Abstract
Purpose
The purpose of our study was to evaluate the therapeutic effect of VIP on human retinal endothelial cells (HREC) under high glucose conditions. Diabetes affects almost 250 million people worldwide. Over 40% of diabetics are expected to develop diabetic retinopathy, which remains the leading cause of visual impairment/blindness. Currently, treatment is limited to late stages of retinopathy with no options available for early stages. To this end, the purpose of the current study is to evaluate the therapeutic effect of vasoactive intestinal peptide (VIP) on HREC under high glucose conditions.
Methods
Primary HREC were cultured in normal (5 mM) or high (25 mM) glucose medium +/− VIP treatment. Protein levels of TNF-α, resolvin D1 (RvD1), formyl peptide receptor 2 (FPR2), G protein-coupled receptor 32 (GPR32), VEGF, and VIP receptors, VPAC1 and VPAC2 were measured.
Results
High glucose-induced changes in TNF-α and RvD1 were restored to control levels with VIP treatment. RvD1 receptors, FPR2 and GPR32, were partially rescued with VIP treatment. VPAC2 expression appeared to be the major receptor involved in VIP signaling in HREC, as VPAC1 receptor was not detected. In addition, VIP did not induce HREC secretion of VEGF under high glucose conditions.
Conclusions
Our results demonstrate that VIP’s therapeutic effect on HREC, occurs in part, through the balance between the pro-inflammatory cytokine, TNF-α, and the pro-resolving mediator, RvD1. Although VPAC1 is considered the major VIP receptor, VPAC2 is predominantly expressed on HREC under both normal and high glucose conditions.
Keywords: neuropeptide, resolvins, diabetic retinopathy, treatment
Introduction
Diabetic retinopathy (DR) continues as the leading cause of irreversible blindness in the United States resulting in over 10,000 new cases annually1. With both type 1 and type 2 diabetics at risk, over 40% of all adult diabetic patients are expected to develop this visually debilitating disease. Despite the fact that diabetes is projected to reach epidemic levels by 2030, there remains no available treatment for early stage DR, save for maintaining glycemic control. Hallmark features of DR are of both vascular and neural natures, including leukocyte adhesion to retinal vasculature, vascular occlusions, endothelial cell damage and pericyte and photoreceptor loss with underlying degenerative and inflammatory changes2. Inflammation has been linked to DR as early as the 1960’s when it was found that diabetic patients, who were administered salicylates for rheumatoid arthritis, demonstrated a lower incidence of retinopathy3. However, only more recently has the inflammatory response come to the forefront as a major contributing factor to the development and progression of DR.
TNF-α is a well-characterized cytokine known to play a role in a wide spectrum of biological activities, predominately pro-inflammatory in nature. It has been reported that TNF-α levels are increased in retinas of both type 1 and type 2 diabetic rodents, as well as during the development of DR4. It has been indicated as a major cytokine involved in driving leukocyte adhesion. Furthermore, it has been shown that this molecule induces endothelial and pericyte cell injury and apoptotic cell death2; key events in the progression of DR.
In contrast, resolvins are a family of protective, pro-resolving compounds produced by docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) biosynthetic pathways5. RvD1 is derived from D-series ω-3 polyunsaturated fatty acids and binds to G protein-coupled receptors ALX/FPR2 and GPR326, leading to reduced polymorphonuclear leukocytes (PMN) infiltration and increased nonphlogistic phagocytosis of apoptotic PMN7. A protective effect of RvD1 against diabetic neovascularization has been demonstrated, in part through the suppression of the pro-inflammatory cytokine TNF-α8.
VIP is an endogenous immunoregulatory neuropeptide synthesized by neurons throughout the central and peripheral nervous systems, in addition to immune cells9. Focusing on the retina, VIP immunoreactivity has been detected in amacrine cells and other interneurons of the inner nuclear layer (INL) and inner plexiform layer (IPL)10,11. The immunoregulatory activities of VIP are mediated predominately by two G protein-coupled receptors, VPAC1/VIPR1 and VPAC2/VIPR2. VPAC1 is constitutively expressed in lymphocytes, macrophages, dendritic cells, microglia, monocytes and mast cells, whereas VPAC2 is thought to require activation12. VPAC1 serves as the major immunoregulatory receptor for VIP in various immune cells, while VPAC2 is thought to play a role in immune homeostasis and tissue restoration13,14.
