Abstract
We report a theranostic nanoparticle that can express ultrasound (US) imaging and simultaneous therapeutic functions for cancer treatment. We developed doxorubicin-loaded calcium carbonate (CaCO3) hybrid nanoparticles (DOX-CaCO3-MNPs) through a block copolymer templated in situ mineralization approach. The nanoparticles exhibited strong echogenic signals at tumoral acid pH by producing carbon dioxide (CO2) bubbles and showed excellent echo persistence. In vivo results demonstrated that the DOX-CaCO3-MNPs generated CO2 bubbles at tumor tissues sufficient for echogenic reflectivity under a US field. In contrast, the DOX-CaCO3-MNPs located in the liver or tumor-free subcutaneous area did not generate the CO2 bubbles necessary for US contrast. The DOX-CaCO3-MNPs could also trigger the DOX release simultaneously with CO2 bubble generation at the acidic tumoral environment. The DOX-CaCO3-MNPs displayed effective antitumor therapeutic activity in tumor-bearing mice. The concept described in this work may serve as a useful guide for development of various theranostic nanoparticles for US imaging and therapy of various cancers.
Keywords: theranostics, carbon dioxide, mineralization, nanobubble, ultrasound, drug delivery
Graphical abstract
Ultrasound (US) is a noninvasive real-time imaging modality that has many advantages, such as safety, low cost, and easy access to the public.1–3 Currently, gas-filled echogenic microbubbles are used as a US contrast agent to improve the US image quality.4–9 They are primarily used for imaging of various organs and blood vessels but are not equipped with functionalities for imaging at tumor tissues. Recently, microbubbles with surface-conjugated ligands for specific molecular markers were developed as targeted contrast agents for tracking biological processes of tumor angiogenesis.10,11 This targeted US imaging, based on the microbubbles, may play a significant role that can broaden the application boundary of a US imaging to tumor diagnosis. However, due to their large size (ca. 1–8 µm), the microbubbles are predominantly located in vascular systems after i.v. administration and do not permeate into host tumoral environments by tissue extravasation.1,12 For this reason, the application of current micron-size bubbles is limited to the imaging of intravascular processes.10–14 Moreover, the gas-filled microbubbles suffer from inherent drawbacks, such as low stability and short half-life in blood due to rapid diffusion of gas species through defects of the microbubbles.15,16
Recently, we proposed a “gas-generating” polymeric nanoparticle system that could generate carbon dioxide (CO2) bubbles by hydrolysis of carbonate side chains and showed its potential as a US contrast agent.17 This gas-generating solid nanoparticle may overcome the limitations of current gas-filled microbubbles because gas-generating nanoparticles may circulate stably in the bloodstream and can effectively accumulate in tumor tissues by the enhanced permeation and retention effect. In addition, the gas-generating nanoparticles continuously generate CO2 nanobubbles that coalesce into microbubbles, allowing for the persistence of strong echogenic US imaging. Although polycarbonate-based gas-generating nanoparticles expressed several advantages, they have a weakness in effective tumor imaging in that they did not have gas-generating properties in response to tumoral environments.
In recent years, theranostic nanoparticles, which integrate imaging and therapeutic functionalities, have played a central role in the improvement of tumor treatment.18–23 Diverse theranostic nanoparticles for tumor imaging have been a major research target for various imaging tools, such as positron emission tomography, magnetic resonance, computed tomography, and optical imaging.24–27 However, the development of a theranostic nanoparticle that expresses US imaging and therapeutic functions for tumor treatment is an unexplored field. Therefore, it is challenging to develop theranostic nanoparticles that not only generate gas bubbles for tumoral US imaging but also simultaneously provide therapeutic activity for tumor treatment.
Herein, we aim to develop a gas-generating calcium carbonate (CaCO3) mineralized nanoparticle (CaCO3-MNP) that can generate CO2 bubbles and trigger the release of anticancer drugs at tumoral acidic pH for US imaging and simultaneous therapy of tumors. The extracellular pH of tumor tissues (6.8–7.2) is lower than that of normal tissues (pH 7.4) because of up-regulated glycolysis, which produces lactates and protons in an extracellular environment.28,29 We chose the lower pH of tumor tissues as a target for tumoral US imaging and therapeutic strategies.
RESULTS AND DISCUSSION
Block Copolymer Mediated Mineralization for Doxorubicin-Loaded CaCO3 Nanoparticles
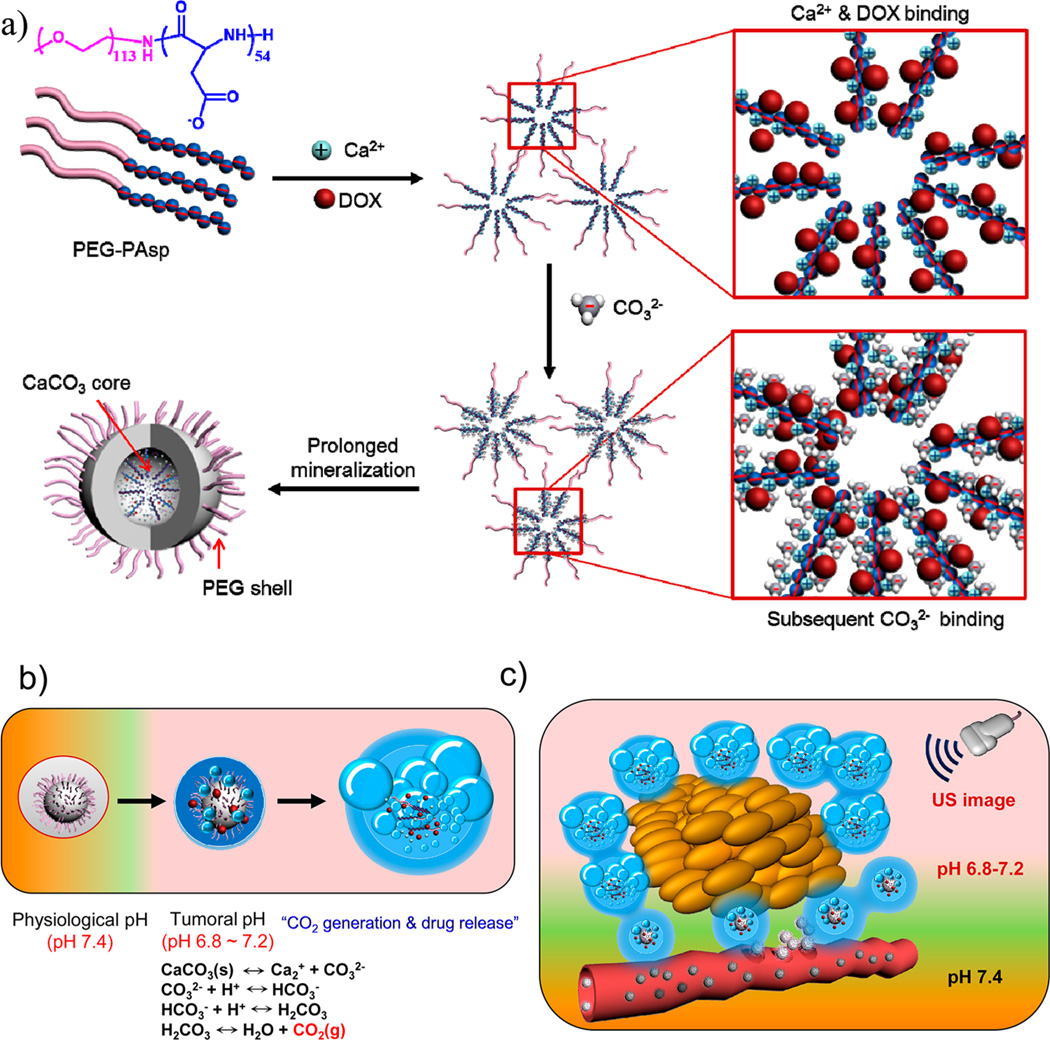
Our recent research has been focused on biomimetic mineralization of various templates, such as assembly of nanoparticles and mesoporous nanoparticles, to generate pH-controlled drug-releasing nanocarriers for efficient tumor therapy.30–32 In this work, our key strategy is to prepare doxorubicin (DOX)-loaded mineralized nanoparticles (DOX-CaCO3-MNPs) by block copolymer templated CaCO3 mineralization in the presence of calcium cations (Ca2+), carbonate anions (CO32−), and positively charged DOX. Figure 1 shows the overall process for fabrication of the DOX-CaCO3-MNPs and how the mineralized nanoparticle enables US imaging at tumoral environments (pH 6.8–7.2) and facilitates DOX release at tumor tissues. As shown in Figure 1b, the inorganic mineral CaCO3 is known to react with protons and dissolve to generate CO2 gas in an acid environment.33 For preparation of mineralized nanoparticles, the block copolymer (poly(ethylene glycol)-b-poly(l-aspartic acid) (PEG-PAsp)) was added during CaCO3 mineralization (Figure 1a). The anionic PAsp block of PEG-PAsp can act as a mineral-nucleating and growth template by participating in the formation of the CaCO3 core. The PEG block forms a hydrated shell and may contribute to colloidal stability in the aqueous phase. In brief, an aqueous Ca2+ solution was added to a stirred solution of PEG-PAsp to enable electrostatic interaction of Ca2+ with anionic aspartate moieties of PEG-PAsp, and DOX, which is positively charged, was subsequently added to the solution mixture. In the second step, an aqueous solution of CO32− was added dropwise to the solution mixture to induce formation of double ionic layers around the PAsp moieties. This induced ionic supersaturation triggered the growth of CaCO3 minerals. The prolonged mineralization induced coalescence of mineralized species for the DOX-CaCO3-MNPs. During PEG-PAsp templated mineralization, DOX could be readily loaded in situ within the CaCO3 core through ionic interaction. To remove residual ionic species and DOX, the solution mixture was dialyzed against excess water (pH 8.0). To prevent uncontrolled rapid growth of CaCO3 in the aqueous phase and selectively induce mineral growth around the acidic PAsp blocks, the stoichiometric molar ratio of [Asp]/[Ca2+]/[CO32−] was fixed at 1:1:1. It was found that a higher or lower feed molar ratio of [Asp] to [Ca2+]/[CO32−], such as 1:2:2 or 1:0.5:0.5, resulted in either the formation of large, irregular aggregates or the ineffective formation of mineralized particles (Figure S1a in the Supporting Information).
