Supplemental Digital Content is available in the text.
Keywords: respiratory syncytial virus, lower respiratory tract infections, respiratory syncytial virus hospitalization, moderate-late preterm infants
Abstract
Background:
Moderate-late preterm infants, 33–35 weeks’ gestational age (wGA), are at increased risk for respiratory syncytial virus hospitalization (RSVH). The objective of this study is to quantify the burden of RSVH in moderate-late preterm infants.
Methods:
A pooled analysis was conducted on RSVH from 7 prospective, observational studies in the Northern Hemisphere from 2000 to 2014. Infants’ 330–356 wGA without comorbidity born during the respiratory syncytial virus season who did not receive respiratory syncytial virus immunoprophylaxis were enrolled. Data for the first confirmed RSVH during the season (+1 month) were analyzed. Incidence and hospitalization rate per 100 patient-seasons, intensive care unit admission and length of stay (LOS), oxygen support, mechanical ventilation and overall hospital LOS were assessed.
Results:
The pooled analysis comprised 7,820 infants; 267 experienced a confirmed RSVH at a median age of 8.4 weeks. The crude pooled RSVH incidence rate was 3.41% and the rate per 100 patient-seasons was 4.52. Median hospital LOS was 5.7 days. A total of 22.2% of infants required intensive care unit admission for a median LOS of 8.3 days. A total of 70.4% received supplemental oxygen support for a median of 4.9 days, and 12.7% required mechanical ventilation for a median of 4.8 days.
Conclusions:
The burden of RSVH in moderate-late, 33–35 weeks’ wGA preterm infants without comorbidities born during the viral season in Northern Hemisphere countries is substantial. Severe cases required prolonged and invasive supportive therapy.
Respiratory syncytial virus (RSV) is an important cause of lower respiratory tract infection (LRTI) in infants.1,2 In 2005, the estimated global burden of new episodes of RSV LRTIs in children <5 years of age was over 33 million, with at least 3.4 million episodes representing severe RSV LRTI necessitating hospitalization.1 RSV hospitalizations (RSVHs) have a major impact on healthcare resources3–5 and costs,6–8 and have been associated with recurrent wheeze and possibly asthma.9,10
Infants born at 33–35 weeks’ gestational age (wGA) (moderate-late preterm) are an important risk group for severe RSVH and sequelae.3–5,7,11–13 Horn et al.3 found that moderate-late preterm infants had the highest rate of intubation and longest hospital and intensive care unit (ICU) length of stay (LOS) compared with ≤32 or ≥36 wGA infants, potentially linked to critical lung underdevelopment between 32 and 35 wGA and immunologic immaturity. Prophylaxis with palivizumab has been shown to reduce RSVH in moderate-late preterm infants by up to 82%14,15; however, these infants are considered eligible for prophylaxis only under specific circumstances according to different national guidelines from different countries.16–20
Defining and understanding the burden of severe RSV infection can aid judicious use of palivizumab prophylaxis,21,22 within the context of limited healthcare resources.23 Data specific to the epidemiology and disease burden of RSV in Northern Hemisphere moderate-late preterm infants are currently limited by the small number of RSVH available in individual studies. The primary objective was to quantify the epidemiology and burden of severe RSV LRTI in a homogeneous dataset of 33–35 wGA (moderate-late preterm) infants in the Northern Hemisphere born and experiencing first RSVH within the same RSV season (+1 month) as their birth.
MATERIALS AND METHODS
Study Selection
An electronic Medline and Embase search was performed using the following search terms: respiratory syncytial virus (RSV), infection, disease, illness, epidemiology, hospitalization, late preterm, premature and gestational age. Filters included the time span of January 1, 1998, to January 1, 2015, English language, and humans. The Cochrane Central Register of Controlled Trials and Database of Systematic Reviews were searched for reviews on RSV infection. All identified reports were checked for references to additional controlled trials or pertinent citations. A pooled analysis was performed on RSVH data from 33 to 35 wGA (defined as 33 weeks and 0 days to 35 weeks and 6 days) moderate-late preterm infants born in the Northern Hemisphere. Gestational age in the selected studies was largely based on standard criteria of ultrasonographic dating in the early phase of pregnancy and/or the number of weeks elapsed between the first day of the last menstrual period and the date of delivery. To avoid potential regional or local biases, studies were only included if they had a multicenter, observational, prospective design; assessed >1000 preterm infants at risk for severe RSV disease; included infants with laboratory-confirmed RSV infection; evaluated data on RSVH morbidity and contained data available for analysis by January 2015. Severe RSV infection was defined as the need for hospitalization. Studies were excluded if >15% of infants received palivizumab prophylaxis to standardize the cohort and avoid the potential confounding effect of prophylaxis. The study did not include more than one dataset from a country (excluding multinational studies) to avoid the perception of bias toward any one country. If more than one dataset met the inclusion criteria for a particular country, then the most recent dataset was selected.
Data Extraction and Infant Selection
All data from studies meeting the inclusion criteria were anonymized before they were transferred to the central database for analysis (Table 1). After receipt of the data from these studies, to ensure homogeneity, infants were excluded from analyses if they had received palivizumab, had a relevant comorbidity, such as congenital heart disease, bronchopulmonary dysplasia, Down syndrome or immunodeficiency, or had incomplete data.
TABLE 1.
