Supplemental Digital Content is available in the text.
Keywords: Bangladesh, acute gastroenteritis, rotavirus, hospital-based surveillance, rotavirus vaccination
Abstract
Background:
In anticipation of introduction of a rotavirus vaccine into the national immunization program of Bangladesh, active hospital-based surveillance was initiated to provide prevaccine baseline data on rotavirus disease.
Methods:
Children 5 years of age and younger admitted with acute gastroenteritis (AGE) (≥3 watery or looser-than-normal stools or ≥1 episode of forceful vomiting) at 7 hospitals throughout Bangladesh were identified. Clinical information and stool specimens were collected from every 4th patient. Specimens were tested for rotavirus antigen by enzyme immunoassays; 25% of detected rotaviruses were genotyped.
Results:
From July 2012 to June 2015, rotavirus was detected in 2432 (64%) of 3783 children hospitalized for AGE. Eight enrolled children died, including 4 (50%) who were rotavirus positive. Rotavirus was detected year-round in Bangladesh with peak detection rates of >80% during November–February. Most (86%) rotavirus AGE cases were 6–23 months of age. Sixty-nine percent of children with rotavirus had severe disease (Vesikari score, ≥11). Among 543 strains genotyped, G1P[8] (31%) and G12P[8] (29%) were the most common.
Conclusions:
Rotavirus is a major cause of morbidity in Bangladeshi children, accounting for nearly two-thirds of AGE hospitalizations. These data highlight the potential value of rotavirus vaccination in Bangladesh, and will be the key for future measurement of vaccine impact.
Rotavirus, the most common cause of severe diarrhea in infants and young children, remains a significant cause of morbidity and mortality worldwide.1 In 2010, ~200,000 global diarrhea deaths among children 5 years of age and younger were attributed to rotavirus, with ~25% occurring in Southeast Asia.1 In Bangladesh, single-site studies have demonstrated a considerable burden of rotavirus on healthcare systems; rotavirus was the etiology of gastroenteritis in 20% of children seeking care in a diarrhea treatment facility in urban Dhaka,2 and in 33% of the hospitalizations because of gastroenteritis in rural Matlab.3
Two live attenuated oral rotavirus vaccines, RotaTeq (RV5; Merck, Whitehouse Station, NJ), a pentavalent (G1, G2, G3, G4, P[8]) bovine-human reassortant vaccine, and Rotarix (RV1; GSK Biologicals, Rixensart, Belgium), a monovalent (G1P[8]) human strain vaccine, are currently recommended by the World Health Organization for introduction into national immunization programs worldwide.4 A key factor in the decision by countries to introduce rotavirus vaccines has been demonstrating high rotavirus disease burden through establishment of surveillance for rotavirus diarrhea. In addition, prevaccine data on disease burden have been crucial for monitoring vaccine impact after vaccine introduction. Assessing vaccine impact in resource-limited settings is particularly important given the lower efficacy of RV1 and RV5 shown in clinical trials in Africa and Asia, compared with that seen in Europe and America.5–10
In anticipation of introduction of a rotavirus vaccine into the routine immunization schedule in Bangladesh, this study aims to provide baseline data on the burden of rotavirus gastroenteritis and circulating strains at sentinel hospitals nationwide. Because the most recent verbal autopsy data from the Bangladesh Demographic and Health Survey noted an 85% reduction in diarrhea-specific mortality between 2004 and 2011 (from 7 to 1 per 1000 live births),11 we specifically aimed at measuring the contribution of rotavirus to healthcare utilization.
