JH. Respiratory roles of genioglossus, sternothyroid, and sternohyoid muscles during sleep. Exp Neurol. 1985;90:118–128. doi: 10.1016/0014-4886(85)90045-7. [DOI] [PubMed] [Google Scholar]
- 342.Megirian D, Sherrey JH. Respiratory functions of the laryngeal muscles during sleep. Sleep. 1980;3:289–298. doi: 10.1093/sleep/3.3-4.289. [DOI] [PubMed] [Google Scholar]
- 343.Mengod G, Pompeiano M, Martínez-Mir MI, Palacios JM. Localization of the mRNA for the 5-HT2 receptor by in situ hybridization histochemistry. Correlation with the distribution of receptor sites. Brain Res. 1990;524:139–143. doi: 10.1016/0006-8993(90)90502-3. [DOI] [PubMed] [Google Scholar]
- 344.Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: An overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- 345.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am J Respir Crit Care Med. 1996;153:1880–1887. doi: 10.1164/ajrccm.153.6.8665050. [DOI] [PubMed] [Google Scholar]
- 347.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Millhorn DE, Hökfelt T, Szymeczek CL, Bayliss DA, Seroogy KB. Cellular, molecular and developmental aspects of chemical synaptic transmission. In: Haddad GG, Farber JP, editors. Developmental Neurobiology of Breathing. Dekker; New York: 1991. [Google Scholar]
- 349.Mody P, Rukhadze I, Kubin L. Rats subjected to chronic-intermittent hypoxia have increased density of noradrenergic terminals in the trigeminal sensory and motor nuclei. Neurosci Lett. 2011;505:176–179. doi: 10.1016/j.neulet.2011.10.015. doi: 10.1016/j.neulet.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Moore MW, Akladious A, Hu Y, Azzam S, Feng P, Strohl KP. Effects of orexin 2 receptor activation on apnea in the C57BL/6J mouse. Respir Physiol Neurobiol. 2014;200:118–125. doi: 10.1016/j.resp.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Morales FR, Boxer P, Chase MH. Behavioral state-specific inhibitory postsynaptic potentials impinge on cat lumbar motoneurons during active sleep. Exp Neurol. 1987;98:418–435. doi: 10.1016/0014-4886(87)90252-4. [DOI] [PubMed] [Google Scholar]
- 352.Morales FR, Chase MH. Postsynaptic control of lumbar motoneuron excitability during active sleep in the chronic cat. Brain Res. 1981;225:279–295. doi: 10.1016/0006-8993(81)90836-2. [DOI] [PubMed] [Google Scholar]
- 353.Morales FR, Chase MH. Repetitive synaptic potentials responsible for inhibition of spinal cord motoneurons during active sleep. Exp Neurol. 1982;78:471–476. doi: 10.1016/0014-4886(82)90065-6. [DOI] [PubMed] [Google Scholar]
- 354.Morales FR, Engelhardt JK, Soja PJ, Pereda AE, Chase MH. Motoneuron properties during motor inhibition produced by microinjection of carbachol into the pontine reticular formation of the decerebrate cat. J Neurophysiol. 1987;57:1118–1129. doi: 10.1152/jn.1987.57.4.1118. [DOI] [PubMed] [Google Scholar]
- 355.Morales M, Battenberg E, de Lecea L, Sanna PP, Bloom FE. Cellular and subcellular immunolocalization of the type 3 serotonin receptor in the rat central nervous system. Mol Brain Res. 1996;36:251–260. doi: 10.1016/0169-328x(96)88406-3. [DOI] [PubMed] [Google Scholar]
- 356.Morin-Surun MP, Champagnat J, Denavit-Saubié M, Moyanova S. The effects of acetylcholine on bulbar respiratory related neurones. Consequences of anaesthesia by pentobarbital. Naunyn-Schmiedebergs Arch Pharmacol. 1984;325:205–208. doi: 10.1007/BF00495944. [DOI] [PubMed] [Google Scholar]
- 357.Morrison DL, Launois SH, Isono S, Feroah TR, Whitelaw WA, Remmers JE. Pharyngeal narrowing and closing pressures in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;148:606–611. doi: 10.1164/ajrccm/148.3.606. [DOI] [PubMed] [Google Scholar]
- 358.Morrison JL, Sood S, Liu H, Park E, Liu X, Nolan P, Horner RL. Role of inhibitory amino acids in control of hypoglossal motor outflow to genioglossus muscle in naturally sleeping rats. J Physiol (Lond) 2003;552:975–991. doi: 10.1113/jphysiol.2003.052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Morrison JL, Sood S, Liu X, Liu H, Park E, Nolan P, Horner RL. Glycine at hypoglossal motor nucleus: Genioglossus activity, CO2 responses, and the additive effects of GABA. J Appl Physiol. 2002;93:1786–1796. doi: 10.1152/japplphysiol.00464.2002. [DOI] [PubMed] [Google Scholar]
- 360.Morrison JL, Sood S, Liu H, Park E, Nolan P, Horner RL. GABAA receptor antagonism at the hypoglossal motor nucleus increases genioglossus muscle activity in NREM but not REM sleep. J Physiol (Lond) 2003;548:569–583. doi: 10.1113/jphysiol.2002.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Mortimore IL, Douglas NJ. Palatopharyngeus has respiratory activity and responds to negative pressure in sleep apnoeics. Eur Respir J. 1996;9:773–778. doi: 10.1183/09031936.96.09040773. [DOI] [PubMed] [Google Scholar]
- 362.Mortimore IL, Douglas NJ. Palatal muscle EMG response to negative pressure in awake sleep apneic and control subjects. Am J Respir Crit Care Med. 1997;156:867–873. doi: 10.1164/ajrccm.156.3.9608008. [DOI] [PubMed] [Google Scholar]
- 363.Mukai T, Nagao Y, Nishioka S, Hayashi T, Shimizu S, Ono A, Sakagami Y, Watanabe S, Ueda Y, Hara M, Tokudome K, Kato R, Matsumura Y, Ohno Y. Preferential suppression of limbic Fos expression by intermittent hypoxia in obese diabetic mice. Neurosci Res. 2013;77:202–207. doi: 10.1016/j.neures.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 364.Mutoh T, Bonham AC, Joad JP. Substance P in the nucleus of the solitary tract augments bronchopulmonary C fiber reflex output. Am J Physiol. 2000;279:R1215–R1223. doi: 10.1152/ajpregu.2000.279.4.R1215. [DOI] [PubMed] [Google Scholar]
- 365.Nakamura Y, Goldberg LJ, Chandler SH, Chase MH. Intracellular analysis of trigeminal motoneuron activity during sleep in the cat. Science. 1978;199:204–207. doi: 10.1126/science.202025. [DOI] [PubMed] [Google Scholar]
- 366.Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J Appl Physiol. 2007;102:241–248. doi: 10.1152/japplphysiol.00679.2006. [DOI] [PubMed] [Google Scholar]
- 367.Nakaya Y, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. J Comp Neurol. 1994;347:249–274. doi: 10.1002/cne.903470208. [DOI] [PubMed] [Google Scholar]
- 368.Nasse J, Travers JB. Adrenoceptor modulation of oromotor pathways in the rat medulla. J Neurophysiol. 2014;112:580–593. doi: 10.1152/jn.00091.2014. doi: 10.1152/jn.00091.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Netick A, Orem J, Dement W. Neuronal activity specific to REM sleep and its relationship to breathing. Brain Res. 1977;120:197–207. doi: 10.1016/0006-8993(77)90900-3. [DOI] [PubMed] [Google Scholar]
- 370.Neuzeret PC, Gormand F, Reix P, Parrot S, Sastre JP, Buda C, Guidon G, Sakai K, Lin JS. A new animal model of obstructive sleep apnea responding to continuous positive airway pressure. Sleep. 2011;34:541–548. doi: 10.1093/sleep/34.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Neuzeret P-C, Sakai K, Gormand F, Petitjean T, Buda C, Sastre J-P, Parrot S, Guidon G, Lin J-S. Application of histamine and serotonin to the hypoglossal nucleus increases genioglossus activity across the wake-sleep cycle. J Sleep Res. 2009;18:113–121. doi: 10.1111/j.1365-2869.2008.00708.x. [DOI] [PubMed] [Google Scholar]
- 372.Nguyen AT, Jobin V, Payne R, Beauregard J, Naor N, Kimoff RJ. Laryngeal and velopharyngeal sensory impairment in obstructive sleep apnea. Sleep. 2005;28:585–593. doi: 10.1093/sleep/28.5.585. [DOI] [PubMed] [Google Scholar]
- 373.Nicholas AP, Pieribone VA, Arvidsson U, Hökfelt T. Serotonin-, substance P- and glutamate/aspartate-like immunoreactivities in medullo-spinal pathways of rat and primate. Neuroscience. 1992;48:545–559. doi: 10.1016/0306-4522(92)90401-m. [DOI] [PubMed] [Google Scholar]
- 374.Nicholas CL, Bei B, Worsnop C, Malhotra A, Jordan AS, Saboisky JP, Chan JK, Duckworth E, White DP, Trinder J. Motor unit recruitment in human genioglossus muscle in response to hypercapnia. Sleep. 2010;33:1529–1538. [PMC free article] [PubMed] [Google Scholar]
