Supplemental Digital Content is available in the text.
Abstract
Background:
Combination of fillers and botulinum toxin for aesthetic applications is increasingly popular. Patient demographics continue to diversify, and include an expanding population receiving maintenance treatments over decades.
Methods:
A multinational panel of plastic surgeons and dermatologists convened the Global Aesthetics Consensus Group to develop updated guidelines with a worldwide perspective for hyaluronic acid fillers and botulinum toxin. This publication considers strategies for combined treatments, and how patient diversity influences treatment planning and outcomes.
Results:
Global Aesthetics Consensus Group recommendations reflect increased use of combined treatments in the lower and upper face, and some midface regions. A fully patient-tailored approach considers physiologic and chronologic age, ethnically associated facial morphotypes, and aesthetic ideals based on sex and culture. Lower toxin dosing, to modulate rather than paralyze muscles, is indicated where volume deficits influence muscular activity. Combination of toxin with fillers is appropriate for several indications addressed previously with toxin alone. New scientific data regarding hyaluronic acid fillers foster an evidence-based approach to selection of products and injection techniques. Focus on aesthetic units, rather than isolated rhytides, optimizes results from toxin and fillers. It also informs longitudinal treatment planning, and analysis of toxin nonresponders.
Conclusions:
The emerging objective of injectable treatment is facial harmonization rather than rejuvenation. Combined treatment is now a standard of care. Its use will increase further as we refine the concept that aspects of aging are intimately related, and that successful treatment entails identifying and addressing the primary causes of each.
CLINICAL QUESTION/LEVEL OF EVIDENCE:
Therapeutic, V.

Combination of hyaluronic acid fillers and botulinum toxin type A is becoming increasingly popular. Recent comparative studies have established the safety and enhanced efficacy compared with solo treatment of combined treatments for lower face rejuvenation.1,2 A 2008 North American consensus cited combination treatment as important for the lower face and sometimes appropriate for the upper face.3 French consensus recommendations in 20114,5 and a panel of five experts from Canada, Europe, and South America in 20136 affirmed the value of the combined approach.
Surveys from the American Society for Aesthetic Plastic Surgery, the American Society for Dermatologic Surgery, and the International Society for Aesthetic Plastic Surgery7–9 report steady increases in the number of toxin and hyaluronic acid filler procedures performed each year. These and other procedural surveys10 illuminate another significant trend: the growing diversity of patients’ ethnicity, sex, and age. The 2014 American Society for Aesthetic Plastic Surgery survey reported that approximately 22 percent of all cosmetic procedures were performed on ethnic minorities. Men were the recipients of approximately 11.5 percent of botulinum toxin procedures and 8 percent of hyaluronic acid filler procedures.7 As worldwide experience accrues, another important patient group has emerged—those receiving repeated treatments over years or decades.
CONSENSUS OBJECTIVES AND METHODOLOGY
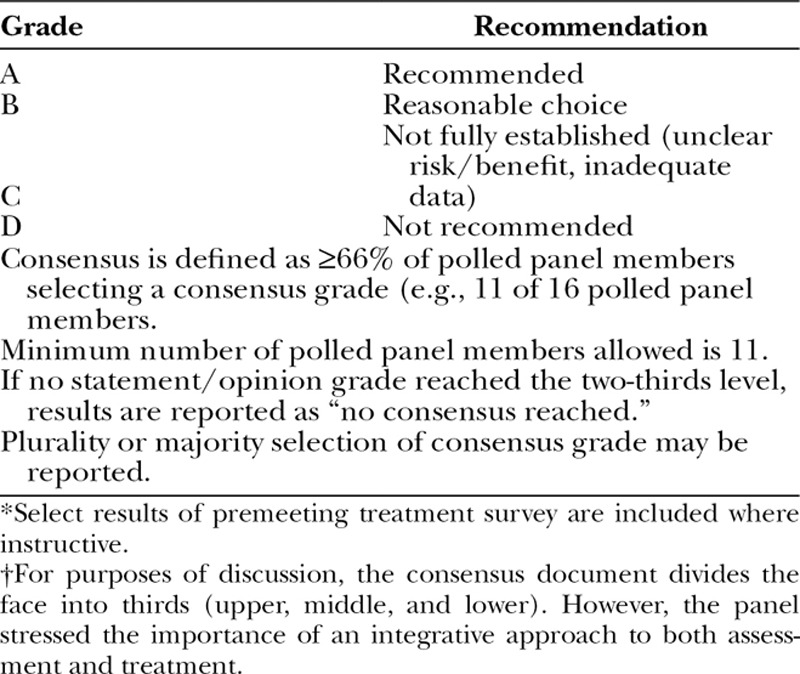
From January 17 through 19, 2014, a multidisciplinary group of key opinion leaders in the core aesthetic specialties from Asia, Australia, Europe, and North and South America convened the Global Aesthetics Consensus Group to formulate updated guidelines for aesthetic use of hyaluronic acid fillers and botulinum toxin type A. This publication provides recommendations for combined treatments. It also presents concepts for treatment planning and implementation in diverse patient populations. The Global Aesthetics Consensus Group is well suited for these objectives by virtue of its own geographic and ethnic diversity. The methodology used for determining consensus is summarized in Table 1.
Table 1.
Methodology for Global Aesthetics Consensus Group Panel Consensus: Grading of Statements and Opinions Developed during the Conference*†
EVOLVING CONCEPTS IN FACIAL AGING
Facial aging occurs at all levels. The epidermis, dermis, subcutis, and bone undergo remodeling throughout life; degradation of existing tissue is balanced with generation of new tissue. With age, regenerative properties decline and the balance of remodeling becomes disrupted, such that there is a net loss of tissue that is recognized as resorption. From a quantitative perspective, volume loss is significant from the deeper tissue layers, with deflation and descent of depleted subcutaneous fat compartments and loss of bone (Fig. 1).11–18 Total collagen content of the skin also decreases. There is qualitative degeneration of tissue components, including dermal collagen and elastin.19,20
Fig. 1.
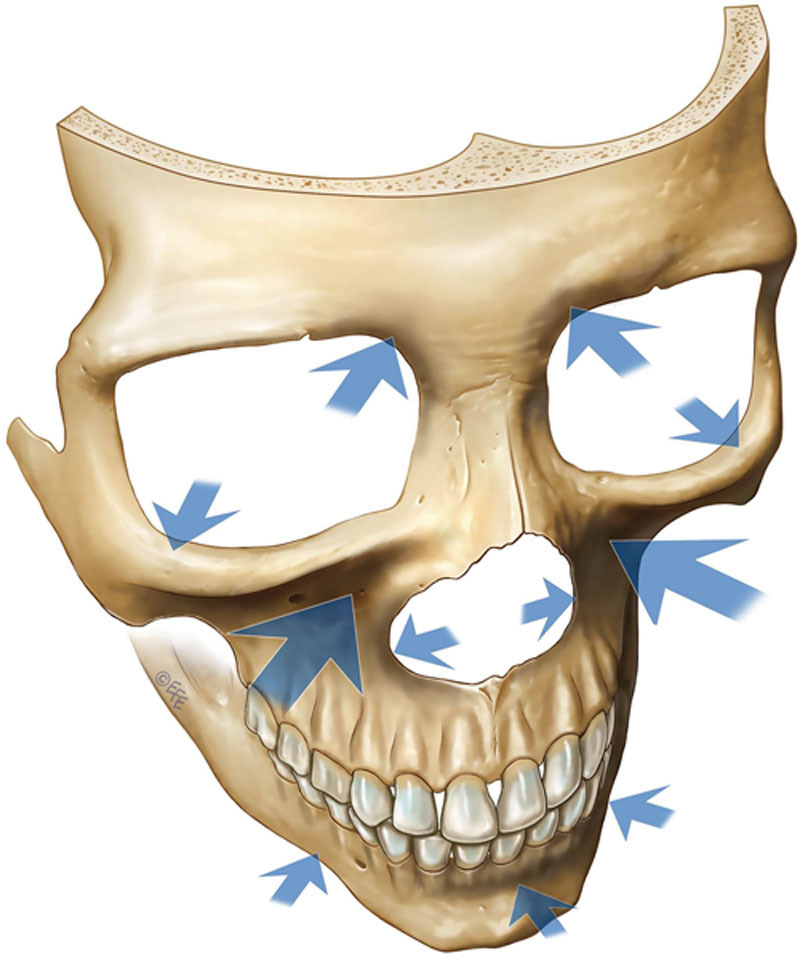
Age-related bony changes are mainly in the periorbital and midcheek zones, including the superomedial and inferolateral aspects of the orbit, the medial suborbital and piriform areas of the maxilla, and the prejowl area of the mandible. Arrows indicate the areas of the facial skeleton susceptible to resorption with aging. The size of the arrow correlates with the amount of resorption. (From Mendelson B, Wong CH. Changes in the facial skeleton with aging: Implications and clinical applications in facial rejuvenation. Aesthetic Plast Surg. 2012;36:753–760. Reprinted with permission from Springer International Publishing AG. © EFE.)