MATERIALS AND METHODS
Starting in July 2012, an active hospital-based rotavirus surveillance system, consisting of 7 tertiary hospitals located in all 7 divisions of Bangladesh (Fig. 1), was initiated in 3 phases. These hospitals were chosen because they have a high number of pediatric gastroenteritis admissions each year. Rotavirus surveillance was started at the Dhaka, Rajshahi and Sylhet sites in July 2012, extended to Chittagong and Rangpur in February 2013, and further extended to Khulna and Barisal in August 2013. Surveillance followed the World Health Organization protocol for rotavirus surveillance in hospital settings.12 At each hospital, from 8:30 am to 4:00 pm each day (except for weekends and holidays), field assistants identified children 5 years of age and younger admitted to pediatric wards with diarrhoea by reviewing admission logbooks and screened them for acute gastroenteritis (AGE) symptoms. AGE was defined as the occurrence of ≥3 watery or looser-than-normal stools or ≥1 episode of forceful vomiting within a 24-hour period, with symptoms lasting ≤7 days. Surveillance physicians enrolled every 4th child listed who met the surveillance case definition. Compared with other sites, diarrhea-associated hospitalizations in Rangpur were low in number so, only at this site, the protocol was modified after 3 months to enroll every child admitted with AGE. Surveillance physicians collected demographic and clinical information of enrolled children from the parents and hospital records using a standard questionnaire and assessed the extent of dehydration following clinical criteria in World Health Organization diarrhea treatment guidelines.13 In addition, discharge and death logbooks were reviewed to ascertain the outcome of children admitted with diarrhoea but not enrolled in the surveillance.
FIGURE 1.
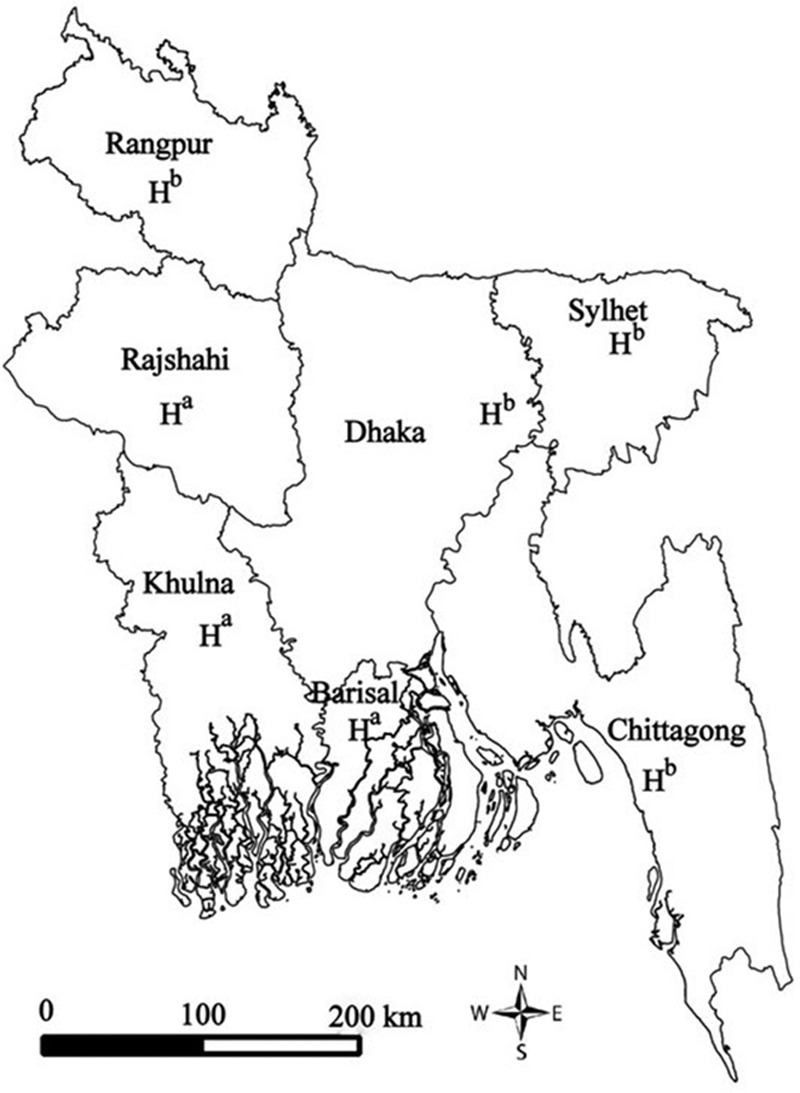
Locations of 7 sentinel hospitals where rotavirus surveillance was conducted in Bangladesh, July 2012 to June 2015. H represents the location of the hospital within each division. aPublic hospital; bprivate hospital.
