Abstract
Idiopathic pulmonary fibrosis is a progressive and fatal fibrotic disease of the lungs with unclear etiology. Prior efforts to treat idiopathic pulmonary fibrosis that focused on anti-inflammatory therapy have not proven to be effective. Recent insight suggests that the pathogenesis is mediated through foci of dysregulated fibroblasts driven by profibrotic cytokine signaling. TGF-β and PDGF are 2 of the most potent of these cytokines. In the current study, we investigated the role of TGF-β–induced fibrosis mediated by activation of the Abelson (Abl) tyrosine kinase. Our data indicate that fibroblasts respond to TGF-β by stimulating c-Abl kinase activity independently of Smad2/3 phosphorylation or PDGFR activation. Moreover, inhibition of c-Abl by imatinib prevented TGF-β–induced ECM gene expression, morphologic transformation, and cell proliferation independently of any effect on Smad signaling. Further, using a mouse model of bleomycin-induced pulmonary fibrosis, we found a significant inhibition of lung fibrosis by imatinib. Thus, Abl family members represent common targets for the modulation of profibrotic cytokine signaling.
Introduction
Idiopathic pulmonary fibrosis (IPF) is an inexorably progressive form of interstitial lung disease with no known etiology (1, 2). Persons diagnosed with IPF can expect an unremitting and progressive clinical course with median survival less than 3 years from diagnosis (3). Our understanding of the pathogenesis of IPF has increased dramatically over the past decade. Current consensus points to a model of early injury/inflammation leading to dysregulation of tissue repair driven by profibrotic cytokines (4). The hypothesized initial step of injury/inflammation has for years been targeted using anti-inflammatory and immune-suppressive therapies without proven efficacy and with potential adverse effects to patients with IPF (5). Recently, intense interest has focused on profibrotic cytokines such as TGF-β, PDGF, and TNF-α (6). These profibrotic cytokines cause fibroblast transformation, proliferation, and accumulation leading to production and deposition of ECM, tissue destruction, and loss of lung function (7). Several independent investigations have shown that inhibition of TGF-β, PDGF, and other cytokines is an effective means to potentially inhibit IPF (8–10). Unfortunately, the vast majority of these experimental strategies have practical and safety issues that limit their potential for future treatment of IPF.
Although inhibition of PDGF-mediated tyrosine kinase signaling ameliorates lung injury–induced fibrosis, many investigations implicate the serine/threonine signaling pathway initiated by TGF-β as responsible for initiation, maintenance, and termination of tissue fibrosis (11–15). The fact that PDGF (in tyrosine kinase–induced fibrosis) and TGF-β (in serine/threonine kinase–induced fibrosis) have been assumed to activate distinct downstream targets has led authors to investigate independent and mutually exclusive signaling mechanisms in which lung fibrosis is the common end result. A more likely possibility is that multiple cytokines synergistically function through stimulation of common mediators (16). In that regard, the c-Abelson (c-Abl) proto-oncogene has a potential role in regulating cytokine-mediated fibrosis, since it functions in cell cycle regulation and cytoskeletal remodeling and is a downstream target responsive to PDGFR activation (17). Although regulation of c-Abl is poorly understood, imatinib mesylate (Gleevec; Novartis Pharmaceuticals Corp.) was found to potently inhibit Abl family kinases and is FDA approved for treatment of chronic myelogenous leukemia (18–20). As imatinib was initially developed as a PDGFR inhibitor, it is reasonable to hypothesize (a) that imatinib will inhibit PDGF-stimulated fibrosis, and (b) that if TGF-β–induced fibrosis is mediated through c-Abl, imatinib would represent a single therapy capable of inhibiting activity of both TGF-β and PDGF, the 2 key profibrotic growth factors that drive dysregulated tissue fibrosis in IPF. Therefore, the present study was designed to determine whether c-Abl is a downstream target of TGF-β signaling and whether imatinib might be an effective treatment in a mouse lung fibrosis model. The results show that (a) TGF-β stimulates c-Abl kinase activity independent of Smad2 and Smad3; (b) TGF-β activation of c-Abl is not dependent on PDGFR tyrosine phosphorylation or expression; (c) inhibition or loss of the c-Abl kinase prevents TGF-β–mediated morphologic alterations, expression of ECM genes, and cell proliferation; and (d) imatinib mesylate obviates the fibrotic changes associated with the bleomycin (BLM) model of lung fibrosis.
Results
The c-Abl kinase is a direct target of TGF-β signaling.
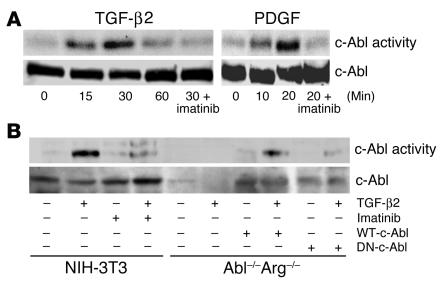
TGF-β stimulation of fibroblasts results in a multitude of effects, including proliferation, migration, cytoskeletal reorganization, and transformation, which eventually lead to tissue fibrosis (16, 21). Similarly, c-Abl activation has been shown to generate analogous cellular phenotypes (17, 22–24). The multitude of biologic similarities between TGF-β and c-Abl led us to hypothesize that c-Abl might be a downstream mediator of TGF-β signaling in fibroblasts (25). To explore this we assessed the kinase activity of c-Abl following TGF-β stimulation (Figure 1A). Addition of TGF-β resulted in enhanced c-Abl kinase activity within 15 minutes, which returned to basal levels after 1 hour. A similar response was observed in AKR-2B and Balb/c-3T3 cells, although we were unable to detect c-Abl activation in epithelial cultures (data not shown). As previously determined, c-Abl kinase activity increases with PDGF stimulation, peaking at approximately 10–20 minutes (25). Thus, c-Abl appears to be a new cell type–specific target of TGF-β signaling, activated in a subset of mesenchymal cell lines.
Figure 1.
TGF-β2 stimulates c-Abl kinase activity. (A) NIH-3T3 fibroblasts were grown to confluence, placed in 0.1% FBS DMEM overnight, and stimulated with 10 ng/ml TGF-β2 or 25 ng/ml PDGF-AB for the indicated times. Parallel plates were pretreated for 20 minutes with 10 μg/ml imatinib prior to growth factor addition. Following c-Abl immunoprecipitation, in vitro kinase activity was determined as described in Methods. Similar results were obtained with AKR-2B fibroblasts and TGF-β1 (data not shown). Lower half: Prior to immunoprecipitation and kinase assay, 100 μg of protein was used for c-Abl Western analysis. (B) NIH-3T3 cells, Abl–/–Arg–/– MEFs, or Abl–/–Arg–/– fibroblasts stably expressing (+) WT-c-Abl or dominant negative c-Abl (DN-c-Abl) were plated at 2.0 106 cells per 100-mm plate and grown overnight in 20% FBS DMEM. The medium was changed to 0.5% FBS DMEM for 24 hours, and the cultures were left untreated (–) or stimulated (+) with 10 ng/ml TGF-β2 for 30 minutes. Parallel plates were pretreated for 20 minutes with 10 μg/ml imatinib prior to growth factor addition. c-Abl kinase activity (top) and Western analysis (bottom) were determined as in A.
