Abstract
The prefrontal cortex (PFC) represents and executes the highest forms of goal-directed behavior, and has thereby attained a central neuroanatomical position in most pathophysiological conceptualizations of motivational disorders, including alcohol use disorder (AUD). Excessive, intermittent exposure to alcohol produces an allostatic dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis along with heightened forebrain glucocorticoid signaling that can damage PFC architecture and function. Negative affective states intimately associated with the transition to alcohol dependence result not only from a dysregulated HPA axis, but also from the inability of a damaged PFC to regulate subcortical stress and reinforcement centers, including the ventral striatum and amygdala. Several cognitive symptoms commonly associated with severe AUD, ranging from poor risk management to the cognitive/affective dimension of pain, are likely mediated by altered function of key anatomical elements that modulate PFC executive function, including contributions from the cingulate cortex and insula. Future therapeutic strategies for severe AUD should focus on attenuating the deleterious effects of excessive stress hormone activity on cognitive/affective and motivational behaviors gated by the PFC.
Keywords: Alcohol Use Disorder, Anterior Cingulate Cortex, Glucocorticoids, Insula, Pain, Prefrontal Cortex
Prefrontal Cortex Vulnerability in the Manifestation of Alcohol Use Disorder
Recent epidemiological data incorporating DSM-V criteria indicated that 13.9% of the U.S. population met the criteria for alcohol use disorder (AUD) over the past year [1]. Alcohol dependence (or severe alcohol use disorder) can be conceptualized as a chronic, relapsing disease characterized by the emergence of negative emotional states (e.g., dysphoria, anxiety, pain) and a profound narrowing of motivation toward the escalation of alcohol intake [2]. Consequently, the progression from recreational, limited consumption to uncontrolled, escalated drinking is proposed to involve a transition from positive to negative reinforcement mechanisms. In some cases, powerful negative subjective experiences can precede (e.g., post-traumatic stress disorder) or accompany (e.g., chronic pain) excessive alcohol drinking, and can themselves have a powerful influence on reward and reinforcement mechanisms, possibly facilitating the transition to dependence in vulnerable individuals [3–4]. This review focuses on a conceptualization of our understanding of prefrontal cortex (PFC) neuroadaptation as cause and consequence of alcohol dependence based on its central role in regulating a multitude of limbic and executive functions [5]. Many of these functions are mediated via complex information processing within several key frontocortical areas, often in association with an executive regulation of subcortical circuitry and broader somatic physiological states. In healthy individuals, these interactive mechanisms between brain and body are facilitated via adaptive and homeostatic stress hormone function. Based on its principle regulation of and particular vulnerability to systemic stress signaling, the PFC has been hypothesized to drive the emerging allostatic dysregulation of brain stress and reinforcement systems that promote the alcohol-dependent state [6].
Influence of Excessive Alcohol Exposure and Stress Hormones on Prefrontal Function
A role for central stress hormone and neuropeptide signaling has emerged as a conceptual bridge between chronic alcohol exposure, affective and cognitive disruption, and propensity to relapse to excessive drinking patterns. As the key integrative link between central brain and systemic stress adaptation, the hypothalamic-pituitary-adrenal (HPA) axis is responsible for initiating the physiological stress response in mammals. This response is regulated by negative and positive feedback mechanisms, where glucocorticoids (GCs) released from the adrenal gland can inhibit or stimulate (respectively) their own genomic and non-genomic actions by binding to glucocorticoid receptors (GRs) in the brain [7–8]. Moderate to high levels of GRs are located throughout PFC areas, and these regulate the HPA axis in a subregion-dependent manner. Lesions of the dorsomedial PFC in rats increase corticotropin-releasing factor (CRF) release from the hypothalamic paraventricular nucleus in response to acute restraint stress, while lesions of the ventromedial PFC decrease stress-induced CRF release from the hypothalamus [9]. These findings suggest that global PFC impairment can contribute to dysregulation of the HPA axis, particularly as repeated or prolonged (chronic) stressors produce HPA axis dysfunction and target end organ pathology over time. Both chronic stress and chronic glucocorticoid administration promote dendritic reorganization and morphological changes including reduced spine volume, surface area, and length in the PFC of rats [10–11]. As a specific type of stressor, alcohol intoxication and withdrawal activate the HPA axis to elevate circulating glucocorticoids in both rodents and humans [12–13]. This effect often has disparate effects both between and within target end organs, including the brain. In one study, circulating glucocorticoids were markedly increased during acute alcohol withdrawal in rodents, but this peripheral elevation returned to normal within a 24-hour period [14]. In contrast, glucocorticoids remained elevated in specific brain regions including the PFC after two months of alcohol withdrawal [14]. This long-lasting increase in central brain glucocorticoids may promote a blunted HPA axis response [13,15], sensitization of negative reinforcement circuitry [8], and neurotoxicity in vulnerable brain regions including the PFC [16]; all considered hallmarks of severe AUD.