Field assistants collected bulk stool specimens (4 mL) from each child on the day of enrollment and immediately stored specimens in a –70°C liquid nitrogen dry shipper after collection. The study team shipped stool samples in these containers to the International Centre for Diarrheal Disease Research (icddr,b) virology laboratory in Dhaka every 2 weeks, where a commercially available enzyme-linked immunoassay (EIA) (Prospect; Oxoid Diagnostics Ltd, Basingstoke Hants, United Kingdom) was used to test for rotavirus antigen. At icddr,b, stool samples were stored at –70°C. Every 3 months, G and P genotyping of ~25% of rotavirus-positive specimens were done using methods described previously.14 EIA, genotyping and sequencing have been done at icddr,b virology laboratory in Dhaka.
We obtained written informed consent from the enrolled children’s parents or guardians. The study protocol was reviewed and ethics approval obtained from the ethical review committee of icddr,b.
We calculated the overall and site-specific proportions of rotavirus-associated AGE hospitalizations by dividing the number of rotavirus-positive stools by the total number of samples collected and tested for rotavirus. To estimate the number of rotavirus-associated hospitalizations, the overall proportion of rotavirus-associated AGE hospitalizations was applied to the total number of AGE admissions during the surveillance period. We compared clinical and demographic characteristics and outcomes between children testing positive versus negative for rotavirus. This included an assessment for differences in clinical severity of AGE between the 2 groups, using the 20-point Vesikari scale; illnesses with scores <7 were classified as mild, illnesses with scores ≥7 and ≤10 as moderate and illnesses with scores ≥11 as severe.15 Proportions were compared using χ2 and Cochran–Armitage trend tests, and continuous variables were compared using the Wilcoxon rank sum test.
RESULTS
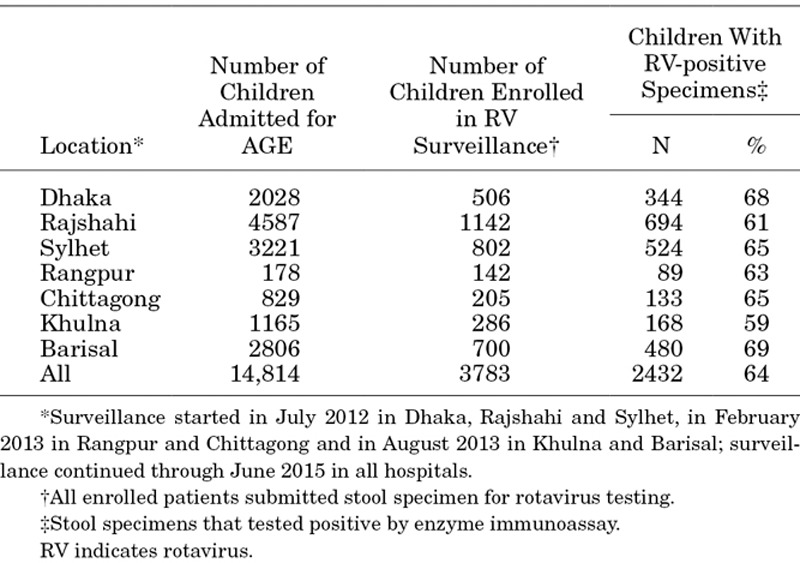
During 212 hospital months of surveillance, 129,156 children 5 years of age and younger were admitted to the pediatric wards of participating hospitals, of whom 14,814 (12%) were hospitalized with AGE. Surveillance staff collected clinical data and stool specimens from 26% (3783/14,814) of children admitted with AGE (Table 1). EIA testing identified rotavirus antigen in the stools of 64% (2432/3783) of enrolled children; detection rates varied by site from 59% to 69% (Table 1). Extrapolation of rotavirus-testing results to the untested AGE cases yielded an estimated total of 9728 rotavirus AGE admissions. Thus, about 8% (9728/129,156) of all pediatric admissions at participating hospitals were attributable to rotavirus-associated AGE during the study period.
TABLE 1.