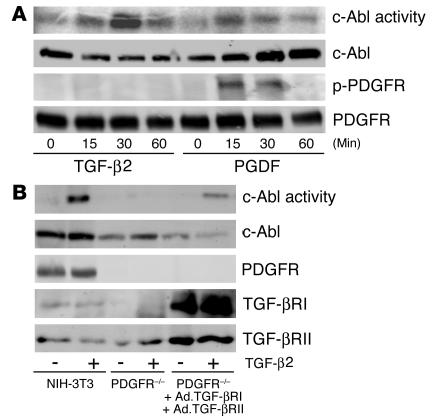
In order to document that the activity measured in Figure 1A did not reflect a contaminating kinase in the c-Abl immunoprecipitation, we examined mouse embryo fibroblasts (MEFs) with the c-Abl and Arg genes (2 mammalian Abl family members) deleted (26). While Abl–/–Arg–/– fibroblasts did not generate any kinase activity in the c-Abl immunoprecipitation that was capable of phosphorylating the c-Crk substrate in response to TGF-β, stable expression of WT, but not dominant negative, c-Abl restored TGF-β responsiveness (Figure 1B). Further, while imatinib pretreatment of cultured fibroblasts abolished c-Abl activity stimulated by TGF-β or PDGF (Figure 1), it had no effect on the level of TGF-β secretion. When TGF-β1 levels were measured in the conditioned medium by ELISA, control and imatinib-treated cells contained 167.4 pg/ml and 164.3 pg/ml TGF-β1, respectively (not significantly different). Therefore, in addition to being activated by receptor tyrosine kinases, the c-Abl kinase is also coupled to signals emanating from the serine/threonine TGF-β receptor complex.–––– –––Previous publications have shown that TGF-β is capable of inducing PDGF expression and secretion (27). Since PDGF similarly activates the c-Abl kinase (25) (Figure 1A), we wished to determine whether c-Abl activation reflected a direct or an indirect response to TGF-β. This was addressed by 2 distinct approaches. First, NIH-3T3 cells were treated with TGF-β or PDGF, and lysates were examined for c-Abl kinase activity and PDGF-β receptor tyrosine phosphorylation. As shown in Figure 2A, TGF-β stimulated c-Abl activity with no detectable effect on PDGFR phosphorylation. In contrast, addition of PDGF simultaneously activated both responses. Second, to further document an independent action of TGF-β and PDGF on c-Abl kinase activity, MEFs harboring a knockout in the PDGF-α and -β receptor genes (F cells) (28) were tested for functional TGF-β signaling. Initial characterization showed that the F cells expressed undetectable levels of the type I TGF-β receptor and were unresponsive to TGF-β ligand as evidenced by the absence of c-Abl and Smad2/3 activation (Figure 2B and data not shown). However, coinfection with adenovirus expressing the type I and type II TGF-β receptors restored TGF-β–stimulated c-Abl activity (Figure 2B). Most importantly, c-Abl kinase activation was coincident with TGF-β–dependent signaling and occurred (a) in the absence of detectable PDGF-β receptor tyrosine phosphorylation (Figure 2A), and (b) in PDGF-α and -β receptor KO cells (Figure 2B).
Figure 2.
TGF-β2 activation of c-Abl is independent of PDGFR signaling. (A) NIH-3T3 cells were treated for the indicated times with TGF-β2 (10 ng/ml) or PDGF-AB (25 ng/ml). Cell lysates were prepared and processed for c-Abl kinase activity (first panel), total c-Abl protein (second panel), tyrosine-phosphorylated PDGF-β receptor (p-PDGFR; third panel), or total PDGF-β receptor (fourth panel) as described in Methods. (B) NIH-3T3 cells were treated as described in Figure 1. PDGF-α and -β receptor–null F cells were seeded at 1.5 × 106 cells per 100-mm dish. Following overnight growth, the medium was replaced with serum-free DMEM alone (PDGFR–/–) or adenovirus (MOI 100) expressing the type I and type II TGF-β receptors (Ad.TGF-βRI and Ad.TGF-βRII, respectively) for 24 hours. Cultures were left untreated (–) or stimulated (+) with 10 ng/ml TGF-β2 for 30 minutes and processed for c-Abl kinase activity (first panel), or total c-Abl (second panel), PDGF-β receptor (third panel), type I TGF-β receptor (TGF-βRI; fourth panel), or type II TGF-β receptor (TGF-βRII; fifth panel) protein.
TGF-β–stimulated c-Abl activation and Smad activation are independently regulated.
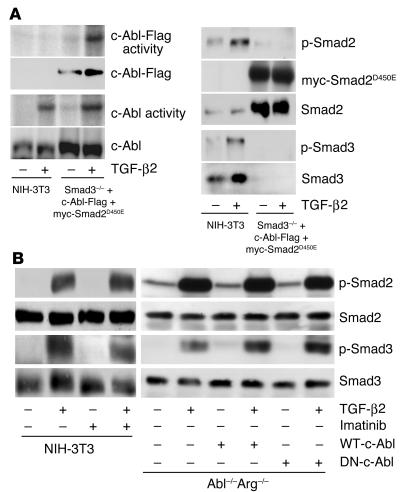
Smad proteins are mediators of TGF-β signaling through receptor-induced phosphorylation and nuclear translocation. Although Smad-dependent signaling is responsible for the expression of numerous TGF-β target genes, Smad-independent pathways have been reported to link TGF-β to cytoskeletal alterations and MAPK signaling (29–32). As c-Abl is similarly associated with a variety of morphologic changes, we examined (a) whether Smad2 or Smad3 activity is necessary for TGF-β stimulation of c-Abl (Figure 3A), and, conversely, (b) whether the c-Abl kinase was required for Smad2 and/or Smad3 activation (Figure 3B). To address the first of these questions MEFs with the Smad3 gene deleted were transiently transfected with a dominant negative Smad2 construct and epitope-tagged c-Abl. While neither Smad2 nor Smad3 phosphorylation occurred following TGF-β addition (Figure 3A, right), the kinase activity of endogenous or transfected c-Abl was unaffected (Figure 3A, left). In order to determine whether c-Abl was needed for TGF-β–mediated Smad2 and/or Smad3 activation, Smad phosphorylation was assessed in the presence of imatinib and in MEFs with both the c-Abl and the Arg genes deleted. As shown in Figure 3B, when normalized for total Smad protein, Smad2 and Smad3 are similarly phosphorylated by TGF-β regardless of the presence of imatinib (Figure 3B, left) or Abl family member genes (Figure 3B, right). Thus, c-Abl and Smad proteins are separately activated by TGF-β, and c-Abl represents a new Smad2- and Smad3-independent target of TGF-β signaling.