Data Extracted From Each Dataset
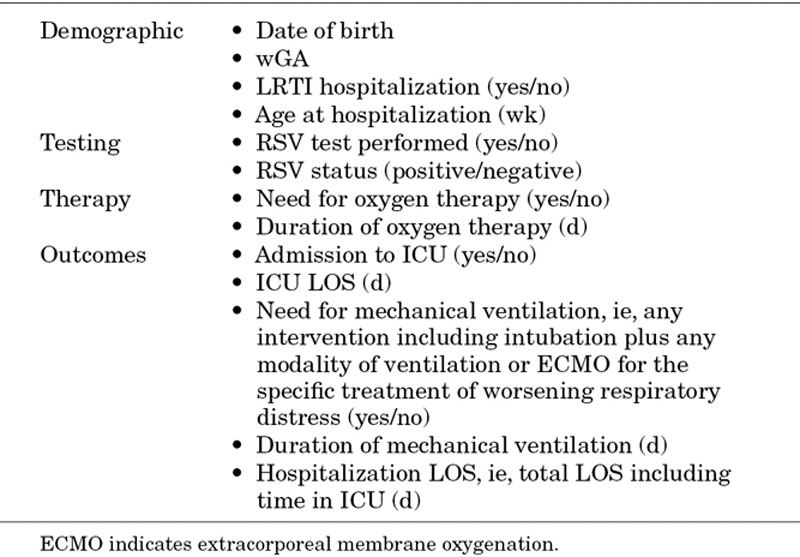
Data Analysis
Descriptive Analyses
Descriptive enumerative analyses were carried out in all datasets including totals, total missing values and range of values by variable. Where appropriate, frequency and dispersive characteristics were investigated, including median and the interquartile range. Reference RSV seasons were taken from each study. Distribution of first RSVH through the RSV season (+1 month) was calculated both as an incidence rate and as the rate per 100 patient-seasons.
Pooled Analysis
A pooled analysis was conducted of homogenous data across the 7 included datasets (Fig. 1). Since the Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) database contained only infants born and hospitalized for RSV within the same RSV season (+1 month),24 to maximize homogeneity, all datasets were standardized to this criterion. Weighted average analyses using inverse variance weighting in a fixed effects model was applied. Missing values within the data were classified as “not recorded” rather than zero and the absence of data was confirmed with the authors of the individual studies. Variances were calculated for each variable across all datasets. Calculated descriptive measurements were weighted by the inverse variance associated with the variables in each dataset. This allowed the calculation of weighted averages.
FIGURE 1.
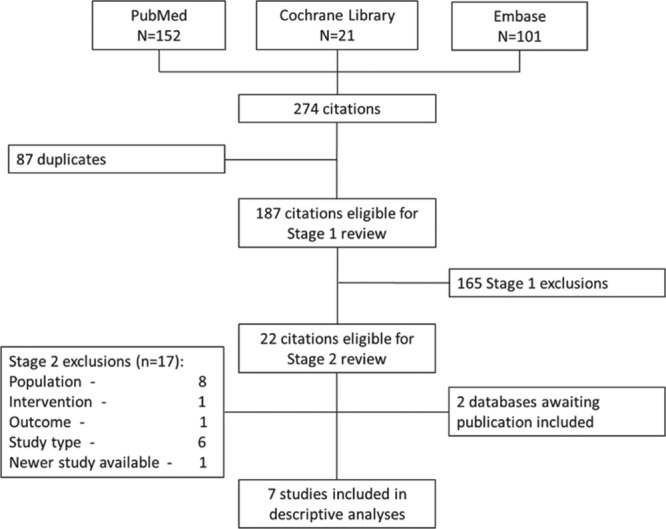
PRISMA of systematic review process. Stage 1 review: 2 reviewers assessed citations for inclusion/exclusion based on the title and abstract. Stage 2 review: 2 reviewers assessed each citation for inclusion/exclusion based on the full text. Reasons for exclusion were categorized as follows: population (eg, outside 33–35 weeks’ gestational age range), intervention (eg, study including >15% of infants receiving prophylaxis), study type (eg, retrospective) or new study available (ie, 2 studies available from the same country whereupon the newer one would be included). For both stages, if a consensus could not be reached, a third experienced reviewer made the decision. PRISMA indicates Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
RSVH rates by gestational age in individual studies and the combined database were compared using the chi square test. The rates of supplemental oxygen use, admission to ICU and mechanical ventilation use in RSVH infants were compared across the individual datasets using the chi square test, while the duration of these outcomes was compared using one-way analysis of variance.
An analysis was undertaken to determine if hospital resource use differed between earlier and more contemporary studies by splitting the combined database into 2 halves and comparing outcomes using t tests and Mann-Whitney U tests, as appropriate.
Heterogeneity Tests
Heterogeneity (equality of variance) tests across datasets for key variables were undertaken using nonparametric Levene tests to determine whether data were suitable for pooled analyses. Cox regression analyses were also used to test homogeneity. The variables and outcomes of interest were then assembled into a combined database.
All analyses were performed using SPSS for Windows version 15.0 (SPSS Inc, Chicago, IL), Microsoft Access SQL (Microsoft, Redmond, WA) and Microsoft Access/Excel VBScript (Microsoft, Redmond, WA).
