Abstract
Hypothesis
The compound action potential (CAP) is a purely neural component of the cochlea’s response to sound, and may provide information about the existing neural substrate in cochlear implant (CI) subjects that can help account for variance in speech perception outcomes.
Background
Measurement of the ‘total response’ (TR), or sum of the magnitudes of spectral components in the ongoing responses to tone bursts across frequencies, has been shown to account for 40–50% of variance in speech perception outcomes. The ongoing response is composed of both hair cell and neural components. This correlation may be improved with the addition of the CAP.
Methods
Intraoperative round window electrocochleography (ECochG) was performed in adult and pediatric CI subjects (n=238). Stimuli were tones of different frequencies (250 Hz–4 kHz) at 90 dB nHL. The CAP was assessed in two ways, as an amplitude and with a scaling factor derived from a function fitted to the response. The results were correlated with CNC word scores at 6 months post-implantation (n=51).
Results
Only about half of the subjects had a measurable CAP at any frequency. The consonant-nucleus-consonant (CNC) word scores correlated weakly with both amplitude (r2=0.20, p < 0.001) and scaling factor (r2=0.25, p < 0.01). In contrast, the TR alone accounted for 43% of the variance, and addition of either CAP measurement in multiple regression did not account for additional variance.
Conclusions
The underlying pathology in CI patients causes the CAP to be often absent and highly variable when present. The TR is a better predictor of speech perception outcomes than the CAP.
Introduction
There is a wide range of speech perception outcomes in patients with cochlear implants (CIs). Many factors have been shown to influence these outcomes, including duration of deafness, age, and audiometric and surgical factors1–4. However such factors have only been shown to account for a small amount of the variability among patients, typically less than 20% for single factors and only slightly increased with multiple variables included. A factor that may help explain more of the variability is residual cochlear physiology. Given that cochlear implants function by stimulating residual neural elements, it is intuitive that having more surviving nerve fibers should lead to improved outcomes. However, this intuition has generally not been confirmed by the literature. Most anatomic studies that have counted spiral ganglion cells post-mortem have shown no correlation or even negative correlation between surviving nerve fibers and speech perception5–8. An exception is a recent study that showed a positive correlation between SCG counts and outcomes in six bilaterally implanted patients9.
Evidence for the influence of residual cochlear physiology on speech perception has recently come from electrocochleography (ECochG). Using ECochG, the electrical response of the cochlea to sound can be recorded prior to implantation. Measurements of the “total response” (TR), accounted for 40–50% of variance in speech recognition outcomes at 6 months10,11. The TR is the sum of the spectral magnitudes of the ongoing, or steady-state, response to a series of tone bursts of different frequencies. A limitation is that TR does not discriminate between hair cell (cochlear microphonic, or CM) and neural responses (auditory nerve neurophonic, or ANN) that are combined in the ongoing response. The major aim of this study was to determine if the compound action potential (CAP), a purely neural response, can be used to improve the ability of ECochG to account for variance in speech perception across subjects.
The CAP is the product of the summed, synchronous firing of action potentials from auditory nerve fibers in response to the onset or offset of a signal12–14. It is a purely neural potential, unlike the TR. Earl and Chertoff15 demonstrated in gerbils that by fitting a modeled CAP that includes a scaling parameter reflecting neural survival to measured CAPs, it was possible to predict the extent of experimentally induced damage to the auditory nerve more accurately than if they measured the CAP amplitude alone. For the present study, we evaluated different ways to measure the CAP following intraoperative recording in CI subjects, including the analytic method of Earl and Chertoff (2010), in both children and adults. We then correlated these measures to consonant-nucleus-consonant (CNC) word scores 6-month post-implantation in adult CI subjects, both alone and in combination with the TR.
Materials and Methods
All pediatric and adult CI candidates at the study institution were potential subjects. Non-English speaking subjects, subjects undergoing revision surgery, and subjects with malformed cochlear anatomy were excluded. The procedures were in accordance with the ethical standards of the study site’s institutional review board, and informed consent was obtained for all subjects before implantation (UNC Protocol No. 05-2616).
ECochG to acoustic stimuli was recorded intraoperatively from the round window (RW) just before electrode array insertion. Because the CAP has not been previously studied in the general population of CI subjects, the first step was to characterize and develop methods to quantify the CAP across adult and pediatric subjects (n=242, 112 adults and 130 children). The relationship between the CAP and postoperative speech perception outcomes was then evaluated in adult subjects with word score outcomes (n=54).
Surgery and Recording Setup
Cochlear implantation was performed by four neurotologists at the study institution. Before incision, surface electrodes were placed on the forehead and the contralateral mastoid, and a sound tube attached to a foam insert was placed in the ear canal. A standard transmastoid facial recess approach was used to access the cochlea. Once the RW niche was visible in the surgical field, a sterile monopolar probe (Neurosign, Magstim Co, Wales, UK) was placed adjacent to the RW membrane. All included recordings had impedances below 16 kOhms on each electrode. Acoustic stimuli were delivered from an Etymotic ER-3b speaker. Recordings took 5–10 minutes of surgical time prior to array insertion.