Figure 3.
TGF-β2 activation of c-Abl signaling and TGF-β2 activation of Smad signaling are independent. (A) Left: Smad3 KO MEFs (Smad3–/–) were transfected with Flag-tagged c-Abl (c-Abl–Flag) and dominant negative Smad2 (myc-Smad2D450E) as described in Methods. NIH-3T3 cells were treated as described in Figure 1. Following 30-minute stimulation in the absence (–) or presence (+) of 10 ng/ml TGF-β2, kinase activity of the transfected (c-Abl–Flag activity) or endogenous (c-Abl activity) c-Abl protein was determined using anti-Flag or K12 serum respectively. The corresponding total protein is indicated in the second and fourth panels. Right: NIH-3T3 or Smad2/3 KO cells were treated with (+) or without (–) TGF-β as described for the left panels. Following 30-minute stimulation, lysates containing equivalent protein were Western-blotted for the indicated proteins. (B) Left: NIH-3T3 cells were treated with (+) or without (–) TGF-β2 and/or imatinib as described in Figure 1. Following 30-minute stimulation, lysates containing equivalent protein were Western-blotted for phospho-Smad2 (p-Smad2), phospho-Smad3 (p-Smad3), or the corresponding total protein. Right: Abl–/–Arg–/– MEFs or Abl–/–Arg–/– fibroblasts stably expressing (+) WT-c-Abl or dominant negative c-Abl were treated with (+) or without (–) TGF-β2 for 30 minutes and processed for the indicated Smad protein as described for the left panels.
c-Abl is required for fibroblast morphologic alteration and proliferation mediated by TGF-
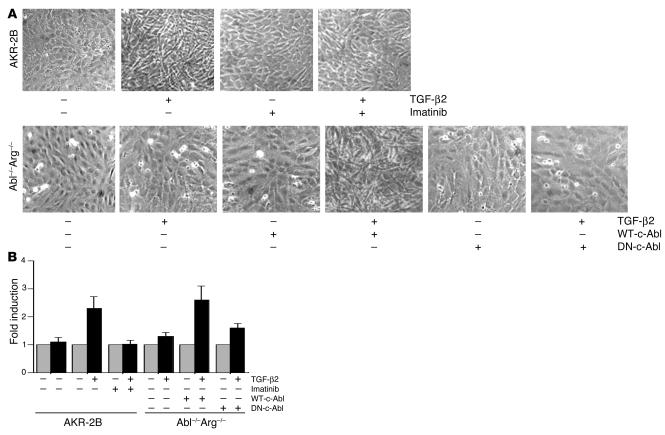
β. The pathologic and etiologic hallmark of IPF is the development of fibroblastic foci, the presence of which is an independent predictor of worsening lung function and increased mortality (33). TGF-β is one of the key stimulatory cytokines that cause transformation of fibroblasts to spindle-shaped myofibroblasts which have been proposed as the pathogenic cell type of fibroblastic foci (5). Since c-Abl is integrally involved in actin cytoskeletal remodeling (34, 35), and TGF-β stimulates c-Abl kinase activity (Figures 1–3), we tested the c-Abl inhibitor imatinib for its potential to limit TGF-β–induced fibroblast morphologic transformation (Figure 4A). Fibroblasts stimulated with TGF-β underwent marked changes in appearance to polygonal, spindle-shaped sheets of polarized cells typical of the myofibroblast phenotype as described by Evans et al. (36). The presence of imatinib effectively inhibited TGF-β–induced fibroblast transformation, while imatinib alone had little effect on unstimulated fibroblasts. Phalloidin staining of similarly treated cells demonstrated polarized filamentous actin primarily localized to the cell surface in TGF-β–treated cells, while imatinib plus TGF-β resulted in cells expressing cytoplasmic, short, and disrupted actin fragments, similar to unstimulated controls (data not shown). Moreover, imatinib did not decrease the total amount of α-SMA induced by TGF-β, as this is a Smad-dependent transcriptional response independent of c-Abl (37) (data not shown).
Figure 4.
c-Abl is required for TGF-β–induced morphologic alteration and cell proliferation. (A) AKR-2B cells, Abl–/–Arg–/– MEFs, or Abl–/–Arg–/– fibroblasts stably expressing (+) WT-c-Abl or dominant negative c-Abl (DN-c-Abl) were grown to confluence as described in Methods. Cultures were stimulated with DMEM alone or supplemented (+) with 5 μg/ml imatinib and/or 10 ng/ml TGF-β2. Following 48-hour incubation, representative areas were photographed at 20 phase. (B) Triplicate plates from A were trypsinized, and cells were counted with a hemocytometer.
While the preceding data implicate c-Abl as a key mediator of cytoskeletal rearrangement mediated through TGF-β (23), MEFs with the c-Abl and Arg genes deleted were used to directly document a role for c-Abl in TGF-β morphologic transformation. As shown in the lower panel of Figure 4A, addition of TGF-β had no appreciable effect on the morphology of Abl–/–Arg–/– cells. However, stable expression of WT c-Abl rendered the cultures responsive to TGF-β morphologic alterations. Specificity of this response was further documented by the inability of dominant negative c-Abl to induce a similar phenotype. Thus, TGF-β morphologic transformation is (a) inhibited by imatinib, and (b) dependent on expression of WT c-Abl (Figure 4A).
The cellular response to TGF-β is dependent on the cell context. Although TGF-β generally stimulates growth of mesenchymal cells, it inhibits the growth of epithelial and other cell types. To assess the role of TGF-β–activated c-Abl in fibroblast proliferation, the cultures photographed in Figure 4A were trypsinized, and the cell number was determined (Figure 4B). While TGF-β stimulated an approximately 2.5-fold increase in cell number, addition of imatinib reduced this to basal levels (Figure 4B). Furthermore, an analogous response was observed when Abl–/–Arg–/– cells were examined: that is, no effect of TGF-β in the absence of c-Abl expression and a 2.5- to 3.0-fold increase in cell proliferation when the Abl-depleted cultures were reconstituted with WT c-Abl and treated with TGF-β (Figure 4B). These findings implicate c-Abl as an important mediator of TGF-β–stimulated fibroblast proliferation.
TGF-β activation of matrix gene expression is dependent on c-Abl.
Since c-Abl activation represents a novel target coupling serine/threonine receptor kinases to nonreceptor tyrosine kinase signaling, we next addressed 2 general questions: first, does TGF-β similarly stimulate c-Abl activity in human lung fibroblasts; and second, would the expression of TGF-β–induced genes associated with fibrotic changes be dependent on c-Abl activation? In agreement with the results obtained with NIH-3T3 mouse cells (Figures 1–3), TGF-β activated the c-Abl kinase in human IMR90 fibroblasts within 15 minutes of addition (Figure 5A). Moreover, besides stimulating c-Abl in NIH-3T3 and IMR90 cells (Figures 1 and 55A), TGF-β activates c-Abl in Balb/c-3T3 and AKR-2B fibroblasts, but it has no stimulatory effect on Mv1Lu, HeLa, or MDCK epithelial cell lines (data not shown).
