Abstract
Objective
Weight gain during the menopausal transition is common. Although studies have suggested that weight gain is more likely related to aging than menopause, there is a reduction in resting energy expenditure with surgical or natural menopause which is independent of age and changes in body composition. The underlying mechanisms could include a reduction in core body temperature.
Methods
Data were obtained from two related studies. Sample size was 23 men and 25 women (12 premenopausal,13 postmenopausal). In the Clinical Research Unit, core temperature was measured every minute for 24 hours (CorTemp System,HQ Inc.).
Results
Mean 24-hour core body temperature was 0.25 ± 0.06 °C lower in postmenopausal than premenopausal women (p=0.001). Mean 24-hour core temperature was 0.34 ± 0.05 °C lower in men than in premenopausal women (p<0.001).
Conclusions
Postmenopausal women, like men, had lower core body temperatures than premenopausal women. This may have implications for midlife weight gain.
Keywords: temperature, menopause, women, obesity, metabolism, weight
Introduction
Weight gain during the menopausal transition is a common symptom. Controversy exists as to whether perimenopausal weight gain is due to changes in energy metabolism related to aging or changes in sex hormones related to menopause. Many studies have suggested that aging is the primary driver of weight gain in perimenopausal women, rather than menopause per se.1-4 However, there is a reduction in resting energy expenditure at menopause which has been shown to be independent of changes in body composition.5 In a rodent model, this decreased energy expenditure occurs as a result of the surgical loss of ovarian function rather than aging.6 In premenopausal women, acute sex hormone suppression with GnRH antagonist therapy reduces resting energy expenditure below baseline follicular phase levels.7
The mechanisms underlying changes in energy expenditure at menopause could include a reduction in core body temperature. Indeed, body temperature is an understudied variable linked to energy metabolism.8 It has been estimated that 40-80% of a typical mammal's basal metabolic rate is spent in the cost of maintaining homeothermy.9 In addition, small decreases in body temperature coincide with large decreases in energy expenditure, as seen in previous studies of caloric restriction in humans.10, 11 Therefore, a small decrease in core temperature with menopause could lead to significantly decreased energy expenditure and weight gain over time.
Body temperature has long been known to decline with extremes of age in men. However, recent data suggest that women may also experience a decrease in body temperature around ages 40-60.12 The more abrupt decline in body temperature observed in women could be due to menopause. In contrast, the gradual decline in temperature that occurs in men could correlate with the more gradual sex hormone changes of andropause.
Prior studies have measured core body temperature in postmenopausal women. Using an ingested core temperature sensor (the CorTemp sensor), Freedman et. al. found that core body temperature is significantly lower over 24 hours and during sleep in postmenopausal women who experience hot flashes than in asymptomatic postmenopausal women.13 Freedman and Subramanian assessed core temperature in premenopausal women and postmenopausal women with and without hot flashes using a rectal probe over 2-4 sessions lasting 5 hours each.14 The mean rectal temperature was significantly lower in postmenopausal women with hot flashes than in asymptomatic postmenopausal and premenopausal women.
Our study is the first known study to assess 24-hour core body temperature in premenopausal versus postmenopausal women using the ingested CorTemp sensor. We hypothesized that 24-hour core temperature would be lower in postmenopausal women than in premenopausal women, which could have implications for energy metabolism in midlife.
Methods
Subjects
Subjects' data were obtained from two separate but related studies of core temperature conducted by the same investigators using the same methods. The first study, conducted in 2009-2010, was designed to explore potential differences in core body temperature between lean (BMI: 18.5-24.9 kg/m2) and obese (BMI: 30.0-39.9 kg/m2) men and women, ages 25-40.15 Subjects were lean men (n = 6), lean women (n = 6), obese men (n = 6) and obese women (n = 6). All subjects were weight stable, with no weight change of > 2% for at least 2 months. The second study, conducted in 2011-2013, was primarily designed to examine changes in core body temperature with weight loss. This study included overweight and obese (BMI: 27 to 39.9 kg/m2) men (n = 11) and postmenopausal women (n = 13), ages 18-65. For this report, only baseline data were included. Subjects were weight stable; they had no weight change of > 2% in the 2 months prior to study enrollment and were within 3% of their maximal body weights. The sample size drawn from the combined studies was 23 men and 25 women, ages 23-64 (See Table 1). A wide range of ethnic backgrounds was represented.