Stimulation and Recording
A Bio-logic Navigator Pro (Natus Medical, Inc., San Carlos, CA) generated acoustic stimuli and recorded electrophysiological signals. Saline was introduced into the RW niche to reduce impedance in some cases. Stimuli were tone bursts (250, 500, 750, 1000, 2000, and 4000 Hz), alternating in condensation and rarefaction starting phases, with up to 250 repetitions of each phase. In cases with large responses, fewer averages were often used. Rise/fall times were one cycle or 1 ms, whichever was longer, shaped by a Blackman window. Plateau durations were 20 ms (250–750 Hz), 10 ms (1000 and 2000 Hz) or 5 ms (4000 Hz). Recording epochs were 32 ms (250–2000 Hz) or 10.66 ms (4000 Hz). The high-pass filter setting was 10 Hz, and low-pass setting were 5000 Hz (250–1000Hz), 10000 Hz (2 kHz) or 15,000 Hz (4 kHz). Stimulation rates were 17.3 (250–2000 Hz) or 23.3 (4000 Hz) per sec. All stimuli were presented at 90 dB nHL (87–107 dB SPL depending on frequency).
Data Analysis
The data from condensation and rarefaction phases were stored separately. An average ‘difference’ trace was determined by subtracting, and a ‘sum’ trace by adding, the response to the condensation and rarefaction phases and dividing by 2. The TR from each case represents the magnitude of the fast Fourier transform (FFT) of the stimulus frequency and its harmonics during the ongoing portion of the response, summed across stimulus frequencies. Each response peak was considered significant if it exceeded the noise level by 3 standard deviations. The noise and its variance were determined from 6 bins, 3 on each side of the signal starting 2 bins away from the peak.
The sum tracing was used for all CAP measurements. First, the CAP amplitude was measured as a negative deviation from a baseline, and was considered significant if the first negative deflection (N1) was below both the baseline and the ongoing response by more than 3 standard deviations. For this measurement, filters and windows often needed to be adjustable as the latency and morphology of the CAP varied considerably. Even with the addition of flexible filters and windows, the morphology of the CAP varied enough that many cases were resistant to this objective analysis. In cases where a CAP was visually present but not objectively detected the CAP was measured with a cursor as the maximum deviation from the pre-response baseline. For 250 Hz a CAP was difficult to detect either automatically or visually, so this frequency was not used in the CAP measurement.
The second measurement technique was based on a model (Eq. 1) developed by Goldstein and Kiang13, which represents a convolution of the single unit response waveform U(t) and the probability density function P(t) of firing in a population of nerve fibers whose size is represented by the scaling factor N. Functional equations for U(t) and P(t) were developed by Chertoff14 and are shown in equations 2 and 3. The single unit response waveform, U(t), is modeled as a decaying sine wave where k represents rate of decay and f represents oscillation frequency of the wave. The probability density function, P(t), is written as a gamma function, where β governs the width of the density function and α represents delay after stimulus onset. Time in ms is represented by t, γ is a constant with a value of 2, and ω=2π f. Nonlinear regression (MATLAB, NLINFIT) was used to model the CAP using the convolution equation 1-yielding a fitted CAP. Once a best-fit was obtained, the parameter of most interest was the scaling factor N. In many cases, the original sum tracing required a smoothing function (triangular smooth over one period of the stimulus frequency was used) for the regression to be successful. An R2 value was obtained for each fitted CAP. A fit with an R2 value of 0.75 or greater was considered acceptable.
| Eq 1 |
| Eq 2 |
| Eq 3 |
The CAP amplitudes and scaling factor N were summed across all frequencies where CAP was present to give a “total CAP amplitude” (TCA) and “total N’ (TN).
Speech Perception
The CNC word test consists of phonemically balanced monosyllabic words and was administered as part of the speech perception assessment by audiologists. It was scored as a percent correct out of 50 words, and the results 6 months after CI activation was the outcomes measure. Because it consists of isolated words, the CNC word test is typically more difficult than other speech perception measures, reducing ceiling effects16. The test was administered in the sound field, with the subject in a sound-isolated booth facing the speaker at 0° azimuth. Lists were presented at 60 dB SPL.
Statistics
Single and multiple linear regression was performed using IBM SPSS Statistics 23.
