Abstract
Objective
Atherosclerosis is characterized by frequent communication between infiltrating leukocytes and vascular cells, through chemokine and cytokine networks. IL-17C is detectable within atherosclerotic lesions; however the potential involvement of this cytokine has not been examined. Thus we sought to investigate the role of IL-17C in atherosclerosis.
Approach and Results
The expression of IL-17 cytokines was profiled within Apoe−/− aortas and Il17c expression was elevated. Flow cytometry experiments revealed a major population of aortic IL-17C-producing smooth muscle cells. Next, we generated Il17c−/−Apoe−/− mice and demonstrated that atherosclerotic lesion and collagen content was diminished within WD-fed Il17c−/−Apoe−/− aortas and aortic roots in comparison to Apoe−/− controls. Smooth muscle cells and fibroblasts were mainly responsible for the reduced Col1A1 expression in the aorta of Il17c−/−Apoe−/− mice. Importantly, IL-17C treated Apoe−/− aortas upregulated Col1A1 expression ex vivo. Il17c−/−Apoe−/− mice displayed a proportional reduction in aortic macrophages, neutrophils, T cells, Th1, and Tregs, without corresponding changes in the peripheral immune composition. Examination of aortic IL-17A+ TCRγδ T cells and Th17 cells demonstrated a stark reduction in the percentage and number of these subsets within Il17c−/−Apoe−/− versus Apoe−/− mice. Explanted 12 week WD Apoe−/− aortas treated with IL-17C resulted in the induction of multiple vascular chemokines and cytokines. Th17 cells demonstrated attenuated migration towards supernatants from cultures Il17c−/−Apoe−/− smooth muscle cells and short-term homing experiments revealed diminished recruitment of Th17 cells to the aorta of Il17c−/−Apoe−/− recipients.
Conclusions
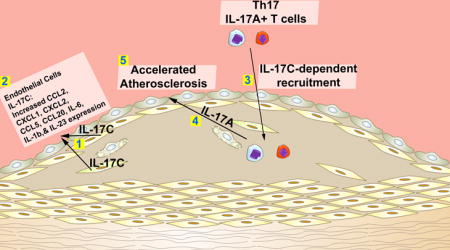
Smooth muscle cell-derived IL-17C plays a pro-atherogenic role by supporting the recruitment of Th17 cells to atherosclerotic lesions.
Keywords: Atherosclerosis, Inflammation, Leukocytes, Smooth muscle cells, Cytokines, Migration
Graphical abstract
Introduction
Cardiovascular diseases (CVDs) are the leading cause of global deaths, and atherosclerosis associated-CVDs were responsible for 13.5 million out of 17.3 million CVDs worldwide in 2008.1 The formation of atherosclerotic plaques is a chronic arterial inflammatory process that is characterized not only by the formation of lipid-rich plaques,2 but also regular communication between arterial hematopoietic cells and vascular cells through cytokine and chemokine networks. T cells, as a part of the adaptive immune response, actively participate in regulating local and systemic inflammation during atherogenesis. In this respect, the pro- and anti-atherogenic roles of IFNγ+ T helper 1 (Th1) and T regulatory (Treg) cells are well established. However, the roles of IL-17A+ T cells, including T helper 17 cells (Th17) and IL-17A+ TCRγδ T cells have been controversial, due to conflicting results on the functions of IL-17A in atherosclerosis.3–8
The Interleukin-17 cytokine family consists of six family members, (IL-17A-IL-17F), which vary in their distribution, biological roles, and sequence homology.9,10 The roles and biological functions of IL-17A and IL-17F-producing cells in inflammation have been well studied. However, the functions of other members of the IL-17 family (IL-17B-D) were unclear until recently.9 Recent work on the biology of IL-17C has revealed that IL-17C signals through a heteromeric-receptor consisting of the IL-17RE subunit and the common IL-17RA subunit;11,12 which is known to be shared with IL-17A, IL-17F, and IL-17B. IL-17C signaling results in the induction of chemokines and pro-inflammatory cytokines, including Cxcl1, Cxcl2, Ccl20, Tnf, and Il1b within IL-17RA and IL-17RE-expressing colonic epithelial cells and keratinocytes.12,13 Th17 cells which express IL-17RA and IL-17RE up-regulate IL-17A, IL-17F, and IL-22 production in response to IL-17C,11 indicating that IL-17C might play a role in Th17 differentiation or maintenance. Importantly, IL-17RA and IL-17RE are expressed within Apoe−/−aortas,4 suggesting that the receptor complex for IL-17C signaling is present within the aorta.
Due to the plethora of types of cells that express IL-17RA and IL-17RE, IL-17C is likely to play pleiotropic roles in host-defense, autoimmune, or inflammatory pathologies.11,14,15 IL-17C is expressed by colonic epithelial cells and plays key roles in host survival by maintaining mucosal barrier integrity in a Citrobacter Rodentium infection model12 and dextran sulfate sodium-induced colitis models.13,16 IL-17C is elevated in psoriatic lesions and it significantly affects the abundance of F4/80+ macrophages within inflamed psoriatic plaques.13,14,17 Similarly, IL-17C is required for the development of MOG-induced experimental autoimmune encephalomyelitis (EAE). In the context of atherosclerosis, recent work from our group demonstrated that several IL-17 cytokines are present within atherosclerotic Apoe−/− aortas,18 including IL-17A, IL-17C, and IL-17F, suggesting that IL-17C might also play a role in atherogenesis.
In addition to IL-17C, IL-17A is an important pro-inflammatory cytokine that is required to efficiently control bacterial and fungal infections, but also participates in major autoimmune diseases.9,19 Several studies have reported elevated levels of Th17 and IL-17A+ TCRγδ T cells within atherosclerotic Apoe−/− and Ldlr−/− mice,3,18,20–22 in patients with coronary artery disease (CAD) patients and in endarterectomy patients with vulnerable plaques.3,23,24 However the precise role(s) that IL-17A plays have been disputed.3–8 Mechanistic studies in murine models of atherosclerosis have yielded at least two unifying hypotheses, that IL-17A plays a pro-atherogenic role by affecting aortic chemokine and cytokine production and myeloid cell recruitment,3,4,6,8,18,20,22,25 or an atheroprotective role, via regulation of aortic Th1 content, smooth muscle cell content, and collagen deposition.5,21,26
In the present study, we hypothesized that in addition to IL-17A, other IL-17 family members might participate in the pathology of atherosclerosis. We report here that aortic IL-17C is elevated within atherosclerotic Apoe−/− mice in comparison to non-atherosclerotic C57Bl6 controls. Aortic smooth muscle cells (SMCs) are, unexpectedly, prominent sources of arterial IL-17C within Apoe−/− and C57Bl6 mice. To study the role of IL-17C in atherosclerosis, Il17c−/−Apoe−/− mice were generated. Deficiency of IL-17C resulted in moderate reductions in atherosclerotic lesions, lesional collagen content, aortic leukocyte, macrophage, neutrophil, Th1 and Treg cells, and robustly reduced aortic Th17 and IL-17A+TCRγδ+ T cell content, due to defective IL-17C-mediated IL-17A+ T cell recruitment to the aorta. Importantly, these data underscore the significance of communication between aortic IL-17C+ SMCs and arterial IL-17A-producing T cells in order to efficiently promote atherogenesis.
Materials and Methods
Animals
Il17c−/− (kindly provided by Amgen, inc) on the C57Bl6 background were crossed with Apoe−/− mice to generate Il17c−/−Apoe−/− mice. Six week old male and female Apoe−/− and Il17c−/−Apoe−/− were placed on a high fat Western diet (21% fat and 0.15% cholesterol, TD.88137, Harlan Laboratories, Indianapolis) for 12 weeks and used at 18 weeks of age. For en face preparations, Apoe−/− and Il17c−/−Apoe−/− littermates from Il17c+/− Apoe−/− breeders were used. Dedicated Il17c−/−Apoe−/− and Apoe−/− breeders were used subsequently as atherosclerotic lesions were equivalent between Apoe−/− mice from Il17c+/−Apoe−/− and Apoe−/− breeders. For the in vitro chemotaxis assays, 40 week old Il17aiCre/iCreR26R tdTomato/tdTomatoApoe−/− mice were used as a source of CD4+ T cells as CD4+ Th17 cells are IL-17AtdTomato+. All animals were kept in specific-pathogen-free conditions and animal experiments were approved by the Eastern Virginia Medical School Animal Care and Use Committee. Unless noted otherwise, all surgical procedures and organ collections were collected aseptically in order to prevent potential bacterial or fungal contaminants from affecting the results.
