Abstract
Purpose
HSP90, a viable target for cancer treatment, mediates the maturation and stabilization of client oncoproteins. HSP90i are potentially active in a variety of tumors, but therapeutic benefit is confirmed in only a small subset. We explored potential biomarkers across multiple studies of HSP90i in advanced solid tumors.
Methods
Archived tumor specimens from patients treated with HSP90i on 7 different phase I/II trials at MSKCC were identified. Tumor tissue was tested by IHC: ER, PR and AR: ≥1% positive and <1% negative; HSP90 and HSP70: 0, 1+ negative and 2+, 3+ positive; PTEN: 0 negative, 1 reduced and 2 positive; HER2: 0, 1+ negative, 2+ equivocal, 3+ positive; EGFR: 0 negative and 1+, 2+, 3+ positive. The expression of the biomarker panel was correlated with clinical benefit (CB) (defined by ORR or CB by “8 week” scan) using Fisher's exact test.
Results
Adequate tissue was available for 51/158 patients (32%), including 10 different solid tumors. Of these, 71% (36/51) and 51 % (26/51) patients met the criteria to assess CB by best ORR or by “8 week scan” assessment respectively. Breast was the most frequent tumor. Mean duration of HSP90i therapy was 55 days (range, 16-411). There were 16 responses (4 PR; 12 SD); 13/16 responses strongly correlated with HER2+ status (p = 0.001).
Conclusion
Our findings suggest HER2 as a sensitive client and perhaps the only effective biomarker for sensitivity to these HSP90i.
Keywords: HSP90, HER2, Breast cancer, Biomarker
Introduction
Heat shock protein 90 [HSP90], a molecular chaperone, along with several co-chaperones such as HSP70, activator of HSP90 ATPase 1 [Aha1], HSP40, HSP70/HSP90 organizing protein [HOP], P23, cell division cycle protein 37 [CDC37] exerts its role by employing a complex cycle of “client” protein binding and hydrolysis of ATP and regulating the stability and function of these client proteins 1-4. Of the approximate 200 client proteins (for a continually updated list see http://www.picard.ch/downl-loads), several are oncoproteins including kinases (human epidermal growth factor receptor [HER2], epidermal growth factor receptor [EGFR]), steroid receptors (estrogen, progesterone, and androgen receptors [ER, PR and ARs]), metastable signaling proteins (Akt, Raf-1) among others, that represent nodal points in multiple oncogenic signaling pathways 5. Inhibition of the HSP90 chaperone cycle leads to simultaneous degradation of these oncoproteins via the ubiquitin-proteasome pathway, downregulation of these redundant pathways that are essential for cell survival, along with HSP70 induction1,2. Together, these constitute a pharmacodynamics read out or molecular signature of HSP90 inhibition 6,7,8-10.
Since the serendipitous discovery of anti-tumor activity noted with the natural product, geldanamycin, there have been several preclinical studies that evaluated the pharmacologic inhibition of HSP90 and attest to its anti-tumor activity 1-3. In fact, HSP90 has been championed over 20 years by the National Cancer Institute, academic institutions and pharmaceutical companies as a cancer target with several clinical studies evaluating over 17 different first and second generation HSP90 inhibitors. While HSP90 inhibitors were developed in the hope of treating an array of solid and hematological malignancies, clinical activity to date has been limited to cancers that are addicted to a particular oncogene that is a very sensitive HSP90 client (example HER2 in breast cancer or echinoderm microtubule-associated proteinlike 4 [EML4]-anaplastic lymphoma kinase [ALK] positive non-small cell lung cancer [NSCLC]) 11,12. It is also known that tumor cells that become resistant to targeted therapy directed at HSP90 clients remain sensitive to HSP90 inhibitors. For example, for HER2 positive metastatic breast cancer, there is a benefit to continuing trastuzumab (anti-human monoclonal antibody against HER2) beyond progression due to continued dependence on the HER2 pathway 13. Putative mechanisms of trastuzumab resistance include activation of the downstream Phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway due to loss of function of the phosphatase and tensin homolog (PTEN) or direct activating PI3K/AKT mutations 14,15, activation of other receptor tyrosine kinases including IGF-1 receptor, and expression of a truncated (P95) fragment of HER2 that lacks the trastuzumab-binding epitope 16. These mechanisms are also susceptible to HSP90 inhibition by virtue of the fact that they rely on HSP90 clients 17. HSP90 inhibitors have therefore been hypothesized to be active in trastuzumab resistant tumors.