RESULTS
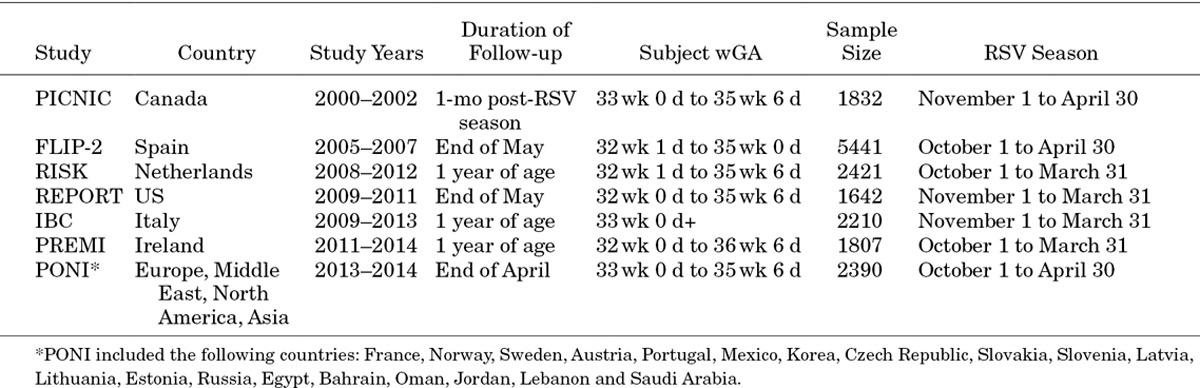
Birth and first confirmed RSVH data on 330–356 wGA (moderate-late preterm) infants born during the period 2000–2014 were collected from 7 separate databases across the Northern Hemisphere. Five studies were identified via the systematic search and a further 2 were found that were awaiting publication (Fig. 1).The datasets included were as follows: second Risk Factors Linked to RSV Infection Requiring Hospitalization in Premature Infants Study (FLIP-2, Spain),11 RISK study (Netherlands),25 PICNIC (Canada),24 RSV Preterm Risk Estimation Measure for RSVH in Ireland (RSV-PREMI, Ireland),13 Italian National Birth Cohort (IBC, Italy),26 RSV Respiratory Events among Preterm Infants Outcomes and Risk Tracking (REPORT, US)5 and Predictors Associated with RSV hOspitalization in Nonprophylaxed, Premature Infants (PONI, multinational).27 Individual study designs and demographics are shown in Tables 2 and 3.
TABLE 2.
Data Derivation
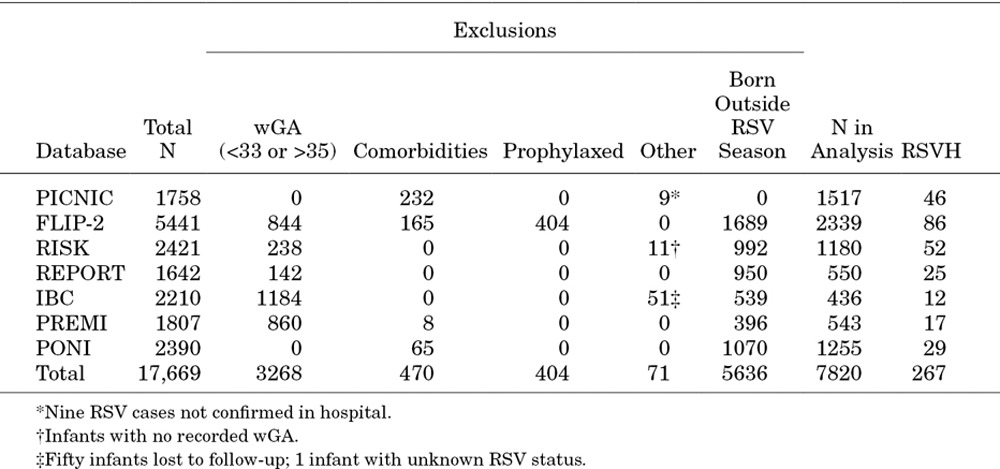
TABLE 3.
Study Designs
Data from 17,669 moderate-late preterm infants were identified from these 7 datasets. Of these, 4213 (23.8%) did not meet the inclusion criteria and were excluded (Fig. 2), primarily for failure to meet the 330–356 wGA criteria (3268, 18.5%), palivizumab prophylaxis (404, 2.3%), presence of comorbidities (470, 2.7%) or incomplete data (71, 0.4%). Of the 13,456 remaining infants, 474 (3.5%) had clinically confirmed RSVH. However, nonparametric Levene tests showed significant heterogeneity between datasets within this group (P = 0.001 for age at admission; P < 0.001 for duration of hospitalization).
FIGURE 2.
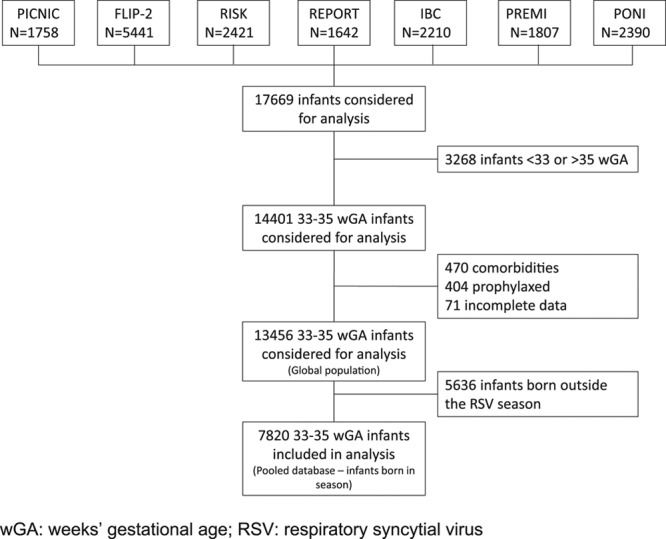
Diagram of derivation of analytical dataset.
The PICNIC database contained only infants born within the RSV season (+1 month).24 In the majority of the other datasets, around 50% cases of RSVH were observed in infants born within the RSV season: FLIP-2 (57.7%), RISK (45.2%), REPORT (46.3%), IBC (75.0%), PREMI (47.2%) and PONI (50.0%). To maximize homogeneity across the datasets, all data were standardized to 330–356 wGA infants born during the RSV season and who experienced their first RSVH during the same RSV season (+1 month). This excluded an additional 5636 infants (41.9%) and resulted in a core database of 7820 infants (58.1%) for analysis (Table 2). Nonparametric Levene tests showed no significant heterogeneity between datasets within this group (P = 0.129 for age at admission; P = 0.150 for duration of hospitalization). A Cox regression analysis confirmed similarity of the datasets with a nonsignificant source covariate (P = 0.609). This approach captured 56% of the total RSVH (267/474).