An expanded Methods section is available in the Online Data Supplement.
Results
IL-17C is elevated in atherosclerotic Apoe−/− aortas, and is produced primarily by aortic smooth muscle cells
While several recent studies have examined the role(s) of IL-17A and IL-17F in atherosclerosis, the potential roles of other IL-17 family members have not been explored. To re-confirm18 that IL-17 cytokine family members are present during atherosclerosis, we initially examined the expression of the IL-17 cytokines in 40 week old aged Apoe−/− and C57Bl6 aortas. For all experiments presented in this study, the aortas were cleaned of all peri-aortic adipose tissue, but contained the aortic adventitia. The aortas with surrounding adventitia were used for flow cytometric or RT-PCR analyses. Several IL-17 cytokines were present and elevated within aged atherosclerotic Apoe−/− aortas, including Il17a, Il17c, and Il17f (Fig.1A). To confirm these results and to determine whether IL-17C expression is altered during atherogenesis, we examined the aortas isolated from 12-week chow diet–fed C57Bl6 and Apoe−/− mice, and the aortas from 12-week WD-fed Apoe−/− mice for IL-17C expression (Figure 1B). IL-17C expression was elevated in CD-fed atherosclerotic Apoe−/− aortas and was further enhanced in 12 week WD-fed Apoe−/− mice (Fig.1B). Next, to determine the sources of aortic IL-17C, we examined 12 week WD Apoe−/− aortas for IL-17C expression by flow cytometry (Fig.1D) and PCR (Fig.1C). In agreement with reports on the biology of IL-17C within mucosal epithelial cells and keratinocytes,11–15 IL-17C was almost exclusively expressed by CD45− non-hematopoietic cells, but not CD45+ hematopoietic cells within the aorta of within 12 week WD-fed Apoe−/− aortas (Fig.1D). To determine the non-hematopoietic cellular sources of aortic IL-17C, we developed a scheme for examining and isolating vascular cell components by FACS (Supplemental Figure 1) with a specific focus at IL-17C production within vascular cell subsets (Fig.1D–F). Leukocytes were excluded from the analysis based on CD45 expression and CD45− vascular cells were subsequently examined for CD31, αSMA, and CD29 positivity (Supplemental Figure 1). CD45−CD31+ endothelial cells (ECs), CD45−CD31−αSMA+ SMCs, CD45−CD31−αSMA−CD29+ fibroblasts, and CD45−CD31−αSMA−CD29− vascular cells were sub-gated and examined for IL-17C production (Fig.1D–F). As expected, SMCs and endothelial cells represented the clear majority of vascular cells recovered from aged C57Bl6, Apoe−/− and 12 week WD-fed Apoe−/− aortas (Fig.1E). Interestingly, CD45−CD31−αSMA+ SMCs and CD45−CD31−αSMA−CD29− vascular cells were the primary producers of IL-17C within the aorta, and a higher percentage of SMCs were IL-17C+ within Apoe−/− aortas in comparison to non-atherosclerotic C57Bl6 aortas (Fig.1F). To confirm our results using a complimentary technique, we isolated primary Apoe−/− aortic SMCs using magnetic separation from 12 week WD-fed Apoe−/− mice and assessed Il17c expression (Fig.1C). In confirmation of our flow cytometry results, Il17c expression was detected in non-hematopoietic cells and further significantly enriched in CD45−CD31−CD29− SMCs (Fig.1C). Collectively these data demonstrate that the vasculature is an important source of IL-17C in atherosclerosis. In addition, these data are the first to report that IL-17C can be detected by flow cytometry using conventional PMA-based re-stimulation techniques.
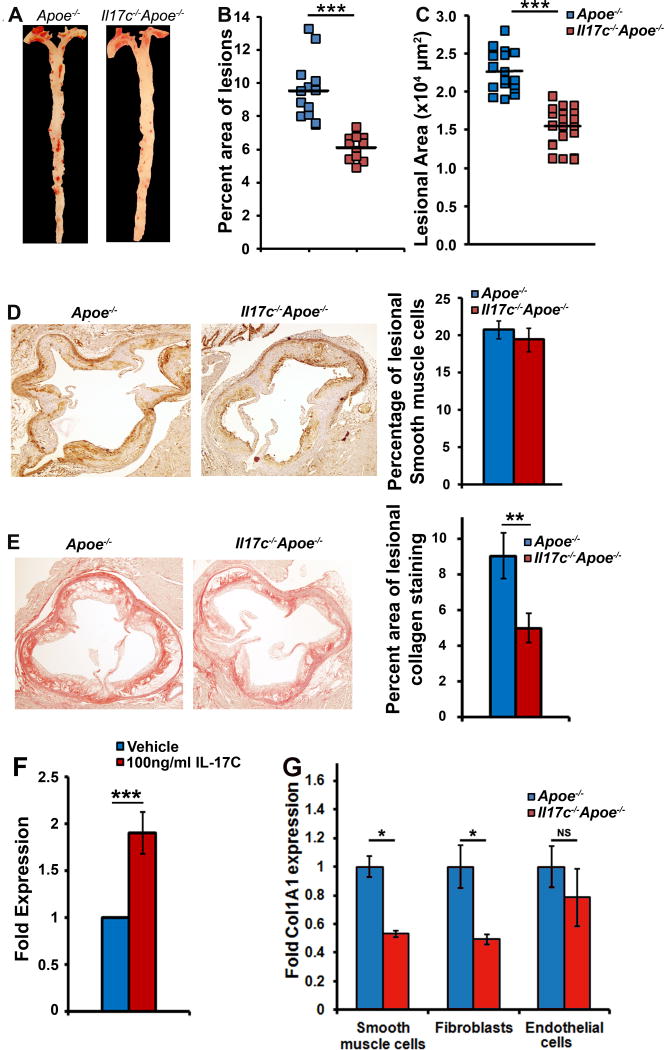
Figure 1. Aortic Il17c is elevated in Apoe−/− mice and IL-17C+ smooth muscle cells and CD45−CD31−αSMA−CD29− vascular cells are the sources of aortic IL-17C within Apoe−/− mice.