Rotavirus Gastroenteritis Hospitalizations Among Children <5 Years of Age, by Site, in Bangladesh, July 2012 through June 2015
Nearly all (96%) of the rotavirus hospitalizations were among children <2 years (Fig. 2), with 57% occurring during the first year of life. Rotavirus hospitalizations occurred year-round, with rotavirus accounting for >10% of AGE admissions during every month of the year. Overall, rotavirus was detected in >80% of AGE cases during the rotavirus peak season (November–February) (Fig. 3). There were no differences in the seasonal patterns among surveillance sites (data not shown).
FIGURE 2.
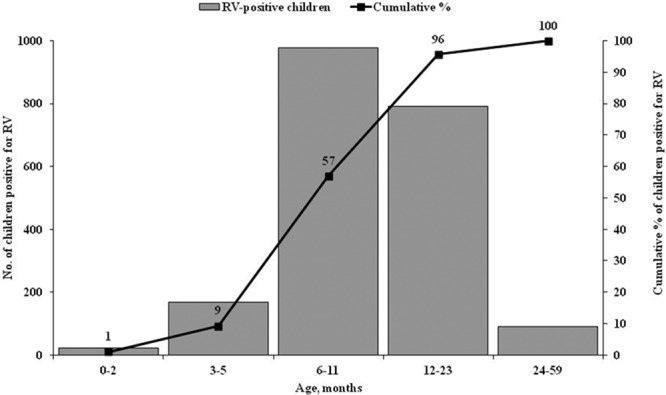
Age distribution of children <5 years of age (N = 2432) who were RV positive and admitted to 7 sentinel hospitals in Bangladesh, July 2012 to June 2015. The black line represents the cumulative percentage of hospitalizations of RV by the end of each of the designated age periods. RV indicates rotavirus.
FIGURE 3.
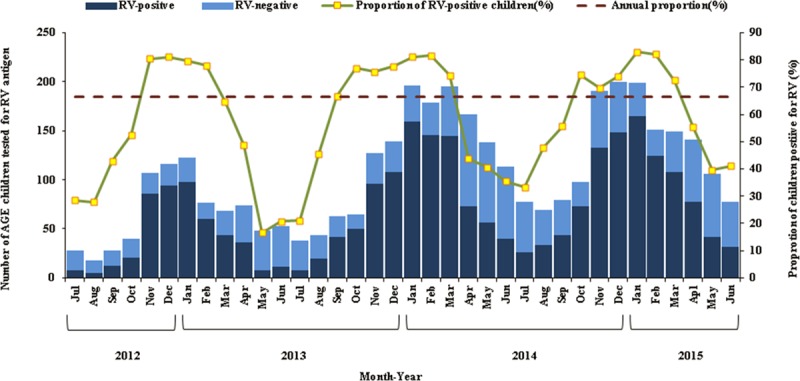
Seasonality of hospitalization (by month of admission) for RV AGE among children <5 years of age at 7 sentinel hospitals in Bangladesh, July 2012 to June 2015. The darker blue bars represent children with AGE in which RV was detected, and the lighter blue bars represent children with AGE in which RV was not detected. The small yellow squares represent the proportion (%) of children admitted for AGE that were RV-positive in the designated calendar month. The horizontal red-dashed line indicates the overall annual proportion of 63% (based on the sum of the monthly median numbers of RV-positive specimens and the sum of the monthly median number of specimens tested during the surveillance period). RV indicates rotavirus.