Recent studies have reported decreased expression of VIP and both VPAC1/VPAC2 receptors in the retina during early stage DR14,15. Moreover, in diabetic macular edema, activation of the protective VIP/PACAP pathway has been shown to prevent the breakdown of the outer blood retinal barrier by mediating tight junction integrity16. However, the modulatory mechanism and potential therapeutic effect of VIP during the development of DR is largely unknown. The current study seeks to demonstrate a potential pro-resolving role for VIP during DR by preliminarily investigating its interaction with TNF-α and RvD1 in HREC under high glucose conditions.
Materials and Methods
Retinal Endothelial Cell Culture
Primary HREC were acquired from Cell System Corporation (CSC, Kirkland, WA). Cells were grown in M131 medium containing microvascular growth supplements (MVGS; Invitrogen, Carlsbad, CA), 10 mg/mL gentamycin, and 0.25 mg/mL amphotericin B. All primary cells were used within six passages. Prior to experimentation, cells were transferred for three days to high (25 mM) or normal (5 mM) glucose medium (M131 medium supplemented with glucose) with MVGS and antibiotics, then quiesced by removing MVGS for 24h. Cells were exposed to VIP (10−9 M) for 4h17,18, followed by rinsing with cold PBS and collection into lysis buffer containing protease and phosphatase inhibitors. Cellular extracts were prepared by sonication, and total protein concentration was determined for analyses as described below.
To evaluate whether VIP acts directly via VPAC2 and/or ALX/FPR2 regarding TNF-α levels, cells were treated with the VIP receptor antagonist, [D-p-Cl-Phe6,Leu17]-VIP (Leu) (VPAC antagonist; R&D Systems, Minneapolis, MN) or a selective antagonist of ALX/FPR2 signaling, WRW4 (Tocris, Pittsburg, PA). Cells cultured under normal and high glucose conditions were exposed to VIP (10−9 M) in the presence of each antagonist (Leu at 2 μM19 or WRW4 at 1 μM20) for 4h, then processed for protein analyses as described above.
Previously, high osmolar conditions have been included as an additional control to determine whether the observed in vitro effects were a result of high glucose treatment or increased osmolarity of the treatment media21. Since it has been established that no differences were observed between high osmolarity and normal glucose, this control was omitted from the current study.
ELISA
Levels for TNF-α and RvD1 were determined using ELISA kits (Thermo Fisher Scientific, Waltham, MA; Cayman Chemical, Ann Arbor, MI). Cells were collected and processed as described above. All samples were centrifuged at 5,000 × g for 5 min and an aliquot of each supernatant was assayed in duplicate or triplicate per the manufacturer’s instruction. Equal protein was loaded into all wells. The reported sensitivities of these assays are as follows: <2.0 pg/mL for TNF-α and 3.3 pg/mL for RvD1.
Western Blotting
Proteins were separated on 4–12% tris-glycine gels (Invitrogen, Carlsbad, CA) and transferred to nitrocellulose membranes. After blocking membranes in TBST (10 mM Tris-HCl buffer, pH 8.0, 150 mM NaCl, 0.1% Tween 20) and 5% (w/v) BSA at r.t. for 60 min, membranes were incubated overnight at 4°C with antigen-specific primary antibodies. The primary antibodies were used as follows: GPR32, ALX/FPR2 and VEGF (Abcam, San Francisco, CA); VPAC1 and VPAC2 (Santa Cruz, Santa Cruz, CA). Blots were then incubated with species-specific HRP-conjugated secondary antibodies for 2 h at r.t. Proteins were visualized by incubation with a chemiluminescence substrate kit (Thermo Fisher Scientific, Waltham, MA). Western blot images were collected (Azure Biosystem C500, Dublin, CA) and target protein levels were quantified (Image Studio Lite software) after normalizing to β-actin. One representative blot is shown. Treatment groups were normalized to β-actin levels and then compared to normal glucose, which was normalized to 1.0.
Statistical analysis
All assays were performed twice from three independent experiments and the data (n = 6/group) are presented as mean ± SEM. Data were analyzed by the Kruskal-Wallis test, followed by Dunn’s testing. P < 0.05 was considered to be statistically significant.