While the activity in HER2 positive breast cancer and chimeric ALK NSCLC was well predicted by preclinical studies, it is unclear and rather disappointing that the preclinical findings have not translated into clinical benefit for many other tumor types that also harbor HSP90-addicted oncoproteins. Identifying biomarkers of response are therefore critical in identifying a susceptible subgroup of patients and further optimizing the full therapeutic potential of HSP90 inhibitors. The objective of our study was to identify potential biomarkers of response to HSP90 inhibitor therapy from archived tumor specimens of patients with advanced solid tumors treated on prospective clinical trials of HSP90 inhibitors. We hypothesized that predictive biomarkers would identify patients who were most likely to benefit from HSP90 inhibitors.
Patients and Methods
Electronic Medical records of patient enrolled on 7 phase I/II clinical trials of various HSP90 inhibitors (17-AAG, 17-DMAG, CNF-2024, IPI-504 and ganetespib) between 1999 and 2011 at Memorial Sloan Kettering Cancer Center (MSKCC) were reviewed by a single investigator. Patients were included if they met the following criteria: 1) archived tumor specimens were available (primary or metastatic), 2) they received at least 2 cycles (1 cycle defined in each individual protocol) of HSP90 inhibitor therapy, 3) they received HSP90 inhibitor therapy within 30 days of a restaging scan, 4) they had at least one re-staging scan after start of HSP90 inhibitor therapy with either complete response (CR), partial response (PR), or stable disease (SD). Notably, the timing of re-staging scan varied with each protocol. Clinical benefit was defined (CR+ PR+ SD) and assessed by a single investigator using RECIST 1.1 criteria using two endpoints: 1) best overall response (ORR) from start of HSP90 inhibitor therapy until disease progression on that particular trial and 2) best response at a defined time-point of 6-10 weeks from start of HSP90 inhibitor therapy henceforth referred as the “8 week scan”.
Archived specimens were analyzed using immunohistochemistry (IHC) and scored by a dedicated study pathologist (blinded to the clinical data) based on the intensity of staining for HSP90, HSP70, ER, PR, AR, HER2, EGFR and loss of PTEN. Antibodies and scoring methods are listed in Table 1. These antibodies were commercially available. PTEN 18, HSP90 and HSP70 staining were validated at MSKCC. The expression of the biomarker panel was compared between patients who derived clinical benefit and those that did not using Fisher's exact test.
Table 1. Summary of the used antibodies, scoring systems and cut-offs.
| Biomarker | Clone | Dilution | Company | Scoring System | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| ER | 1D5 | 1:500 | Dako | >1% | <1% |
| PR | PGR636 | 1:100 | Dako | >1% | <1% |
| AR | AR441 | 1:300 | Abcam | >1% | <1% |
| HER2 | SP3 | 1:50 | Epitomics | 3+ | 0, 1+ |
| EGFR | EP38Y | 1:50 | Thermo Scientific | 1+, 2+, 3+ | 0 |
| HSP70* | C9F3A-5 | 1:50 | Enzo Life Sciences | 2+, 3+ | 0, 1+ |
| HSP90* | Polyclonal | 1:125 | Cell Signaling technology | 2+, 3+ | 0, 1+ |
| PTEN* | 6H2.1 | 1:100 | Dako | 2 | 0, 1 |
Validated at MSKCC, ER estrogen receptor, PR progesterone receptor, AR androgen receptor, HER2 human epidermal growth factor receptor 2, EGFR epidermal growth factor receptor 2, PTEN phosphatase and tensin homolog