Results
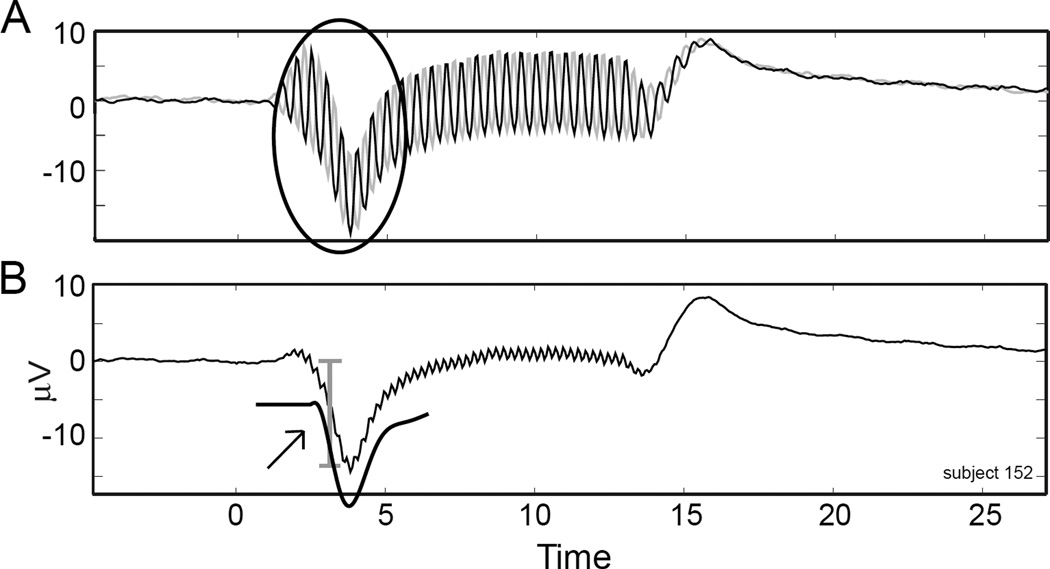
Figure 1 shows an example of a case with a CAP and the measurement methods used. The responses to condensation and rarefaction phases (Figure 1A) show a prominent CAP (circled). The sum of the responses (Figure 1B) removes those components that change with the phase of the stimulus, which includes most of the CM but little of the CAP in most cases. Thus, the sum waveform was used for all measurements. The largest portion and often the only part of the CAP was typically the first deflection below the baseline, so the N1 was taken as the CAP amplitude. The fitted CAP, used to determine N (Eq. 1), is also shown (arrow).
Figure 1. Measuring the Compound Action Potential.
A. Responses to condensation and rarefaction phase stimuli. The compound action potential (CAP) is circled. B. The sum of condensation and rarefaction phases. The “N1”amplitude of the CAP is marked by the bracket. The fitted CAP derived from the response is represented by the solid black line under the tracing (arrow).
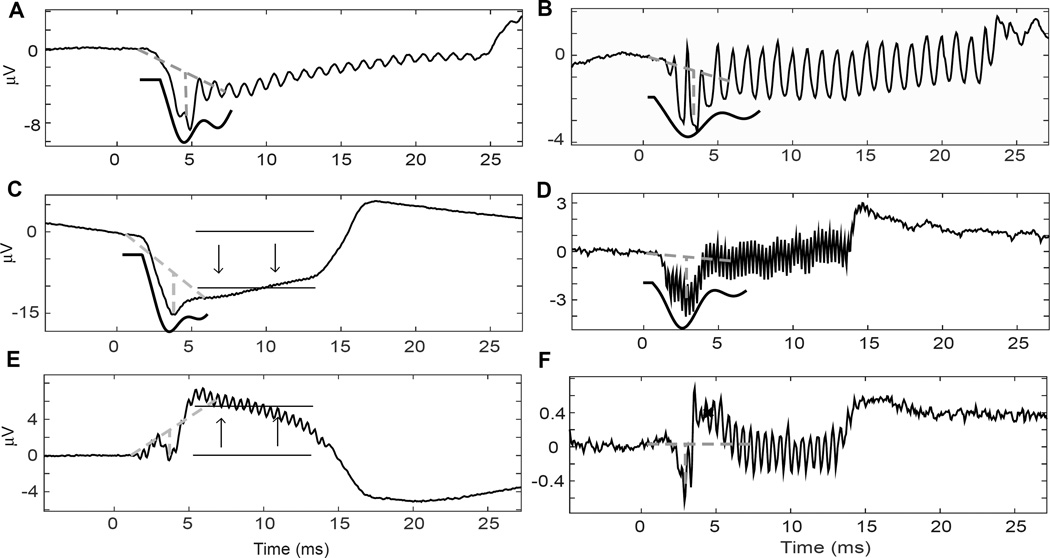
In general the CAPs had a highly variable morphology (Fig. 2). A major difficulty was that the CAPs were often partially obscured by the CM and/or the summating potential (SP, Figure 2A–2E). Because the modeling equation is based on a decaying sine wave, cases where the CAP took a different form proved challenging and sometimes impossible for the regression algorithm (Figure 2E, F). In 15 cases, CAP amplitude could be measured but it was not possible to obtain an “N” value with an R2 above our acceptable threshold using the model.
Figure 2. Examples of CAP morphology in CI patients.
For each case, the dotted lines indicate the baseline and amplitude measurements, and the solid black line is the model fit. A. This response had a well-defined CAP to a low frequency (500 Hz). B. This CAP to 500 Hz was obscured by the fine structure of the response. C. This apparent CAP was associated with a large summating potential (arrows). D. This CAP was shallow and spread in time. E. This CAP was also obscured by a summating potential, which was positive in this case. The fitting program was unable to measure this CAP. F. This CAP had a prominent P1, or positive, component (x). The fitting program was also unable to measure this CAP.