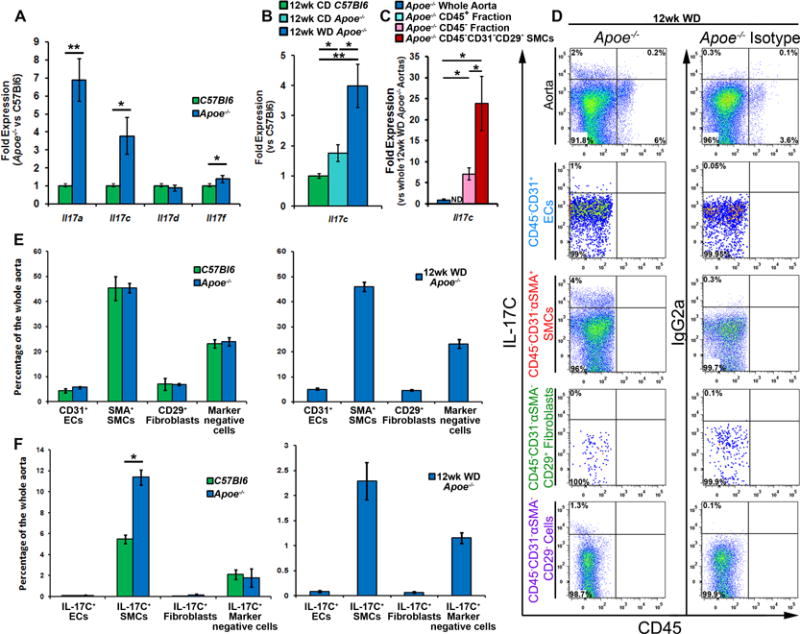
(A) mRNA expression of IL-17 cytokine family members. 40 week old C57Bl6 and Apoe−/− aortas with aortic adventitia were sterilely collected, processed for RT-PCR, and assessed for Il17a, Il17c, Il17e, and Il17f expression. n=6 mice/genotype, 3 independent experiments. (B) mRNA expression of Il17c in sterilely collected 12 week CD C57Bl6, Apoe−/−, and 12 week WD-fed Apoe−/− aortas. N=6 mice/genotype, 3 independent experiments. (C) Expression of Il17c in whole Apoe−/− aortas (Blue), and magnetically sorted Apoe−/− aortic leukocytes (CD45+, teal bar), Apoe−/− vascular cells (CD45− cells, pink bar), and FACS-sorted Apoe−/− CD45−CD31−CD29− smooth muscle cells (SMCs, red bar). n= 12 Apoe−/− mice, 3 independent experiments. (D) Aortic cell suspensions from 40 week old C57Bl6, Apoe−/−, and 12 week WD fed Apoe−/− mice were stained with anti-CD45, CD31, αSMA, CD29 and IL-17C antibodies or appropriate isotype controls and assessed by flow cytometry. Representative IL-17C staining and isotype control staining of non-hematopoietic vascular cells, gated endothelial cells, smooth muscle cells, fibroblasts, and remaining vascular cells within 12 week WD Apoe−/− aortas. The corresponding isotype control for IL-17C within each gate is shown. (E) Quantification of the percentage of different vascular cell subsets and (F) IL-17C positivity within each subset, as a percentage of the entire aorta within 40 week old C57Bl6 (green bars), Apoe−/− (blue bars), and 12 week WD Apoe−/− (blue bars, right) aortas. The data depicts means±SEM. n=14 mice/genotype, 6 independent experiments. Means±SEM are shown, * - p<0.05,** - p<0.01.
IL-17C-deficient conditions reduce atherosclerosis and the number of aortic leukocytes and myeloid cell subsets
To directly assess the role of IL-17C in atherosclerosis, we generated Il17c−/−Apoe−/− mice and compared atherosclerotic lesions between 12 week western diet-fed Apoe−/− and Il17c−/−Apoe−/− mice. Apoe−/− and Il17c−/−Apoe−/− mice showed no difference in body weight, plasma cholesterol, triglyceride, LDL-cholesterol, or HDL-cholesterol content between males or females (Table 1). Diet matched Il17c−/−Apoe−/− mice developed 40% smaller lesions throughout the aorta (Fig 2A–B) and a 32% reduction in aortic root lesions (Fig.2C) in comparison with 12 week western diet-fed Apoe−/− littermates. As some reports have suggested that IL-17A might promote collagen deposition by SMCs5,21,26, and other reports did not observe any effects of IL-17A on lesional collagen content4,6,22, we next sought to determine if IL-17C might affect lesional SMC or collagen content within the aortic root. While we did not detect any difference in the percentage of lesional aortic root SMCs (Fig.2D), we did detect a 40% reduction in total collagen and type 1 collagen fiber content (Fig.2E, data not shown) in 12 week WD-fed Il17c−/−Apoe−/− mice. To confirm these results and to test if IL-17C might play a role in promoting collagen deposition, we treated explanted 12 week WD-fed Apoe−/− aortas with or without IL-17C and assessed pro-collagen expression (Col1a1, Fig.2F). In this system, IL-17C promoted pro-collagen expression. To identify the specific cell type that is responsible for the IL-17C-dependent increased collagen synthesis, we isolated primary Apoe−/− and Il17c−/−Apoe−/− populations of CD45+ aortic leukocytes, CD45−CD31+ endothelial cells, CD45−CD31−CD29− SMCs, and CD45−CD31−CD29+ fibroblasts by FACS. The primary cells were then assessed for Col1a1 expression (Fig.2G). Col1a1 expression was enhanced in IL-17C expressing Apoe−/− SMCs and fibroblasts versus IL-17C-deficient Il17c−/−Apoe−/− SMCs and fibroblasts. These data suggest that IL-17C does play a role in promoting pro-collagen expression in aortic SMCs and fibroblasts, but IL-17C plays a decidedly pro-inflammatory role in atherosclerosis.
Table 1.
12 week Western Diet-fed Apoe−/− and Il17c−/−Apoe−/− body weights, fasting plasma triglyceride, HDL-cholesterol, LDL-cholesterol, and total cholesterol levels. NS – Not significant Il17c−/−Apoe−/− vs Apoe−/− controls, p>0.05
| Body Weight (g) | Triglycerides (mg/dl) | HDL-Cholesterol (mg/dl) | LDL-Cholesterol (mg/dl) | Total Cholesterol (mg/dl) | |
|---|---|---|---|---|---|
| Apoe−/−, 12 weeks WD, fasted | 30.3±0.23 Males 24.5±1.2 Females |
81.6±4.35 | 51.2±2.1 | 576±32.6 | 641±33.4 |
| Il17c−/−Apoe−/−, 12 weeks WD, fasted | 32.2±1.7 MalesNS 26.6±1.1 FemalesNS |
74.3±5.54NS | 48.0±2.3NS | 599±23.1NS | 660±23.8NS |
Figure 2. Deficiency of IL-17C attenuates atherosclerosis in Apoe−/− mice.
(A–B) Apoe−/− and Il17c−/−Apoe−/− mice were fed a WD for 12 weeks and assessed for atherosclerotic plaques. (A) Representative en face Oil Red O staining of Apoe−/− and Il17c−/− Apoe−/− aortas. (B) Quantification of lesion area as a percentage of the area of each aorta. Each symbol represents 1 animal and the horizontal bars represent the population mean. (C) Quantification of the average lesional area within the aortic roots of 12 week WD Apoe−/− and Il17c−/−Apoe−/− mice. (D) Representative aortic root immunohistochemical alpha-smooth muscle actin staining (α-SMA, brown) and the average percent area of lesional α-SMA+ smooth muscle cells. (E) Representative picosirius red staining (red), and the average percent area of collagen fiber positivity within the aortic roots of 12 week WD-fed Apoe−/− and Il17c−/−Apoe−/− mice. (F) Fold induction of Col1a1 expression within 24 hours in response to vehicle control or 100ng/ml IL-17C, within explanted 12 week WD Apoe−/− aortas. n=10 aortas/condition, 4 independent experiments. (G) 12 week WD-fed Apoe−/− and Il17c−/−Apoe−/− aortas were isolated and single cell suspensions were prepared. Aortic single cell suspensions were stained with CD31, CD45, and CD29 antibodies in order to isolate CD45negativeCD31negativeCD29negative SMCs, CD45negativeCD31negativeCD29positive fibroblasts, and CD45negativeCD31positive endothelial cells by FACS for RT-PCR to assess Col1a1 expression. Means±SEM are shown, * - p<0.05,** - p<0.01, *** - p<0.001.
The development of atherosclerotic plaques requires active and sustained recruitment of leukocytes into the aortic wall.27 As prior work from our group4 demonstrated that the IL-17A/IL-17RA pathway promotes the recruitment of leukocytes to the aorta, notably monocytes and neutrophils,4,18 we next sought to characterize the aortic immune composition in Il17c−/−Apoe−/− mice by flow cytometry. In agreement with our en face and aortic root lesion data (Figure 2), Il17c−/−Apoe−/− aortas with the surrounding aortic adventitia displayed a ~35% reduction in both the percentage and number of aortic CD45+ leukocytes, in comparison to Apoe−/− littermates (Figure 3A and 3B). To determine whether IL-17C deficient conditions might impact the abundance of aortic or peripheral myeloid cells and myeloid cell subsets, we examined the percentage and numbers of aortic infiltrating and peripheral CD11b+ myeloid cells, CD68+ macrophages and GR1+ neutrophils. 12 week WD-fed Il17c−/−Apoe−/− mice displayed a significant reduction (~40%) in the percentage and number of aortic CD11b+ myeloid cells, CD11b+CD68++ macrophages, and CD11b+GR1++ neutrophils versus Apoe−/− controls (Fig.3C–D). As IL-17C is known to be produced by colonic epithelial cells and keratinocytes, and IL-17C plays important roles in helping to maintain mucosal immunity,12–15 we next determined whether IL-17C deficient conditions might additionally affect the systemic immune composition. Surprisingly, we did not detect differences in the composition of CD11b+ leukocytes, macrophages, neutrophils, Ly6Chigh monocytes or Ly6Clow monocytes within the blood or spleens of 12 week western diet-fed Il17c−/−Apoe−/− and Apoe−/− mice (Data not shown), suggesting that the effects seen within the aorta are due to the local effects of IL-17C.