RSV-positive Hospitalization Rates
The incidence of RSVH in the homogeneous population varied among the datasets, from 2.31% to 4.55% (Table 4). The individual incidence rates ranged from 3.15 to 5.92 per 100 patient-seasons. In the pooled analysis, 33–35 wGA preterm infants born and hospitalized within the same RSV season showed a crude RSVH incidence rate of 3.41% and a rate per 100 patient-seasons of 4.52, 95% confidence interval (3.83–5.21).
TABLE 4.
Number and Incidence of RSVH in 33–35 wGA Moderate-Late Preterm Infants in Each Dataset
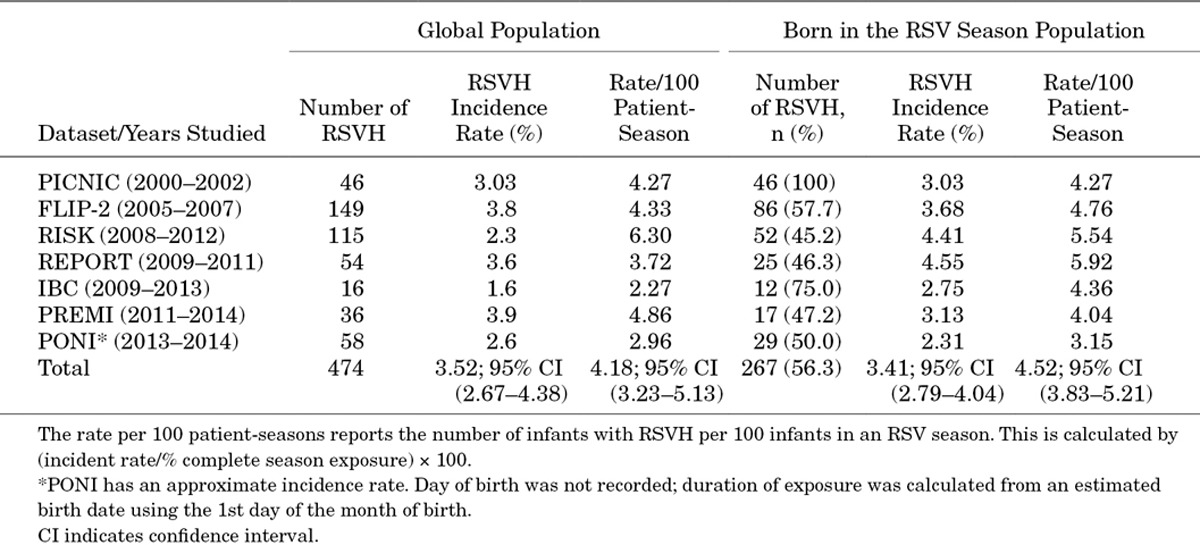
Incidence Rate of RSVH Stratified by Gestational Age
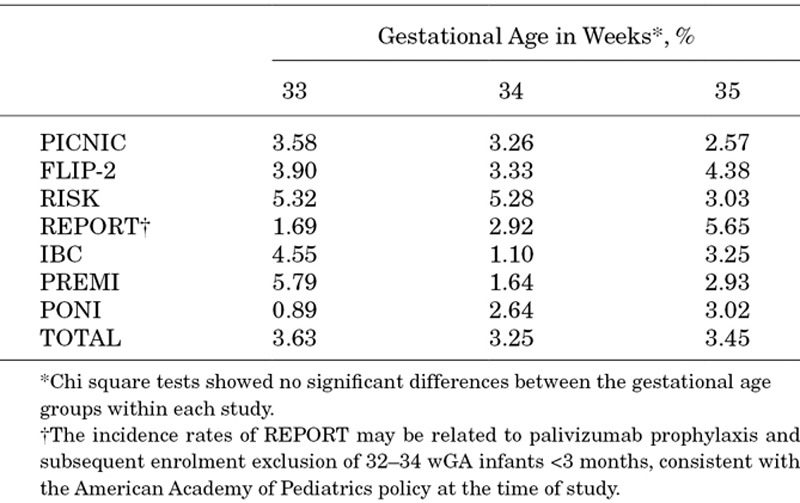
The pooled incidence rate was similar across gestational age groups: 3.63% for 33 wGA; 3.25% for 34 wGA and 3.45% for 35 wGA (Table 5). No consistent trend in RSV incidence by wGA was evident among the different datasets.
TABLE 5.
Incidence Rate of RSVH Stratified by Gestational Age at Birth in 33–35 wGA Infants Born and Hospitalized in the Same RSV Season
Seasonality of RSV-positive Hospitalizations
The rates of RSVH during the RSV season (+1 month) for infants born in that season show a degree of variation between the datasets in terms of the calendar month (Fig. 3). There are fewer hospitalizations during the first month of the season with a peak in December–March and then a steep decline. There was a difference in the peak of the RSV season with time. Taking the midpoint in terms of number of seasons for which data were available, before 2010, 27.3% of cases occurred in December, whereas 27.1% of cases occurred in January after 2010.
FIGURE 3.