- 375.Nicholas CL, Jordan AS, Heckel L, Worsnop C, Bei B, Saboisky JP, Eckert DJ, White DP, Malhotra A, Trinder J. Discharge patterns of human tensor palatini motor units during sleep onset. Sleep. 2012;35:699–707. doi: 10.5665/sleep.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Nishijima T, Sakurai S, Arihara Z, Takahashi K. Plasma orexin-A-like immunoreactivity in patients with sleep apnea hypopnea syndrome. Peptides. 2003;24:407–411. doi: 10.1016/s0196-9781(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 377.Oh JD, Woolf NJ, Roghani A, Edwards RH, Butcher LL. Cholinergic neurons in the rat central nervous system demonstrated by in situ hybridization of choline acetyltransferase mRNA. Neuroscience. 1992;47:807–822. doi: 10.1016/0306-4522(92)90031-v. [DOI] [PubMed] [Google Scholar]
- 378.Okabe S, Hida W, Kikuchi Y, Taguchi O, Takishima T, Shirato K. Upper airway muscle activity during REM and non-REM sleep of patients with obstructive apnea. Chest. 1994;106:767–773. doi: 10.1378/chest.106.3.767. [DOI] [PubMed] [Google Scholar]
- 379.Okabe S, Kubin L. Role of 5HT1 receptors in the control of hypoglossal motoneurons in vivo. Sleep. 1996;19:S150–S153. doi: 10.1093/sleep/19.suppl_10.150. [DOI] [PubMed] [Google Scholar]
- 380.Okabe S, Mackiewicz M, Kubin L. Serotonin receptor mRNA expression in the hypoglossal motor nucleus. Respir Physiol. 1997;110:151–160. doi: 10.1016/s0034-5687(97)00080-7. [DOI] [PubMed] [Google Scholar]
- 381.Okabe S, Woch G, Kubin L. Role of GABAB receptors in the control of hypoglossal motoneurons in vivo. NeuroReport. 1994;5:2573–2576. doi: 10.1097/00001756-199412000-00042. [DOI] [PubMed] [Google Scholar]
- 382.Oliven A, O'Hearn DJ, Boudewyns A, Odeh M, De BW, van de HP, Smith PL, Eisele DW, Allan L, Schneider H, Testerman R, Schwartz AR. Upper airway response to electrical stimulation of the genioglossus in obstructive sleep apnea. J Appl Physiol. 2003;95:2023–2029. doi: 10.1152/japplphysiol.00203.2003. [DOI] [PubMed] [Google Scholar]
- 383.Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Ono T, Ishiwata Y, Inaba N, Kuroda T, Nakamura Y. Hypoglossal premotor neurons with rhythmical inspiratory-related activity in the cat: Localization and projection to the phrenic nucleus. Exp Brain Res. 1994;98:1–12. doi: 10.1007/BF00229103. [DOI] [PubMed] [Google Scholar]
- 385.Orem J. Medullary respiratory neuron activity: Relationship to tonic and phasic REM sleep. J Appl Physiol. 1980;48:54–65. doi: 10.1152/jappl.1980.48.1.54. [DOI] [PubMed] [Google Scholar]
- 386.Orem J. Neuronal mechanisms of respiration in REM sleep. Sleep. 1980;3:251–267. doi: 10.1093/sleep/3.3-4.251. [DOI] [PubMed] [Google Scholar]
- 387.Orem J. The nature of the wakefulness stimulus for breathing. In: Suratt P, Remmers JE, editors. Sleep and Respiration. Wiley-Liss; New York: 1990. [Google Scholar]
- 388.Orem J. Central respiratory activity in rapid eye movement sleep: Augmenting and late inspiratory cells. Sleep. 1994;17:665–673. doi: 10.1093/sleep/17.8.665. [DOI] [PubMed] [Google Scholar]
- 389.Orem J. Excitatory drive to the respiratory system in REM sleep. Sleep. 1996;19:S154–S156. doi: 10.1093/sleep/19.suppl_10.154. [DOI] [PubMed] [Google Scholar]
- 390.Orem J, Anderson CA. Diaphragmatic activity during REM sleep in the adult cat. J Appl Physiol. 1996;73:751–760. doi: 10.1152/jappl.1996.81.2.751. [DOI] [PubMed] [Google Scholar]
- 391.Orem J, Kubin L. Respiratory physiology: Central neural control. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th ed Elsevier-Saunders; Philadelphia: 2005. [Google Scholar]
- 392.Orem J, Lovering AT, Dunin-Barkowski W, Vidruk EH. Endogenous excitatory drive in the respiratory system in rapid eye movement sleep in cats. J Physiol (Lond) 2000;527:365–376. doi: 10.1111/j.1469-7793.2000.00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Orem J, Montplaisir J, Dement WC. Changes in the activity of respiratory neurons during sleep. Brain Res. 1974;82:309–315. doi: 10.1016/0006-8993(74)90611-8. [DOI] [PubMed] [Google Scholar]
- 394.Orem J, Osorio I, Brooks E, Dick TE. Activity of respiratory neurons during NREM sleep. J Neurophysiol. 1985;54:1144–1156. doi: 10.1152/jn.1985.54.5.1144. [DOI] [PubMed] [Google Scholar]
- 395.Orem JM. Respiratory neuronal activity in sleep. In: Edelman NH, Santiago TV, editors. Breathing Disorders of Sleep. Churchill Livingstone; New York: 1986. [Google Scholar]
- 396.Orem JM, Lovering AT, Vidruk EH. Excitation of medullary respiratory neurons in REM sleep. Sleep. 2005;28:801–807. doi: 10.1093/sleep/28.7.801. [DOI] [PubMed] [Google Scholar]
- 397.Pagliardini S, Gosgnach S, Dickson CT. Spontaneous sleep-like brain state alternations and breathing characteristics in urethane anesthetized mice. PLoS ONE. 2013;8:e70411. doi: 10.1371/journal.pone.0070411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Pagliardini S, Greer JJ, Funk GD, Dickson CT. State-dependent modulation of breathing in urethane-anesthetized rats. J Neurosci. 2012;32:11259–11270. doi: 10.1523/JNEUROSCI.0948-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Parisi RA, Edelman NH, Santiago TV. Central respiratory carbon dioxide chemosensitivity does not decrease during sleep. Am Rev Respir Dis. 1992;145:832–836. doi: 10.1164/ajrccm/145.4_Pt_1.832. [DOI] [PubMed] [Google Scholar]
- 400.Parisi RA, Neubauer JA, Frank MM, Edelman NH, Santiago TV. Correlation between genioglossal and diaphragmatic responses to hypercapnia during sleep. Am Rev Respir Dis. 1987;135:378–382. doi: 10.1164/arrd.1987.135.2.378. [DOI] [PubMed] [Google Scholar]
- 401.Parisi RA, Santiago TV, Edelman NH. Genioglossal and diaphragmatic EMG responses to hypoxia during sleep. Am Rev Respir Dis. 1988;138:610–616. doi: 10.1164/ajrccm/138.3.610. [DOI] [PubMed] [Google Scholar]
- 402.Parkis MA, Bayliss DA, Berger AJ. Actions of norepinephrine on rat hypoglossal motoneurons. J Neurophysiol. 1995;74:1911–1919. doi: 10.1152/jn.1995.74.5.1911. [DOI] [PubMed] [Google Scholar]
- 403.Parkis MA, Berger AJ. Clonidine reduces hyperpolarization-activated inward current (Ih) in rat hypoglossal motoneurons. Brain Res. 1997;769:108–118. doi: 10.1016/s0006-8993(97)00677-x. [DOI] [PubMed] [Google Scholar]
- 404.Patel SR, Goodloe R, De G, Kowgier M, Weng J, Buxbaum SG, Cade B, Fulop T, Gharib SA, Gottlieb DJ, Hillman D, Larkin EK, Lauderdale DS, Li L, Mukherjee S, Palmer L, Zee P, Zhu X, Redline S. Association of genetic loci with sleep apnea in European Americans and African-Americans: The Candidate Gene Association Resource (CARe) PLoS ONE. 2012;7:e48836. doi: 10.1371/journal.pone.0048836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol. 2007;102:547–556. doi: 10.1152/japplphysiol.00282.2006. [DOI] [PubMed] [Google Scholar]
- 406.Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain. IV. Autoradiographic mapping of serotonin-2 receptors. Neuroscience. 1987;21:123–139. doi: 10.1016/0306-4522(87)90327-7. [DOI] [PubMed] [Google Scholar]
- 407.Pedroarena C, Castillo P, Chase MH, Morales FR. The control of jaw-opener motoneurons during active sleep. Brain Res. 1994;653:31–38. doi: 10.1016/0006-8993(94)90368-9. [DOI] [PubMed] [Google Scholar]
- 408.Peever JH, Shen L, Duffin J. Respiratory pre-motor control of hypoglossal motoneurons in the rat. Neuroscience. 2002;110:711–722. doi: 10.1016/s0306-4522(01)00594-2. [DOI] [PubMed] [Google Scholar]
- 409.Petrof BJ, Pack AI, Kelly AM. Pharyngeal myopathy of loaded upper airway in dogs with sleep apnea. J Appl Physiol. 1994;76:1746–1752. doi: 10.1152/jappl.1994.76.4.1746. [DOI] [PubMed] [Google Scholar]
- 410.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff T. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Philip P, Gross CE, Taillard J, Bioulac B, Guilleminault C. An animal model of a spontaneously reversible obstructive sleep apnea syndrome in the monkey. Neurobiol Dis. 2005;20:428–431. doi: 10.1016/j.nbd.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 412.Pilowsky PM, Lipski J, Prestidge R, Jiang C. Dual fluorescence combined with a two-color immunoperoxidase technique: A new way of visualizing diverse neuronal elements. J Neurosci Meth. 1991;36:185–193. doi: 10.1016/0165-0270(91)90044-z. [DOI] [PubMed] [Google Scholar]
