Abstract
Background. The memory effect of dexmedetomidine has not been prospectively evaluated in children. We evaluated the feasibility of measuring memory and sedation responses in children during dexmedetomidine sedation for non-painful radiological imaging studies. Secondarily, we quantified changes in memory in relation to the onset of sedation.
Methods. A 10 min bolus of dexmedetomidine (2 mcg kg-1) was given to children as they named simple line drawings every five s. The absence of sedation was identified as any verbal response, regardless of correctness. After recovery, recognition memory was tested with correct Yes/No recognitions (50% novel pictures) and was matched to sedation responses during the bolus period (subsequent memory paradigm).
Results. Of 64 accruals, 30 children (mean [SD]6.1 (1.2) yr, eight male) received dexmedetomidine and completed all study tasks. Individual responses were able to be modelled successfully in the 30 children completing all the study tasks, demonstrating feasibility of this approach. Children had 50% probability of verbal response at five min 40 s after infusion start, whereas 50% probability of subsequent recognition memory occurred sooner at four min five s.
Conclusions. Quantifying memory and sedation effects during dexmedetomidine infusion in verbal children was possible and demonstrated that memory function was present until shortly before verbal unresponsiveness occurred. This is the first study to investigate the effect of dexmedetomidine on memory in children.
Clinical trial registration. NCT 02354378.
Keywords: child, conscious sedation, dexmedetomidine, memory
Editor’s key points
One aim of procedural sedation, at least in some settings, is to provide amnesia.
Dexmedetomidine has been shown to suppress signalling pathways in the hippocampus.
This study found that memory functions can be reliably assessed in young children.
Dexmedetomidine seems to separate the sedation-recall curves, indicating some impairment of memory function.
Dexmedetomidine is frequently administered intravenously for procedural sedation and monitored anaesthesia care for infants, children, and developmentally challenged adolescents and adults.1–9 Dexmedetomidine, an alpha2 adrenergic agonist, was approved in the USA in 2007 for procedural sedation of non-intubated adults. In Europe it was approved in 2010 for sedation of adults in the intensive care units. Off label administration of dexmedetomidine for paediatric sedation for non-invasive radiological imaging has been widely reported10–17, with a loading dose given over approximately a 10 min period.
The effects of dexmedetomidine on memory function have never been studied in children. The clinical scenario of infusing dexmedetomidine over a 10 min period to achieve adequate sedation for MR scanning, provides an opportunity to measure changes in memory as sedation ranges from awake to verbal unresponsiveness. Effects on memory are best defined when sedation levels are increasing relatively slowly as are present when dexmedetomidine sedation is induced over 10 min. In the current study, memory and sedation effects were modelled in each individual during the period of responsiveness as dexmedetomidine was infused. To our knowledge this is the first study of this nature in children.
The primary objective was to determine if data of suitable quality could be obtained in children in this clinical setting to model memory and sedation responses. We used a standardized visual picture naming task18 to assess memory during drug infusion as sedation increased. Memory and sedation effects of dexmedetomidine were secondary end-points of this study. These effects were quantitated by modelling how long after the start of infusion 50% probability of subsequent recognition memory occurred in relation to 50% probability of loss of verbal responsiveness, as a measure of sedation.
Methods
No previous data were available to optimize study design. The study cohort consisted of children undergoing sedation for MRI scanning and recruitment continued consecutively until a sample of 30 children was available for analysis. We did not apply a formal sample size calculation. Feasibility was defined by 25 or more of patients fully completing the recognition memory task and yielding usable parameter estimates for modelling, within a reasonable accrual time frame (approximately 1 yr). This end point was met, and the current manuscript also reports results of modelling memory and sedation thresholds.
Children undergoing sedation for MR imaging lasting more than 15 min were included. A control group of children to determine performance on memory tasks without any sedation underwent MR imaging without sedation. Analysis groups included children who were able to complete all study tasks and required no rescue medications. Inclusion criteria included English-speaking children between four and 14 yr of age, with a minimum weight of eight kg, and able to comprehend and perform the picture naming task. Exclusion criteria included allergy to dexmedetomidine (for those patients requiring sedation), pregnancy, and recent use (within five half-lives) of centrally acting medications that could affect concentration (e.g. diphenhydramine).
The study was approved by the Boston Children’s Hospital Committee on Clinical Investigation and registered at Clinical Trials.gov (NCT02354378).
The picture-naming task used in this study consisted of black and white line drawings obtained from a standardized dataset (International Picture Naming Project)18, which include pictures from the original Snodgrass and Vanderwart set. Pictures were selected so that children of the age range planned in the current study would be able to name them without difficulty.
After informed consent was obtained on the day of the procedure, prospective subjects were tested in the waiting room to see if they understood how to perform the naming task, and were asked to name a series of practice pictures (20 different pictures, one shown every five s). Any verbal response was noted (i.e. "I don’t know" or an incorrect identification of an object was considered an adequate response). Those who failed to provide at least eight responses of the 20 pictures shown were withdrawn from the study. The practice pictures were different from the study pictures used to test memory in the encoding and recognition tasks.
Encoding task
Each subject was brought to the MRI scanner. Children requiring sedation received dexmedetomidine 2 mcg kg−1 intravenously over 10 min, per established and approved institutional protocols. As soon as the infusion was started, 100 study pictures contained in a non-ferrous flipbook, were presented one every five s, until the child was unresponsive (i.e. the child’s eyes were closed).