Figure 5.
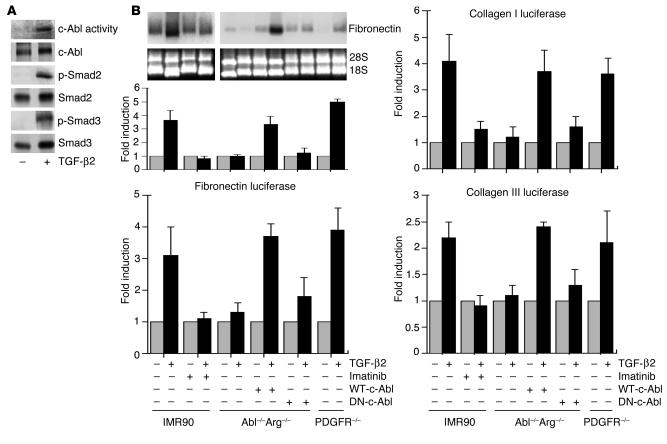
TGF-β stimulates c-Abl–dependent signaling. (A) IMR90 cells were treated with DMEM alone (–) or containing 10 ng/ml TGF-β2 (+). Following 30-minute stimulation, c-Abl kinase activity or Smad2 (p-Smad2) and Smad3 (p-Smad3) phosphorylation was determined. Total c-Abl, Smad2, and Smad3 protein is shown below the corresponding activity. (B) Top blots: IMR90 cells, Abl–/–Arg–/– MEFs, Abl–/–Arg–/– fibroblasts stably expressing (+) WT-c-Abl or dominant negative c-Abl, or PDGF-α and -β receptor–null F cells (PDGFR–/–) were left untreated (–) or treated (+) for 24 hours with TGF-β2 (10 ng/ml) and/or imatinib (10 μg/ml). Imatinib was added 20 minutes before TGF-β2, and fibronectin mRNA accumulation was determined. Bottom blots: Ethidium bromide staining for 28S and 18S ribosomal RNA subunits prior to Northern analysis. Top left graph: The fold fibronectin mRNA stimulation by TGF-β2 is indicated for the various cell types. Results represent the mean ± SE of 2 separate experiments. Bottom left graph: Cell lines as described above were transfected with a fibronectin luciferase construct. The fold induction of 10 ng/ml TGF-β2 with or without 5 μg/ml imatinib was determined as described in Methods and by Penheiter et al. (51). Right graphs: Collagen I (top) and collagen III (bottom) luciferase activity was determined as described for the bottom left graph. Results for all luciferase assays represent the mean ± SE of 2 separate experiments, each done in triplicate.
A hallmark of cytokine-mediated fibrosis is the induction of genes associated with the ECM. For instance, both fibronectin expression and collagen expression are stimulated by both TGF-β and PDGF (38–40). As TGF-β stimulated c-Abl in IMR90 human lung fibroblasts (Figure 5A), we wished to determine (a) whether a similar effect would be observed on fibronectin, collagen I, and collagen III gene activity; (b) whether c-Abl was required for TGF-β–induced ECM gene expression; and (c) whether the effect of TGF-β on ECM proteins was direct, or mediated via PDGFR activity. As shown in Figure 5B (left panels), endogenous fibronectin mRNA accumulation as well as fibronectin promoter luciferase activity was stimulated 3- to 4-fold by TGF-β and reduced to basal levels by imatinib. This response was directly dependent on c-Abl and independent of PDGFRs (Figure 5B, left panels). The data indicate that Abl–/–Arg–/– MEFs require c-Abl for fibronectin gene activity and that the absence of PDGF-α and -β receptors (in F cells; see Figure 2B) has no effect on TGF-β–stimulated fibronectin expression. An identical response is observed for TGF-β induction of the collagen I and III genes (Figure 5B, right panels).
Imatinib prevents BLM-induced pulmonary fibrosis.
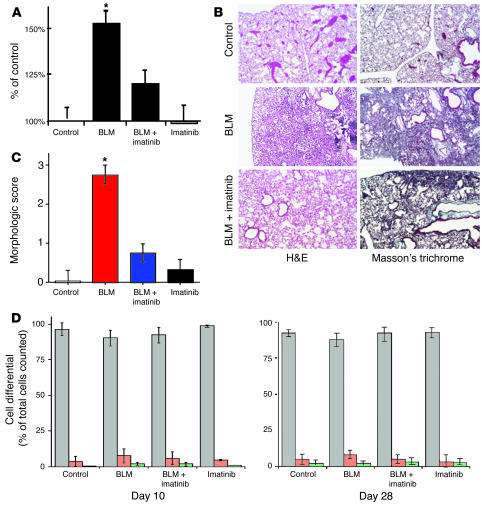
Identification of c-Abl as a novel mediator of TGF-β–induced fibroblast growth, morphologic transformation, and matrix gene expression (i.e., surrogate in vitro profibrotic markers) encouraged us to further investigate the role of c-Abl in an animal model of fibrosis. We reasoned that if (a) TGF-β is a key cytokine controlling pulmonary fibrosis (6, 36, 41), and (b) c-Abl is a downstream target of TGF-β signaling regulating mesenchymal cell proliferation and morphologic alterations (Figures 1–5), then imatinib inhibition of c-Abl would be an effective means to prevent pulmonary fibrosis. To evaluate this hypothesis, we used the murine model of BLM-mediated pulmonary fibrosis as it has been shown to be dependent on TGF-β and PDGF signaling (5, 7, 42). After BLM injury, the extent of lung fibrosis was assessed through OH-proline measurement as a quantitative marker of lung collagen, whose expression by TGF-β was previously shown to be direct and dependent on c-Abl (Figure 5B). Hydroxyproline values in the BLM-treated controls were significantly higher than those in the BLM-treated mice that received imatinib (102.72 ± 4.4 and 80.73 ± 4.6 μg per 10 mg left lung, respectively; P < 0.05), which did not significantly differ from the values in the saline-treated control group (Figure 6A).
Figure 6.
Imatinib reduces hydroxyproline content and prevents histopathologic changes in BLM-treated lungs. (A) Twenty-eight days after intratracheal instillation of BLM or saline, mice were sacrificed (control, n = 12; BLM, n = 15; BLM plus imatinib, n = 14; imatinib, n = 8). The left lung of each animal was used for hydroxyproline assay as described in Methods and previously (52). *P < 0.05 vs. BLM mice receiving imatinib. There was no significant difference (P > 0.05) among control, BLM plus imatinib, and imatinib groups. (B) Mice were treated as in A. The right lung was sectioned and stained with H&E or Masson’s trichrome to visualize collagen. Representative images are shown at ×10 phase. (C) A morphologic score (44) was assigned in a blinded fashion to H&E-stained samples. Zero indicates no fibrosis, 1 indicates occasional small subpleural foci, 2 indicates interalveolar septal thickening and subpleural foci, and 3 indicates continuous interalveolar and subpleural fibrosis. There was no significant difference among the control, BLM plus imatinib, and imatinib groups. (D) Mice were treated as described for A. Immediately prior to explantation trachea were cannulated and lavaged with a total of 10 ml 0.9 normal saline. The collected fluid was centrifuged, and the cell differential was determined. Gray bars indicate percent macrophages, while red and green reflect polymorphonuclear cells and lymphocytes, respectively. Error bars indicate the SE of measurement between samples within the same group.