These processes result in three-dimensional alterations in facial shape and contour; skin laxity, folds, and rhytides; and surface changes, including skin roughness and xerosis. The primary extrinsic causes of skin aging have been characterized as the “three S’s”—sun (ultraviolet radiation), smoking, and stress. They increase oxidative stress by means of reactive oxygen species overload and concomitant antioxidant depletion. Multiple biochemical pathways are triggered. They promote collagen loss by inducing suppression of transforming growth factor-β receptor II; overexpression of matrix metalloproteinases, which are collagenases; and increased inflammation through the nuclear factor kappa B pathway. Ultraviolet radiation also directly damages the skin’s structural proteins. Intrinsic skin aging is related to a progressive age-related decline in antioxidant capacity, coupled with increased reactive oxygen species production from oxidative metabolism in skin cells. This contributes to oxidative stress. Chromosomal analysis of aging cells reveals progressive telomere shortening, associated with tissue damage. The rates of extrinsic and intrinsic aging vary considerably, based on individual exposure to the causative factors and hereditary predisposition.19,20
The multifactorial cause of facial aging provides the rationale for combined treatments. The balance of fillers with toxin depends on individualized patient assessment and functional understanding of what the observations mean. In previous guidelines, toxin has been considered the foundation of treatment to the upper face, with fillers playing a larger role in the middle and lower face.3 Increasingly, however, fillers are seen as improving the effects of toxin—and are particularly indicated as patient age increases. The consensus panel acknowledged that combined treatment has value throughout the face.
Optimizing Treatment Outcomes in Diverse Patient Populations
The objective of aesthetic treatment is often described as facial rejuvenation. A broader perspective, encompassing patient age, ethnicity, and sex, yields the more accurate objective of facial harmonization—through correction of acquired (usually age-related) disharmonies, together with modification of congenital characteristics. In recognition of growing patient diversity in clinical practice, the panel recommended enrollment of more heterogeneous subject populations in injectables studies.
Age
Surveys report that the typical age at which patients seek cosmetic procedures is between 30 and 50 years.7 When clinically indicated, fillers and toxin are of demonstrable benefit to older and younger patients. A retrospective review from 2008 to 2013 of a database endorsed by the American Society of Plastic Surgeons showed that patients older than 65 years had significantly more cosmetic facial procedures performed than did younger patients (62.9 percent versus 12 percent). There was no statistically significant difference in complication rates following all procedures in older patients (mean age, 69.1 years; 1.94 percent) versus younger patients (mean age, 39.2 years; 1.84 percent). This was despite the greater presence of health risks in the older group, including higher body mass index and higher incidence of diabetes mellitus.21
The variable time course and manifestations of aging underscore the importance of both chronologic and physiologic age when planning treatment. Individualized analysis includes assessment of tissue quality, extent and pattern of volume loss from soft and hard tissues, extent and pattern of muscular contraction, and surface skin changes. Selection of appropriate injectables to achieve the best results has been compared metaphorically to an artist’s choices from a palette of paints to create a beautiful picture. As an extension of this metaphor, the face may be considered the “canvas” for aesthetic interventions.
Respect for the aging facial canvas is an evolving and fundamental principle of treatment. A paradigm shift is advocated from the youthful face as the “gold standard,” toward age-appropriate facial harmonization. For example, it is inadvisable to try to restore the midface convexity (ogee curve) of a 50-year-old with depleted facial skeletal support to a level appropriate for a 25-year-old. Attempts to do so by filler injection may produce passable results in repose. But there may be undesirable effects in animation, such as impingement of the midface on the lid-cheek junction when smiling, or a discernible ledge between the submalar region and the lower face. Overvolumization is best avoided by viewing patients before, during, and after injection—not only at rest, but also in animation. A conservative approach is preferable, particularly with newer, longer lasting hyaluronic acid fillers, for which the panel agreed that “less is often more.” Although these principles may seem obvious and even superfluous to state explicitly, they can become obscured if adherence to rigid algorithms and cutaneous landmarks (which shift with age) supersedes a patient-tailored approach.
There is no upper limit for age beyond which patients cease to benefit from volume restoration. Declining tissue quality and consequent skin laxity are indications for combining fillers with lasers, energy-based devices,22 or surgery when appropriate. In older patients, fewer injection sites and smaller toxin doses at each site may be indicated if muscle mass or function is reduced.
In younger patients, age-related disharmonies are not yet prominent. Emphasis is typically on modification of congenital characteristics or of acquired disharmonies that are age-independent, such as from injuries. Treatment of younger patients is commonly described as proactive or “preventive” (e.g., when glabellar or frontal lines that were present since childhood or incipient in early adulthood are injected with toxin).
Gender
Male and female faces are governed by different treatment principles. There are considerable differences in anatomy and in what is aesthetically appealing. Harmonization of the female face entails restoring prominence to the upper and middle thirds with a lower facial taper, to achieve a heart or inverted triangle shape. In contrast, the male face is perceived as harmonious when somewhat longer, with more equal prominence of facial thirds and a well-defined jawline. The average male skull is significantly larger than that of a female skull. The frontal, maxillary, zygomatic, and mandibular bones tend to be broader, squarer, and flatter, and the supraorbital rim is more prominent. Men have greater average muscle mass and a higher density of blood vessels.23 Gender-related differences in epidermal and dermal thickness and fat distribution are considered to be modulated by sex steroids.24,25 Study of composite facial images indicates that greater facial symmetry confers a more gender-typical appearance for men and women.26
Many caveats that apply to treatment of men with injectables pertain to avoidance of facial feminization. The panel cautioned that overvolumization of the male midface produces a feminizing convexity. It is also important not to overfill the temple, as temporal hollowing is aesthetically appealing in many men. Injection of fillers into the lips may be appropriate for some men (e.g., to improve vermilion border definition), but care must be taken to avoid a feminine shape or fullness. Inappropriate elevation of the eyebrows (especially the lateral tails) with toxin will tend to feminize a male face.
Ethnicity
Clinical data with Evidence Levels I and II support the safety and efficacy of botulinum toxin type A and hyaluronic acid fillers in persons of color27–30 (Fig. 2). Guidelines based on patient ethnicity should not obscure the basic tenet that ideals of beauty are largely preserved across time and cultures. Although some quantifiable congenital characteristics are commonly associated with specific ethnicities,31–35 the general principles of individualized analysis and correction apply to every patient. Geographic variations in treatment approaches have evolved as worldwide use of injectables has expanded. This is a manifestation of ethnicity and prevalent facial morphotypes, cultural preferences, and global migration patterns.
Fig. 2.
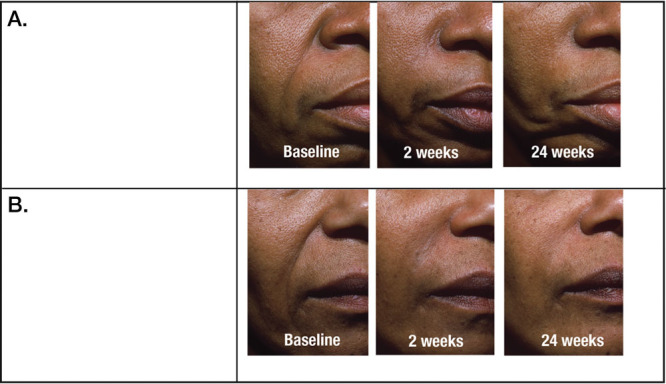
Efficacy of nasolabial fold correction with crosslinked hyaluronic acid filler in patients with Fitzpatrick skin phototype VI. Nasolabial folds are shown before injection and 2 and 24 weeks after injection with Hylacross hyaluronic acid filler. (Above) Before and after injection of Juvéderm Ultra. (Below) Before and after injection of Juvéderm Ultra Plus. (From Grimes PE, Thomas JA, Murphy DK. Safety and effectiveness of hyaluronic acid fillers in skin of color. J Cosmet Dermatol. 2009;8:162–168. Reprinted with permission from John Wiley & Sons.)
Additional considerations pertain to immigrant patients (e.g., the cultural overlays when Asian patients consult with European or American surgeons). Asian, Latin American, African, and other patients are sometimes perceived mistakenly as uniform populations. However, there are significant physical and cultural variations within these continents. Ethnic mixing, because of intermarriage, adds to the multiplicity of facial morphotypes. These considerations inform qualitative and quantitative differences in treatment. Compared with whites, Asians are more likely to display central face retrusion; flattening of the anteromedial midface; recessed piriform fossae; flatter, broader foreheads; retrognathia; and microgenia.34,36 These congenital characteristics account for the prevalence of filler injection to the medial midface, nose, chin, and forehead of younger Asian patients. In older patients, volumization of these regions remains a priority, in conjunction with correction of age-related disharmonies. Skeletal structure affects resting tone and contraction of the overlying musculature. A recent classification of Asian facial morphotypes proposes division into three basic categories to guide treatment strategies with botulinum toxin and fillers.37 Validation of this classification and the associated treatment strategies is now in process.
Variations in incidence and presentation of photoaging among ethnic groups are attributable in part to physical differences, such as variations in fibroblast size and structure,38,39 and in part to differences in lifestyle. Although all ethnic groups eventually manifest signs of photoaging, whites typically have an earlier onset and display more rhytides at a younger age. The old adage that skin of color ages more by folding than wrinkling underscores a key point—that volume loss is ubiquitous to all ethnicities. It therefore follows that restoration of volume and correction of related sequelae, including effects on associated musculature, are fundamental strategies for every patient.
Longitudinal Treatment Planning
Ongoing treatment with hyaluronic acid fillers and botulinum toxin yields cumulative improvements. The greatest benefits are obtained if patients return for treatment when the previous results start to diminish rather than after they disappear completely. Ultimately, treatment may be required less frequently, and doses can often be reduced. A retrospective medical chart review of 194 patients who received a total of 4402 onabotulinumtoxinA treatments to glabellar lines over a mean of 9.1 years demonstrated sustained patient and physician satisfaction.40 Greater perceived benefits were reported in patients treated for longer periods.