Response time was defined as the time from presentation of a study picture to the time the subject verbalized a response. An observer noted correct/incorrect response by key-press on a laptop using E-Prime software (PST, Sharpsburg, PA), which displayed study pictures in the same sequence as those presented in the flip book. As this was a feasibility study in an MR environment, direct measures of reaction time were not obtained, as this would have required specialized equipment not available in the current clinical setting.
After infusion of the dexmedetomidine ‘bolus’, sedation was then maintained with a continuous infusion of 1.5 mcg kg hr−1 i.v. until completion of the imaging study. If necessary, rescue medications (propofol, penotobarbital) were administered to maintain an adequate level of sedation in order to complete the scan. Any patient receiving rescue medication was not included in the analysis group.
Recognition memory task
Recognition memory was tested in the post-anaesthesia care unit (PACU), after discharge criteria were met (minimum Modified Aldrete Score nine, maintained for a minimum of 30 min). Each subject was presented with 100 pictures, one shown every five s; half of these had been shown during induction of sedation. The pictures and sequence were identical for each child.
Any verbal response was scored as positive regardless of correctness of naming. Importantly, during infusion children were asked to name the picture to ensure that the mnemonic processes engaged were consistent across children and pictures were attended to (memory is decremented by lack of attention and modulated by the processing engaged during stimulus presentation).19 For each picture, the child nominated by verbal yes/no response whether it had been shown previously. Responses were coded as true positives (correct recognition of a previously presented picture, ‘hits’, ‘1’), false negatives (‘no’ for pictures that were shown before, ‘0’), and false alarms, FA, for pictures nominated as ‘yes’ but not shown before.
Subsequent memory paradigm
By definition, memory is a retrospective behavioural measure. In other words, when a stimulus is presented, one cannot know if it is remembered until memory for this stimulus is tested at some time later, a procedure called subsequent recognition. Recognition is tested using equal numbers of previously presented and novel (not presented) stimuli to test for guessing, especially relevant in this age group. For example, if a child says "yes" to every picture, a 100% hit rate will be obtained (each previously presented stimulus had a correct response). Thus, a false alarm rate is obtained as an estimate of guessing. The false alarm rate is a "yes" response to a novel stimulus, and the expectation is that there will always be some false alarms. However, if it is unusually high (e.g. greater than 20%), then correct recognition (hits) data are likely unreliable. Two patients who substantially surpassed this threshold were dropped from analysis.
The first presentation of a stimulus occurs during ‘encoding’ and testing for memory of that stimulus occurs during ‘recognition’. Using the results of recognition (whether a stimulus was or was not remembered), one can retrospectively mark a stimulus presentation during encoding as being subsequently remembered or not. This procedure is called a subsequent recognition paradigm. Using this method, one can investigate changes in condition during encoding (such as the presence of a drug) to determine their effect on memory. In the current study, subsequent recognition of previously shown pictures was tested and results were matched with the verbal response for those pictures during induction, the period of memory encoding.
Relation of sedation to subsequent recognition memory
This subsequent memory paradigm allowed us to determine the time from the start of infusion at which 50% probability of subsequent recognition memory occurred and the time at which 50% probability of sedation occurred using time as a predictor variable. We anticipated significant inter-individual variability. Thus, responses were quantitated at 50% probability, which would provide more reliable estimates than smaller or larger probabilities, for example 10% or 90%. At these more extreme points the number of assessable stimuli before or beyond 10% or 90% would be few.
Methodologic considerations
Training of research staff was conducted by the senior investigator (R.V.) who had extensive experience in conducting memory studies in volunteers, to ensure consistency in task administration, competency with E-prime software for picture presentation, and response recording during encoding and recognition.
Statistical analysis
Descriptive statistics were generated to characterize the study samples, using frequencies for categorical variables and means (median) and standard deviations (confidence interval) for continuous variables.
Modeling memory and sedation responses
These involved calculation of a sedation and a memory threshold for each child. The number of stimuli presented during encoding was empirically balanced between sufficient numbers to fit threshold functions, vs too many for children to continue efforts at the naming task.20 We were concerned with a possibility of some stimuli being too difficult to name for children of this age group, as some stimuli (e.g. typewriter) had a very low verbal response rate as accruals began. A control group was accrued to investigate this possibility, and children undergoing MRI scanning without sedation completed the same study paradigm as children receiving dexmedetomidine.
Initial attempts to model responses using an asymmetric Weibull cumulative density function were unsuccessful in the first 10 individuals, and responses were instead modelled using a logit link model of binary responses (response-no response at encoding, hits (1)-false negatives (0) determination at recognition). The model was used to estimate the effect of drug, treating time or concentration as a random effect to estimate child-specific responses, accounting for over-dispersion, and assuming the correlation between individual responses were unstructured. Plots of individual children were generated using generalized linear mixed models. Statistical interaction terms were included in the models to test for a difference in response over time and precited concentration. All statistics were generated using statistical analysis software (SAS, version 9.3; SAS Institute, Cary NC). Mixed models were estimated with the GLIMMIX procedure, all statistical tests were two-sided with an α = 0.05.
Drug concentration modeling
To explore the possibility that dexmedetomidine concentration might be a better predictor of response than time (study picture number), predicted serum concentrations for each individual were modelled using Stanpump software (previously downloaded from at http://anesthesia.stanford.edu/pkpd (last accessed 6/19/2008), current URL http://opentci.org/code/stanpump) using the human study 3-compartment kinetic parameter set. These were modelled against memory and sedation responses similarly to that described in the Statistical Analysis section.