To further validate the effect of imatinib on collagen content in mice treated with transtracheal BLM, histologic analysis of mouse lung tissue from the explanted right lung was performed (Figure 6B, left column). BLM-treated mice showed dense deposition of collagen with destruction of normal tissue architecture and a relative paucity of inflammatory cells, consistent with the final fibrotic stage of BLM lung fibrosis (43). However, BLM-treated mice given imatinib demonstrated less collagen deposition and tissue destruction as shown with both H&E and Masson’s trichrome staining (Figure 6B, right column). Quantitation of the histopathology (44) using a morphologic score where 0 indicates no fibrosis, 1 indicates occasional small subpleural foci, 2 indicates interalveolar septal thickening and subpleural foci, and 3 indicates continuous interalveolar and subpleural fibrosis, demonstrated a significant difference between mice treated with BLM and those given BLM and imatinib (Figure 6C). There was no significant difference between the BLM plus imatinib group and the saline or imatinib-alone controls. Further, the decrease in collagen content is unlikely due to any anti-inflammatory effect of imatinib, as cell-differential counts showed no difference between any of the groups, either at day 10 — when the inflammatory response is most prominent — or at the termination of the study (Figure 6D). The results suggest that the imatinib-mediated reduction in collagen content is likely a reflection of the disruption in cytokine stimulation of fibroblasts by the common mediator c-Abl.
Discussion
Our results indicate that TGF-β stimulates the nonreceptor tyrosine kinase c-Abl independent of Smad2 and/or Smad3 and is an important and previously unrecognized component of TGF-β–mediated fibroblast proliferation and morphologic alteration (Figures 1–5). These in vitro findings were extended to an in vivo mouse model of lung fibrosis whereby treatment with the c-Abl inhibitor imatinib mesylate was shown to be effective in preventing cytokine-induced lung fibrosis (Figure 6). Thus, these results support the hypothesis that the in vitro regulation of mesenchymal cell growth by the serine/threonine TGF-β receptor kinase is mediated, in part, through the nonreceptor c-Abl tyrosine kinase. Furthermore, imatinib mesylate is an effective in vivo therapy for growth factor–dependent fibrosis in an animal model of injury-induced pulmonary scarring.
It is well established that both TGF-β and c-Abl are key regulators of the actin cytoskeleton, cell morphology, and cell migration (14, 16, 22, 34, 35). The commonality of function between c-Abl and TGF-β suggests a relationship in cell signaling. After establishing c-Abl inhibition as an effective means to inhibit in vitro TGF-β–induced fibroblast function, we next wanted to assess our observations in an animal model. Although many animal models of pulmonary fibrosis exist, all with significant shortcomings, the most widely studied and accepted is BLM-induced pulmonary fibrosis. BLM is a chemotherapeutic agent known to cause human and animal lung parenchymal injury and subsequent lung fibrosis (42). BLM induces lung fibrosis by inflammation-induced release of cytokines and subsequent augmentation of fibroblast proliferation and activity, and in this manner, the BLM model is similar to current models of IPF (45). For instance, the pathogenesis of IPF is believed to begin with an initiating event or injury, followed by alveolar epithelial cytokine signaling and cell recruitment. Under the direction of the potent mitogen PDGF, fibroblasts are recruited to lung parenchyma, and in the presence of TGF-β, they undergo transformation to myofibroblasts. What causes the propagation of cytokine signaling is poorly understood. What is clear, however, is that tissue levels and alveolar levels of key cytokines including TGF-β and PDGF remain elevated, causing relentless progressive fibrosis that leads to destruction of tissue architecture and death. Past attempts to treat IPF have focused heavily on treatment of underlying inflammation and suppression of inflammatory cell activity using potent and prolonged anti-inflammatory therapy in conjunction with immune-modulating drugs. Unfortunately, this approach has not proven efficacious for treatment of IPF (5). Additionally, carefully diagnosed cases of IPF showing the histologic features of usual interstitial pneumonia on open-lung pathology specimens do not demonstrate significant alveolar inflammation as a predominant feature (3, 41).
In this context, it has become apparent that cytokine-mediated fibrosis, rather than inflammation, is central to the pathogenesis of IPF, and recent therapy for IPF has been directed at modulating the influence of these cytokines. At the center of the fibrotic process in IPF lungs is the fibroblast, recruited to injured lung by PDGF and/or TGF-β, morphologically transformed to a myofibroblast by TGF-β, and stimulated to form the principal pathologic feature of IPF, the fibroblastic foci. Fibroblastic foci form polarized aggregates of myofibroblasts surrounded by ECM throughout the IPF lung. In areas of active fibrosis and tissue destruction, the extent of fibroblastic foci in IPF lungs is an independent predictor of lung function and mortality (41). Our finding that inhibition of TGF-β–induced c-Abl kinase activity prevents morphologic transformation, cell proliferation, and matrix gene mRNA expression in vitro (Figures 4 and 5B) led us to hypothesize that imatinib might be an effective inhibitor of cytokine-driven lung fibrosis (Figure 6). Moreover, the coincident ability of imatinib to inhibit the PDGFR only strengthens this hypothesis, as TGF-β and PDGF are considered the 2 most profibrotic cytokines driving fibrosis in IPF (6).
IPF is a complex disease requiring the synergistic action of multiple cytokines. Although the exact role(s) of growth factors in the development of lung fibrosis has not been fully elucidated, the profibrotic activity of TGF-β and PDGF is considered essential (6). PDGF is believed responsible for fibroblast recruitment and growth, while TGF-β is implicated in a number of profibrotic processes including fibroblast recruitment, morphologic transformation, and eventual production and deposition of ECM (46). While we have shown that imatinib is an effective inhibitor of TGF-β–induced fibrotic changes (Figures 4, 5B, and 6), TGF-β is capable of inducing PDGF-like activity (27), and imatinib is also a potent inhibitor of PDGFR signaling (19). Therefore experiments were performed to determine whether c-Abl was a direct target of TGF-β signaling or, conversely, was activated in response to induced PDGF. Figure 2 shows the former hypothesis to be correct, as TGF-β has no effect on PDGFR autophosphorylation (at times when c-Abl activation is maximal) and PDGF-α and -β receptor–null F cells (28) are capable of stimulating c-Abl kinase activity in response to TGF-β. Moreover, loss of Abl family members abolishes TGF-β–stimulated proliferation, morphologic transformation, and ECM gene expression (Figures 4 and 5).