Figure 3. IL-17C deficient Apoe−/− mice display diminished aortic leukocyte, myeloid cell, macrophage and neutrophil content.
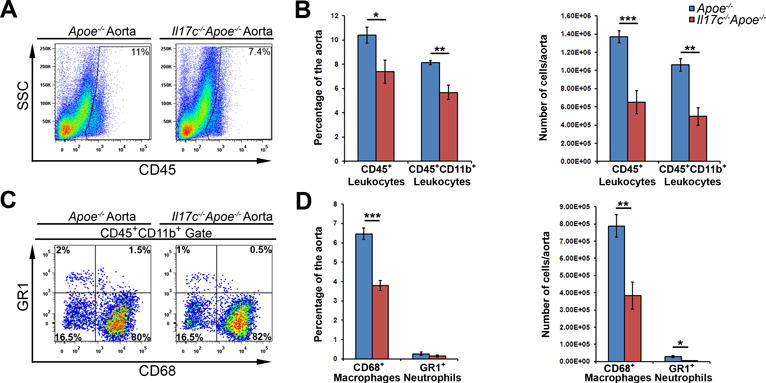
(A–D) 12 week WD-fed Apoe−/− and Il17c−/−Apoe−/− aortas with surrounding aortic adventitia were stained with anti CD45, CD11b, CD68, and Gr1 antibodies and assessed by flow cytometry. (A) Representative aortic CD45 staining and quantification (B) of 12 week WD Apoe−/− (blue) and Il17c−/−Apoe−/− (red) aortic CD45+ leukocyte, and CD45+CD11b+ myeloid cell content. (C) Representative CD68 and Gr1 staining within CD45+CD11b+ gated aortas. (D) Quantification of the percentage and number of CD68+ macrophages and Gr1+ neutrophils per Apoe−/− (blue) and Il17c−/−Apoe−/− (red) aorta. n= 5 Apoe−/− mice, n=8 Il17c−/−Apoe−/− mice, 3 independent experiments. Means±SEM are shown, * - p<0.05, ** - p<0.01, *** - p<0.001.
IL-17C supports the accumulation of pro-atherogenic IL-17A+ T cells within the aortic wall
As recent work by Chang and colleagues11 demonstrated that both Th17 cells and IL-17A+ TCRγδ+ T cells express IL-17RA and IL-17RE, the functional receptor for IL-17C, and that IL-17C helps to regulate pro-inflammatory IL-17A production in a model of experimental autoimmune encephalitis, we next examined the T cell composition of 12 week WD-fed Il17c−/−Apoe−/− and Apoe−/− mice (Fig.4). The percentages of aortic CD3+ and TCRαβ+ CD3+ T cells were similar between Il17c−/−Apoe−/− and Apoe−/− mice (Fig.4A–B), however the absolute number of infiltrating aortic CD3+ and TCRαβ+ CD3+ T cells were decreased by 60% in Il17c−/−Apoe−/− aortas (Fig.4B). To determine if IL-17C might affect the abundance of aortic Th1, Th17, Treg, or cytokine-producing TCRαβ− T cells (which are mostly TCRγδ+ T cells within the aorta, additional data not shown) in 12 week WD Apoe−/− and Il17c−/−Apoe−/− aortas, IL-17A and IFNγ cytokine staining was performed (Fig.4C–E). While the overall percentages of aortic IFNγ+ Th1 (Fig.4C–D), and Foxp3+ Tregs (Fig.4D), were similar between 12 week WD Apoe−/− and Il17c−/−Apoe−/− aortas, the number of these subsets were proportionally lower in Il17c−/−Apoe−/− mice as a consequence of diminished aortic CD3+ T cell counts (Fig.4D). In contrast, we detected a stark reduction (~70%) in both the percentage and number of aortic CD3+TCRαβ+ Th17 cells (Fig.4C–D) and IL-17A+ TCRαβ− T cells (Fig.4E–F) within 12 week WD Il17c−/−Apoe−/− mice.
Figure 4. Disruption of IL-17C results in severely diminished aortic IL-17A+ Th17, IL-17A+ CD3+TCRαβ− T cell content.
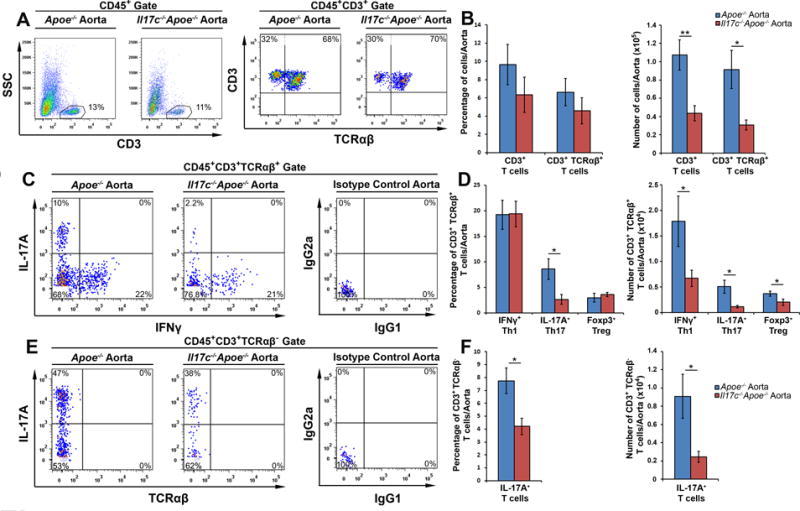
(A–F) 12 week WD-fed Apoe−/− and Il17c−/−Apoe−/− aortas with surrounding aortic adventitia were stained with anti-CD45, CD3, TCRβ, IL-17A, IFNγ, and Foxp3 antibodies and analyzed by flow cytometry (n=7 Apoe−/− mice, n=11, Il17c−/−Apoe−/− mice, 4 independent experiments). (A) Representative CD3 and TCRαβ staining within WD Apoe−/− and Il17c−/−Apoe−/− CD45+ aortic leukocytes and (B) quantification of the percentage and number of CD3+ and CD3+TCRαβ+ T cells/aorta. (C) Representative IFNγ and IL-17A staining in CD3+TCRαβ+ gated aortic T cells and (D) quantification of the percentages and numbers of IFNγ+ Th1, IL-17A+ Th17, and Foxp3+ Tregs/aorta. (E) Representative IFNγ and IL-17A staining within CD3+TCRαβ− gated aortic T cells. (F) Quantification of the percentage and number of CD3+TCRαβ−IFNγ+ T cells and IL-17A+ T cells in 12 week WD Apoe−/− (blue) and Il17c−/−Apoe−/− (red) aortas. Means±SEM are shown. * - p<0.05, ** - p<0.01.
Colonic epithelial cells and keratinocytes are also important sources of IL-17C in vivo, thus we similarly characterized T cell subsets within the spleens, peri-aortic lymph nodes, and peripheral lymph nodes of 12 week WD-fed Apoe−/− and Il17c−/−Apoe−/− mice to determine if a global deficiency of IL-17C might affect peripheral T cell subsets. Despite diminished aortic Th1 and Treg cellularity, and the strong reduction in both the percentage and number of aortic Th17 and IL-17A+ TCRαβ− T cells, we did not observe differences in the percentage or number of these subsets in the spleens, peri-aortic lymph nodes (Supplemental Figure 2), or peripheral lymph nodes (data not shown) of Apoe−/− and Il17c−/−Apoe−/− mice. Together, these data suggest that a global deficiency of IL-17C results in severely impaired accumulation of aortic IL-17A+ T cells within the arterial wall and proportionally reduced aortic macrophage, neutrophil, Th1 and Treg cellularity in Apoe−/− mice.
