Abstract
In humans, reward cues, including drug cues in addicts, are especially effective in biasing attention towards them, so much so they can disrupt ongoing task performance. It is not known, however, whether this happens in rats. To address this question, we developed a behavioral paradigm to assess the capacity of an auditory drug (cocaine) cue to evoke cocaine-seeking behavior, thus distracting thirsty rats from performing a well-learned sustained attention task (SAT) to obtain a water reward. First, it was determined that an auditory cocaine cue (tone-CS) reinstated drug-seeking equally in sign-trackers (STs) and goal-trackers (GTs), which otherwise vary in the propensity to attribute incentive salience to a localizable drug cue. Next, we tested the ability of an auditory cocaine cue to disrupt performance on the SAT in STs and GTs. Rats were trained to self-administer cocaine intravenously using an Intermittent Access self-administration procedure known to produce a progressive increase in motivation for cocaine, escalation of intake, and strong discriminative stimulus control over drug-seeking behavior. When presented alone, the auditory discriminative stimulus elicited cocaine-seeking behavior while rats were performing the SAT, but it was not sufficiently disruptive to impair SAT performance. In contrast, if cocaine was available in the presence of the cue, or when administered non-contingently, SAT performance was severely disrupted. We suggest that performance on a relatively automatic, stimulus-driven task, such as the basic version of the SAT used here, may be difficult to disrupt with a drug cue alone. A task that requires more top-down cognitive control may be needed.
Keywords: addiction, sign-tracking, goal-tracking, cocaine, discriminative stimulus, intermittent access, attention
1. INTRODUCTION
Cues that have been associated with rewards are especially efficacious in drawing attention towards them, to the extent they can disrupt ongoing task performance, and there is considerable individual variation in their ability to do so (Hickey et al., 2010; Anderson, 2016). Indeed, even under conditions in which participants are aware that reward cues are irrelevant to the current task, and should be ignored, such cues remain capable of capturing attention, distracting the individual, and consequently, impairing task performance (Hickey et al., 2010; Anderson et al., 2011a, b). In individuals experiencing addiction, drug cues are especially effective in attracting attention relative to other stimuli and the degree to which drug cues produce an attentional bias is predictive of their ability to induce drug craving and of the likelihood of relapse (Cox et al., 2002; Waters et al., 2003; Marissen et al., 2006). One reason reward cues may bias attention towards them is because they become attributed with incentive motivational properties (incentive salience), and as incentive stimuli they become themselves highly desired, capable of attracting individuals towards them and instigating reward-seeking behavior (Robinson and Berridge, 1993; Cardinal et al., 2002; Robinson et al., 2014).
The ability of a drug cue to gain attention and thus distract an individual from attending to other stimuli necessary for successful task performance has rarely been studied in animals. One goal of the experiments reported here was to develop a procedure to study potential drug cue-induced disruption of ongoing task performance. To do this, rats were first trained to perform a sustained attention task (SAT) which requires constant monitoring of a stimulus array to report the occurrence, or non-occurrence, of a visual signal to obtain a natural reward (McGaughy and Sarter, 1995; Demeter et al., 2008; St Peters et al., 2011). Rats were then independently trained to self-administer cocaine using an Intermittent Access (IntA) procedure known to produce addiction-like behavior (Zimmer et al., 2012; Kawa et al., 2016). This procedure involves cycles of Drug Available and No-Drug Available periods, signaled by a discriminative stimulus (DS+), such that drug-seeking quickly comes under strong discriminative stimulus control. Discriminative stimuli are known to have potent motivational properties, robustly reinstating drug-seeking behavior even after long periods of abstinence (Ciccocioppo et al., 2001; Weiss et al., 2001; Perry et al., 2014). Therefore, we examined the ability of the DS+ (a signal for cocaine availability) to interrupt performance on the SAT, with or without the simultaneous availability of cocaine.
There is considerable individual variation in the extent to which reward cues, including drug cues, are attributed with incentive salience (Flagel et al., 2007; Flagel et al., 2009; Saunders and Robinson, 2010; Yager and Robinson, 2010; Yager et al., 2015). Animals prone to attribute incentive salience to reward cues are called sign-trackers (STs) and those less prone to do so are called goal-trackers (GTs) (for review (Robinson et al., 2014)). Furthermore, STs tend to be more prone to impulsive action than GTs (Lovic et al., 2011) and they have relatively poor attentional control, as indicated by fluctuating levels of attentional performance and attenuated cholinergic neuromodulatory mediation of performance when compared to GTs (Paolone et al., 2013). Therefore, we further asked whether a cocaine cue would influence task performance differently in STs and GTs.
2. METHODS
2.1. Animals
Male Sprague-Dawley rats (Envigo, Indianapolis, IN) weighing 250–274 grams upon arrival were individually housed in Plexiglas cages and kept on a 12-h light/12-h dark cycle (lights on at 0800 hr) with regulated temperature and humidity. After arrival, rats were given 1 week to acclimate to the colony room before experimentation commenced. Food (Rodent Chow, Laboratory Rodent Diet 5001, LabDiet) and water were available ad libitum until either self-administration or SAT training began. At the start of SAT training, animals were water-deprived by restricting water access to a 15 min period after each training session. Water was also provided as a reward during task performance (see below). On days not tested, water access was increased to a total of 60 min. During task training, food was available ad libitum. Starting 2 days before the first day of self-administration the animals were mildly food restricted to maintain a stable body weight throughout testing (Rowland, 2007) and water was available ad libitum. All procedures were approved by the University of Michigan Institutional Animal Care and Use Committee and were conducted in AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care)-accredited laboratories.
2.2. Pavlovian Conditioned Approach with Food as the Unconditioned Stimulus
2.2.1. Apparatus
Med Associates test chambers (20.5 cm × 24.1 cm floor area, 29.2 cm high; Med Associates, St. Albans, VT) were used for Pavlovian training. Each chamber was equipped with a food receptacle located 2.5 cm above the floor in the center of the wall. A catch tray filled with corn-cob bedding was located underneath the floor, which was constructed from stainless steel rods. A red house light was located on the wall opposite the food receptacle and remained on for the duration of training sessions. An illuminated retractable lever (Med Associates) was located approximately 2.5 cm to the left or right of the food receptacle, 6 cm above the floor. The side of the lever with respect to the food receptacle was counter-balanced across boxes. A white LED was flush-mounted on the inside of the lever and was used to illuminate the slot through which the lever protruded. The lever required a ~20 g force to deflect, such that most contacts with the lever were recorded as a ‘lever press’. The pellet dispenser (Med Associates) delivered one-45 mg banana-flavored food pellet (Bio-Serv®, #F0059, Frenchtown, NJ) into the food receptacle at a time. Head entry into the food receptacle was recorded each time a rat broke the infrared photobeam located inside the receptacle (approximately 1.5 cm above the base of the food cup). Each conditioning chamber was located in a sound-attenuating enclosure and background noise was supplied by a ventilating fan to mask outside noise. Data collection was controlled by Med-PC software.
2.2.2. Pavlovian Conditioned Approach
All rats (Experiments 1 and 2) were initially trained using a Pavlovian conditioned approach (PCA) procedure described previously (Flagel et al., 2007). During a 1 week acclimation to the colony room, rats were handled regularly before training procedures commenced. All training sessions were conducted during the 12 hr lights on period. Prior to the start of training, ~20 banana-flavored food pellets were placed into the rats’ home cages to familiarize the animals with this food. For pre-training, rats were placed into the test chamber with a red houselight illuminated, while the lever remained retracted throughout the entire session. Twenty-five food pellets were delivered on a variable interval (30 sec) schedule to determine whether rats were reliably retrieving the pellets from the receptacle. If a rat failed to retrieve all of its food pellets, it received a second pre-training session. By the end of pre-training, all rats consumed all food pellets.
During the Pavlovian training sessions, each individual trial consisted of the insertion of the illuminated lever (CS) into the chamber for 8 s, and immediately following retraction of the lever, the pellet dispenser was activated causing the delivery of a single food pellet (unconditioned stimulus, US) into the food receptacle. The intertrial interval (ITI) started immediately following the retraction of the lever. The CS was presented on a variable interval 90 sec schedule (one presentation of the CS occurred on average every 90 s, but the actual time between CS presentations varied randomly between 30 and 150 sec). Each Pavlovian training session consisted of 25 trials, resulting in a 35–45 min session. Pavlovian training was conducted over 5 consecutive days. We recorded the following events for each trial: (i) number of lever deflections (contacts), (ii) latency to first lever deflection, (iii) number of head entries into the food receptacle (referred to as magazine entries) during presentation of the CS, (iv) latency to the first receptacle entry following CS presentation and (v) number of magazine entries during the ITI. All animals included in analysis consumed all food pellets during each training session. At the end of each training session the animals were returned to their home cages in the animal colony room.
At the conclusion of Pavlovian training, to quantify the propensity of each individual rat to approach the lever-CS vs the food magazine during the CS period (i.e., sign-tracking or goal-tracking) a Pavlovian conditioned approach (PCA) index score was calculated, as previously described in detail (Meyer et al., 2012). Briefly, this PCA index score consisted of averaging three measures of conditioned approach: (i) the probability of contacting either the lever-CS or food magazine during the CS period [P(lever) – P(magazine)]; (ii) the response bias for contacting the lever-CS or the food magazine during the CS period [(#lever-CS contacts − #food magazine contacts)/(#lever-CS contacts + #food magazine contacts)]; and (iii) the latency to contact the lever-CS or the food magazine during the CS period [(food magazine contact latency – lever-CS contact latency)/8]. Averaging these three measures produces PCA index scores along a scale ranging from −1.0 to +1.0, where +1 indicates an animal made a sign-tracking CR on every trial, −1 a goal-tracking CR on every trial, and 0 a 50:50 distribution of sign- and goal-tracking CRs. For the purpose of classification, rats with an averaged PCA index score from days 4 and 5 ranging from −1.0 to −0.5 were operationally defined as GTs (i.e., rats more likely to direct behavior towards food magazine than lever), and rats with a PCA index score between +0.3 and +1.0 were designated as STs (i.e., rats more likely to direct behavior towards the lever-CS than the food magazine). Rats that were within the range of −0.49 to +0.29, whose behavior vacillated between lever-CS and food magazine, were classified as Intermediates and were not used given that our primary interest was comparing rats that strongly differed in their propensity to attribute incentive salience to food cues (Meyer et al., 2012). Of the rats screened for PCA behavior, a subset of 45 STs and 42 GTs were used in experiments described here. Experiment 1 included 21 STs and 24 GTs and Experiment 2 included 24 STs and 18 GTs.