- 413.Piper AJ, Yee BJ. Hypoventilation syndromes. Compr Physiol. 2014;4:1639–1676. doi: 10.1002/cphy.c140008. doi: 10.1002/cphy.c140008. [DOI] [PubMed] [Google Scholar]
- 414.Plowman L, Lauff DC, Berthon-Jones M, Sullivan CE. Waking and genioglossal muscle responses to upper airway pressure oscillation in sleeping dogs. J Appl Physiol. 1990;68:2564–2573. doi: 10.1152/jappl.1990.68.6.2564. [DOI] [PubMed] [Google Scholar]
- 415.Polotsky M, Elsayed-Ahmed AS, Pichard L, Harris CC, Smith PL, Schneider H, Kirkness JP, Polotsky V, Schwartz AR. Effects of leptin and obesity on the upper airway function. J Appl Physiol. 2012;112:1637–1643. doi: 10.1152/japplphysiol.01222.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Polotsky M, Elsayed-Ahmed AS, Pichard L, Richardson RA, Smith PL, Schneider H, Kirkness JP, Polotsky V, Schwartz AR. Effect of age and weight on upper airway function in a mouse model. J Appl Physiol. 2011;111:696–703. doi: 10.1152/japplphysiol.00123.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Pompeiano O. The neurophysiological mechanisms of the postrual and motor events during desynchronized sleep. Res Publ Assoc Res Nerv Ment Dis. 1967;45:351–423. [PubMed] [Google Scholar]
- 418.Powell GL, Rice A, Bennett-Cross SJ, Fregosi RF. Respiration-related dischange of hyoglossus muscle motor units in the rat. J Neurophysiol. 2014;111:361–368. doi: 10.1152/jn.00670.2013. doi: 10.1152/jn.00670.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Proctor DF. The upper airways. I. Nasal physiology and defense of the lungs. Am Rev Respir Dis. 1977;115:97–129. doi: 10.1164/arrd.1977.115.1.97. [DOI] [PubMed] [Google Scholar]
- 420.Puizillout J-J, Ternaux J-P. Variations d'activités toniques, phasiques et respiratoires, au niveau bulbaire pendant l'endormement de la préparation encéphale isolé. Brain Res. 1974;66:67–83. [Google Scholar]
- 421.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, Shahar E, Unruh ML, Samet JM. Sleep-disordered breathing and mortality: A prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Quirion R, Araujo D, Regenold W, Boksa P. Characterization and quantitative autoradiographic distribution of [3H]acetylcholine muscarinic receptors in mammalian brain. Apparent labelling of an M2-like receptor sub-type. Neuroscience. 1989;29:271–289. doi: 10.1016/0306-4522(89)90057-2. [DOI] [PubMed] [Google Scholar]
- 423.Quitadamo C, Fabbretti E, Lamanauskas N, Nistri A. Activation and desensitization of neuronal nicotinic receptors modulate glutamatergic transmission on neonatal rat hypoglossal motoneurons. Eur J Neurosci. 2005;22:2723–2734. doi: 10.1111/j.1460-9568.2005.04460.x. [DOI] [PubMed] [Google Scholar]
- 424.Rainbow TC, Parsons B, Wolfe BB. Quantitative autoradiography of b1- and b2-adrenergic receptors in rat brain. Proc Natl Acad Sci USA. 1984;81:1585–1589. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Ramirez JM, Doi A, Garcia AJ, III, Elsen FP, Koch H, Wei AD. The cellular building blocks of breathing. Compr Physiol. 2012;2:2683–2731. doi: 10.1002/cphy.c110033. doi: 10.1002/cphy.c110033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Ramirez JM, Richter DW. The neuronal mechanisms of respiratory rhythm generation. Curr Op Neurobiol. 1996;6:817–825. doi: 10.1016/s0959-4388(96)80033-x. [DOI] [PubMed] [Google Scholar]
- 427.Rampin O, Pierrefiche O, Denavit-Saubié M. Effects of serotonin and substance P on bulbar respiratory neurons in vivo. Brain Res. 1993;622:185–193. doi: 10.1016/0006-8993(93)90818-8. [DOI] [PubMed] [Google Scholar]
- 428.Rampon C, Luppi P-H, Fort P, Peyron C, Jouvet M. Distribution of glycine-immunoreactive cell bodies and fibers in the rat brain. Neuroscience. 1996;75:737–755. doi: 10.1016/0306-4522(96)00278-3. [DOI] [PubMed] [Google Scholar]
- 429.Ratnavadivel R, Chau N, Stadler D, Yeo A, McEvoy RD, Catcheside PG. Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med. 2009;5:519–524. [PMC free article] [PubMed] [Google Scholar]
- 430.Ray AD, Magalang UJ, Michlin CP, Ogasa T, Krasney JA, Gosselin LE, Farkas GA. Intermittent hypoxia reduces upper airway stability in lean but not obese Zucker rats. Am J Physiol. 2007;293:R372–R378. doi: 10.1152/ajpregu.00038.2007. [DOI] [PubMed] [Google Scholar]
- 431.Reiner PB. Clonidine inhibits central noradrenergic neurons in unanesthetized cats. Eur J Pharmacol. 1985;115:249–257. doi: 10.1016/0014-2999(85)90697-1. [DOI] [PubMed] [Google Scholar]
- 432.Reiner PB. Correlational analysis of central noradrenergic neuronal activity and sympathetic tone in behaving cats. Brain Res. 1986;378:86–96. doi: 10.1016/0006-8993(86)90288-x. [DOI] [PubMed] [Google Scholar]
- 433.Reinke C, Bevans-Fonti S, Drager LF, Shin MK, Polotsky VY. Effects of different acute hypoxic regimens on tissue oxygen profiles and metabolic outcomes. J Appl Physiol. 2011;111:881–890. doi: 10.1152/japplphysiol.00492.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Rekling JC. Excitatory effects of thyrotropin-releasing hormone (TRH) in hypoglossal motoneurons. Brain Res. 1990;510:175–179. doi: 10.1016/0006-8993(90)90749-2. [DOI] [PubMed] [Google Scholar]
- 435.Remmers JE, DeGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 436.Ribeiro-do-Valle LE, Metzler CW, Jacobs BL. Facilitation of masseter EMG and masseteric (jaw-closure) reflex by serotonin in behaving cats. Brain Res. 1991;550:197–204. doi: 10.1016/0006-8993(91)91318-u. [DOI] [PubMed] [Google Scholar]
- 437.Richard CA, Harper RM. Respiratory-related activity in hypoglossal neurons across sleep-waking states in cats. Brain Res. 1991;542:167–170. doi: 10.1016/0006-8993(91)91014-r. [DOI] [PubMed] [Google Scholar]
- 438.Richerson GB, Wang W, Tiwari J, Bradley SR. Chemosensitivity of serotonergic neurons in the rostral ventral medulla. Respir Physiol. 2001;129:175–189. doi: 10.1016/s0034-5687(01)00289-4. [DOI] [PubMed] [Google Scholar]
- 439.Rivera-Garcia AP, Ramirez-Salado I, Corsi-Cabrera M, Calvo JM. Facial muscle activation during sleep and its relation to the rapid eye movements of REM sleep. J Sleep Res. 2011;20:82–91. doi: 10.1111/j.1365-2869.2010.00853.x. [DOI] [PubMed] [Google Scholar]
- 440.Roberts JL, Reed WR, Thach BT. Pharyngeal airway-stabilizing function of sternohyoid and sternothyroid muscles in the rabbit. J Appl Physiol. 1984;57:1790–1795. doi: 10.1152/jappl.1984.57.6.1790. [DOI] [PubMed] [Google Scholar]
- 441.Rosin DL, Talley EM, Lee A, Stornetta RL, Gaylinn BD, Guyenet PG, Lynch KR. Distribution of a2C-adrenergic receptor-like immunoreactivity in the rat central nervous system. J Comp Neurol. 1996;372:135–165. doi: 10.1002/(SICI)1096-9861(19960812)372:1<135::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 442.Rowley JA, Permutt S, Willey S, Smith PL, Schwartz AR. Effect of tracheal and tongue displacement on upper airway airflow dynamics. J Appl Physiol. 1996;80:2171–2178. doi: 10.1152/jappl.1996.80.6.2171. [DOI] [PubMed] [Google Scholar]
- 443.Rukhadze I, Fenik VB, Benincasa KE, Price A, Kubin L. Chronic intermittent hypoxia alters density of aminergic terminals and receptors in the hypoglossal motor nucleus. Am J Respir Crit Care Med. 2010;182:1321–1329. doi: 10.1164/rccm.200912-1884OC. doi: 10.1164/rccm.200912-1884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Rukhadze I, Fenik VB, Branconi JL, Kubin L. Fos expression in pontomedullary catecholaminergic cells following REM sleep-like episodes elicited by pontine carbachol in urethane-anesthetized rats. Neuroscience. 2008;152:208–222. doi: 10.1016/j.neuroscience.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Rukhadze I, Kalter J, Stettner GM, Kubin L. Lingual muscle activity across sleep-wake states in rats with surgically altered upper airway. Front Neurol. 2014;5:61. doi: 10.3389/fneur.2014.00061. doi: 10.3389/fneur.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Rukhadze I, Kamani H, Kubin L. Quantitative differences among EMG activities of muscles innervated by subpopulations of hypoglossal and upper spinal motoneurons during non-REM sleep - REM sleep transitions: A window on neural processes in the sleeping brain. Arch Ital Biol. 2011;149:499–515. doi: 10.4449/aib.v149i4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Rukhadze I, Kubin L. Differential pontomedullary catecholaminergic projections to hypoglossal motor nucleus and viscerosensory nucleus of the solitary tract. J Chem Neuroanat. 2007;33:23–33. doi: 10.1016/j.jchemneu.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 448.Rukhadze I, Kubin L. Mesopontine cholinergic projections to the hypoglossal motor nucleus. Neurosci Lett. 2007;413:121–125. doi: 10.1016/j.neulet.2006.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Ryan S, Nolan P. Superior laryngeal and hypoglossal afferents tonically influence upper airway motor excitability in anesthetized rats. J Appl Physiol. 2005;99:1019–1028. doi: 10.1152/japplphysiol.00776.2004. [DOI] [PubMed] [Google Scholar]