Results
Table 1 reports the patient characteristics of the study groups. Of 64 children who were consented, 54 started dexmedetomidine infusion, five did not need sedation for the procedure (decision made after consent obtained), two required inhalation induction by mask (after practice phase), and three were withdrawn by parental request after practice phase. Of the 54 children who started dexmedetomidine infusion, 12 needed rescue medication to perform the procedure (propofol and/or pentobarbital) and were excluded from the analysis group. Of the remaining 42 children, four dropped out during the encoding task (one computer crash, three unable to complete), and six were unable to fully complete the recognition task. Two additional children were excluded from analysis because of high false alarm rates, being 42 and 72%. Thus, 30 children completed all study tasks, and all 30 individual memory and sedation responses to dexmedetomidine were able to be modelled.
Table 1.
Patient characteristics
| Statistic | activeDEX | Control | |
|---|---|---|---|
| (n=30) | (n=16) | ||
| Age (yr) | mean (SD) | 6.1 (1.2) | 9.5 (1.9) |
| median | 6 | 10 | |
| min - max | 4.0 – 8.0 | 6.0 – 13.0 | |
| Weight (kg) | mean (SD) | 23.9 (6.6) | 38.9 (20.1) |
| median | 22.3 | 35.2 | |
| min - max | 13.8 – 38.4 | 19.0 – 103.0 | |
| Male | n (%) | 8 (26.7%) | 8 (50.0%) |
| Region Scanned (number of pts) | Brain | 15 | 7 |
| Brain-Spine | – | 2 | |
| Spine | 4 | 2 | |
| Head/Neck | 1 | – | |
| Abd/Pelvis/Chest | 6 | 2 | |
| Extremities | 4 | 3 | |
| Cancer related diagnosis (number of pts) | 4 | 4 | |
| Length of scan (min) | mean (SD) | 52.2 (17.1) | 60.6 (18.1) |
| median | 47.5 | 60 | |
| min - max | 25.0 – 85.0 | 45.0 – 105.0 | |
| Time – Scan start to discharge (minutes) | mean (SD) | 108.1 (26.9) | 66.6 (18.1) |
| median | 109 | 60 | |
| min - max | 51.0 – 165.0 | 45.0 – 105.0 | |
| Time from encoding to recognition (min) | mean (SD) | 140.1 (22.8) | 85.9 (36.6) |
| median | 135.5 | 71.5 | |
| min - max | 102.0 – 196.0 | 43.0 – 162.0 |
The control group consisted of 16 children, who were tested to determine if any of the picture stimuli were difficult to recognize, and this was not the case. As these children were able to undergo MR scanning without sedation, it is not surprising that they were older and weighed more. Patients stayed after the procedure as long as was tolerable for them and their legal guardians. Despite this, recognition testing was done somewhat earlier than in the dexmedetomidine group. As patients were not randomized to groups, no statistical inferences were made between control and drug groups.
Encoding responses
The primary measure of sedation was a verbal response. However, there were a number of different verbal responses made to naming a picture, which include correct naming, incorrect naming, ‘I don’t know’ naming, and the lack of a response even though the patient still had their eyes open. It is possible that encoding was affected by the nature of the response (e.g. correct naming might be more memorable than non-response), and this possibility was explored, with results reported in Table 2. There were few non-correct naming responses (all categories other than correctly named response), and comparison with hit rates for correctly named pictures revealed no difference (t(29) = 1.79, P=0.09) for patients receiving dexmedetomidine. The patients in the control group demonstrated significantly better hit rates for noncorrectly named stimuli (P=0.003), though only about 10% of items were incorrectly named, and hit rates for all categories were close to 1.0.
Table 2.
Encoding and subsequent memory responses. ˆthis hit rate includes all non-correct responses with eyes open (incorrect naming, ‘I don’t know’, and no response). **P<0.01 vs correct naming
| Statistic | N pictures | Hit rate | N pictures | Hit rate | |
|---|---|---|---|---|---|
| DEX | DEX | CONTROL | CONTROL | ||
| (max = 50) | (correct rcgs - FA) | (max = 50) | (correct rcgs - FA) | ||
| Number of Pictures seen during encoding | mean (SD) | 35.1 (6.6) | 0.63 (0.14) | 50 | 0.94 (0.05) |
| median | 35 | ||||
| min - max | 22 - 46 | ||||
| Correct Naming | mean (SD) | 26.8 (7.9) | 0.66 (0.13) | 38.9 (5.9) | 0.93 (0.06) |
| median | 28 | 41 | |||
| min - max | Oct-40 | 27 – 47 | |||
| Incorrect Naming | mean (SD) | 2.3 (1.5) | 0.57 (0.27) ˆ NS | 5.6 (3.0) | 0.97 (0.04) ˆ ** |
| median | 2 | 5 | |||
| min - max | 0 - 7 | 13-Feb | |||
| ‘I don’t Know’ | mean (SD) | 3.9 (4.0) | 1.2 (1.7) | ||
| median | 2 | 0 | |||
| min - max | 0 – 13 | 0 – 5 | |||
| No response with eyes open | mean (SD) | 2.1 (2.7) | 1.8 (2.5) | ||
| median | 1 | 1 | |||
| min - max | 0 - 10 | 0 – 8 | |||
| Stimuli presented after eyes closed | mean (SD) | 14.9 (6.6) | 0.04 (0.06) | N/A | |
| median | 15 | ||||
| min - max | 28-Apr | ||||
| Discrimination index d’ | mean (SD) | 2.37 (0.62) | 3.91 (0.50) | ||
| median | 2.4 | 3.98 | |||
| min - max | 1.23 – 3.51 | 3.13 – 4.650 | |||
| Response Bias Criterion | mean (SD) | 0.66 (0.33) | 0.20 (0.26) | ||
| c | median | 0.69 | 0.15 | ||
| min - max | 0.00 – 1.35 | −0.14 – 0.67 |
Recognition responses
Though two children in the dexmedetomidine group with high false alarm (FA) rates were excluded, FAs still occurred in the remaining subjects. These were at a low rate (5.7 [4.9]%, 1.0 [1.0]% in the control group), which did not change through the length of the recognition list (first vs second half, t(48) = 0.99 P=0.33). Thus fatigue during the recognition task did not appear to be a consideration in task performance. Hit rates (correct recognitions of previously seen pictures, see Table 2) were corrected for the FA rate for each individual. Any hits for stimuli presented after eyes closed were considered false alarms, and were included in the false alarm rate.