2.3. Experiment 1: Individual variation in reinstatement of drug-seeking by an auditory Pavlovian cocaine cue (CS)
2.3.1. Overview
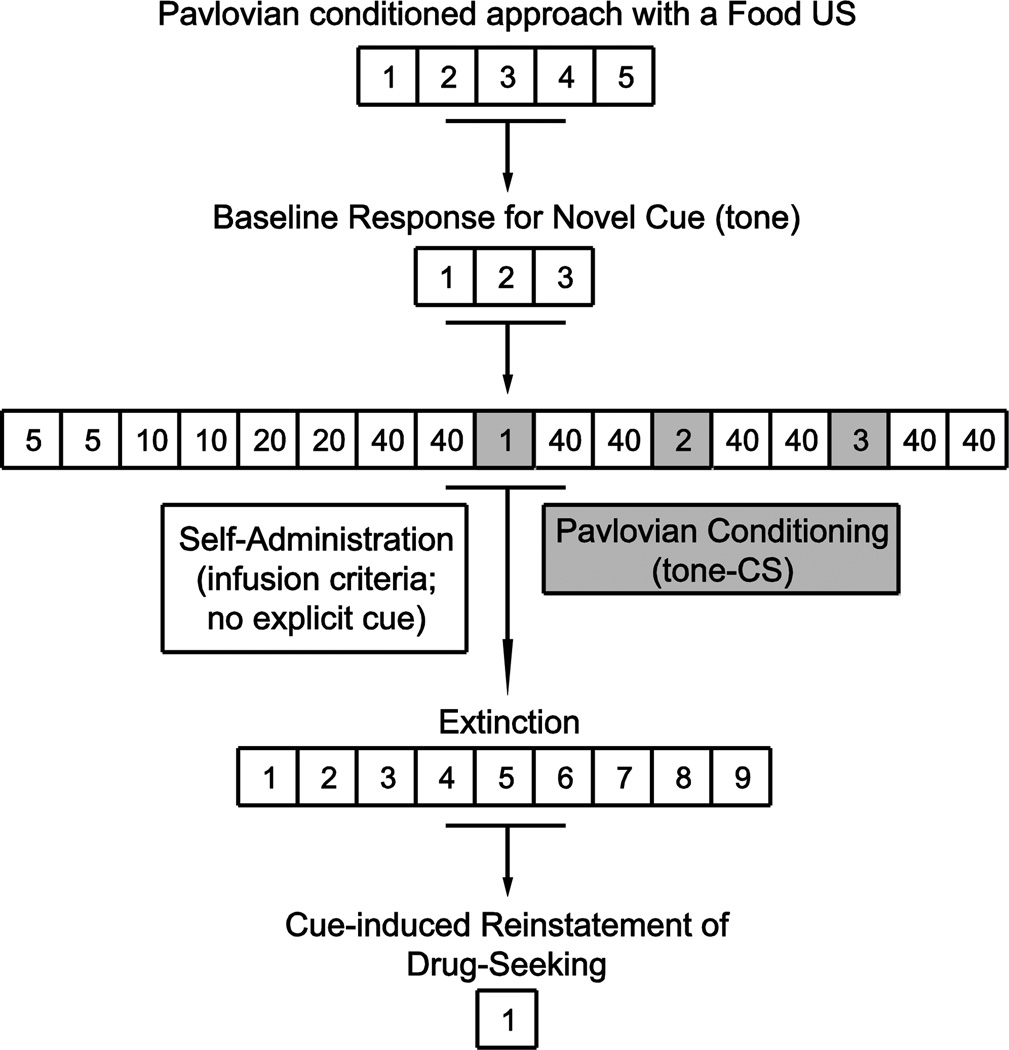
In the subsequent experiment (Experiment 2), we planned to use an auditory cue as a distractor, because rats do not approach an auditory cue (Cleland and Davey, 1983), which would make any differential effect in STs versus GTs more easily interpretable. That is, if we used a cue that was approached by STs but not GTs it would not be surprising if it disrupted ongoing performance to a greater degree in STs than GTs. It is known, however, that the form of the CS is important in determining the extent to which it acquires motivational value, when a food reward is used, such that an auditory cue is a less effective incentive stimulus (Meyer et al., 2014; Beckmann et a., 2015; Singer et al., In Press). Thus, we first wanted to determine whether an auditory stimulus (a tone-CS) paired with cocaine reward acquired sufficient motivational value to motivate renewed cocaine-seeking behavior, and whether it did so equally in STs and GTs. A summary of the experimental design for Experiment 1 is shown in Figure 1.
Figure 1.
Schematic illustration of the experimental design for Experiment 1. Each numbered box represents an individual session/day. Following Pavlovian training with a food unconditioned stimulus (US) and lever (CS), rats were classified as STs or GTs and then tested for responses for a novel cue (tone). Next, they were trained to self-administer cocaine (US) in the absence of any explicit cue. During subsequent Pavlovian conditioning sessions (grey), rats received non-contingent tone (CS)-cocaine (US) pairings. Following cocaine self-administration and Pavlovian conditioning, all animals underwent extinction training followed by a cue-induced reinstatement of drug-seeking test. Depending on the experimental stage, an active nose poke produced the cocaine-US (self-administration), no US (extinction), or the CS but no US (reinstatement).
2.3.2. Intravenous Catheter Surgery
Following Pavlovian training using food as the US (described above), a chronic indwelling catheter was implanted into the right jugular vein of all rats, as described previously (Crombag et al., 2000). Briefly, animals were anesthetized initially with 5% isoflurane in an anesthetic chamber (Anesco/Surgivet) then maintained with 2% isoflurance using a nose cone. Gas was carried via oxygen at a flow rate of 0.6 L/min. Post-operative pain was managed with carprofen (Rimadyl; 5 mg/kg, s.c.). The catheter exited through the dorsal skin surface between scapulae. Following surgery, catheters were flushed daily with 0.2 mL of sterile saline containing 5 mg/mL gentamicin sulfate (Vedco) to prevent occlusions and minimize infections. Catheter patency was tested periodically by intravenous (IV) injection of 0.15 mL of methohexital sodium (10 mg/mL in sterile water; JHP Pharmaceuticals). Animals were removed from analysis if they failed to become ataxic after infusion.
2.3.3. Apparatus
Cocaine self-administration, extinction and reinstatement testing was conducted in behavioral chambers similar to those used to screen animals for Pavlovian conditioned approach but with different manipulanda and programmable features. The food magazine and lever were removed from the chamber and replaced with two nose poke ports located 3 cm above the floor on the left and right sides of the wall opposite the houselight. In addition, a tone generator was placed on the same wall as the houselight. The side of the active nose poke port was counterbalanced between rats. A syringe pump, located outside the sound attenuating chamber and connected to the rats’ catheter back ports, delivered cocaine infusions. The infusion tubing was suspended into the chamber via a swivel mechanism, which allowed free movement in the chamber. A pine scented air-freshener was placed in the chamber, red houselight was used, and the floor was made of stainless steel bars. Contexts differed between Pavlovian conditioning and self-administration sessions to reduce any effect of context conditioning acquired during Pavlovian conditioning from influencing responding during the reinstatement test. For Pavlovian conditioning sessions, the nose poke ports were removed from the chambers, a vanilla scented air-freshener was placed in the chamber, a white houselight was used, and the floor was made of wire mesh.
2.3.4. Baseline Responding
Following recovery (7 days) from IV catheter surgery, rats underwent 3 sessions during which they had an opportunity to make instrumental responses for the presentation of stimulus tone (2 s). These habituation sessions were conducted to decrease otherwise high levels of responding to a novel stimulus. Once animals had fewer than 20 responses, cocaine self-administration training started.
2.3.5. Self-Administration Training
Rats were trained to make an instrumental response (nose poke in the active port) to receive an intravenous injection of cocaine hydrochloride (NIDA) dissolved in 0.9% sterile saline (0.2 or 0.4 mg/kg/infusion in 50 µL over 2.8 s) on a fixed ratio 1 (FR1) schedule of reinforcement with a 20 s time-out after each infusion. Responses into the active nose poke during the time-out, or into the inactive port at any time during the session, had no programmed consequence. Importantly, no explicit discrete cue was associated with drug infusion during self-administration sessions. Rather than restricting time of session, rats were required to earn a fixed number of infusions during each session (infusion criteria, IC), which increased across days (0.4 mg/kg/infusion for IC5, IC10; 0.2 mg/kg/infusion for IC20, IC40). Session length was determined by number of infusions (rather than total time) to ensure rats received the same amount of drug exposure during training.
2.3.6. Pavlovian training procedures with cocaine as the US
Following acquisition of stable self-administration behavior, the nose poke ports were removed and rats underwent three sessions of Pavlovian training with cocaine as the US and a tone (~78 dB) as the CS. Each of these Pavlovian conditioning sessions were separated by two days of self-administration at IC40 (Fig. 1). Prior to Pavlovian training, rats were assigned to either Paired (CS and US presented together) or Unpaired (US explicitly not paired with presentation of the CS) groups based on length of time to complete self-administration sessions averaged over the last 2 days of training at IC40 (ST-Paired, n = 11, ST-Unpaired, n = 10, GT-Paired, n = 12, GT-Unpaired, n = 12). This Pavlovian training occurred over 3 total sessions, each of which consisted of 12 trials on a variable interval schedule with a mean ITI of 900 s (840–960 s). For rats in the Paired group, the tone-CS was presented for 20 s and cocaine delivery (0.2 mg/kg/infusion over 2.8 s) coincided with the onset of the CS. No action was required to initiate either cocaine infusion or tone presentation. Rats in the Unpaired groups received non-contingent cocaine infusions that were explicitly not paired with tone presentation - cocaine was administered on a variable interval schedule with a mean ITI of 180 s after the CS presentation ended. Following the third and final Pavlovian conditioning session, rats were again allowed to self-administer cocaine at IC40 to re-stabilize behavior. Thus, in this experiment the CS (tone) that predicted cocaine delivery was not present during self-administration, but was associated with cocaine in three separate Pavlovian training sessions, and the Pavlovian context was distinct from the self-administration context (as described above).
2.3.7. Extinction and Reinstatement of Drug-Seeking Behavior
After the last self-administration session at IC40, rats underwent 9 daily 1 hr sessions of extinction training. During extinction, a response into either nose port had no consequence and there were no CS presentations. The day after the final extinction session, rats underwent an additional day of testing identical to extinction except on this day a response in the active nose poke was reinforced by a brief (5 s) presentation of the cocaine cue (tone-CS) and activation of the infusion pump but no cocaine delivery. A response in the inactive nose poke port had no consequence.
2.3.8. Statistical Analysis
Linear mixed-models (LMM) analysis was used for all repeated measures data, including active and inactive responses, and infusion rate during acquisition of self-administration, and comparing responses made during the final extinction session and reinstatement test. The best fitting model of repeated measures covariance was determined by the lowest Akaike information criterion score (West, 2006). Significant main effects and interactions are indicated with uncorrected degrees of freedom and were followed by planned pairwise comparison (Bonferroni t-test). Alpha was set at p < 0.05.