IL-17C promotes aortic chemokine and cytokine expression, and helps to recruit Th17 cells to atherosclerotic plaques
Several studies have shown that IL-17A promotes arterial vascular chemokine, cytokine expression, and supports the recruitment of monocytes and neutrophils to atherosclerotic lesions.3,4,18,20,23,28–31 Thus, we next sought to determine the effects of IL-17C on aortic chemokine and cytokine expression (Fig.5A). 12 week WD-fed Apoe−/− aortas were explanted for 24 hours with or without IL-17C and assessed for Ccl2, Ccl7, Ccl20, Cx3cl1, Cxcl1, Cxcl2, Cxcl5, Il6, Il23a, and Il1b expression by RT-PCR. In this system, IL-17C broadly supported the expression of these cytokines and chemokines (Fig.5A), including the pro-Th17 chemokine Ccl20, and cytokines Il6, Il23, and Il1b. Thus, as IL-17C helped induce aortic pro-Th17 chemokine and cytokine expression, and Il17c−/−Apoe−/− mice displayed a specific reduction in aortic Th17 and IL-17A+ TCRαβ− T cell content (Fig.4), we hypothesized that IL-17C+ smooth muscle cells might support the accumulation of Th17 and IL-17A+ TCRαβ− T cells within the arterial wall. To test this hypothesis, we performed IL-17C-dependent Th17 chemotaxis experiments in vitro, in order to clarify whether Th17 cells directly or indirectly migrate to IL-17C. In brief, we generated a new strain of IL-17A lineage tracing Il17aicre/icreR26RtdTomato/tdTomatoApoe−/− mice in our laboratory, in which the expression of IL-17A is detected via R26RtdTomato/tdTomato expression. While IL-17AtdTomato+ CD4+ T cells efficiently migrate to IL-17C-producing Il17c+/+Apoe−/− aortic SMC supernatants, Th17 cell migration to Il17c−/−Apoe−/− aortic smooth muscle cell supernatants was significantly reduced (Fig.5B). Thus IL-17C does play a role in supporting the recruitment of Th17 cells in chemotaxis assays. To further examine the role of IL-17C in the regulation of Th17 cell homing vivo, we adoptively transferred fluorescently labeled Apoe−/− splenocytes to 12 week WD-fed Apoe−/− and Il17c−/− Apoe−/− mice for 72 hours, and tracked the migration of Apoe−/− splenic CD4+ and Th17 cells to recipient spleens (Fig.5C–D), peri-aortic lymph nodes (data not shown), and aortas (Fig.5E–F). An equivalent percentage and number of transferred CD4+ T cells, and Th17 cells migrated to the recipient Apoe−/− and Il17c−/−Apoe−/− spleens (Fig.5C–D) and PALNs (data not shown). In contrast, a significantly lower percentage and number of transferred CD4+ T cells, and Th17 cells successfully accumulated in the aortas of 12 week western diet Il17c−/−Apoe−/− mice versus diet matched Apoe−/− recipients (Fig.5E–F). These data demonstrate that IL-17C had no effect on the peripheral maintenance or accumulation of Th17 cells but did affect the accumulation of Th17 cells within the atherosclerotic aortic wall. Therefore, these data demonstrate that IL-17C plays a pro-atherogenic role by supporting the accumulation of pro-atherogenic Th17 cells within the aorta, which can in turn affect the recruitment of monocytes and neutrophils to the plaque.4
Figure 5. IL-17C supports aortic chemokine expression and supports the recruitment of IL-17A+ Th17 cells to the aorta.
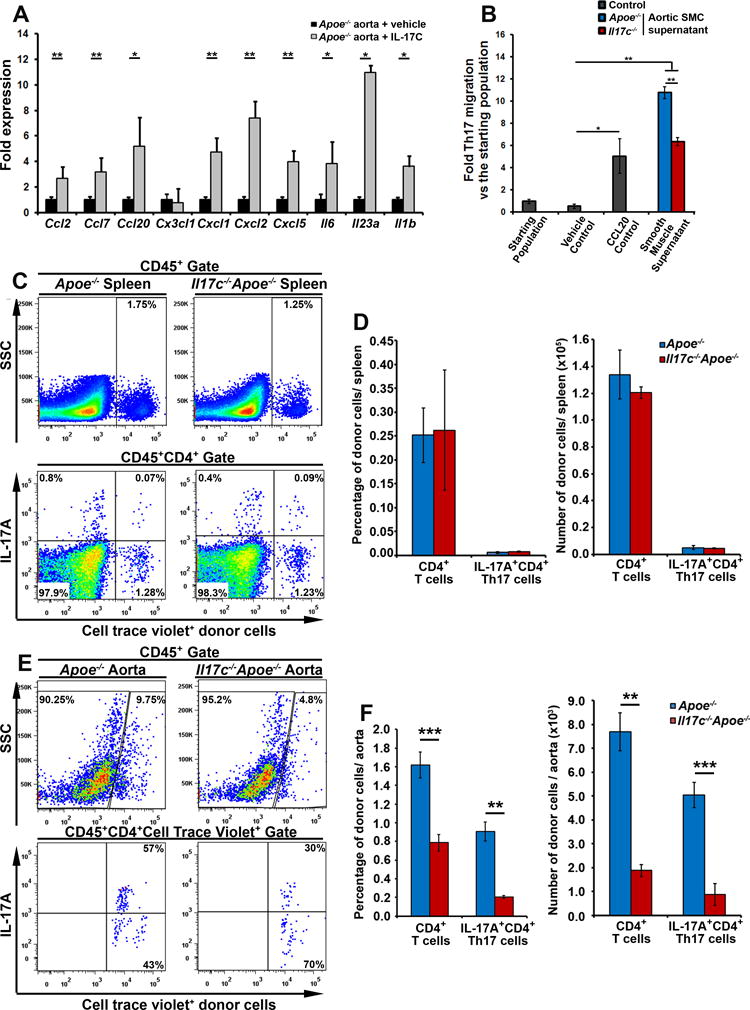
A) 12 week WD Apoe−/− aortas were sterilely explanted and untreated (black bars) or cultured with 100 ng/ml IL-17C (grey bars) for 24 hours before being collected and processed for RT-PCR. Fold induction of Ccl2, Ccl7, Cxc3cl1, Cxcl1, Cxcl2, Cxcl5, Ccl20, Il6, Il23a, and Il1b expression. n=9 Apoe−/− mice, 3 independent experiments. (B) 12 week WD-fed Apoe−/− and Il17c−/−Apoe−/− aortic smooth muscle cells (CD45−CD31−CD29− aortic cells) were sterilely FACS sorted and cultured for an hour in vitro to collect cell supernatants. CD4+ T cells were isolated in parallel from 40 week CD Il17aicre/icre R26RtdTomato/tdTomato Apoe−/− mice. Il17aicre/icreR26RtdTomato/tdTomatoApoe−/− CD4+ T cells migrated towards either a migration media (vehicle control), or 1000 ng/ml rCCL20, or Apoe−/− or Il17c−/−Apoe−/− aortic smooth muscle cell supernatants for 2 hrs. The transmigrated cells were collected and assessed for IL-17AtdTomato+ Th17 cells by flow cytometry and normalized to the percentage of Th17 cells in the starting population. n= 5 independent experiments, all assays were performed in triplicate. (C–F) 30×106 12 week WD Apoe−/− splenocytes were labeled with cell trace violet (CTV) and adoptively transferred to 12 week WD Apoe−/− and Il17c−/−Apoe−/− mice for 72 hours. The migration of CTV+CD4+ T cells, and CTV+ Th17 cells within the recipients was assessed by flow cytometry. Representative flow cytometry plots for CTV+ splenocytes, and CD4+ Th17 cell migration to recipient spleens (C) and aortas (E) are shown. Quantification of the percentage and number of donor CD4+ T cells and Th17 cells within the spleens (D) and aortas (F) of recipient 12 week WD Apoe−/− (blue) and Il17c−/−Apoe−/− (red) recipients. n= 5 recipients/genotype, 4 independent experiments. Bars depict means±SEM. * - p<0.05, ** - p<0.01, *** - p<0.001.