Figure 1.
Schematic illustration of (a) fabrication process of DOX-CaCO3-MNPs, (b) mechanism of CO2 generation and drug release, and (c) bubble generation and drug release after accumulation of DOX-CaCO3-MNPs at tumor tissues.
Using the optimal stoichiometric feed ratio ([Asp]/[Ca2+]/[CO32−] = 1:1:1), we obtained uniform and spherical DOX-loaded nanoparticles, as analyzed by transmission electron microscopy (TEM) (Figure 2a). The particle size and morphology of the DOX-CaCO3-MNPs were dependent on mineralization time (Figure S1b and Table S1 in the Supporting Information).
Figure 2.
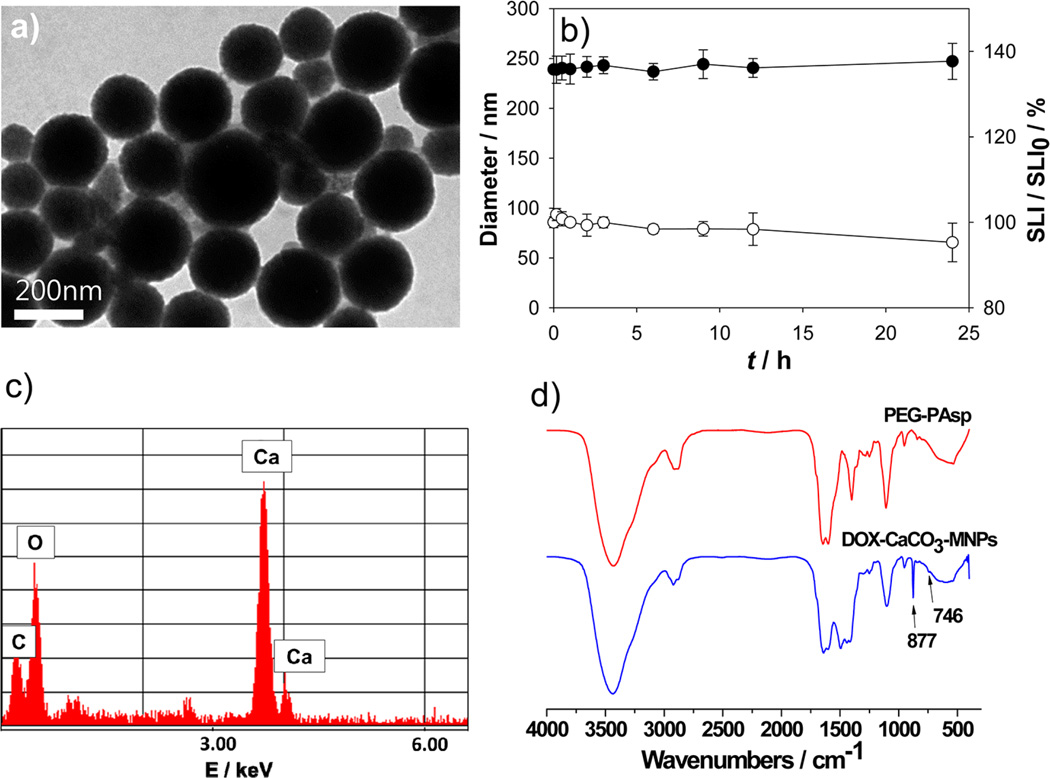
(a) TEM image of DOX-CaCO3-MNPs after mineralization for 12 h. (b) Time-dependent changes of the mean diameter (●) and the ratio of scattered light intensities (○) of DOX-CaCO3-MNPs in the serum-containing PBS solution at 37 °C (n = 3). (c) TEM-associated EDX spectra. (d) FT-IR spectra of PEG-PAsp and DOX-CaCO3-MNPs. SLI refers to scattering light intensity.
As mineralization time increased from 0.5 to 12 h, the mean hydrodynamic diameter of the DOX-CaCO3-MNPs increased from 145.2 ± 55.6 to 240.8 ± 8.1 nm. In addition, morphology of the DOX-CaCO3-MNPs was also affected by mineralization time. At 0.5 h, the DOX-CaCO3-MNPs adopted an irregular shape, most likely due to immature mineral growth and coalescence. The TEM image showed broad distribution of particle sizes, ranging from approximately 10 to 200 nm. In contrast, after increasing mineralization time (from 1 to 12 h), the morphology of the DOX-CaCO3-MNPs was found to be a regular spherical shape, which was reflected in narrower polydispersities (Table S1 in the Supporting Information). Using dynamic light scattering, the stability of the DOX-CaCO3-MNPs in a serum solution (50% fetal bovine serum (FBS) in a phosphate buffered saline (PBS) solution) was estimated. The DOX-CaCO3-MNPs exhibited excellent colloidal stability in serum solutions, as judged by the constant mean diameter and scattered light intensities (SLI) for up to 24 h (Figure 2b).
To estimate the effect of PEG-PAsp on formation of the stable DOX-CaCO3-MNPs, the mineralization was performed in the presence or absence of PEG-PAsp. After CaCO3 mineralization, the solution was centrifuged at 1500 rpm for 5 min. During the mineralization process, the solution without PEG-PAsp produced a large amount of precipitates in the bottom because of uncontrolled nucleation and mineral growth in the aqueous phase (Figure S2 in the Supporting Information). On the other hand, the mineralized solution prepared in the presence of PEG-PAsp maintained a homogeneous dispersion even after centrifugation. These results suggest that PEG-PAsp plays a critical role in the mineralization process and formation of the nanosized DOX-CaCO3-MNPs with colloidal stability.
TEM-associated energy-dispersive X-ray spectroscopy (EDX) analysis showed that the DOX-CaCO3-MNPs predominantly contained Ca, C, and O, which belong to CaCO3 (Figure 2c). Fourier transform infrared (FT-IR) analysis showed that DOX-loaded mineralized nanoparticles showed stretching bands at 877 and 746 cm−1, indicating successful formation of CaCO3 minerals in a vaterite form (Figure 2d).34 The X-ray powder diffraction (XRD) pattern also showed that DOX-CaCO3-MNPs exhibited characteristic peaks of hexagonal vaterite polymorphs (Figure S3 in the Supporting Information).
pH-Dependent CO2 Generation from DOX-CaCO3-MNPs
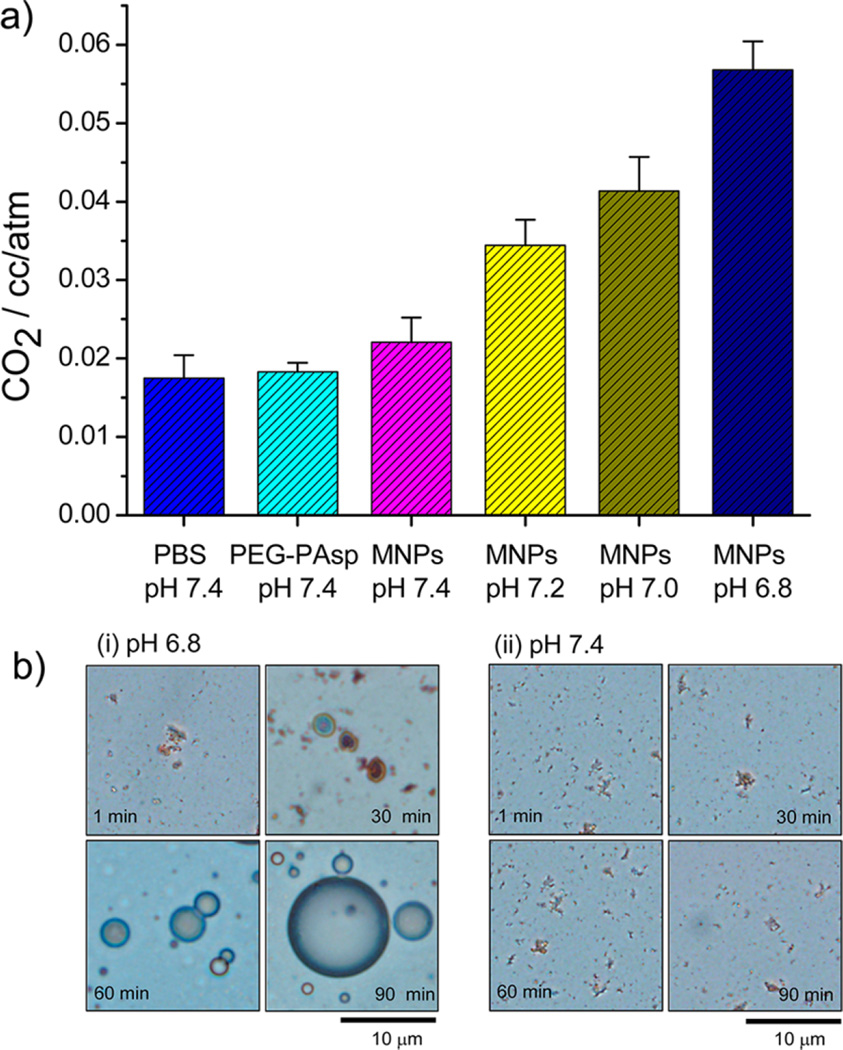
The quantitative analysis of CO2 gas generation was examined using a mass spectrometer. The amount of CO2 was estimated by monitoring the peak at 44 Da.17 As controls, the amount of residual CO2 present in a buffer solution (pH 7.4) and a PEG-PAsp-containing buffer solution (pH 7.4) was measured. As shown in Figure 3a, the amount of CO2 from the PEG-PAsp solution (0.018 cc/atm) was almost the same as the amount of residual CO2 in the buffer solution, reasonably indicating that PEG-PAsp had no effect on CO2 generation. To analyze the dependency of pH on CO2 generation, the amount of generated CO2 from the DOX-CaCO3-MNPs was measured under pH controls (from pH 7.4 to 6.8). Although CO2 was generated from the DOX-CaCO3-MNPs at pH 7.4, the level was as low as 0.022 cc/atm, a negligible amount similar to control groups. In contrast, as the pH level decreased, the amount of CO2 generated from the DOX-CaCO3-MNPs dramatically increased to 0.034, 0.041, and 0.057 cc/atm for pH 7.2, 7.0, and 6.8, respectively. Compared to the amount of CO2 generated at pH 7.4, these CO2 amounts corresponded to an increase of 54.5, 86.4, and 159.0%, respectively. The increase in CO2 generation at lower pH level may be due to faster dissolution of the CaCO3 mineral phase. This pH-dependent CO2 generation from CaCO3 phases can be explained by the inherent solution behavior of CaCO3. According to the chemical equilibrium of CaCO3 in the aqueous phase (Figure 1b), three major carbonate species (CO32−, HCO3−, and H2CO3) exist, and their distribution varies depending on solution pH. It is worth noting that the level of H2CO3 (carbonic acid), the major source for CO2 generation, becomes dramatically dominant below pH 7.0, while it exists at an insignificant level at pH 7.4. This distinguished gas-generating feature of the DOX-CaCO3-MNPs at weakly acidic tumoral pH supports our assumption that CaCO3 mineralized nanoparticles may sense the acidic pH of tumors, causing CO2 bubble generation.