Importantly, each of these symptoms of alcohol dependence can be reliably modeled in rodents using chronic, intermittent ethanol vapor (CIEV) exposure, which produces a wide range of both somatic and motivational symptoms of dependence [17] in both rats and mice. During alcohol withdrawal, a functional increase in central brain GR signaling is associated with escalated alcohol drinking in dependent rats [18], even following periods of protracted abstinence [19]. Greater nuclear localization of the GR in the PFC has been reported following withdrawal from chronic alcohol drinking [14]. This potentiation of central brain GR signaling is thought to contribute to negative reinforcement mechanisms associated with the transition to alcohol dependence [8]. In support of this theory, systemic administration of the GR antagonist mifepristone reduces alcohol drinking in rats given voluntary access to ethanol without influencing water consumption [20]. This effect was specific to the GR, because systemic administration of the mineralocorticoid receptor (MR) antagonist, spironolactone, did not reduce voluntary ethanol intake. Additionally, systemic delivery of mifepristone reduces escalated drinking associated with dependence in both rat models and early clinical trials [21]. These investigations suggest that increased GR signaling from excessive alcohol exposure underlies the transition from initial alcohol use to the uncontrolled and excessive alcohol intake associated with AUD.
The PFC mediates a number of cognitive functions involved in decision-making, memory formation and retention, emotion, and attention, all functions impaired in individuals suffering from AUD [22]. In preclinical models, CIEV exposure in mice alters trauma-related synaptic plasticity in the PFC [23] and impairs cognitive flexibility on attentional set shifting [24]. In addition to the evidence that increased GR signaling contributes to escalated alcohol drinking in dependence, there is evidence that dysregulation of GCs exacerbates neurocognitive deficits associated with AUD [25–26]. Central stress signaling may also play a critical role in cognitive dysfunction associated with binge alcohol consumption. In a rodent model of binge drinking, increased recruitment of Fos- and CRF-positive neurons in the PFC of rats was associated with impaired working memory on a Y-maze task during withdrawal [27]. In another model of chronic alcohol exposure, pronounced memory deficits were found in mice during alcohol withdrawal [28]. In the same study, object recognition and odor discrimination memory impairments were attenuated following systemic administration of mifepristone. In summary, these findings indicate that PFC dysregulation and potentiation of GR-mediated stress signaling from excessive alcohol exposure contributes to the emergence of cognitive deficits and promotes escalation of alcohol drinking associated with dependence.
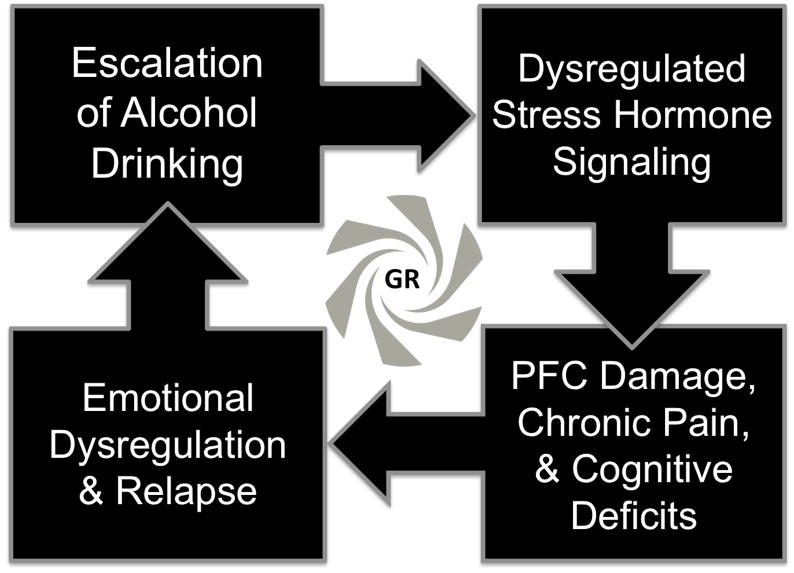
As one key motivational consequence of cognitive deficits, compromised PFC function leads to a failure of self-regulation processes that can facilitate relapse in individuals suffering from AUD [29]. This failure to self-regulate is associated with a pathological imbalance between corticolimbic circuits, most notably between the PFC, ventral striatum, and amygdala [30], and may be summarized as a narrowing of behavioral repertoire toward compulsive alcohol-seeking behavior, even in the face of adverse consequences. As one recent example of this complex phenomenon, stimulation of glutamatergic afferents from the PFC to the ventral striatum was shown to mediate aversion-resistant alcohol consumption [31]. In comparison, connectivity between the PFC and amygdala may contribute to the escalation of drinking following traumatic experiences [3]. Individuals transitioning to severe AUD may progress from dysregulated stress signaling, through neurodegeneration and loss of cognitive control over negative affect, to increased vulnerability to relapse during attempted abstinence (Figure 1). Cognitive deficits in certain patients may thus be inextricably linked to negative reinforcement processes that escalate the severity of AUD.