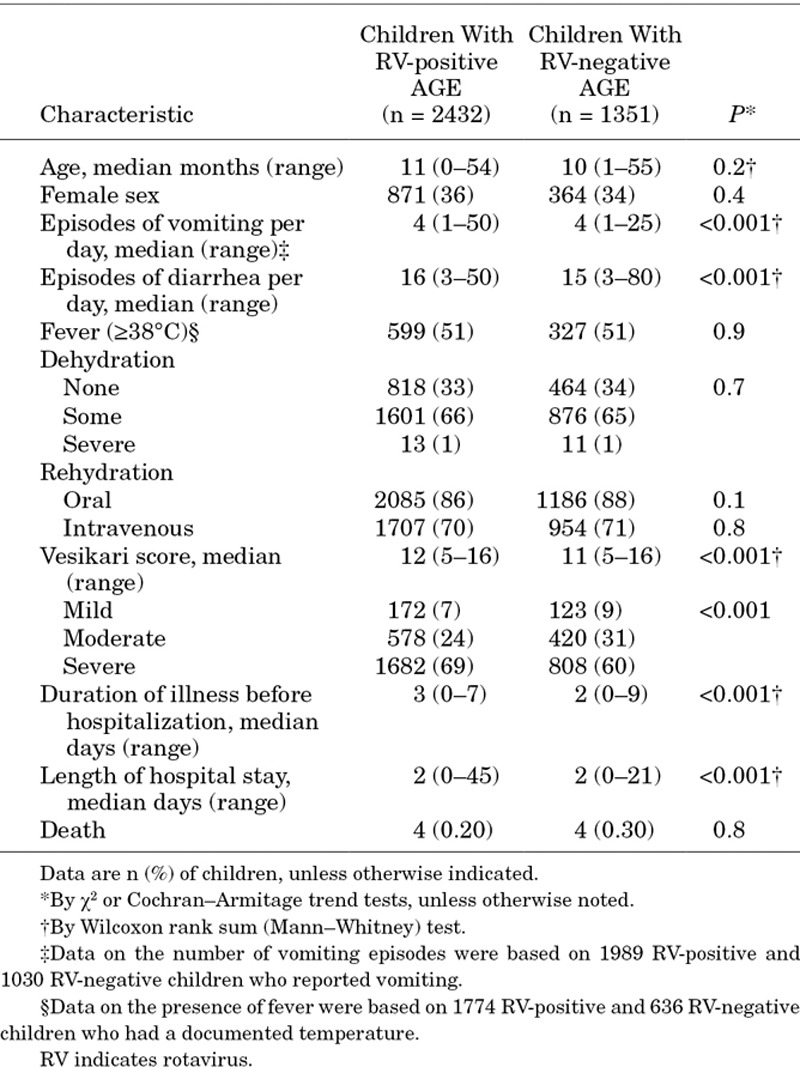
The median Vesikari score among children with rotavirus-confirmed AGE was slightly higher for those with compared to those without laboratory-confirmed rotavirus infection (12 vs. 11; P < 0.001). The duration of illness before hospitalization was longer for children with rotavirus infection compared with children who did not have evidence of rotavirus infection (3 vs. 2 days; P < 0.001), but that there were no differences in the length of hospital stay (2 days) (Table 2). Among the children enrolled over the study period, 8 (0.25%) died in the hospital. Rotavirus was detected in the stool of 50% (4/8) of those who died from AGE, and 3 of the 4 rotavirus-associated deaths occurred in children 6–23 months of age (the remaining death was in a 3-month old infant). According to discharge and death logbooks, 104 children were admitted with diarrhea and died in hospital during the study period; 69% were not be screened or enrolled because they were admitted after surveillance hours, or because death occurred soon after arrival at the hospital (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/C564)
TABLE 2.