The long-term restorative potential of diluted (reconstituted) Hylacross hyaluronic acid filler (Juvéderm Ultra; Allergan, Inc., Irvine, Calif.) is indicated by a retrospective evaluation of more than 350 patients whose clinical improvement after superficial injection of facial fine lines persisted beyond the time frame that could be accounted for by space-filling effects alone.41 The scientific rationale is provided by a rat study, in which enzyme-linked immunosorbent assay and reverse-transcriptase polymerase chain reaction showed up-regulation of types I and III collagen and elastin expression after intracutaneous injection of Hylacross filler, but not after control injection of saline.42
A recent prospective study of onabotulinumtoxinA raised the possibility of biomechanical restoration, demonstrating increased skin pliability and elastic recoil after injection of the lateral orbital, forehead, and glabellar regions of 40 women.43 Because botulinum toxin spreads in a three-dimensional manner after injection, the mechanism of action could be intramuscular or related to the overlying skin. Previous anecdotal reports of improvement in skin texture and turgor after intradermal toxin injection44–46 have been attributed to intracutaneous fluid retention, by means of effects on acetylcholine receptors, which exist on keratinocytes, melanocytes, and cells of the sebaceous glands.47
Several Global Aesthetics Consensus Group recommendations describe lower toxin doses than in previous guidelines. This is to modulate rather than obliterate muscular activity, with developing understanding that combining toxin with fillers is more appropriate for some indications in which toxin alone was advocated in the past. Some panelists expressed concern that overtreatment with toxin, especially with short retreatment intervals, could produce muscular atrophy that would exacerbate volume loss. Blinded, placebo-controlled studies of botulinum toxin type A for moderate to severe crow’s feet show little clinical difference between the two most efficacious doses, although higher doses tended to provide greater patient satisfaction.48,49 Longevity is used in studies as an endpoint of efficacy. In clinical practice, a more qualitative focus on functional and aesthetic appropriateness of the results may serve patients better. With counseling, the philosophy of more frequent visits to achieve and maintain consistently good, rather than overdone, results is readily understood by patients by extrapolation from other health and beauty arenas.
Response to Repeated Treatments with Botulinum Toxin Type A
The response rate to botulinum toxin type A for aesthetic applications is very high. In cases of apparent nonresponse or partial response to any toxin formulation, practitioners should first consider causes such as inappropriate patient selection, dosing, or placement of injection sites. If volume loss is a significant contributor to rhytides, they will respond better to combined fillers and toxin than to toxin alone. It is obvious that underdosing or incorrect placement of toxin in target muscles can impair treatment response. Overdosing with resultant recruitment of adjacent muscles can give the illusion of nonresponse, as can injection into muscles that are not the primary cause of what needs to be addressed.
From an evidence-based perspective, it is challenging to evaluate reports of secondary treatment failure with toxin, because they are typically anecdotal and retrospective. Pertinent details are likely to be missing if the practitioner who is consulted for nonresponse did not perform the treatment. Although a number of case reports describe secondary treatment failure in the presence of neutralizing antibodies, there is no high-level evidence to implicate antibodies as the actual cause.50 Table 2 summarizes challenges in interpreting current data.51–56 Ongoing monitoring of the increasing number of patients who receive repeated treatments will allow conclusions that are evidence-based, rather than circumstantial.
Table 2.
Secondary Treatment Failure with Botulinum Toxin Type A for Aesthetic Indications: Evidence-Based and Experiential Analysis

Application of Science to Optimize Clinical Efficacy and Safety
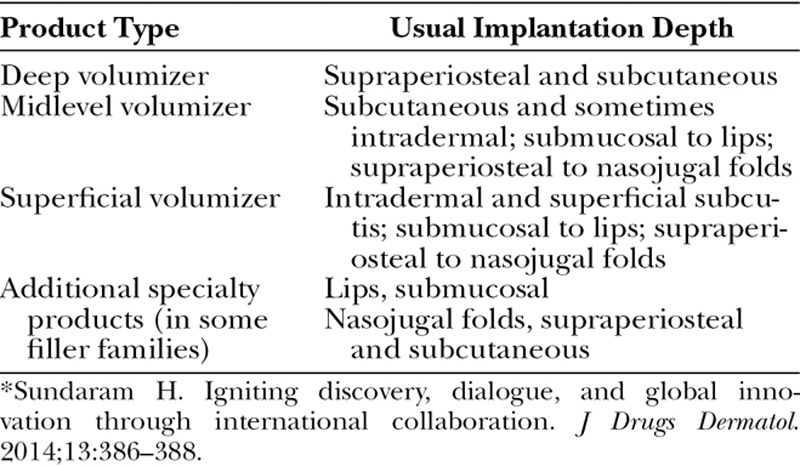
New and emerging hyaluronic acid fillers are arranged in families that are designed for layered tissue implantation. Examples include cohesive polydensified matrix (Belotero; Merz, Frankfurt am Main, Germany), Optimal Balance Technology (Emervel; Galderma, Lausanne, Switzerland), Resilient Hyaluronic Acid (Teosyal; Teoxane, Geneva, Switzerland), and Vycross (Allergan). Table 3 summarizes typical products in a filler family.57 Complete families are available in Europe, Canada, Latin America, and parts of Asia-Pacific and the Middle East. The United States and other regions currently have partial families.
Table 3.
Typical Products in a Hyaluronic Acid Filler Family Designed for Layered Implantation, and Their Usual Implantation Depths*
The intended clinical applications of each product are informed by its rheologic (flow-related) properties, which depend on its manufacturing process.58,59 Deep volumizers typically have higher elasticity (G′), to confer firmness and resistance to applied force; and higher viscosity, to confer resistance to spread. For example, deep volumizer Vycross (Voluma) has higher elasticity than midlevel Vycross (Volift), which has higher elasticity than the superficial volumizer (Volbella).59–61 Elasticity is higher in Vycross than in Hylacross products.59,61 Complex viscosity (η*), which accurately measures whole gel behavior, is higher in deep volumizer Vycross than in Hylacross.61
Science-based selection of filler products and injection techniques allows a more evidence-based approach toward safety and efficacy. Treatment outcomes can be predicted by a product’s scientific design, in the context of its target tissue and the techniques that are used to implant it.57 Two controlled, split-face studies of Evidence Level II fulfill the rheologic prediction that optimal nasolabial fold correction requires a significantly smaller volume of a higher than a lower elasticity filler.62,63 Histopathologic and ultrasonographic studies directly correlate a filler’s viscosity and cohesivity to its pattern of spread and tissue integration after in vivo, intradermal implantation.64–66
The initial focus was on elasticity as a primary determinant of tissue “lift” when fillers were implanted deeply as boluses. It was subsequently proposed that the “lift capacity” of a hyaluronic acid filler is determined not only by elasticity but also by cohesivity.67 A certain level of cohesivity (i.e., the tendency of the filler not to dissociate because of affinity of its molecules for each other) can be considered a prerequisite to maintain filler integrity during and after implantation. A standardized, five-point visual scale for hyaluronic acid filler cohesivity was recently developed and validated.68 Convergence of these data with seminal studies of facial fat compartments and bony anatomy11–16,69–73 elucidates the primary objective of volume replacement as inflation of age-deflated soft tissues, and thus restoration of three-dimensional tissue support. A filler’s elasticity, cohesivity, and water binding all contribute to this multilevel process. It is a composite of layered tissue expansion, with vertical and horizontal vectors; and tissue projection, with more vertical, bolus-type vectoring predominantly from deep tissues.22,61
The scientific balance of each hyaluronic acid product parallels its clinical characteristics. Higher elasticity increases tissue projection by providing firmness and resistance to muscular and gravitational forces. Cohesivity confers more three-dimensional tissue expansion. As an example of how scientific balance influences clinical behavior, it is instructive to compare Vycross and Hylacross fillers. The lower cohesivity of Vycross has been described as providing more malleability.60 The lower water uptake of Vycross, as measured by in vitro gel swelling assay, is attributed to tighter hyaluronic acid cross-linking.60 Additional factors contribute to tissue swelling after in vivo implantation of hyaluronic acid fillers. Dilution-reconstitution of Hylacross41 decreases tissue swelling, presumably because of preinjection saturation of some water-binding sites.
In vitro resistance of Hylacross filler to degradation by ovine testicular hyaluronidase has been attributed to its cross-linking, cohesivity, and total hyaluronic acid concentration.74 The longevity of Vycross filler is attributed to cross-linking, independent of concentration.60 From a safety perspective, it is noteworthy that these fillers are degraded transarterially after implantation into cadaveric arteries—by exogenous hyaluronidase injected into surrounding soft tissue, or by immersion of closed arterial segments in hyaluronidase at therapeutic doses.75 [See Video, Supplemental Digital Content 1, which demonstrates transarterial degradation of Vycross and Hylacross fillers, by injection of exogenous hyaluronidase into the surrounding soft tissues, in a fresh, frozen cadaver model in real time. In section 1 of this video, dyed Hylacross filler (Juvéderm Ultra) is injected into the facial artery lateral to the oral commissure, and product flows to the mid cheek. Digital pressure on the lower part of the vessel demonstrates that the filler gel distends and completely occludes the vessel. Ovine hyaluronidase at a concentration of 150 IU/ml (Hyalase; Sanofi-Aventis, Gentilly, France) is infiltrated around, but not directly into, the vessel containing Hylacross filler. Digital pressure and simulated tissue massage are applied. The Hylacross filler within the artery is depolymerized because of transarterial enzymatic action of the hyaluronidase. Both the vascular distention and obstruction are removed as the filler product is broken down and disperses. In section 2 of this video, dyed Vycross filler (Juvéderm Volift) is injected into the superficial temporal artery, and readily flows along the vessel to escape from the cut end of the vessel. Ovine hyaluronidase (150 IU/ml) infiltrated around, but not directly into, the artery breaks down and disperses the Vycross filler within it. In a more graphic demonstration of enzymatic depolymerization, droplets of hyaluronidase that are applied directly to extruded Vycross filler rapidly break it down and disperse it. In section 3 of this video, dyed Vycross filler (Juvéderm Volbella) is injected into the facial artery at the lower nasolabial fold, and extrudes from the cut end of a downstream arterial branch in the alar fold danger zone. This video demonstrates how injection of hyaluronic acid filler into a vessel of significant diameter can result in its spread to distant locations along the vascular tree of that vessel. The action of hyaluronidase is rapid and profound, despite infiltration into the surrounding soft tissue, rather than direct intravascular infiltration. This is demonstrated by dispersal of the filler within the vessel after a short period. Hyaluronidase also has a rapid effect when applied directly to hyaluronic acid filler lying free within the soft tissue. Courtesy of Dr. Mark Magnusson, Dr. Tim Papadopoulos, and the Australasian Society of Aesthetic Plastic Surgery. This content was developed for the Society’s Anatomical and Live Injecting Workshop, in association with the Australasian Society of Aesthetic Plastic Surgery Annual Non-Surgical Symposium. © 2014 Australasian Society of Aesthetic Plastic Surgery, available in the “Related Videos” section of the full-text article on PRSJournal.com or, for Ovid users, available at, http://links.lww.com/PRS/B683.]