Figure 3.
(a) Amount of CO2 generated from DOX-CaCO3-MNPs at various pH and (b) optical micrographs of CO2-generating profiles of sub-micron-size aggregates of DOX-CaCO3-MNPs incubated in PBS ((i) pH 6.8 and (ii) pH 7.4) for 90 min.
We visualized the generation of CO2 bubbles from the DOX-CaCO3-MNPs using optical microscopy. Because the individual DOX-CaCO3-MNPs are nanosized and cannot be identified using optical microscopy, we used a sub-micron-size aggregate of the DOX-CaCO3-MNPs of approximately 1 µm to visualize the direct bubble formation at tumoral pH. It is interesting to note that, at 30 min postincubation at pH 6.8, the sub-micron-size aggregate expanded to form larger bubbles of approximately 3 µm (Figure 3b). As the incubation time increased to 90 min, the bubble diameter increased to approximately 10 µm, possibly due to the expansion or coalescence of nanobubbles into microbubbles. These micron-size bubbles may serve as echogenic contrast agents by displaying enhanced reflection at tumor tissues. As expected from a quantification assay in Figure 3a, we could not observe any substantial microbubble formation from DOX-CaCO3-MNPs at pH 7.4 (Figure 3b).
pH-Dependent In Vitro Echogenic Properties of DOX-CaCO3-MNPs
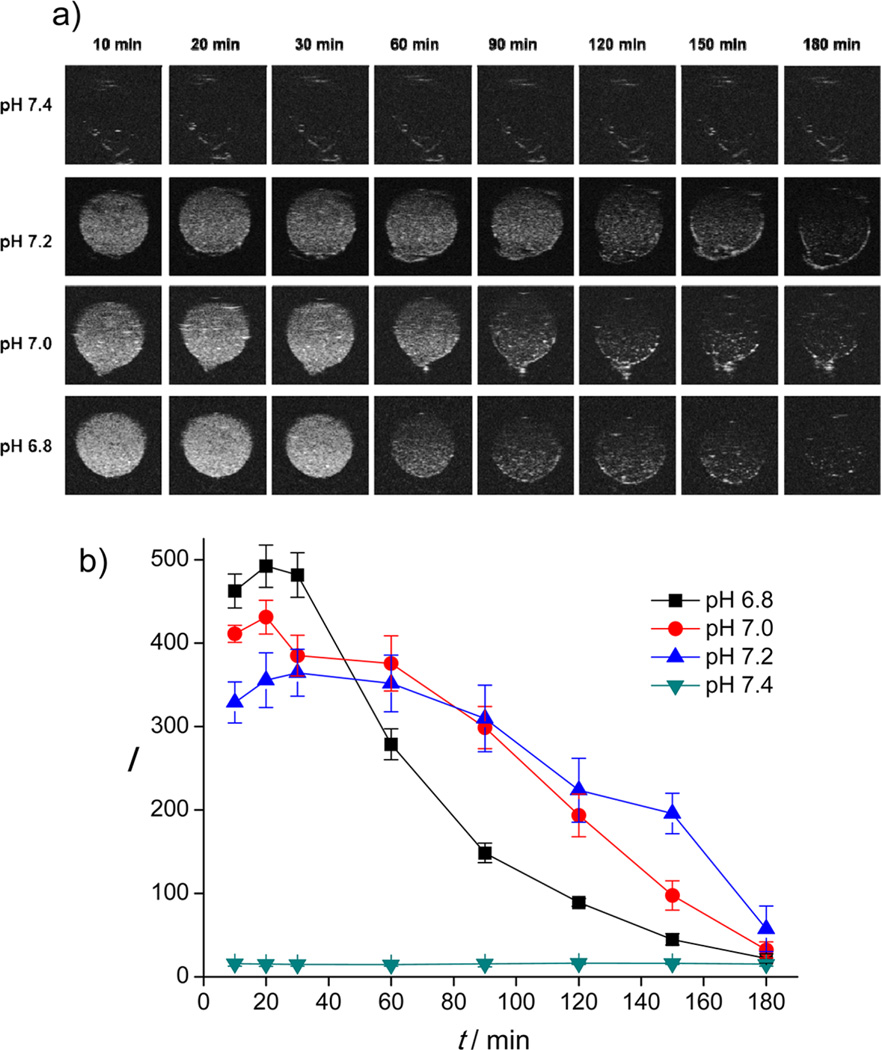
Next, we monitored the in vitro echogenic performance of the DOX-CaCO3-MNPs as a function of pH using an agar gel phantom test at 37 °C. As shown in Figure 4a, the DOX-CaCO3-MNPs at pH 7.4 showed no significant contrast signals under a US field, most likely because the DOX-CaCO3-MNPs did not generate CO2 bubbles sufficient for echogenic reflection. This observation is consistent with the results of the pH-dependent quantification assay of CO2 generation (Figure 3a). In contrast, US contrast images from the DOX-CaCO3-MNPs were significantly enhanced at weakly acidic pH conditions (pH 7.2, 7.0, and 6.8). At pH 7.2, the echo intensity persisted for more than 150 min, and the half-life of echo signals is up to 106 min (Figure 4b). Notably, as the pH level decreased from 7.2 to 7.0 and 6.8, the echo intensity of the DOX-CaCO3-MNPs was initially enhanced, while the half-life of US signals decreased to 83.0 and 48.1 min, respectively. This is a well understood phenomenon in which the higher echo intensity at lower pH levels is ascribed to facilitated formation of larger amounts of CO2 bubbles due to faster dissolution of CaCO3 phases, and the echo signals faded away earlier than at higher pH. The excellent echo persistence at weak acidic pH can be attributed to the retarded ionization of CaCO3 solid phases.35 The contrast enhancement that arises from the DOX-CaCO3-MNPs at tumoral pH indicates that generation of CO2 bubbles was responsible for echogenic US resonation. This gas-generating feature at a broad range of weak acidic pH may be suitable for US imaging of tumors, which have tissue heterogeneity and diverse pH levels (pH 6.8–7.2).
Figure 4.
(a) In vitro US images and (b) time-dependent US contrast intensity from DOX-CaCO3-MNPs at various pH. I refers to the intensity in arbitrary units, and t refers to time.
In Vivo US Imaging of a Tumor with DOX-CaCO3-MNPs
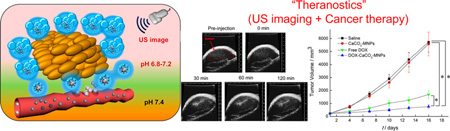
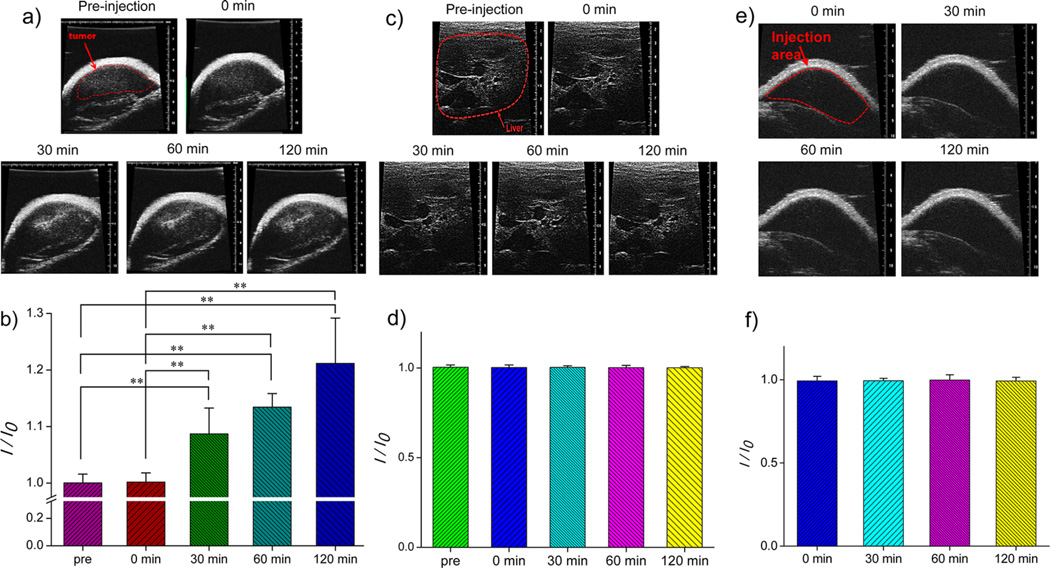
To demonstrate potential of the DOX-CaCO3-MNPs for US imaging of a tumor, we administered an intratumoral injection of a DOX-CaCO3-MNP dispersion to squamous cell carcinoma (SCC-7) tumor-xenograft-bearing C3H/HeN nude mice and monitored US images as a function of time (Figure 5a,b). Immediately after injection, we could not observe any contrast enhancement at tumor tissues. Interestingly, enhancement in US images was observed within 30 min after injection. In addition, this contrast enhancement lasted up to 120 min (Figure 5b). This long echo persistence was consistently supported by in vitro imaging data (Figure 4a). We also observed localized spots of enhanced contrast, possibly due to adequate accumulation of mineralized nanoparticles in the tumor tissues.
Figure 5.