Figure 1.
Hypothesized neurobiological progression of alcohol use disorder (AUD) severity. Excessive drinking disrupts homeostatic stress signaling, and resulting excessive glucocorticoid receptor (GR) activity at the level of the prefrontal cortex (PFC) and downstream regions may produce a loss of cognitive control over negative affect, leading to increased vulnerability to relapse to heavy drinking patterns during attempted abstinence.p
Alcohol Dependence and Affective Pain: Role of the Insula and Cingulate Cortex
Chronic pain is estimated to affect approximately 20% of adults worldwide [32], a number that will likely increase over the next several decades given the aging global population. Thus, the neurobiological interaction of pain and substance misuse represents a critical area of research and public interest [4]. While the analgesic efficacy of alcohol has long been described [33], withdrawal from chronic use often results in increased pain sensitivity (or hyperalgesia) as part of a constellate alcohol withdrawal syndrome [34]. Such data suggest that excessive drinking in alcoholics may in part be motivated by a desire to self-medicate hyperalgesia. Self-reports of alcohol use specifically for pain management are common. Problem drinkers of both sexes report more severe pain symptoms compared to non-drinkers, and also a higher incidence of using alcohol to manage their pain [35]. This evidence of bidirectional causation between these two pathologies has lead to the conceptualization of alcohol dependence as a chronic pain disorder [36], as addiction and pain share a common neurocircuitry that may be compromised in either or both conditions.
The insula and the anterior cingulate cortex (ACC) are key components of a distinct neural network within the expansive, complex executive control system of the PFC. Communication between these regions is suggested to facilitate recognition of a stimulus and attribution of emotional salience to it. Of particular interest is the role of these regions in pain processing, as well as their more recently discovered association with alcohol dependence. Pain is a multidimensional experience, possessing both sensory and affective-motivational components [37]. The insula and ACC have been identified as key regions for supraspinal processing of the affective dimension through studies of these regions both in isolation and as a network. Imaging studies have identified increased activity in the insula and ACC with the anticipation of pain, and have correlated perceived pain intensity with degree of concurrent activity in the insula and ACC in human subjects [38–39]. In rodents, selective lesions as well as electrical stimulation of the ACC have been shown to reduce pain aversion, without altering the sensory element of painful stimuli [40].
The insula and ACC exhibit bidirectional neural projections and functional studies have reported a high level of co-activation during both cognitive task-based and resting-state imaging. Cognitive-task based imaging measures regional co-activity in the brain during completion of cognitively demanding tasks. Additionally, resting-state imaging can be used to study the network connectivity and co-activity of different brain regions. Resting-state fMRI analyses have led to the identification of a precise network based in the insula and ACC that extends to several sub-cortical regions now referred to as the salience network (SN). The SN model was developed from the integration of multiple fMRI studies that ultimately lead to the hypothesis that this particular circuitry recognizes and assimilates interoceptive and external information, recruits and de-recruits additional executive networks to engage the appropriate cognitive processes (focusing attention to stimuli, utilizing working memory), and finally regulates a behavioral response [41]. New research has provided evidence that alcohol dependence dysregulates activity of the insula-ACC salience network in humans, as indicated by fMRI data acquired during risk-taking trials. This is hypothesized to cause impairments in executive network switching, compromising the ability to make appropriate cognitively demanding decisions [42]. Salience network dysfunction may specifically contribute to the maintenance of alcohol dependence by making an individual unable to clearly discern risk in their behavior, such as the decision to seek out and consume excessive amounts of alcohol despite adverse consequences. Moreover, this network may be particularly vulnerable in AUD patients during stressful conditions due to ACC dysfunction [43].