Characteristics and Clinical Symptoms of RV-positive and -negative Children <5 Years of Age Hospitalized With AGE at 7 Sentinel Hospitals in Bangladesh, July 2012 to June 2015
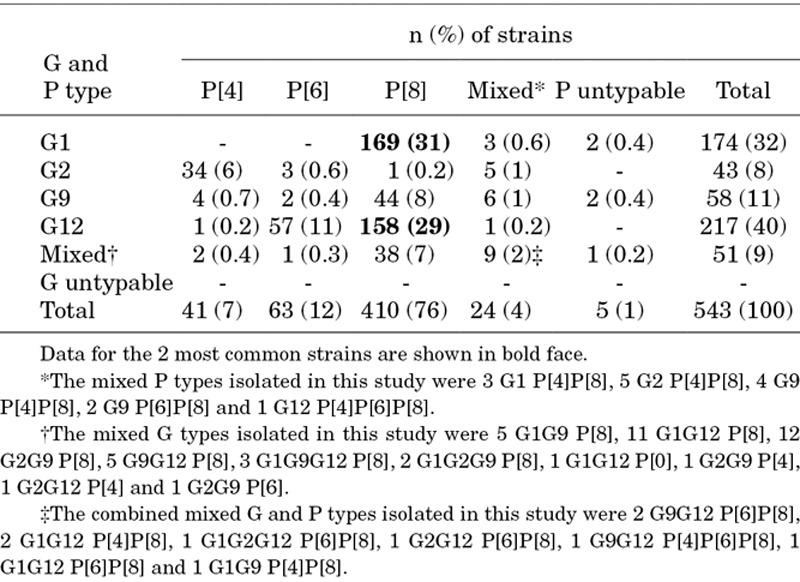
Genotyping was performed on 22% (543/2432) of positive rotavirus stool samples (Table 3). Four G genotypes (G1, G2, G9 and G12) and 3 P genotypes (P4, P6 and P8) were identified. G12 (40%) and G1 (32%) were the most prevalent G types, and P[8] (76%) and P[6] (12%) were the most prevalent P types. G1P[8] (31%) and G12P[8] (29%) were the most commonly identified strains. Mixed rotavirus strains were seen in 13% of the children; 5 (1%) of specimens were untypable. All detected G and P genotypes were observed in sites across the 7 divisions of Bangladesh.
TABLE 3.
Distribution of Rotavirus G and P Genotypes Isolated in Samples From Children <5 Years of Age With Rotavirus Acute Gastroenteritis in Bangladesh, July 2012 to June 2015
DISCUSSION
This study confirms the considerable burden of rotavirus AGE among children 5 years of age and younger in Bangladesh. We found that rotavirus accounted for ~64% of all childhood hospitalizations for AGE or ~8% of all pediatric hospitalizations at participating hospitals during the study period. Rotavirus was detected year-round among children with AGE, and during the peak winter months from November to February, the detection rates of rotavirus among children hospitalized with AGE exceeded 80%. Children with rotavirus AGE had illness that was somewhat more severe than those with AGE from other causes. Children 6–23 months of age accounted for ~85% of all rotavirus AGE hospitalizations, indicating that rotavirus vaccines, which are administered in the first few months of life, have the potential to reduce the burden of rotavirus AGE substantially. Collectively, our findings demonstrate the potential value of rotavirus vaccination in reducing the tremendous morbidity from rotavirus AGE in Bangladeshi children.
Earlier single-site studies in Bangladesh have reported lower rotavirus prevalence in children admitted for AGE, ranging from 20% to 42%.2,3,16,17 The higher prevalence estimate for rotavirus we report may be attributed to a variety of factors, including the types of healthcare facilities where surveillance took place (eg, urban vs. rural, referral vs. primary hospitals), as well as differences in laboratory procedures (polymerase chain reaction and EIA) and surveillance methodologies (case definitions, sample of children tested). Perhaps more importantly, improvements in sanitation and hygiene in Bangladesh18 may have disproportionately reduced the bacterial and parasitic causes of diarrhea (which are mainly transmitted through contaminated food and water), and may have had less of an impact on rotavirus diarrhea (which is largely spread person-to-person), resulting in an increase in the proportion of diarrhea attributable to rotavirus.19,20 This is supported by data from a longitudinal evaluation of hospitalizations data from icddr,b showing that the proportion of diarrhea cases attributable to rotavirus nearly doubled during 2002–2004 compared with 1993–1995 (42% vs. 22%).21
This study demonstrated substantial rotavirus strain diversity within Bangladesh. As opposed to previous years (1991–2012), when G1, G2 and G9 were the predominant strains detected in Bangladesh,22,23 G12 was also commonly detected genotype in the last two and a half years, consistent with the recent global spread of this emergent genotype.24 We found that 31% of detected strains were homotypic to strains in both vaccines (ie, matched in both the G and P types), 44% were partially heterotypic (matched only the G or P type) and 12% were fully heterotypic (ie, did not match the G or P type). In addition, ~14% of the strains were mixed or untypable using current primers. Although both natural and vaccine-induced protection against a range of genotypes has been shown in developed settings,25,26 the considerable variability in circulating rotavirus strains in Bangladesh warrants an assessment of the effect prevalent and mixed genotypes may have on the overall effectiveness of the vaccine after it is introduced into Bangladesh’s immunization schedule, as well as close monitoring of the impact the vaccine could have on strain selection and the incidence of locally circulating strains.