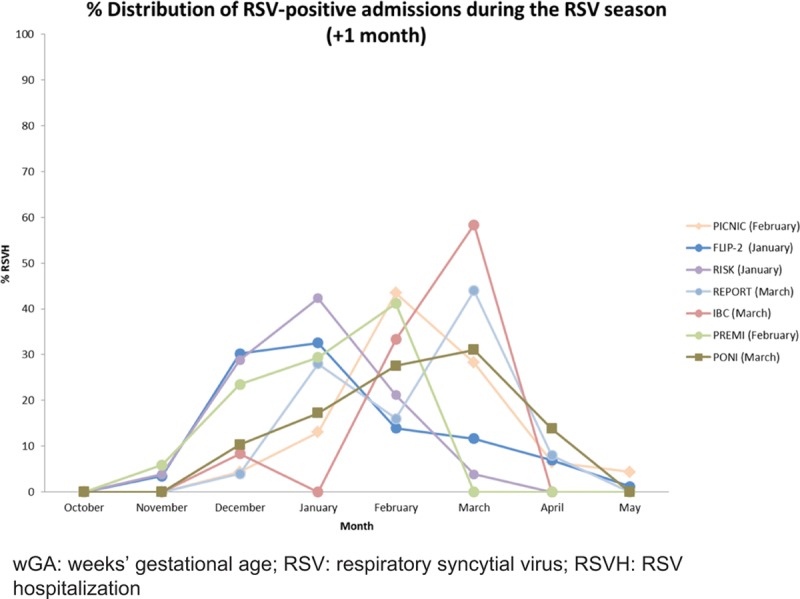
RSVH distribution in 33–35 wGA preterm infants during the RSV season (+1 month). Peak month shown in parentheses.
Age at First Confirmed RSV LRTI Hospitalization for Infants Born and Hospitalized in the Same RSV Season
The median age for infants born and hospitalized for their first confirmed RSV LRTI in the same RSV season ranged from 7.1 (RISK) to 11.0 (REPORT) weeks (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/C555). The weighted median age at first confirmed RSVH within the pooled analysis was 8.4 weeks.
Healthcare Resource Use During Hospitalization
There was significant variation in the proportion of infants who received supplemental oxygen across the 5 datasets, ranging from 32.0 (REPORT) to 88.2% (PREMI). The median duration of supplemental oxygen showed consistency across datasets at 4–5 days (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/C555). In the pooled analysis, a total of 70.4% of infants received supplemental oxygen for a median of 4.9 days.
The percentage of RSV-positive infants admitted to ICU ranged from 15.4% to 41.4%, with a median ICU LOS across the datasets of 3.5–7.0 days. The pooled analyses showed that 22.2% of moderate-late preterm infants were admitted to the ICU as part of their total RSVH, for a median duration of 8.3 days.
The requirement for mechanical ventilation while in hospital varied from 6.5% to 35.3%, with a median duration of 3–8 days. In the pooled analysis, a total of 12.7% of infants received mechanical ventilation for a median of 4.8 days. Five studies documented overall hospital LOS. The median ranged from 3 to 9 days (P = 0.005 across datasets), with the pooled LOS being 5.7 days.
When split into studies conducted from 2000 to 2007 (N = 132 RSVH) and from 2008 to 2014 (N = 135 RSVH), there were no significant differences in terms of hospital resource use (P = 0.500 for duration of respiratory support; P = 0.958 for duration of mechanical ventilation; P = 0.659 for ICU LOS; P > 0.999 for total LOS).
DISCUSSION
The results of this analysis illustrated that the burden of RSV LRTI in 33–35 wGA (moderate-late preterm) infants born during the RSV season in Northern Hemisphere countries is substantial, although RSVH rates varied between studies. Overall, the incidence rates of RSVH per 100 patient-seasons ranged from 3.15 (PONI) to 5.92 (REPORT), with a pooled rate of 4.52.
The pooled median hospital LOS was 5.7 days, with 22.2% of infants requiring admission to ICU for a median of 8.3 days. Among those infants with RSVH, up to 88% required supplemental oxygen (PREMI) and up to 35% required mechanical ventilation (PREMI), with the pooled analysis showing that 70.4% of all RSVH infants received oxygen and 12.7% received mechanical ventilation. In the 6 datasets reporting on mortality (PONI excluded mortality data from their analyses), there were no RSV-associated deaths.
Our findings on the effect of prematurity on hospital resource use and outcomes are consistent with previous observations.3,28 Diez-Domingo et al28 recently, systematically searched the literature and found that between 17.8% and 48.4% of 33–35 wGA infants with RSVH are admitted to the ICU, and a substantial proportion of all infants with RSVH required assisted ventilation for recurrent apnea, labored breathing, hypoxemia and respiratory failure (~10%), but this figure doubled in preterm infants (20% in 32 wGA RSVH infants).
Moderate-late preterm infants, 33–36 wGA, with a history of RSV cost the US healthcare system almost 5 times more over the first year of life than premature infants with no history of RSV.29 The use of palivizumab to prevent RSVH has been shown to be highly cost-effective in some studies and not cost-effective in others, depending on the modeling approaches, type of analyses performed, assumptions used and cost-effectiveness thresholds applied.30 In moderate-late preterm infants, the cost-effectiveness of palivizumab has been shown to be improved with the inclusion of risk factors for RSVH.31,32 In addition to increasing healthcare utilization and causing considerable distress for infants and children, hospitalization can significantly disrupt the lives of the families of affected infants, leading to increased emotional and financial burdens.33–36
The pooled analysis of data from 33 to 35 wGA preterm infants born and hospitalized for RSV within the same season highlight the risk for early burden of RSVH, which is linked to longer term respiratory morbidity in this gestational age group.9 The data suggest that moderate-late preterm infants are a potential target group for intervention: 32–35 wGA infants have smaller airways and greater lung immaturity than full-term infants,37,38 although they are often considered physiologically and functionally close to full-term infants.