- 450.Rye DB. Contributions of the pedunculopontine region to normal and altered REM sleep. Sleep. 1997;20:757–788. doi: 10.1093/sleep/20.9.757. [DOI] [PubMed] [Google Scholar]
- 451.Rye DB, Lee HJ, Saper CB, Wainer BH. Medullary and spinal efferents of the pedunculopontine tegmental nucleus and adjacent mesopontine tegmentum in the rat. J Comp Neurol. 1988;269:315–341. doi: 10.1002/cne.902690302. [DOI] [PubMed] [Google Scholar]
- 452.Saboisky JP, Butler JE, Fogel RB, Taylor JL, Trinder JA, White DP, Gandevia SC. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol. 2006;95:2213–2221. doi: 10.1152/jn.00940.2005. [DOI] [PubMed] [Google Scholar]
- 453.Saboisky JP, Jordan AS, Eckert DJ, White DP, Trinder JA, Nicholas CL, Gautam S, Malhotra A. Recruitment and rate-coding strategies of the human genioglossus muscle. J Appl Physiol. 2010;109:1939–1949. doi: 10.1152/japplphysiol.00812.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Saha S, Appenteng K, Batten TFC. Light and electron microscopical localisation of 5-HT-immunoreactive boutons in the rat trigeminal motor nucleus. Brain Res. 1991;559:145–148. doi: 10.1016/0006-8993(91)90297-9. [DOI] [PubMed] [Google Scholar]
- 455.Sahin M, Durand DM, Haxhiu MA. Chronic recordings of hypoglossal nerve activity in a dog model of upper airway obstruction. J Appl Physiol. 1999;87:2197–2206. doi: 10.1152/jappl.1999.87.6.2197. [DOI] [PubMed] [Google Scholar]
- 456.Sakai K. Discharge properties of presumed cholinergic and noncholinergic laterodorsal tegmental neurons related to cortical activation in non-anesthetized mice. Neuroscience. 2012;224:172–190. doi: 10.1016/j.neuroscience.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 457.Sakai K, Sastre J-P, Kanamori N, Jouvet M. State-specific neurons in the ponto-medullary reticular formation with special reference to the postural atonia during paradoxical sleep in the cat. In: Pompeiano O, Ajmone Marsan C, editors. Brain Mechanisms of Perceptual Awareness and Purposeful Behavior. Raven; New York: 1981. [Google Scholar]
- 458.Sakai K, Takada T, Nakayama H, Kubota Y, Nakamata M, Satoh M, Suzuki E, Akazawa K, Gejyo F. Serotonin-2A and 2C receptor gene polymorphisms in Japanese patients with obstructive sleep apnea. Int Med. 2005;44:928–933. doi: 10.2169/internalmedicine.44.928. [DOI] [PubMed] [Google Scholar]
- 459.Sakurai S, Nishijima T, Takahashi S, Yamauchi K, Arihara Z, Takahashi K. Low plasma orexin-A levels were improved by continuous positive airway pressure treatment in patients with severe obstructive sleep apnea-hypopnea syndrome. Chest. 2005;127:731–737. doi: 10.1378/chest.127.3.731. [DOI] [PubMed] [Google Scholar]
- 460.Sakurai T. Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep Med Rev. 2005;9:231–241. doi: 10.1016/j.smrv.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 461.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 462.Sant'Ambrogio G, Tsubone H, Sant'Ambrogio FB. Sensory information from the upper airway: Role in the control of breathing. Respir Physiol. 1995;102:1–16. doi: 10.1016/0034-5687(95)00048-i. [DOI] [PubMed] [Google Scholar]
- 463.Sapin E, Lapray D, rod A, Goutagny R, ger L, Ravassard P, ment O, Hanriot L, Fort P, Luppi P-H. Localization of the brainstem GABAergic neurons controlling paradoxical (REM) sleep. PLoS ONE. 2009;4:e4272. doi: 10.1371/journal.pone.0004272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Sauerland EK, Harper RM. The human tongue during sleep: Electromyographic activity of the genioglossus muscle. Exp Neurol. 1976;51:160–170. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- 465.Sauerland EK, Orr WC, Hairston LE. EMG patterns of oropharyngeal muscles during respiration in wakefulness and sleep. EMG Clin Neurophysiol. 1981;21:307–316. [PubMed] [Google Scholar]
- 466.Scammell TE, Winrow CJ. Orexin receptors: Pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2011;51:243–266. doi: 10.1146/annurev-pharmtox-010510-100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, Fremeau RT., Jr Distribution of α2-adrenergic receptor subtype gene expression in rat brain. Mol Brain Res. 1994;21:133–149. doi: 10.1016/0169-328x(94)90386-7. [DOI] [PubMed] [Google Scholar]
- 468.Schmid K, Böhmer G, Gebauer K. GABAB receptor mediated effects on central respiratory system and their antagonism by phaclofen. Neurosci Lett. 1989;99:305–310. doi: 10.1016/0304-3940(89)90464-3. [DOI] [PubMed] [Google Scholar]
- 469.Schwab RJ, Gefter WB, Hoffman EA, Gupta KB, Pack AI. Dynamic upper airway imaging during awake respiration in normal subjects and patients with sleep disordered breathing. Am Rev Respir Dis. 1993;148:1385–1400. doi: 10.1164/ajrccm/148.5.1385. [DOI] [PubMed] [Google Scholar]
- 470.Schwab RJ, Kim C, Bagchi S, Keenan BT, Comyn FL, Wang S, Tapia IE, Huang S, Traylor J, Torigian DA, Bradford RM, Marcus CL. Understanding the anatomic basis for obstructive sleep apnea syndrome in adolescents. Am J Respir Crit Care Med. 2015;191:1295–1309. doi: 10.1164/rccm.201501-0169OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Schwartz AR, Eisele DW, Hari A, Testerman R, Erickson D, Smith PL. Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol. 1996;81:643–652. doi: 10.1152/jappl.1996.81.2.643. [DOI] [PubMed] [Google Scholar]
- 472.Schwartz AR, Smith PL, Oliven A. Electrical stimulation of the hypoglossal nerve: A potential therapy. J Appl Physiol. 2014;116:337–344. doi: 10.1152/japplphysiol.00423.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Schwarz PB, Peever JH. Noradrenergic control of trigeminal motoneurons in sleep: Relevance to sleep apnea. Adv Exp Med Biol. 2010;669:281–284. doi: 10.1007/978-1-4419-5692-7_57. [DOI] [PubMed] [Google Scholar]
- 474.Schwarz PB, Yee N, Mir S, Peever JH. Noradrenaline triggers muscle tone by amplifying glutamate-driven excitation of somatic motoneurones in anaesthetized rats. J Physiol (Lond) 2008;586:5787–5802. doi: 10.1113/jphysiol.2008.159392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Segizbaeva MO, Pogodin MA, Aleksandrova NP. Effects of body positions on respiratory muscle activation during maximal inspiratory maneuvers. Adv Exp Med Biol. 2013;756:355–363. doi: 10.1007/978-94-007-4549-0_43. [DOI] [PubMed] [Google Scholar]
- 476.Sekizawa S, Joad JP, Bonham AC. Substance P presynaptically depresses the transmission of sensory input to bronchopulmonary neurons in the guinea pig nucleus tractus solitarii. J Physiol (Lond) 2003;552:547–559. doi: 10.1113/jphysiol.2003.051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Selvaratnam SR, Parkis MA, Funk GD. Devalopmental modulation of mouse hypoglossal nerve inspiratory output in vitro by noradrenergic receptor agonists. Brain Res. 1998;805:104–115. doi: 10.1016/s0006-8993(98)00673-8. [DOI] [PubMed] [Google Scholar]
- 478.Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: A retro- and anterograde transport and immunohistochemical study. J Comp Neurol. 1992;323:387–410. doi: 10.1002/cne.903230307. [DOI] [PubMed] [Google Scholar]
- 479.Sériès F, Cormier Y, Desmeules M, La Forge J. Effects of respiratory drive on upper airways in sleep apnea patients and normal subjects. J Appl Physiol. 1989;67:973–979. doi: 10.1152/jappl.1989.67.3.973. [DOI] [PubMed] [Google Scholar]