Since these results clearly indicate c-Abl as a direct target of TGF-β receptor signaling and a necessary component of TGF-β’s actions in vitro, we next examined whether imatinib mesylate, an Abl kinase inhibitor, would be effective in preventing the fibrotic changes associated with BLM-induced lung injury. Figure 6 documents that the morphologic alterations and increased OH-proline content seen following BLM challenge are significantly reduced by imatinib treatment. While these in vivo results are consistent with our in vitro data and the hypothesis that BLM-mediated lung fibrosis results from TGF-β signaling through the c-Abl kinase, we believe that a more inclusive interpretation better accounts for the present findings and previous publications (10, 47, 48): since imatinib also inhibits the PDGFR (in addition to Abl family proteins), and lung fibrosis is likely mediated by the synergistic action of multiple cytokines, the potential to inhibit 2 key growth factors regulating the fibrotic process (i.e., TGF-β and PDGF) leads us to reason that imatinib is a potential antifibrotic therapy for patients with IPF (46).
In conclusion, TGF-β activation of c-Abl is an important (and previously unrecognized) Smad-independent component of TGF-β signaling and mediator of TGF-β–driven fibroblast proliferation and ECM gene expression. Addition of imatinib attenuated BLM-induced pulmonary fibrosis and could prove to be an effective antifibrotic therapy in the treatment of IPF and/or other fibrotic disorders.
Methods
Materials.
TGF-β was purchased from Austral Biologicals, while PDGF-AB was from Sigma-Aldrich. Unless otherwise stated, all other reagents were obtained from Sigma-Aldrich.
Cell culture.
NIH-3T3 and AKR-2B MEFs were grown in high-glucose DMEM supplemented with 10% FBS (BioSource International Inc.). Prior to use, 1 × 106 cells were seeded on 100-mm culture plates, grown to confluence, washed with PBS, and serum-starved for 24 hours in 0.1% FBS and DMEM. MEF lines null for c-Abl and Arg (49) or the PDGF-α and -β receptors (28) were cultured in 10% FBS DMEM, while IMR90 human lung fibroblasts were grown in MEM containing 10% FBS and supplemented with 0.1 mM nonessential amino acids and 1.0 mM sodium pyruvate. Abl–/–Arg–/– MEF lines stably expressing WT or kinase-inactive (dominant negative) c-Abl were kindly provided by Pamela Woodring (Salk Institute, La Jolla, California, USA) and propagated in 10% FBS DMEM (23).
Smad2/3 KO cells were produced by transfection (using Lipofectamine 2000; Invitrogen Corp.) of Smad3 KO MEFs with 5 μg dominant negative Smad2 (Myc-Smad2D450E) and Flag-tagged c-Abl expression plasmids for 4 hours. Following overnight incubation in 20% FBS DMEM, the cultures were placed in serum-free DMEM for 24 hours and stimulated as indicated for 30 minutes.
Imatinib mesylate preparation.
Imatinib was purchased from the Mayo Clinic pharmacy in 100-mg capsules and solubilized in distilled/deionized H2O. The particulate matter was removed by centrifugation at 2,500 g (twice) and the supernatant was lyophilized with 90% recovery. Reverse-phase high-pressure liquid chromatography and mass spectrometry indicated greater than 99% purity of the lyophilized material.
c-Abl kinase assays.
Cultures were stimulated for the indicated time and then lysed for 30 minutes at 4°C in 750 μl of kinase lysis buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1% Triton X, 0.1% SDS, 1% sodium deoxycholate, 0.1 TIU/ml aprotinin, 50 μg/ml PMSF, 1 mM sodium vanadate, 1 mg/ml leupeptin). Extracts were clarified, and equivalent protein (∼500 μg) was incubated overnight at 4°C with anti-Abl (K12; Santa Cruz Biotechnology Inc.) or anti-Flag (Sigma-Aldrich). Immune complexes were collected with protein A-Sepharose (Sigma-Aldrich) and washed twice in kinase lysis buffer and twice in kinase buffer (25 mM Tris [pH 7.4], 10 mM MgCl2, 1 mM DTT) prior to incubation in 40 μl kinase buffer containing 5 μM ATP, 2 μg GST-Crk, and 0.5 μCi/Rxn [γ32P]ATP. The kinase reaction was allowed to proceed for 5 minutes at 37°C, stopped with 40 μl (×2 concentration) Laemmli buffer, and visualized by autoradiography after SDS-PAGE. Total c-Abl protein was detected using an antibody from BD Biosciences — Pharmingen (no. 554148).
Western blotting.
To assess Smad activation, NIH-3T3 cells were treated as indicated and lysed (50 mM Tris [pH 7.4], 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EGTA, 1 mM Na3VO4, Complete Protease Inhibitor [Roche Diagnostics Corp.]), and equivalent protein (∼100 μg) was probed with a phosphospecific Smad2 antibody (no. 06-829; Upstate Biotechnology Inc.) or a rabbit anti–phospho-Smad3 antibody generated in our laboratory to the peptide COOH-GSPSIRCSpSVpS. This antibody shows little cross-reactivity with phospho-Smad2 (50). The same blot was stripped and probed for total Smad protein (Smad2, no. 06-829; Upstate Biotechnology Inc.; and Smad3, no. 51-1500; Zymed Laboratories Inc.).
Tyrosine phosphorylation of the PDGF-β receptor was determined on cellular lysates prepared from cultures stimulated with TGF-β (10 ng/ml) or PDGF-AB (25 ng/ml). Following immunoprecipitation (1.0 mg protein) with an antibody (sc-1627; Santa Cruz Biotechnology Inc.) to the PDGF-β receptor, Western analysis was performed using an anti–phospho-tyrosine (no. 06-427; Upstate Biotechnology Inc.). The blot was stripped, and total receptor protein was assessed using the same antibody.
Luciferase assays.
Cultures were plated in 6-well dishes at 3 × 105 cells per well 24 hours prior to transfection. Two micrograms of the indicated luciferase reporter and 0.5 μg of CMV–β-galactosidase were transfected per well with LipofectAMINE (Invitrogen Corp.). TGF-β–stimulated luciferase activity was determined as described previously on normalized samples (51).
Morphologic assessment.
AKR-2B fibroblasts were plated at 2.5 × 105 cells per 6-well dish and incubated at 37°C for 24 hours. Confluent cultures were placed in serum-free MCDB 402 (JRH Biosciences) for 48 hours and stimulated by the addition of fresh serum-free medium alone or containing 10 ng/ml TGF-β2 with or without 5 μg/ml imatinib. Cells were then incubated for a further 48 hours at 37°C and imaged by light microscopy. Abl–/–Arg–/– MEFs or Abl–/–Arg–/– fibroblasts stably expressing WT or dominant negative c-Abl were plated at 2.0 × 105 cells per 6-well dish in 20% FBS DMEM. Following overnight growth, the medium was replaced with 0.5% FBS DMEM for 24 hours and left untreated or stimulated with 10 ng/ml TGF-β2 for an additional 48 hours.