Results
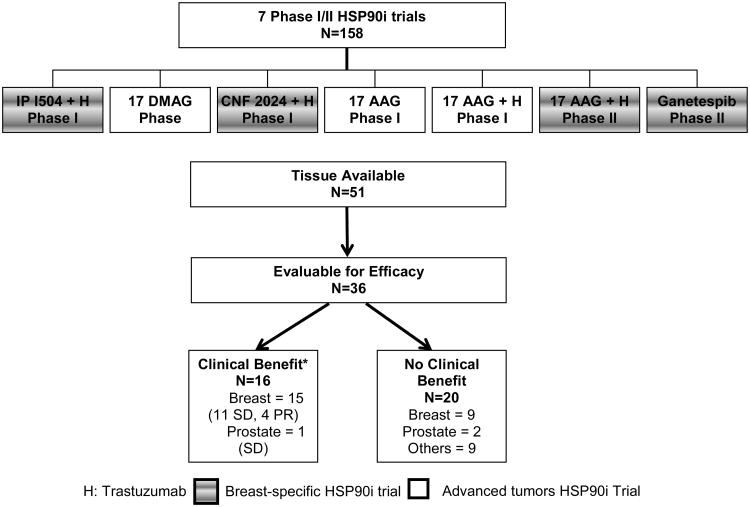
A total of 158 patients were enrolled on 7 unique phase I/II HSP90 inhibitors trials (Table 2). Of these, 4 trials were specific for metastatic breast cancer patients (3 in combination with trastuzumab for HER2 positive metastatic breast cancer and 1 monotherapy trial in unselected metastatic breast cancer) and the remaining 3 were conducted in patients with advanced solid malignancies (Figure 1, Table 2). Adequate archived tissue was available for 32% (51/158) patients. Of these, 71% (36/51) patients met the criteria to assess clinical benefit by best ORR and 51 % (26/51) for clinical benefit by “8 week scan” assessment. Patient demographics for the 36 evaluable patients are detailed in Table 3. Median age at primary cancer diagnosis was 50 years (range, 24-74 years). Median performance status by the Karnofsky Performance Scale was 90% (range 80-100%). As expected, the majority of the included patients (66%) had breast cancer. Medians lines of chemotherapy in the metastatic setting was 2 (range 0-7). Median number of days for which a patient was on the HSP90 inhibitor therapy was 55 (range, 16-411 days).
Table 2. Overall patient population.
| HSP90 Inhibitor |
Phase | Dosing schedule |
Cycle | Total enrolled by disease type N = 158 |
Tissue available N=51 |
Evaluable for efficacy# N=36 |
Clinical benefit by best ORR N=16 |
|---|---|---|---|---|---|---|---|
| 17-AAG9 | I |
|
21 days | 54
|
17
|
12 | 2 SD
|
| 17-AAG + trastuzumab weekly continuously28 | I | Weekly IV × 4 Q4 weeks | 28 days | 13
|
3
|
2 | 1 SD Breast |
| 17-DMAG (unpublished) | I | Weekly IV × 3 Q4 weeks | 28 days | 26
|
6
|
3 | 0 |
| *CNF-2024 + trastuzumab Q3 weeks29 | I | Oral daily | 21 days | Breast: 11 | Breast: 3 | 2 | 1 PR 1 SD |
| *17-AAG + trastuzumab weekly continuously11 | II | Weekly IV × 4 Q4 weeks | 28 days | Breast: 28 | Breast: 15 | 11 | 2 PR 7 SD |
| *IPI-504 + trastuzumab30 | II | Weekly IV × 3 Q3 weeks | 21 days | Breast: 4 | Breast: 1 | 1 | 0 |
| *Ganetespib31 | II | Weekly IV × 3 Q4 weeks | 28 days | Breast: 22 | Breast: 6 | 5 | 1 PR 1 SD |
Clinical Benefit by best ORR,
Breast cancer specific metastatic trials, ORR overall response rate, PR partial response, SD stable disease
Figure 1. Overall patient population.
* Clinical Benefit by Best Overall Response
Table 3. Demographics for evaluable patients (N=36).
| Variable | N (%) |
|---|---|
|
| |
| Age at primary diagnosis (yrs), median (range) | 50 (24-74) |
|
| |
| Sex | |
| Male | 10 (28%) |
| Female | 26 (72%) |
|
| |
| Median KPS | 90 (80-100) |
|
| |
| Tumor Types | |
| Breast | 24 (67%) |
| Prostate | 3 (8%) |
| Others | 9 (25%) |
| TCC Bladder | 2 |
| TCC Ureter | 1 |
| Adrenocortical carcinoma | 1 |
| Leiomyosarcoma | 1 |
| Melanoma | 1 |
| NSCLC | 1 |
| Squamous cell of Tongue | 1 |
| Colon | 1 |
|
| |
| Lines of prior chemotherapy in metastatic setting, median (range) | 2 (0-7) |
|
| |
| Duration of HSP90 inhibitor therapy (days), median (range) | 55 (16-411) |
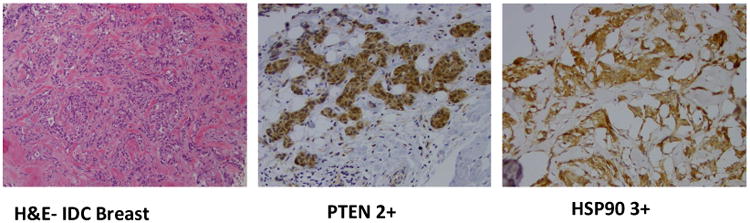
IHC results for evaluable patients (N=36) are shown in table 4. Micrographs of hematoxylin and eosin staining and IHC showing positivity for PTEN (2+) and HSP90 (3+) in a patient with invasive ductal carcinoma of the breast are illustrated in Figure 2.
