Abstract
Purpose
The cell adhesion molecule integrin αvβ3 is an important player in the process of tumor angiogenesis and metastasis. Abegrin™, a fully humanized anti-integrin αvβ3 monoclonal antibody, was currently in clinical trials for cancer therapy. Herein, we labeled Abegrin™ with 111In, evaluated the in vitro and in vivo characteristics, and investigated whether the expression of integrin αvβ3 in tumors could be imaged with 111In-labeled Abegrin™.
Methods
The binding affinity and specificity of Abegrin™ was analyzed using U87MG glioblastoma cells. Abegrin™ was coupled with 1,4,7,10-tetraazadodecane-N,N,N’,N”-tetraacetic acid (DOTA) for 111In radiolabeling. γ Imaging of 111In-DOTA–Abegrin™ was carried out in nude mice bearing both integrin αvβ3-positive U87MG and integrin αvβ3-negative HT-29 tumors. Biodistribution and blocking studies of 111In-DOTA–Abegrin™ were investigated in U87MG tumor-bearing nude mice.
Results
Abegrin™ exhibited high-binding affinity to human integrin αvβ3 expressed on U87MG cells (Kd of 0.35±0.06 nM). The antibody retained antigen-binding affinity/specificity after DOTA conjugation. γ Imaging showed that the tumor uptake of 111In-DOTA–Abegrin™ in integrin αvβ3-positive U87MG tumors was much higher than that in integrin αvβ3-negative HT-29 tumors. In the HT-29 tumors, Abegrin™ was mainly nonspecifically accumulated around the blood vessels, while in the U87MG tumors, besides the nonspecific tumor retention, Abegrin™ also specifically bound the human integrin αvβ3 expressed on the tumor cells. Biodistribution and blocking studies exhibited that the U87MG tumor uptake of 111In-DOTA–Abegrin™ decreased from 14.12±0.44 to 6.93±0.94 percentage of injected dose per gram of tissue after coinjection of excess dose of cold Abegrin™, which confirmed the in vivo integrin αvβ3 binding specificity of 111In-DOTA–Abegrin™.
Conclusions
Abegrin™ showed specific binding to human integrin αvβ3 expressed on the tumor cells. 111In-DOTA–Abegrin™ can specifically target the human integrin αvβ3 expression in the nude mouse model. 111In-DOTA–Abegrin™ has a potential for clinical translation as an agent for integrin αvβ3-positive tumor imaging, evaluating tumor angiogenic status and monitoring the therapeutic efficacy of Abegrin™-based cancer therapy.
Keywords: Tumor, Integrin αvβ3, 111In, Abegrin™, Imaging
Introduction
Integrins are a family of cell surface adhesion receptors consisting of two heterodimeric subunits (α and β), with function to promote invasion and support survival, as well as to regulate cell adhesion [1, 2]. As an important member of this family, integrin αvβ3 is generally not found in normal tissues but is highly expressed on activated endothelial cells during tumor angiogenesis, as well as some tumor cells, such as late-stage glioblastomas, breast and prostate tumors, malignant melanomas, and ovarian carcinomas [3–5]. Increased expression of integrin αvβ3 is closely associated with tumor biological activities, including angiogenesis, cell adhesion, invasion, and metastasis [6, 7]. Inhibition of integrin αvβ3 function using blocking monoclonal antibodies, peptide antagonists, and small peptide mimetics matrix has been shown to be a promising strategy for antiangiogenic cancer therapy [8–10].
In the past decade, radiolabeled arginine-glycine-aspartic acid (RGD) peptides and analogs have been wildly investigated for noninvasive imaging of tumor integrin αvβ3 expression in both animal models and clinical trials [11–14], based on the recognition between RGD and integrin αvβ3. In addition to RGD peptides, anti-integrin αvβ3 antibodies were also generated and evaluated as integrin αvβ3-targeting vehicles. Abegrin™, also known as MEDI-522 or Vitaxin™, is a humanized monoclonal antibody against human integrin αvβ3, which is currently in clinical trials for the treatment of stage IV metastatic melanoma and androgen-independent prostate cancer [15, 16]. Unlike other antibodies, Abegrin™ recognizes the integrin αv and β3 subunits as one entity [16, 17], which makes it much more specific for integrin αvβ3 [18]. In a recent clinical study, Abegrin™ was well tolerated with no evidence of immunogenicity in patients with advanced solid tumors, which guaranteed its further clinical investigation [19].