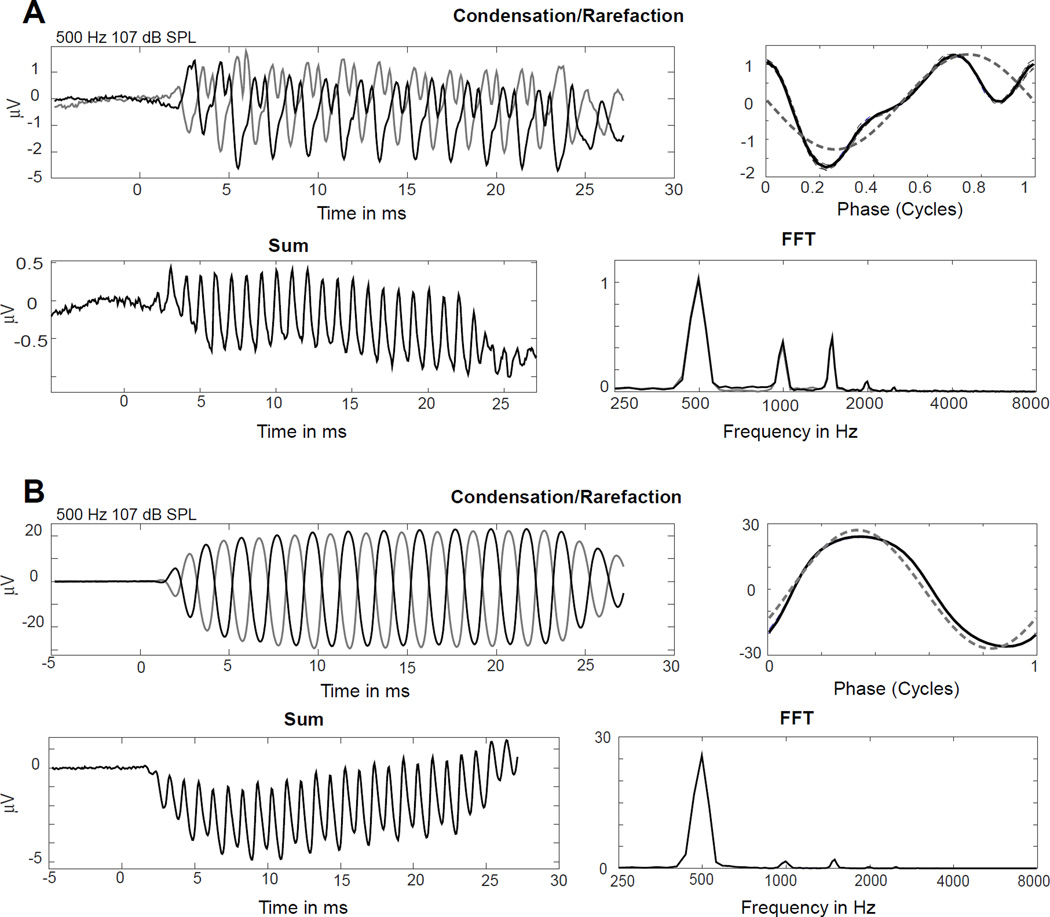
A CAP was detected to at least one frequency in 50% of subjects (121/242). The CAP was slightly more common in adults (56%) than children (46%). In contrast, a significant ongoing response was present in almost all cases (>90%). The lack of a CAP was not conclusive evidence of the lack of neural responses. Some cases without a CAP showed nerve activity in the form of the ANN. The ANN is the evoked correlate of auditory nerve phase locking. It produces unique distortion patterns in the ECochG tracing. Figure 3A is an example with an absent CAP but a clear ANN based on distortion patterns. The ANN was found in 15 subjects that had no discernable CAP.
Figure 3. The presence or absence of an ANN.
A. This case had no CAP (sum, bottom left), but had a strong auditory nerve neurophonic (ANN), indicated by the distortions in the ‘average cycle; (upper right) and in the FFT (bottom right). B. This case had neither CAP (sum, bottom left) nor ANN, as indicated by relative lack of distortions (upper right and FFT, lower right)).
The remaining 107 cases without evidence of CAP had no evidence of ANN either. Cases without CAPs were somewhat more likely to have a small TR. However, there were many cases at all levels of TR that did not have a CAP. One example of a case with a large TR but no CAP is shown in Figure 3B.
Correlations of TR, TCA and TN
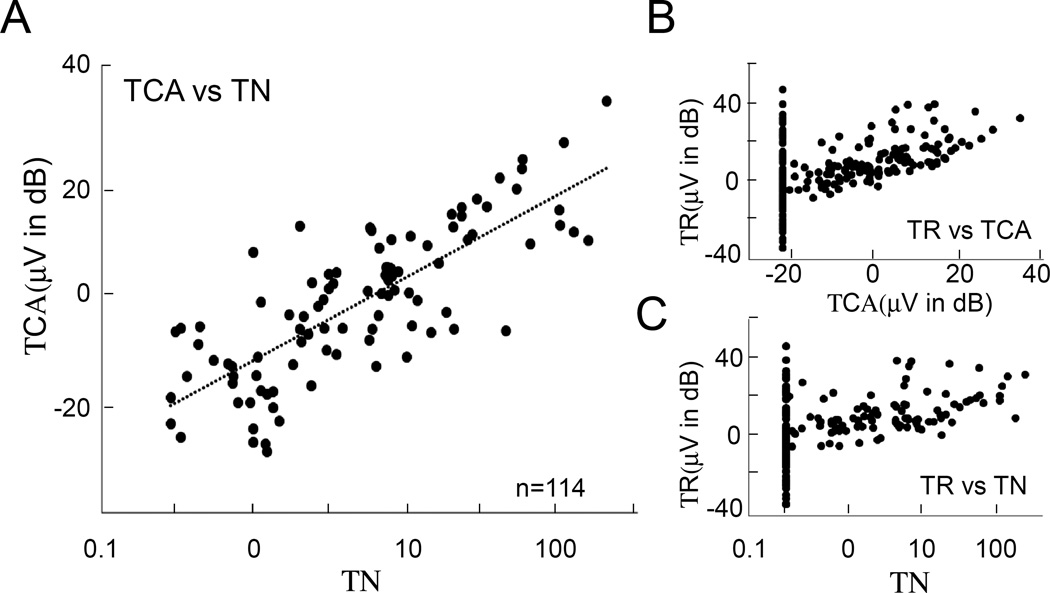
For comparison with the TR, which is the sum of responses to different frequencies, the “total CAP amplitude (TCA)” and “total N (TN)” were determined from the summed amplitudes and N values across frequencies for each case. Our two CAP measuring methods, the TCA and TN, were highly correlated (Figure 4A, r2 = 0.62 only including cases with present CAPs). For cases that had CAPs, their amplitudes and N components scaled with the size of the TR (Figure 4B, 3C).