No trends were reported in comparison of relative RSVH incidence by wGA, and the pooled data shown in Table 5 illustrated similar overall means of between 3.25% and 3.63%. A bias against the most preterm infants might be expected in the data: those born at 33 wGA would receive extended neonatal hospitalization compared with 35 wGA infants and therefore experience a shorter duration of RSV seasonal exposure. However, the data reported here did not reflect any bias in this direction. In terms of seasonality, there appeared to be a trend for the majority of cases of RSVH to peak during December/January but it is difficult to draw any generalizations as the start date and length of the RSV season, as well as RSVH rates in developed countries, can vary from year-to-year.39–43
A key strength of our study is that data were collected from 7 large, methodologically similar but independent multicenter, observational, prospective studies that enrolled moderate-late preterm infants from several countries across the Northern Hemisphere. The combined analysis lends more robust credence that the burden of RSV illness in this population is substantial and similar to the morbidities experienced by preterm infants <32 wGA.3
There were several important limitations in this study. The combined dataset was affected by the focus of each individual study. FLIP-2, RISK and PICNIC concentrated exclusively on RSV-positive hospitalizations, whereas PONI, REPORT and IBC evaluated all LRTI admissions. FLIP-2, RISK and RSV-PREMI collated hospitalizations over a calendar year, whereas PICNIC focused on births during the RSV season. PONI, RSV-PREMI and IBC used a wider span of gestational age ranges than the other studies. Consequently, studies that were large in terms of initial patient numbers had reduced numbers of RSVH for 33–35 infants, particularly those born within the RSV season. RSV testing was not standardized in many hospitals involved in these studies. Furthermore, rapid antigen testing, shell vial culture and viral culture predominated in earlier studies, which are typically less sensitive than currently available polymerase chain reaction–based assays. This may have resulted in an underestimate of the true burden of RSV LRTI, and may also explain the variation in RSVH rates between the datasets. Variations in the frequency of recognized biologic/medical (eg, male sex) and social/environmental (eg, day care attendance) risk factors for RSVH could also have influenced the respective rates of RSVH across the datasets.5,11,13,24–27 Coinfections are another factor that could have influenced the severity of disease, but such data were not available for most of the datasets and were not analyzed.
The studies were multinational, with variance in hospitalization practices (including admission criteria, criteria for oxygen use, admission to ICU and type of respiratory support used) and data extending over >10 years, which could be affected by time-related changes in medical practice. However, this practice variation may still be prevalent, because the use of oxygen in the management of bronchiolitis relative to accepted criteria for hospitalization vary significantly, inclusive of the criteria for ICU admission.44–46 An additional limitation in these analyses is that the data were restricted to those infants born and/or hospitalized in the same RSV season. Also, infants who received palivizumab prophylaxis and those with significant comorbidities (eg, chronic lung disease and congenital heart disease) were excluded and only infants with laboratory-confirmed RSV infection were included in the analyses. Thus, although this resulted in a homogeneous subset of 7820 infants, important at risk infants were excluded from the analysis of these 7 multinational studies. Data from a broader study covering the entire first year of life would provide a complementary understanding of the “real life” clinical situation. Additional analyses could also further investigate the effect of chronologic age and chronologic versus developmental age on the incidence and severity of RSV illness in these infants.
This study contributes important data on the burden of RSV LRTI in 33–35 wGA (moderate-late preterm) infants in Northern Hemisphere countries. Knowledge of the health risk and burden of RSVHs in high-risk infants could guide the cost-effective use of prophylaxis with palivizumab or future RSV vaccines. Identification of higher risk moderate-late preterm infants for prophylaxis could reduce the substantial clinical and economic burden of RSV in these infants.
ACKNOWLEDGMENTS
The authors would like to thank the following individuals for their valuable contributions to the dataset transfers and quality control for analysis, respectively: Dr. Fulvio Adorni (IBC), Institute of Biomedical Technologies, Milan, Italy; Michel Bordeleau (PICNIC), JSS Medical Research, Montreal, Canada; Dr. Joan Murphy (RSV-PREMI), Coombe Women and Infants University Hospital, Dublin, Ireland; Dr. Massimo Musicco (IBC), National Research Council, Milan, Italy; Dr. Xionghua Wu (REPORT), MedImmune, MD, US, as well the Dutch RSV Neonatal Network for their participation in the RISK study. The authors would also like to thank Dr. Kristina Unnebrink, AbbVie Deutschland GmbH & Co. KG for her review and Dr. Joanne Smith, Strategen, Basingstoke, UK, for her invaluable assistance with the statistical analysis and editing of this manuscript. Dr. John Fullarton had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supplementary Material
Footnotes
The authors wrote the manuscript and are solely responsible for its content. AbbVie reviewed and approved this manuscript.