(a) In vivo US imaging of the SCC-7 tumor by intratumoral injection of DOX-CaCO3-MNPs, (b) histogram of a US intensity profile of the tumor as a function of time (I0 is the ultrasound intensity of preinjection), (c) in vivo US imaging of the liver by the injection of DOX-CaCO3-MNPs into the liver, (d) histogram of a US intensity profile of the liver as a function of time (I0 is the ultrasound intensity of preinjection), (e) in vivo US imaging of subcutaneously injected areas of DOX-CaCO3-MNPs, and (f) histogram of a US intensity profile of subcutaneous areas as a function of time (I0 is the ultrasound intensity immediately after injection). Images and data at 0 min were obtained immediately after injection. Statistical analysis: one-way ANOVA + Tukey’s post hoc test (**P < 0.01).
In vivo results indicate that the DOX-CaCO3-MNPs generated nanobubbles at tumor tissues, which coalesced into micron-size bubbles, which could generate sufficient echogenic reflectivity under a US field. To verify the imaging ability at tumor tissues, we injected a DOX-CaCO3-MNP dispersion to the liver or subcutaneously to the back of tumor-free C3H/HeN mice and examined the time-dependent US imaging patterns (Figure 5c,d and Figure 5e,f). We could not observe any contrast enhancement at the liver and the subcutaneous tissues for 120 min. This suggests that the DOX-CaCO3-MNPs located in the liver and subcutaneous area at physiological pH did not generate the CO2 bubbles necessary for US contrast.
To confirm the pH-dependent generation of the echogenic signals, we measured pH of the SCC-7 tumor tissues using a pH meter and a probe for semisolid materials. pH values of the liver and the subcutaneous tissues were also measured as control experiments. Estimation of pH in human and animal tumors by insertion of pH electrodes was well-documented, and in general, the tumors were known to be more acidic than normal tissues with mean pH values of ca. 7.0 in tumors and 7.5 in normal tissues.36 As shown in Figure S4 in the Supporting Information, pH of the SCC-7 tumor tissue was found to be 6.8 ± 0.1, whereas pH of the liver and the subcutaneous tissues were 7.3 ± 0.05 and 7.4 ± 0.04, respectively. The acidic pH is known as the main factor that can dissolve CaCO3 to generate CO2 molecules. Although there may exist various factors in vivo, these experimental supporting data based on in vivo pH measurements would verify that our nanoparticles generated CO2 bubbles at an acidic pH environment of the tumor tissues. Based on this proof-of-concept study, CaCO3 mineralized nanoparticles can provide the opportunity for visualization of the low pH environment in tumors by a noninvasive and real-time US imaging system.
pH-Controlled DOX Release and CaCO3 Dissolution Behavior
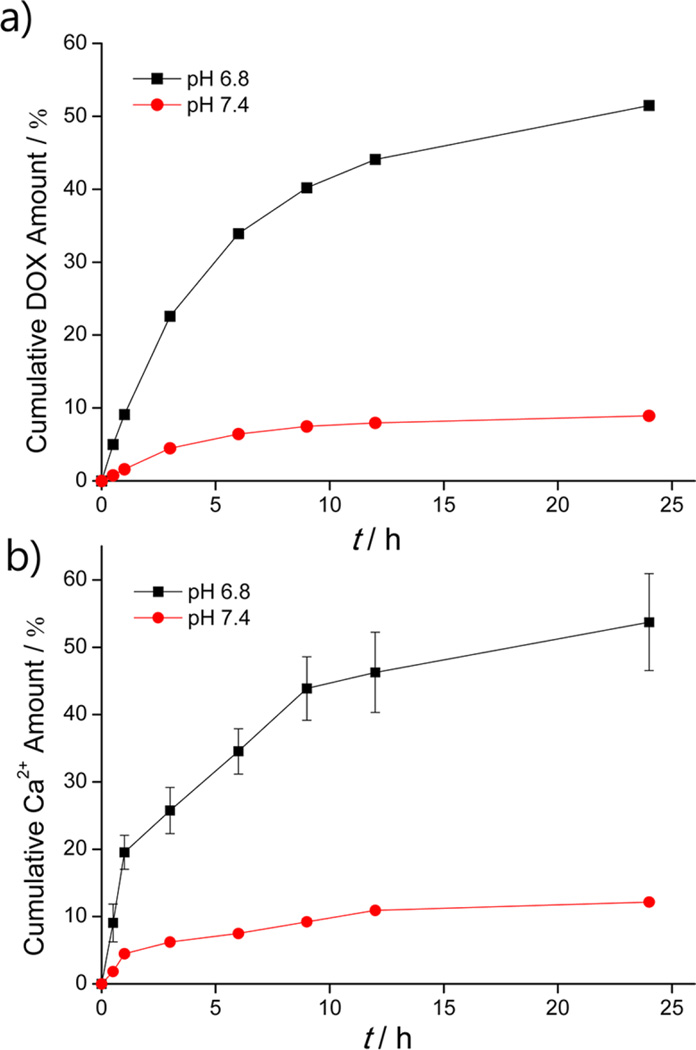
The block copolymer templated CaCO3 mineralization in this work is beneficial as it is a simple process that can produce nanocarriers capable of loading diverse ionic cancer therapeutics in situ within the CaCO3 core. In our system, the CaCO3 mineral serves not only as a CO2 bubble-generating precursor for US imaging but also as a cargo that can stably load anticancer drugs and release these drugs under pH control. To verify the drug-releasing ability of the DOX-CaCO3-MNPs at tumoral pH, we examined the profiles of DOX release in aqueous buffer solutions of pH 7.4 and 6.8 (Figure 6a). The loading content of DOX in the DOX-CaCO3-MNPs was 7.1 wt %. It is well-documented that, when the pH level decreases, aqueous solubility of CaCO3 exponentially increases. CaCO3 is known to react with acids to produce carbonic acid, which decreases the concentration of CO32−, thereby making CaCO3 more soluble.35 The release of DOX from the DOX-CaCO3-MNPs was significantly inhibited at pH 7.4. This indicates that the DOX-CaCO3-MNPs can maintain the solid nanoparticle structure at physiological pH due to low solubility of CaCO3. In contrast, at pH 6.8, the DOX-CaCO3-MNPs exhibited rapid DOX release rate, with 44% of DOX released within 12 h, indicating the facilitated dissolution of DOX-loaded CaCO3 phases. To further analyze this pH-dependent release behavior, the kinetics of Ca2+ dissolution from the DOX-CaCO3-MNPs was also investigated (Figure 6b).
Figure 6.
(a) DOX release profiles from DOX-CaCO3-MNPs at different pH values of 6.8 and 7.4 (n = 3) and (b) kinetics of calcium dissolution from DOX-CaCO3-MNPs under pH control (n = 3).
At pH 7.4, the DOX-CaCO3-MNPs showed a retarded release of Ca2+ ions of less than 12%. In contrast, at pH 6.8, we found accelerated dissolution of CaCO3 mineral phases. The rapid dissolution of CaCO3 minerals at the lower pH is a reasonable finding, as the aqueous solubility of CaCO3 increases proportionally to the concentration of proton ions.35 It is of great interest to note that this pH-dependent kinetics of CaCO3 dissolution corresponds directly to the DOX release. This result suggests that the DOX-CaCO3-MNPs may stably hold DOX in blood and trigger DOX release at the target tumor tissue.
In Vitro Cytotoxicity of DOX-CaCO3-MNPs
The cytotoxicity of the DOX-CaCO3-MNPs was evaluated on SCC-7 cells using a cell counting kit-8 assay. Importantly, the DOX-free CaCO3-MNPs did not show any noticeable cytotoxicity on the SCC-7 cells for concentrations up to 500 µg/mL (Figure S5 in the Supporting Information). In vitro cytotoxicity of free DOX and the DOX-CaCO3-MNPs was also estimated. Free DOX significantly decreased the viability of SCC-7 cancer cells in a dose-dependent manner (Figure S6 in the Supporting Information). Although the DOX-CaCO3-MNPs also exhibited a cytotoxic effect, SCC-7 cells treated with the DOX-CaCO3-MNPs retained higher viabilities than cells exposed to free DOX (Table S2 in the Supporting Information). The somewhat lower toxicity of the DOX-CaCO3-MNPs, compared to free DOX, was most likely due to the gradual release of DOX within acidic compartments of the cell (endo/lysosomes) because the DOX release, under our in vitro experimental condition, was triggered by CaCO3 dissolution within cellular acidic environment.
Cellular Uptake and Intracellular Distribution of DOX-CaCO3-MNPs
To monitor cellular uptake of DOX-CaCO3-MNPs, we prepared fluorescein isothiocyanate (FITC)-incorporated DOX-CaCO3-MNPs. The intracellular distribution of FITC-incorporated DOX-CaCO3-MNPs was monitored by confocal laser scanning microscopy (CLSM). After 1 h incubation, the SCC-7 cells treated with FITC-incorporated DOX-CaCO3-MNPs displayed a green and red fluorescence at the cytosol and a negligible red fluorescence within the nuclei (Figure S7 in the Supporting Information). This indicates the uptake of the nanoparticles and their location at the acidic endo/lysosomal compartments. After a further incubation of 5 h, a stronger DOX fluorescence was found in nuclei than in cytosols, possibly due to the DOX release triggered by dissolution of DOX-loaded CaCO3 cores in the acidic compartments. On the other hand, the majority of green FITC fluorescence was still found in the cytosol, not in the nuclei. This indicates that the residual FITC-incorporated nanoparticles or FITC-labeled PEG-PAsp, generated by the nanoparticle dissolution in endo/lysosomes, stayed at the cytosols.
As an additional experiment to verify the cellular uptake of the CaCO3-MNPs, the dissolution of the CaCO3 cores within acidic cellular environments was visualized using an acetoxymethyl ester derivative of Rhod-2 (Rhod-2/AM), a membrane-permeable derivative of a calcium-specific dye Rhod-2. Upon cellular internalization, Rhod-2/AM is rapidly hydrolyzed to Rhod-2 by endogenous esterases, and Rhod-2 binds with free Ca2+ to form the Rhod-2-Ca2+ complex, which generates the red fluorescence.37,38 As shown in Figure S8 in the Supporting Information, the cells of the Rhod-2/AM-treated positive control displayed a weak red fluorescence due to the native Ca2+ in the cytosol. It is noted that the cells treated with CaCO3-MNPs exhibited a stronger red fluorescence, compared with the positive control and PEG-PAsp-treated cells. In addition, as the concentration of CaCO3-MNPs increased from 100 to 300 µg/mL, the stronger fluorescence of the Rhod-2-Ca2+ complex was observed. This result strongly supports that the CaCO3-MNPs were internalized within cells and localized within acidic endo/lysosomes to dissolve CaCO3 cores, thereby generating Ca2+ ions.