Additional insight into recognition performance was provided by signal detection theory.21 This method provides measures describing two overlapping normally distributed curves representing internal evidence, for an item being truly experienced before (old) or not (new). The separation between peaks for old and new is a measure of how easy it is to discriminate old and new items (similar items are harder to discriminate, e.g. ‘snow’/’show’ or ‘snow’/‘powder’ vs unrelated items e.g. ‘fork’/‘shoe’), and is reported as d’ (a value greater than 1.0 indicates good recognition performance). The overlap between curves is a ‘grey zone,’ where it is unclear if an item is old or new. In this case, where internal evidence is relatively equal between old or new, a feeling of how familiar an item is (‘gut reaction’) sets an internal criterion for judgement. Some people need little familiarity before indicating ‘old’ (liberal bias, c < 0) whereas others require a high degree of familiarity before indicating old (conservative bias, c > 0). These signal detection measures of recognition response were calculated for stimuli that were presented during encoding when the child was not asleep. In the dexmedetomidine group, distribution of hits data were no different from normality (Kolmogorov-Smirnov(30) = 0.103, P=0.2), and d' was 2.4 (0.6), indicative of good yes/no recognition performance, in line with the generally low FA rate. The criterion of response bias, c was 0.66 (0.33), indicative of a very conservative response (e.g. if unsure, the item was categorized as unrecognized). Based on the low FA rate which did not vary over the recognition task, memory response was modelled using correct/no recognitions (binary response) over the period of induction of sedation.
The control group demonstrated high hit rates and good discrimination (d’). (see Table 2) Response bias criterion (c) was still conservative, but not as conservative as patients receiving dexmedetomidine. However, direct comparison is not warranted, as groups were not randomly assigned. Additionally, recognition hit rates were very high in the control group, and the FA rate was very low (near ceiling/basement effects). Any differences should be considered as hypothesis generating only. We tested for correlation between age and criterion (c) within group only, and found no significant correlation in either dexmedetomidine group (P=0.10) or control group (P=0.11).
Response modeling
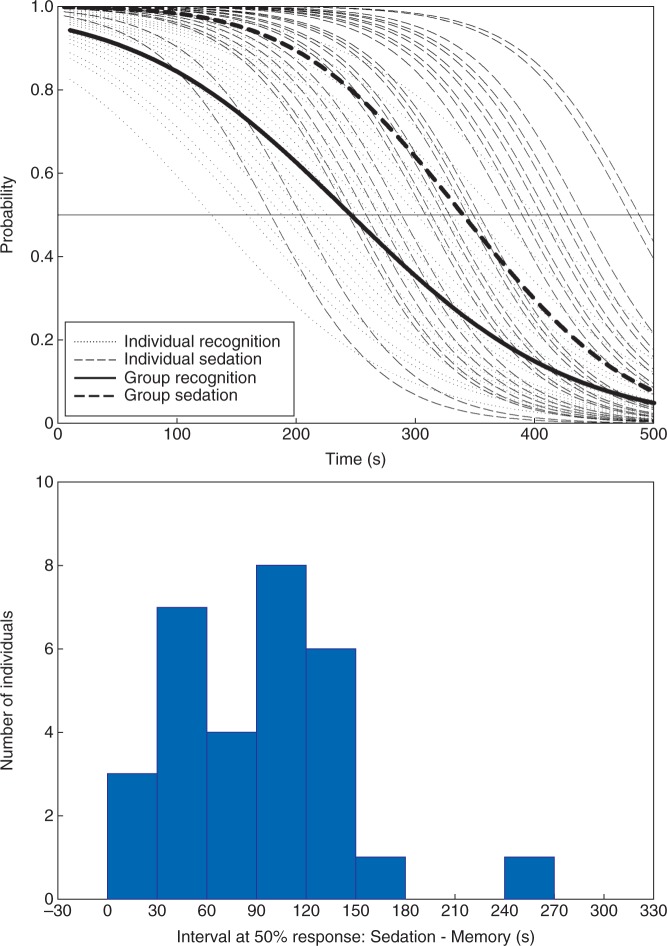
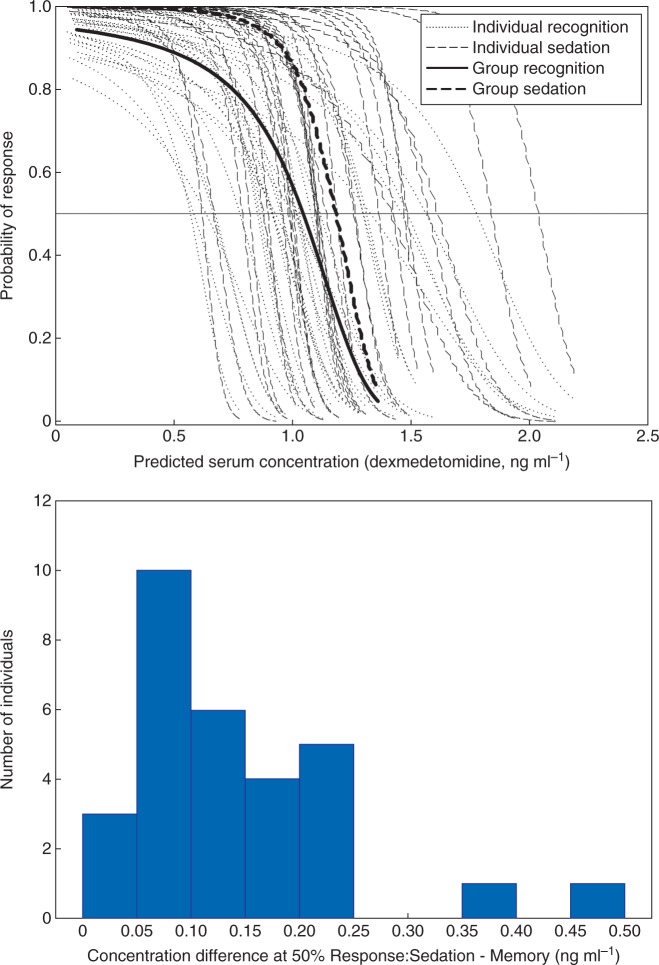
The threshold end-point was 50% probability of subsequent recognition memory or 50% probability of sedation, with time as a predictor variable. Time was a significant predictor of both sedation and memory responses, (P<0.001, standard error P<0.005). Confidence intervals could be calculated for the primary end-point of response probability, but because of the nature of time being a predictor variable, a similar measure could not be determined for time. The time to achieve 50% (CI 45.8% - 52.2%) sedation was 340 s, and was longer than the 245 s to achieve 50% (CI 47.7% – 53.0%) probability of no recognition (see Fig. 1). To illustrate the variability in responses, individual differences between 50% sedation and 50% memory effect are illustrated in the histogram portion of Fig. 1. Most (23/30) patients lost memory within two min before the loss of verbal response. Modelling responses against dexmedetomidine concentrations revealed similar relationships, with concentration being a significant predictor of memory and sedation response (P<0.001, standard error P<0.03, Fig. 2). Attempts to model sedation using verbal response times were unsuccessful, the models did not converge.