- 480.Series F, Simoneau J-A, St. Pierre S, Marc I. Characteristics of the genioglossus and musculus uvulae in sleep apnea hypopnea syndrome and in snorers. Am J Respir Crit Care Med. 1996;153:1870–1874. doi: 10.1164/ajrccm.153.6.8665048. [DOI] [PubMed] [Google Scholar]
- 481.Shah NH, Aizenman E. Voltage-gated potassium channels at the crossroads of neuronal function, ischemic tolerance, and neurodegeneration. Transl Stroke Res. 2014;5:38–58. doi: 10.1007/s12975-013-0297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Shao XM, Feldman JL. Acetylcholine modulates respiratory pattern: Effects mediated by M3-like receptors in pre-Bötzinger complex inspiratory neurons. J Neurophysiol. 2000;83:1243–1252. doi: 10.1152/jn.2000.83.3.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Shao XM, Feldman JL. Mechanisms underlying regulation of respiratory pattern by nicotine in pre-Bötzinger complex. J Neurophysiol. 2001;85:2461–2467. doi: 10.1152/jn.2001.85.6.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Shao XM, Feldman JL. Pharmacology of nicotinic receptors in pre-Bötzinger complex that mediate modulation of respiratory pattern. J Neurophysiol. 2002;88:1851–1858. doi: 10.1152/jn.2002.88.4.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Shao Y, Sutin J. Noradrenergic facilitation of motor neurons: Localization of adrenergic receptors in neurons and nonneuronal cells in the trigeminal motor nucleus. J Neurosci. 1986;6:30–37. doi: 10.1016/0014-4886(91)90038-e. [DOI] [PubMed] [Google Scholar]
- 486.Shao Y, Sutin J. Expression of adrenergic receptors in individual astrocytes and motor neurons isolated from the adult rat brain. Glia. 1992;6:108–117. doi: 10.1002/glia.440060205. [DOI] [PubMed] [Google Scholar]
- 487.Sharma A, Punhani T, Fone KCF. Distribution of the 5-hydroxytryptamine2c receptor protein in adult rat brain and spinal cord determined using a receptor-directed antibody: Effect of 5,7-dihydroxytryptamine. Synapse. 1997;27:45–56. doi: 10.1002/(SICI)1098-2396(199709)27:1<45::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 488.Shaw C-F, Cohen MI, Barnhardt R. Inspiratory-modulated neurons of the rostrolateral pons: Effects of pulmonary afferent input. Brain Res. 1989;485:179–184. doi: 10.1016/0006-8993(89)90681-1. [DOI] [PubMed] [Google Scholar]
- 489.Shepard JW, Jr, Thawley SE. Localization of upper airway collapse during sleep in patients with obstructive sleep apnea. Am Rev Respir Dis. 1990;141:1350–1355. doi: 10.1164/ajrccm/141.5_Pt_1.1350. [DOI] [PubMed] [Google Scholar]
- 490.Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 491.Sherrey JH, Megirian D. State dependence of upper airway respiratory motoneurons: Functions of the cricothyroid and nasolabial muscles of the unanesthetized rat. EEG Clin Neurophysiol. 1977;43:218–228. doi: 10.1016/0013-4694(77)90129-8. [DOI] [PubMed] [Google Scholar]
- 492.Sherrey JH, Megirian D. Respiratory EMG activity of the posterior cricoarytenoid, cricothyroid and diaphragm muscles during sleep. Respir Physiol. 1980;39:355–365. doi: 10.1016/0034-5687(80)90066-3. [DOI] [PubMed] [Google Scholar]
- 493.Sherrey JH, Pollard MJ, Megirian D. Respiratory functions of the inferior pharyngeal constrictor and sternohyoid muscles during sleep. Exp Neurol. 1986;92:267–277. doi: 10.1016/0014-4886(86)90140-8. [DOI] [PubMed] [Google Scholar]
- 494.Shiromani PJ, Lai YY, Siegel JM. Descending projections from the dorsolateral pontine tegmentum to the paramedian reticular nucleus of the caudal medulla in the cat. Brain Res. 1990;517:224–228. doi: 10.1016/0006-8993(90)91030-k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Sica AL, Greenberg HE, Ruggiero DA, Scharf SM. Chronic-intermittent hypoxia: A model of sympathetic activation in the rat. Respir Physiol. 2000;121:173–184. doi: 10.1016/s0034-5687(00)00126-2. [DOI] [PubMed] [Google Scholar]
- 496.Sieck GC, Harper RM. Pneumotaxic area neuronal discharge during sleep-waking states in the cat. Exp Neurol. 1980;67:79–102. doi: 10.1016/0014-4886(80)90162-4. [DOI] [PubMed] [Google Scholar]
- 497.Sieck GC, Trelease RB, Harper RM. Sleep influences on diaphragmatic motor unit discharge. Exp Neurol. 1984;85:316–335. doi: 10.1016/0014-4886(84)90143-2. [DOI] [PubMed] [Google Scholar]
- 498.Siegel JM. Narcolepsy. Scient Amer. 2000;282:58–63. [Google Scholar]
- 499.Siegel JM, Wheeler RL, McGinty D. Activity of medullary reticular formation neurons in the unrestrained cat during waking and sleep. Brain Res. 1999;179:49–60. doi: 10.1016/0006-8993(79)90488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Silvani A, Bastianini S, Berteotti C, Franzini C, Lenzi P, Lo Martire V, Zoccoli G. Dysregulation of heart rhythm during sleep in leptindeficient obese mice. Sleep. 2010;33:355–361. doi: 10.1093/sleep/33.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Singer JH, Bellingham MC, Berger AJ. Presynaptic inhibition of glutamatergic synaptic transmission to rat motoneurons by serotonin. J Neurophysiol. 1996;76:799–807. doi: 10.1152/jn.1996.76.2.799. [DOI] [PubMed] [Google Scholar]
- 502.Sirieix C, Gervasoni D, Luppi P-H, Leger L. Role of the lateral paragigantocellular nucleus in the network of paradoxical (REM) sleep: An electrophysiological and anatomical study in the rat. PLoS One. 2012;7:e28724. doi: 10.1371/journal.pone.0028724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Smith CA, Henderson KS, Xi L, Chow CM, Eastwood PR, Dempsey JA. Neural-mechanical coupling of breathing in REM sleep. J Appl Physiol. 1997;83:1923–1932. doi: 10.1152/jappl.1997.83.6.1923. [DOI] [PubMed] [Google Scholar]
- 504.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: A brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Soja PJ, Finch DM, Chase MH. Effect of inhibitory amino acid antagonists on masseteric reflex suppression during active sleep. Exp Neurol. 1987;96:178–193. doi: 10.1016/0014-4886(87)90179-8. [DOI] [PubMed] [Google Scholar]
- 506.Soja PJ, López-Rodríguez F, Morales FR, Chase MH. The postsynaptic inhibitory control of lumbar motoneurons during the atonia of active sleep: Effect of strychnine on motoneuron properties. J Neurosci. 1991;11:2804–2811. doi: 10.1523/JNEUROSCI.11-09-02804.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Sood S, Liu X, Liu H, Horner RL. Genioglossus muscle activity and serotonergic modulation of hypoglossal motor output in obese Zucker rats. J Appl Physiol. 2007;102:2240–2250. doi: 10.1152/japplphysiol.01229.2006. [DOI] [PubMed] [Google Scholar]
- 508.Sood S, Liu X, Liu H, Nolan P, Horner RL. 5-HT at hypoglossal motor nucleus and respiratory control of genioglossus muscle in anesthetized rats. Respir Physiol Neurobiol. 2003;138:205–221. doi: 10.1016/j.resp.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 509.Sood S, Morrison JL, Liu H, Horner RL. Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am J Respir Crit Care Med. 2005;172:1338–1347. doi: 10.1164/rccm.200502-258OC. [DOI] [PubMed] [Google Scholar]
- 510.Stanchina ML, Malhotra A, Fogel RB, Ayas N, Edwards JK, Schory K, White DP. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am J Respir Crit Care Med. 2002;165:945–949. doi: 10.1164/ajrccm.165.7.2108076. [DOI] [PubMed] [Google Scholar]
- 511.Stanchina ML, Malhotra A, Fogel RB, Trinder J, Edwards JK, Schory K, White DP. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep. 2003;26:851–856. doi: 10.1093/sleep/26.7.851. [DOI] [PubMed] [Google Scholar]