2.4. Experiment 2: Individual variation in the ability of cocaine and/or a cocaine cue to disrupt performance on a sustained attention task
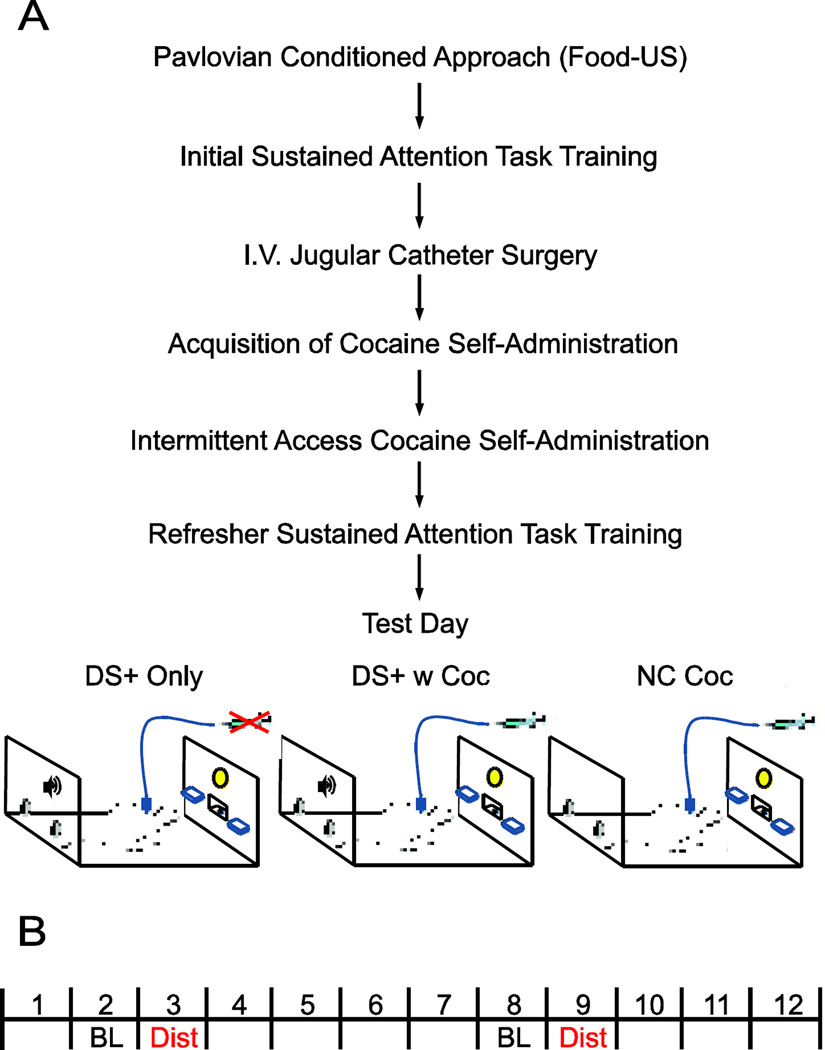
Humans find it difficult to resist attending to reward cues despite negative consequences, including interference with their ability to perform an ongoing task (for review (Anderson, 2016)). Although there is a large literature on attentional biases for reward cues, including drug cues, in humans, this has rarely been studied in animals. Thus, the purpose of Experiment 2 was to determine whether a cue (DS+) associated with cocaine availability would distract and/or disengage rats’ attention from ongoing performance in a sustained attention task, shifting them to seek and/or take cocaine, and whether this effect would differ in STs and GTs. Experiment 2 had several different stages, as outlined in Figure 2A. Below is a description of each stage in experimental order that occurred following PCA training using food as the US to identify STs and GTs (described above).
Figure 2.
Schematic illustration of the experimental design for Experiment 2. A, Following Pavlovian training with a food unconditioned stimulus (US) and lever (CS), rats were classified as STs or GTs and then started training on the sustained attention task (SAT). Once animals reached asymptotic performance at the task, rats were implanted with intravenous jugular catheters and trained to self-administer cocaine (US) in the absence of any explicit cue. Once animals had acquired cocaine self-administration, they entered a second cocaine self-administration procedure called Intermittent Access (IntA; DS+ = tone; DS− = white noise). After IntA training, animals went back to SAT before one of three distractor test day conditions (independent groups of rats were used for each distractor test day condition): DS+ Only, DS+ w Cocaine or Non-Contingent Cocaine (NC-coc). B, Each test day was comprised of 12 - 5 min blocks, including Blocks 3 and 9 with the distractor specific for test day (Dist), and Blocks 2 and 8 which served as baseline (BL) blocks for comparisons.
2.5. Sustained Attention Task (SAT)
2.5.1. Apparatus
Each chamber was equipped with an intelligence panel consisting of one panel light, two retractable levers (6 cm above floor), and a centered water dispenser (40 – 45 uL of water per delivery; 2.5 cm above floor). A white houselight was located on the back wall. A catch tray filled with corn-cob bedding was located underneath the floor, which was constructed from stainless steel rods. Each conditioning chamber was located in a sound-attenuating enclosure and background noise was supplied by a ventilating fan to mask outside noise.
2.5.2. Initial SAT Training
After PCA training, rats identified as STs and GTs (above) were initially trained to lever press using a modified FR1 schedule for water reinforcement. Prior to SAT training, animals were placed into the operant chamber with both levers extended. Each lever press was reinforced with the exception that if the total number of lever presses on either side exceeded greater than 5 more than the total presses on the other lever, that lever became inactive until the number of presses on the lever presses were made equal. This procedure removed any side bias persisting from PCA training. After at least 3 consecutive, daily sessions of >120 reinforced lever presses, animals entered the first stage of task training. Animals were then trained to discriminate between signal (1 s illumination of central panel light) and non-signal (no illumination) events. Two seconds after either one of these events, two levers were extended into the chamber. On signal trials, a response on the left lever was reinforced and termed a “hit”, whereas a response on the right lever was not reinforced and termed a “miss”. On non-signal trials, a response on the right lever was reinforced and termed a “correct rejection”, whereas a response on the left lever was not reinforced and termed a “false alarm”. Half the animals were trained using the reverse set of rules. If no response occurred within 4 s, the levers were retracted and the trial was recorded as an omission. Signal and non-signal trials were presented in pseudorandom order for 81 trials each (total of 162 trials) per session. The intertrial interval (ITI) was 12 +/− 3 s. During this initial stage of task training, incorrect responses were followed by up to three correction trials in which the previous trial type was repeated. In the event of an incorrect response on the third correction trial, a forced trial was initiated in which only the correct lever was extended for up to 90 s or until the animal made a response. When the forced trial was a signal trial, the central panel light remained illuminated while the appropriate lever was extended. The houselight was not illuminated during this training stage. After at least 5 consecutive days of stable performance, defined as > 70% hits and > 70% correct rejections, multiple signal durations (500, 50, 25 ms) were introduced. Trial type (signal or non-signal) and signal duration continued to be pseudo-randomly determined for each trial. The pseudo-random selection of trial type and signal duration was designed to ensure that approximately half of the trials were signal, and that similar numbers of 500, 50 and 25 ms signals were presented during the session. The houselight remained off, correction and forced trials were discontinued and the event rate was increased by reducing the ITI to 9 +/− 3 s. After at least 5 consecutive days of stable performance, defined as > 70% hits to 500 ms cues, > 70% correct rejections and < 20% omissions, animals moved to the final stage of training. In the final version of the task, the houselight was illuminated throughout the session and session length was set to 40 min to allow for post hoc analysis of performance over eight 5 min blocks of trials. The final step is essential for assessing sustained attention performance, as it requires the animals to constrain their behavior and maintain persistent attention on the front intelligence panel due to the houselight now being illuminated. Once animals reached this final stage of training, they remained on task for an additional 30 sessions before undergoing IV jugular catheter surgery (described above).
2.5.3. Behavioral Measures and Data Analysis
Previous studies have supported the validity of performance measures in terms of indicating attention/signal detection performance in rats (McGaughy and Sarter, 1995; Demeter et al., 2008), mice (St Peters et al., 2011) and humans (Demeter et al., 2008). For each session, hits, misses, correct rejections, false alarms and omissions were recorded. The relative number of hits (hits/(hits + misses)) was calculated for each signal duration (Hits500,50,25) as were the relative number of correct rejections (correct rejections/(correct rejections + false alarms)). Errors of omission were recorded separately. Unpaired and paired t-tests were used to compare ST and GT, and the effect of cocaine within STs or GTs, respectively. Alpha was set at p < 0.05.
2.6. Cocaine self-administration
2.6.1. Apparatus
The initial training to acquire of cocaine self-administration and then IntA self-administration training were both conducted in behavioral chambers similar to those described above for cocaine self-administration (Experiment 1), including two nose poke ports located 3 cm above the floor on the left and right sides of the wall on the same side as the white houselight. In addition, tone and white noise generators were placed on the same wall as the white houselight. The side of the active nose poke port was counterbalanced between rats. A syringe pump, located outside the sound attenuating chamber and connected to the rats’ catheter back ports, delivered cocaine infusions.
2.6.2. Acquisition of Cocaine Self-Administration
Following SAT training, chronic indwelling catheters were implanted into the jugular vein of all rats (described above) and animals were given 7 days to recover. Then, rats were trained to make an instrumental response (nose poke) to receive an intravenous injection of cocaine (0.2 or 0.4 mg/kg/infusion over 2.8 s) on a FR1 with 20 s time-out schedule of reinforcement. Responses into the active nose poke during the time-out, or into the inactive port, had no programmed consequence, but were recorded. Importantly, no experimenter-provided explicit cue was associated with drug infusion during self-administration sessions. Rather than restricting length of session, rats were required to earn a fixed number of infusions during each session (infusion criteria, IC), which increased across days (0.4 mg/kg/infusion for IC5, IC10; 0.2 mg/kg/infusion for IC20, IC40). Session length was determined by number of infusions to ensure rats received the same amount of drug. Animals had 3 sessions for IC5, IC10 and IC20 and 5 sessions for IC40.
2.6.3. Intermittent Access (IntA) Cocaine Self-Administration Procedure
Following acquisition of cocaine self-administration, rats continued to self-administer cocaine, but now using an IntA self-administration procedure, because this is known to produce addiction-like behavior (Zimmer et al., 2012; Kawa et al., 2016). Rats were placed into the behavioral chamber with the houselight illuminated for two minutes before the beginning of the first 5-min Drug Available period, which was signaled by a discriminative stimulus (DS+, tone, ~78 dB). In the presence of the DS+ a response into the active port caused the administration of a single infusion of cocaine hydrochloride (0.2 mg/kg/infusion; NIDA) dissolved in sterile saline (0.9%) on a FR1 schedule. There was no experimenter defined CS. Each response produced a 2.8 s infusion of cocaine. Apart from this 2.8 s infusion interval during which the pump was on, no timeouts were imposed to limit the number or timing of infusions within a Drug Available period. After the 5 min Drug Available period, the tone (DS+) turned off and white noise (DS−, ~78 dB) turned on signaling a 25 min No-Drug Available period. During the No-Drug Available period, nose pokes were recorded but they had no consequence. After 25 min the white noise was turned off and the tone was turned on again to signal another 5 min Drug Available period. Each IntA session consisted of 8 Drug Available and 8 No-Drug Available periods resulting in a 4 hr session. This self-administration procedure results in a series of spikes in brain cocaine concentrations, rapidly rising and returning back to baseline levels prior to the next Drug Available period (Zimmer et al., 2012). There were a total of 14 IntA self-administration sessions.
2.6.4. Statistical Analysis for IntA
Linear mixed-models (LMM) analysis was used for all repeated measures data. The best fitting model of repeated measures covariance was determined by the lowest Akaike information criterion score (West, 2006). Significant main effects and interactions are indicated with corrected degrees of freedom. Alpha was set at p < 0.05.
2.7. Retraining on the SAT
Once animals completed IntA cocaine self-administration training, all animals returned to the SAT for 7 days. In order to refresh task rules, animals were put onto the second stage of training (described above) for 3 days then moved to an extended version (60 min: 12 - 5 min blocks) of the final stage of the task for 4 days. Task performance following IntA cocaine experience was calculated by averaging % hits and correct rejections over the final 3 days of this retraining.
2.8. Distractor Test Days
2.8.1. Overview
After animals had completed the week of refresher SAT sessions, independent groups of animals were tested for the impact of the presentation of the discriminative stimulus (DS+) with or without the availability of cocaine self-administration on task performance. Independent groups of STs and GTs were subdivided into 3 different distractor test day conditions: (i) DS+ only; (ii) DS+ with cocaine available; (iii) Non-contingent (NC) cocaine (see below and Fig. 2).