The cellular uptake of DOX-CaCO3-MNPs was monitored by CLSM. The acidic cellular compartment was labeled with green fluorescent LysoTracker as an indicator. As shown in Figure S9a in the Supporting Information, after 1 h incubation of SCC-7 cells with free DOX, almost all the DOX molecules were located in the nucleus, indicating fast accumulation by diffusion across the cellular membrane. In contrast, SCC-7 cells treated with DOX-CaCO3-MNPs, after 1 h incubation, exhibited a bright red DOX fluorescence within the acidic compartments and a negligible red fluorescence within the nuclei (Figure S9b in the Supporting Information). After 6 h of incubation, a strong fluorescence was found in the nuclei, which can be ascribed to the released DOX from internalized DOX-CaCO3-MNPs (Figure S9c in the Supporting Information). This indicates that a triggering factor for DOX release was the dissolution of DOX-loaded CaCO3 minerals in acidic cellular compartments. Therefore, it might be suggested that the CaCO3 core can effectively hold DOX before cellular uptake, and the DOX release can be facilitated within endo/lysosomes by the dissolution of the CaCO3 core. From the in vitro experiments, we confirmed that the DOX-CaCO3-MNPs have the ability to inhibit proliferation of SCC-7 cells by delivering DOX within the cells.
In Vivo Antitumor Efficacy of DOX-CaCO3-MNPs
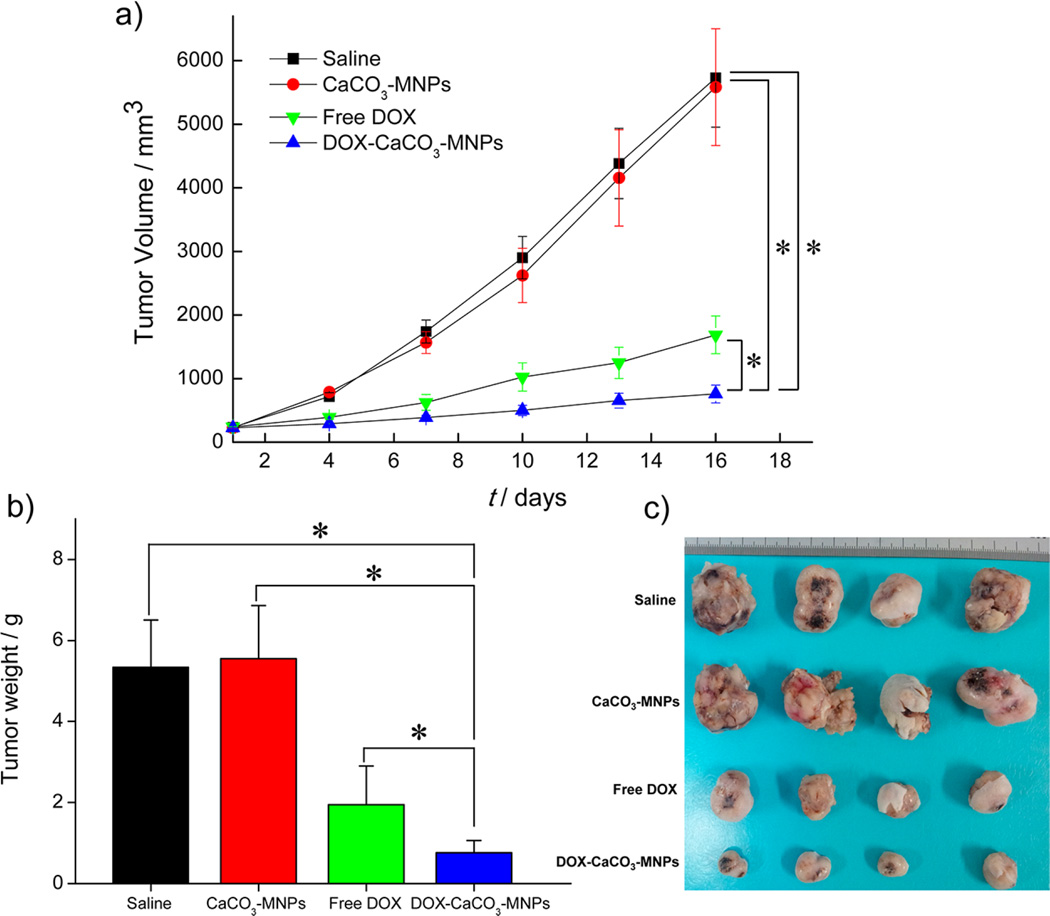
The anti-cancer therapeutic efficacy of the DOX-CaCO3-MNPs was examined in SCC-7 tumor-bearing mice. Free DOX (10 mg/kg) and the DOX-CaCO3-MNPs (10 mg/kg of DOX) were administered with single intratumoral injection. Figure 7a shows the changes in tumor volumes in nude male mice after injection of saline, the CaCO3-MNPs, free DOX, the DOX-CaCO3-MNPs. The DOX-free control groups (saline and CaCO3-MNPs) did not show any noticeable inhibition of tumor growth. In contrast, the DOX-CaCO3-MNPs are significantly more efficacious in tumor reduction than control groups and the free DOX. At 16 days postinjection, free DOX suppressed the tumor weight by 61% and the DOX-CaCO3-MNPs decreased the tumor weight by 85%, compared to the saline-treated mice (Figure 7b,c). In our experimental design, the anticancer efficacy was evaluated for a few weeks because this time period is enough to confirm whether our newly developed theranostic nanoparticles exhibit the improved efficacy for cancer therapy, compared to the free anticancer drug.
Figure 7.
(a) Changes in tumor volumes after injection of saline, CaCO3-MNPs, free DOX, and DOX-CaCO3-MNPs; (b) tumor weights after 16 days post-treatment; and (c) images of excised tumors of each group with controls (saline and CaCO3-MNPs), free DOX, and DOX-CaCO3-MNPs after 16 days post-treatment (n = 4). The results represent the means ± SD (n = 4). Statistical analysis: one-way ANOVA + Tukey’s post hoc test (*P < 0.05).
This enhanced antitumor therapeutic efficacy of the DOX-CaCO3-MNPs might be explained by the delayed clearance of the DOX-CaCO3-MNPs at the tumor site due to the enhanced retention properties of the nanoparticles.32 To estimate time-dependent DOX distribution after intratumoral injection, we obtained noninvasive real-time fluorescence imaging of DOX. The tumor tissues, treated with free DOX, generated strong red fluorescence for 12 h initially, and thereafter, the fluorescence intensity rapidly decreased to 23.8% of the initial intensity (Figure S10a,b in the Supporting Information). In contrast, for DOX-CaCO3-MNP-treated tumors, the strong fluorescence of DOX was maintained for up to 8 days and 74.1% of the initial intensity was maintained. At 8 days postinjection, the tumor tissues were isolated for observation of ex vivo images. It was found that the total fluorescence photon counts in the DOX-CaCO3-MNP-treated tumors were significantly much higher than the tumors treated with free DOX (Figure S10c,d in the Supporting Information). This real-time imaging experiment for DOX distribution strongly supported the prolonged retention of DOX-CaCO3-MNPs at tumor tissues and the continuous release of DOX from the DOX-CaCO3-MNPs.
This is consistent with long-lasting echo signals of the DOX-CaCO3-MNPs at tumor tissues, which were evaluated using in vivo US imaging of tumors (Figure 5a). Furthermore, DOX was released simultaneously with CO2 bubble generation at the acidic tumoral environment and could be rapidly taken up by tumor cells, allowing for the enhanced antitumor effect.
CONCLUSIONS
In summary, we successfully constructed DOX-loaded CaCO3 (vaterite) mineralized nanoparticles through a block copolymer templated in situ mineralization approach. The nanoparticles with well-defined morphology and size were obtained by controlling the feed ratio of ions (Ca2+ and CO32−) to anionic Asp units and mineralization time. The nanoparticles generated CO2 bubbles at tumoral pH and simultaneously triggered the release of DOX. The DOX-CaCO3-MNPs have great potential as a theranostic agent for simultaneous US imaging and therapy of cancers. The approach suggested in this work may provide a valuable direction for useful theranostic nanoparticles with improved US imaging and anticancer therapeutic functions.
MATERIALS AND METHODS
Materials
α-Methoxy-ω-aminopoly(ethylene glycol) (CH3O-PEG-NH2), Mn = 5000 g/mol, was purchased from IDBIOCHEM Inc. (Seoul, Korea).β-Benzyl l-aspartate (BAsp) and doxorubicin hydrochloride (DOX-HCl) were purchased from Sigma Co. (St. Louis, MO) and used without further purification. Triphos-gene was purchased from Aldrich Co. (Milwaukee, WI) and used as received. N,N-Dimethylformamide (DMF) was dried and distilled over calcium hydride. Calcium chloride (CaCl2) and sodium carbonate (Na2CO3) were of the reagent grade. β-Benzyl l-aspartate N-carboxyanhydride (BAsp-NCA) of high purity was synthesized by the Fuchs-Farthing method using triphosgene.39 All other chemicals were of analytical grade and used without further purification.
Synthesis of PEG-b-PAsp Copolymer (PEG-PAsp)
A poly(ethylene glycol)-b-poly(l-aspartic acid) copolymer (PEG-PAsp) that has EG units of 113 and Asp units of 54 was synthesized by a modified procedure established in our laboratory.40 In brief, to a stirred solution of CH3O-PEG-NH2 (1.5 g, 0.3 mmol) in dry DMF (10 mL) was added BAsp-NCA (4.5 g, 18 mmol) at 35 °C under nitrogen. After 24 h, PEG-PBAsp was isolated by repeated precipitation from DMF into diethyl ether. Yield: 83%. Finally, hydrolytic deprotection of benzyl groups in PEG-PBAsp was performed by treating the block copolymer (2 g) with 0.1 N NaOH (200 mL).The aqueous solution was then dialyzed using a membrane (molecular weight cutoff (MWCO): 1000) for 24 h, followed by freeze-drying. The polydispersity index (Mw/Mn), estimated by gel permeation chromatography, was 1.06.