- 512.Stanek E, Cheng S, Takatoh J, Han BX, Wang F. Monosynaptic premotor circuit tracing reveals neural substrates for oro-motor coordination. eLife. 2014;3:e02511. doi: 10.7554/eLife.02511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Steenland HW, Liu H, Horner RL. Endogenous glutamatergic control of rhythmically active mammalian respiratory motoneurons in vivo. J Neurosci. 2008;28:6826–6835. doi: 10.1523/JNEUROSCI.1019-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Steenland HW, Liu H, Sood S, Liu X, Horner RL. Respiratory activation of the genioglossus muscle involves both non-NMDA and NMDA glutamate receptors at the hypoglossal motor nucleus in vivo. Neuroscience. 2006;138:1407–1424. doi: 10.1016/j.neuroscience.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 515.Steinbusch HWM. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- 516.Steriade M, Datta S, Paré D, Oakson G, Curró Dossi R. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Stettner GM, Fenik VB, Kubin L. Effect of chronic intermittent hypoxia on noradrenergic activation of hypoglossal motoneurons. J Appl Physiol. 2012;112:305–312. doi: 10.1152/japplphysiol.00697.2011. doi: 10.1152/japplphysiol.00697.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Stettner GM, Kubin L. Antagonism of orexin receptors in the posterior hypothalamus reduces hypoglossal and cardiorespiratory excitation from the perifornical hypothalamus. J Appl Physiol. 2013;114:119–130. doi: 10.1152/japplphysiol.00965.2012. doi: 10.1152/japplphysiol.00965.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Stettner GM, Lei Y, Benincasa HK, Kubin L. Evidence that adrenergic ventrolateral medullary cells are activated whereas precerebellar lateral reticular nucleus neurons are suppressed during REM sleep. PLoS One. 2013;8:e62410. doi: 10.1371/journal.pone.0062410. doi: 10.1371/journal.pone.0062410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Stettner GM, Rukhadze I, Mann GL, Lei Y, Kubin L. Respiratory modulation of lingual muscle activity across sleep-wake states in rats. Respir Physiol Neurobiol. 2013;188:308–317. doi: 10.1016/j.resp.2013.05.033. doi: 10.1016/j.resp.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Strohl KP, Olson LG. Concerning the importance of pharyngeal muscles in the maintenance of upper airway patency during sleep. Chest. 1987;92:918–920. doi: 10.1378/chest.92.5.918. [DOI] [PubMed] [Google Scholar]
- 522.Strollo PJ, Gillespie MB, Soose RJ, Maurer JT, de Vries N, Cornelius J, Hanson RD, Padhya TA, Steward DL, Woodson BT, Verbraecken J, Vanderveken OM, Goetting MG, Feldman N, Chabolle F, Badr MS, Randerath W, Strohl KP, Stimulation Therapy for Apnea Reduction (STAR) Trial Group Upper airway stimulation for obstructive sleep apnea: Durability of the treatment effect at 18 months. Sleep. 2015;38:1593–1598. doi: 10.5665/sleep.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Sullivan CE, Murphy E, Kozar LF, Phillipson EA. Waking and ventilatory responses to laryngeal stimulation in sleeping dogs. J Appl Physiol. 1978;45:681–689. doi: 10.1152/jappl.1978.45.5.681. [DOI] [PubMed] [Google Scholar]
- 524.Sun QJ, Berkowitz RG, Goodchild AK, Pilowsky PM. Serotonin inputs to inspiratory laryngeal motoneurons in the rat. J Comp Neurol. 2002;451:91–98. doi: 10.1002/cne.10329. [DOI] [PubMed] [Google Scholar]
- 525.Sun QJ, Pilowsky P, Llewellyn-Smith IJ. Thyrotropin-releasing hormone inputs are preferentially directed towards respiratory motoneurons in rat nucleus ambiguus. J Comp Neurol. 1995;362:320–330. doi: 10.1002/cne.903620303. [DOI] [PubMed] [Google Scholar]
- 526.Sunanaga J, Deng BS, Zhang W, Kanmura Y, Kuwaki T. CO2 activates orexin-containing neurons in mice. Respir Physiol Neurobiol. 2009;166:184–186. doi: 10.1016/j.resp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 527.Svanborg E. Impact of obstructive apnea syndrome on upper airway respiratory muscles. Respir Physiol Neurobiol. 2005;147:263–272. doi: 10.1016/j.resp.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 528.Swale DR, Kharade SV, Denton JS. Cardiac and renal inward rectifier potassium channel pharmacology: Emerging tools for integrative physiology and therapeutics. Cur Op Pharmacol. 2014;15:7–15. doi: 10.1016/j.coph.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Tago H, McGeer PL, McGeer EG, Akiyama H, Hersh LB. Distribution of choline acetyltransferase immunopositive structures in the rat brainstem. Brain Res. 1989;495:271–297. doi: 10.1016/0006-8993(89)90221-7. [DOI] [PubMed] [Google Scholar]
- 530.Taguchi O, Kubin L, Pack AI. Evocation of postural atonia and respiratory depression by pontine carbachol in the decerebrate rat. Brain Res. 1992;595:107–115. doi: 10.1016/0006-8993(92)91458-q. [DOI] [PubMed] [Google Scholar]
- 531.Takahash K, Lin J-S, Sakai K. Neuronal activity of orexin and nonorexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153:860–870. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 532.Takahashi K, Kayama Y, Lin JS, Sakai K. Locus coeruleus neuronal activity during the sleep-waking cycle in mice. Neuroscience. 2010;169:1115–1126. doi: 10.1016/j.neuroscience.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 533.Takahashi K, Lin JS, Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J Neurosci. 2006;26:10292–10298. doi: 10.1523/JNEUROSCI.2341-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Takakusaki K, Ohta Y, Mori S. Single medullary reticulospinal neurons exert postsynaptic inhibitory effects via inhibitory interneurons upon alpha-motoneurons innervating cat hindlimb muscles. Exp Brain Res. 1989;74:11–23. doi: 10.1007/BF00248276. [DOI] [PubMed] [Google Scholar]
- 535.Takata M. Two types of inhibitory postsynaptic potentials in the hypoglossal motoneurons. Progr Neurobiol. 1993;40:385–411. doi: 10.1016/0301-0082(93)90016-l. [DOI] [PubMed] [Google Scholar]
- 536.Tallaksen-Greene SJ, Elde R, Wessendorf MW. Regional distribution of serotonin and substance P co-existing in nerve fibers and terminals in the brainstem of the rat. Neuroscience. 1993;53:1127–1142. doi: 10.1016/0306-4522(93)90495-2. [DOI] [PubMed] [Google Scholar]
- 537.Talley EM, Rosin DL, Lee A, Guyenet PG, Lynch KR. Distribution of α2A-adrenergic receptor-like immunoreactivity in the rat central nervous system. J Comp Neurol. 1996;372:111–134. doi: 10.1002/(SICI)1096-9861(19960812)372:1<111::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 538.Talley EM, Sadr NN, Bayliss DA. Postnatal development of serotonergic innervation, 5-HT1A receptor expression, and 5-HT responses in rat motoneurons. J Neurosci. 1997;17:4473–4485. doi: 10.1523/JNEUROSCI.17-11-04473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Tamisier R, Gilmartin GS, Launois SH, Pepin JL, Nespoulet H, Thomas R, Levy P, Weiss JW. A new model of chronic intermittent hypoxia in humans: Effect on ventilation, sleep, and blood pressure. J Appl Physiol. 2009;107:17–24. doi: 10.1152/japplphysiol.91165.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing pre-Bötzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci. 2008;11:538–540. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Tangel DJ, Mezzanotte WS, Sandberg EJ, White DP. Influences of NREM sleep on the activity of tonic vs. inspiratory phasic muscles in normal men. J Appl Physiol. 1992;73:1058–1066. doi: 10.1152/jappl.1992.73.3.1058. [DOI] [PubMed] [Google Scholar]
- 542.Tangel DJ, Mezzanotte WS, White DP. Influences of NREM sleep on activity of palatoglossus and levator palatini muscles in normal men. J Appl Physiol. 1995;78:689–695. doi: 10.1152/jappl.1995.78.2.689. [DOI] [PubMed] [Google Scholar]
- 543.Tantucci C, Mehiri S, Duguet A, Similowski T, Arnulf I, Zelter M, Derenne J-P, Milic-Emili J. Application of negative expiratory pressure during expiration and activity of genioglossus in humans. J Appl Physiol. 1998;84:1076–1082. doi: 10.1152/jappl.1998.84.3.1076. [DOI] [PubMed] [Google Scholar]
- 544.Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: Alterations in glucose metabolism and inflammation. Proc Am Thor Soc. 2008;5:207–217. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- 545.Thach BT. Potential central nervous cystem involvement in sudden unexpected infant deaths and the sudden infant death syndrome. Compr Physiol. 2015;5:1061–1068. doi: 10.1002/cphy.c130052. doi: 10.1002/cphy.c130052. [DOI] [PubMed] [Google Scholar]
- 546.Thor KB, Blitz-Siebert A, Helke CJ. Autoradiographic localization of 5HT1 binding sites in autonomic areas of the rat dorsomedial medulla oblongata. Synapse. 1992;10:217–227. doi: 10.1002/syn.890100305. [DOI] [PubMed] [Google Scholar]
- 547.Thor KB, Helke CJ. Serotonin- and substance p-containing projections to the nucleus tractus solitarii of the rat. J Comp Neurol. 1987;265:275–293. doi: 10.1002/cne.902650210. [DOI] [PubMed] [Google Scholar]