Video.
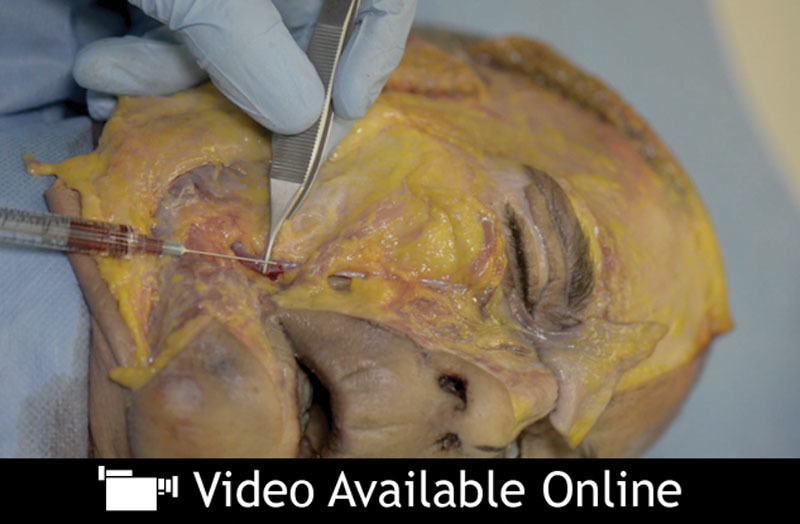
Supplemental Digital Content 1 demonstrates transarterial degradation of Vycross and Hylacross fillers, by injection of exogenous hyaluronidase into the surrounding soft tissues, in a fresh, frozen cadaver model in real time. Courtesy of Dr. Mark Magnusson, Dr. Tim Papadopoulos, and the Australasian Society of Aesthetic Plastic Surgery. This content was developed for the Society’s Anatomical and Live Injecting Workshop, in association with the Australasian Society of Aesthetic Plastic Surgery Annual Non-Surgical Symposium. © 2014 Australasian Society of Aesthetic Plastic Surgery, available in the “Related Videos” section of the full-text article on PRSJournal.com or, for Ovid users, available at, http://links.lww.com/PRS/B683.
CONSENSUS RECOMMENDATIONS FOR COMBINED TREATMENT
Figure 3 presents results of the premeeting panel surveys. Table 4 lists key points for the upper, mid, and lower face. Tables 5 through 7 provide consensus toxin dosages and representative filler product selections for combined treatment. The panel did not recommend specific changes in dosing or product selections compared to solo treatment, which is discussed in other Global Aesthetics Consensus Group publications.76,77 They noted that the frequency of re-treatment may decrease when fillers and toxin are combined. Table 8 summarizes consensus recommendations and position statements.78
Fig. 3.
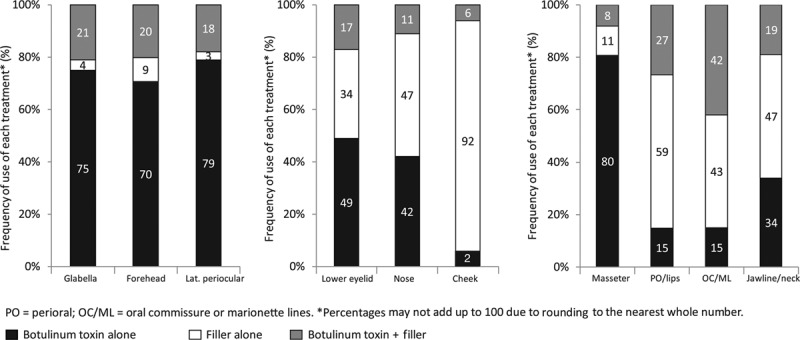
Consensus panel’s practice patterns in the (left) upper face, (center) midface, and (right) lower face, based on premeeting surveys. Percentage use of botulinum toxin alone, filler alone, and botulinum toxin plus filler are shown for each facial zone. Because percentages are rounded to the nearest whole number, they may not add up to 100 percent. PO, perioral; OC, oral commissure; ML, marionette lines.
Table 4.
Key Points from Premeeting Panel Survey and Consensus Proceedings Regarding the Upper Face, Midface, and Lower Face
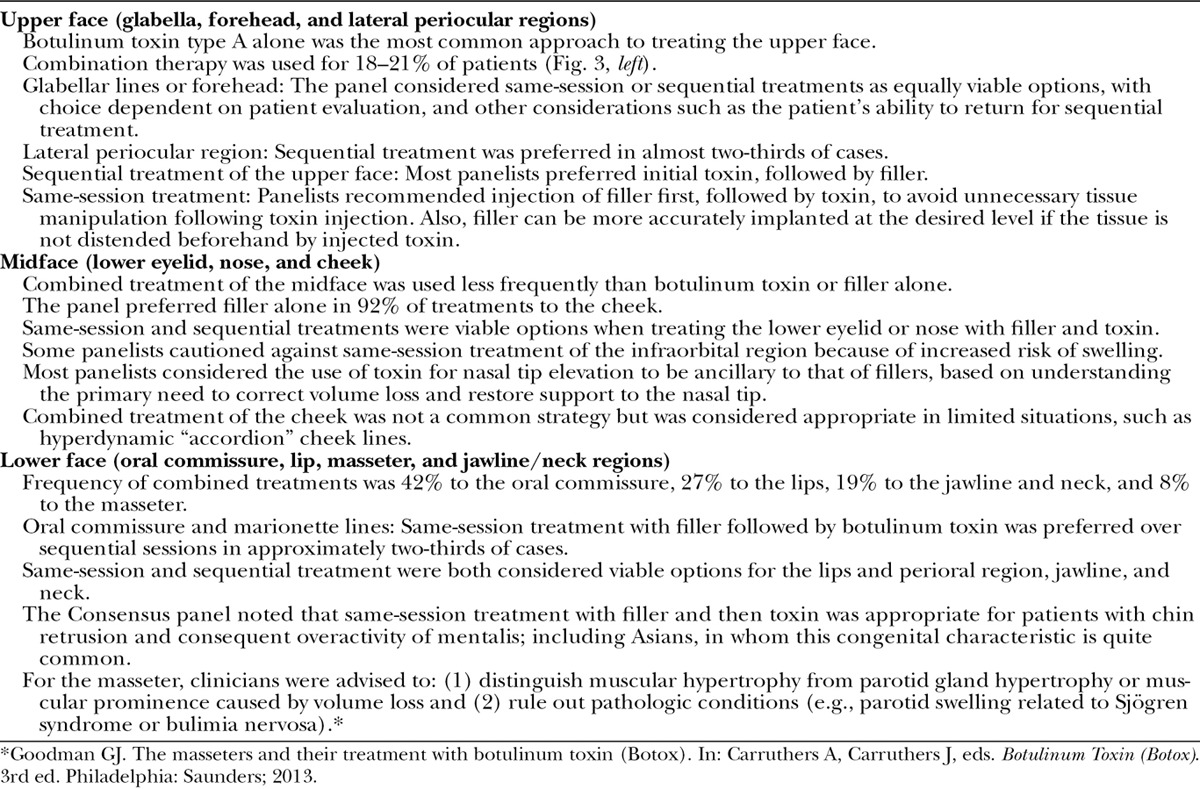
Table 5.