Figure 4. Population data.
A. Total CAP Amplitude (TCA) vs. total N (TN). These were highly correlated. B, C. Both measurements of the CAP vs. total response (TR). They scaled with total response, but many cases had no CAP despite a strong TR.
Correlation of TR, TCA and TN with CNC word score test after 6 months
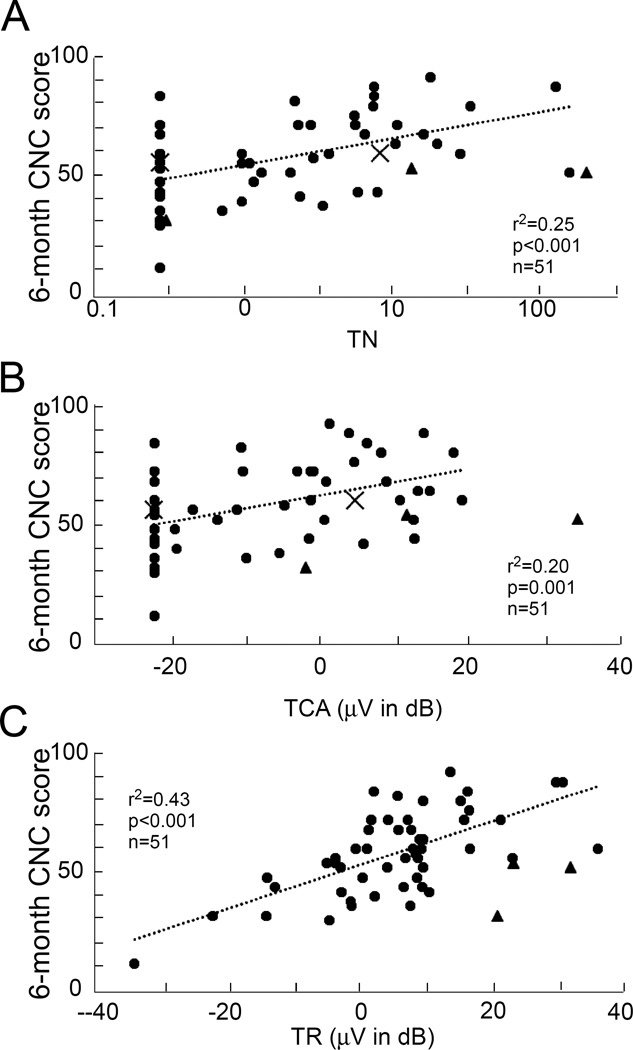
The CNC word scores at 6 months were obtained for 54 adult patients. When plotted against TR (Figure 5A), there were 3 cases (grey triangles) that were not included in the correlation with outcomes in previous studies10,11 and were also excluded here. These had a large TR and considerable indication of neural activity in the form of a CAP and/or ANN, but only average speech perception outcomes. The ECochG results suggest the reason for merely average performance had little to do with their cochlear physiology (see Discussion). These subjects had Meniere’s disease (n=1), auditory nerve spectrum disorder (n=1) and unknown (n=1) causes of their hearing loss.
Figure 5. Outcomes data.
A. TR vs 6-month CNC score. The data points marked by grey triangles and “X” are outliers. Those marked by triangles are excluded from the regression analysis, while those marked by “X” are included (see text). B. TN vs CNC score. C. TCA vs CNC score.
Two cases (x in Figure 5A) that were excluded in previous studies10,11 were included here. These cases had a large TR but comparatively little evidence of neural activity, so that the large TR might not indicate a strong neural substrate for electrical stimulation. The inclusion of a nerve-specific measure like the CAP might prove especially useful in accounting for the modest outcome compared with that expected from the TR alone.
In 18 of the 51 cases there was no evidence of an acoustic CAP, but most subjects performed well with the implants. Both TCA and the TN parameters had significant positive correlations with outcomes based on CNC scores. TN had a stronger correlation with CNC score (Figure 5B) than that between TCA and CNC score (Figure 5C). The correlation of TR with CNC scores for the same 51 cases was much higher than either of these (Figure 5A). Multiple regression using the TR and TCA, or TR and TN did not improve prediction of CNC scores over that of TR alone.