- 548.Tian C, Zhu R, Zhu L, Qiu T, Cao Z, Kang T. Potassium channels: Structures, diseases, and modulators. Chem Biol Drug Des. 2014;83:1–26. doi: 10.1111/cbdd.12237. [DOI] [PubMed] [Google Scholar]
- 549.Tojima H, Kubin L, Kimura H, Davies RO. Spontaneous ventilation and respiratory motor output during carbachol-induced atonia of REM sleep in the decerebrate cat. Sleep. 1992;15:404–414. doi: 10.1093/sleep/15.5.404. [DOI] [PubMed] [Google Scholar]
- 550.Torterolo P, Chase MH. The hypocretins (orexins) mediate the “phasic” components of REM sleep: A new hypothesis. Sleep Sci. 2014;7:19–29. doi: 10.1016/j.slsci.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Travers JB, Norgren R. Afferent projections to the oral motor nuclei in the rat. J Comp Neurol. 1983;220:280–298. doi: 10.1002/cne.902200303. [DOI] [PubMed] [Google Scholar]
- 552.Travers JB, Rinaman L. Identification of lingual motor control circuits using two strains of preudorabies virus. Neuroscience. 2002;115:1139–1151. doi: 10.1016/s0306-4522(02)00489-x. [DOI] [PubMed] [Google Scholar]
- 553.Travers JB, Yoo JE, Chandran R, Herman K, Travers SP. Neurotransmitter phenotypes of intermediate zone reticular formation projections to the motor trigeminal and hypoglossal nuclei in the rat. J Comp Neurol. 2005;488:28–47. doi: 10.1002/cne.20604. [DOI] [PubMed] [Google Scholar]
- 554.Trimmer JS. Subcellular localization of K+ channels in mammalian brain neurons: Remarkable precision in the midst of extraordinary complexity. Neuron. 2015;85:238–256. doi: 10.1016/j.neuron.2014.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Trinder J, Jordan AS, Nicholas CL. Discharge properties of upper airway motor units during wakefulness and sleep. Progr Brain Res. 2014;212:59–75. doi: 10.1016/B978-0-444-63488-7.00004-5. [DOI] [PubMed] [Google Scholar]
- 556.Trinder J, Woods M, Nicholas CL, Chan JK, Jordan AS, Semmler JG. Motor unit activity in upper airway muscles genioglossus and tensor palatini. Respir Physiol Neurobiol. 2013;188:362–369. doi: 10.1016/j.resp.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 557.Ugolini G. Specificity of rabies virus as a transneuronal tracer of motor networks: Transfer from hypoglossal motoneurons to connected second-order and higher order central nervous system cell groups. J Comp Neurol. 1995;356:457–480. doi: 10.1002/cne.903560312. [DOI] [PubMed] [Google Scholar]
- 558.Umemiya M, Berger AJ. Presynaptic inhibition by serotonin of glycinergic inhibitory synaptic currents in the rat brain stem. J Neurophysiol. 1995;73:1192–1200. doi: 10.1152/jn.1995.73.3.1192. [DOI] [PubMed] [Google Scholar]
- 559.Vander Maelen CP, Aghajanian GK. Serotonin-induced depolarization of rat facial motoneurons in vivo: Comparison with amino acid transmitters. Brain Res. 1982;239:139–152. doi: 10.1016/0006-8993(82)90838-1. [DOI] [PubMed] [Google Scholar]
- 560.Vanni-Mercier G, Sakai K, Lin JS, Jouvet M. Mapping of cholinoceptive brainstem structures responsible for the generation of paradoxical sleep in the cat. Arch Ital Biol. 1989;127:133–164. [PubMed] [Google Scholar]
- 561.Veasey SC. Serotonin agonists and antagonists in obstructive sleep apnea: Therapeutic potential. Am J Respir Med. 2003;2:21–29. doi: 10.1007/BF03256636. [DOI] [PubMed] [Google Scholar]
- 562.Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: Protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27:194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- 563.Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Veasey SC, Panckeri KA, Hoffman EA, Pack AI, Hendricks JC. The effects of serotonin antagonists in an animal model of sleep-disordered breathing. Am J Respir Crit Care Med. 1996;153:776–786. doi: 10.1164/ajrccm.153.2.8564132. [DOI] [PubMed] [Google Scholar]
- 565.Veasey SC, Zhan G, Fenik P, Pratico D. Long-term intermittent hypoxia: Reduced excitatory hypoglossal nerve output. Am J Respir Crit Care Med. 2004;170:665–672. doi: 10.1164/rccm.200403-261OC. [DOI] [PubMed] [Google Scholar]
- 566.Vibert JF, Foutz AS, Caille D, Hugelin A. Respiratory rhythm multistability during sleep-wake states. Brain Res. 1988;448:403–405. doi: 10.1016/0006-8993(88)91286-3. [DOI] [PubMed] [Google Scholar]
- 567.Vilaro MT, Palacios JM, Mengod G. Multiplicity of muscarinic autoreceptor subtypes? Comparison of the distribution of cholinergic cells and cells containing mRNA for five subtypes of muscarinic receptors in the rat brain. Mol Brain Res. 1994;21:30–46. doi: 10.1016/0169-328x(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 568.Vincent SR, Kimura H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience. 1992;46:755–784. doi: 10.1016/0306-4522(92)90184-4. [DOI] [PubMed] [Google Scholar]
- 569.Volgin DV, Fay R, Kubin L. Postnatal development of serotonin 1B, 2A and 2C receptors in brainstem motoneurons. Eur J Neurosci. 2003;17:1179–1188. doi: 10.1046/j.1460-9568.2003.02545.x. [DOI] [PubMed] [Google Scholar]
- 570.Volgin DV, Lu JW, Stettner GM, Mann GL, Ross RJ, Morrison AR, Kubin L. Time- and behavioral state-dependent changes in posterior hypothalamic GABAA receptors contribute to the regulation of sleep. PLoS ONE. 2014;9:e86545. doi: 10.1371/journal.pone.0086545. doi: 10.1371/journal.pone.0086545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Volgin DV, Mackiewicz M, Kubin L. α1B receptors are the main postsynaptic mediators of adrenergic excitation in brainstem motoneurons, a single-cell RT-PCR study. J Chem Neuroanat. 2001;22:157–166. doi: 10.1016/s0891-0618(01)00124-7. [DOI] [PubMed] [Google Scholar]
- 572.Volgin DV, Rukhadze I, Kubin L. Hypoglossal premotor neurons of the intermediate medullary reticular region express cholinergic markers. J Appl Physiol. 2008;105:1576–1584. doi: 10.1152/japplphysiol.90670.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Volgin DV, Saghir M, Kubin L. Developmental changes in the orexin 2 receptor mRNA in hypoglossal motoneurons. NeuroReport. 2002;13:433–436. doi: 10.1097/00001756-200203250-00014. [DOI] [PubMed] [Google Scholar]
- 574.Volgin DV, Stettner GM, Kubin L. Circadian dependence of receptors that mediate wake-related excitatory drive to hypoglossal motoneurons. Respir Physiol Neurobiol. 2013;188:301–307. doi: 10.1016/j.resp.2013.04.024. doi: 10.1016/j.resp.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Voss MD, de Castro D, Lipski J, Pilowsky PM, Jiang C. Serotonin immunoreactive boutons form close appositions with respiratory neurons of the dorsal respiratory group in the cat. J Comp Neurol. 1990;295:208–218. doi: 10.1002/cne.902950205. [DOI] [PubMed] [Google Scholar]
- 576.Vranish JR, Bailey EF. A comprehensive assessment of genioglossus electromyographic activity in healthy adults. J Neurophysiol. 2015;113:2692–2699. doi: 10.1152/jn.00975.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Wang S, Benamer N, Zanella S, Kumar NN, Shi Y, Bevengut M, Penton D, Guyenet PG, Lesage F, Gestreau C, Barhanin J, Bayliss DA. TASK-2 channels contribute to pH sensitivity of retrotrapezoid nucleus chemoreceptor neurons. J Neurosci. 2013;33:16033–16044. doi: 10.1523/JNEUROSCI.2451-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Wang S, Shi Y, Shu S, Guyenet PG, Bayliss DA. Phox2b-expressing retrotrapezoid neurons are intrinsically responsive to H+ and CO2. J Neurosci. 2013;33:7756–7761. doi: 10.1523/JNEUROSCI.5550-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Weber F, Chung S, Beier KT, Xu M, Luo L, Dan Y. Control of REM sleep by ventral medulla GABAergic neurons. Nature. 2015;526:435–438. doi: 10.1038/nature14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Wess J, Blin N, Yun J, Schoneberg T, Liu J. Molecular aspects of muscarinic receptor assembly and function. Progr Brain Res. 1996;109:153–162. doi: 10.1016/s0079-6123(08)62097-x. [DOI] [PubMed] [Google Scholar]
- 581.Wheatley JR, Mezzanotte WS, Tangel DJ, White DP. Influence of sleep on genioglossus muscle activation by negative pressure in normal men. Am Rev Respir Dis. 1993;148:597–605. doi: 10.1164/ajrccm/148.3.597. [DOI] [PubMed] [Google Scholar]