Radiolabeled monoclonal antibodies have been developed for both diagnosis and therapy of tumors. We recently reported the radioimmunotherapy of 90Y-labeled Abegrin™ in the glioblastoma tumor model [8]. The 90Y-DOTA–Abegrin™ showed promising therapeutic efficacy in the integrin αvβ3-positive tumor models as monitored by 2-deoxy-2-[F-18]fluoro-D-glucose (FDG) and 3’-[F-18] fluoro-3’-deoxythymidine (FLT)-based micro-PET imaging. 99mTc-labeled hLM609-I, the earlier version of Abegrin™, was previously tested for the in vivo imaging of tumor vasculature. However, the imaging was unsuccessful due to the instability of 99mTc labeling [20]. Cai et al. recently evaluated the in vitro and in vivo characterizations of 64Cu (t1/2 = 12.7 h; β+, 17%; β− , 39%)-labeled Abegrin™ in nude mouse tumor models. The probe showed high and specific tumor uptake in integrin αvβ3-positive tumors, which makes the 64Cu-labeled tracer promising for evaluating the pharmacokinetics, tumor targeting efficacy, and dose optimization of Abegrin™ [21]. Comparing with 64Cu, 111In (t1/2 = 2.8 days; γ, 173 keV, 89%; 247 keV, 94%) possesses more suitable half-life for antibody-based tumor imaging, which allows a long-term observation of the in vivo behaviors and clinical imaging with a gamma camera or single photon emission computed tomography (SPECT). Nowadays, a series of 111In-labeled antibodies have been used in clinical trials for tumor imaging and monitoring the therapeutic efficacy of anticancer drugs [22–25]. In this study, we labeled Abegrin™ with 111In using 1,4,7,10-tetraazadodecane-N,N′,N′′,N′′-tetraacetic acid (DOTA) as the chelator, tested the in vitro immunoreactivity and affinity, and investigated the in vivo tumor targeting properties in integrin αvβ3-positive U87MG tumors and also in integrin αvβ3-negative HT-29 tumors.
Materials and Methods
Materials and Reagents
The humanized anti-integrin αvβ3 monoclonal antibody (mAb) Abegrin™ (MEDI-522 or Vitaxin™) was obtained from MedImmune, Inc. (Gaithersburg, MD). Macrocyclic chelating agent DOTA was purchased from Macrocyclics, Inc. (Dallas, TX). 1-Ethyl-3-[3-(dimethylamino)-propyl] carbodiimide (EDC) and N-hydroxysulfonosuccinimide (SNHS) were purchased from Aldrich (St. Louis, MO). 111InCl3 was obtained from Perkin-Elmer Life and Analytical Sciences (North Billarica, MA). PD-10 desalting columns were purchased from GE Healthcare (Piscataway, NJ). All water and buffers used for DOTA conjugation and radiolabeling were passed through a Chelex 100 (Sigma-Aldrich, St. Louis, MO) column to minimize the trace metal contaminants.
Cell Culture and Animal Models
U87MG human glioblastoma cells and HT-29 human colon cancer cells were obtained from American Type Culture Collection (Manassas, VA). U87MG cells were cultured in low glucose Dulbecco’s Modified Eagle’s Medium (DMEM) [21], and HT-29 cells were cultured in high-glucose DMEM culture medium [26]. Both cell lines were cultured in medium supplemented with 10% (v/v) fetal bovine serum at 37°C in a humidified atmosphere with 5% CO2. Female BALB/c nude mice (4~5 weeks of age) were purchased from the Department of Experimental Animal, Peking University Health Science Center. All animal experiments were performed in accordance with guidelines of Peking University Health Science Center Animal Care Committee (Institutional Animal Care and Use Committee at Peking University). U87MG tumor model was established by subcutaneous injection of 2×106 U87MG tumor cells into the right upper flanks of nude mice. When the tumor volume reached 200~300 mm3 (3~4 weeks after inoculation), the U87MG tumor-bearing nude mice were used for biodistribution studies. To establish another tumor model bearing both U87MG and HT-29 tumor xenografts, the U87MG cells (2×106) were first inoculated subcutaneously into the right upper flanks of mice. After about 15 days, HT-29 cells (5×106) were inoculated subcutaneously into left upper flanks of the same nude mice. In this condition, the two tumors reached a similar volume (200~300 mm3 ) after another 13 days, and then they were used for in vivo studies.
Saturation Binding Assay
The binding affinity of mAb Abegrin™ for integrin αvβ3 and the available binding sites per U87MG cell were measured by saturation binding assay [27]. Briefly, 125I-Abegrin™ was generated by incubating Abegrin™ (50 µg) with Na125I (37 MBq) in a vial coated with Iodogen (Sigma-Aldrich, St. Louis, MO) and then purified by a PD-10 column using a previously described method [28]. U87MG cells were seeded in a Filter multiscreen DV plate (96-well; pore size, 0.65 µm; Millipore) and then incubated with increasing concentrations of 125I-Abegrin™ (from 0.033 to 16.67 nM) in binding buffer (20 mM Tris, pH 7.4, 150 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, 1 mM MnCl2, and 0.1% BSA). The total volume was adjusted to 200 µL using binding buffer. For each concentration, nonspecific binding was determined in the presence of an excess (>100-fold) unlabeled mAb Abegrin™. After 4 h of incubation at 4°C, the plate was filtered through a multiscreen vacuum manifold and washed five times with cold phosphate-buffered saline (PBS). The hydrophilic polyvinylidenedifluoride (PVDF) filters were collected, and the radioactivity was determined using a NaI (Tl) γ-counter (Wallac 1470-002, Perkin-Elmer, Finland). A saturation binding curve and Scatchard transformation were obtained by a nonlinear regression analysis, and the dissociation constant (Kd) value of 125I-Abegrin™ and the binding sites per U87MG cell were determined using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA). Each data point represents the average value from triplicate wells.