2.8.2. Apparatus
Distractor test days were conducted in behavioral chambers outfitted for both SAT and cocaine self-administration. The front wall had the SAT intelligence panel: central cue light and 2 retractable levers on either side of a water receptacle. The back wall was configured for cocaine self-administration: two nose poke ports, the white houselight, and tone and white noise generators. As described earlier, a syringe pump was located outside the sound attenuating chamber and was connected to the rats’ catheter back ports to deliver cocaine infusions (if necessary).
2.8.3. Distractor Test Day Conditions
The 3 distractor test day conditions are depicted in Fig. 2: (i) DS+ Only; (ii) DS+ with Cocaine Available; (iii) Non-Contingent (NC) Cocaine. Note the presence or absence of DS+ (tone) and/or cocaine availability. Each test day was comprised of a 60 min session in which thirsty rats could perform the SAT throughout to obtain water reward. Blocks with distractors (i.e., DS+ presentation) along with baseline blocks used for behavioral comparisons are indicated for test day conditions in Figure 2B. For DS+ Only condition (ST, n=5; GT, n=7), the DS+ was presented during the 3rd and 9th 5-min blocks and the DS− during the 5th and 11th 5-min blocks, but under extinction conditions. That is, during this test an active response (nose poke in the active port) had no consequence. DS+ with Cocaine Available condition (ST, n=9; GT, n=5) had identical conditions as in DS+ Only, except active response during DS+ presentation (Blocks 3 and 9) resulted in a single cocaine infusion (0.2 mg/kg/infusion in 25 µL delivered over 2.8 s; same dose used during IntA) and the DS− was not presented during the session. Similar to IntA, there were no drug infusion timeouts other than during an infusion. Finally, in the NC Cocaine condition (ST, n=10; GT, n=6), during blocks 3 and 9, cocaine was passively infused (0.2 mg/kg/infusion in 25 µL delivered over 2.8 s) 5 times over each of these 5 min block, but no DS+ was presented. These 5 infusions were similar to the amount consumed by animals during the final Test Day condition (DS+ w coc; discussed below). Nose poke responses were recorded but had no consequence. For all distractor test day conditions, data collection included SAT performance (hits, misses, correct rejections, false alarms and number of trial omissions), and drug-seeking and -taking behavior (cocaine infusions, active and inactive responses).
2.8.4. Statistical Analysis for Distractor Test Days
Linear mixed-models (LMM) analysis was used for all repeated measures data. The best fitting model of repeated measures covariance was determined by the lowest Akaike information criterion score (West, 2006). We narrowed our analysis on active and inactive responses (cocaine self-administration behavioral parameters), and total number of hits, correct rejections and omissions (SAT behavioral parameters) to focus only on pertinent 5-min blocks depending on test day condition, including during DS presentations with or without cocaine availability, non-contingent cocaine infusions and baseline blocks which immediately preceded DS+ presentation and/or cocaine availability/infusions. In contrast to earlier SAT performance parameters being reported as a relative number (e.g., hits/hits+misses), total number of hits and correct rejections were used to demonstrate SAT performance during test days due to the high number of omissions and thus, the relatively low number of hits and correct rejection, particularly during DS+ w coc/NC-coc blocks. For between block comparisons, each test day was divided into halves with each half containing a baseline measure compared to a manipulation(s) (DS+/DS− alone, DS+ with coc available or non-contingent cocaine). Narrowing our focus to these blocks was done to investigate our specific hypotheses and to avoid a large number of unnecessary comparisons between blocks. Significant main effects and interactions are indicated with corrected degrees of freedom and were followed by planned pairwise comparison (Bonferroni t-test). Alpha was set at p < 0.05.
3. RESULTS
3.1. Experiment 1
3.1.1. Individual Variation in Pavlovian Conditioned Approach Behavior to a Food Cue
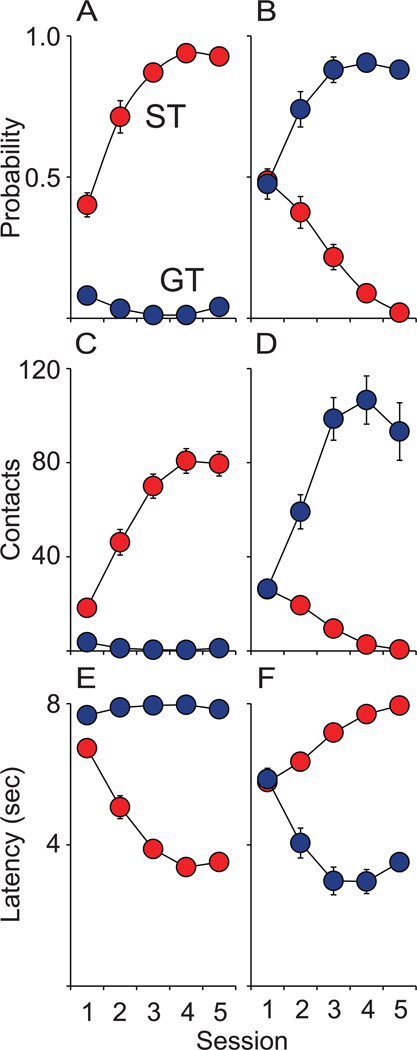
Two distinct phenotypes emerged as a result of Pavlovian training using food as the US, as reported previously (Flagel et al., 2009; Meyer et al., 2012). For a subset of rats, the presentation of the lever-CS came to evoke a sign-tracking conditioned response, consisting of a reliable and rapid approach to the lever-CS (Fig. 3A and E) and vigorous engagement with it (Fig. 3C). For another subset of rats, the presentation of the lever- CS rarely elicited approach to the lever-CS. Rather, presentation of the lever-CS came to evoke a goal-tracking conditioned response that consisted of reliable and rapid approach to the food magazine (Fig. 3B and F) and numerous entries into it (Fig. 3D). These magazine- or lever-directed behaviors increased across training days for GTs and STs, respectively. For classification purposes, a PCA Index score was calculated (described above) for each rat by averaging PCA Index scores for training days 4 and 5. The STs and GTs used in the current study had PCA Index scores between 0.309 to 0.918 (mean ± SEM; 0.696 ± 0.027) and −0.515 to −0.946 (mean ± SEM; −0.802 ± 0.019), respectively.
Figure 3.
Pavlovian conditioned approach behavior towards a lever (CS; left column) vs the location of food delivery (Food Cup; right column) was measured over 5 training sessions to determine a sign-tracker (ST, n = 42 total) or goal-tracker (GT, n = 45 total) phenotype. Mean ± SEM for (A) probability of approaching the lever-CS during the 8 s CS period, (B) probability of approaching the food magazine during the 8 s CS period, (C) number of lever contacts, (D) number of food magazine entries during the 8 s CS period, (E) latency to first lever contact after CS presentation, and (F) latency to the first food magazine entry after CS presentation.
3.1.2. Acquisition of Cocaine Self-Administration and Extinction of Drug-Seeking Behavior
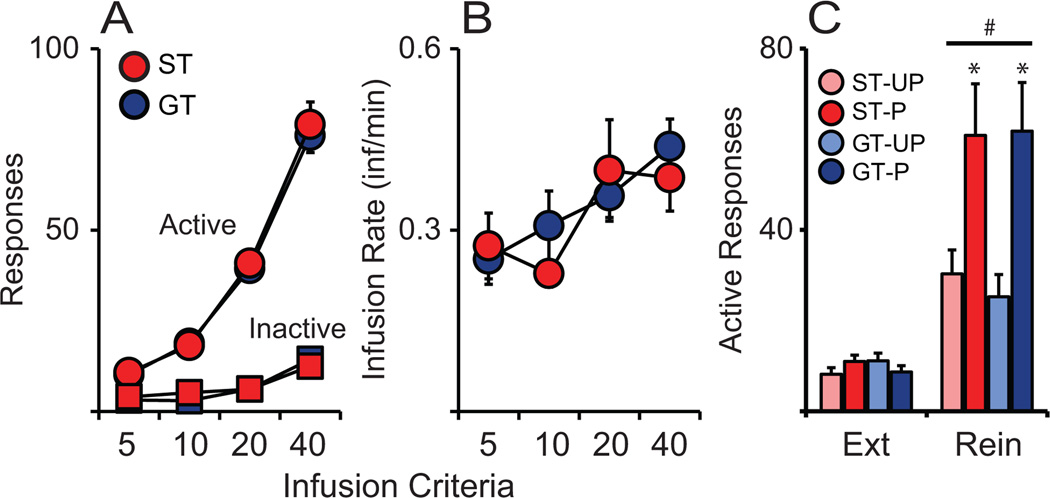
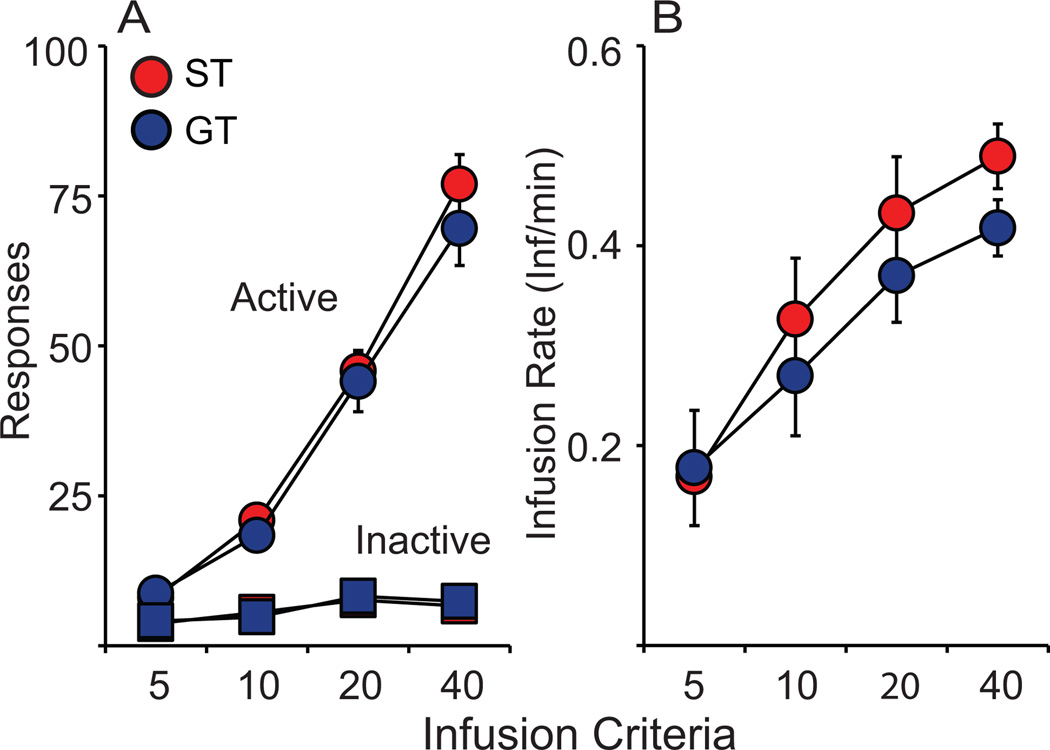
Following PCA training, rats were trained to self-administer cocaine using an infusion criterion (IC) procedure (Saunders and Robinson 2010). All animals received the same number response-reinforcer pairings during training which required fixed number of drug infusions each session (IC5, 10, 20, 40). Thus, any differences in the acquisition of self-administration would be evident in active/inactive responses or infusion rate (average number of cocaine infusions taken per minute). The number of active and inactive responses increased across training phases (Active, F(3,44) = 151.465, p < 0.001; Inactive, F(3,44) = 14.009, p < 0.001; Fig. 4A) similarly in both STs and GTs (Active, F(1,44) = 0.155, p = 0.696; Inactive, F(1,44) = 0.057, p = 0.813; Fig. 4A). Despite the increase in inactive response across IC training, it is clear that both groups learned to discriminate active and inactive ports. Similar to responses, there was an increase of infusion rate across training (IC, F(3,44) = 4.769, p = 0.006; Fig. 4B) in both STs and GTs (F(1,44) = 0.123, p = 0.728; Fig. 4B). Thus, we confirmed previous findings that STs and GTs do not differ in the acquisition of cocaine self-administration behavior under these conditions (Saunders and Robinson, 2010).