Consensus Recommendations and Expert Panel Opinion Regarding Combination Treatment of the Upper Face*
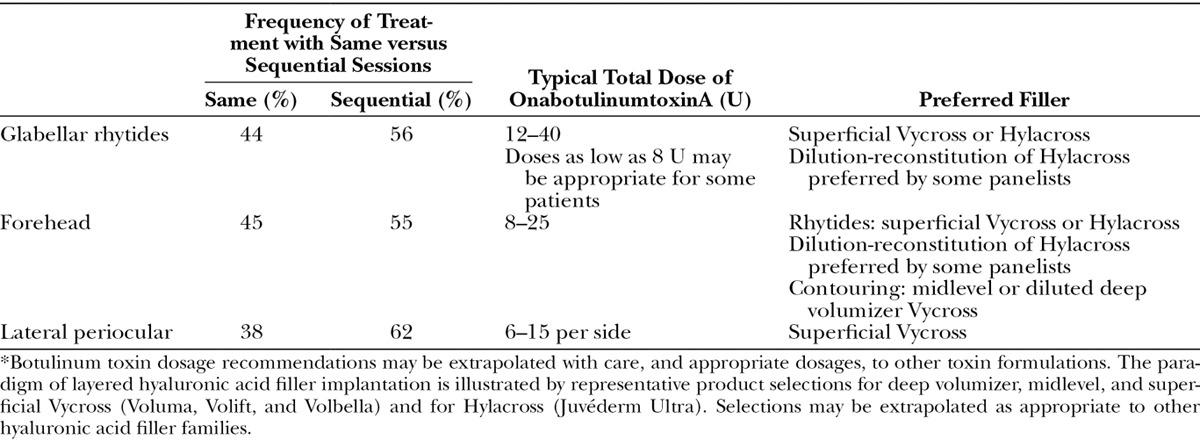
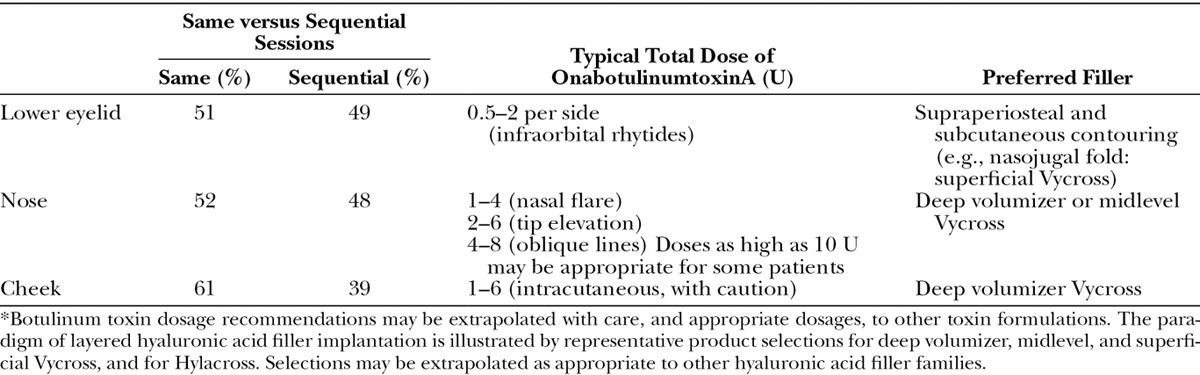
Table 7.
Consensus Recommendations and Expert Panel Opinion Regarding Combination Treatment of the Lower Face*
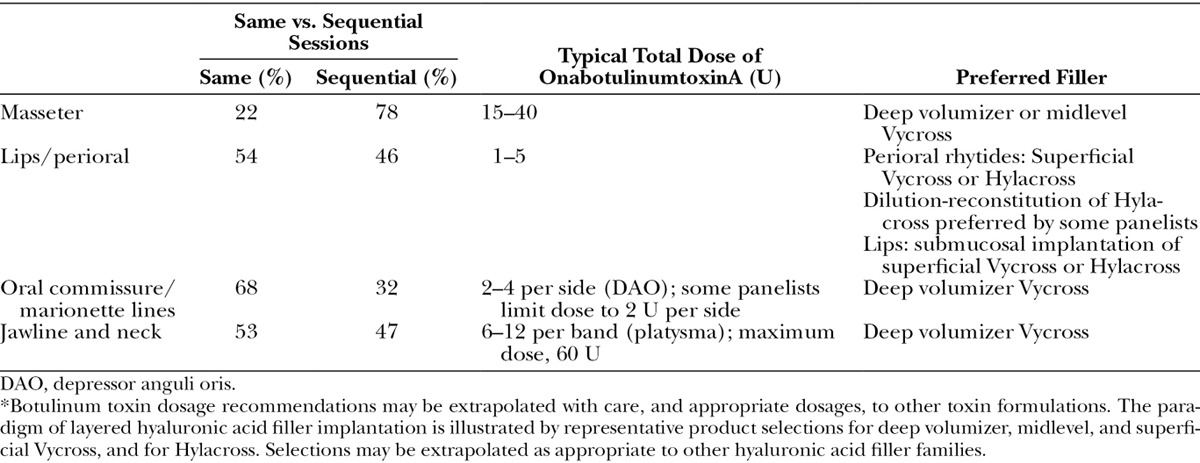
Table 8.
Global Aesthetics Consensus Group Recommendations and Position Statements for Combined Treatment with Hyaluronic Acid Fillers and Botulinum Toxin Type A in Diverse Patient Populations
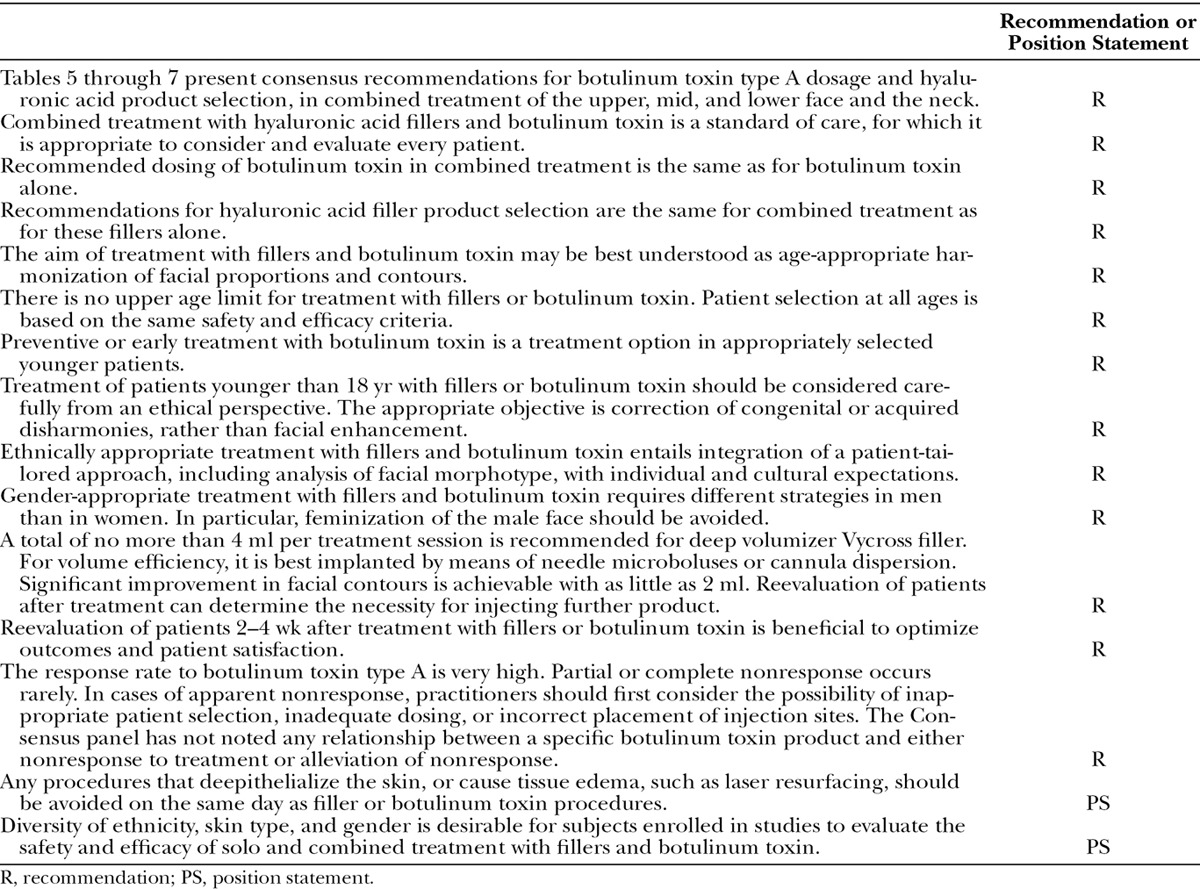
Table 6.
Consensus Recommendations and Expert Panel Opinion Regarding Combination Treatment of the Middle Face*
CONCLUSIONS
Combined treatment was originally advocated to address aspects of facial aging that were regarded as distinct. Volume restoration with fillers ameliorated facial folds and contours. Weakening of overcontracting muscles with botulinum toxin improved hyperdynamic rhytides. The lower face was viewed as most appropriate for combined treatments. The potential for synergy and importance of individualized treatment planning were recognized. The Global Aesthetics Consensus Group’s recommendations reflect growing use of combined treatment for the lower face, and also for the upper face and certain areas of the midface. The principle of individualization has been extended to a fully patient-tailored philosophy. This integrates considerations of physiologic and chronologic age, sex, ethnicity and its associated facial morphotypes, and cultural overlays. Lower dosing of botulinum toxin modulates the activity of excessively contracting muscles rather than paralyzing them. The contribution of volume deficits in the soft and hard tissues to muscular activity is increasingly recognized. Research continues to deepen our understanding of how scientific balances in hyaluronic acid fillers influence their indications and clinical performance.
The current rationale for combining fillers and toxin is that they address intimately related processes. When viewed from this new perspective, the identification of various patterns of muscular activity (e.g., in the formation of glabellar79 or nasal oblique lines80) has significant implications. Rather than serving as a mandate to target all overcontracting muscles with toxin, it provides the imperative to seek and carefully identify the primary cause of muscular recruitment. A focus on aesthetic units, rather than isolated stigmata of aging such as rhytides, is necessary to optimize results from both fillers and toxin. The evolving concepts discussed in this publication direct the clinician toward the creation of panfacial harmony and balance.