Discussion
Regular communication through cytokine and chemokine networks between arterial hematopoietic and vascular cells plays a critical role in atherosclerosis. Various aortic infiltrating cell populations have been described to produce cytokines and chemokines in atherogenesis and many groups have studied the effects of manipulating or knocking out cytokines in atherosclerosis, as reviewed elsewhere.27 Recent efforts to examine the role of IL-17A-secreting Th17 cells have been a subject of debate,3–6, 8 although Th17 cells are clearly present in both murine and human atherosclerotic plaques.3,18,20–24 However the IL-17 cytokine family consists of five additional family members (IL-17B-IL-17F), and recent work has demonstrated that IL-17 cytokines may also participate in autoimmune and inflammatory pathologies. Thus we sought to examine whether other IL-17 cytokines, in addition to IL-17A, might participate in atherogenesis.
We observed here that several IL-17 cytokines are present within atherosclerotic Apoe−/− aortas, including Il17a, Il17c, Il17e, and Il17f. However, Il17c was surprisingly the most abundant IL-17 cytokine within Apoe−/− atherosclerotic aortas, and that a significant portion of aortic vascular cells produce IL-17C, ranging from 2–12% of the entire aorta. To determine which vascular cells might express IL-17C we developed a gating scheme to look at different vascular cell subsets and IL-17C production by those subsets via flow cytometry. These experiments revealed that a major population of aortic SMCs produces IL-17C, ex vivo. To further confirm these results, we utilized RT-PCR analysis as a complimentary method to assess the primary sources of IL-17C. Using highly purified populations of CD45+ hematopoietic and sorted subsets of CD45− non-hematopoietic cells we demonstrated robust expression of Il17c in SMCs, but not in CD45+ cells. These results are noteworthy for several reasons. To date, the identification of the cellular sources of IL-17C in vivo, and the examination of cytokine production within SMCs has been technically limited to western blot, histological, or PCR-based assays. We report here that with a careful gating strategy, aortic SMCs can be examined for cytokine production ex vivo and that IL-17C can be detected by conventional PMA, Ionophore-based cytokine flow cytometry techniques. Additionally, the data presented here represent the first report to demonstrate that aortic SMCs are an important source of IL-17C in vivo. SMCs play critical roles in atherosclerosis, not only in stabilizing atherosclerotic plaques through collagen deposition and the formation of fibrous caps, but also in promoting inflammation by producing pro-inflammatory cytokines.32 Evidence demonstrate that SMCs may assume a pro-inflammatory phenotype in sterile inflammation and participate in atherosclerosis. SMCs may produce several cytokines and chemokines in atherosclerosis, including pro-inflammatory IL-8, CCL2, CXCL1, IL-1β, IL-6, atheroprotective IL-19, and now, IL-17C as well. While the specific mechanisms of IL-17C induction within SMCs remain to be determined, prior work on IL-17C and SMC biology suggest that several pathways might be involved. SMCs respond to oxLDL via the lectin-like oxidized low density lipoprotein scavenger receptor (LOX-1) in culture by producing MCP-1, CXCL1, TNFα, and VCAM-1,32–34 likely in an NFκB, NFAT, p38 and JNK-dependent manner;32 suggesting that oxLDL or oxidized lipids might promote IL-17C expression within atherosclerosis as well. Additionally, as PMA and Ionomycin C are known to activate T cells in a PKC- NFκB, NFAT, MAPK-dependent manner, PMA and Ionomycin C-stimulation may induce IL-17C expression in aortic SMCs in a PKC-dependent fashion. In support of this notion, IL-17C is quickly induced within the gut in response to TLR 2 and TLR 5 in an NFκB-dependent fashion.13 Ramirez-Carrozzi et.al.,13 focused exclusively on the role of IL-17C in mucosal immunity, however similar mechanisms may be at work in sterile atherogenic inflammation. SMCs express TLR proteins and TLR2, TLR3, CD36, and TLR4 have been demonstrated to participate in both sterile35,36 and infectious atherogenesis.28,37 While the experiments here were conducted in sterilely, there are clear links between P. Gingivalis and C. Pneumoniae infection, atherosclerosis, and aortic IL-17A+ T cell accumulation. Thus the potential induction of aortic IL-17C via TLR signaling within SMCs during sterile or infectious atherogenesis may be an important link between the vasculature and the adaptive immune response.
To examine the role of IL-17C in atherosclerosis, we generated Il17c−/−Apoe−/− mice, which exhibited a ~35% reduction in atherosclerotic lesions in comparison to diet matched 12 week western diet Apoe−/− controls. These results are in line with recent studies on IL-17C-producing cells in the gut and skin, and mechanistic studies in autoimmune pathologies provided insight on the potential mechanisms of action for IL-17C in atherosclerosis.11–15 In the context of colitis and psoriasis, epithelial cell and keratinocyte-derived IL-17C induced an array of chemokines that are also IL-17A-responsive, such as CXCL1, CXCL2, and CCL20, and supported the local accumulation of F4/80+ macrophages.13,14 These studies provided insight on the potential functions of aortic IL-17C in atherosclerosis. While IL-17C clearly displayed pro-atherogenic role, IL-17C also supported the synthesis of collagen by SMCs and fibroblasts highlighting an important aspect of IL-17C biology. IL-17C signals through a heteromeric receptor consisting of the IL-17RE subunit and the common IL-17RA subunit,11,12 via the MAPK and NFκB pathways11 in an Act1-dependent fashion.13 Interestingly, the IL-17C/IL-17RE-IL-17RA and IL-17A/IL-17RC-IL-17RA signaling pathways share several similarities, including the common co-receptor IL-17RA, the adaptor protein Act1, MAPK and NFκB signaling intermediates, and a set of target transcripts, including IL-1β, IL-6, IL-8, CXCL1, CXCL2, CCL2, and CCL20, suggesting that IL-17C and IL-17A may exert similar actions on myeloid cell and CCL20-dependent Th17 recruitment, in vivo. In atherosclerosis, IL-17RA, IL-17RE, and IL-17RC are all expressed, suggesting that IL-17 ligand regulation plays a major role in controlling IL-17 signaling. This notion is in line with prior data which demonstrated that the severity of atherosclerotic plaques corresponded with the presence of IL-17A+ T cells in the aortic arches of Apoe−/− mice.4 Mechanistic studies in murine models of atherosclerosis have yielded at least two unifying hypotheses, that IL-17A plays a pro-atherogenic role by affecting aortic chemokine and cytokine production and myeloid cell recruitment3,4,6,8,18,20,22,25 or an atheroprotective role, via regulation of aortic Th1 content, SMC content, and collagen deposition.5,21,26 More recent work has further proposed that IL-17A might directly affect activation of vasculature38 and plaque macrophage functions as well39,40. As Il17c−/−Apoe−/− mice demonstrated an athero-protective phenotype, with reduced IL-17A+ T cell content and Th17 recruitment to the aorta, and a decrease in total lesional collagen, our findings here further support a pro-atherogenic role of Th17 cells.
The recruitment of myeloid cells to nascent plaques plays a critical role in the initiation and progression of atherosclerotic plaques, and IL-17A promotes the accumulation of monocytes and neutrophils within atherosclerotic lesions3,4,6,8,18,20,22,25. Thus we sought to determine whether a global deficiency of IL-17C might affect the aortic or circulating myeloid cell immune composition. These experiments revealed a proportional 40–50% decrease in aortic myeloid cells, including macrophages and neutrophils highlighting a strong correlation between atherosclerotic plaque lesion size and macrophage and neutrophil content within the aortic wall. As IL-17C helps maintain mucosal immunity and is abundant within the inflamed aorta and skin,14 it was important to examine whether IL-17C might affect the peripheral immune system. However the peripheral immune composition was unchanged, suggesting that the local actions of SMC-derived arterial IL-17C were responsible for the protective phenotype of Il17c−/−Apoe−/− mice. As specific Il17cflox/flox mice are not currently available, we used global IL-17C-deficient Apoe−/− mice in this study. Further studies utilizing a SMC-specific IL-17C-deficient mouse model might reveal additional effects of SMC-derived IL-17C on atherosclerosis.