Figure 4.
There were no differences between STs (n = 21) and GTs (n = 24) in the acquisition of cocaine acquisition in Experiment 1 as indicated by similar (A) number of active and inactive responses, and (B) number of cocaine infusion/min for infusion criteria 5 and 10 (0.4 mg/kg/inf) and 20 and 40 (0.2 mg/kg/inf). Paired groups demonstrated greater cue-induced reinstatement (rein) of drug-seeking behavior than Unpaired groups using a classically conditioned auditory cue as indicated by number of active responses made during the cue-induced reinstatement test compared to final day of extinction (ST-UP, n = 10; ST-P, n = 11; GT-UP, n = 12; GT-P, n = 12).* indicates significant difference from unpaired group within phenotype during reinstatement; # indicates significant difference between reinstatement test and extinction within group. Values represent means ± SEM.
Following stable responding at IC40, rats underwent three Pavlovian conditioning sessions with cocaine as the US (described above). After each Pavlovian conditioning session, rats were returned to self-administration (IC40) to re-stabilize behavior. Following the final day of self-administration at IC40, rats underwent extinction training (9 sessions) during which an active response no longer resulted in a cocaine infusion. All groups (ST-unpaired, ST-paired, GT-unpaired, GT-paired) extinguished drug-seeking behavior (number of active responses) at a similar rate (F(3,44) = 1.436, p = 0.242; data not shown), as reported previously (Yager and Robinson, 2013; Saunders et al., 2014), and all groups extinguished to the same low level of responding (Fig. 4C).
3.1.3. Pavlovian Cocaine Cue-induced Reinstatement of Cocaine-Seeking Behavior
Following extinction training, all rats were tested for the ability of response-dependent presentation of an auditory Pavlovian cocaine cue (tone-CS) to reinstate drug-seeking behavior. Despite evidence that the form of a CS is important in determining the extent to which it acquires motivational value (Meyer et al., 2014), it has not been previously determined whether an auditory cue would reinstate cocaine-seeking differently in STs and GTs. During reinstatement, no cocaine was delivered, but a response into the active port produced a brief presentation of the tone-CS, which had been previously either paired or unpaired with cocaine. We used LMM to compare responses made during the final extinction session and reinstatement test (Factors: Session, Pairing and Phenotype). Both STs and GTs (Phenotype, F(1,44) = 0.119, p = 0.732; Fig. 4C) reinstated responding demonstrated by an increased number of active responses in response to the Pavlovian cocaine cue compared to the final extinction session (Session, F(1,44) = 57.915, p < 0.001; Fig. 4C). It is common to observe low, yet significant, reinstatement of drug-seeking behavior even in Unpaired groups because cocaine has a relatively long duration of action, so it is difficult to completely unpair a CS and the drug US, unless the ITI is very long (beyond the half-life of the drug). Here, we utilized a 15 min ITI, which may have been short enough that during Pavlovian conditioning sessions, brain levels of cocaine may have still been slightly elevated during CS presentation; thus, permitting some association between the CS and US in the Unpaired groups. Despite a small reinstatement effect in the Unpaired groups, pairing a tone-CS with cocaine caused greater reinstatement of drug-seeking behavior (F(1,44) = 14.891, p < 0.001; Fig. 4C). We observed a significant Session × Pairing interaction (F(1,44) = 13.255, p = 0.001; Fig. 4C). Paired STs and GTs showed significantly greater reinstatement of responding than corresponding Unpaired groups (ST, p = 0.029; GT, p = 0.007; Fig. 4C).
Experiment 1 established two findings of note: (i) an auditory cue (tone) paired with cocaine is attributed with sufficient incentive motivational value to reinstate cocaine-seeking behavior and (ii) an auditory cue (tone) paired with cocaine has equally strong motivational properties in STs and GTs, as these groups did not differ in the ability of the tone-CS to reinstate cocaine-seeking behavior.
3.2. Experiment 2
3.2.1. Cocaine Self-Administration Acquisition
Once animals reached 30 sessions at the last stage of the SAT training and displayed stable asymptotic performance levels, animals had IV jugular catheters implanted (described above). Following 7 days of recovery, rats were trained to self-administer cocaine using infusion criterion (IC5, 10, 20, 40) procedure similar to that utilized in Experiment 1. As expected, STs and GTs made similar number active (F(1,41) = 0.753, p = 0.390; Fig. 5A) and inactive (F(1,41) = 0.095, p = 0.759; Fig. 5A) responses, and displayed similar infusion rates (F(1,41) = 0.920, p = 0.343; Fig. 5B). As expected, as IC increased, active (F(3,41) = 122.926, p < 0.001; Fig. 5A) and inactive responses increased (F(3,41) = 5.529, p = 0.003; Fig. 5A) as did infusion rate (F(3,41) = 25.953, p < 0.001; Fig. 5B). These results are consistent with Experiment 1 and previous reports (Saunders and Robinson, 2010).
Figure 5.
There were no differences between STs (n = 24) and GTs (n = 18) in the acquisition of cocaine acquisition in Experiment 2 as indicated by (A) number of active and inactive responses, and (B) number of cocaine infusion/min for infusion criteria 5 and 10 (0.4 mg/kg/inf), and 20 and 40 (0.2 mg/kg/inf). Values represent means ± SEM.
3.2.2. Intermittent Access (IntA) Cocaine Self-Administration
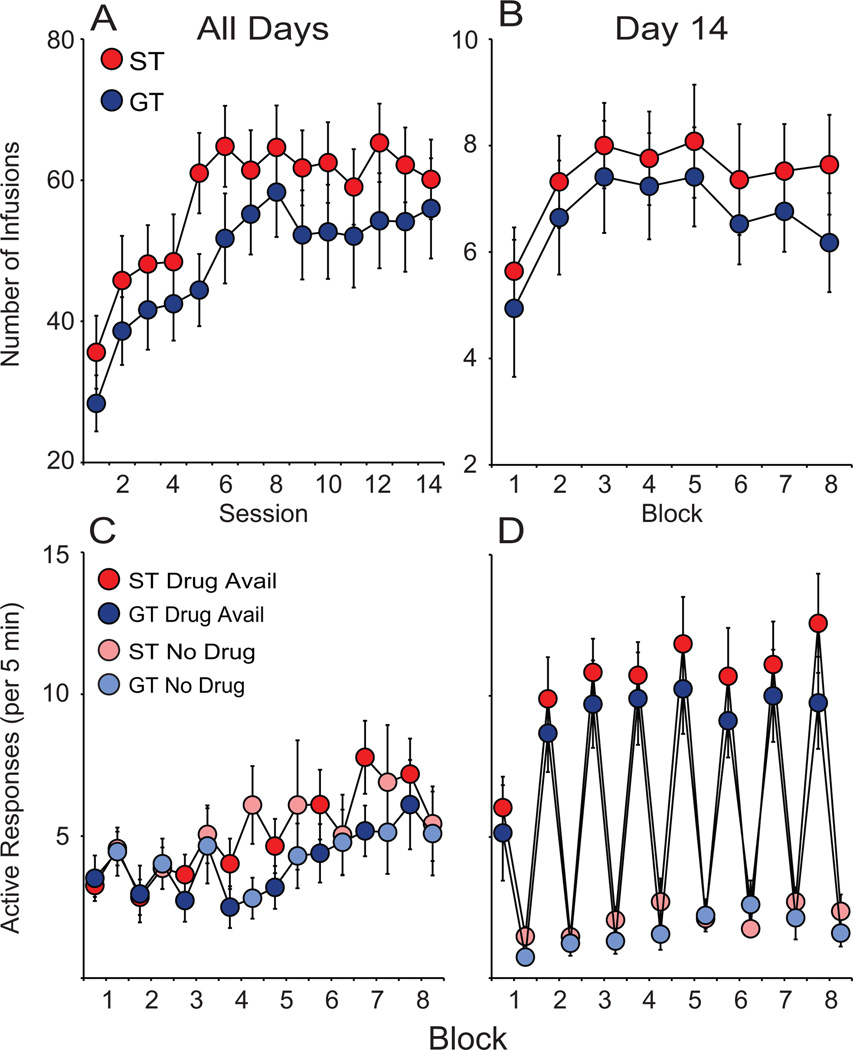
IntA self-administration lasted for a total of 14 sessions. Both groups escalated cocaine intake (number of infusions) from the 1st to 14th session (F(13,41) = 8.000, p < 0.001; Fig. 6A) in a similar manner as STs and GTs did not significantly differ in number of infusions over these 14 sessions (F(1,41) = 2.336, p = 0.134; Fig. 6A), consistent with a previous study (Kawa et al., 2016). Thus, STs and GTs received a comparable amount of cocaine throughout self-administration training (IC training and IntA). On day 14, there was, again, no ST/GT difference detected in number of infusions (F(1,41) = 0.619, p = 0.436; Fig. 6B).
Figure 6.
Acquisition and expression of Intermittent Access (IntA) cocaine self-administration in STs (n = 24) and GTs (n = 18). Both STs and GTs escalated cocaine intake and consumed similar amounts of cocaine over IntA training as indicated by (A) number of cocaine infusions during each of the 14 IntA sessions and (B) number of infusions during each block on day 14, the final IntA session. Both STs and GTs learned to discriminate between Drug Available periods (dark colors) and No-Drug Available periods (light circles) as indicated by number of active response per 5 min during the (C) first day of IntA compared to the end of IntA training on day 14 (D). Values represent means ± SEM.
Figures 6C and 6D show the number of active responses during Drug Available and No-Drug Available periods (averaged/5 min) for sessions 1 and 14, respectively. During session 1, the animals’ active responses were relatively constant during both DS+ and DS− periods because they had not yet learned to discriminate Drug Available vs. No-Drug Available periods. By session 14, animals had learned to distinguish between the DS+ and DS− periods as indicated by a large reduction in active responses during DS− periods compared to DS+ periods (Fig. 6D).