Fig 1.
Individual probabilities (faint plots) of verbal responses (sedation) and subsequent recognition memory with time (s) as the predictor variable during the induction of dexmedetomidine sedation. Group average plots are indicated in bold. Probability of recognition memory consistently decayed before onset of sedation, and the distribution of time intervals between 50% probabilities are shown in part (B). Positive values indicate that 50% recognition memory occurred sooner than 50% sedation.
Fig 2.
Individual probabilities (faint plots) of verbal responses (sedation) and subsequent recognition memory with predicted serum dexmedetomidine concentration (ng ml-1) as the predictor variable during the induction of dexmedetomidine sedation. Group average plots are indicated in bold. Probability of recognition memory consistently decayed at lower predicted concentrations than onset of sedation, and the distribution of concentrations differences between 50% probabilities are shown in part (B). Positive values indicate that 50% recognition memory occurred at a lower concentration than 50% sedation.
Discussion
This is the first investigation we are aware of reporting the memory effects of dexmedetomidine in children of this age. The common use of dexmedetomidine for procedural sedation in infants and children, particularly for non-painful, diagnostic radiological imaging studies, allowed an opportunity to examine more precisely the relationship of drug induced sedation to memory function in the clinical setting.5 22–27 The feasibility of measuring memory and sedation responses in this setting was demonstrated by the fact that individual responses could be modelled in the 30 children included in the final data analysis. Roughly equal numbers of patients were excluded from final analysis on the basis of rescue medication needed to achieve adequate sedation for the procedure, and inability to complete the necessary encoding and recognition tasks. Events during encoding of a memory were related to the presence or absence of recognition memory at a later time, using the subsequent memory paradigm.
Sedation affects memory processes ‘upstream’ from ones that are affected by the amnestic actions of drugs. Sedation prevents memory formation on the basis of inattention, with no information being processed from working memory to longer lasting memory (encoding impairment). Amnestic doses of midazolam or propofol, for example, allow a memory to be formed, which is rapidly lost over time (forgetting).28–30 As the memory effect of amnestic drugs is changing rapidly during a 10-30 min period after encoding, subsequent recognition memory testing is best done beyond this time frame, and in current study was done after imaging and recovery from sedation.29 31 Thus, amnesia in the sense of lack of memory for events occurring at drug concentrations causing little sedation, is most evident when drug concentrations are increasing slowly, or are held constant over increasing steps such as in studies using volunteers.28 29 32–35 Clinical constraints limited how slowly dexmedetomidine could be infused, and measures of memory and sedation were constantly changing over time, which we attempted to model using binary classifications of sedation and memory responses.