Future Therapeutic Directions Targeting Alcohol-Driven PFC Dysfunction
In addition to the promise of mifepristone in treating excessive drinking [21] and cognitive deficits [44] in those suffering from AUD, downstream products of GR-mediated transcriptional regulation in the PFC may offer valuable treatment targets for consideration [45]. For example, while repeated cycles of excessive drinking and withdrawal produce an accumulation of damage in the PFC, trophic support mechanisms are also likely compromised due to decreased levels of brain-derived neurotrophic factor and nerve growth factor [46]. The emerging role of alcohol-induced neuroimmune dysregulation [47] and epigenetic modification [48] within the PFC also represents a fertile area for future research and medication development. Finally, the influence of sex on the interaction of stress, excessive drinking, and PFC function should be explored further [49], particularly given the unique relationship between PFC glucocorticoid signaling and alcohol-induced neurotoxicity in females [50]. Given the potential link between cognitive deficits and negative affect dysregulation, targeting these two conditions together in AUD patients may represent a superior conceptual strategy for medication development.
Highlights.
The prefrontal cortex (PFC) is a key gate for alcohol use disorder (AUD) symptoms.
AUD symptoms regulated by the PFC include compulsive drinking and pain.
PFC glucocorticoid receptor (GR) signaling facilitates negative affect and relapse.
Reducing GR signaling restores PFC functionality and may reduce heavy drinking.
Acknowledgments
Preparation of this review was generously supported by the LSUHSC-New Orleans Comprehensive Alcohol-HIV/AIDS Research Center (P60AA009803), as well as training (T32AA007577; ARP, MAM) and research (R00AA020839; SE) grants from the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Conflict of Interest Statement
All authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of dsm-5 alcohol use disorder: Results from the national epidemiologic survey on alcohol and related conditions iii. JAMA Psychiatry. 2015;72(8):757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards S, Koob GF. Neurobiology of dysregulated motivational systems in drug addiction. Future Neurol. 2010;5(3):393–401. doi: 10.2217/fnl.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards S, Baynes BB, Carmichael CY, Zamora-Martinez ER, Barrus M, Koob GF, Gilpin NW. Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Transl Psychiatry. 2013;3:e296. doi: 10.1038/tp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LeBlanc DM, McGinn MA, Itoga CA, Edwards S. The affective dimension of pain as a risk factor for drug and alcohol addiction. Alcohol. 2015;49(8):803–809. doi: 10.1016/j.alcohol.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5**.George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2010;35(2):232–247. doi: 10.1016/j.neubiorev.2010.05.002. A comprehensive and valuable review of prefrontal cortical areas and their afferent and efferent functional connections with respect to drug addiction-related symptomatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6*.Lu YL, Richardson HN. Alcohol, stress hormones, and the prefrontal cortex: A proposed pathway to the dark side of addiction. Neuroscience. 2014;277:139–151. doi: 10.1016/j.neuroscience.2014.06.053. Describes the manifestation and potentially harmful implications of neuroendocrine tolerance that occurs during the transition from alcohol use to dependence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myers B, McKlveen JM, Herman JP. Neural regulation of the stress response: The many faces of feedback. Cell Mol Neurobiol. 2012 doi: 10.1007/s10571-012-9801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards S, Little HJ, Richardson HN, Vendruscolo LF. Divergent regulation of distinct glucocorticoid systems in alcohol dependence. Alcohol. 2015;49(8):811–816. doi: 10.1016/j.alcohol.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26(50):12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49(3):245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 11.Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507(1):1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabakoff B, Jafee RC, Ritzmann RF. Corticosterone concentrations in mice during ethanol drinking and withdrawal. J Pharm Pharmacol. 1978;30(6):371–374. doi: 10.1111/j.2042-7158.1978.tb13259.x. [DOI] [PubMed] [Google Scholar]