IL-17C has been reported to play a key role in promoting IL-17A production within IL-17RE-expressing Th17 and IL-17A+ TCRγδ+ T cells and key roles in the pathology of experimental autoimmune encephalitis11 and psoriasis.14 We detected here an overall reduction in the total number of aortic infiltrating CD3+ and CD3+TCRαβ+ T cells, Th1, and Treg cells within WD Il17c−/−Apoe−/− mice. However, when we examined Th17 and IL-17+TCRαβ− T cells, we observed a disproportionate 70–80% decrease in the percentage and number of cells within Il17c−/−Apoe−/− aortas versus Apoe−/− controls. Several mechanisms could be responsible for a diminished aortic IL-17A+ T cell response, ranging from defective migration, to altered differentiation, proliferation, or survival. We reasoned that if changes in Th17 differentiation, proliferation, or survival were responsible for diminished aortic Th17 content, Il17c−/−Apoe−/− mice would display similar defects peripherally. As peripheral T cell subsets were unaltered between Il17c−/−Apoe−/− and Apoe−/− mice and IL-17C broadly supported pro-Th17 chemokine/cytokine expression in ex vivo experiments, we hypothesized that aortic IL-17C might support Th17 cell recruitment during atherogenesis. In our adoptive transfer experiments, donor CD4+IL-17A+ Th17 cells did not migrate as well to the aortas of Il17c−/−Apoe−/− vs Apoe−/− recipients. Interestingly, equivalent amounts of donor CD4+IFNγ+ Th1 cells accumulated in Il17c−/−Apoe−/− and Apoe−/− recipient aortas (data not shown).
Thus, these results demonstrate that the reduction of aortic Th17 cells in Il17c−/−Apoe−/− mice are due to the inefficient recruitment of Th17 cells, rather than a defect in T cell recruitment or peripheral Th17 differentiation or accumulation. Together these results demonstrate that in sterile conditions, IL-17C is produced and acts locally on the aortic wall to promote pro-inflammatory chemokines and cytokines, and the efficient recruitment of pro-inflammatory monocytes, neutrophils, and Th17 cells. These observations are important as little is known about the recruitment of Th17 cells in atherosclerosis. Th17 cells are known to express CCR6 and CCR4, however as Th17 recruitment was unchanged within Ccr6−/−Ldlr−/− mice, CCR6 may not be necessary for the accumulation of aortic Th17 cells.41 Thus, IL-17C is likely initially produced within the arterial wall and serves to promote the accumulation of Th17 and IL-17A+TCRγδ+ T cells. Once Th17 cells migrate to the aortic wall, IL-17A may support the production of pro-inflammatory chemokines, and monocyte and neutrophil recruitment to nascent atherosclerotic plaques; a process that is critical for the initiation and progression of atherosclerotic plaques.3, 4
Altogether, our data demonstrates a novel pro-atherogenic role for vascular IL-17C and highlight a dynamic between arterial IL-17C and Th17 and IL-17A+ TCRγδ+ T cells. These data suggest a model in which aortic IL-17C might act in an autocrine/paracrine manner on nearby vascular cells to support the recruitment of IL-17A+ T cells and suggest that in unison, both IL-17A and IL-17C help to recruit additional myeloid cells, thereby affecting the cellularity of atherosclerotic lesions.
Supplementary Material
Highlights.
IL-17C expression is elevated in atherosclerotic aortas
Aortic smooth muscle cells are the major producers of IL-17C in the aortic wall
Il17c−/−Apoe−/− western diet fed mice develop smaller lesions in comparison with Apoe−/− mice.
IL-17c induces the expression of various chemokines within the aorta and support Th17 and TCRγδ+ T cells recruitment to the aortic wall
IL-17A and IL-17C help to recruit additional myeloid cells, thereby affecting the cellularity of atherosclerotic lesions
Acknowledgments
We thank Amgen Inc. for providing us with Il17c−/− breeding pairs. We also thank Mr. Raaj Talauliker, Mr. Chris McGary, and Ms. Chih Wu for their technical assistance.
Sources of Funding. This work was supported by the NHLBI RO1HL107522 (to E. Galkina) and the AHA pre-doctoral fellowship 11PRE7520041 (to M.Butcher).
Non-standard Abbreviations and Acronyms
- Apoe
Apolipoprotein E
- IL-17A
Interleukin-17A
- IL-17C
Interleukin-17C
- IL-17RA
Interleukin-17 Receptor A
- IL-17RE
Interleukin 17 Receptor E
- Ldlr
Low-density lipoprotein receptor
- WD
Western Diet
- MΦ
Macrophages
- PLN
Peripheral Lymph Node
- PALN
Peri-aortic Lymph Node
- SMC
Smooth muscle cells
- Th
T helper
- TCR
T cell receptor
- CD
Cluster of Differentiation
- FACS
Fluorescence activated cell sorting
Footnotes
Disclosures. None
Reference List
- 1.Mendis S, Puska P, Norrving B, et al. Global atlas on cardiovascular disease prevention and control; Published by the World Health Organization in collaboration with the World Heart Federation and the World Stroke Organization. Geneva: World Health Organization; 2009. [Google Scholar]
- 2.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38(6):1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butcher M, Galkina E. Current views on the functions of Interleukin-17A-producing cells in atherosclerosis. Thromb Haemost. 2011;106(5):787–795. doi: 10.1160/TH11-05-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butcher MJ, Gjurich BN, Phillips T, Galkina EV. The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ Res. 2012;110(5):675–687. doi: 10.1161/CIRCRESAHA.111.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danzaki K, Matsui Y, Ikesue M, Ohta D, Ito K, Kanayama M, Kurotaki D, Morimoto J, Iwakura Y, Yagita H, Tsutsui H, Uede T. Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32(2):273–280. doi: 10.1161/ATVBAHA.111.229997. [DOI] [PubMed] [Google Scholar]
- 6.Madhur MS, Funt SA, Vinh A, Chen W, Lob HE, Iwakura Y, Blinder Y, Rahman A, Quyyumi AA, Harrison DG. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31(7):1565–1572. doi: 10.1161/ATVBAHA.111.227629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taleb S, Tedgui A, Mallat Z. Interleukin-17: friend or foe in atherosclerosis? Curr Opin Lipidol. 2010;21(5):404–408. doi: 10.1097/MOL.0b013e32833dc7f9. [DOI] [PubMed] [Google Scholar]
- 8.Usui F, Kimura H, Ohshiro T, Tatsumi K, Kawashima A, Nishiyama A, Iwakura Y, Ishibashi S, Takahashi M. Interleukin-17 deficiency reduced vascular inflammation and development of atherosclerosis in Western diet-induced apoE-deficient mice. Biochem Biophys Res Commun. 2012;420(1):72–77. doi: 10.1016/j.bbrc.2012.02.117. [DOI] [PubMed] [Google Scholar]
- 9.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9(8):556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pappu R, Ramirez-Carrozzi V, Sambandam A. The interleukin-17 cytokine family: critical players in host-defence and inflammatory diseases. Immunology. 2011;134(1):8–16. doi: 10.1111/j.1365-2567.2011.03465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang SH, Reynolds JM, Pappu BP, Chen G, Martinez GJ, Dong C. Interleukin-17C promotes Th17 cell responses and autoimmune disease via interleukin-17 receptor E. Immunity. 2011;35(4):611–621. doi: 10.1016/j.immuni.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song X, Zhu S, Shi P, Liu Y, Levin SD, Qian Y. IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nat Immunol. 2011;12(12):1151–1158. doi: 10.1038/ni.2155. [DOI] [PubMed] [Google Scholar]
- 13.Ramirez-Carrozzi V, Sambandam A, Luis E, et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol. 2011;12(12):1159–1166. doi: 10.1038/ni.2156. [DOI] [PubMed] [Google Scholar]