Table 4. Immunohistochemistry analysis for evaluable patients (N=36).
| POSITIVE | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| NEGATIVE | ER | PR | AR | HER2 | EGFR | HSP90 | HSP70 | PTEN | |
| ER | X | 0 | 10 | 9 | 14 | 15 | 4 | 5 | |
| PR | 8 | X | 16 | 16 | 14 | 21 | 5 | 7 | |
| AR | 3 | 1 | X | 7 | 8 | 9 | 2 | 3 | |
| HER2 | 3 | 2 | 8 | X | 12 | 9 | 4 | 5 | |
| EGFR | 12 | 5 | 13 | 16 | X | 15 | 2 | 4 | |
| HSP90 | 4 | 1 | 5 | 4 | 6 | X | 0 | 2 | |
| HSP70 | 12 | 5 | 17 | 18 | 12 | 27 | X | 7 | |
| PTEN | 10 | 4 | 14 | 16 | 11 | 18 | 4 | X | |
ER estrogen receptor, PR progesterone receptor, AR androgen receptor, HER2 human epidermal growth factor receptor 2, EGFR epidermal growth factor receptor 2, HSP90 heat shock protein 90, HSP70 heat shock protein 70, PTEN phosphatase and tensin homolog
Figure 2. Micrographs showing hematoxylin and Eosin eosin staining and IHC example showing positivity for PTEN (2+) and HSP90 (3+) in a patient with invasive ductal carcinoma (IDC) of the breast.
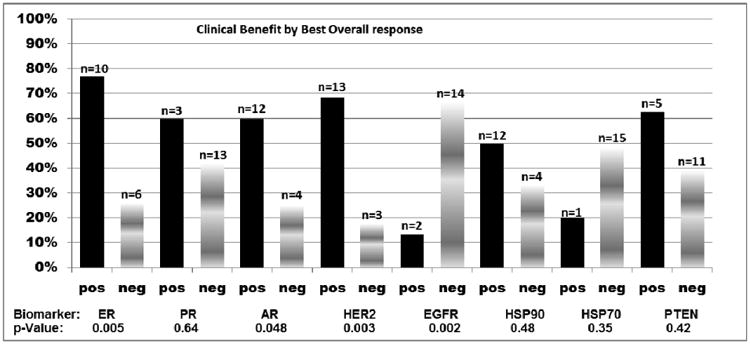
Clinical benefit as defined by best ORR was noted in 44% of the patients (16/36). Specifically, 11% (4/36) achieved PR and the remaining 33% achieved SD (12/36) (Figure 1, Table 2, Figure 3A). It is important to note that all 4 patients with PR and all but one patient with SD had metastatic breast cancer. Ten of the 36 patients had no scan performed at 8 weeks (6-10 weeks) and were therefore excluded from analysis of clinical benefit by the “8-week scan” (Figure 3B). Of these 26 patients, 46% (12/26) had clinical benefit from HSP90 inhibitor therapy with PR in 8% (2/26) and SD in 38% (10/26) (Figure 3B).
Figure 3A. Association of biomarker analysis with clinical benefit by best overall response (N=16*).
*Of note, 16/36 patients evaluable for efficacy had clinical benefit and are included in Figure 3 A.
Figure 3B. Association of biomarker analysis with clinical benefit using “8-week scan” (N=12*).
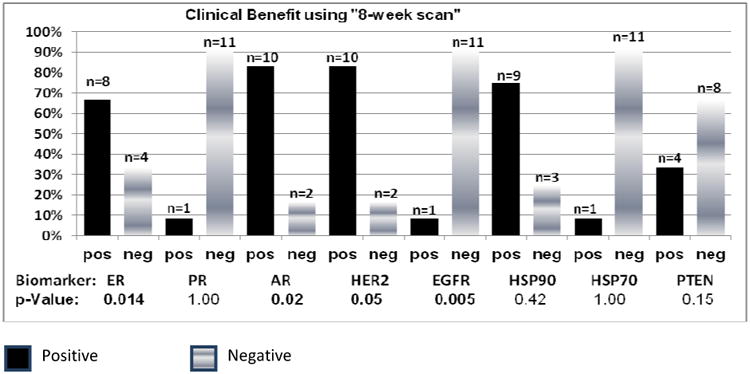
* 10/36 pts had no scan at 8 weeks (+/- 2 weeks) and were excluded. Of the remaining 26, 12 had clinical benefit by the 8 week scan and are included in Figure 3 B.