DOTA Conjugation and 111In Radiolabeling
Abegrin™ was conjugated with DOTA as previously described [21]. Briefly, DOTA was activated by EDC and SNHS at pH 5.5 for 30 min at 4°C with a molar ratio of 10:5:4 (DOTA/EDC/SNHS). The DOTA–N-hydroxysulfosuccinimidyl (OSSu) was then added to Abegrin™ at the molar ratio of DOTA–OSSu/Abegrin™=200:1 in the bicarbonate buffer (pH = 9.0). After incubating overnight at 4°C, the DOTA–Abegrin™ conjugates were then purified by PD-10 column and concentrated by Centricon filter (Millipore, Bedford, MA), and the final concentration was determined using a micro-BCA protein assay kit (Pierce Biotechnology).
For 111In radiolabeling, 74 MBq of 111InCl3 (2 mCi) was diluted in 300 µL of 0.2 M sodium acetate buffer (pH 5.5) and added to 50 µg of DOTA–Abegrin™. The reaction mixture was incubated for 1 h at 39°C with constant shaking. 111In-DOTA–Abegrin™ was then purified by PD-10 column using PBS as the mobile phase.
Immunoreactivity and Specificity
The immunoreactivity of DOTA–Abegrin™ and intact Abegrin™ to integrin α vβ3 was evaluated by whole-cell displacement assay [29]. The U87MG cells were incubated with 125I-Abegrin in the presence of increasing concentrations of Abegrin™ or DOTA–Abegrin™ in a 96-well filter plate. After 2 h of reaction at 4°C, the plates were washed, then the PVDF filters were collected, and measured in a γ-counter as described above. The best-fit 50% inhibitory concentration (IC50) values were calculated by fitting the data with nonlinear regression using Graph Pad Prism 4.0 (GraphPad Software, Inc.). Experiments were performed twice with triplicate samples.
The integrin binding specificity of 111In-DOTA–Abegrin™ was tested using U87MG cells. U87MG cells were seeded in six-well plates and incubated overnight at 37°C to allow adherence. After washing with PBS, 250 µL of the 111In-DOTA–Abegrin solution (10 ng/mL in culture medium) was added to the wells of the plate with or without an excess amount of cold Abegrin™ (5 µg/mL). The cells were then incubated at 4°C for 2 h, after which the cells were washed and then collected by trypsinizing with 0.25% trypsin solution. The cell-associated radioactivity was measured in γ-counter. Results were expressed as percentage of the total added dose per million cells (%AD/106 cells). Experiments were carried out twice with the triplicate samples.
Immunofluorescent Staining
The immunofluorescent staining of tumor cells were performed as we previously described [21, 30]. Briefly, U87MG or HT-29 cells were incubated with Abegrin™ (2 µg/mL) for 1 h at room temperature. After washing, the cells were visualized with Cy3-labeled donkey antihuman IgG (1:500) under the microscope (Carl Zeiss Axiovert 200 M, Carl Zeiss, Thornwood, NY).
U87MG and HT-29 tumor tissue sections (5 µm-thick) were cut from the frozen blocks, mounted on coated slides, fixed in acetone for 10 min, and allowed to dry in the air for 30 min. The sections were blocked with 10% donkey serum for 1 h at room temperature. For murine integrin αvβ3 and human integrin αvβ3 double staining, the sections were incubated with hamster antimouse integrin β3 (1:100; BD Biosciences, San Jose, CA) and Abegrin™ (10 µg/mL) for 1 h at room temperature. After incubating with Cy3-conjugated donkey antihamster secondary antibody (1:200; Jackson ImmunoResearch Laboratories) and fluorescein isothiocyanate (FITC)-conjugated donkey antihuman secondary antibody (1:200), the tumor sections were examined under the microscope (Carl Zeiss Axiovert 200 M, Thornwood, NY).
Integrin αvβ3 Expression on Tumor Cells
The U87MG and HT-29 cells grown in 24-well plates (1 × 105/well) were incubated with ~80,000 cpm of 125I-Abegrin™. For each cell line, nonspecific binding was determined in the presence of an excess (>100-fold) unlabeled Abegrin™. The total volume was adjusted to 250 µL with 1% bovine serum albumin (in PBS). After incubating for 3 h at 4°C, the cells in the plate were washed gently with PBS for three times and then solubilized with 2 M NaOH. The cell-associated activity was measured in a γ-counter. Experiments were carried out twice with triplicate wells. Results were expressed as percentage of added dose per million cells (%AD/106 cells).
Planar γ Imaging
Each nude mouse bearing both U87MG and HT-29 tumor xenografts was injected with ~11.1 MBq (300 µCi) of 111In-DOTA–Abegrin™ via tail vein (n = 3). Animals were anesthetized with intraperitoneal injection of sodium pentobarbital at a dose of 45.0 mg/kg and then placed prone on a two-headed camera (SIEMENS, E. CAM) equipped with a parallel-hole, middle-energy, and high-resolution collimator. Posterior images were acquired at 2, 24, 48, and 120 h postinjection (p.i.) and stored digitally in a 128×128 matrix. The acquisition count limits were set at 200 K.