Preparation of DOX-Loaded CaCO3 Mineralized Nanoparticles (DOX-CaCO3-MNPs)
DOX-CaCO3-MNPs were prepared by a PEG-PAsp templated in situ mineralization technique. PEG-PAsp (100 mg) was dissolved in 4 mL of distilled water (pH 8.0) for 1 h, and then an aqueous CaCl2 solution (53.2 mg, 0.48 mmol) of 2 mL was first added to a stirred solution and equilibrated for 2 h under stirring at 800 rpm. The DOX solution (10 mg, 2 mL) was subsequently added and stirred in the dark at room temperature for 2 h, and an aqueous solution (2 mL) of Na2CO3 (50.8 mg, 0.48 mmol) was then slowly dropped into the reaction mixture. For a stoichiometric ratio, the molar concentration ratio of [Asp]/[Ca2+]/[CO32−] was 1:1:1. The mixed solution was stirred magnetically at 800 rpm at room temperature for 12 h. The solution was centrifuged and dialyzed to remove unreacted ionic species, and the dialysate was lyophilized to obtain the DOX-CaCO3-MNPs. To determine the drug loading content and loading efficiency, DOX-CaCO3-MNPs were stirred in 1 N HCl solution for 30 min. The fluorescence emission intensity was measured at 588 nm (excitation at 480 nm). The drug loading content was calculated based on the standard curve obtained using DOX.
Characterization of DOX-CaCO3-MNPs
Dynamic light scattering measurements were performed using a 90 plus particle size analyzer (Brookhaven Instruments Corporation). The scattered light of a vertically polarized He–Ne laser (632.8 nm) was measured at an angle of 90° and was collected on an auto-correlator. The hydrodynamic diameters (d) of micelles were calculated by using the Stokes–Einstein equation.41 The polydispersity factor of DOX-CaCO3-MNPs, represented as µ2/Γ2, where µ2 is the second cumulant of the decay function and Γ is the average characteristic line width, was calculated from the cumulant method.41 The morphology of DOX-CaCO3-MNPs was examined by transmission electron microscopy (JEM-2000EX, JEOL Tokyo, Japan). DOX-CaCO3-MNPs were analyzed by TEM-associated EDX to reveal atomic components. EDX measurement was carried out using JEOL JEM-2100F equipped with an EDAX Genesis series TEM at 200 kV. XRD measurements were performed with a Rigaku D/max-RB apparatus (Tokyo, Japan) powder diffractometer and image-plate photography using graphite-monochromatized Cu Kα radiation (λ = 1.542 Å). Data were collected from 10 to 60° with a step size of 0.05 and step time of 5 s. FT-IR spectra of samples were measured with PerkinElmer FT-IR system (Spectrum GX) at room temperature under a stream of nitrogen gas. The mixed powder of samples and KBr was pressed for pellets of the IR window and then dried in a vacuum oven for 24 h. FT-IR spectra of polymer sample were measured at a resolution of 4 cm−1.
Stability of DOX-CaCO3-MNPs
The physical stability of the DOX-CaCO3-MNPs was evaluated in serum-containing solutions. The effect of serums on the physical stability of DOX-CaCO3-MNPs in aqueous media was estimated by dynamic light scattering analysis. For the stability in serum conditions, the stability of DOX-CaCO3-MNPs (1 mg/mL) in the serum-containing PBS solution (50% FBS, pH 7.4) was examined. The mean diameter of the nanoparticles was monitored, and scattered light intensity (SLI) was compared to the initial scattered light intensity (SLI0).
Quantification of CO2 Generation from DOX-CaCO3-MNPs as a Function of pH
The amount of CO2 generated from DOX-CaCO3-MNPs was measured with a quadrupole mass spectrometer (Prisma QME 200, Germany) equipped with a Faraday cup detector. The vapor was analyzed with an emission current of 0.058 mA, an electron energy of 8 eV, and resolution of 750. A sample chamber was vacuumed with a turbo molecular pump (Pfeiffer vacuum, CA, USA) and measured at room temperature. Phosphate buffer solutions at various pH (7.4, 7.2, 7.0, and 6.8, 2 mL) and 20 mg of DOX-CaCO3-MNPs were placed in the sample with two branched chambers. The buffer solution in the chamber was frozen under vacuum conditions and thawed. As control experiments, the amounts of CO2 present in the buffer solution (pH 7.4) and the PEG-PAsp-containing buffer solution (pH 7.4) were calculated. For measurement of CO2 generated from DOX-CaCO3-MNPs, DOX-CaCO3-MNPs were exposed to each thawed buffer solution with variable pH. After 30 min exposure to buffer solutions, the sample was frozen and then vacuumed. The amount of generated CO2 was calculated with an inlet volume for mass measurement.
In Vitro US Imaging at Various pH
In vitro US imaging of DOX-CaCO3-MNPs was performed in phosphate buffer solutions at various pH (7.4, 7.2, 7.0, and 6.8). An optically transparent phantom gel plate, made by embedding a 500 µL Eppendorf tube in the agarose gel (3%, w/v) and then removing the tube after the phantom gel had cooled, was used as the in vitro experiment. Aqueous nanoparticle solutions (25 mg/mL) were prepared at various pH. US images were obtained using Visual-Sonics Vevo 770 (high-resolution micro-imaging system, Visual-Sonics, Toronto, Canada) with the RMV 706 transducer at 40 MHz of a static state using a contrast mode. The change of US intensity for each sample was measured up to 180 min, and the US intensity of the water as a control was subtracted from the sample intensity as the normalizing process.
Visualization of CO2 Bubbles Generated from DOX-CaCO3-MNPs
A drop of nanoparticle solution (5 mg/mL) was immediately placed on slide glass after dispersion of DOX-CaCO3-MNPs in phosphate buffer solutions (pH 6.8 and 7.4). The CO2 bubble image from DOX-CaCO3-MNPs was monitored for 90 min at room temperature using an optical microscope (IX71; Olympus Co. Ltd., Japan) equipped with 40 × focal lens (optical magnification: 400 ×).
In Vivo US Imaging of a Xenograft Tumor
All animal treatments and surgical procedures followed approved protocols and were performed in accordance with the Institutional Animal Care and Use Committee at Kyung Hee University. The SCC-7 xenograft tumor was grown in 7-week-old male athymic C3H/HeN nude mice (20 g, Institute of Medical Science, Tokyo) by inoculating 1.0 × 106 SCC-7 cells into the dorsal side of mice. When tumors grew to approximately 250 ± 20 mm3 in volume, US imaging of DOX-CaCO3-MNPs was investigated. Before US imaging, mice were anesthetized by inhalation of 2% isoflurane with 1% oxygen. Anesthetized mice were placed on a thermostatically controlled heating pad to maintain body temperature. Then, 100 µL of PBS solution (pH 7.4) containing DOX-CaCO3-MNPs (25 mg/mL, 10 mg/kg of DOX equivalent) was administered by an intratumoral injection. The tumor was imaged with the VisualSonics Vevo 770 in vivo high-resolution micro-imaging system with the RMV 706 transducer at 40 MHz of a static state using a contrast mode. For normalized US signals, US intensity of the background was subtracted from the intensity from DOX-CaCO3-MNPs injected in the tumor region and then divided by US intensity of the background.42 As a control in vivo experiment, the DOX-CaCO3-MNP dispersion was administered into the liver or subcutaneous tissues in the lower backs of anesthetized tumor-free nude mice, and then time-dependent US imaging was monitored following the identical procedure mentioned above. The statistical analysis was performed using an one-way ANOVA ± Tukey’s post hoc test.
Measurement of Tumor pH
SCC-7 tumor-bearing C3H/HeN nude mice (male, n = 2) were under general anesthetics with Zoletil + xylazine (30 mg/kg + 10 mg/kg, IP). Tumor sites were surgically opened using a sterile razor blade, and the tumoral pH was measured using a pH meter (Thermo Electron Corporation, Waltham, MA, USA) equipped with an electrode for semisolid types of materials (Istek, Seoul, Korea). Three random spots of each tumor were measured to compare the mean tumor pH to the mean pH of the liver and subcutaneous tissues. The pH of the liver and subcutaneous tissues was measured using an identical method used for measurement of tumor pH.
DOX Release from DOX-CaCO3-MNPs
In vitro release profiles of DOX from DOX-CaCO3-MNPs were examined in phosphate buffer solutions (pH 7.4 and 6.8). DOX-CaCO3-MNPs of 1 mg/mL were dispersed in aqueous buffer solutions (1 mL) and transferred to a dialysis membrane bag (MWCO: 3500 g/mol). The release experiment was initiated by placing the dialysis bag in 10 mL of release media. The release medium was shaken at a speed of 150 rpm at 37 °C. At predetermined time intervals, samples (10 mL) were withdrawn and replaced with an equal volume of the fresh medium. The concentration of released DOX in the samples was determined by measurement of fluorescence emission intensity at 588 nm (excitation at 480 nm) based on the standard curve obtained using DOX.
Calcium Dissolution of DOX-CaCO3-MNPs
In vitro calcium dissolution experiments of DOX-CaCO3-MNPs were performed in the aqueous buffer solutions (pH 7.4 and 6.8). The DOX-CaCO3-MNP solution (1 mg/mL) was sealed in a dialysis membrane bag (MWCO: 3500) and was shaken at 150 rpm at 37 °C in release media. The release medium (10 mL) was withdrawn at predetermined time intervals and replaced with an equal volume of the fresh medium. The release rate of calcium ions was monitored by taking 100 µL of the sample and diluting it in the Arsenazo III solution (2 mL, 0.2 mM) in HEPES-buffered saline (where [HEPES] = 20 mM and [NaCl] = 150 mM at pH 7.4). The absorbance of the solution at 656 nm was then measured, and the concentration of calcium ions was calculated based on the standard curve.