- 582.Wheatley JR, Tangel DJ, Mezzanotte WS, White DP. Influence of sleep on alae nasi EMG and nasal resistance in normal men. J Appl Physiol. 1993;75:626–632. doi: 10.1152/jappl.1993.75.2.626. [DOI] [PubMed] [Google Scholar]
- 583.Wheatley JR, Tangel DJ, Mezzanotte WS, White DP. Influence of sleep on response to negative airway pressure of tensor palatini muscle and retropalatal airway. J Appl Physiol. 1993;75:2117–2124. doi: 10.1152/jappl.1993.75.5.2117. [DOI] [PubMed] [Google Scholar]
- 584.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–1370. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 585.White DP. The pathogenesis of obstructive sleep apnea: Advances in the past 100 years. Am J Respir Cell Mol Biol. 2006;34:1–6. doi: 10.1165/rcmb.2005-0317OE. [DOI] [PubMed] [Google Scholar]
- 586.White DP, Younes MK. Obstructive sleep apnea. Compr Physiol. 2012;2:2541–2594. doi: 10.1002/cphy.c110064. doi: 10.1002/cphy.c110064. [DOI] [PubMed] [Google Scholar]
- 587.White SR, Fung SJ, Jackson DA, Imel KM. Serotonin, norepinephrine and associated neuropeptides: Effects on somatic motoneuron excitability. Progr Brain Res. 1996;107:183–199. doi: 10.1016/s0079-6123(08)61865-8. [DOI] [PubMed] [Google Scholar]
- 588.Widdicombe JG. Reflexes from the upper respiratory tract. Compr Physiol. 2011;(Suppl. 11):363–394. [Google Scholar]
- 589.Wiegand DA, Latz B, Zwillich CW, Wiegand L. Geniohyoid muscle activity in normal men during wakefulness and sleep. J Appl Physiol. 1990;69:1262–1269. doi: 10.1152/jappl.1990.69.4.1262. [DOI] [PubMed] [Google Scholar]
- 590.Wiegand DA, Latz B, Zwillich CW, Wiegand L. Upper airway resistance and geniohyoid muscle activity in normal men during wakefulness and sleep. J Appl Physiol. 1990;69:1252–1261. doi: 10.1152/jappl.1990.69.4.1252. [DOI] [PubMed] [Google Scholar]
- 591.Wiegand L, Zwillich CW, Wiegand D, White DP. Changes in upper airway muscle activation and ventilation during phasic REM sleep in normal men. J Appl Physiol. 1991;71:488–497. doi: 10.1152/jappl.1991.71.2.488. [DOI] [PubMed] [Google Scholar]
- 592.Wilkinson V, Malhotra A, Nicholas CL, Worsnop C, Jordan AS, Butler JE, Saboisky JP, Gandevia SC, White DP, Trinder J. Discharge patterns of human genioglossus motor units during sleep onset. Sleep. 2008;31:525–533. doi: 10.1093/sleep/31.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Winter WC, Gampper T, Gay SB, Suratt PM. Enlargement of the lateral pharyngeal fat pad space in pigs increases upper airway resistance. J Appl Physiol. 1995;79:726–731. doi: 10.1152/jappl.1995.79.3.726. [DOI] [PubMed] [Google Scholar]
- 595.Winzer-Serhan UH, Raymon HK, Broide RS, Chen Y, Leslie FM. Expression of a2 adrenoceptors during rat brain development - I. α2A messenger RNA expression. Neuroscience. 1997;76:241–260. doi: 10.1016/s0306-4522(96)00368-5. [DOI] [PubMed] [Google Scholar]
- 596.Wirth KJ, Steinmeyer K, Ruetten H. Sensitization of upper airway mechanoreceptors as a new pharmacologic principle to treat obstructive sleep apnea: Investigations with AVE0118 in anesthetized pigs. Sleep. 2013;36:699–708. doi: 10.5665/sleep.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Withington-Wray DJ, Mifflin SW, Spyer KM. Intracellular analysis of respiratory-modulated hypoglossal motoneurons in the cat. Neuroscience. 1988;25:1041–1051. doi: 10.1016/0306-4522(88)90057-7. [DOI] [PubMed] [Google Scholar]
- 598.Woch G, Davies RO, Pack AI, Kubin L. Behavior of raphe cells projecting to the dorsomedial medulla during carbachol-induced atonia in the cat. J Physiol (Lond) 1996;490:745–758. doi: 10.1113/jphysiol.1996.sp021182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Woch G, Kubin L. Non-reciprocal control of rhythmic activity in respiratory-modulated XII motoneurons. NeuroReport. 1995;6:2085–2088. doi: 10.1097/00001756-199510010-00031. [DOI] [PubMed] [Google Scholar]
- 600.Woch G, Ogawa H, Davies RO, Kubin L. Behavior of hypoglossal inspiratory premotor neurons during the carbachol-induced, REM sleep-like suppression of upper airway motoneurons. Exp Brain Res. 2000;130:508–520. doi: 10.1007/s002219900244. [DOI] [PubMed] [Google Scholar]
- 601.Worsnop C, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol. 1998;85:908–920. doi: 10.1152/jappl.1998.85.3.908. [DOI] [PubMed] [Google Scholar]
- 602.Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- 603.Wu MF, John J, Boehmer LN, Yau D, Nguyen GB, Siegel JM. Activity of dorsal raphe cells across the sleep-waking cycle and during cataplexy in narcoleptic dogs. J Physiol (Lond) 2004;554:202–215. doi: 10.1113/jphysiol.2003.052134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Yamada Y, Yamamura K, Inoue M. Coordination of cranial motoneurons during mastication. Respir Physiol Neurobiol. 2005;147:177–189. doi: 10.1016/j.resp.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 605.Yamuy J, Fung SJ, Xi M, Morales FR, Chase MH. Hypoglossal motoneurons are postsynaptically inhibited during carbachol-induced rapid eye movement sleep. Neuroscience. 1999;94:11–15. doi: 10.1016/s0306-4522(99)00355-3. [DOI] [PubMed] [Google Scholar]
- 606.Yasuda K, Robinson DM, Selvaratnam SR, Walsh CW, McMorland AJ, Funk GD. Modulation of hypoglossal motoneuron excitability by NK1 receptor activation in neonatal mice in vitro. J Physiol (Lond) 2001;534:447–464. doi: 10.1111/j.1469-7793.2001.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Yoo PB, Durand DM. Effects of selective hypoglossal nerve stimulation on canine upper airway mechanics. J Appl Physiol. 2005;99:937–943. doi: 10.1152/japplphysiol.00652.2004. [DOI] [PubMed] [Google Scholar]
- 608.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–658. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 609.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–633. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 610.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: Eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 611.Yu MS, Jung NR, Choi KH, Choi K, Lee BJ, Chung YS. An animal model of obstructive sleep apnea in rabbit. Laryngoscope. 2014;124:789–796. doi: 10.1002/lary.24398. [DOI] [PubMed] [Google Scholar]
- 612.Zanella S, Viemari JC, Hilaire G. Muscarinic receptors and α2-adrenoceptors interact to modulate the respiratory rhythm in mouse neonates. Respir Physiol Neurobiol. 2007;157:215–225. doi: 10.1016/j.resp.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 613.Zaninetti M, Tribollet E, Bertrand D, Raggenbass M. Presence of functional neuronal nicotinic acetylcholine receptors in brainstem motoneurons of the rat. Eur J Neurosci. 1999;11:2737–2748. doi: 10.1046/j.1460-9568.1999.00689.x. [DOI] [PubMed] [Google Scholar]
- 614.Zhan G, Shaheen F, Mackiewicz M, Fenik P, Veasey SC. Single cell laser dissection with molecular beacon polymerase chain reaction identifies 2A as the predominant serotonin receptor subtype in hypoglossal motoneurons. Neuroscience. 2002;113:145–154. doi: 10.1016/s0306-4522(02)00137-9. [DOI] [PubMed] [Google Scholar]
- 615.Zhang GH, Liu ZL, Zhang BJ, Geng WY, Song NN, Zhou W, Cao YX, Li SQ, Huang ZL, Shen LL. Orexin A activates hypoglossal motoneurons and enhances genioglossus muscle activity in rats. Br J Pharmacol. 2014;171:4233–4246. doi: 10.1111/bph.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Zhang H, Ye JY, Hua L, Chen ZH, Ling L, Zhu Q, Wang LM, Zheng L, Zhang YH. Inhomogeneous neuromuscular injury of the genioglossus muscle in subjects with obstructive sleep apnea. Sleep Breath. 2015;19:539–545. doi: 10.1007/s11325-014-1044-3. [DOI] [PubMed] [Google Scholar]
- 617.Zhang SX, Wang Y, Gozal D. Pathological consequences of intermittent hypoxia in the central nervous system. Compr Physiol. 2012;2:1767–1777. doi: 10.1002/cphy.c100060. doi: 10.1002/cphy.c100060. [DOI] [PubMed] [Google Scholar]
- 618.Zhu Y, Fenik P, Zhan G, Mazza E, Kelz M, Aston-Jones G, Veasey SC. Selective loss of catecholaminergic wake active neurons in a murine sleep apnea model. J Neurosci. 2007;27:10060–10071. doi: 10.1523/JNEUROSCI.0857-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Zifa E, Fillion G. 5-hydroxytryptamine receptors. Pharmacol Rev. 1992;44:401–458. [PubMed] [Google Scholar]