ER estrogen receptor, PR progesterone receptor, AR androgen receptor, HER2 human epidermal growth factor receptor 2, EGFR epidermal growth factor receptor 2, HSP90 heat shock protein 90, HSP70 heat shock protein 70, PTEN phosphatase and tensin homolog
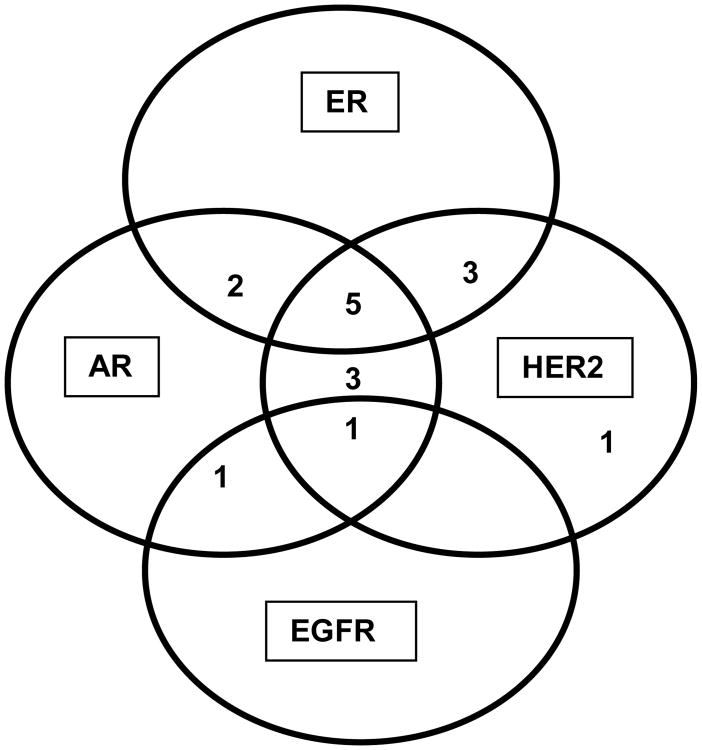
Biomarkers were individually associated with clinical benefit as assessed by best ORR and by “8 week scan”. As noted, a trend for clinical benefit was noted for ER, AR and HER2 positivity and EGFR negativity (Figures 3A and 3B). Clinical benefit was also evaluated amongst patients with selected combinations of biomarker expression (ER, AR, HER2 and EGFR) (Figure 4A) and also among patients with ER/AR and HER2 (Figure 4B). Interestingly a small subset of patients with ER positivity/AR positivity/HER2negativity derived clinical benefit (Figure 4B). We also performed an additional subset analysis of biomarker correlation for patients who had objective tumor responses defined as PR+CR (N=4). Notably, all 4 patients with PR were HER2 positive and EGFR negative and ER and HER2 positivity correlated with response with p –values of 0.47 each.
Figure 4A. ER, AR, HER2 and EGFR expression amongst patients with clinical benefit.
Figure 4B. Clinical benefit amongst patients with selected combinations of biomarkers (ER, AR and HER2).