- 13.Adinoff B, Martin PR, Bone GH, Eckardt MJ, Roehrich L, George DT, Moss HB, Eskay R, Linnoila M, Gold PW. Hypothalamic-pituitary-adrenal axis functioning and cerebrospinal fluid corticotropin releasing hormone and corticotropin levels in alcoholics after recent and long-term abstinence. Arch Gen Psychiatry. 1990;47(4):325–330. doi: 10.1001/archpsyc.1990.01810160025004. [DOI] [PubMed] [Google Scholar]
- 14*.Little HJ, Croft AP, O’Callaghan MJ, Brooks SP, Wang G, Shaw SG. Selective increases in regional brain glucocorticoid: A novel effect of chronic alcohol. Neuroscience. 2008;156(4):1017–1027. doi: 10.1016/j.neuroscience.2008.08.029. Provided a novel neurobiological mechanism of alcohol-induced regulation of brain glucocorticoids relevant to cognition and AUD. Increased brain glucocorticoids were observed in both male and female mice and rats following chronic alcohol administration. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Rivier C. An initial, three-day-long treatment with alcohol induces a long-lasting phenomenon of selective tolerance in the activity of the rat hypothalamic-pituitary-adrenal axis. J Neurosci. 1997;17(22):8856–8866. doi: 10.1523/JNEUROSCI.17-22-08856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson HN, Chan SH, Crawford EF, Lee YK, Funk CK, Koob GF, Mandyam CD. Permanent impairment of birth and survival of cortical and hippocampal proliferating cells following excessive drinking during alcohol dependence. Neurobiol Dis. 2009;36(1):1–10. doi: 10.1016/j.nbd.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vendruscolo LF, Roberts AJ. Operant alcohol self-administration in dependent rats: Focus on the vapor model. Alcohol. 2014;48(3):277–286. doi: 10.1016/j.alcohol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32(22):7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Repunte-Canonigo V, Shin W, Vendruscolo LF, Lefebvre C, van der Stap L, Kawamura T, Schlosburg JE, Alvarez M, Koob GF, Califano A, Sanna PP. Identifying candidate drivers of alcohol dependence-induced excessive drinking by assembly and interrogation of brain-specific regulatory networks. Genome Biol. 2015;16:68. doi: 10.1186/s13059-015-0593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koenig HN, Olive MF. The glucocorticoid receptor antagonist mifepristone reduces ethanol intake in rats under limited access conditions. Psychoneuroendocrinology. 2004;29(8):999–1003. doi: 10.1016/j.psyneuen.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 21**.Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, McGinn MA, Zamora-Martinez ER, Belanoff JK, Hunt HJ, Sanna PP, et al. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest. 2015;125(8):3193–3197. doi: 10.1172/JCI79828. Translational evidence of mifepristone efficacy to reduce excessive drinking in both a preclinical model of severe alcohol use disorder and in alcohol-dependent human subjects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: A substrate of disruption and repair. Psychopharmacology (Berl) 2005;180(4):583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- 23**.Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, et al. Chronic alcohol remodels prefrontal neurons and disrupts nmdar-mediated fear extinction encoding. Nat Neurosci. 2012;15(10):1359–1361. doi: 10.1038/nn.3204. Demonstrates the drastic consequences of chronic alcohol exposure on prefrontal function and the development of trauma-related anxiety. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One. 2012;7(5):e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Errico AL, King AC, Lovallo WR, Parsons OA. Cortisol dysregulation and cognitive impairment in abstinent male alcoholics. Alcohol Clin Exp Res. 2002;26(8):1198–1204. doi: 10.1097/01.ALC.0000025885.23192.FF. [DOI] [PubMed] [Google Scholar]
- 26.Rose AK, Shaw SG, Prendergast MA, Little HJ. The importance of glucocorticoids in alcohol dependence and neurotoxicity. Alcohol Clin Exp Res. 2010;34(12):2011–2018. doi: 10.1111/j.1530-0277.2010.01298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Koob GF. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci U S A. 2012;109(44):18156–18161. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacquot C, Croft AP, Prendergast MA, Mulholland P, Shaw SG, Little HJ. Effects of the glucocorticoid antagonist, mifepristone, on the consequences of withdrawal from long term alcohol consumption. Alcohol Clin Exp Res. 2008;32(12):2107–2116. doi: 10.1111/j.1530-0277.2008.00799.x. [DOI] [PubMed] [Google Scholar]
- 29.Baumeister RF. Ego depletion and self-regulation failure: A resource model of self-control. Alcohol Clin Exp Res. 2003;27(2):281–284. doi: 10.1097/01.ALC.0000060879.61384.A4. [DOI] [PubMed] [Google Scholar]