The relation of subsequent recognition memory to sedation responses over time or concentration are close and roughly parallel. Individual comparisons between 50% probabilities of subsequent recognition memory and sedation revealed a consistent separation. In the current study, the leftward shift of the subsequent recognition memory response curve in relation to sedation produced the separation, and could be a result of encoding impairment, forgetting of encoded material by the time recognition testing takes place, or a conservative response bias. In the current study it is impossible to clearly differentiate between encoding failure (on the basis of sedation) and forgetting (amnestic effect), but a conservative response bias was clearly documented. A conservative response bias, the child nominating ‘no’ during recognition if unsure, would have the effect of shifting the recognition memory response leftward, increasing separation from sedation. It is interesting that the response bias criterion (c) was quite different between the dexmedetomidine group, which was highly conservative, and the control group, being less conservative. There seemed to be no strong relationship with age within group, and other factors may have accounted for this difference. The response bias criterion may vary with difficulty of the task, being more conservative as the task difficulty increases, depending on context.36 Task difficulty was likely more difficult in the dexmedetomidine group, as these children were younger, and were trying to recall pictures whose recognition memory was impaired by the drug. However, measures of response bias are not well worked out in dynamic situations such as were present during encoding in the dexmedetomidine group, and the difficulty of recognition likely varied for different pictures.37
Additionally, the measure used for sedation will affect the relation between memory and sedation responses. In this initial study a crude measure of sedation, lack of verbal response, was used. It is highly likely that a more sensitive measure of sedation such as reaction time, or eye movement tracking would have resulted in a leftward shift of the sedation response curve (sedation occurring sooner, or at lower drug concentrations), decreasing the separation between memory and sedation responses. Children, likely anxious as a result of being in the hospital, were thus sedated in a very quiet and peaceful setting by practitioners who possessed a large experience administering dexmedetomidine. In such a calm setting, small degrees of sedation might impair memory substantially more than if the child was, for example, in a noisy operating room. This again would also tend to shift the memory response curve leftward. Residual anxiety, known to impair memory, could also act to inhibit sedation, again increasing separation between memory and sedation responses.38 39 Thus a number of factors are at play that may explain the temporal or concentration separation between subsequent recognition memory and sedation in the current study.
There are some limitations to our study, unavoidable when performing and designing memory tests on children. For example the need for presenting 100 images in the recovery period over an eight min test period to assess recognition memory, may have resulted in fatigue and non-compliance with the tasks. However, 30 of 42 children fully completed the study and provided data which could be successfully modelled individually. No formal testing for cognitive disability was performed other that the requirement for successful completion of a practice memory task for inclusion in the study. The possibility exists of unrecognized cognitive impairment which could result in poor memory performance in certain individuals, and might explain some of the variability in individual responses. We excluded analysis of two patients who had exceptionally high FA rates, as we felt that this was indicative of confusion regarding how to perform the recognition task. However, an alternative explanation is that these patients used a very liberal response bias. Formal testing for outliers may be warranted in this patient population in the future. Predicted dexmedetomidine concentrations were used to explore the possibility that less variability would be present. Though no major difference was apparent from when time was used as the predictor variable, the dose response appeared to be quite steep, indicating that drug concentration may be a more accurate predictor. Whether variability was related to individual sensitivity or rather inaccurate effect site modeling is difficult to determine. Actual serum and effect site concentrations may be substantially different from predicted serum concentrations. Few pharmacokinetic studies have been done in children, and most were done in the critical care setting.40 To our knowledge no study has determined rate constants for a sedation pharmacodynamic effect in children (though one study determined a ke0 of ∼ 10 min for a cardiovascular effect).41 A definitive PK-PD study children of similar age undergoing minor procedures would be difficult to conduct.
In conclusion, the slow bolus infusion of dexmedetomidine allowed more detailed measures of memory and sedation responses in verbal paediatric patients. In a quiet setting, dexmedetomidine produced impairment of recall, as measured by subsequent recognition memory, some min before loss of responsiveness. Children provided conservative recognition responses, needing a greater degree of confidence to categorize previously experienced pictures as recognized, particularly in the dexemedetomidine group. More controlled studies will be needed to establish whether dexmedetomidine has amnestic properties similar to midazolam or propofol. The requirements for these studies would seem to necessitate stepped or very slowly ramped target concentrations, and possibly dexmedetomidine drug assays. More accurate information about memory might be obtained if step concentrations were used, providing a ‘block’ (stationary) design which could be analysed using signal detection theory more reliably. Such a study would be difficult to do in children of this age. With the current state of knowledge pertaining to dexmedetomidine sedation, practitioners would be prudent to assume some memory function is present until sedation is sufficient to prevent response to verbal stimulation.
Authors’ contributions
Study design/planning: K.P.M., E.K., R.P., R.V., V.Y., J.R.
Study conduct: K.P.M., E.K., R.P., R.V., V.Y., M.M.
Data analysis: F.R., K.P.M., E.K., R.P., R.V., J.R.
Writing paper: K.P.M., E.K., R.P., R.V., F.R., V.Y.
Revising paper: all authors.