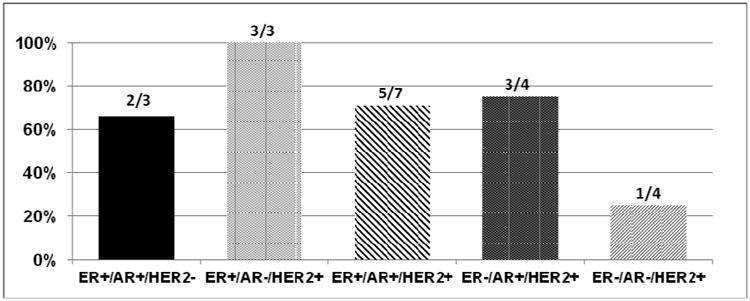
ER estrogen receptor, AR androgen receptor, HER2 human epidermal growth factor receptor 2, EGFR epidermal growth factor receptor 2
Discussion
To our knowledge, this is the first retrospective study exploring potential predictive biomarkers for patients treated with HSP90 inhibitors. This exploratory analysis supports the association for clinical benefit with HSP90 inhibitor therapy with ER positivity, AR positivity, HER2 positivity and EGFR negativity. A significant association for objective response with HSP90 inhibitor therapy was noted with ER positivity and HER2 positivity. However, the most important biomarker of benefit was HER2. 81% of the patients who derived clinical benefit by best ORR had HER2 positive tumors. All 4 patients with tumors which expressed PR also had HER2 positive metastatic breast cancer. Preclinically, 17-AAG doses significantly below the maximum tolerated dose potently inhibited HER2 with no effect on Akt despite both being HSP90 client proteins17,19. Akt could be degraded only with higher repeated dosing of 17-AAG, suggesting that only a limited subset of proteins are amenable to degradation in vivo 20. While our retrospective analysis includes patients with advanced solid tumors, 24/36 evaluable patients, all objective responses and all but 1 SD were noted in breast cancer; predominantly HER2 positive breast cancer. Our findings are consistent with these prior pre-clinical data suggesting that HER kinases are exquisitely sensitive targets of HSP90 inhibition 21,22.
While a very small subset, it was rather interesting to see clinical benefit in a subset that was HER2 negative but ER and AR positive. This is relevant because although androgen signaling has been extensively studied in prostate cancer, there is emerging evidence that AR is expressed in 70-90% of primary breast tumors 23,24. Furthermore, comprehensive microarray analysis has determined that mammary tumor cells can be divided into three subgroups based on steroid receptor activity: luminal (ER+, AR+), basal (ER-/AR-), and apocrine (ER-, AR+) 25. Additionally, receptors or molecules involved in endocrine resistance include HER2, AKT, mutant p53, Raf-1 and IGF-1R, among others, rely on the HSP90 machinery for activity and stability and HSP90 inhibitors might therefore be relevant in this context. This has formed the basis of combining HSP90 inhibitors with endocrine therapy.
High expression of HSP90 has been noted in breast cancer lines and also in breast tumor tissue when compared to normal breast tissue. Moreover, HSP90 was identified as an independent prognostic marker in breast cancer, being associated with a shorter survival 26. However, it did not predict for response in our limited dataset. Additionally, similar to the experience noted with HSP70 induction in peripheral blood mononuclear cells 6, baseline HSP70 expression also failed to predict clinical responses in our limited samples.
There are several limitations of this study including its retrospective nature, small number of tissue specimens, heterogeneous population of patients treated with several different HSP90 inhibitors at varying doses and schedules. While the assays were validated at MSKCC, it is possible that using other technologies such as automated, quantitative analysis (AQUA) of tissue microarrays could have perhaps yielded more accurate results for HSP90 and HSP70 expression 26,27. Furthermore, tumor tissue was not adequate to test several other relevant clients such as Akt, Raf-1 or other markers of HER2 resistance such as PI3K mutation, IGFR-1, p95HER2.
Nonetheless, prospective validation of these biomarkers in a homogeneous group of patients is warranted to further aid the clinical development of HSP90 inhibitors. However, to our knowledge, the current ongoing efforts directed at combination trials in triple negative, endocrine receptor positive or HER2 positive metastatic breast cancer do not have any planned correlative analyses of these biomarkers, most likely because they are in early phases of clinical development (NCT02474173, NCT02060253, NCT01677455, NCT01560416).
MICROABSTRACT.
There is a lack of predictive biomarkers that can help determine a subset of patients that might most benefit from treatment with HSP90 inhibitors. This retrospective tissue analysis performed on advanced solid tumor patients (majority with breast cancer) treated with various HSP90 inhibitors suggests that HER2 might be the only effective biomarker of sensitivity to these HSP90 inhibitors.
Clinical Practice Points.
There is augmenting interest in pursuing HSP90 inhibitors as an anti-cancer strategy and various second generation HSP90 inhibitors have moved into clinical setting.
However, there is still much work to be done as the success of these inhibitors in unselected patient population is limited. Predictive biomarkers are desperately needed for appropriate patient selection and further advancement of these inhibitors in clinic.
This exploratory retrospective analysis performed in patient tissues treated with various HSP90 inhibitors in 7 different clinical trials of advanced solid tumors, predominantly breast cancer, suggested that the biomarker of most benefit was HER2. A trend for clinical benefit was also noted for ER, AR and HER2 positivity and EGFR negativity.