Funding support for this pooled database analysis was provided by AbbVie. E.J.A., X.C.-E., M.B., M.L., M.S.-P. and B.P. have received research funding and/or compensation as advisor/lecturer from AbbVie. E.J.A. has received research funding from MedImmune. B.R.-G. and J.F., working for Strategen, have previously received payment from AbbVie for work on various projects. E.R., P.V. and F.C. are former employees of AbbVie and may hold AbbVie stock or stock options. G.N. is an employee of AbbVie and may hold AbbVie stock or stock options. The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain S, Williams DJ, Arnold SR, et al. CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372:835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horn SD, Smout RJ. Effect of prematurity on respiratory syncytial virus hospital resource use and outcomes. J Pediatr. 2003;143(5 suppl):S133–S141. doi: 10.1067/s0022-3476(03)00509-2. [DOI] [PubMed] [Google Scholar]
- 4.Figueras-Aloy J, Carbonell-Estrany X, Quero J IRIS Study Group. Case-control study of the risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born at a gestational age of 33-35 weeks in Spain. Pediatr Infect Dis J. 2004;23:815–820. doi: 10.1097/01.inf.0000136869.21397.6b. [DOI] [PubMed] [Google Scholar]
- 5.Ambrose CS, Anderson EJ, Simões EA, et al. Respiratory syncytial virus disease in preterm infants in the U.S. born at 32-35 weeks gestation not receiving immunoprophylaxis. Pediatr Infect Dis J. 2014;33:576–582. doi: 10.1097/INF.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shefali-Patel D, Paris MA, Watson F, et al. RSV hospitalisation and healthcare utilisation in moderately prematurely born infants. Eur J Pediatr. 2012;171:1055–1061. doi: 10.1007/s00431-012-1673-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forbes ML, Hall CB, Jackson A, et al. Comparative costs of hospitalisation among infants at high risk for respiratory syncytial virus lower respiratory tract infection during the first year of life. J Med Econ. 2010;13:136–141. doi: 10.3111/13696990903583404. [DOI] [PubMed] [Google Scholar]
- 8.Palmer L, Hall CB, Katkin JP, et al. Respiratory outcomes, utilization and costs 12 months following a respiratory syncytial virus diagnosis among commercially insured late-preterm infants. Curr Med Res Opin. 2011;27:403–412. doi: 10.1185/03007995.2010.542744. [DOI] [PubMed] [Google Scholar]
- 9.Carbonell-Estrany X, Pérez-Yarza EG, García LS, et al. IRIS (Infección Respiratoria Infantil por Virus Respiratorio Sincitial) Study Group. Long-term burden and respiratory effects of respiratory syncytial virus hospitalization in preterm infants-the SPRING study. PLoS One. 2015;10:e0125422. doi: 10.1371/journal.pone.0125422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bacharier LB, Cohen R, Schweiger T, et al. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2012;130:91–100.e3. doi: 10.1016/j.jaci.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Figueras-Aloy J, Carbonell-Estrany X, Quero-Jiménez J, et al. IRIS Study Group. FLIP-2 Study: risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born in Spain at a gestational age of 32 to 35 weeks. Pediatr Infect Dis J. 2008;27:788–793. doi: 10.1097/INF.0b013e3181710990. [DOI] [PubMed] [Google Scholar]
- 12.Carbonell-Estrany X, Quero J IRIS Study Group. Hospitalization rates for respiratory syncytial virus infection in premature infants born during two consecutive seasons. Pediatr Infect Dis J. 2001;20:874–879. doi: 10.1097/00006454-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Sheridan-Pereira M, Murphy J, Sloan J, et al. Respiratory syncytial virus preterm (32-36 completed weeks of gestation) risk estimation measure for RSV hospitalization in Ireland: A prospective study. Pediatr Infect Dis J. 2016;35:19–24. doi: 10.1097/INF.0000000000000918. [DOI] [PubMed] [Google Scholar]
- 14.Notario G, Vo P, Gooch K, et al. Respiratory syncytial virus-related hospitalization in premature infants without bronchopulmonary dysplasia: subgroup efficacy analysis of the IMpact-RSV trial by gestational age group. Pediatric Health Med Ther. 2014;5:43–48. [Google Scholar]
- 15.Blanken MO, Rovers MM, Molenaar JM, et al. Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368:1791–1799. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 16.Figueras Aloy J, Carbonell Estrany X Comité de Estándares de la SENeo. [Update of recommendations on the use of palivizumab as prophylaxis in RSV infections]. An Pediatr (Barc) 2015;82:199.e1–199.e2. doi: 10.1016/j.anpedi.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Bollani L, Baraldi E, Chirico G, et al. Italian Society of Neonatology. Revised recommendations concerning palivizumab prophylaxis for respiratory syncytial virus (RSV). Ital J Pediatr. 2015;41:97. doi: 10.1186/s13052-015-0203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Association of Scientific Medical Societies (DE) Leitlinie zur Prophylaxe von schweren Erkrankungen durch Respiratory Syncytial Virus (RSV) bei Risikokindern. AWMF-LL. 2012:048–012 28. Oktober. [Google Scholar]
- 19.Robinson JL, Le Saux N Canadian Paediatric Society, Infectious Diseases and Immunization Committee. Preventing hospitalizations for respiratory syncytial virus infection. Paediatr Child Health. 2015;20:321–333. doi: 10.1093/pch/20.6.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:e620–e638. doi: 10.1542/peds.2014-1666. [DOI] [PubMed] [Google Scholar]
- 21.The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 22.Feltes TF, Cabalka AK, Meissner HC, et al. Cardiac Synagis Study Group. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143:532–540. doi: 10.1067/s0022-3476(03)00454-2. [DOI] [PubMed] [Google Scholar]
- 23.Langley GF, Anderson LJ. Epidemiology and prevention of respiratory syncytial virus infections among infants and young children. Pediatr Infect Dis J. 2011;30:510–517. doi: 10.1097/INF.0b013e3182184ae7. [DOI] [PubMed] [Google Scholar]