Finally, for the adults with CNC scores, the presence or absence of a CAP across the different frequencies are tallied as a function of the audiometric thresholds in Table 1. There were fewer than 50% of cases with CAPs for all frequencies. There were fewer subjects with CAPs when the audiometric thresholds were highest, as might be expected. However, even where thresholds were relatively good, i.e. where the hearing loss was mild to severe, only about half the subjects had CAPs.
Table 1.
Proportions of subjects showing CAP responses grouped by frequency and audiometric thresholds. Data are number of subjects with CAP per total number of subjects for each frequency and threshold group. Only recordings where there was a significant responses to the tone frequencies in the steady state were included.
| Frequency | Threshold <=40 dB HL |
Threshold 41– 60 dB HL |
Threshold 61– 80 dB HL |
Threshold >80 dB HL |
Total |
Percent with CAP |
|---|---|---|---|---|---|---|
| 250 | 5/12 | 3/12 | 0/9 | 1/16 | 9/49 | 18 |
| 500 | 4/5 | 6/11 | 6/12 | 7/21 | 23/49 | 47 |
| 1000 | 1/2 | 2/3 | 9/19 | 5/25 | 17/49 | 35 |
| 2000 | 0/0 | 2/2 | 6/9 | 1/10 | 9/21 | 43 |
| 4000 | 0/1 | 1/1 | 2/6 | 0/3 | 3/11 | 27 |
| Total | 10/20 | 14/29 | 23/55 | 14/75 | 61/179 | 34% |
|
Percent with CAP |
50 | 48 | 51 | 19 |
Discussion
Only ~50% of subjects showed evidence of a CAP to any frequency. When present, the CAP morphology was extremely variable. Two measurement techniques, TCA and TN, were highly correlated with each other but had different strengths in some cases. The lack of a CAP in many subjects, its variability when present, and its high correlation with TR significantly limited the CAP’s utility in helping to account for speech perception outcomes.
CAP prevalence in the CI population
About half of CI subjects had no CAP, and yet most subjects in the CNC scores group had a measurable TR and did well with their implants. Cases with good outcomes despite no CAP or ANN must have nerve fibers that can be electrically stimulated even if they do not contribute a response to sound. In this respect the CAP is similar to the audiogram, because a behavioral response to sound also requires a functioning chain of hair cell and auditory nerve connections, while electrical stimulation requires only ganglion cells. Correlations between the audiogram and speech perception outcomes tend to be poor17,18 so a similar result for the CAP was not unexpected. The CAP and the audiogram differ in that CAP measurements are of magnitude rather than threshold, and because responses to low frequencies that lead to behavioral responses may produce relatively poor CAPs due to slow rise times that reduce synchrony. This is the reason that finding a CAP to 250 Hz is rare (Table 1). In CI subjects the largest responses, and those seen in most subjects, are to 1000 Hz or lower frequencies10,19. Although outside the scope of this study, the large data set will allow for future reports comparing the CAP and other attributes of the ECochG recordings for different etiologies of hearing loss in children and adults.
CAP morphology in the CI population
In animal models with normal hearing, the CAP typically has a negative deflection below the baseline (N1), followed by a positive deflection (P1), and then possible further fluctuations. This pattern is the basis of the modeling equation used here14,21. Few of the CAPs in the CI subjects had the “typical” pattern (compare Figures 1 and 2). This morphologic variability and the overall dearth of CAPs discussed above can probably be largely explained by anatomic and physiologic characteristics of the CI population. The CAP depends on the simultaneous firing of many nerve fibers throughout the length of the cochlea. In normal hearing cases at high intensities, the CAP is thought to originate primarily from the base of the cochlea14, which is the area most heavily damaged in most CI subjects22–24. In a study applying the same convolution model used here to humans who were noise exposed to cause temporary deafness, the authors found that noise exposed subjects had flattening and widening of the CAP complex, and concluded that this reflected a temporary loss of synchrony in their subjects’ nerve fibers25. This widening is similar to many of the CAPs we observed in our study (examples Fig 2B, 3D). In the case of CI subjects, this may reflect some loss of synchrony due to hearing loss, due to slow rise times of the low frequency stimuli, and due to the responses originating from the apex where the traveling wave slows.
The variable CAP morphology in this population posed challenges when using modeling. In particular, CAPs that had very steep rise and fall and CAPs with large positive components did not work with the decaying sine wave model of the unit potential used in the convolution equation (see Figure 1D and Figure 2E, 2F). Still, most CAPs we observed were successfully fitted using the analytic equation.
The ANN as a neural marker
A subset of patients did not have any evidence of CAP but showed direct evidence of nerve activity through an observable ANN. In these cases, there was a population of nerve fibers phase-locking to the stimulus that did not respond synchronously enough to form an onset CAP. The ANN is present at low frequencies where neurons can phase-lock to the stimulus, typically below 1500 Hz26–28. This could give the ANN an advantage as a neural marker in CI subjects that have residual hearing at predominantly low frequencies. Unfortunately, it is difficult to measure because it forms a mixed response with the CM. At this time there is no technique that can cleanly separate the two, and therefore a method to quantify the ANN is lacking.
The CAP as a predictor of speech perception outcomes
The N component of the modeled CAP correlated slightly better with outcomes than amplitude measurements, cconsistent with Earl and Chertoff’s (2010) findings in experimentally deafened gerbils. Amplitude measurements were less predictive of speech perception outcomes, but had the advantage of being a simpler measurement that could be applied to more cases.