While retrospective in a small subset of patients, our findings are in line with the preclinical data that HER2 is the most sensitive client protein of HSP90 inhibition and requires prospective validation in larger studies.
Acknowledgments
We would like to thank all the patients and their families who participated in these clinical trials.
Footnotes
Conflict Of Interest: The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zuehlke A, Johnson JL. Hsp90 and co-chaperones twist the functions of diverse client proteins. Biopolymers. 2010 Mar;93(3):211–217. doi: 10.1002/bip.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann N Y Acad Sci. 2007 Oct;1113:202–216. doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- 3.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005 Oct;5(10):761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 4.Jhaveri K, Ochiana SO, Dunphy MP, et al. Heat shock protein 90 inhibitors in the treatment of cancer: current status and future directions. Expert Opin Investig Drugs. 2014 May;23(5):611–628. doi: 10.1517/13543784.2014.902442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jhaveri K, Modi S. HSP90 inhibitors for cancer therapy and overcoming drug resistance. Adv Pharmacol. 2012;65:471–517. doi: 10.1016/B978-0-12-397927-8.00015-4. [DOI] [PubMed] [Google Scholar]
- 6.Kummar S, Gutierrez ME, Gardner ER, et al. Phase I trial of 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), a heat shock protein inhibitor, administered twice weekly in patients with advanced malignancies. Eur J Cancer. 2010 Jan;46(2):340–347. doi: 10.1016/j.ejca.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajan A, Kelly RJ, Trepel JB, et al. A phase I study of PF-04929113 (SNX-5422), an orally bioavailable heat shock protein 90 inhibitor, in patients with refractory solid tumor malignancies and lymphomas. Clin Cancer Res. 2011 Nov 1;17(21):6831–6839. doi: 10.1158/1078-0432.CCR-11-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerji U, O'Donnell A, Scurr M, et al. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol. 2005 Jun 20;23(18):4152–4161. doi: 10.1200/JCO.2005.00.612. [DOI] [PubMed] [Google Scholar]
- 9.Solit DB, Ivy SP, Kopil C, et al. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. Clin Cancer Res. 2007 Mar 15;13(6):1775–1782. doi: 10.1158/1078-0432.CCR-06-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banerji U, Walton M, Raynaud F, et al. Pharmacokinetic-Pharmacodynamic Relationships for the Heat Shock Protein 90 Molecular Chaperone Inhibitor 17-Allylamino, 17-Demethoxygeldanamycin in Human Ovarian Cancer Xenograft Models. Clinical Cancer Research. 2005 Oct 1;11(19):7023–7032. doi: 10.1158/1078-0432.CCR-05-0518. 2005. [DOI] [PubMed] [Google Scholar]
- 11.Modi S, Stopeck A, Linden H, et al. HSP90 inhibition is effective in breast cancer: a phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res. 2011 Aug 1;17(15):5132–5139. doi: 10.1158/1078-0432.CCR-11-0072. [DOI] [PubMed] [Google Scholar]
- 12.Jhaveri K, Miller K, Rosen L, et al. A Phase I Dose-Escalation Trial of Trastuzumab and Alvespimycin Hydrochloride (KOS-1022; 17 DMAG) in the Treatment of Advanced Solid Tumors. Clinical Cancer Research. 2012 Sep 15;18(18):5090–5098. doi: 10.1158/1078-0432.CCR-11-3200. 2012. [DOI] [PubMed] [Google Scholar]
- 13.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized Study of Lapatinib Alone or in Combination With Trastuzumab in Women With ErbB2-Positive, Trastuzumab-Refractory Metastatic Breast Cancer. Journal of Clinical Oncology. 2010 Mar 1;28(7):1124–1130. doi: 10.1200/JCO.2008.21.4437. 2010. [DOI] [PubMed] [Google Scholar]