ACKNOWLEDGMENTS
The consensus meeting and editorial assistance for manuscript development were organized by Educational Awareness Solutions (Norwalk, Conn.) and supported by Allergan, Inc. (Irvine, Calif.). The authors express their deep appreciation to Daniel Cassuto, M.D., and Taro Kono, M.D., for expert guidance and input during development of this article; to Mark Magnusson, M.B.B.S., F.R.A.C.S., Tim Papadopoulos, M.B.B.S., F.R.A.C.S., and the Australasian Society of Aesthetic Plastic Surgery for the video content associated with this article; and to John F. Kross, M.Sc., D.M.D., of Educational Awareness Solutions, for editorial support.
APPENDIX: GLOBAL AESTHETICS CONSENSUS GROUP
The Global Aesthetics Consensus Group comprises the following members: André Vieira Braz, M.D. (dermatology), Rio de Janeiro, Brazil; Jean D. A. Carruthers, M.D. (ophthalmology), Vancouver, British Columbia, Canada; Koenraad L. De Boulle, M.D. (dermatology), Aalst, Belgium; Steven Fagien, M.D. (ophthalmic plastic surgery), Boca Raton, Florida; Greg J. Goodman, M.D. (dermatology), Carlton, Victoria, Australia; Soo-Keun Lee, M.D., Ph.D. (dermatology), Seoul, Republic of Korea; Steven Liew, M.B.B.S., F.R.A.C.S. (plastic surgery), Sydney, New South Wales, Australia; Gary Monheit, M.D. (dermatology), Birmingham, Alabama; Hervé Raspaldo, M.D. (facial plastic surgery), Cannes, France; Rod J. Rohrich, M.D. (plastic surgery), Dallas, Texas; Gerhard Sattler, M.D. (dermatology), Darmstadt, Germany; Massimo Signorini, M.D. (plastic surgery), Milan, Italy; Hema Sundaram, M.D. (dermatology), Rockville, Maryland; Arthur Swift, M.D., C.M. (plastic surgery), Montreal, Quebec, Canada; Ada R. Trindade de Almeida, M.D. (dermatology), Sao Paulo, Brazil; and Yan Wu, M.D., Ph.D. (dermatology), Beijing, People’s Republic of China.
Supplementary Material
Footnotes
Disclosure: André Vieira Braz, M.D. states that he is a consultant and speaker with Allergan, Galderma, and Palomar. Jean D. A. Carruthers, M.D. states that she is a consultant and researcher with Allergan, Merz and Kythera. Koenraad L. De Boulle, M.D. states that he is a consultant, member of the speaker’s board, and recipient of honoraria and grants for studies and lectures for Allergan; a consultant for Kythera and Genévrier; and a consultant and member of the speaker’s bureau for Johnson & Johnson. Steven Fagien, M.D. states that he is a consultant/investigator for Allergan, Galderma, Merz, Kythera and Aquavit. Greg J. Goodman, M.D. states that he has no financial interests that would conflict with the manuscript; he serves on advisory boards and speaker panels for Allergan, Galderma, and Merz that may be seen to have an interest in the outcome of a consensus document. Soo-Keun Lee, M.D. states that he is a consultant for Allergan. Steven Liew, F.R.A.C.S. states that he serves on advisory boards for Allergan, Galderma, and Kythera and has received honoraria from Allergan and Galderma for delivering local and international workshops and lectures and trials. Gary Monheit, M.D. states that he is a consultant and clinical investigator for Allergan Corporation (Juvéderm), Ipsen/Medicis (Dysport), MELA Sciences, Inc. (MelaFind), Revance, Kythera, Galderma, Mentor, and Merz; a consultant for Myoscience and Qmed; and a clinical investigator for Dermik Laboratories (Sculptra) and Contura (Aquamid). Hervé Raspaldo, M.D. states that he is a consultant for Allergan and Galderma. Rod Rohrich, M.D. states that he receives instrument royalties from Eriem Surgical, Inc and book royalties from CRC Press and Taylor and Francis Publishing. Gerhard Sattler, M.D. states that he is a clinical investigator for Allergan, Galderma, Merz, Regeneron and Novartis; a participant in advisory board meetings for Allergan, Galderma and Merz; and a speaker for Allergan, Galderma and Merz. Massimo Signorini, M.D. states that he is a medical expert for and takes part in advisory boards and workshops for Allergan, Inc. Hema Sundaram, M.D. states that she serves as a clinical investigator and/or consultant for Allergan, CosmoFrance, Evolus/Strathspey Crown, Galderma, HaoHai Healthcare, IBSA, Kythera, Merz, and Teoxane. Arthur Swift, M.D. states that he is a speaker, clinical researcher and advisor to Allergan, Galderma and Merz. Ada R. Trindade de Almeida, M.D. states that she is an advisor for Allergan, Merz, Galderma, Roc and Mantecorp and has participated in clinical trials for Allergan. Dr. Trindade is also a speaker for Allergan and Theraskin. Yan Wu, M.D. states that she serves as a clinical investigator for Allergan, GlaxoSmithKline, Lanzhou Biological Products Institute and Freda Biopharm (Shandong hyaluronic acid filler).
Supplemental digital content is available for this article. Direct URL citations appear in the text; simply type the URL address into any Web browser to access this content. Clickable links to the material are provided in the HTML text of this article on the Journal’s Web site (www.PRSJournal.com).
REFERENCES
- 1.Custis T, Beynet D, Carranza D, Greco J, Lask GP, Kim J. Comparison of treatment of melomental fold rhytides with cross-linked hyaluronic acid combined with onabotulinumtoxina and cross-linked hyaluronic acid alone. Dermatol Surg. 2010;36(Suppl 3):1852–1858. doi: 10.1111/j.1524-4725.2010.01741.x. [DOI] [PubMed] [Google Scholar]
- 2.Carruthers A, Carruthers J, Monheit GD, Davis PG, Tardie G. Multicenter, randomized, parallel-group study of the safety and effectiveness of onabotulinumtoxinA and hyaluronic acid dermal fillers (24-mg/ml smooth, cohesive gel) alone and in combination for lower facial rejuvenation. Dermatol Surg. 2010;36(Suppl 4):2121–2134. doi: 10.1111/j.1524-4725.2010.01705.x. [DOI] [PubMed] [Google Scholar]
- 3.Carruthers JD, Glogau RG, Blitzer A Facial Aesthetics Consensus Group Faculty. Advances in facial rejuvenation: Botulinum toxin type a, hyaluronic acid dermal fillers, and combination therapies. Consensus recommendations. Plast Reconstr Surg. 2008;121(Suppl):5S–30S; quiz 31S. doi: 10.1097/PRS.0b013e31816de8d0. [DOI] [PubMed] [Google Scholar]
- 4.Raspaldo H, Baspeyras M, Bellity P, et al. Consensus Group. Upper- and mid-face anti-aging treatment and prevention using onabotulinumtoxin A: The 2010 multidisciplinary French consensus. Part 1. J Cosmet Dermatol. 2011;10:36–50. doi: 10.1111/j.1473-2165.2010.00544.x. [DOI] [PubMed] [Google Scholar]
- 5.Raspaldo H, Niforos FR, Gassia V, et al. Consensus Group. Lower-face and neck antiaging treatment and prevention using onabotulinumtoxin A: The 2010 multidisciplinary French consensus. Part 2. J Cosmet Dermatol. 2011;10:131–149. doi: 10.1111/j.1473-2165.2011.00560.x. [DOI] [PubMed] [Google Scholar]
- 6.Carruthers J, Fournier N, Kerscher M, Ruiz-Avila J, Trindade de Almeida AR, Kaeuper G. The convergence of medicine and neurotoxins: A focus on botulinum toxin type A and its application in aesthetic medicine. A global, evidence-based botulinum toxin consensus education initiative: Part II. Incorporating botulinum toxin into aesthetic clinical practice. Dermatol Surg. 2013;39:510–525. doi: 10.1111/dsu.12148. [DOI] [PubMed] [Google Scholar]
- 7.American Society for Aesthetic Plastic Surgery. 2014 cosmetic surgery national data bank statistics. Available at: http://www.surgery.org/sites/default/files/2014-Stats.pdf. Accessed June 21, 2015. [DOI] [PubMed]
- 8.American Society for Dermatologic Surgery. ASDS survey: Skin cancer, cosmetic procedures jump 22 percent in 2013. 2013 ASDS survey on dermatologic procedures. Available at: https://www.asds.net/_Media.aspx?id=7744. Accessed December 23, 2014.
- 9.Surgical Aesthetics: The Business of Cosmetic Plastic Surgery. ISAPS global statistics on cosmetic procedures released. Available at: http://surgicalaestheticsmagazine.com/isaps-global-statistics-cosmetic-procedures-released. Accessed September 2, 2014.
- 10.American Society of Plastic Surgeons. 2015 plastic surgery statistics report. Available at: http://www.plasticsurgery.org/Documents/news-resources/statistics/2015-statistics/cosmetic-procedure-trends-2015.pdf. Accessed March 15, 2016.