- 24.Law BJ, Langley JM, Allen U, et al. The Pediatric Investigators Collaborative Network on Infections in Canada study of predictors of hospitalization for respiratory syncytial virus infection for infants born at 33 through 35 completed weeks of gestation. Pediatr Infect Dis J. 2004;23:806–814. doi: 10.1097/01.inf.0000137568.71589.bd. [DOI] [PubMed] [Google Scholar]
- 25.Blanken MO, Koffijberg H, Nibbelke EE, et al. Dutch RSV Neonatal Network. Prospective validation of a prognostic model for respiratory syncytial virus bronchiolitis in late preterm infants: a multicenter birth cohort study. PLoS One. 2013;8:e59161. doi: 10.1371/journal.pone.0059161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanari M, Prinelli F, Adorni F, et al. Study Group of Italian Society of Neonatology on Risk Factors for RSV Hospitalization. Risk factors for bronchiolitis hospitalization during the first year of life in a multicenter Italian birth cohort. Ital J Pediatr. 2015;41:40. doi: 10.1186/s13052-015-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Straňák Z, Saliba E, Kosma P, et al. Predictors of RSV LRTI hospitalization in infants born at 33 to 35 weeks gestational age: a large multinational study (PONI). PLoS One. 2016;11:e0157446. doi: 10.1371/journal.pone.0157446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Díez-Domingo J, Pérez-Yarza EG, Melero JA, et al. Social, economic, and health impact of the respiratory syncytial virus: a systematic search. BMC Infect Dis. 2014;14:544. doi: 10.1186/s12879-014-0544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makari D, Hoopes JM, White N. Impact of RSV: implications for managed care. Manag Care. 2009;18(1 suppl 2):2–7. [PubMed] [Google Scholar]
- 30.Andabaka T, Nickerson JW, Rojas-Reyes MX, et al. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev. 2013:CD006602. doi: 10.1002/14651858.CD006602.pub4. [DOI] [PubMed] [Google Scholar]
- 31.Carbonell-Estrany X, Lázaro y de Mercado P. Health economics and RSV. Paediatr Respir Rev. 2009;10(suppl 1):12–13. doi: 10.1016/S1526-0542(09)70006-5. [DOI] [PubMed] [Google Scholar]
- 32.Lanctôt KL, Masoud ST, Paes BA, et al. The cost-effectiveness of palivizumab for respiratory syncytial virus prophylaxis in premature infants with a gestational age of 32-35 weeks: a Canadian-based analysis. Curr Med Res Opin. 2008;24:3223–3237. doi: 10.1185/03007990802484234. [DOI] [PubMed] [Google Scholar]
- 33.Leidy NK, Margolis MK, Marcin JP, et al. The impact of severe respiratory syncytial virus on the child, caregiver, and family during hospitalization and recovery. Pediatrics. 2005;115:1536–1546. doi: 10.1542/peds.2004-1149. [DOI] [PubMed] [Google Scholar]
- 34.Miedema CJ, Kors AW, Tjon A Ten WE, et al. Medical consumption and socioeconomic effects of infection with respiratory syncytial virus in The Netherlands. Pediatr Infect Dis J. 2001;20:160–163. doi: 10.1097/00006454-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Leader S, Yang H, DeVincenzo J, et al. Time and out-of-pocket costs associated with respiratory syncytial virus hospitalization of infants. Value Health. 2003;6:100–106. doi: 10.1046/j.1524-4733.2003.00220.x. [DOI] [PubMed] [Google Scholar]
- 36.Robbins JM, Tilford JM, Gillaspy SR, et al. Parental emotional and time costs predict compliance with respiratory syncytial virus prophylaxis. Ambul Pediatr. 2002;2:444–448. doi: 10.1367/1539-4409(2002)002<0444:peatcp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 37.Colin AA, McEvoy C, Castile RG. Respiratory morbidity and lung function in preterm infants of 32 to 36 weeks’ gestational age. Pediatrics. 2010;126:115–128. doi: 10.1542/peds.2009-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kugelman A, Colin AA. Late preterm infants: near term but still in a critical developmental time period. Pediatrics. 2013;132:741–751. doi: 10.1542/peds.2013-1131. [DOI] [PubMed] [Google Scholar]
- 39.Weigl JA, Puppe W, Schmitt HJ. Incidence of respiratory syncytial virus-positive hospitalizations in Germany. Eur J Clin Microbiol Infect Dis. 2001;20:452–459. doi: 10.1007/s100960100527. [DOI] [PubMed] [Google Scholar]
- 40.Fjaerli HO, Farstad T, Bratlid D. Hospitalisations for respiratory syncytial virus bronchiolitis in Akershus, Norway, 1993-2000: a population-based retrospective study. BMC Pediatr. 2004;4:25. doi: 10.1186/1471-2431-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall CB, Weinberg GA, Blumkin AK, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132:e341–e348. doi: 10.1542/peds.2013-0303. [DOI] [PubMed] [Google Scholar]
- 42.Panozzo CA, Fowlkes AL, Anderson LJ. Variation in timing of respiratory syncytial virus outbreaks: lessons from national surveillance. Pediatr Infect Dis J. 2007;26(11 suppl):S41–S45. doi: 10.1097/INF.0b013e318157da82. [DOI] [PubMed] [Google Scholar]
- 43.Haynes AK, Prill MM, Iwane MK, et al. Centers for Disease Control and Prevention (CDC) Respiratory syncytial virus–United States, July 2012-June 2014. MMWR Morb Mortal Wkly Rep. 2014;63:1133–1136. [PMC free article] [PubMed] [Google Scholar]
- 44.Schuh S, Freedman S, Coates A, et al. Effect of oximetry on hospitalization in bronchiolitis: a randomized clinical trial. JAMA. 2014;312:712–718. doi: 10.1001/jama.2014.8637. [DOI] [PubMed] [Google Scholar]
- 45.King D, Dicks RA, Wacogne ID. Infants with artificially elevated pulse oximetry levels less likely to be hospitalised during an episode of mild to moderate bronchiolitis. Arch Dis Child Educ Pract Ed. 2016;101:162–163. doi: 10.1136/archdischild-2016-310570. [DOI] [PubMed] [Google Scholar]
- 46.Baraldi E, Lanari M, Manzoni P, et al. Inter-society consensus document on treatment and prevention of bronchiolitis in newborns and infants. Ital J Pediatr. 2014;40:65. doi: 10.1186/1824-7288-40-65. [DOI] [PMC free article] [PubMed] [Google Scholar]