BLM-induced fibrosis.
Female 129tsvems mice (The Jackson Laboratory) 5–8 weeks in age were used for all animal experiments according to standard care and methodology approved by the Mayo Clinic Institutional Animal Care and Use Committee. Anesthetized mice were tracheostomized, and 0.1 U of BLM diluted in 75 μl of 0.9 normal saline, or 75 μl of 0.9 normal saline alone, was aspirated transtracheally under direct visualization. The following day, mice receiving BLM were randomly assigned to treatment with imatinib (50 mg/kg/d in PBS) via i.p. injection, or injection with an equal volume of PBS. Mice receiving 0.9 normal saline were randomized and treated in a similar fashion. Both groups were treated for 28 consecutive days.
Collagen synthesis.
Twenty-eight days after intratracheal instillation of BLM or saline, mice were sacrificed. The left lung of each animal was used for hydroxyproline assay as described previously (52). Briefly, explanted left lung was washed in PBS, weighed, minced, and diluted in 10 ml PBS per milligram lung weight. One-hundred-microliter samples were then hydrolyzed in 12 M HCl, and duplicate samples were analyzed by OH-proline assay and expressed as micrograms OH-proline content per 10 milligrams left lung.
Data analysis.
Data comparisons between experimental and control groups were analyzed using the Student’s t test and expressed as means ± SE. P values less than 0.05 were considered significant.
Acknowledgments
We thank Rina Plattner and Ann-Marie Prendergast for the GST-Crk substrate and helpful advice. Joseph Standing provided excellent technical assistance with the animal studies. Anita Roberts provided the Smad3 KO MEFs; Azedine Atfi, the Myc-Smad2D450E; Anthony Koleske and Pamela Woodring, the MEFs deleted and reconstituted with Abl family members; and Philippe Soriano and Andrius Kazlauskas, the PDGFR-null F cells. This work was supported by Public Health Service grants GM54200 and GM55816 from the National Institute of General Medical Sciences and funds from the Robert N. Brewer Family Foundation.
Footnotes
Craig E. Daniels and Mark C. Wilkes contributed equally to this work.
Nonstandard abbreviations used: Abl, Abelson; BLM, bleomycin; IPF, idiopathic pulmonary fibrosis; MEF, mouse embryo fibroblast.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am. J. Respir. Crit. Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 2.Coultas DB, Sumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am. J. Respir. Crit. Care Med. 1994;150:967–972. doi: 10.1164/ajrccm.150.4.7921471. [DOI] [PubMed] [Google Scholar]
- 3.Bjoraker JA, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 1998;157:199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 4.Strieter RM. Mechanisms of pulmonary fibrosis: conference summary. Chest. 2001;120(1 Suppl.):77S–85S. doi: 10.1378/chest.120.1_suppl.s77-a. [DOI] [PubMed] [Google Scholar]
- 5.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann. Intern. Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 6.Lasky JA, Brody AR. Interstitial fibrosis and growth factors. Environ. Health Perspect. 2000;108:751–762. doi: 10.1289/ehp.00108s4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sime PJ, O’Reilly KM. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin. Immunol. 2001;99:308–319. doi: 10.1006/clim.2001.5008. [DOI] [PubMed] [Google Scholar]
- 8.Kaartinen V, Voncken JW, Warburton SC, Heisterkamp BD, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-beta3 indicates defects of epithelial-mesenchymal interaction. Nat. Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- 9.Kolb M, et al. Transient transgene expression of decorin in the lung reduces the fibrotic response to bleomycin. Am. J. Respir. Crit. Care Med. 2001;163:770–777. doi: 10.1164/ajrccm.163.3.2006084. [DOI] [PubMed] [Google Scholar]
- 10.Nakao A, et al. Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J. Clin. Invest. 1999;104:5–11. doi: 10.1172/JCI6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baarends WM, et al. A novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the müllerian duct. Development. 1994;120:189–197. doi: 10.1242/dev.120.1.189. [DOI] [PubMed] [Google Scholar]
- 12.Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc. Natl. Acad. Sci. U. S. A. 1991;88:6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gharaee-Kermani M, Phan SH. Role of cytokines and cytokine therapy in wound healing and fibrotic diseases. Curr. Pharm. Des. 2001;7:1083–1103. doi: 10.2174/1381612013397573. [DOI] [PubMed] [Google Scholar]
- 14.Branton MH, Kopp JB. TGF-beta and fibrosis. Microbes Infect. 1999;15:1349–1365. doi: 10.1016/s1286-4579(99)00250-6. [DOI] [PubMed] [Google Scholar]
- 15.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N. Engl. J. Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 16.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N. Engl. J. Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 17.Van Etten RA. Cycling, stressed-out and nervous: cellular functions of c-Abl. Trends Cell Biol. 1999;9:179–186. doi: 10.1016/s0962-8924(99)01549-4. [DOI] [PubMed] [Google Scholar]
- 18.Sachdev P, Zeng L, Wang LH. Distinct role of phosphatidylinositol 3-kinase and Rho family GTPases in Vav3-induced cell transformation, cell motility, and morphological changes. J. Biol. Chem. 2002;277:17638–17648. doi: 10.1074/jbc.M111575200. [DOI] [PubMed] [Google Scholar]
- 19.Savage DG, Antman KH. Imatinib mesylate: a new oral targeted therapy. N. Engl. J. Med. 2002;346:683–693. doi: 10.1056/NEJMra013339. [DOI] [PubMed] [Google Scholar]
- 20.Cohen MH, et al. Approval summary for imatinib mesylate capsules in the treatment of chronic myelogenous. Clin. Cancer Res. 2002;8:935–942. [PubMed] [Google Scholar]
- 21.Oft M, et al. TGF-beta1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 1996;10:2462–2477. doi: 10.1101/gad.10.19.2462. [DOI] [PubMed] [Google Scholar]
- 22.Salgia R, et al. The BCR/ABL oncogene alters the chemotactic response to stromal-derived factor-1 alpha. Blood. 1999;94:4233–4246. [PubMed] [Google Scholar]
- 23.Woodring PJ, et al. Modulation of the F-actin cytoskeleton by c-Abl tyrosine kinase in cell spreading and neurite extension. J. Cell Biol. 2002;156:879–892. doi: 10.1083/jcb.200110014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Etten RA, Jackson P, Baltimore D. The mouse type IV c-abl gene product is a nuclear protein, and activation of transforming ability is associated with cytoplasmic localization. Cell. 1989;58:669–678. doi: 10.1016/0092-8674(89)90102-5. [DOI] [PubMed] [Google Scholar]