Table 1. Characteristics of the study population, mean and (SEM).
| Characteristic | Premenopausal Women (n=12) | Postmenopausal Women (n=13) | Men (n=23) |
|---|---|---|---|
| Age (years) | 32.0 (1.7) ¥€ | 56.2 (1.6) ¥✤ | 37.6 (2.1)✤€ |
| BMI (kg/m2) | 28.3 (1.7) ¥ | 34.8 (1.1) ¥ | 31.9 (1.3) |
| Mass (kg) | 76.2 (5.4)✤€ | 92.8 (3.3)¥ | 101.5 (19.9)€ |
| Lean Mass (kg) | 48.7 (1.2)€ | 46.7 (1.5)✤ | 68.9 (1.5)✤€ |
| Fat Mass (kg) | 27.4 (3.4)¥ | 44.5 (2.5)¥ | 31.3 (2.8) |
| Race/Ethnicity | 5W; 2H; 3B; 1A;1U | 6W;2H;4B;1A | 14W;4H;4B;1U |
For p < 0.05 between pre- and post-menopausal women
For p < 0.05 between postmenopausal women and men.
For p < 0.05 between premenopausal women and men. For race/ethnicity of study subjects: W, white non-Hispanic; H, white Hispanic; B, black or African American; A, Asian; U, declined to identify.
Potential volunteers were excluded if they had a medical condition or took medications which might affect core temperature or the use of the CorTemp System. Premenopausal women had a history of regular menstrual cycles (25-30 days duration) and were admitted during days 1-7 of their menstrual cycle (follicular phase). Both studies were approved by Northwestern's institutional review board.
Procedure
Detailed procedures have been published previously.15 In brief, subjects were admitted for a 24-hour stay at the Northwestern Clinical Research Unit (CRU). Participants were asked to wear light clothing and a comfortable room-temperature (∼ 70° F) environment was maintained. At 9 a.m., each subject swallowed a pill-sized CorTemp sensor (CorTemp H.Q. Inc., Palmetto, FL). The sensor passed through the subject's gastrointestinal tract and sent a core temperature reading every minute to an external monitor worn at the waist. The monitor recorded the subjects' temperatures during standardized activities including rest, sleep, and meals. Participants also exercised on a stationary bicycle for 20 minutes, with a goal of achieving at least 10 consecutive minutes between a level of 5 (hard) and 7 (very hard) on the Rate of Perceived Exertion scale.
The Institute of Medicine (IOM) predictive equations were utilized to estimate each subject's total energy expenditure, which was provided in the form of three meals, each containing 15% protein, 35% fat, and 50% carbohydrate.16
Body composition was measured using the QDR 4500 Acclaim Series Elite DEXA scan (Hologic Europe, Bedford, MA).
Data analysis
Outlier temperature data points due to incorrect signals from CorTemp monitors were excluded if they were greater than two standard deviations from the mean. Mean temperature and standard deviation were calculated for each subject during four time intervals: exercise (20 minutes), during dinner (30 minutes), sleep (excluding the first and last half hour), and 24 hours (excluding the first 4 hours after the pill was swallowed to ensure passage into the small intestine). Data analyses were conducted using SPSS v. 22 (IBM). Means and standard error of the mean (SEM) were calculated for subject characteristics (Table 1). Independent samples t-tests were performed to assess for significant differences between study groups. Pearson's correlations were utilized to explore relationships between continuous variables. Significance was taken as p < 0.05.