Acknowledgements
The authors acknowledge K.A.D. DM-Stat, Inc., Malden, Massachusetts, and Y.L. Department of Psychiatry and Behavioural Sciences, Memorial Sloan–Kettering Cancer Center, New York, New York, USA for their contributions. The authors also acknowledge A.B. Department of Anesthesiology, Perioperative, and Pain Medicine at Boston Children’s Hospital, Boston, MA and S.O. Department of Anesthesiology Memorial Sloan–Kettering Cancer Center, New York, New York, USA for their highly valued assistance in the preparation of this manuscript.
Declaration of interest
None declared.
Funding
K.P.M. received an unrestricted educational grant and investigator initiated support from Hospira (Hospira, Lake Forest, IL). This study was supported in part by NIH/NCI P30 CA008748 (MSK Cancer Center Support Grant).
References
- 1.Practice guidelines for sedation and analgesia by non-anesthesiologists. A report by the American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Anesthesiology 1996; 84: 459–71 [DOI] [PubMed] [Google Scholar]
- 2.Cravero JP, Beach ML, Blike GT, Gallagher SM, Hertzog JH, Pediatric Sedation Research C. The incidence and nature of adverse events during pediatric sedation/anesthesia with propofol for procedures outside the operating room: a report from the pediatric sedation research consortium. Anesth Analg 2009; 108: 795–804 [DOI] [PubMed] [Google Scholar]
- 3.Faigel DO, Baron TH, Goldstein JL, et al. Guidelines for the use of deep sedation and anesthesia for GI endoscopy. Gastrointest Endosc 2002; 56: 613–7 [DOI] [PubMed] [Google Scholar]
- 4.Godwin SA, Caro DA, Wolf SJ, et al. Clinical policy: procedural sedation and analgesia in the emergency department. Ann Emerg Med 2005; 45: 177–96 [DOI] [PubMed] [Google Scholar]
- 5.Mason KP. Sedation trends in the 21st century: the transition to dexmedetomidine for radiological imaging studies. Paediatr Anaesth 2010; 20: 265–72 [DOI] [PubMed] [Google Scholar]
- 6.Mason KP, Zurakowski D, Karian VE, Connor L, Fontaine PJ, Burrows PE. Sedatives used in pediatric imaging: comparison of IV pentobarbital with IV pentobarbital with midazolam added. AJR Am J Roentgenol 2001; 177: 427–30 [DOI] [PubMed] [Google Scholar]
- 7.Kamat PP, McCracken CE, Gillespie SE, et al. Pediatric critical care physician-administered procedural sedation using propofol: a report from the pediatric sedation research consortium database. Pediatr Crit Care Med 2015; 16: 11–20 [DOI] [PubMed] [Google Scholar]
- 8.Cravero JP. Pediatric sedation with propofol-continuing evolution of procedural sedation practice. J Pediatr 2012; 160: 714–6 [DOI] [PubMed] [Google Scholar]
- 9.Lamperti M. Adult procedural sedation: an update. Curr Opin Anaesthesiol 2015; 28: 662–7 [DOI] [PubMed] [Google Scholar]
- 10.Mason KP, Lubisch N, Robinson F, Roskos R, Epstein MA. Intramuscular dexmedetomidine: an effective route of sedation preserves background activity for pediatric electroencephalograms. J Pediatr 2012; 161: 927–32 [DOI] [PubMed] [Google Scholar]
- 11.Mason KP, Prescilla R, Fontaine PJ, Zurakowski D. Pediatric CT sedation: comparison of dexmedetomidine and pentobarbital. AJR Am J Roentgenol 2011; 196: W194–8 [DOI] [PubMed] [Google Scholar]
- 12.Mason KP, Robinson F, Fontaine P, Prescilla R. Dexmedetomidine offers an option for safe and effective sedation for nuclear medicine imaging in children. Radiology 2013; 267: 911–7 [DOI] [PubMed] [Google Scholar]
- 13.Mason KP, Zgleszewski SE, Dearden JL, et al. Dexmedetomidine for pediatric sedation for computed tomography imaging studies. Anesth Analg 2006; 103: 57–62 [DOI] [PubMed] [Google Scholar]
- 14.Yuen VM, Hui TW, Irwin MG, Yuen MKA. Comparison of intranasal dexmedetomidine and oral midazolam for premedication in pediatric anesthesia: a double-blinded randomized controlled trial. Anesth Analg 2008; 106: 1715–21 [DOI] [PubMed] [Google Scholar]
- 15.Li BL, Yuen VM, Song XR, et al. Intranasal dexmedetomidine following failed chloral hydrate sedation in children. Anaesthesia 2014; 69: 240–4 [DOI] [PubMed] [Google Scholar]
- 16.Yuen VM, Hui TW, Irwin MG, et al. A randomised comparison of two intranasal dexmedetomidine doses for premedication in children. Anaesthesia 2012; 67: 1210–6 [DOI] [PubMed] [Google Scholar]
- 17.Patino M, Samuels P, Mahmoud M. Pediatric sedation outside the operating room. Int Anesthesiol Clin 2013; 51: 127–46 [DOI] [PubMed] [Google Scholar]
- 18.Szekely A, Jacobsen T, D'amico S, et al. A new on-line resource for psycholinguistic studies. J Mem Lang 2004; 51: 247–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craik FI, Govoni R, Naveh-Benjamin M, Anderson ND. The effects of divided attention on encoding and retrieval processes in human memory. J Exp Psychol Gen 1996; 125: 159–80 [DOI] [PubMed] [Google Scholar]