Results
VIP reduced levels of high glucose-induced TNF-α
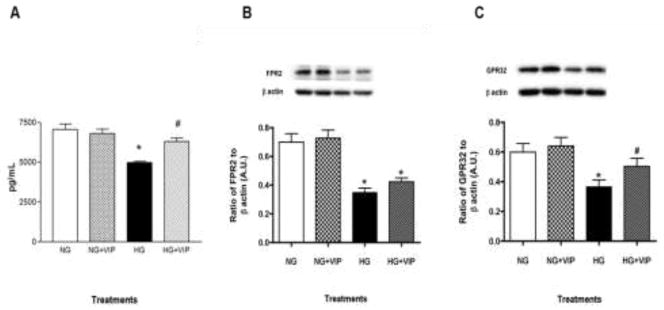
Changes in TNF-α protein levels were assessed under high glucose conditions and after VIP treatment, as shown in Figure 1. As expected, TNF-α protein levels were significantly increased by HREC under high glucose compared to normal glucose conditions (control). This effect was abrogated with VIP treatment, whereby TNF-α levels were similar to controls. No significant effect was observed with VIP treatment in cells cultured in normal glucose.
Figure 1.
TNF-α protein levels as detected by ELISA. HREC were cultured under normal glucose (NG, 5 mM) and high glucose (HG, 25 mM) conditions +/− VIP treatment (10−9 M) for 4 hours. Data shown are representative of three independent experiments in duplicate (n = 6) and are expressed as mean ± SEM. *P < 0.05 vs NG, #P < 0.05 vs HG.
Effect of VIP on pro-resolving mediators
Our previous research on the cornea has demonstrated VIP’s therapeutic effect to be both anti-inflammatory and pro-resolving17,22–24. As such, we next addressed a potential mechanism by which VIP might mediate these pro-resolving effects by looking at RvD1 and corresponding receptors, ALX/FPR2 and GPR32. As illustrated in Figure 2A, RvD1 levels were significantly reduced in high glucose versus normal glucose conditions. However, levels were increased with VIP treatment in high glucose. No notable changes were observed in HREC after VIP treatment under normal conditions.
Figure 2.
HREC were cultured under normal glucose (NG, 5 mM) and high glucose (HG, 25 mM) conditions +/− VIP treatment (10−9 M) for 4 hours. Protein levels of RvD1 (A) were measured by ELISA and its receptors ALX/FPR2 (B) and GPR32 (C) were detected by Western blot. Data shown are representative of three independent experiments in duplicate (n = 6) and are expressed as mean ± SEM. *P < 0.05 vs NG, #P < 0.05 vs HG.
In addition, RvD1 receptors ALX/FPR2 and GPR32 were significantly down-regulated (approximately 50% and 40%, respectively) after high glucose exposure compared to normal glucose controls (Fig. 2B and C, respectively), which is consistent with the changes observed in their ligand, RvD1. Further, VIP treatment enhanced protein levels of both receptors, albeit only GPR32 was significantly increased (28%) over high glucose only. ALX/FPR2 and GPR32 levels after VIP treatment of normoglycemic cells remained comparable to controls.
VIP receptor expression by HREC
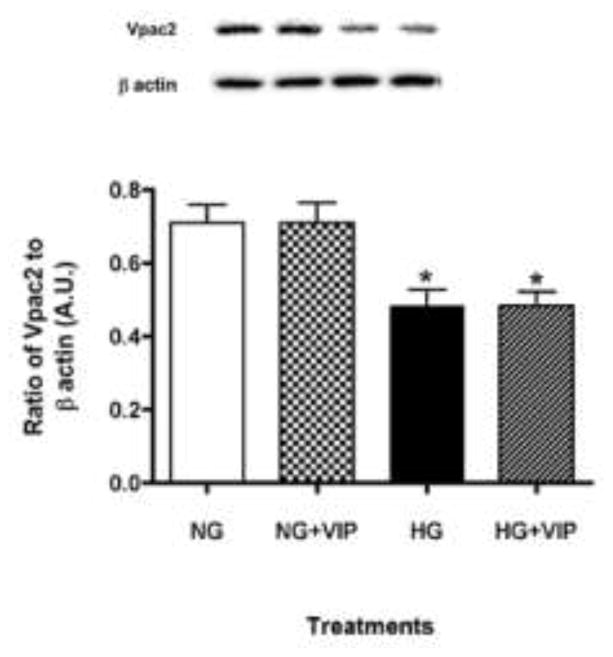
As the two predominant receptors of VIP, levels of VPAC1 and VPAC2 were evaluated in HREC. Although described as the major VIP receptor on most cell types12, VPAC1 was not detected in HREC in either normal or high glucose. In contrast, VPAC2 was constitutively expressed under both high and normal glucose conditions (Fig. 3). However, VPAC2 protein levels were significantly reduced in high glucose versus normal glucose conditions, despite VIP treatment.