Results
Mean (± SEM) 24-hour core body temperature was 0.25 ± 0.06 °C lower in postmenopausal women than in premenopausal women (p = 0.001) (See Fig. 1). Mean core temperature during sleep was 0.28 ± 0.08 °C lower in postmenopausal women than premenopausal women (p = 0.002), and similar differences were found during dinner and exercise (both p's < 0.05) (See Fig. 1). Similarly, mean core body temperature was significantly lower at all timepoints (all p's < 0.05) among our postmenopausal women (mean BMI 34.8 ± 1.1) than among the subset of obese premenopausal women (mean BMI 33.9 ± 0.2). Mean 24-hour core temperature was 0.34 ± 0.05 °C lower in men than premenopausal women (p < 0.001), with similar differences found during sleep, dinner, and exercise (all p's < 0.01) (See Fig. 1). Mean core temperature was not significantly different between postmenopausal women and men (See Fig. 1). There was a significant correlation between age and 24-hour core body temperature for women (r = 0.615, p = 0.001) (See Fig. 2) but not men in our cohort of individuals under age 65.
Figure 1.
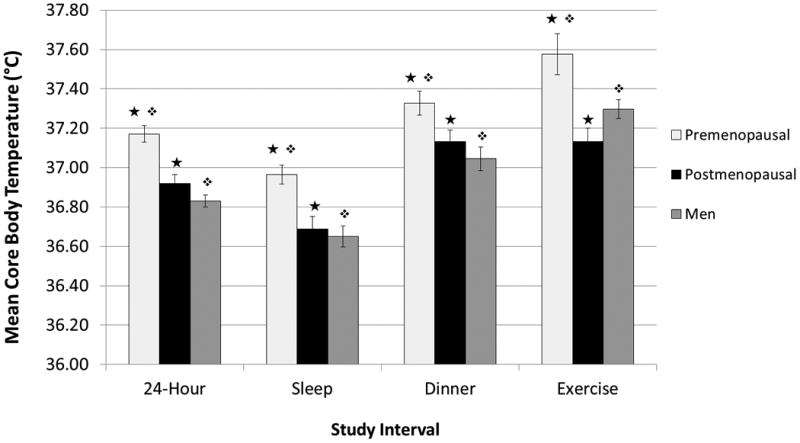
Mean core body temperatures in premenopausal women, postmenopausal women, and men during the activities of the study. Mean core body temperature was lower in postmenopausal women than premenopausal women and more similar to the temperature of men. Symbols denote significantly different groups (p < 0.05).
Figure 2.
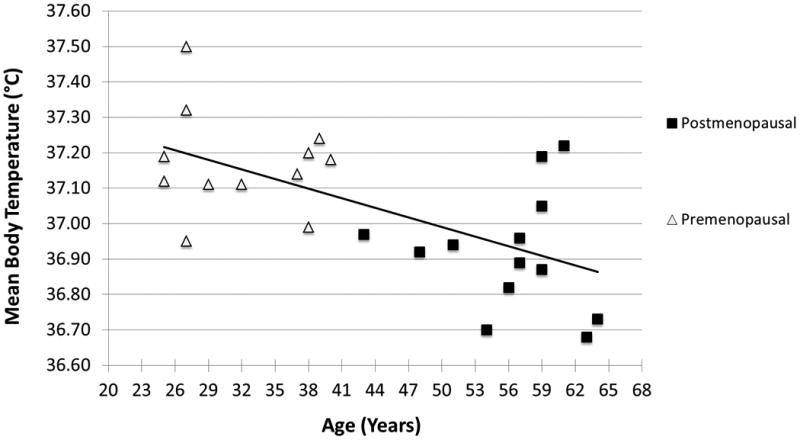
There was a significant correlation between age and 24-hour core body temperature for women (r = 0.615, p = 0.001).