- 11.Coleman SR, Grover R. The anatomy of the aging face: Volume loss and changes in 3-dimensional topography. Aesthet Surg J. 2006;26(Suppl):S4–S9. doi: 10.1016/j.asj.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Mendelson B, Wong CH. Changes in the facial skeleton with aging: Implications and clinical applications in facial rejuvenation. Aesthetic Plast Surg. 2012;36:753–760. doi: 10.1007/s00266-012-9904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw RB, Jr, Katzel EB, Koltz PF, Kahn DM, Girotto JA, Langstein HN. Aging of the mandible and its aesthetic implications. Plast Reconstr Surg. 2010;125:332–342. doi: 10.1097/PRS.0b013e3181c2a685. [DOI] [PubMed] [Google Scholar]
- 14.Lambros V. Models of facial aging and implications for treatment. Clin Plast Surg. 2008;35:319–327. doi: 10.1016/j.cps.2008.02.012. discussion 317. [DOI] [PubMed] [Google Scholar]
- 15.Lambros V. Observations on periorbital and midface aging. Plast Reconstr Surg. 2007;120:1367–1376. doi: 10.1097/01.prs.0000279348.09156.c3. discussion 1377. [DOI] [PubMed] [Google Scholar]
- 16.Pessa JE, Zadoo VP, Yuan C, et al. Concertina effect and facial aging: Nonlinear aspects of youthfulness and skeletal remodeling, and why, perhaps, infants have jowls. Plast Reconstr Surg. 1999;103:635–644. doi: 10.1097/00006534-199902000-00042. [DOI] [PubMed] [Google Scholar]
- 17.Albert AM, Ricanek K, Jr, Patterson E. A review of the literature on the aging adult skull and face: Implications for forensic science research and applications. Forensic Sci Int. 2007;172:1–9. doi: 10.1016/j.forsciint.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Goodman GJ, Roberts S. “Home of Younger Skin” (HOYS) program: Defining the change in apparent skin age after facial treatment with botulinum toxin and dermal fillers. Clin Cosmet Investig Dermatol. 2012;5:93–99. doi: 10.2147/CCID.S34705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundaram H, Mehta RC, Norine JA, et al. Topically applied physiologically balanced growth factors: A new paradigm of skin rejuvenation. J Drugs Dermatol. 2009;8(5 Suppl Skin Rejuenation):4–13. [PubMed] [Google Scholar]
- 20.Schwartz E, Cruickshank FA, Christensen CC, Perlish JS, Lebwohl M. Collagen alterations in chronically sun-damaged human skin. Photochem Photobiol. 1993;58:841–844. doi: 10.1111/j.1751-1097.1993.tb04981.x. [DOI] [PubMed] [Google Scholar]
- 21.Yezhelyev MV, Gupta V, Winocour J, Shack BR, Grotting JC, Higdon KK. Safety of cosmetic procedures in elderly and octogenarian patients. [Abstract] Plast Reconstr Surg. 2014;134(Suppl 1):98. [Google Scholar]
- 22.Sundaram H, Kiripolsky M. Nonsurgical rejuvenation of the upper eyelid and brow. Clin Plast Surg. 2013;40:55–76. doi: 10.1016/j.cps.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Keaney TC, Alster TS. Botulinum toxin in men: Review of relevant anatomy and clinical trial data. Dermatol Surg. 2013;39:1434–1443. doi: 10.1111/dsu.12302. [DOI] [PubMed] [Google Scholar]
- 24.Dao H, Jr, Kazin RA. Gender differences in skin: A review of the literature. Gend Med. 2007;4:308–328. doi: 10.1016/s1550-8579(07)80061-1. [DOI] [PubMed] [Google Scholar]
- 25.Giacomoni PU, Mammone T, Teri M. Gender-linked differences in human skin. J Dermatol Sci. 2009;55:144–149. doi: 10.1016/j.jdermsci.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Little AC, Jones BC, Waitt C, et al. Symmetry is related to sexual dimorphism in faces: Data across culture and species. PLoS One. 2008;3:e2106. doi: 10.1371/journal.pone.0002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumann LS, Shamban AT, Lupo MP, et al. Juvederm vs. Zyplast Nasolabial Fold Study Group. Comparison of smooth-gel hyaluronic acid dermal fillers with cross-linked bovine collagen: A multicenter, double-masked, randomized, within-subject study. Dermatol Surg. 2007;33(Suppl 2):S128–S135. doi: 10.1111/j.1524-4725.2007.33352.x. [DOI] [PubMed] [Google Scholar]
- 28.Grimes PE, Shabazz D. A four-month randomized, double-blind evaluation of the efficacy of botulinum toxin type A for the treatment of glabellar lines in women with skin types V and VI. Dermatol Surg. 2009;35:429–435. doi: 10.1111/j.1524-4725.2009.01063.x. discussion 435. [DOI] [PubMed] [Google Scholar]
- 29.Grimes PE, Thomas JA, Murphy DK. Safety and effectiveness of hyaluronic acid fillers in skin of color. J Cosmet Dermatol. 2009;8:162–168. doi: 10.1111/j.1473-2165.2009.00457.x. [DOI] [PubMed] [Google Scholar]
- 30.Downie JB, Grimes PE, Callender VD. A multicenter study of the safety and effectiveness of hyaluronic acid with a cohesive polydensified matrix for treatment of nasolabial folds in subjects with Fitzpatrick skin types IV, V, and VI. Plast Reconstr Surg. 2013;132(Suppl 2):41S–47S. doi: 10.1097/PRS.0b013e318299ff53. [DOI] [PubMed] [Google Scholar]
- 31.Farkas LG, Forrest CR, Litsas L. Revision of neoclassical facial canons in young adult Afro-Americans. Aesthetic Plast Surg. 2000;24:179–184. doi: 10.1007/s002660010029. [DOI] [PubMed] [Google Scholar]
- 32.Milgrim LM, Lawson W, Cohen AF. Anthropometric analysis of the female Latino nose: Revised aesthetic concepts and their surgical implications. Arch Otolaryngol Head Neck Surg. 1996;122:1079–1086. doi: 10.1001/archotol.1996.01890220045008. [DOI] [PubMed] [Google Scholar]
- 33.Husein OF, Sepehr A, Garg R, et al. Anthropometric and aesthetic analysis of the Indian American woman’s face. J Plast Reconstr Aesthet Surg. 2010;63:1825–1831. doi: 10.1016/j.bjps.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 34.Sim RS, Smith JD, Chan AS. Comparison of the aesthetic facial proportions of southern Chinese and white women. Arch Facial Plast Surg. 2000;2:113–120. doi: 10.1001/archfaci.2.2.113. [DOI] [PubMed] [Google Scholar]
- 35.Porter JP, Lee JI. Facial analysis: Maintaining ethnic balance. Facial Plast Surg Clin North Am. 2002;10:343–349. doi: 10.1016/s1064-7406(02)00030-5. [DOI] [PubMed] [Google Scholar]
- 36.Le TT, Farkas LG, Ngim RC, Levin LS, Forrest CR. Proportionality in Asian and North American Caucasian faces using neoclassical facial canons as criteria. Aesthetic Plast Surg. 2002;26:64–69. doi: 10.1007/s00266-001-0033-7. [DOI] [PubMed] [Google Scholar]
- 37.Sundaram H, Huang PH, Hsu NJ, et al. Aesthetic applications of botulinum toxin type A in Asians: An international, multidisciplinary pan-Asian consensus. Plast Reconstr Surg Glob Open. doi: 10.1097/GOX.0000000000000507. (manuscript in preparation) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor SC. Skin of color: Biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(2 Suppl XXXUnderstanding):S41–S62. doi: 10.1067/mjd.2002.120790. [DOI] [PubMed] [Google Scholar]
- 39.Rawlings AV. Ethnic skin types: Are there differences in skin structure and function? Int J Cosmet Sci. 2006;28:79–93. doi: 10.1111/j.1467-2494.2006.00302.x. [DOI] [PubMed] [Google Scholar]
- 40.Trindade de Almeida A, Carruthers J, Cox SE, Goldman MP, Wheeler S, Gallagher CJ. Patient satisfaction and safety with aesthetic onabotulinumtoxinA after at least 5 years: A retrospective cross-sectional analysis of 4,402 glabellar treatments. Dermatol Surg. 2015;41(Suppl 1):S19–S28. doi: 10.1097/DSS.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 41.Fagien S, Cassuto D. Reconstituted injectable hyaluronic acid: Expanded applications in facial aesthetics and additional thoughts on the mechanism of action in cosmetic medicine. Plast Reconstr Surg. 2012;130:208–217. doi: 10.1097/PRS.0b013e318254b3f6. [DOI] [PubMed] [Google Scholar]
- 42.Paliwal S, Fagien S, Sun X, Holt T, Van Epps D, Messina DJ. Skin extracellular matrix (ECM) stimulation following injection of a hyaluronic acid–based dermal filler in a rat model.. Poster presented at: American Society for Dermatologic Surgery (ASDS) Annual Meeting; October 3–6, 2013; Chicago, Ill. [Google Scholar]
- 43.Bonaparte JP, Ellis D. Skin biomechanical changes after injection of onabotulinum toxin A: Prospective assessment of elasticity and pliability. Otolaryngol Head Neck Surg. 2014;150:949–955. doi: 10.1177/0194599814526558. [DOI] [PubMed] [Google Scholar]
- 44.Chang SP, Tsai HH, Chen WY, Lee WR, Chen PL, Tsai TH. The wrinkles soothing effect on the middle and lower face by intradermal injection of botulinum toxin type A. Int J Dermatol. 2008;47:1287–1294. doi: 10.1111/j.1365-4632.2008.03895.x. [DOI] [PubMed] [Google Scholar]
- 45.Shah AR. Use of intradermal botulinum toxin to reduce sebum production and facial pore size. J Drugs Dermatol. 2008;7:847–850. [PubMed] [Google Scholar]