Figure 3.
VPAC2 receptor levels. HREC were cultured under normal glucose (NG, 5 mM) and high glucose (HG, 25 mM) conditions +/− VIP treatment (10−9 M) for 4 hours. Protein levels of VPAC2 were examined by Western blot. Data shown are representative of three independent experiments in duplicate (n = 6) and are expressed as mean ± SEM. *P < 0.05 vs NG, #P < 0.05 vs HG.
VIP-induced effects are carried out by VPAC2 receptors
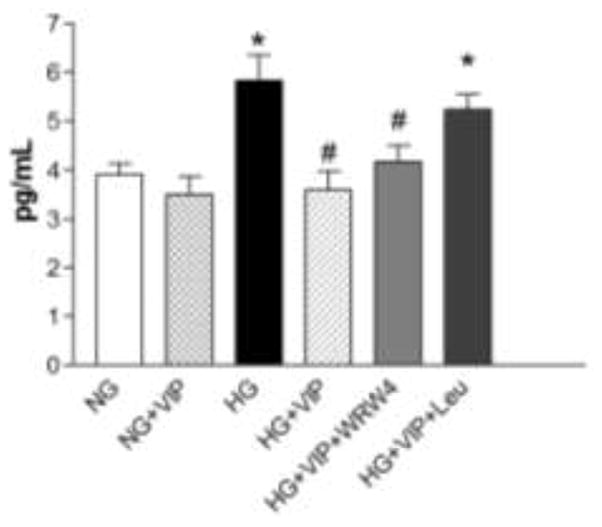
Despite that VPAC2 was predominantly expressed on HREC, we next confirmed whether the VIP-induced changes in TNF-α levels were directly mediated by this receptor. To do so, HREC were cultured under high glucose conditions followed by exposure to a receptor antagonist for either ALX/FPR2 (WRW4) or VPAC2 (Leu) prior to VIP treatment. As shown in Figure 4, results indicate that VIP-induced down-regulation of TNF-α was abrogated in the presence of the VPAC2 antagonist, but not the ALX/FPR2 antagonist. In fact, TNF-α levels from HREC cultured in HG +VIP+Leu were comparable to HG only and significantly higher than NG, NG+VIP and HG+VIP. While the ALX/FPR2 antagonist, WRW4, did result in a slight increase in TNF-α levels compared to HG+VIP, this difference was not statistically significant.
Figure 4.
TNF-α protein levels as detected by ELISA. HREC were cultured under normal glucose (NG, 5 mM) and high glucose (HG, 25 mM) conditions +/− ALX/FPR2 antagonist (WRW4) or VPAC antagonist (Leu) +/− VIP treatment (10−9 M). Data shown are representative of three independent experiments in duplicate (n = 6) and are expressed as mean ± SEM. *P < 0.05 vs NG, #P < 0.05 vs HG.
VIP treatment prevents high glucose-induced increases in VEGF
Considerable clinical effort has been put forth to inhibit VEGF, as it is well known to cause retinal permeability and neovascularization in diabetes. As VIP was previously reported to enhance growth factor production24, we investigated VEGF protein levels after VIP treatment under both normal and high glucose conditions to evaluate this potential side effect. As depicted in Figure 5, VIP treatment did not have any effect on VEGF levels under normal glucose conditions. Remarkably, high glucose-induced VEGF levels, however, were significantly reduced after VIP treatment.
Figure 5.
Protein levels of VEGF as detected by Western blot. HREC were cultured under normal glucose (NG, 5 mM) and high glucose (HG, 25 mM) conditions +/− VIP treatment (10−9 M) for 4 hours. Data shown are representative of three independent experiments in duplicate (n = 6) and are expressed as mean ± SEM. *P < 0.05 vs NG, #P < 0.05 vs HG.