Some limitations should be considered. First, because the surveillance system is based in tertiary hospitals, we likely captured a select group of children with severe disease, and the etiologic distribution of diarrheal illnesses may be different in other hospital settings in Bangladesh (eg, in urban and primary care centers). However, at the same time, our surveillance systematically excluded children who died with diarrhea because these children were often admitted after-hours or died quickly after admission. Because of this, suggesting that our approach is not well-suited for studying deaths from rotavirus, and our data likely underestimate the total number of rotavirus-related deaths, as well as case-fatality ratios. Second, year-to-year variation in rotavirus activity is known to occur, and the proportion of diarrhea attributable to rotavirus depends on the prevalence of other enteric pathogens and health-seeking behaviors and thus we may have captured a period of high rotavirus activity. However, rotavirus prevalence estimates were consistent across years and settings. Third, because controls were not included in this evaluation, we may have overestimated the proportion of cases attributable to rotavirus. However, any overestimation is expected to be small, as EIA rarely detects asymptomatic infections, and the attributable fraction of rotavirus detection with EIA has been shown to be >90%.27–29
ACKNOWLEDGMENTS
We thank all the study participants for their time and support. We are also grateful to our implementation partner, surveillance hospital sites and The Institute of Epidemiology, Disease Control and Research (IEDCR) under the Ministry of Health and Family Welfare of Bangladesh Government. Our technical assistance partner: US Centers for Disease Control and Prevention (CDC).
Supplementary Material
Footnotes
The views expressed herein are those of the author(s) and do not necessarily reflect the views of the US Agency for International Development or US Governments.
This work was supported by USAID-Bangladesh, US Agency for International Development, under the terms of an Interagency Agreement with US Centers for Disease Control and Prevention (CDC); agreement no. 1U51GH001209-01. International Centre for Diarrheal Disease Research (icddr,b) is also grateful to the government of Bangladesh, Canada, Sweden, and the United kingdom for providing core/unrestricted support. The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Walker CL, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unicomb LE, Kilgore PE, Faruque SG, et al. Anticipating rotavirus vaccines: hospital-based surveillance for rotavirus diarrhea and estimates of disease burden in Bangladesh. Pediatr Infect Dis J. 1997;16:947–951. doi: 10.1097/00006454-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Zaman K, Yunus M, Faruque AS, et al. Surveillance of rotavirus in a rural diarrhoea treatment centre in Bangladesh, 2000–2006. Vaccine. 2009;27(suppl 5):F31–F34. doi: 10.1016/j.vaccine.2009.08.063. [DOI] [PubMed] [Google Scholar]
- 4.WER. Rotavirus vaccines:an update. Wkly Epidemiol Rec. 2009;84:533–540. [PubMed] [Google Scholar]
- 5.Linhares AC, Velázquez FR, Pérez-Schael I, et al. Human Rotavirus Vaccine Study Group. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1181–1189. doi: 10.1016/S0140-6736(08)60524-3. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Human Rotavirus Vaccine Study Group. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 7.Vesikari T, Matson DO, Dennehy P, et al. Rotavirus Efficacy and Safety Trial (REST) Study Team. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 8.Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:606–614. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 9.Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 10.Zaman K, Dang DA, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–623. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 11.National Institute of Population Research and Training (NIPORT), Mitra and Associates, and ICF International. Bangladesh Demographic and Health Survey 2013. Dhaka, Bangladesh, Calverton, MD: NIPORT, Mitra and Associates, and ICF International; 2011. Available at: http://dhsprogram.com/pubs/pdf/fr265/fr265.pdf. Accessed February 9, 2016. [Google Scholar]