- 30.Koob GF, Volkow ND. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW. Cortical activation of accumbens hyperpolarization-active nmdars mediates aversion-resistant alcohol intake. Nat Neurosci. 2013;16(8):1094–1100. doi: 10.1038/nn.3445. Optogenetic interrogation of the prefrontal circuitry responsible for drinking in the face of adverse consequences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health. 2011;11:770. doi: 10.1186/1471-2458-11-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riley JL, King C. Self-report of alcohol use for pain in a multi-ethnic community sample. Journal of Pain. 2009;204:423–429. doi: 10.1016/j.jpain.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, Schulteis G, Koob GF. Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: Alleviation by crf(1) receptor antagonism. Neuropharmacology. 2012;62(2):1142–1151. doi: 10.1016/j.neuropharm.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brennan PL, Schutte KK, Moos RH. Pain and use of alcohol to manage pain: Prevalence and 3-year outcomes among older problem and non-problem drinkers. Addiction. 2005;100(6):777–786. doi: 10.1111/j.1360-0443.2005.01074.x. [DOI] [PubMed] [Google Scholar]
- 36.Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev. 2012;36(10):2179–2192. doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6(7):533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. Dissociating pain from its anticipation in the human brain. Science. 1999:1979–1981. doi: 10.1126/science.284.5422.1979. 2845422. [DOI] [PubMed] [Google Scholar]
- 39.Casey KL. Concepts of pain mechanisms: The contribution of functional imaging of the human brain. Prog Brain Res. 2000;129:277–287. doi: 10.1016/S0079-6123(00)29020-1. [DOI] [PubMed] [Google Scholar]
- 40.Fuchs PN, Peng YB, Boyette-Davis JA, Uhelski ML. The anterior cingulate cortex and pain processing. Front Integr Neurosci. 2014;8:35. doi: 10.3389/fnint.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menon V, Uddin LQ. Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu X, Sundby K, Bjork JM, Momenan R. Alcohol dependence and altered engagement of brain networks in risky decisions. Front Hum Neurosci. 2016;10:142. doi: 10.3389/fnhum.2016.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seo D, Lacadie CM, Sinha R. Neural correlates and connectivity underlying stress-related impulse control difficulties in alcoholism. Alcohol Clin Exp Res. 2016;40(9):1884–1894. doi: 10.1111/acer.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donoghue K, Rose A, Coulton S, Milward J, Reed K, Drummond C, Little H. Double-blind, 12 month follow-up, placebo-controlled trial of mifepristone on cognition in alcoholics: The mifcog trial protocol. BMC Psychiatry. 2016;16:40. doi: 10.1186/s12888-016-0757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costin BN, Wolen AR, Fitting S, Shelton KL, Miles MF. Role of adrenal glucocorticoid signaling in prefrontal cortex gene expression and acute behavioral responses to ethanol. Alcohol Clin Exp Res. 2013;37(1):57–66. doi: 10.1111/j.1530-0277.2012.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernandez GM, Stewart WN, Savage LM. Chronic drinking during adolescence predisposes the adult rat for continued heavy drinking: Neurotrophin and behavioral adaptation after long-term, continuous ethanol exposure. PLoS One. 2016;11(3):e0149987. doi: 10.1371/journal.pone.0149987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crews FT, Vetreno RP. Neuroimmune basis of alcoholic brain damage. Int Rev Neurobiol. 2014;118:315–357. doi: 10.1016/B978-0-12-801284-0.00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barbier E, Johnstone AL, Khomtchouk BB, Tapocik JD, Pitcairn C, Rehman F, Augier E, Borich A, Schank JR, Rienas CA, Van Booven DJ, et al. Dependence-induced increase of alcohol self-administration and compulsive drinking mediated by the histone methyltransferase prdm2. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49**.Retson TA, Sterling RC, Van Bockstaele EJ. Alcohol-induced dysregulation of stress-related circuitry: The search for novel targets and implications for interventions across the sexes. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:252–259. doi: 10.1016/j.pnpbp.2015.05.009. This review sets the stage for future investigations into sex as a highly relevant variable at the intersection of stress and AUD treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilhelm CJ, Hashimoto JG, Roberts ML, Bloom SH, Beard DK, Wiren KM. Females uniquely vulnerable to alcohol-induced neurotoxicity show altered glucocorticoid signaling. Brain Res. 2015;1601:102–116. doi: 10.1016/j.brainres.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]