Discussion
Studies have indicated TNF-α is an important mediator of the retinal pathology observed under hyperglycemic conditions, including leukocyte adherence in retinal blood vessels25, retinal endothelial cell apoptosis26, pericyte loss and capillary degeneration27,28 and vascular permeability and leukostasis29. It has been previously shown by Jiang et al. that TNF-α levels are increased in HREC cultured under hyperglycemic conditions26. Therefore, as an initial step toward characterizing the retino-protective effects of VIP during DR, we examined whether VIP can regulate levels of this potent pro-inflammatory cytokine. As expected, high glucose significantly induced TNF-α expression in HREC, suggesting its involvement in the diabetic inflammatory response. VIP abrogated this effect, thus returning TNF-α to control levels. This anti-inflammatory effect of VIP has been reported in other diabetic systems; as VIP was shown to inhibit TNF-α induced apoptosis in acinar cells isolated from submandibular glands of non-obese diabetic mice with salivary dysfunction through functional VPAC1 receptors coupled to the protein kinase A signaling pathway30. The current study suggests that VIP, as an alternate treatment against DR, may effectively ameliorate the initial pro-inflammatory cytokine release observed in vivo; however this effect appears to be VPAC1-independent and associated with the pro-resolving molecule, RvD1.
The balance between TNF-α and RvD1 appears to be an important factor in disease pathogenesis. Previous research also indicates that TNF-α can suppress RvD1 expression8. Using a uveitis model, topical ocular application of RvD1 was shown to reduce levels of TNF-α, resulting in improved disease outcome31. Likewise, in a diabetic mouse model, RvD1 expression was enhanced with the reduction of TNF-α following etanercept treatment, leading to a reduction of pathological retinal angiogenesis8. This resolvin has been demonstrated to reduce angiogenesis and protect against retinopathy8. Therefore, we next sought RvD1 as a potential mechanism by which VIP might mediate its pro-resolving effects. To this end, the current study showed that high glucose conditions decreased RvD1 levels in HREC. More importantly, VIP treatment effectively up-regulated RvD1 production after high glucose exposure similar to observed normal glucose levels. In addition, high glucose-induced reduction of ALX/FPR2 and GPR32 was partially rescued after VIP treatment. Both of these RvD1 receptors are important in carrying out the resolution of acute inflammation by mediating PMN recruitment31, and promoting D1-miRNA circuits33. These data support the idea that VIP’s pro-resolving effects are carried out, at least in part, via lipid mediator circuits. Further, the inverse relationship between TNF-α and RvD1 enhance the efficacy of VIP as a potential therapeutic.
Although VPAC1 is broadly expressed on different cell types, most notably immune cells12, and has received considerable attention for its anti-inflammatory effects, it was not detected in HREC. VPAC1 has been detected in rat brain microvascular endothelial cells34, as well as transformed murine endothelial cells derived from heart (H5V)35, indicating that a lack of VPAC1 detection could be unique to retinal endothelial cells. In contrast, VPAC2 appeared to be constitutively expressed under normal glucose conditions, yet decreased after exposure to high glucose. We have previously shown in a bacterial keratitis model that VPAC2 is more strongly correlated with tissue homeostasis and disease resolution19. Regarding diabetes, Ma et al. have indicated that VPAC2 activation leads to improved glucose and lipid metabolism, while increasing insulin sensitivity in db/db mice36. In the current study, we demonstrate that VPAC2, not VPAC1, is expressed by HREC, thus suggesting a potential mechanism by which VIP treatment could ameliorate disease progression of DR. Although VIP treatment was not able to rescue high glucose-induced down-regulation of VPAC2, it is possible that this receptor could be associated with RvD1 expression/activation. In this regard, VIP-induced changes in TNF-α levels were abrogated by a VPAC antagonist. These findings support the notion that the observed VIP-mediated effects regarding this pro-inflammatory mediator are carried out primarily both of this receptor pathway, which will be further explored in future in vivo studies.