3.2.3. SAT Performance
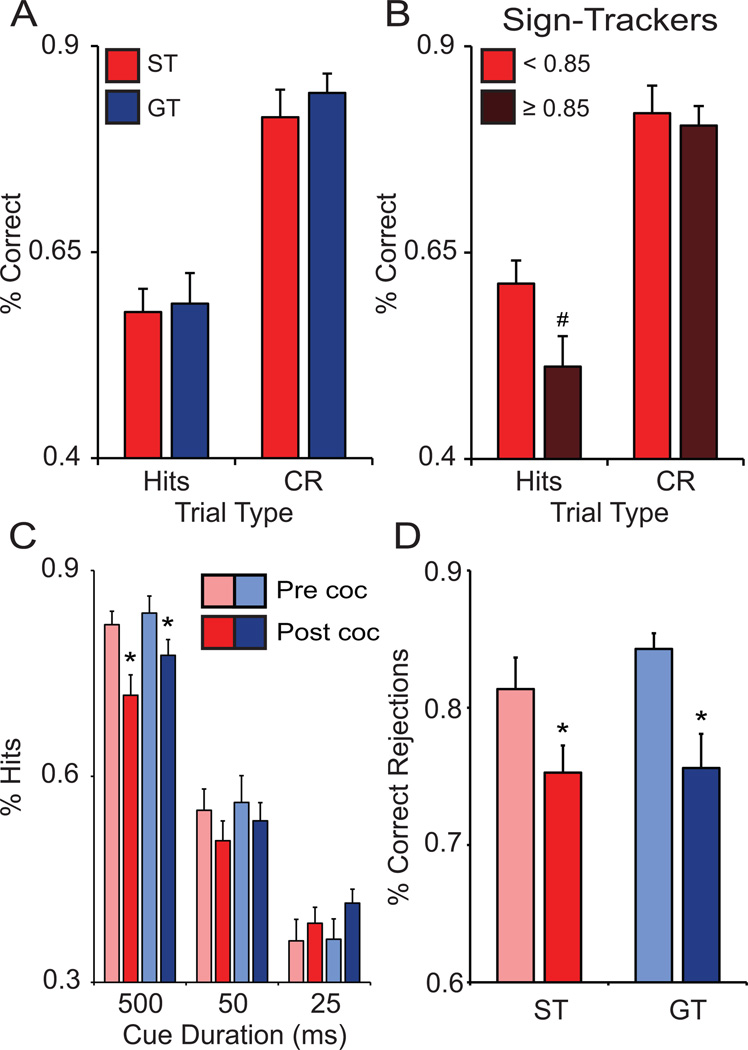
Following PCA training (outlined above), all rats started training at the SAT. SAT performance was analyzed two ways: (i) comparing performance at the end of initial training (before any cocaine self-administration experience) and (ii) comparing performance during SAT retraining (after cocaine self-administration experience) to performance prior to cocaine self-administration. Initial SAT training occurred over several weeks as rats went through multiple phases of training to acquire task rules and reach asymptotic performance levels at the final stage (described above). Overall, STs and GTs did not differ in task performance at the end of SAT training prior to cocaine experience (Fig. 7A). However, once STs were subdivided based on PCA index leading to the identification of ‘extreme’ STs (PCA Index ≥ 0.85; mean ± SEM, 0.88 ± 0.01, n = 8), these animals displayed significantly lower levels of performance (t(22) = 2.216, p = 0.0187; Fig. 7B) compared to STs with a PCA Index of < 0.85 (mean ± SEM, 0.74 ± 0.2, n = 16). This confirms our previous finding that ‘extreme’ STs have relatively poor attentional control (Paolone et al., 2013).
Figure 7.
Performance of the sustained attention task before and after cocaine self-administration experience. Prior to cocaine experience, there were no differences between STs (n = 24) and GTs (n = 18) according to (A) % hits (averaged across signal durations: 500, 50, 25 ms) and % correct rejections (CR). However, when STs were subdivided based on PCA Index value, extreme STs (PCA index ≥ 0.85, n = 8) performed more poorly than other STs (PCA index < 0.85, n = 16) as indicated by (B) % hits but not % CR. Cocaine self-administration experience caused a decrease in performance in both STs and GTs as indicated by (C) % hits (signal durations: 500, 50, 25 m) and (D) CR. * indicates significant difference within phenotype compared to pre-cocaine experience performance. # indicates significant difference from STs with a PCA index < 0.85. Values represent means ± SEM.
Previously, Briand et al (2008) showed that a “Long Access” cocaine self-administration procedure (6 hrs/day) produced persistent impairments in cognitive function manifested by poor performance on a similar task requiring sustain attention. We employed a different self-administration procedure (IntA rather than Long Access), yet we found a similar impairment on performance of SAT in all animals when tested after a few days off cocaine. Compared to performance at the end of initial SAT training, the combination of initial cocaine self-administration training followed by 14 days of IntA experience decreased attention performance in both STs (n = 24) and GTs (n = 18) with no significant effect on % trial omissions (t(23/17) > −1.908, p > 0.074; data not shown). Specifically, cocaine experience significantly decreased % hits500 (t(23/17) > 2.812, p < 0.011; Fig. 7C) and % correct rejections (t(23/17) > 2.883, p < 0.032; Fig. 7D). Although, we did not include an appropriate No-Drug experimental group to compare IntA animals to, Briand et al (2008) found that animals that underwent intravenous jugular catheter surgery and did not perform the attention task for months did not show deficits in task performance. Furthermore, we did not test how long this deficit persisted following cocaine self-administration experience.
3.2.4. Distractor Test Days
Independent groups of animals were used for each distractor test day condition. As described above, block comparisons were made for behavioral measures for each half of the test day: DS+ Only, DS+ with Cocaine Available and Non-Contingent Cocaine. In the first two conditions there was no effect of the distractor during the initial block, and all effects were confined to the second distractor block, so analyses and presented results are given just for the 2nd half of each distractor test day. There was an effect of non-contingent cocaine infusion on SAT performance during the first block (described below).
DS+ Only
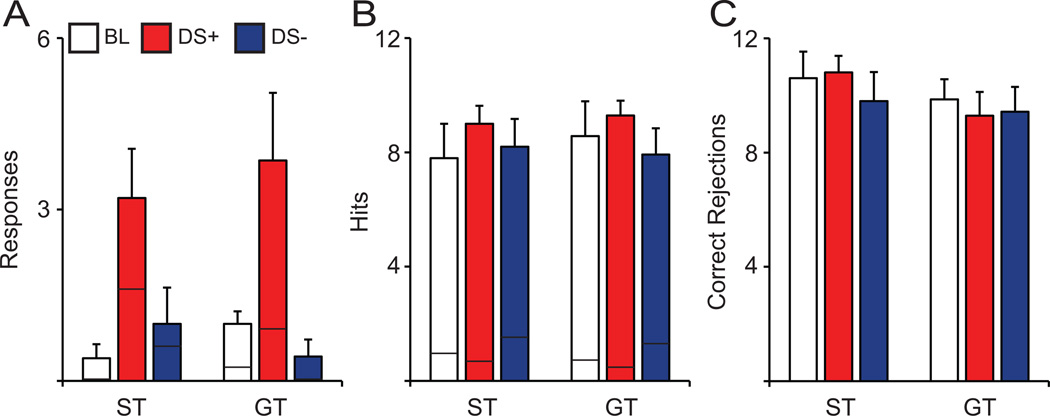
Our initial hypothesis was that the presentation of a discriminative stimulus that signals the availability of cocaine (DS+) would have a detrimental effect on performance of the SAT, by causing disengagement from the task and spurring drug-seeking behavior. During the DS+ only condition, while the animal was performing the SAT, either the DS+ (tone) or DS− (white noise) were presented for 5 min on 2 separate occasions. For comparisons, baseline behavior was taken during the 5 min block immediately preceding each respective DS+ presentation. For analysis, we broke the test day session into two halves: 1st, Baseline in Block 2, DS+ in Block 3, DS− in Block 5; 2nd, Baseline in Block 8, DS+ in Block 9, DS− in Block 11 (Fig. 2B). Behavioral data from the 2nd half of the test day is presented in Fig. 8A–C. The second DS+ presentation (Block 9) caused a significant increase in active (F(2,11) = 6.115, p = 0.010; Fig. 8A) and inactive responses (F(2,11) = 4.385, p = 0.035; Fig. 8A) in both STs and GTs (F(1,11) < 0.421, p > 0.521; Fig. 8A). There was no significant Block × Phenotype interaction (F(2,11) < 1.360, p > 0.283; Fig. 8A) detected for either active or inactive responses, despite an apparent increase in active responses in response to the DS+ compared to baseline and DS− presentation.
Figure 8.
In the second half of each test session, the DS+ presentation spurred drug-seeking behavior in both STs (n = 5) and GTs (n = 7) as indicated by an increase in (A) number of active responses (inactive depicted by black lines) but did not affect cue detection as no changes were observed in (B) hits and trial omissions (black lines) or (C) correct rejections. Values represent means ± SEM.
Although the second DS+ presentation appeared to evoke cocaine-seeking behavior, this did not impair performance on the SAT. Neither DS+ nor DS− had any effect on hits (F(2,11) < 1.394, ps > 0.263; Fig. 8B), correct rejections (F(2,11) < 0.317, ps > 0.733; Fig. 8C) or omissions (F(2,11) < 1.810, ps > 0.212; Fig. 8B). Moreover, STs and GTs were similarly unaffected by either the first or second presentations of DS+/DS− (hits: F(1,11) < 0.744, ps > 0.397; correct rejections: F(1,11) < 1.033, ps > 0.332; omissions: F(1,11) < 1.698, ps > 0.209). Thus, even though DS+ presentation appeared to distract animals away from the SAT, such that it evoked cocaine-seeking behavior, it was not sufficient to impair performance. Animals still managed to correctly detect signal and non-signal events, and respond accordingly.
DS+ with Cocaine Available (DS+ w coc)
The DS+ alone did not have an effect on SAT performance despite causing a relatively small, yet significant increase in drug-seeking behavior. In our second Distractor Test Day condition, cocaine was made available for self-administration during two separate DS+ presentations (Blocks 3 and 9). During these blocks, an active response resulted in a cocaine infusion identical to IntA training (0.2 mg/kg/infusion, FR1 schedule with no timeout period between infusions). For analysis, behavioral measures during each DS+ with cocaine available block were compared to the appropriate baseline behavior (5 min block immediately preceding it).