- 14.Nahta R, Esteva F. HER2 therapy: Molecular mechanisms of trastuzumab resistance. Breast Cancer Research. 2006;8(6):215. doi: 10.1186/bcr1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berns K, Horlings HM, Hennessy BT, et al. A Functional Genetic Approach Identifies the PI3K Pathway as a Major Determinant of Trastuzumab Resistance in Breast Cancer. Cancer Cell. 2007 Oct 16;12(4):395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 16.Chandarlapaty S, Scaltriti M, Angelini P, et al. Inhibitors of HSP90 block p95-HER2 signaling in Trastuzumab-resistant tumors and suppress their growth. Oncogene. 2009 Oct 26;29(3):325–334. doi: 10.1038/onc.2009.337. online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basso AD, Solit DB, Munster PN, Rosen N. Ansamycin antibiotics inhibit Akt activation and cyclin D expression in breast cancer cells that overexpress HER2. Oncogene. 2002 Feb 14;21(8):1159–1166. doi: 10.1038/sj.onc.1205184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakr RA, Barbashina V, Morrogh M, et al. Protocol for PTEN expression by immunohistochemistry in formalin-fixed paraffin-embedded human breast carcinoma. Appl Immunohistochem Mol Morphol. 2010 Jul;18(4):371–374. doi: 10.1097/PAI.0b013e3181d50bd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solit DB, Rosen N. Hsp90: a novel target for cancer therapy. Current topics in medicinal chemistry. 2006;6(11):1205–1214. doi: 10.2174/156802606777812068. [DOI] [PubMed] [Google Scholar]
- 20.Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. The Journal of biological chemistry. 2002 Oct 18;277(42):39858–39866. doi: 10.1074/jbc.M206322200. [DOI] [PubMed] [Google Scholar]
- 21.Munster PN, Marchion DC, Basso AD, Rosen N. Degradation of HER2 by ansamycins induces growth arrest and apoptosis in cells with HER2 overexpression via a HER3, phosphatidylinositol 3′-kinase-AKT-dependent pathway. Cancer Res. 2002 Jun 1;62(11):3132–3137. [PubMed] [Google Scholar]
- 22.Chandarlapaty S, Sawai A, Ye Q, et al. SNX2112, a synthetic heat shock protein 90 inhibitor, has potent antitumor activity against HER kinase-dependent cancers. Clin Cancer Res. 2008 Jan 1;14(1):240–248. doi: 10.1158/1078-0432.CCR-07-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall RE, Aspinall JO, Horsfall DJ, et al. Expression of the androgen receptor and an androgen-responsive protein, apolipoprotein D, in human breast cancer. Br J Cancer. 1996 Oct;74(8):1175–1180. doi: 10.1038/bjc.1996.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuenen-Boumeester V, Van der Kwast TH, Claassen CC, et al. The clinical significance of androgen receptors in breast cancer and their relation to histological and cell biological parameters. Eur J Cancer. 1996 Aug;32A(9):1560–1565. doi: 10.1016/0959-8049(96)00112-8. [DOI] [PubMed] [Google Scholar]
- 25.Farmer P, Bonnefoi H, Becette V, et al. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 2005 Jul 7;24(29):4660–4671. doi: 10.1038/sj.onc.1208561. [DOI] [PubMed] [Google Scholar]
- 26.Pick E, Kluger Y, Giltnane JM, et al. High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res. 2007 Apr 1;67(7):2932–2937. doi: 10.1158/0008-5472.CAN-06-4511. [DOI] [PubMed] [Google Scholar]
- 27.Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med. 2002 Nov;8(11):1323–1327. doi: 10.1038/nm791. [DOI] [PubMed] [Google Scholar]
- 28.Modi S, Stopeck AT, Gordon MS, et al. Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: a phase I dose-escalation study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007 Dec 1;25(34):5410–5417. doi: 10.1200/JCO.2007.11.7960. [DOI] [PubMed] [Google Scholar]
- 29.Modi S, Ismail-Khan R, Munster P, Lucas M, Galluppi GR, Tangri S, Chen Y, Yamashita M, Storgard CM, Moulder S. Phase 1 dose-escalation study of the heat shock protein 90 inhibitor BIIB021 with trastuzumab in HER2+ metastatic breast cancer. CTRC-AACR San Antonio Breast Cancer Symposium, 2010. 2010 [Google Scholar]
- 30.Modi S, Saura C, Henderson C, et al. A multicenter trial evaluating retaspimycin HCL (IPI-504) plus trastuzumab in patients with advanced or metastatic HER2-positive breast cancer. Breast Cancer Res Treat. 2013 May;139(1):107–113. doi: 10.1007/s10549-013-2510-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jhaveri K, Chandarlapaty S, Lake D, et al. A phase II open-label study of ganetespib, a novel heat shock protein 90 inhibitor for patients with metastatic breast cancer. Clin Breast Cancer. 2014 Jun;14(3):154–160. doi: 10.1016/j.clbc.2013.12.012. [DOI] [PubMed] [Google Scholar]