In light of our previous research highlighting VIP’s ability to enhance growth factor expression during corneal wound healing and reconstitution of the extracellular matrix23, we next determined whether this neuropeptide increases VEGF levels. VEGF has been implicated as a major causative factor in diabetic macular edema, retinal neovascularization and related complications37. Remarkably, HREC expression of VEGF was significantly down-regulated in high glucose with VIP treatment compared to high glucose only. Similar to the cornea, which must remain clear for accurate visual processing, VIP treatment does not appear to induce angiogenesis via VEGF expression. These findings are essential in moving forward with investigating VIP as an alternative therapy for DR.
Overall, the current study reports the expression of VPAC2, but not VPAC1, on retinal endothelial cells. Additionally, it indicates a novel regulatory role for VIP over RvD1 levels. Taken together, these findings provide rationale to further explore the therapeutic potential of VIP in the development and progression of DR. This neuropeptide not only reduced anti-inflammatory mediators, but is tied to important lipid mediator circuits, as well. In addition, these data suggest that VPAC2 (not VPAC1) and ALX/FPR2 are the major receptors involved in VIP signaling in HREC.
Highlights.
VIP treatment reduces high glucose-induced TNF-α levels in HREC
High glucose reduces RvD1 expression by HREC
VIP treatment restores RvD1 levels in HREC exposed to high glucose
ALX/FPR2 and GPR32 are expressed on HREC
VPAC2 is the predominant receptor expressed on HREC
Acknowledgments
NIH grants R01 EY023226 (EAB), R01 EY022045 (JJS), P30EY004068 (Core Grant), Research to Prevent Blindness (RPB)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fong DS, et al. Diabetic retinopathy. Diabetes Care. 2004;27(10):2540–53. doi: 10.2337/diacare.27.10.2540. [DOI] [PubMed] [Google Scholar]
- 2.Joussen AM, et al. TNF-alpha mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol Vis. 2009;15:1418–28. [PMC free article] [PubMed] [Google Scholar]
- 3.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30(5):343–58. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joussen AM, et al. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J. 2002;16(3):438–40. doi: 10.1096/fj.01-0707fje. [DOI] [PubMed] [Google Scholar]
- 5.Serhan CN, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196(8):1025–37. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnamoorthy S, et al. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107(4):1660–5. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maddox JF, et al. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J Biol Chem. 1997;272(11):6972–8. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- 8.Connor KM, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13(7):868–73. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henning RJ, Sawmiller DR. Vasoactive intestinal peptide: cardiovascular effects. Cardiovasc Res. 2001;49(1):27–37. doi: 10.1016/s0008-6363(00)00229-7. [DOI] [PubMed] [Google Scholar]
- 10.Terubayashi H, et al. VIP (vasoactive intestinal polypeptide)-like immunoreactive amacrine cells in the retina of the rat. Exp Eye Res. 1983;36(5):743–9. doi: 10.1016/0014-4835(83)90111-2. [DOI] [PubMed] [Google Scholar]
- 11.Park SJ, et al. Function and Circuitry of VIP+ Interneurons in the Mouse Retina. J Neurosci. 2015;35(30):10685–700. doi: 10.1523/JNEUROSCI.0222-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004;56(2):249–90. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- 13.Ganea D, Hooper KM, Kong W. The neuropeptide vasoactive intestinal peptide: direct effects on immune cells and involvement in inflammatory and autoimmune diseases. Acta Physiol (Oxf) 2015;213(2):442–52. doi: 10.1111/apha.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giunta S, et al. Early changes in pituitary adenylate cyclase-activating peptide, vasoactive intestinal peptide and related receptors expression in retina of streptozotocin-induced diabetic rats. Peptides. 2012;37(1):32–9. doi: 10.1016/j.peptides.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Troger J, et al. Substance P and vasoactive intestinal polypeptide in the streptozotocin-induced diabetic rat retina. Invest Ophthalmol Vis Sci. 2001;42(5):1045–50. [PubMed] [Google Scholar]
- 16.Scuderi S, et al. Ameliorative effect of PACAP and VIP against increased permeability in a model of outer blood retinal barrier dysfunction. Peptides. 2013;39:119–24. doi: 10.1016/j.peptides.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Szliter EA, et al. Vasoactive intestinal peptide balances pro- and anti-inflammatory cytokines in the Pseudomonas aeruginosa-infected cornea and protects against corneal perforation. J Immunol. 2007;178(2):1105–14. doi: 10.4049/jimmunol.178.2.1105. [DOI] [PubMed] [Google Scholar]