In Vivo Microdistribution of FITC–Abegrini™ in Tumors
Abegrin™ was conjugated with FITC–NHS (Pierce, Rockford, IL, USA) according to the standard protocol and purified with PD-10 column. The F/P ratio (fluorochrome/protein ratio, i.e., FITC-to-Abegrin™ ratio) of FITC–Abegrin™ after purification was measured based on UV absorbance using the following equation: F/P = 3:1 × A495/[A280 – 0:31 × A495]. The F/P ratio was calculated to be 4.70. The FITC–Abegrin™ was passed through a 0.22-µm Millipore filter and stored at 4°C. A nude mouse bearing both the U87MG and HT-29 tumor xenografts was injected with 200 µg FITC–Abegrin™ via tail vein. At 24 h postinjection, the mouse was anesthetized, sacrificed, and perfused through the heart with 25 mL PBS. The tumors were removed, frozen, and cut into 5-µm-thick slices. The slices were stained with rat antimouse CD31 antibody (1:100; BD Bio-sciences) and then visualized with Cy3-conjugated donkey antirat secondary antibody (1:200; Jackson Immuno-Research Laboratories, Inc.).
Biodistribution and Blocking Studies
Female nude mice bearing U87MG xenografts were injected with 0.74 MBq (20 µCi) of 111In-DOTA–Abegrin™ via tail vein to evaluate the distribution of the tracer in the major organs of mice. At 4, 24, 72, 120, and 168 h p.i., mice were sacrificed. Blood, tumor, major organs, and tissues were collected, wet-weighted, and measured using a γ counter. The results were presented as percentage of injected dose per gram of tissue (%ID/g). Values are expressed as means±SD for a group of four animals (n=4 per group). A blocking study was also performed in a group of four mice by coinjecting 111In-DOTA–Abegrin™ with 400 µg unlabeled Abegrin™. At 24 h p.i., all four animals were sacrificed for determination of organ biodistribution as described above.
Results
Saturation Binding Assay
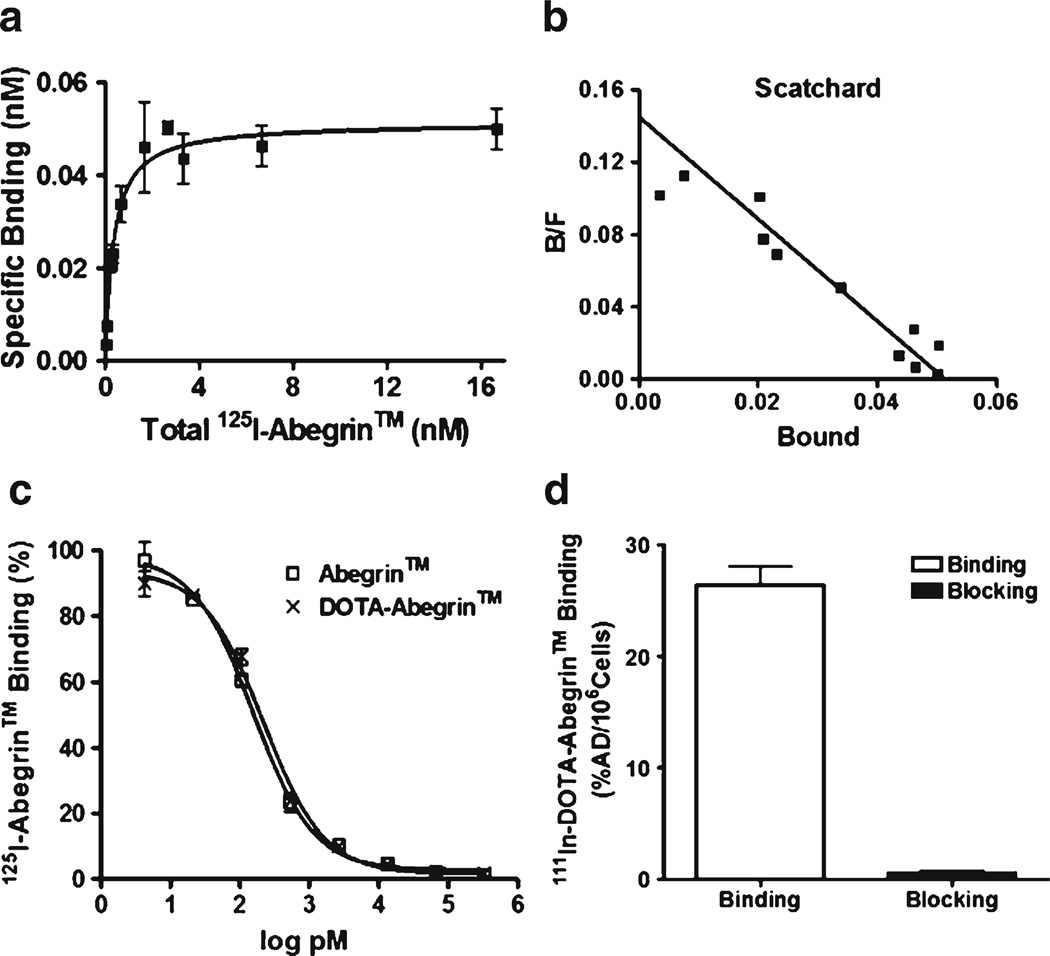
The saturation binding curve and the Scatchard transformation of 125I-Abegrin™ on U87MG cells are shown in Fig. 1a, b. The Kd value for 125I-Abegrin™ was determined to be 0.35±0.06 nM, and the maximum number of binding sites (Bmax) was 2.5 × 105 receptors per U87MG cell, indicating the high affinity of Abegrin™ for integrin αvβ3 and high expression level of integrin αvβ3 on the U87MG cell surface.