Although the correlations of TCA and TN with outcomes were significant they were weak, and the main hope was that any indication of nerve activity could improve the correlation over that available from the TR alone. However, when added into multivariate regression with the TR neither metric for measuring the CAP improved the correlation. Thus, at this time, TR remains the best ECochG metric to predict speech perception outcomes in CI subjects.
Why does the TR, consisting of the CM and ANN (when present), account for so much more of the outcome variance? Unlike the CAP or audiogram, the CM is a measure of hair cell rather than neural health, so it seems counterintuitive that it should better predict outcomes than the neural activity itself. We have previously hypothesized that the inclusion of these hair cell responses provides an indication of overall cochlear health, and a healthy environment may be more likely to support nerve fibers amenable to electrical stimulation10,11. The CAP, like the audiogram, excludes information from hair cells.
In principle, the electrically evoked CAP, or eCAP, which is a direct measure of auditory nerve elements available for stimulation, should show a better correlation than an indirect measure of cochlear health. However, attempts to correlate eCAPs with speech perception outcomes have largely failed, showing weaker correlations than the CAPs in this study and much weaker correlations than TR.20 A possible reason is the very limited dynamic range with eCAPs, compared to the TR, which varies by over 60 dB across subjects.
The prediction of speech recognition outcomes is one of the most promising applications of ECochG. Data from EcochG can be used to identify subjects that are performing worse than expected based on their residual physiology (such as the “grey triangle” outliers in Fig. 5A). This could assist clinicians towards evaluating other potential influences on speech perception. However, there are some cases where it has consistently proven challenging to interpret ECochG. Cases where the degree of hair cell and neural response do not agree are among the most problematic. Finding a way to measure the degree of neural activity in the signal should aid the interpretation of ECochG recordings in these subjects. Though the CAP alone was not sufficient to define the neural response in ECochG, it may still prove useful in conjunction with other neural markers, like the ANN, as part of a more comprehensive measurement.
Acknowledgments
Douglas C. Fitzpatrick: Contractual research support from MED-EL Corporation and research grant support from the NIH-NIDCD.
Kevin Brown: Contractual research support from Cochlear Corporation and MED-EL Corporation.
Craig A. Buchman: Contractual research support from Cochlear Corporation and MED-EL Corporation. Consultant for Advanced Bionics and Cochlear Corporations.
Oliver F. Adunka: Contractual research support from Advanced Bionics, Cochlear Corporation, and MED-EL Corporation. Consultant for MED-EL Corporation and Cochlear Corporation.
Margaret T Dillon: Contractual research support from MED-EL Corporation.
Meredith L Anderson: Contractual research support from MED-EL Corporation.
The authors would like to thank Steven Pulver for his excellent technical assistance. Supported by NIH, NIDCD 5T32DC005360-12, and a grant from the Med El Corporation.
Footnotes
Disclosures:
The remaining authors have nothing to disclose
References
- 1.Lazard DS, Vincent C, Venail F, et al. Pre-, per- and postoperative factors affecting performance of postlinguistically deaf adults using cochlear implants: a new conceptual model over time. PLoS. One. 2012;7(11):e48739. doi: 10.1371/journal.pone.0048739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Incesulu A, Nadol JB., Jr Correlation of acoustic threshold measures and spiral ganglion cell survival in severe to profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1998;107(11 Pt 1):906–911. doi: 10.1177/000348949810701102. [DOI] [PubMed] [Google Scholar]
- 3.Tomblin JB, Barker BA, Spencer LJ, Zhang X, Gantz BJ. The effect of age at cochlear implant initial stimulation on expressive language growth in infants and toddlers. J Speech Lang Hear. Res. 2005;48(4):853–867. doi: 10.1044/1092-4388(2005/059). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holden LK, Finley CC, Firszt JB, et al. Factors Affecting Open-Set Word Recognition in Adults With Cochlear Implants. Ear Hear. 2013 doi: 10.1097/AUD.0b013e3182741aa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan AM, Handzel O, Burgess BJ, Damian D, Eddington DK, Nadol JB., Jr Is word recognition correlated with the number of surviving spiral ganglion cells and electrode insertion depth in human subjects with cochlear implants? Laryngoscope. 2005;115(4):672–677. doi: 10.1097/01.mlg.0000161335.62139.80. [DOI] [PubMed] [Google Scholar]
- 6.Xu HX, Kim GH, Snissarenko EP, Cureoglu S, Paparella MM. Multi-channel cochlear implant histopathology: are fewer spiral ganglion cells really related to better clinical performance? Acta Otolaryngol. 2012;132(5):482–490. doi: 10.3109/00016489.2011.647361. [DOI] [PubMed] [Google Scholar]
- 7.Fayad J, Linthicum FH, Jr, Otto SR, Galey FR, House WF. Cochlear implants: Histopathologic findings related to performance in 16 human temporal bones. Ann Otol Rhinol Laryngol. 1991;100(10):807–811. doi: 10.1177/000348949110001004. [DOI] [PubMed] [Google Scholar]