Discussion
In this post-hoc analysis, we observed that postmenopausal women, like men, have lower core body temperatures than premenopausal women. Postmenopausal women were on average 0.25°C cooler, which is equivalent to a 3.25% reduction in energy expenditure, or 65 fewer kilocalories burned per day. For a typical postmenopausal woman, 50 years old, 150 lbs. (68 kg) and 5 ft. 4 in. (162.5 cm) tall, this would lead to 6.7 lbs. (3.04 kg) of weight gain in one year, assuming no metabolic adaptation to weight gain. On the other hand, it is well known that energy expenditure increases in response to weight gain; thus the amount of weight accumulated over time may be more modest.17 If confirmed in future studies, however, our findings are likely to have important implications for cardiometabolic health. Relatively small weight gains of 5 kg or less in adulthood have been associated with an increased risk of type 2 diabetes, cardiovascular disease, and stroke in women as well as men. 18-21
Importantly, the differences in core temperature in this study cannot be attributed to differences in body composition or size. The regulation of core body temperature is a function of the hypothalamic set-point, and as such it is thought to be independent of body surface area, fat mass, and lean mass.22
Our findings add to what has already been reported by Freedman and Subramanian, who found that postmenopausal women with hot flashes had significantly lower core temperatures than asymptomatic postmenopausal and premenopausal women.14 Notably, we did not assess for the presence of hot flashes in our subjects.
Our study had several other limitations. We may not have observed a decline in core temperature with increasing age in men because our cohort included few men above age 50 and none above age 65, when we would expect to see the greatest decreases in core temperature in men. Another limitation is that all the female subjects ages 41 and older were postmenopausal, so we were not able to differentiate the effects of age and menopausal status on core temperature. Finally, season was not standardized in this study; all volunteers were studied in a research unit under thermoneutral conditions, however, to minimize any potential environmental effect on core body temperature.
Conclusions
Although exploratory in nature, our post-hoc analyses of two of our previous studies suggest that postmenopausal women have lower core body temperatures than premenopausal women and their temperatures are more similar to the temperatures of men. These preliminary observations may have implications for energy metabolism, perimenopausal weight gain, and cardiometabolic risk and thus merit further study.
Acknowledgments
Funding: The Joseph and Bessie Feinberg Foundation and David Kabiller, The project described was supported, in part, by the Northwestern University Clinical and Translational Science Institute, Grant Number UL1TR000150 from the National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award, and by the National Center for Research Resources, Grant Number UL1RR025741. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The Clinical and Translational Science Award (CTSA) is a registered trademark of the U.S. Department of Health and Human Services (DHHS).
Footnotes
This work was presented in part in abstract form at the Endocrine Society Annual Meeting, June 2014.
Conflicts of Interest/Financial Disclosure: Dr. Neff received research funding from GI Dynamics, performed consulting work for Eisai, Inc and received an honorarium for speaking for Pfizer India.
References
- 1.Wing RR, Matthews KA, Kuller LH, Meilahn EN, Plantinga PL. Weight gain at the time of menopause. Arch Intern Med. 1991;151(1):97–102. [PubMed] [Google Scholar]
- 2.Wildman R, Tepper P, Crawford S, Finkelstein J, Sutton-Tyrrell K, Thurston R, et al. Do changes in sex steroid hormones precede or follow increases in body weight during the menopause transition? Results from the Study of Women's Health Across the Nation J Clin Endocrinol Metab. 2012;97(9):E1695–704. doi: 10.1210/jc.2012-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sowers M, Zheng H, Tomey K, Karvonen-Gutierrez C, Jannausch M, Li X, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92(3):895–901. doi: 10.1210/jc.2006-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo SS, Zeller C, Chumlea WC, Siervogel RM. Aging, body composition, and lifestyle: the Fels Longitudinal Study. Amer J Clin Nutr. 1999;70(3):405–11. doi: 10.1093/ajcn/70.3.405. [DOI] [PubMed] [Google Scholar]