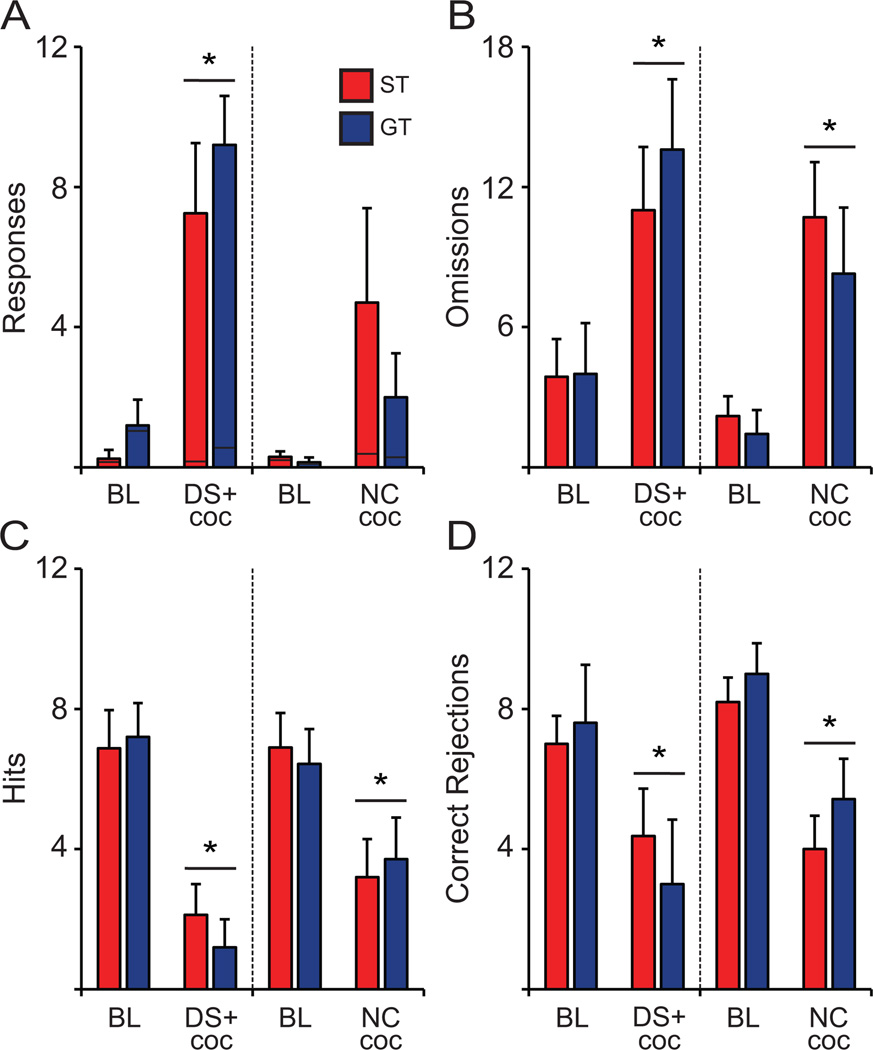
Presentation of the DS+ resulted in a large increase in active (F(1,12) = 27.532, p < 0.001; Fig. 9A) but not inactive responses (F(1,12) = 1.132, p = 0.303; Fig. 9A), with each active response producing an injection of cocaine, and did so to the same extent in STs and GTs (2nd, F(1,12) = 1.029, p = 0.330, Fig. 9A; 1st, F(1,12) = 0.0.572, p = 0.465, data not shown) compared to baseline. DS+ presentation, paired with cocaine availability, significantly impaired performance on the SAT (according to all behavioral measures) in both STs and GTs (F(1,12) < 0.527, ps > 0.483; Fig. 9B–D), as indicated by a decrease in the number of hits and correct rejections, and an increase in trial omissions (F(1,12) > 25.578, ps < 0.001; Fig. 9B–D). Thus, in contrast to the presentation of the DS+ only, the availability of cocaine to self-administer during the second DS+ presentation caused animals to almost entirely neglect the SAT.
Figure 9.
In the second half of each test session, DS+ w Cocaine (coc) and Non-Contingent cocaine (NC coc) spurred drug-seeking as indicated by an increase in (A) active responses (inactive depicted by black lines) and disrupted the sustained attention task performance as indicated by an increase in (B) trial omissions and decreases in number of total (C) hits and (D) correct rejections by STs (DS+ w coc, n = 9; NC coc, n = 10) and GTs (DS+ w coc, n = 5; NC coc, n = 6). * indicates significant difference of DS+ w coc or NC Coc compared to baseline (BL). Values represent means ± SEM.
Non-Contingent Cocaine (NC Coc)
Based on our findings in the other two distractor test day conditions, it was necessary to investigate whether an injection of cocaine, in the absence of the DS+, also disrupted SAT performance. During the DS+ with cocaine available, STs and GTs received 4.25 ± 1.0 and 5.2 ± 0.66 infusions during the 2nd DS+ presentation respectively, which served as the rationale for administering 5 non-contingent cocaine infusions during the NC coc condition. Animals received 5 non-contingent cocaine infusions during 2 separate blocks (Blocks 3 and 9) in the absence any discriminative stimulus. Baseline behavior was again taken during the 5 min block immediately preceding these non-contingent cocaine infusion blocks. Similar to other test day conditions, data shown are from the second half of the session: Block 9 and preceding baseline block (Block 8).
The second set of cocaine infusions in Block 9 caused a near significant increase in active (F(1,14) = 3.367, p = 0.089, Fig. 9A) but not inactive responses (F(1,14) = 0.0, p = 1.0, Fig. 9A). Non-contingent cocaine caused similar drug-seeking behavior in STs and GTs (2nd, F(1,14) = 2.022, p = 0.178, Fig. 9A; 1st, F(1,14) = 0.840, p = 0.374, data not shown). In regards to SAT performance, both the first (Block 3) and second (Block 9) set of non-contingent cocaine infusions caused a significant reduction in task performance as indicated by decreased numbers of hits and correct rejections, and increased trial omissions (Block 3: F(1,14) > 6.730, ps < 0.015, data not shown; Block 9: F(1,14) > 9.154, ps < 0.006, Fig 9B–D). In line with the effect of DS+ with cocaine availability, non-contingent cocaine infusions significantly reduced SAT performance equally in both STs and GTs during Blocks 3 or 9 (F(1,14) < 1.261, ps > 0.282, Fig. 9B–D).
4. DISCUSSION
In humans, reward cues are particularly effective in biasing attention towards them, to the extent they can impair ongoing task performance and, in the case of drug cues in those experiencing addiction, evoke drug-seeking behavior (Cox et al., 2002; Waters et al., 2003; Marissen et al., 2006; Field and Cox, 2008; Hickey et al., 2010; Anderson et al., 2011a, b; Anderson, 2016). To ultimately investigate the neuronal mechanisms by which drug cues shift individuals away from performing tasks that meet basic needs (e.g., obtaining food or water), we set out to develop a behavioral paradigm for such research in rats. Thus, the first objective of the current study was to develop an animal model to determine whether cues associated with self-administration of a drug (cocaine) would shift the attention of thirsty rats from ongoing performance of a water-rewarded sustained attention task (SAT) towards drug-seeking behavior. Our second objective was to test the hypothesis that STs – which are prone to attribute incentive salience to reward cues and have relatively poorer cognitive control – would be more susceptible to drug cue-elicited distraction than GTs. We found: (i) a classically conditioned auditory cue elicited cocaine-seeking equally in STs and GTs; (ii) cocaine self-administration experience using an Intermittent Access (IntA) procedure produced an escalation of drug intake; (iii) a DS+ gained robust stimulus control over IntA self-administration behavior in both STs and GTs; (iv) IntA cocaine self-administration experience impaired subsequent performance on the SAT in both STs and GTs; (iv) presentation of a cocaine cue (DS+) alone elicited cocaine seeking behavior but this was not sufficient to disrupt performance on the SAT; (v) when cocaine was available to self-administer when the DS+ was presented or when cocaine was given non-contingently, SAT performance was severely disrupted, as rats engaged in cocaine-seeking behavior.
Impact of cocaine cues
Contexts and cues associated with cocaine can gain considerable control over motivated behavior, reinstating drug-seeking behavior by inducing a conditioned motivational state and acting as powerful conditioned reinforcers (Kalivas and McFarland, 2003; Shaham et al., 2003; See, 2005; Milton and Everitt, 2012; Bossert et al., 2013; Robinson et al., 2014). Here, we used a DS+ (rather than a CS), which signals drug availability and precedes both the behavioral response and any drug effect. A DS+ gains robust stimulus control over drug-seeking behavior (Zimmer et al., 2012; Calipari et al., 2013; Calipari et al., 2014; Kawa et al., 2016) and its presentation has been shown to reinstate drug-seeking behavior both immediately and after months of abstinence (Alleweireldt et al., 2001; Ciccocioppo et al., 2001; Yun and Fields, 2003), and does so to a greater degree than a Pavlovian CS (Yun and Fields, 2003). Therefore, we used an IntA cocaine self-administration procedure - which is known to produce addiction-like behavior (Zimmer et al., 2012; Kawa et al., 2016) - to imbue the DS+ with motivational properties. We then asked whether the DS+, which predicted drug availability, would come to gain the animal’s attention to the extent that it would impair performance on the SAT.
In a test in which the DS+ was presented alone, it did cause rats to briefly disengage from the SAT, as indicated by an increase in drug-seeking behavior (i.e., increased responses on the cocaine-associated port). However, these attentional shifts away from the SAT were insufficient to disrupt ongoing task performance, as the animals fluidly drifted back and forth between the two behavioral options (SAT vs. drug-seeking). The magnitude of DS-induced drug-seeking was also relatively small, compared to that typically seen during DS-induced reinstatement tests which lack any other behavioral option (Alleweireldt et al., 2001; Ciccocioppo et al., 2001; Yun and Fields, 2003).
The resistance to significant distraction by the DS+ may have been related to the nature of the SAT used here. The basic SAT assesses stimulus-driven responding, but does not require a significant degree of executive control (Sarter et al., 2016). Moreover, animals were extensively trained on this task (6 days/week for ~ 3 months training) probably further enhancing the habitual nature of lever selection and water port visitations. Thus, although the DS captured the rats’ attention, as indicated by increased cocaine-seeking, they remained capable of simultaneously performing the SAT, in part by timing DS+ evoked responses to occur during intertrial intervals of the SAT. These findings highlight the challenges involved in developing rodent paradigms to assess the cognitive impact of drug stimuli and that tasks requiring greater top-down cognitive control for successful performance may be needed to obtain such effects.
Lack of individual differences
Another aim of the present experiment was to determine if the cocaine cue would have the same effect on STs and GTs, which are known to differ in the extent to which they attribute incentive salience to reward cues, including drug cues (Robinson et al., 2014). In most of our previous studies, we assessed the motivational properties of discrete localizable cues, such as a lever- or light-CS. However, in the current study we chose to use a tone DS for a couple reasons. Although STs attribute more incentive salience to a discrete localizable CS (Beckmann and Chow, 2015; Singer et al 2016), an auditory cue is an equally efficacious conditioned reinforcer in STs and GTs (Meyer et al., 2012), which we confirmed in Experiment 1 here. Therefore, we reasoned that any potential group difference in the propensity to be distracted by the cocaine cue (DS) in the present studies would presumably not be because STs attributed greater incentive salience to it, but because they might be more susceptible to attention disruptors compared to GTs because of their relatively poor attentional control (Paolone et al., 2013) and susceptibility to impulsive action (Lovic et al., 2011). On the other hand, we also considered the possibility that GTs might be more susceptible to distraction by a DS, because DSs share properties with contextual stimuli, setting the occasion for appropriate responding (Rosas et al., 2013; Bouton et al., 2014; Thrailkill and Bouton, 2015). Unlike discrete cues, a drug-associated context has been found to exert greater control over behavior in GTs than STs (Saunders et al., 2014; Ahrens et al., 2016). However, we found no differences between STs and GTs, even under conditions that did disrupt SAT performance (i.e., when cocaine was available). This lack of ST/GT difference may be due to groups being equally motivated by the auditory DS+, or because IntA experience eliminated any pre-existing difference in the potentially disruptive efficacy of the cue (see (Kawa et al., 2016) for more discussion).