- 46.Rose AE, Goldberg DJ. Safety and efficacy of intradermal injection of botulinum toxin for the treatment of oily skin. Dermatol Surg. 2013;39:443–448. doi: 10.1111/dsu.12097. [DOI] [PubMed] [Google Scholar]
- 47.Kurzen H, Schallreuter KU. Novel aspects in cutaneous biology of acetylcholine synthesis and acetylcholine receptors. Exp Dermatol. 2004;13(Suppl 4):27–30. doi: 10.1111/j.1600-0625.2004.00258.x. [DOI] [PubMed] [Google Scholar]
- 48.Lowe NJ, Ascher B, Heckmann M, Kumar C, Fraczek S, Eadie N Botox Facial Aesthetics Study Team. Double-blind, randomized, placebo-controlled, dose-response study of the safety and efficacy of botulinum toxin type A in subjects with crow’s feet. Dermatol Surg. 2005;31:257–262. doi: 10.1111/j.1524-4725.2005.31070. [DOI] [PubMed] [Google Scholar]
- 49.Kowalski J, Ravelo A, Saulay M, Fraczek S. Patient self-perceptions and satisfaction with botulinum toxin type-A treatment for moderate to severe crow’s feet: Results from a placebo-controlled clinical study.. Paper presented at: 63rd Annual Meeting of the American Academy of Dermatology; February 18–22, 2005; New Orleans, La. [Google Scholar]
- 50.Torres S, Hamilton M, Sanches E, Starovatova P, Gubanova E, Reshetnikova T. Neutralizing antibodies to botulinum neurotoxin type A in aesthetic medicine: Five case reports. Clin Cosmet Investig Dermatol. 2013;7:11–17. doi: 10.2147/CCID.S51938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benecke R. Clinical relevance of botulinum toxin immunogenicity. BioDrugs. 2012;26:e1–e9. doi: 10.2165/11599840-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamm C, Benecke R. Individualized management of cervical dystonia with different serotypes of botulinum toxin: Recent therapeutic advances and risk development of neutralizing antibodies. Eur Neurol J. 2010;2:49–54. [Google Scholar]
- 53.Naumann M, Carruthers A, Carruthers J, et al. Meta-analysis of neutralizing antibody conversion with onabotulinumtoxinA (Botox) across multiple indications. Mov Disord. 2010;25:2211–2218. doi: 10.1002/mds.23254. [DOI] [PubMed] [Google Scholar]
- 54.Jankovic J, Vuong KD, Ahsan J. Comparison of efficacy and immunogenicity of original versus current botulinum toxin in cervical dystonia. Neurology. 2003;60:1186–1188. doi: 10.1212/01.wnl.0000055087.96356.bb. [DOI] [PubMed] [Google Scholar]
- 55.Yablon SA, Brashear A, Gordon MF, et al. Formation of neutralizing antibodies in patients receiving botulinum toxin type A for treatment of poststroke spasticity: A pooled-data analysis of three clinical trials. Clin Ther. 2007;29:683–690. doi: 10.1016/j.clinthera.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 56.Aoki KR, Merlino G, Spanoyannis A, Wheeler L. Botox (botulinum toxin type A) purified neurotoxin complex prepared from the new bulk toxin retains the same preclinical efficacy as the original but with reduced immunogenicity.. Poster presented at: 51st Annual Meeting of the American Academy of Neurology; April 17–24, 1999; Toronto, Ontario, Canada. Poster 06.109. [Google Scholar]
- 57.Sundaram H. Igniting discovery, dialogue, and global innovation through international collaboration. J Drugs Dermatol. 2014;13:386–388. [PubMed] [Google Scholar]
- 58.Kablik J, Monheit GD, Yu L, Chang G, Gershkovich J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35(Suppl 1):302–312. doi: 10.1111/j.1524-4725.2008.01046.x. [DOI] [PubMed] [Google Scholar]
- 59.Sundaram H, Voigts B, Beer K, Meland M. Comparison of the rheological properties of viscosity and elasticity in two categories of soft tissue fillers: Calcium hydroxylapatite and hyaluronic acid. Dermatol Surg. 2010;36(Suppl 3):1859–1865. doi: 10.1111/j.1524-4725.2010.01743.x. [DOI] [PubMed] [Google Scholar]
- 60.Pierre S, Liew S, Bernardin A. Basics of dermal filler rheology. Dermatol Surg. 2015;41(Suppl 1):S120–S126. doi: 10.1097/DSS.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 61.Sundaram H, Cassuto D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013;132(Suppl 2):5S–21S. doi: 10.1097/PRS.0b013e31829d1d40. [DOI] [PubMed] [Google Scholar]
- 62.Moers-Carpi M, Vogt S, Santos BM, Planas J, Vallve SR, Howell DJ. A multicenter, randomized trial comparing calcium hydroxylapatite to two hyaluronic acids for treatment of nasolabial folds. Dermatol Surg. 2007;33(Suppl 2):S144–S151. doi: 10.1111/j.1524-4725.2007.33354.x. [DOI] [PubMed] [Google Scholar]
- 63.Monheit GD, Baumann LS, Gold MH, et al. Novel hyaluronic acid dermal filler: Dermal gel extra physical properties and clinical outcomes. Dermatol Surg. 2010;36(Suppl 3):1833–1841. doi: 10.1111/j.1524-4725.2010.01780.x. [DOI] [PubMed] [Google Scholar]
- 64.Flynn TC, Sarazin D, Bezzola A, Terrani C, Micheels P. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg. 2011;37:637–643. doi: 10.1111/j.1524-4725.2010.01852.x. [DOI] [PubMed] [Google Scholar]
- 65.Micheels P, Besse S, Flynn TC, Sarazin D, Elbaz Y. Superficial dermal injection of hyaluronic acid soft tissue fillers: Comparative ultrasound study. Dermatol Surg. 2012;38:1162–1169. doi: 10.1111/j.1524-4725.2012.02471.x. [DOI] [PubMed] [Google Scholar]
- 66.Micheels P, Sundaram H, Besse S, et al. Ultrasonographic and histological comparative study of 3 hyaluronic acid gels.. Lecture presented at: International Master Class on Aging Skin (IMCAS) Annual Meeting; January 31–February 2, 2014; Paris, France. Poster 10911. [Google Scholar]
- 67.Borrell M, Leslie DB, Tezel A. Lift capabilities of hyaluronic acid fillers. J Cosmet Laser Ther. 2011;13:21–27. doi: 10.3109/14764172.2011.552609. [DOI] [PubMed] [Google Scholar]
- 68.Sundaram H, Rohrich RJ, Liew S, et al. Cohesivity of hyaluronic acid fillers: Development and clinical implications of a novel assay, pilot validation with a five-point grading scale, and evaluation of six U.S. Food and Drug Administration-approved fillers. Plast Reconstr Surg. 2015;136:678–686. doi: 10.1097/PRS.0000000000001638. [DOI] [PubMed] [Google Scholar]
- 69.Rohrich RJ, Pessa JE. Discussion: Aging of the facial skeleton: Aesthetic implications and rejuvenation strategies. Plast Reconstr Surg. 2011;127:384–385. doi: 10.1097/PRS.0b013e3181fad698. [DOI] [PubMed] [Google Scholar]
- 70.Rohrich RJ, Pessa JE, Ristow B. The youthful cheek and the deep medial fat compartment. Plast Reconstr Surg. 2008;121:2107–2112. doi: 10.1097/PRS.0b013e31817123c6. [DOI] [PubMed] [Google Scholar]
- 71.Rohrich RJ, Arbique GM, Wong C, Brown S, Pessa JE. The anatomy of suborbicularis fat: Implications for periorbital rejuvenation. Plast Reconstr Surg. 2009;124:946–951. doi: 10.1097/PRS.0b013e3181b17b76. [DOI] [PubMed] [Google Scholar]
- 72.Rohrich R, Pessa JE. The fat compartments of the face: Anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119:2219–2227. doi: 10.1097/01.prs.0000265403.66886.54. discussion 2228–2231. [DOI] [PubMed] [Google Scholar]
- 73.Rohrich RJ, Pessa JE. The anatomy and clinical implications of perioral submuscular fat. Plast Reconstr Surg. 2009;124:266–271. doi: 10.1097/PRS.0b013e3181811e2e. [DOI] [PubMed] [Google Scholar]
- 74.Jones D, Tezel A, Borrell M. In vitro resistance to degradation of hyaluronic acid dermal fillers by ovine testicular hyaluronidase. Dermatol Surg. 2010;36:804–809. [Google Scholar]
- 75.DeLorenzi C. Transarterial degradation of hyaluronic acid filler by hyaluronidase. Dermatol Surg. 2014;40:832–841. doi: 10.1097/DSS.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 76.Sundaram H, Signorini M, Liew S, et al. Global Aesthetics Consensus Group: Botulinum toxin type A. Evidence-based review, emerging concepts, and consensus recommendations for aesthetic use, including updates on complications. Plast Reconstr Surg. 2016;137:518e–529e. doi: 10.1097/01.prs.0000475758.63709.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liew S, Sundaram H, Carruthers J, et al. Global Aesthetics Consensus Group: Hyaluronic acid soft-tissue fillers: Evidence- and opinion-based review and consensus recommendations. Plast Reconstr Surg. doi: 10.1097/PRS.0000000000002184. (submitted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goodman GJ. The masseters and their treatment with botulinum toxin (Botox). In: Carruthers A, Carruthers J, editors. Botulinum Toxin (Botox) 3rd ed. Philadelphia: Saunders; 2013. [Google Scholar]
- 79.de Almeida AR, da Costa Marques ER, Banegas R, Kadunc BV. Glabellar contraction patterns: A tool to optimize botulinum toxin treatment. Dermatol Surg. 2012;38:1506–1515. doi: 10.1111/j.1524-4725.2012.02505.x. [DOI] [PubMed] [Google Scholar]
- 80.Tamura BM, Odo MY, Chang B, Cucé LC, Flynn TC. Treatment of nasal wrinkles with botulinum toxin. Dermatol Surg. 2005;31:271–275. doi: 10.1111/j.1524-4725.2005.31072. [DOI] [PubMed] [Google Scholar]