- 5.Lovejoy J, Champagne C, de Jonge L, Xie H, Smith S. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes. 2008;32(6):949–58. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers NH, Perfield JW, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150(5):2161–8. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day DS, Gozansky WS, Pelt REV, Schwartz RS, Kohrt WM. Sex hormone suppression reduces resting energy expenditure and β-adrenergic support of resting energy expenditure. J Clin Endocrinol Metab. 2005;90(6):3312–17. doi: 10.1210/jc.2004-1344. [DOI] [PubMed] [Google Scholar]
- 8.Landsberg L, Young JB, Leonard WR, Linsenmeier RA, Turek FW. Is obesity associated with lower body temperatures? Core temperature: a forgotten variable in energy balance. Metabolism. 2009;58(6):871–6. doi: 10.1016/j.metabol.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Girardier L, Stock M, editors. Mammalian thermogenesis. London: Chapman and Hall; Mammalian thermogenesis: an introduction; pp. 1983pp. 1–8. [Google Scholar]
- 10.Keys A, Brozek J, Henschel A, Mickelsen O, Taylor H. The biology of human starvation. Minneapolis: The University of Minnesota Press; 1950. [Google Scholar]
- 11.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Meyer DEL, Rood J, et al. Effect of 6-mo. calorie restriction on biomarkers of longevity, metabolic adaptation and oxidative stress in overweight subjects. JAMA. 2006;295(13):1539–48. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waalen J, Buxbaum JN. Is older colder or colder older? The association of age with body temperature in 18,630 individuals. J Gerontol A Biol Sci Med Sci. 2011;66A(5):487–92. doi: 10.1093/gerona/glr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman RR, Norton D, Woodward S, Cornelissen G. Core body temperature and circadian rhythm of hot flashes in menopausal women. J Clin Endocrinol Metab. 1995;80(8):2354–8. doi: 10.1210/jcem.80.8.7629229. [DOI] [PubMed] [Google Scholar]
- 14.Freedman RR, Subramanian M. Effects of symptomatic status and the menstrual cycle on hot flash-related thermoregulatory parameters. Menopause. 2005;12(2):156–9. doi: 10.1097/00042192-200512020-00009. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann M, Rodriguez S, Zeiss D, Wachsberg K, Kushner R, Landsberg L, et al. 24-h core temperature in obese and lean mean and women. Obesity. 2012;20(8):1585–90. doi: 10.1038/oby.2011.380. [DOI] [PubMed] [Google Scholar]
- 16.Food and Nutrition Board of the Institute of Medicine Dietary referance intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients) Washington, D.C: The National Academies Press; 2005. [DOI] [PubMed] [Google Scholar]
- 17.Galgani J, Ravussin E. Energy metabolism, fuel selection and body weight regulation. Int J Obes. 0000;32(S7):S109–S19. doi: 10.1038/ijo.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao W, Yeung E, Tobias DK, Hu FB, Vaag AA, Chavarro JE, et al. Long-term risk of type 2 diabetes mellitus in relation to BMI and weight change among women with a history of gestational diabetes mellitus: a prospective cohort study. Diabetologia. 2015;58(6):1212–9. doi: 10.1007/s00125-015-3537-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willett W, Manson J, Stampfer M, Colditz G, Rosner B, Speizer F, et al. Weight, weight change, and coronary heart disease in women: risk within the ‘normal’ weight range. JAMA. 1995;273(6):461–5. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]
- 20.Stevens J, Erber E, Truesdale KP, Wang C-H, Cai J. Long- and short-term weight change and incident coronary heart disease and ischemic stroke: The Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2013;178(2):239–48. doi: 10.1093/aje/kws461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Mutsert R, Sun Q, Willett WC, Hu FB, van Dam RM. Overweight in early adulthood, adult weight change, and risk of type 2 diabetes, cardiovascular diseases, and certain cancers in men: a cohort study. Am J Epidemiol. 2014;179(11):1353–65. doi: 10.1093/aje/kwu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol. 2011;301(5):R1207–28. doi: 10.1152/ajpregu.00109.2011. [DOI] [PubMed] [Google Scholar]