- 18.Delgado M, Ganea D. Inhibition of endotoxin-induced macrophage chemokine production by vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide in vitro and in vivo. J Immunol. 2001;167(2):966–75. doi: 10.4049/jimmunol.167.2.966. [DOI] [PubMed] [Google Scholar]
- 19.Xie Y, et al. Vasoactive intestinal peptide transactivates the androgen receptor through a protein kinas A-dependent extracellular signal-regulated kinase pathway in prostate cancer LNCaP cells. Mol Pharmacol. 2007;72(1):73–85. doi: 10.1124/mol.107.033894. [DOI] [PubMed] [Google Scholar]
- 20.Bae YS, et al. Identification of peptides that antagonize formyl peptide receptor-like 1-mediated signaling. J Immunol. 2004;173(1):607–14. doi: 10.4049/jimmunol.173.1.607. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Soderland C, Steinle JJ. Regulation of retinal endothelial cell apoptosis through activation of the IGFBP-3 receptor. Apoptosis. 2013;18(3):361–368. doi: 10.1007/s10495-012-0793-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger EA, et al. VIP promotes resistance in the Pseudomonas aeruginosa-infected cornea by modulating adhesion molecule expression. Invest Ophthalmol Vis Sci. 2010;51(11):5776–82. doi: 10.1167/iovs.09-4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berger EA, et al. Effects of VIP on corneal reconstitution and homeostasis following Pseudomonas aeruginosa induced keratitis. Invest Ophthalmol Vis Sci. 2012;53(12):7432–9. doi: 10.1167/iovs.12-9894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang X, et al. VIP and growth factors in the infected cornea. Invest Ophthalmol Vis Sci. 2011;52(9):6154–61. doi: 10.1167/iovs.10-6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joussen AM, et al. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol. 2002;160(2):501–9. doi: 10.1016/S0002-9440(10)64869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang Y, et al. TNFalpha and SOCS3 regulate IRS-1 to increase retinal endothelial cell apoptosis. Cell Signal. 2012;24(5):1086–92. doi: 10.1016/j.cellsig.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behl Y, et al. Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol. 2008;172(5):1411–8. doi: 10.2353/ajpath.2008.071070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behl Y, et al. FOXO1 plays an important role in enhanced microvascular cell apoptosis and microvascular cell loss in type 1 and type 2 diabetic rats. Diabetes. 2009;58(4):917–25. doi: 10.2337/db08-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang H, et al. TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest Ophthalmol Vis Sci. 2011;52(3):1336–44. doi: 10.1167/iovs.10-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calafat M, et al. Vasoactive intestinal peptide inhibits TNF-alpha-induced apoptotic events in acinar cells from nonobese diabetic mice submandibular glands. Arthritis Res. doi: 10.1186/ar2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ther. 2009;11(2):R53.29. doi: 10.1186/ar2671. [DOI] [PMC free article] [PubMed] [Google Scholar]; Settimio R, et al. Resolvin D1 reduces the immunoinflammatory response of the rat eye following uveitis. Mediators Inflamm. 2012;2012:318621.30. doi: 10.1155/2012/318621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norling LV, et al. Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: receptor-dependent actions. Arterioscler Thromb Vasc Biol. 2012;32(8):1970–8. doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Recchiuti A, et al. MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB J. 2011;25(2):544–60. doi: 10.1096/fj.10-169599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J, et al. Vasoactive intestinal peptide increases VEGF expression to promote proliferation of brain vascular endothelial cells via the cAMP/PKA pathway after ischemic insult in vitro. Peptides. 2013;42:105–11. doi: 10.1016/j.peptides.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Castorina A, et al. Effects of PACAP and VIP on hyperglycemia-induced proliferation in murine microvascular endothelial cells. Peptides. 2010;31(12):2276–83. doi: 10.1016/j.peptides.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Ma Y, et al. A recombinant slow-release PACAP-derived peptide alleviates diabetes by promoting both insulin secretion and actions. Biomaterials. 2015;51:80–90. doi: 10.1016/j.biomaterials.2015.01.064. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, et al. Vascular endothelial growth factor-A: a multifunctional molecular player in diabetic retinopathy. Int J Biochem Cell Biol. 2009;41(12):2368–71. doi: 10.1016/j.biocel.2009.07.011. [DOI] [PubMed] [Google Scholar]