Fig. 1.
Saturation binding curve (a) and Scatchard transformation (b) of 111In-DOTA–Abegrin™ to U87MG cells; c the binding of 125I-Abegrin™ to U87MG cells in the presence of increasing concentrations of DOTA–Abegrin™ or Abegrin™; d Binding of 111In-DOTA–Abegrin™ to U87MG cells with or without an excess dose of cold Abegrin™. Each data point represents the mean±SD of triplicate measurements.
Immunoreactivity and Binding Specificity
The immunoreactivity of DOTA–Abegrin™ was compared with Abegrin™ by competition binding assay using 125I-Abegrin™ as a radioligand. As shown in Fig. 1c, no obvious difference was observed between the DOTA–Abegrin™ and Abegrin™, indicating that DOTA conjugation had minimal effect on the immunoreactivity of Abegrin™.
The binding of 111In-DOTA–Abegrin™ to U87MG cells can be significantly inhibited by adding an excess dose of Abegrin™, demonstrating the in vitro specific binding of 111In-DOTA–Abegrin™ to integrin αvβ3 on U87MG cells (Fig. 1d).
Integrin αvβ3 Expression on Tumor Cells
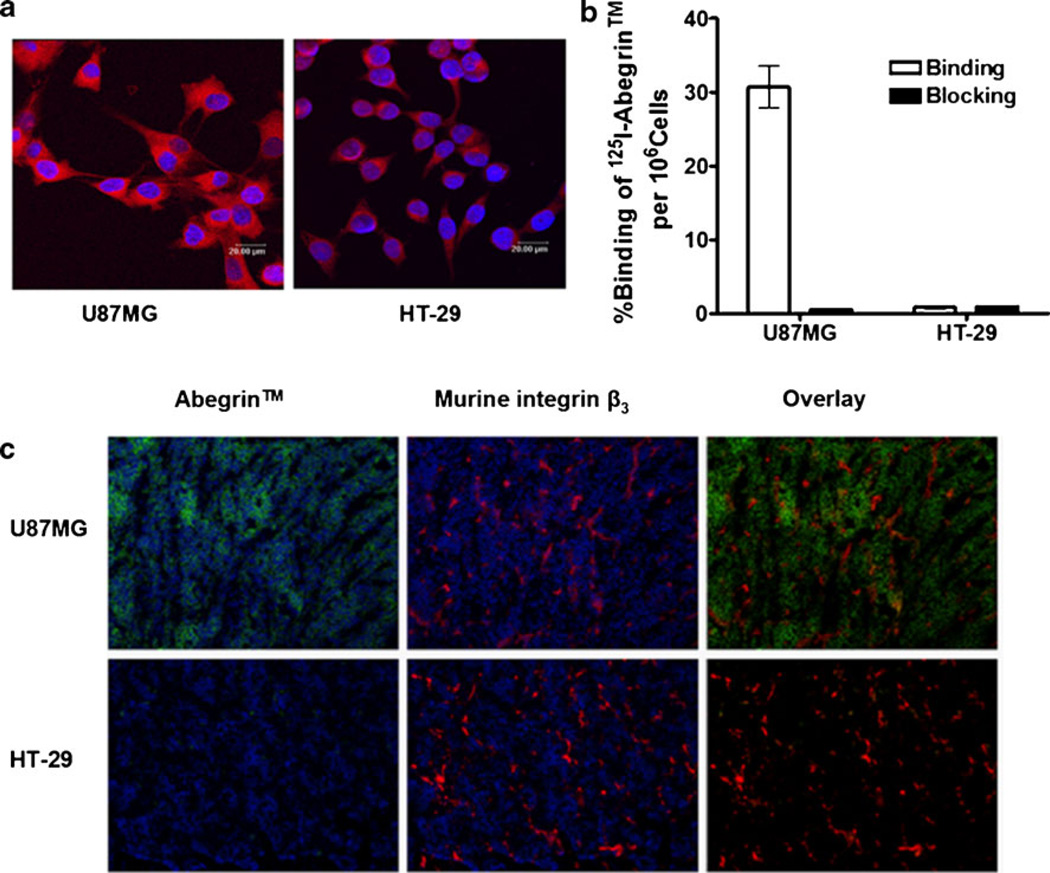
The expression of integrin αvβ3 on U87MG and HT-29 cells was detected by fluorescent staining and radioligand binding assay. As shown in Fig. 2a, Abegrin™ bound strongly to U87MG cells but not HT-29 cells. Prominently, the specific staining of Abegrin™ was observed mostly on the cell membrane as integrin αvβ3 is mainly expressed on the cell surface. The binding fraction of 125I-Abegrin™ on U87MG was about 30%AD/106 cells, and the cell-bound 125I-Abegrin™ was significantly blocked by adding an excess dose of cold Abegrin™, indicating the specific binding of 125I-Abegrin™ on U87MG cells (Fig. 2b). In contrast, HT-29 cells can bind 125I-Abegrin™ only at an extremely low level, which was consistent with the result shown in Fig. 2a. Overall, integrin αvβ3 is highly expressed on U87MG cells but lowly or nonexpressed on HT-29 cells.