Conclusions
Standard drug access settings are typically void of alternative competitors for attention (i.e., stimuli, opportunities, etc.). The absence of available alternate (salient) stimuli to engage with not only encourages drug self-administration (Carroll et al., 1989; Nader and Woolverton, 1991; Carroll and Lac, 1993) but also contrasts the richness of a human drug user’s environment which involves the presence of potentially competing tasks and behavioral activities. Several studies have modified drug access settings to incorporate choice between rewards, and in doing so, showed that drug-seeking behavior is drug- and setting-specific (Montanari et al., 2015). Indeed, the majority of animals prefer a desirable nondrug reward over drug reward when faced with a forced choice (Aigner and Balster, 1978; Lenoir et al., 2007; Cantin et al., 2010; Maguire et al., 2013; Tunstall et al., 2014; Banks et al., 2015; Caprioli et al., 2015). Here, we attempted to further “increase the costs of drug taking” as rats were expected to terminate SAT performance in the presence of cocaine or a cocaine cue. As already discussed, the cocaine cue alone failed to disrupt SAT performance and this was likely due to the nature of the task. However, when cocaine was available, and the rats had the option to either take cocaine or perform the SAT to obtain water, they largely took cocaine and SAT performance was severely disrupted. It will be important moving forward to further advance the current approach by assessing the effects of drug cues on the performance of tasks that require a greater degree of executive top-down control, and that cannot be performed with the high degree of automaticity, as with the basic SAT used here. Given that most tasks require extensive training in rodents to achieve reliable levels of performance, this is a challenging objective. In order to study the brain mechanisms that reliably allow drug cues to incite drug-seeking behavior even while the individual is engaged in tasks and activities that generate essential nondrug rewards, more realistic scenarios revealing the power of drug cues in vulnerable subjects are urgently needed.
Highlights.
A classically conditioned auditory cue elicited cue-induced drug seeking in STs and GTs
Intermittent Access cocaine self-admin produced escalation of drug intake
Intermittent Access cocaine self-admin produced robust stimulus control over self-admin behavior
Auditory cocaine cue elicited cocaine-seeking but did not disrupt performance of a sustained attention task
Cocaine availability, contingent on the discriminative stimulus or noncontingently, severely disrupted attention task performance
Acknowledgments
This research was supported by grants from National Institute on Drug Abuse to TER and MS (PO1 DA031656), and Canadian Institutes of Health Research to KKP (201311MFE-321581-165632).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Ahrens AM, Singer BF, Fitzpatrick CJ, Morrow JD, Robinson TE. Rats that sign-track are resistant to Pavlovian but not instrumental extinction. Behavioural brain research. 2016;296:418–430. doi: 10.1016/j.bbr.2015.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigner TG, Balster RL. Choice behavior in rhesus monkeys: cocaine versus food. Science. 1978;201:534–535. doi: 10.1126/science.96531. [DOI] [PubMed] [Google Scholar]
- Alleweireldt AT, Weber SM, Neisewander JL. Passive exposure to a contextual discriminative stimulus reinstates cocaine-seeking behavior in rats. Pharmacology, biochemistry, and behavior. 2001;69:555–560. doi: 10.1016/s0091-3057(01)00573-1. [DOI] [PubMed] [Google Scholar]
- Anderson BA. What is abnormal about addiction-related attentional biases? Drug and alcohol dependence. 2016 doi: 10.1016/j.drugalcdep.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, Yantis S. Value-driven attentional capture. Proceedings of the National Academy of Sciences of the United States of America. 2011a;108:10367–10371. doi: 10.1073/pnas.1104047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, Yantis S. Learned value magnifies salience-based attentional capture. PloS one. 2011b;6:e27926. doi: 10.1371/journal.pone.0027926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Hutsell BA, Schwienteck KL, Negus SS. Use of Preclinical Drug vs. Food Choice Procedures to Evaluate Candidate Medications for Cocaine Addiction. Current treatment options in psychiatry. 2015;2:136–150. doi: 10.1007/s40501-015-0042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology. 2013;229:453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Todd TP, Leon SP. Contextual control of discriminated operant behavior. Journal of experimental psychology Animal learning and cognition. 2014;40:92–105. doi: 10.1037/xan0000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Zimmer BA, Roberts DC, Jones SR. Temporal pattern of cocaine intake determines tolerance vs sensitization of cocaine effects at the dopamine transporter. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:2385–2392. doi: 10.1038/npp.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Siciliano CA, Zimmer BA, Jones SR. Intermittent cocaine self-administration produces sensitization of stimulant effects at the dopamine transporter. The Journal of pharmacology and experimental therapeutics. 2014;349:192–198. doi: 10.1124/jpet.114.212993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F, Vouillac C, Ahmed SH. Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PloS one. 2010;5:e11592. doi: 10.1371/journal.pone.0011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Zeric T, Thorndike EB, Venniro M. Persistent palatable food preference in rats with a history of limited and extended access to methamphetamine self-administration. Addiction biology. 2015;20:913–926. doi: 10.1111/adb.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience and biobehavioral reviews. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Autoshaping i.v. cocaine self-administration in rats: effects of nondrug alternative reinforcers on acquisition. Psychopharmacology. 1993;110:5–12. doi: 10.1007/BF02246944. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Nygaard SL. A concurrently available nondrug reinforcer prevents the acquisition or decreases the maintenance of cocaine-reinforced behavior. Psychopharmacology. 1989;97:23–29. doi: 10.1007/BF00443407. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland GG, Davey GC. Autoshaping in the rat: The effects of localizable visual and auditory signals for food. Journal of the experimental analysis of behavior. 1983;40:47–56. doi: 10.1901/jeab.1983.40-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox WM, Hogan LM, Kristian MR, Race JH. Alcohol attentional bias as a predictor of alcohol abusers' treatment outcome. Drug and alcohol dependence. 2002;68:237–243. doi: 10.1016/s0376-8716(02)00219-3. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Maren S, Robinson TE. The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behavioural brain research. 2000;116:1–22. doi: 10.1016/s0166-4328(00)00243-6. [DOI] [PubMed] [Google Scholar]
- Demeter E, Sarter M, Lustig C. Rats and humans paying attention: cross-species task development for translational research. Neuropsychology. 2008;22:787–799. doi: 10.1037/a0013712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug and alcohol dependence. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology. 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- Hickey C, Chelazzi L, Theeuwes J. Reward guides vision when it's your thing: trait reward-seeking in reward-mediated visual priming. PloS one. 2010;5:e14087. doi: 10.1371/journal.pone.0014087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kawa AB, Bentzley BS, Robinson TE. Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology. 2016 doi: 10.1007/s00213-016-4393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PloS one. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovic V, Saunders BT, Yager LM, Robinson TE. Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behavioural brain research. 2011;223:255–261. doi: 10.1016/j.bbr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, France CP. Effect of delay on self-administration of remifentanil under a drug versus drug choice procedure in rhesus monkeys. The Journal of pharmacology and experimental therapeutics. 2013;347:557–563. doi: 10.1124/jpet.113.208355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marissen MA, Franken IH, Waters AJ, Blanken P, van den Brink W, Hendriks VM. Attentional bias predicts heroin relapse following treatment. Addiction. 2006;101:1306–1312. doi: 10.1111/j.1360-0443.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology. 1995;117:340–357. doi: 10.1007/BF02246109. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Cogan ES, Robinson TE. The form of a conditioned stimulus can influence the degree to which it acquires incentive motivational properties. PloS one. 2014;9:e98163. doi: 10.1371/journal.pone.0098163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PloS one. 2012;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ. The persistence of maladaptive memory: addiction, drug memories and anti-relapse treatments. Neuroscience and biobehavioral reviews. 2012;36:1119–1139. doi: 10.1016/j.neubiorev.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Montanari C, Stendardo E, De Luca MT, Meringolo M, Contu L, Badiani A. Differential vulnerability to relapse into heroin versus cocaine-seeking as a function of setting. Psychopharmacology. 2015;232:2415–2424. doi: 10.1007/s00213-015-3877-2. [DOI] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Effects of increasing the magnitude of an alternative reinforcer on drug choice in a discrete-trials choice procedure. Psychopharmacology. 1991;105:169–174. doi: 10.1007/BF02244304. [DOI] [PubMed] [Google Scholar]
- Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M. Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:8321–8335. doi: 10.1523/JNEUROSCI.0709-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CJ, Zbukvic I, Kim JH, Lawrence AJ. Role of cues and contexts on drug-seeking behaviour. British journal of pharmacology. 2014;171:4636–4672. doi: 10.1111/bph.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain research Brain research reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: Individual differences. Neuropharmacology. 2014;76(Pt B):450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas JM, Todd TP, Bouton ME. Context Change and Associative Learning. Wiley interdisciplinary reviews Cognitive science. 2013;4:237–244. doi: 10.1002/wcs.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland NE. Food or fluid restriction in common laboratory animals: balancing welfare considerations with scientific inquiry. Comparative medicine. 2007;57:149–160. [PubMed] [Google Scholar]
- Sarter M, Lustig C, Blakely RD, Koshy Cherian A. Cholinergic genetics of visual attention: Human and mouse choline transporter capacity variants influence distractibility. Journal of physiology, Paris. 2016 doi: 10.1016/j.jphysparis.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biological psychiatry. 2010;67:730–736. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, O'Donnell EG, Aurbach EL, Robinson TE. A cocaine context renews drug seeking preferentially in a subset of individuals. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:2816–2823. doi: 10.1038/npp.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. European journal of pharmacology. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Singer BF, Bryan MA, Popov P, Scarff R, Carter C, Wright E, Aragona BJ, Robinson TE. The sensory features of a food cue influence its ability to act as an incentive stimulus and evoke dopamine release in the nucleus accumbens core. Learning and Memory. 2016 doi: 10.1101/lm.043026.116. (In Press) In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Peters M, Cherian AK, Bradshaw M, Sarter M. Sustained attention in mice: expanding the translational utility of the SAT by incorporating the Michigan Controlled Access Response Port (MICARP) Behavioural brain research. 2011;225:574–583. doi: 10.1016/j.bbr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrailkill EA, Bouton ME. Contextual control of instrumental actions and habits. Journal of experimental psychology Animal learning and cognition. 2015;41:69–80. doi: 10.1037/xan0000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall BJ, Riley AL, Kearns DN. Drug specificity in drug versus food choice in male rats. Experimental and clinical psychopharmacology. 2014;22:364–372. doi: 10.1037/a0037019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Bradley BP, Mogg K. Attentional shifts to smoking cues in smokers. Addiction. 2003;98:1409–1417. doi: 10.1046/j.1360-0443.2003.00465.x. [DOI] [PubMed] [Google Scholar]
- Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL, Ben-Shahar O. Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2001;25:361–372. doi: 10.1016/S0893-133X(01)00238-X. [DOI] [PubMed] [Google Scholar]
- West BT, Welch KB, Galecki AT. Linear Mixed Models: A Practical Guide Using Statistical Software. First. Taylor & Francis; 2006. [Google Scholar]
- Yager LM, Robinson TE. Cue-induced reinstatement of food seeking in rats that differ in their propensity to attribute incentive salience to food cues. Behavioural brain research. 2010;214:30–34. doi: 10.1016/j.bbr.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology. 2013;226:217–228. doi: 10.1007/s00213-012-2890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Pitchers KK, Flagel SB, Robinson TE. Individual variation in the motivational and neurobiological effects of an opioid cue. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40:1269–1277. doi: 10.1038/npp.2014.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun IA, Fields HL. Basolateral amygdala lesions impair both cue- and cocaine-induced reinstatement in animals trained on a discriminative stimulus task. Neuroscience. 2003;121:747–757. doi: 10.1016/s0306-4522(03)00531-1. [DOI] [PubMed] [Google Scholar]
- Zimmer BA, Oleson EB, Roberts DC. The motivation to self-administer is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:1901–1910. doi: 10.1038/npp.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]