- 12.WorldHealth Organization. Generic Protocols for (i) Hospital-Based Surveillance to Estimate the Burden of Rotavirus Gastroenteritis in Children and (ii) a Community-Based Survey on Utilization of Health Care Services for Gastroenteritis in Children: Field Test Version. 2002. [Google Scholar]
- 13.World Health Organization. The Treatment of Diarrhoea. A Manual for Physicians and Other Senior Health Workers. 2005 Available at: http://www.who.int/maternal_child_adolescent/documents/9241593180/en/. Accessed September 12, 2015. [Google Scholar]
- 14.Rahman M, Sultana R, Ahmed G, et al. Prevalence of G2P[4] and G12P[6] rotavirus, Bangladesh. Emerg Infect Dis. 2007;13:18–24. doi: 10.3201/eid1301.060910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis. 1990;22:259–267. doi: 10.3109/00365549009027046. [DOI] [PubMed] [Google Scholar]
- 16.Sarker MH, Das SK, Ahmed S, et al. Changing characteristics of rotavirus diarrhea in children younger than five years in urban Bangladesh. PLoS One. 2014;9:e105978. doi: 10.1371/journal.pone.0105978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siddique AK, Ahmed S, Iqbal A, et al. Epidemiology of rotavirus and cholera in children aged less than five years in rural Bangladesh. J Health Popul Nutr. 2011;29:1–8. doi: 10.3329/jhpn.v29i1.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.UNICEF. Sanitation, Hygiene Education and Water Supply in Bangladesh (SHEWA,B)-Factsheet. 2012. Aug, Available at: http://www.unicef.org/bangladesh/SHEWAB—factsheet—-—FINAL-21 April12.pdf. Accessed October 21, 2015. [Google Scholar]
- 19.Parashar UD, Gibson CJ, Bresee JS, et al. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tate JE, Burton AH, Boschi-Pinto C, et al. WHO-coordinated Global Rotavirus Surveillance Network. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–141. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka G, Faruque AS, Luby SP, et al. Deaths from rotavirus disease in Bangladeshi children: estimates from hospital-based surveillance. Pediatr Infect Dis J. 2007;26:1014–1018. doi: 10.1097/INF.0b013e318125721c. [DOI] [PubMed] [Google Scholar]
- 22.Afrad MH, Matthijnssens J, Afroz SF, et al. Differences in lineage replacement dynamics of G1 and G2 rotavirus strains versus G9 strain over a period of 22 years in Bangladesh. Infect Genet Evol. 2014;28:214–222. doi: 10.1016/j.meegid.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Rahman M, Alamgir AS, Saiada F, et al. Co-circulation of G1, G2 and G9 rotaviruses in hospitalized patients in Bangladesh during 2006–2009. Hum Vaccin. 2011;7:929–933. doi: 10.4161/hv.7.9.15988. [DOI] [PubMed] [Google Scholar]
- 24.Bányai K, László B, Duque J, et al. Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: insights for understanding the impact of rotavirus vaccination programs. Vaccine. 2012;30(suppl 1):A122–A130. doi: 10.1016/j.vaccine.2011.09.111. [DOI] [PubMed] [Google Scholar]
- 25.Leshem E, Lopman B, Glass R, et al. Distribution of rotavirus strains and strain-specific effectiveness of the rotavirus vaccine after its introduction: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:847–856. doi: 10.1016/S1473-3099(14)70832-1. [DOI] [PubMed] [Google Scholar]
- 26.Velázquez FR, Matson DO, Calva JJ, et al. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med. 1996;335:1022–1028. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 27.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 28.Phillips G, Lopman B, Tam CC, et al. Diagnosing rotavirus A associated IID: Using ELISA to identify a cut-off for real time RT-PCR. J Clin Virol. 2009;44:242–245. doi: 10.1016/j.jcv.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Tate JE, Mijatovic-Rustempasic S, Tam KI, et al. Comparison of 2 assays for diagnosing rotavirus and evaluating vaccine effectiveness in children with gastroenteritis. Emerg Infect Dis. 2013;19:1245–1252. doi: 10.3201/eid1908.130461. [DOI] [PMC free article] [PubMed] [Google Scholar]