Fig. 2.
a Immunofluorescence staining of U87MG and HT-29 cells using Abegrin™ as the primary antibody; b Binding of 125I-Abegrin™ to U87MG or HT-29 cells with or without an excess dose of cold Abegrin™ (n=3, bars represent means±SD); c human integrin αvβ3 (Abegrin™ as the primary antibody) and mouse integrin β3 staining of U87MG and HT-29 tumor sections.
Immunofluorescence Staining of U87MG and HT-29 Tumor Tissues
U87MG and HT-29 frozen tumor sections were stained for human integrin αvβ3 and mouse integrin αvβ3. As shown in Fig. 2c, U87MG tumors were found to be positive for human integrin αvβ3, while HT-29 tumors did not express human integrin αvβ3, which was consistent with the above cell results. Both U87MG and HT-29 tumor tissues expressed murine integrin αvβ3 due to the generation of newborn tumor blood vessels in the xenografted tumors. There was no colocalization between Abegrin™ staining and murine integrin αvβ3 for U87MG tumors because Abegrin™ recognizes only the human tumor cells, but not the mouse tumor vasculature.
Planar γ Imaging
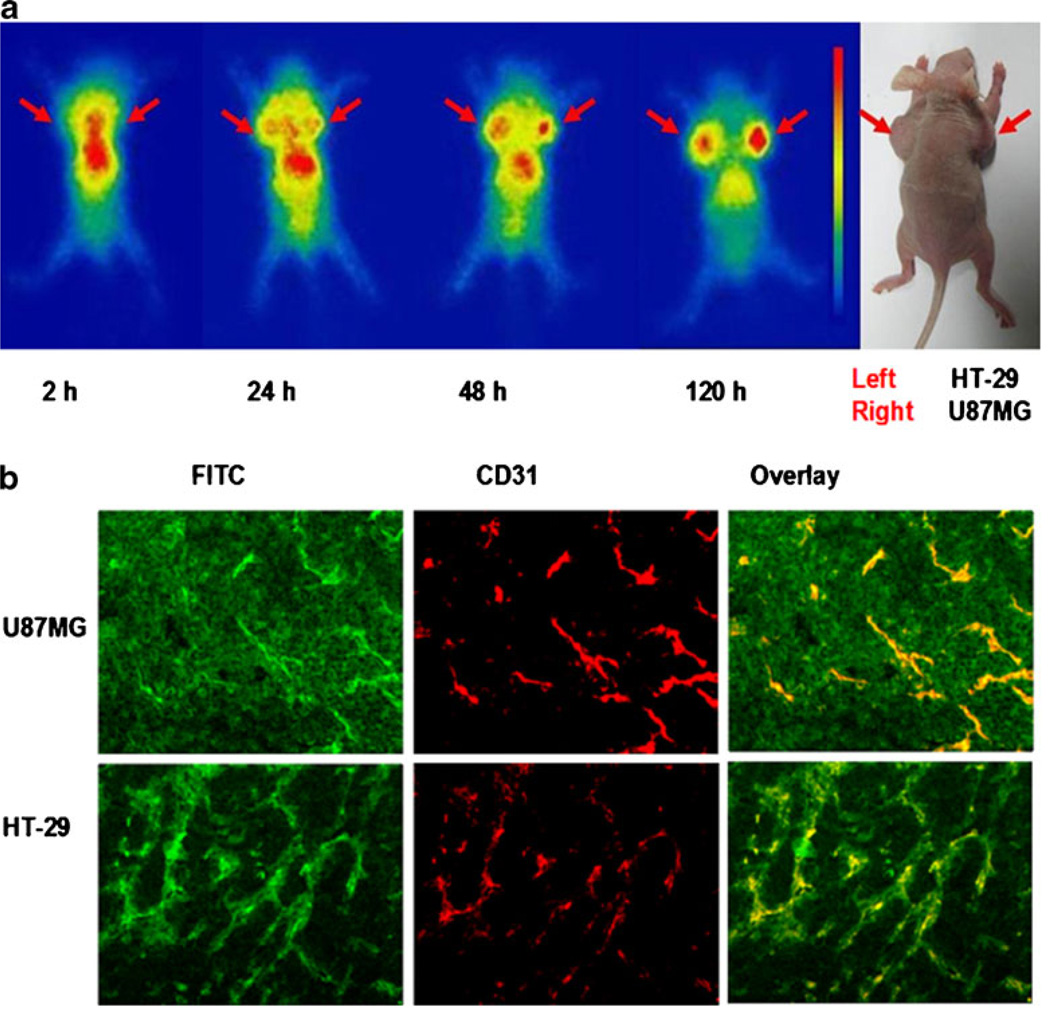
The representative planar γ images acquired at several time points after injection of 111In-DOTA–Abegrin™ are shown in Fig. 3a. Note that although both posterior and anterior images could be acquired simultaneously by the two-headed camera, we only showed the representative posterior images here because they were closer to the mice and had less blurred images than the anterior images. At 2 h post-injection, radioactivity was mainly accumulated in the heart and abdomen. The U87MG (right) and HT-29 (left) tumors were gradually visualized from 24 h postinjection. With the clearance of the radiotracer from normal organs, the tumor visualization was clearer, and the activity accumulation in U87MG tumors was higher than that in HT-29 tumors. At 120 h postinjection, the U87MG tumors showed high contrast of activity accumulation, which was significantly higher than that of HT-29 tumors. Liver is also visible due to the liver clearance of 111In-DOTA–Abegrin™.