- 8.Fayad JN, Linthicum FH., Jr Multichannel cochlear implants: relation of histopathology to performance. The Laryngoscope. 2006;116(8):1310–1320. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- 9.Seyyedi M, Viana LM, Nadol JB., Jr Within-subject comparison of word recognition and spiral ganglion cell count in bilateral cochlear implant recipients. Otol Neurotol. 2014;35(8):1446–1450. doi: 10.1097/MAO.0000000000000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzpatrick DC, Campbell AT, Choudhury B, et al. Round window electrocochleography just before cochlear implantation: relationship to word recognition outcomes in adults. Otol Neurotol. 2014;35(1):64–71. doi: 10.1097/MAO.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClellan JH, Formeister EJ, Merwin WH, 3rd, et al. Round window electrocochleography and speech perception outcomes in adult cochlear implant subjects: comparison with audiometric and biographical information. Otol Neurotol. 2014;35(9):e245–e252. doi: 10.1097/MAO.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 12.Chertoff M, Lichtenhan J, Willis M. Click- and chirp-evoked human compound action potentials. J Acoust. Soc Am. 2010;127(5):2992–2996. doi: 10.1121/1.3372756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein MH, Kiang NYS. Synchrony of neural activity in electric responses evoked by transient acoustic stimuli. J. Acoust. Soc. Am. 1958;30:107–114. [Google Scholar]
- 14.Chertoff ME. Analytic treatment of the compound action potential: estimating the summed post-stimulus time histogram and unit response. J Acoust. Soc Am. 2004;116(5):3022–3030. doi: 10.1121/1.1791911. [DOI] [PubMed] [Google Scholar]
- 15.Earl BR, Chertoff ME. Predicting auditory nerve survival using the compound action potential. Ear Hear. 2010;31(1):7–21. doi: 10.1097/AUD.0b013e3181ba748c. [DOI] [PubMed] [Google Scholar]
- 16.Gifford RH, Shallop JK, Peterson AM. Speech recognition materials and ceiling effects: considerations for cochlear implant programs. Audiol Neurootol. 2008;13(3):193–205. doi: 10.1159/000113510. [DOI] [PubMed] [Google Scholar]
- 17.Choudhury B, Fitzpatrick DC, Buchman CA, et al. Intraoperative round window recordings to acoustic stimuli from cochlear implant patients. Otol Neurotol. 2012;33(9):1507–1515. doi: 10.1097/MAO.0b013e31826dbc80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blamey P, Artieres F, Baskent D, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: an update with 2251 patients. Audiol Neurootol. 2013;18(1):36–47. doi: 10.1159/000343189. [DOI] [PubMed] [Google Scholar]
- 19.Blamey P, Arndt P, Bergeron F, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiol. Neurootol. 1996;1(5):293–306. doi: 10.1159/000259212. [DOI] [PubMed] [Google Scholar]
- 20.Abbas PJ, Brown CJ. Assessment of responses to cochlear implant stimulation at different levels of the auditory pathway. Hear Res. 2015;322:67–76. doi: 10.1016/j.heares.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chertoff ME, Lichtenhan JT, Tourtillott BM, Esau KS. The influence of noise exposure on the parameters of a convolution model of the compound action potential. J Acoust. Soc Am. 2008;124(4):2174–2185. doi: 10.1121/1.2967890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felder E, Schrott-Fischer A. Quantitative evaluation of myelinated nerve fibres and hair cells in cochleae of humans with age-related high-tone hearing loss. Hear. Res. 1995;91(1–2):19–32. doi: 10.1016/0378-5955(95)00158-1. [DOI] [PubMed] [Google Scholar]
- 23.Otte J, Schuknecht HF, Kerr AG. Ganglion cell populations in normal and pathological human cochleae. Implications for cochlear implantation. 1978. Laryngoscope. 2015;125(5):1038. doi: 10.1002/lary.25219. [DOI] [PubMed] [Google Scholar]
- 24.Choudhury B, Adunka OF, Awan O, Pike JM, Buchman CA, Fitzpatrick DC. Electrophysiologic consequences of flexible electrode insertions in gerbils with noise-induced hearing loss. Otol Neurotol. 2014;35(3):519–525. doi: 10.1097/MAO.0b013e31829bdf2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lichtenhan JT, Chertoff ME. Temporary hearing loss influences post-stimulus time histogram and single neuron action potential estimates from human compound action potentials. J Acoust. Soc Am. 2008;123(4):2200–2212. doi: 10.1121/1.2885748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snyder RL, Schreiner CE. The auditory neurophonic: basic properties. Hear. Res. 1984;15(3):261–280. doi: 10.1016/0378-5955(84)90033-9. [DOI] [PubMed] [Google Scholar]
- 27.Henry KR. Auditory nerve neurophonic recorded from the round window of the Mongolian gerbil. Hear. Res. 1995;90(1–2):176–184. doi: 10.1016/0378-5955(95)00162-6. [DOI] [PubMed] [Google Scholar]
- 28.Forgues M, Koehn HA, Dunnon AK, et al. Distinguishing hair cell from neural potentials recorded at the round window. J Neurophysiol. 2014;111(3):580–593. doi: 10.1152/jn.00446.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after "temporary" noise-induced hearing loss. J Neurosci. 2009;29(45):14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin HW, Furman AC, Kujawa SG, Liberman MC. Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol. 2011;12(5):605–616. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear. Res. 2015;330(Pt B):191–199. doi: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]