Cytotoxicity of DOX-CaCO3-MNPs
SCC-7 cells were originally obtained from the American Type Culture Collection (Rockville, MD) and cultured in RPMI 1640 medium (Gibco BRL, Gaithers-burg, MD) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (Gibco BRL) and 1% (v/v) penicillin-streptomycin (Gibco BRL). Cells were cultured in a humidified incubator at 37 °C with 5% CO2. The culture medium was replaced every 2 days. Cells were seeded at a density of 5 × 103 cells/well in 96-well flat-bottomed plates for 1 day. Cells were washed twice with PBS and incubated with various concentrations CaCO3-MNPs, free DOX, and DOX-CaCO3-MNPs for 24 h at 37 °C. The cells were washed twice with PBS to eliminate the remaining drugs, and the medium was replaced with cell counting kit-8 solutions (Dojindo Laboratories, Kumamoto). The absorbance of individual wells was measured at 450 nm by a microplate reader (Biorad Elizer, PA). The data are expressed as the percentages of viable cells compared to the survival of a control group. The IC50 value was calculated as the concentration of DOX, yielding 50% inhibition of cell proliferation compared to the untreated control.
Monitoring of Cellular Uptake and Intracellular Distribution of FITC-Incorporated DOX-CaCO3-MNPs
To monitor the cellular uptake of DOX-CaCO3-MNPs, green fluorescent FITC was incorporated into the DOX-CaCO3-MNPs. FITC was labeled on the primary amine terminal of PEG-PAsp block copolymers as follows: To a stirred aqueous solution (pH 7.4) of PEG-PAsp (400 mg, 0.04 mml) was added an aqueous solution (pH 7.4) of FITC (0.8 mg, 0.002 mmol). After being stirred for 24 h at room temperature in the dark, the reaction mixture was dialyzed using a membrane bag (MWCO: 1000 g/mol) to remove the unreacted FITC, followed by lyophilization. The labeling ratio of FITC to PEG-PAsp was estimated by a standard calibration curve and was found to be 2.0%. FITC-incorporated DOX-CaCO3-MNPs was prepared following the identical procedure used for DOX-CaCO3-MNPs, except for using FITC-labeled PEG-PAsp. To monitor cellular uptake, SCC-7 cells (2 × 104 cells/mL per well) were seeded onto a covered glass-bottom dish (SPL Life Sciences) in 2 mL of DMEM supplemented with 10% FBS and 1% antibiotics. After incubation for 24 h (37 °C, 5% CO2), the medium was carefully aspirated and replaced with 1 mL of medium containing 5 µg/mL DOX equivalent of FITC-incorporated DOX-CaCO3-MNPs. The cells were incubated for 1 and 6 h and washed three times with PBS. The CLSM images of SCC-7 cells treated with FITC-incorporated DOX-CaCO3-MNPs were obtained using a confocal laser scanning microscope.
Visualization of Dissolved Calcium from DOX-CaCO3-MNPs in SCC-7 Cells
SCC-7 cells (2 × 104 cells per well) were seeded onto a covered glass-bottom dish (SPL Life Sciences) in 2 mL of phenol red-free DMEM cell culture medium supplemented with 10% FBS and 1% antibiotics and incubated for 24 h. Rhod-2/AM (50 µg) was dissolved in DMSO (20 µL) and was added to 6.7 mL of phenol red-free DMEM cell culture medium before usage. The Rhod-2/AM solution (2 mL, 6.6 mM) was added to the cell culture chamber and incubated for 30 min. After being washed by PBS three times, the cells were incubated in 1 mL of phenol red-free DMEM cell culture medium containing 100 or 300 µg CaCO3-MNPs for 6 h. After being washed by PBS three times, the cells were fixed with 3.7% formaldehyde for 5 min. Finally, the cells were washed three times with PBS, and then the fluorescence images of Rhod-2-labeled SCC-7 cells treated with CaCO3-MNPs were obtained using a fluorescence microscopy (λex = 550 nm, λem = 580 nm, IX71, Olympus, Japan).
Intracellular Distribution of DOX-CaCO3-MNPs
SCC-7 cells (2 × 104 cells/mL per well) were seeded onto a covered glass-bottom dish (SPL Life Sciences) in 2 mL of DMEM supplemented with 10% FBS and 1% antibiotics (penicillin 100 U/mM, streptomycin 0.1 mg/mL). After incubation for 24 h (37 °C, 5% CO2), the medium was carefully aspirated and replaced with 1 mL of medium containing 5 µg/mL DOX equivalent of DOX-CaCO3-MNPs and LysoTracker (50 nM). The cells were incubated for 1 and 6 h and then washed three times with PBS. The CLSM images of LysoTracker-labeled SCC-7 cells treated with DOX-CaCO3-MNPs were obtained using a confocal laser scanning microscope (C1si, Nikon, Japan) by green fluorescing (λex = 470–490 nm) and red fluorescing (λex = 520–550 nm).
Antitumor Effect of DOX-CaCO3-MNPs on SCC-7 Tumor Xenografts
Athymic C3H/HeN nude mice (20–25 g, Harlan, Indianapolis, IN) were used for animal experiments. The tumor model was established by subcutaneously inoculating 1.0 × 106 SCC-7 cells into the left back of a mouse. When tumors grew to approximately 200–250 mm3, saline (the control group, n = 4), CaCO3-MNPs (the control group, n = 4), free DOX at 10 mg/kg (n = 4), and DOX-CaCO3-MNPs at 10 mg/kg of DOX (n = 4) were administered by a single intratumoral injection. Tumor volumes were calculated as a × b2/2, where a and b are the largest and smallest diameter, respectively.43 The statistical analysis was performed using an one-way ANOVA + Tukey’s post hoc test.
In Vivo Imaging of DOX Clearance Pattern of DOX-CaCO3-MNPs
All animal treatments and surgical procedures followed approved protocols and were performed in accordance with an NIH-approved protocol. The SCC-7 xenograft tumor was grown in female athymic C3H/HeN nude mice (20–25 g, Harlan, Indianapolis, IN) by inoculating 1.0 × 106 SCC-7 cells into in the right flank side of mice. When tumors reached a size between 250 and 300 mm3 in volume, 100 µL of PBS solution (pH 7.4) containing free DOX (500 µg/mL, 2 mg/kg of DOX equivalent) or DOX-CaCO3-MNPs (2 mg/kg of DOX equivalent) was administered by an intratumoral injection. The tumor was monitored by using a Maestro all-optical imaging system (Cambridge Research & Instrumentation, Woburn, MA) using blue filter set as the excitation at predetermined time points (immediately after injection (0 h), 12 h, 0.5, 1, 3, 5, and 8 days). For all imaging procedures, mice were anesthetized with 3–4% isoflurane using a nose-cone manifold and restrained on the imaging stage. After 8 days of imaging, the animals were sacrificed. The tumors were harvested for ex vivo imaging. Tumors were quantified by ROI analyses on both in vivo and ex vivo results using the software provided by the vendor.
Statistical Analysis
The statistical significance of differences between experimental and control groups was determined using an one-way ANOVA followed by Tukey’s post hoc test. P value of <0.05 or <0.01 were considered significant, and significant differences are shown by asterisks in the figures.
Supplementary Material
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (Nos. 2013R1A2A2A01009239 and 2012R1A5A2051388).
Footnotes
Conflict of Interest: The authors declare no competing financial interest.
Supporting Information Available: Details of synthesis and experimental methods. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES AND NOTES
- 1.Lindner JR. Microbubbles in Medical Imaging: Current Applications and Future Directions. Nat. Rev. Drug Discovery. 2004;3:527–533. doi: 10.1038/nrd1417. [DOI] [PubMed] [Google Scholar]
- 2.Stewart VR, Sidhu PS. New Directions in Ultrasound: Microbubble Contrast. Br. J. Radiol. 2006;79:188–194. doi: 10.1259/bjr/17790547. [DOI] [PubMed] [Google Scholar]
- 3.Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Molecular Imaging in Drug Development. Nat. Rev. Drug Discovery. 2008;7:591–607. doi: 10.1038/nrd2290. [DOI] [PubMed] [Google Scholar]
- 4.Qin SP, Caskey CF, Ferrara KW. Ultrasound Contrast Microbubbles in Imaging and Therapy: Physical Principles and Engineering. Phys. Med. Biol. 2009;54:R27–R57. doi: 10.1088/0031-9155/54/6/R01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara K, Pollard R, Borden M. Ultrasound Microbubble Contrast Agents: Fundamentals and Application to Gene and Drug Delivery. Annu. Rev. Biomed. Eng. 2007;9:415–447. doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- 6.Schutt EG, Klein DH, Mattrey RM, Riess JG. Injectable Microbubbles as Contrast Agents for Diagnostic Ultrasound Imaging: The Key Role of Perfluorochemicals. Angew. Chem., Int. Ed. 2003;42:3218–3235. doi: 10.1002/anie.200200550. [DOI] [PubMed] [Google Scholar]
- 7.Kiessling F, Gaetjens J, Palmowski M. Application of Molecular Ultrasound for Imaging Integrin Expression. Theranostics. 2011;1:127–134. doi: 10.7150/thno/v01p0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernot S, Klibanov AL. Microbubbles in Ultrasound-Triggered Drug and Gene Delivery. Adv. Drug Delivery Rev. 2008;60:1153–1166. doi: 10.1016/j.addr.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanna V, Pintus G, Bandiera P, Anedda R, Punzoni S, Sanna B, Migaleddu V, Uzzau S, Sechi M. Development of Polymeric Microbubbles Targeted to Prostate-Specific Membrane Antigen as Prototype of Novel Ultrasound Contrast Agents. Mol. Pharmaceutics. 2011;8:748–757. doi: 10.1021/mp100360g. [DOI] [PubMed] [Google Scholar]