- 14.Johnston A, Fritz Y, Dawes SM, Diaconu D, Al-Attar PM, Guzman AM, Chen CS, Fu W, Gudjonsson JE, McCormick TS, Ward NL. Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation. J Immunol. 2013;190(5):2252–2262. doi: 10.4049/jimmunol.1201505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kusagaya H, Fujisawa T, Yamanaka K, Mori K, Hashimoto D, Enomoto N, Inui N, Nakamura Y, Wu R, Suda T, Chida K. Toll-like receptor-mediated airway IL-17C enhances epithelial host defense in an autocrine/paracrine manner. Am J Respire Cell Mol Biol. 2014;50(1):30–39. doi: 10.1165/rcmb.2013-0130OC. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds JM, Martinez GJ, Nallaparaju KC, Chang SH, Wang YH, Dong C. Cutting edge: regulation of intestinal inflammaiton and barrier function by IL-17C. J Immunol. 2012;189(9):4226–4230. doi: 10.4049/jimmunol.1103014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin DA, Towne JE, Kricorian G, Klekotka P, Gudjonsson JE, Krueger JG, Russell CB. The Emerging Role of IL-17 in the Pathogenesis of Psoriasis: Preclinical and Clinical Findings. J Invest Dermatol. 2012;133:17–26. doi: 10.1038/jid.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121(15):1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reynolds JM, Angkasekwinai P, Dong C. IL-17 family member cytokines: regulation and function in innate immunity. Cytokine Growth Factor Rev. 2010;21(6):413–423. doi: 10.1016/j.cytogfr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Bockler D, Katus HA, Dengler TJ. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol. 2009;183(12):8167–8175. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 21.Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, Herbin O, Esposito B, Perez N, Yasukawa H, Van SJ, Yoshimura A, Tedgui A, Mallat Z. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009;206(10):2067–2077. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Es T, van Puijvelde GH, Ramos OH, Segers FM, Joosten LA, van den Berg WB, Michon IM, de V P, van Berkel TJ, Kuiper J. Attenuated atherosclerosis upon IL-17R signaling disruption in LDLr deficient mice. Biochem Biophys Res Commun. 2009;388(2):261–265. doi: 10.1016/j.bbrc.2009.07.152. [DOI] [PubMed] [Google Scholar]
- 23.Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119(10):1424–1432. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erbel C, Dengler TJ, Wangler S, Lasitschka F, Bea F, Wambsganss N, Hakimi M, Bockler D, Katus HA, Gleissner CA. Expression of IL-17A in human atherosclerotic lesions is associated with increased inflammation and plaque vulnerability. Basic Res Cardiol. 2011;106(1):125–134. doi: 10.1007/s00395-010-0135-y. [DOI] [PubMed] [Google Scholar]
- 25.Gao Q, Jiang Y, Ma T, Zhu F, Gao F, Zhang P, Guo C, Wang Q, Wang X, Ma C, Zhang Y, Chen W, Zhang L. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol. 2010;185(10):5820–5827. doi: 10.4049/jimmunol.1000116. [DOI] [PubMed] [Google Scholar]
- 26.Gistera A, Robertson AK, Andersson J, Ketelhuth DF, Ovchinnikova O, Nilsson SK, Lundberg AM, Li MO, Flavell RA, Hansson GK. Transforming growth factor-b signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci Transl Med. 2013;5(196):196ra100. doi: 10.1126/scitranslmed.3006133. [DOI] [PubMed] [Google Scholar]
- 27.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai Y, Kobayashi R, Hashizume-Takizawa T, Kurita-Ochiai T. Porphyromonas gingivalis infection enhances Th17 responses for development of atherosclerosis. Arch Oral Biol. 2014;59(11):1183–1191. doi: 10.1016/j.archoralbio.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Koltsova EK, Kim G, Lloyd KM, Saris CJ, von Vietinghoff S, Kronenberg M, Ley K. Interleukin-27 receptor limits atherosclerosis in Ldlr−/− mice. Circ Res. 2012;111(10):1274–1285. doi: 10.1161/CIRCRESAHA.112.277525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Vietinghoff S, Koltsova EK, Mestas J, Diehl CJ, Witztum JL, Ley K. Mycophenolate mofetil decreases atherosclerotic lesion size by depression of aortic T-lymphocyte and interleukin-17-mediated macrophage accumulation. J Am Coll Cardiol. 2011;57(21):2194–2204. doi: 10.1016/j.jacc.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Chen J, Liu X, Awar L, McMickle A, Bai F, Nagarajan S, Yu S. IL-17 induces expression of vascular cell adhesion molecule through signalling pathway of NF-kB, but not Akt1 and TAK1 in vascular smooth muscle cells. Scand J Immunol. 2013;77(4):230–237. doi: 10.1111/sji.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orr AW, Hastings NE, Blackman BR, Wamhoff BR. Complex regulation and function of the inflammatory smooth muscle cell phenotype in atherosclerosis. J Vasc Res. 2009;47(2):168–180. doi: 10.1159/000250095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barlic J, Zhang Y, Murphy PM. Atherogenic lipids induce adhesion of human coronary artery smooth muscle cells to macrophages by up-regulating chemokine CX3CL1 on smooth muscle cells in a TNFalpha-NFkappaB-dependent manner. J Biol Chem. 2007;282(26):19167–19176. doi: 10.1074/jbc.M701642200. [DOI] [PubMed] [Google Scholar]
- 34.Hofnagel O, Luechtenborg B, Stolle K, Lorkowski S, Eschert H, Pienz G, Robenek H. Proinflammatory cytokines regulate LOX-1 expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24(10):1789–1795. doi: 10.1161/01.ATV.0000140061.89096.2b. [DOI] [PubMed] [Google Scholar]
- 35.Ishibashi M, Sayers S, D’Armiento JM, Tall AR, Welch CL. TLR3 deficiency protects against collagen degradation and medial destruction in murine atherosclerotic plaques. Atherosclerosis. 2013;229(1):52–61. doi: 10.1016/j.atherosclerosis.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee GL, Chang YW, Wu JY, Wu ML, Wu KK, Yet SF, Kuo CC. TLR 2 induces vascular smooth muscle cell migration through cAMP response element-binding protein-mediated interleukin-6 production. Arterioscler Thromb Vasc Biol. 2012;32(11):2751–2760. doi: 10.1161/ATVBAHA.112.300302. [DOI] [PubMed] [Google Scholar]
- 37.Chen S, Shimada K, Zhang W, Huang G, Crother TR, Arditi M. IL-17A is proatherogenic in high-fat diet-induced and Chlamydia pneumoniae infection-accelerated atherosclerosis in mice. J Immunol. 2010;185(9):5619–5627. doi: 10.4049/jimmunol.1001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffin GK, Newton G, Tarrio ML, Bu DX, Maganto-Garcia E, Azcutia V, Alcaide P, Grabie N, Luscinskas FW, Croce KJ, Lichtman AH. IL-17 and TNF-a sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol. 2012;188(12):6287–6299. doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erbel C, Akhavanpoor M, Okuyucu D, Wangler S, Dietz A, Zhao L, Stellos K, Little KM, Lasitschka F, Doesch A, Hakimi M, Dengler TJ, Giese T, Blessing E, Katus HA, Gleissner CA. IL-17A influences essential funcitons of the monocyte/macrophage lineage and is involved in advanced murine and human atherosclerosis. J Immunol. 2014;193(9):4344–4355. doi: 10.4049/jimmunol.1400181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kotla S, Singh NK, Heckle MR, Tigyi GJ, Rao GN. The transcription factor CREB enhances interleukin-17A production and inflammation in a mouse model of atherosclerosis. Sci Signal. 2013;6(293):ra83. doi: 10.1126/scisignal.2004214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manthey HD, Cochain C, Barnsteiner S, Karshovska E, Pelisek J, Koch M, Chaudhari SM, Busch M, Eckstein HH, Weber C, Koenen RR, Zernecke A. CCR6 selectively promotes monocyte mediated inflammation and atherogenesis in mice. Thromb Haemost. 2013;110(6):1267–1277. doi: 10.1160/TH13-01-0017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.