Fig. 3.
a Nude mice bearing both U87MG and HT-29 tumor xenografts were injected intravenously with ~300 µCi of 111In-DOTA–Abegrin™, and then planar γ images were acquired at 2, 24, 48, and 120 h postinjection. Arrows indicate the tumor location; b FITC–Abegrin™ was injected intravenously into nude mice bearing both U87MG and HT-29 tumor xenografts. At 24 h postinjection, the tumors were cut into slices and then stained with antimouse CD31 antibody.
In Vivo Microdistribution of FITC–Abegrin™ in Tumors
To investigate the in vivo microdistribution of Abegrin™ in tumors, we labeled Abegrin™ with FITC. FITC–Abegrin™ was injected into nude mouse bearing both U87MG and HT-29 tumors, and the mouse was sacrificed at 24 h postinjection. The tumors and normal organs slices were costained with CD31. We performed the in vivo micro-distribution study at 24 h p.i. because the tumor uptake of Abegrin™ at 24 h p.i. could reach a high level (similar to that at 72 h p.i., see below the ex vivo biodistribution data). Most importantly, we believe that the in vivo stability of FITC–Abegrin™ at 24 h p.i. was much better than that at 72 h p.i. so that the fluorescent signal determined by microdistribution study may truly reflect the Abegrin™ location in tumors. As shown in Fig. 3b, both U87MG and HT-29 tumors showed predominant CD31 positive staining, indicating the high density of tumor vasculature in both of two tumors. The distribution of FITC–Abegrin™ in HT-29 tumor was almost localized around the tumor vasculature as determined by the good overlay of FITC with CD31. In contrast, besides the localization around the blood vessels, FITC–Abegrin™ also showed predominant tumor cell staining on U87MG tumors, indicating that FITC–Abegrin™ could bind to the U87MG tumor cells in vivo.
Biodistribution Studies
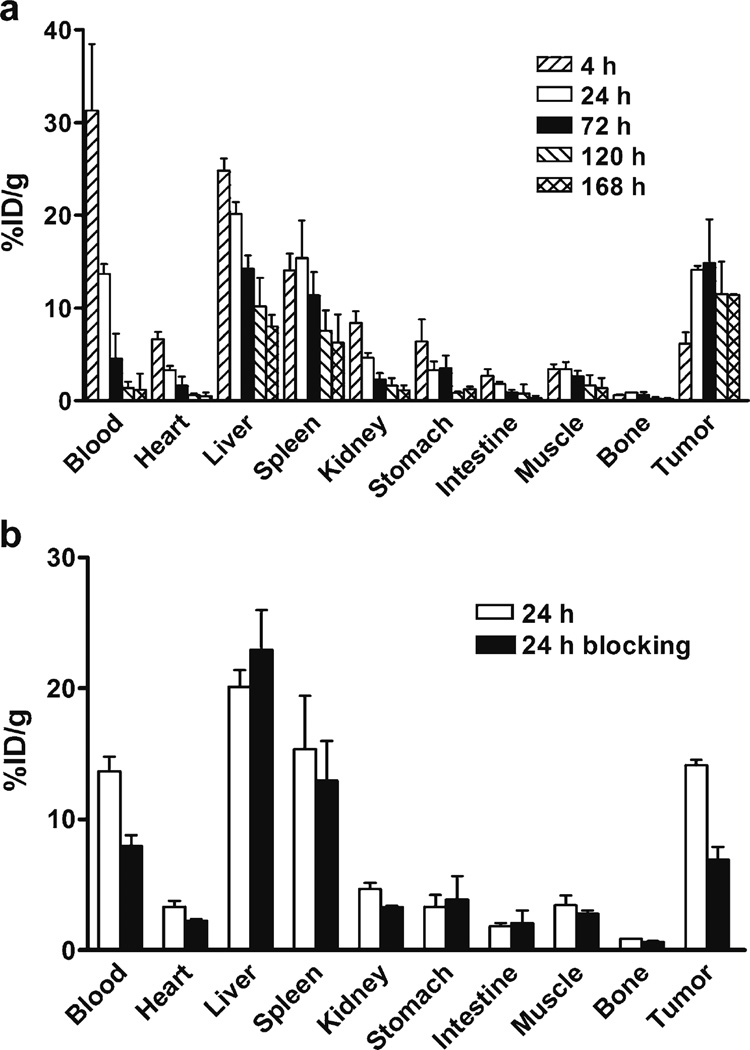
Biodistribution of 111In-DOTA–Abegrin™ was determined in nude mice bearing U87MG tumors, and the results are shown in Fig. 4a. The uptake of 111In-DOTA–Abegrin™ in U87MG tumors increased steadily from 4 h to 72 h p.i and then decreased from 72 h to 168 h p.i.(tumor uptake value was 6.19±1.16, 14.12±0.44, 14.85±4.73, 11.47±3.51, and 11.40±0.07%ID/g at 4, 24, 72, 120, and 168 h, respectively). Radioactivity levels of 111In-DOTA–Abegrin™ in blood were 31.31±7.16%ID/g at 4 h p.i, followed by a rather rapid clearance by the end of 168 h (1.17±1.02%ID/g). 111In-DOTA–Abegrin™ also showed predominant liver and spleen uptake, while the kidney uptake was relatively low. At 72 h p.i, the uptake of 111In-DOTA–Abegrin™ was 14.21±1.47%ID/g for liver, 11.35±2.51%ID/g for spleen, and 2.29±0.71%ID/g for kidney, respectively. The bone uptake of 111In-DOTA–Abegrin™ was very low (less than 1%ID/g at any time point), indicating the in vivo stability of the tracer. With the clearance of 111In-DOTA–Abegrin™ from blood and normal organs, the radioactive ratios of tumor to nontumor increased with time. At 168 h p.i, the ratios were 53.10±15.31 for blood, 1.45±0.24 for liver, 2.13±1.05 for kidney, 11.21±6.30 for muscle, and 49.57±14.75 for bone, respectively.