- 25.Plattner R, Kadlec L, DeMali KA, Kazlauskas A, Pendergast AM. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 1999;13:2400–2411. doi: 10.1101/gad.13.18.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pendergast A. The Abl family kinases: mechanisms of regulation and signaling. Adv. Cancer Res. 2002;85:51–100. doi: 10.1016/s0065-230x(02)85003-5. [DOI] [PubMed] [Google Scholar]
- 27.Leof EB, et al. Induction of c-sis mRNA and activity similar to platelet-derived growth factor by transforming growth factor beta: a proposed model for indirect mitogenesis involving autocrine activity. Proc. Natl. Acad. Sci. U. S. A. 1986;83:2453–2457. doi: 10.1073/pnas.83.8.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrews A, et al. Platelet-derived growth factor plays a key role in proliferative vitreoretinopathy. Invest. Ophthalmol. Vis. Sci. 1999;40:2683–2689. [PubMed] [Google Scholar]
- 29.Hocevar BA, Brown TL, Howe PH. TGF-β induces fibronectin synthesis through a c-Jun N-termial kinase-dependent, Smad4-independent pathway. EMBO J. 1999;18:1345–1356. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johansson N, et al. Expression of collagenase-3 (MMP-13) and collagenase-1 (MMP-1) by transformed keratinocytes is dependent on the activity of p38 mitogen-activated protein kinase. J. Cell Sci. 2000;113:227–235. doi: 10.1242/jcs.113.2.227. [DOI] [PubMed] [Google Scholar]
- 31.Engel ME, McDonnell MA, Law BK, Moses HL. Interdependent SMAD and JNK signaling in TGF-β mediated transcription. J. Biol. Chem. 1999;274:37413–37420. doi: 10.1074/jbc.274.52.37413. [DOI] [PubMed] [Google Scholar]
- 32.Kim J-T, Joo C-K. Involvement of cell-cell interactions in the rapid stimulation of Cas tyrosine phosphorylation and Src kinase activity by transforming growth factor-β1. J. Biol. Chem. 2002;277:31938–31948. doi: 10.1074/jbc.M201178200. [DOI] [PubMed] [Google Scholar]
- 33.Nicholson AG, et al. The relationship between individual histologic features and disease progression in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2002;166:173–177. doi: 10.1164/rccm.2109039. [DOI] [PubMed] [Google Scholar]
- 34.Kadlec L, Pendergast AM. The amphiphysin-like protein 1 (ALP1) interacts functionally with the cABL tyrosine kinase and may play a role in cytoskeletal regulation. Proc. Natl. Acad. Sci. U. S. A. 1997;94:12390–12395. doi: 10.1073/pnas.94.23.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Escalante M, et al. Phosphorylation of c-Crk II on the negative regulatory Tyr222 mediates nerve growth factor-induced cell spreading and morphogenesis. J. Biol. Chem. 2000;275:24787–24797. doi: 10.1074/jbc.M000711200. [DOI] [PubMed] [Google Scholar]
- 36.Evans RA, Tian YC, Steadman R, Phillips AO. TGF-beta1-mediated fibroblast-myofibroblast terminal differentiation: the role of smad proteins. Exp. Cell Res. 2003;282:90–100. doi: 10.1016/s0014-4827(02)00015-0. [DOI] [PubMed] [Google Scholar]
- 37.Hu B, Wu Z, Phan SH. Smad3 mediates TGF-beta-induced alpha-smooth muscle actin expression. Am. J. Respir. Crit. Care Med. 2003;29:397–404. doi: 10.1165/rcmb.2003-0063OC. [DOI] [PubMed] [Google Scholar]
- 38.Butt RP, Laurent GJ, Bishop JE. Collagen production and replication by cardiac fibroblasts is enhanced in response to diverse classes of growth factors. Eur. J. Cell Biol. 1995;68:330–335. [PubMed] [Google Scholar]
- 39.Bissell DM. Chronic liver injury, TGF-beta, and cancer. Exp. Mol. Med. 2001;33:179–190. doi: 10.1038/emm.2001.31. [DOI] [PubMed] [Google Scholar]
- 40.Raines EW, Koyama H, Carragher NO. The extracellular matrix dynamically regulates smooth muscle cell responsiveness to PDGF. Ann. N. Y. Acad. Sci. 2000;902:39–51. doi: 10.1111/j.1749-6632.2000.tb06299.x. [DOI] [PubMed] [Google Scholar]
- 41.Pardo A, Selman M. Idiopathic pulmonary fibrosis: new insights in its pathogenesis. Int. J. Biochem. Cell Biol. 2002;34:1534–1538. doi: 10.1016/s1357-2725(02)00091-2. [DOI] [PubMed] [Google Scholar]
- 42.Adamson IY, Bowden DJ. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am. J. Pathol. 1974;77:185–197. [PMC free article] [PubMed] [Google Scholar]
- 43.Cutroneo KR, Sterling KM., Jr A molecular basis for bleomycin-induced pulmonary fibrosis. Chest. 1986;89(3 Suppl.):121S–122S. doi: 10.1378/chest.89.3_supplement.121s. [DOI] [PubMed] [Google Scholar]
- 44.Tran PL, et al. Prevention of bleomycin-induced pulmonary fibrosis after adenovirus-mediated transfer of the bacterial bleomycin resistance gene. J. Clin. Invest. 1997;99:608–617. doi: 10.1172/JCI119203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moseley PL, Hemken C, Hunninghake GW. Augmentation of fibroblast proliferation by bleomycin. J. Clin. Invest. 1986;78:1150–1154. doi: 10.1172/JCI112695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rice AB, Moomaw CR, Morgan DL, Bonner JC. Specific inhibitors of platelet-derived growth factor or epidermal growth factor receptor tyrosine kinase reduce pulmonary fibrosis in rats. Am. J. Pathol. 1999;155:213–221. doi: 10.1016/S0002-9440(10)65115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J. Clin. Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munger JS, et al. The integrin αvβ6 binds and activates latent TGFβ1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 49.Koleske AJ, et al. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21:1259–1272. doi: 10.1016/s0896-6273(00)80646-7. [DOI] [PubMed] [Google Scholar]
- 50.Wilkes MC, Murphy SJ, Garamszegi N, Leof EB. Cell-type-specific activation of PAK2 by transforming growth factor β independent of Smad2 and Smad3. Mol. Cell. Biol. 2003;23:8878–8889. doi: 10.1128/MCB.23.23.8878-8889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Penheiter SG, et al. Internalization-dependent and -independent requirements for transforming growth factor β receptor signaling via the Smad pathway. Mol. Cell. Biol. 2002;22:4750–4759. doi: 10.1128/MCB.22.13.4750-4759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christensen PJ, Goodman RE, Pastoriza L, Moore B, Toews GB. Induction of lung fibrosis in the mouse by intratracheal instillation of fluorescein isothiocyanate is not T-cell-dependent. Am. J. Pathol. 1999;155:1773–1779. doi: 10.1016/S0002-9440(10)65493-4. [DOI] [PMC free article] [PubMed] [Google Scholar]