- 20.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein ARA. Simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996; 49: 1373–9 [DOI] [PubMed] [Google Scholar]
- 21.Wickens TD, Elementary signal detection theory. Oxford; New York: Oxford University Press, 2002 [Google Scholar]
- 22.Mason KP, Lubisch NB, Robinson F, Roskos R. Intramuscular dexmedetomidine sedation for pediatric MRI and CT. AJR Am J Roentgenol 2011; 197: 720–5 [DOI] [PubMed] [Google Scholar]
- 23.Mason KP, Turner DP, Houle TT, Fontaine PJ, Lerman J. Hemodynamic response to fluid management in children undergoing dexmedetomidine sedation for MRI. AJR Am J Roentgenol 2014; 202: W574–9 [DOI] [PubMed] [Google Scholar]
- 24.Mason KP, Zurakowski D, Connor L, et al. Infant sedation for MR imaging and CT: oral versus intravenous pentobarbital. Radiology 2004; 233: 723–8 [DOI] [PubMed] [Google Scholar]
- 25.Mason KP, Zurakowski D, Zgleszewski SE. et al. High dose dexmedetomidine as the sole sedative for pediatric MRI. Paediatr Anaesth 2008; 18: 403–11 [DOI] [PubMed] [Google Scholar]
- 26.Ngamprasertwong P, Mahmoud M. Anesthesia for MRI enterography in children. J Clin Anesth 2014; 26: 249.. [DOI] [PubMed] [Google Scholar]
- 27.Mahmoud M, Gunter J, Donnelly LF, Wang Y, Nick TG, Sadhasivam S. A comparison of dexmedetomidine with propofol for magnetic resonance imaging sleep studies in children. Anesth Analg 2009; 109: 745–53 [DOI] [PubMed] [Google Scholar]
- 28.Veselis RA, Pryor KO, Reinsel RA, Mehta M, Pan H, Johnson R., Jr. Low-dose propofol-induced amnesia is not due to a failure of encoding: left inferior prefrontal cortex is still active. Anesthesiology 2008; 109: 213–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pryor KO, Reinsel RA, Mehta M, Li Y, Wixted JT, Veselis RA. Visual P2-N2 complex and arousal at the time of encoding predict the time domain characteristics of amnesia for multiple intravenous anesthetic drugs in humans. Anesthesiology 2010; 113: 313–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veselis RA, Pryor KO, Reinsel RA, Li Y, Mehta M, Johnson R., Jr. Propofol and midazolam inhibit conscious memory processes very soon after encoding: an event-related potential study of familiarity and recollection in volunteers. Anesthesiology 2009; 110: 295–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veselis RA, Reinsel RA, Feshchenko VA, Johnson R., Jr. Information loss over time defines the memory defect of propofol: a comparative response with thiopental and dexmedetomidine. Anesthesiology 2004; 101: 831–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pryor KO, Veselis RA, Reinsel RA, Feshchenko VA. Enhanced visual memory effect for negative versus positive emotional content is potentiated at sub-anaesthetic concentrations of thiopental. Br J Anaesth 2004; 93: 348–55 [DOI] [PubMed] [Google Scholar]
- 33.Veselis RA, Reinsel RA, Feshchenko VA, Wronski M. The comparative amnestic effects of midazolam, propofol, thiopental, and fentanyl at equisedative concentrations. Anesthesiology 1997; 87: 749–64 [DOI] [PubMed] [Google Scholar]
- 34.Ghoneim MM, Hinrichs JV. Drugs, memory, and sedation: specificity of effects. Anesthesiology 1997; 87: 734–6 [DOI] [PubMed] [Google Scholar]
- 35.Ghoneim MM, Ali MA, Block RI. Appraisal of the quality of assessment of memory in anesthesia and psychopharmacology literature. Anesthesiology 1990; 73: 815–20 [DOI] [PubMed] [Google Scholar]
- 36.Pendergrass R, Olfman D, Schmalstig M, Seder K, Light LL. Age, criterion flexibility, and associative recognition. J Gerontol B Psychol Sci Soc Sci 2012; 67: 36–42 [DOI] [PubMed] [Google Scholar]
- 37.Brown S, Steyvers M. The dynamics of experimentally induced criterion shifts. J Exp Psychol Learn Mem Cogn 2005; 31: 587–99 [DOI] [PubMed] [Google Scholar]
- 38.Ghoneim MM, Block RI. Immediate peri-operative memory. Acta Anaesthesiol Scand 2007; 51: 1054–61 [DOI] [PubMed] [Google Scholar]
- 39.Stait ML, Leslie K, Bailey R. Dreaming and recall during sedation for colonoscopy. Anaesth Intensive Care 2008; 36: 685–90 [DOI] [PubMed] [Google Scholar]
- 40.Potts AL, Anderson BJ, Warman GR, Lerman J, Diaz SM, Vilo S. Dexmedetomidine pharmacokinetics in pediatric intensive care–a pooled analysis. Paediatr Anaesth 2009; 19: 1119–29 [DOI] [PubMed] [Google Scholar]
- 41.Potts AL, Anderson BJ, Holford NH, Vu TC, Warman GR. Dexmedetomidine hemodynamics in children after cardiac surgery. Paediatr Anaesth 2010; 20: 425–33 [DOI] [PubMed] [Google Scholar]