- 10.Willmann JK, Kimura RH, Deshpande N, Lutz AM, Cochran JR, Gambhir SS. Targeted Contrast-Enhanced Ultrasound Imaging of Tumor Angiogenesis with Contrast Microbubbles Conjugated to Integrin-Binding Knottin Peptides. J. Nucl. Med. 2010;51:433–440. doi: 10.2967/jnumed.109.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iagaru A, Gambhir SS. Imaging Tumor Angiogenesis: The Road to Clinical Utility. Am. J. Roentgenol. 2013;201:W183–W191. doi: 10.2214/AJR.12.8568. [DOI] [PubMed] [Google Scholar]
- 12.Wilson SR, Burns PN. Microbubble-Enhanced US in Body Imaging: What Role? Radiology. 2010;257:24–39. doi: 10.1148/radiol.10091210. [DOI] [PubMed] [Google Scholar]
- 13.Willmann JK, Paulmurugan R, Chen K, Gheysens O, Rodriguez-Porcel M, Lutz AM, Chen IY, Chen X, Gambhir SS. US Imaging of Tumor Angiogenesis with Microbubbles Targeted to Vascular Endothelial Growth Factor Receptor Type 2 in Mice. Radiology. 2008;246:508–518. doi: 10.1148/radiol.2462070536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiessling F, Gaetjens J, Palmowski M. Application of Molecular Ultrasound for Imaging Integrin Expression. Theranostics. 2011;1:127–134. doi: 10.7150/thno/v01p0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloch SH, Wan M, Dayton PA, Ferrara KW. Optical Observation of Lipid- and Polymer-Shelled Ultrasound Microbubble Contrast Agents. Appl. Phys. Lett. 2004;84:631–633. [Google Scholar]
- 16.Min HS, Kang E, Koo H, Lee J, Kim K, Park R-W, Kim I-S, Choi Y, Kwon IC, Han M. Gas-Generating Polymeric Microspheres for Long-Term and Continuous In Vivo Ultrasound Imaging. Biomaterials. 2012;33:936–944. doi: 10.1016/j.biomaterials.2011.09.082. [DOI] [PubMed] [Google Scholar]
- 17.Kang E, Min HS, Lee J, Han MH, Ahn HJ, Yoon IC, Choi K, Kim K, Park K, Kwon IC. Nanobubbles from Gas-Generating Polymeric Nanoparticles: Ultrasound Imaging of Living Subjects. Angew. Chem., Int. Ed. 2010;49:524–528. doi: 10.1002/anie.200903841. [DOI] [PubMed] [Google Scholar]
- 18.Janib SM, Moses AS, MacKay JA. Imaging and Drug Delivery Using Theranostic Nanoparticles. Adv. Drug Delivery Rev. 2010;62:1052–1063. doi: 10.1016/j.addr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tassa C, Shaw SY, Weissleder R. Dextran-Coated Iron Oxide Nanoparticles: A Versatile Platform for Targeted Molecular Imaging, Molecular Diagnostics, and Therapy. Acc. Chem. Res. 2011;44:842–852. doi: 10.1021/ar200084x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ambrogio MW, Thomas CR, Zhao Y-L, Zink JI, Stoddart JF. Mechanized Silica Nanoparticles: A New Frontier in Theranostic Nanomedicine. Acc. Chem. Res. 2011;44:903–913. doi: 10.1021/ar200018x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melancon MP, Zhou M, Li C. Cancer Theranostics with Near-Infrared Light-Activatable Multimodal Nanoparticles. Acc. Chem. Res. 2011;44:947–956. doi: 10.1021/ar200022e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan D, Caruthers SD, Hu G, Senpan A, Scott MJ, Gaffney PJ, Wickline SA, Lanza GM. Ligand-Directed Nanobialys as Theranostic Agent for Drug Delivery and Manganese-Based Magnetic Resonance Imaging of Vascular Targets. J. Am. Chem. Soc. 2008;130:9186–9187. doi: 10.1021/ja801482d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taratula O, Schumann C, Naleway MA, Pang AJ, Chon KJ, Taratula O. A Multifunctional Theranostic Platform Based on Phthalocyanine-Loaded Dendrimer for Image-Guided Drug Delivery and Photodynamic Therapy. Mol. Pharmaceutics. 2013;10:3946–3958. doi: 10.1021/mp400397t. [DOI] [PubMed] [Google Scholar]
- 24.Xiao Y, Hong H, Matson VZ, Javadi A, Xu W, Yang Y, Zhang Y, Engle JW, Nickles RJ, Cai W. Gold Nanorods Conjugated with Doxorubicin and cRGD for Combined Anticancer Drug Delivery and PET Imaging. Theranostics. 2012;2:757–768. doi: 10.7150/thno.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoo D, Lee J-H, Shin T-H, Cheon J. Theranostic Magnetic Nanoparticles. Acc. Chem. Res. 2011;44:863–874. doi: 10.1021/ar200085c. [DOI] [PubMed] [Google Scholar]
- 26.Kim D, Jeong YY, Jon S. A Drug-Loaded Aptamer-Gold Nanoparticle Bioconjugate for Combined CT Imaging and Therapy of Prostate Cancer. ACS Nano. 2010;4:3689–3696. doi: 10.1021/nn901877h. [DOI] [PubMed] [Google Scholar]
- 27.Choi KY, Jeon EJ, Yoon HY, Lee BS, Na JH, Min KH, Kim SY, Myung SJ, Lee S, Chen X, et al. Theranostic Nanoparticles Based on PEGylated Hyaluronic Acid for the Diagnosis, Therapy and Monitoring of Colon Cancer. Biomaterials. 2012;33:6186–6193. doi: 10.1016/j.biomaterials.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerweck LE, Seetharaman K. Cellular pH Gradient in Tumor versus Normal Tissue: Potential Exploitation for the Treatment of Cancer. Cancer Res. 1996;56:1194–1198. [PubMed] [Google Scholar]
- 29.Min KH, Kim JH, Bae SM, Shin H, Kim MS, Park S, Lee H, Park RW, Kim IS, Kim K, et al. Tumoral Acidic pH-Responsive MPEG-Poly(β-amino ester) Polymeric Micelles for Cancer Targeting Therapy. J. Controlled Release. 2010;144:259–266. doi: 10.1016/j.jconrel.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 30.Lee HJ, Kim SE, Kwon IK, Park C, Kim C, Yang J, Lee SC. Spatially Mineralized Self-Assembled Polymeric Nanocarriers with Enhanced Robustness and Controlled Drug-Releasing Property. Chem. Commun. 2010;46:377–379. doi: 10.1039/b913732g. [DOI] [PubMed] [Google Scholar]
- 31.Min KH, Lee HJ, Kim K, Kwon IC, Jeong SY, Lee SC. The Tumor Accumulation and Therapeutic Efficacy of Doxorubicin Carried in Calcium Phosphate-Reinforced Polymer Nanoparticles. Biomaterials. 2012;33:5788–5797. doi: 10.1016/j.biomaterials.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 32.Rim HP, Min KH, Lee HJ, Jeong SY, Lee SC. pH-Tunable Calcium Phosphate Covered Mesoporous Silica Nanocontainers for Intracellular Controlled Release of Guest Drugs. Angew. Chem., Int. Ed. 2011;50:8853–8857. doi: 10.1002/anie.201101536. [DOI] [PubMed] [Google Scholar]
- 33.Choi B, Park H, Hwang S, Park J. Preparation of Alginate Beads for Floating Drug Delivery: Effects of CO2 Gas Forming Agents. Int. J. Pharm. 2002;239:81–91. doi: 10.1016/s0378-5173(02)00054-6. [DOI] [PubMed] [Google Scholar]
- 34.Naka K, Tanaka Y, Chujo Y. Effect of Anionic Starburst Dendrimers on the Crystallization of CaCO3 in Aqueous Solution: Size Control of Spherical Vaterite Particles. Langmuir. 2002;18:3655–3658. [Google Scholar]
- 35.Goss SL, Lemons KA, Kerstetter JE, Bogner RH. Determination of Calcium Salt Solubility with Changes in pH and P(CO(2)), Simulating Varying Gastrointestinal Environments. J. Pharm. Pharmacol. 2007;59:1485–1492. doi: 10.1211/jpp.59.11.0004. [DOI] [PubMed] [Google Scholar]
- 36.Wike-Hooley JL, Haveman J, Reinhold JS. The Relevance of Tumour pH to the Treatment of Malignant Disease. Radiother. Oncol. 1984;2:343–366. doi: 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]
- 37.Nido PJD, Glynn P, Buenaventura P, Salama G, Koretsky AP. Fluorescence Measurement of Calcium Transients in Perfused Rabbit Heart Using Rhod 2. Am. J. Physiol. 1998;274:728–741. doi: 10.1152/ajpheart.1998.274.2.H728. [DOI] [PubMed] [Google Scholar]
- 38.Fonteriz RI, Fuente SDI, Moreno A, Lobaton CD, Montero M, Alvarez J. Monitoring Mitochondrial [Ca2+] Dynamics with Rhod-2, Ratiometric Pericam and Aequorin. Cell Calcium. 2010;48:61–69. doi: 10.1016/j.ceca.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Daly WH, Poché D. The Preparation of N-Carboxy-anhydrides of a-Amino Acids Using Bis(trichloromethyl)-carbonate. Tetrahedron Lett. 1988;29:5859–5862. [Google Scholar]
- 40.Lee SJ, Min KH, Lee HJ, Koo AN, Rim HP, Jeon BJ, Jeong SY, Heo JS, Lee SC. Ketal Cross-Linked Poly-(ethylene glycol)-Poly(amino acid)s Copolymer Micelles for Efficient Intracellular Delivery of Doxorubicin. Biomacromolecules. 2011;12:1224–1233. doi: 10.1021/bm101517x. [DOI] [PubMed] [Google Scholar]
- 41.Harada A, Kataoka K. Novel Polyion Complex Micelles Entrapping Enzyme Molecules in the Core: Preparation of Narrowly-Distributed Micelles from Lysozyme and Poly(ethylene glycol)-Poly(aspartic acid) Block Copolymer in Aqueous Medium. Macromolecules. 1998;31:288–294. [Google Scholar]
- 42.Decano JL, Moran AM, Ruiz-Opazo N, Herrera VL. Molecular Imaging of Vasa Vasorum Neovascularization via DEspR-Targeted Contrast-Enhanced Ultrasound Microimaging in Transgenic Atherosclerosis Rat Model. Mol. Imaging Biol. 2011;13:1096–1106. doi: 10.1007/s11307-010-0444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Min KH, Park K, Kim Y-S, Bae SM, Lee S, Jo HG, Park R-W, Kim I-S, Jeong SY, Kim K, et al. Hydro-phobically Modified Glycol Chitosan Nanoparticles-Encapsulated Camptothecin Enhance the Drug Stability and Tumor Targeting in Cancer Therapy. J. Controlled Release. 2008;127:208–218. doi: 10.1016/j.jconrel.2008.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.