Fig. 4.
a Biodistribution of 111In-DOTA–Abegrin™ in U87MG tumor-bearing nude mice at 4, 24, 72, 120, and 168 h post-injection; b biodistribution of 111In-DOTA–Abegrin™ in U87MG tumor-bearing nude mice with and without coinjection of an excess dose of cold Abegrin™ as a blocking agent at 24 h postinjection. Data are expressed as %ID/g±SD (n=4 per group).
The tumor-targeting specificity of 111In-DOTA–Abegrin™ was detected by blocking study. The coinjection of an excess dose of cold Abegrin™ with 111In-DOTA–Abegrin™ resulted in a significantly reduced tumor uptake at 24 h p.i. (from 14.12±0.44 to 6.93±0.94%ID/g, n=4, PG0.01), indicating that the radioactivity uptake in U87MG tumors was at least partially integrin αvβ3-mediated. The cold Abegrin™ did not totally block the tumor uptake of 111In-DOTA–Abegrin™, which might be caused by the nonspecific targeting of large molecules, e.g., enhanced permeability and retention (EPR) effect [31]. The blocking study did not significantly affect the radioactivity uptake in other normal organs, such as heart, liver, and spleen, demonstrating that 111In-DOTA–Abegrin™ did not specifically target the normal organs. The blood uptake also decreased after blocking, which might result from the enhanced blood clearance of 111In-DOTA–Abegrin™ when coinjected with an excess dose of Abegrin™ (Fig. 4b).
Discussion
In the present study, the in vitro and in vivo integrin αvβ3 targeting properties of Abegrin™, a fully humanized monoclonal antibody against integrin αvβ3, was investigated, and the 111In-labeled Abegrin™ (111In-DOTA–Abegrin™) was evaluated as a SPECT tracer for molecular imaging of integrin αvβ3 expression.
Recently, Cai et al. investigated the effect of DOTA conjugation with different DOTA/Abegrin™ reaction ratios (20:1, 50:1, 100:1, 200:1, and 1,000:1) on the tumor uptake of the resulting 64Cu-labeled Abegrin™ [21]. The result showed that the ratio of 200:1 was enough for the conjugation and radiolabeling. We conjugated Abegrin™ with DOTA at a reaction ratio of 200:1 for 111In labeling, and the result revealed that the conjugation of DOTA under this condition did not impair the integrin αvβ3 binding specificity and affinity of Abegrin™ (Fig. 1c). It has been well documented for the relatively low in vivo thermodynamic and kinetic stability of 64Cu-DOTA [32–34]. The instability of the 64Cu-DOTA conjugates would result in demetallization and subsequent accumulation in nontarget tissues such as liver [35]. Compared with 64Cu-DOTA–Abegrin™, the liver uptake of 111In-DOTA–Abegrin™ was significantly reduced, possibly because the DOTA-chelating ability for 111In is much stronger than that for 64Cu.
Integrin αvβ3 is highly expressed not only on some tumor cells but also on the activated endothelial cells of the newborn blood vessels [7, 36]. U87MG (human integrin αvβ3-positive) and HT-29 (human integrin αvβ3-negative) tumor cells were inoculated into nude mice; therefore, the two tumor tissues both expressed murine integrin αvβ3 on the tumor neovasculature (Fig. 2c). Abegrin™ cross-reacts with integrin αvβ3 originated from rabbit, chicken, and hamster, but not from mouse [21] so Abegrin™ can recognize only the human integrin αvβ3 on the tumor cells but not the murine integrin αvβ3 on the tumor vasculature. In the human integrin αvβ3-negative HT-29 tumors, FITC–Abegrin™ almost accumulated around the tumor vasculature (Fig. 3b), which was most likely due to the nonspecific targeting of EPR (enhanced permeability and retention [31, 37]) effect of tumors, instead of specific tumor vasculature targeting. In the human integrin αvβ3-positive U87MG tumors, besides the specific targeting of Abegrin™ to the tumor cells, a large amount of antibody molecules also remained untargeted to the vasculature (Fig. 3b), indicating that the accumulation of Abegrin™ in integrin αvβ3-positive tumors was caused by both specific and nonspecific targeting.
Comparing with radiolabeled RGD tracers, 111In-labeled anti-integrin αvβ3 antibody exhibited a quite different pharmacokinetics, such as the slower blood clearance, higher liver and spleen uptake, and high passive tumor targeting. The high nonspecific targeting of Abegrin™ in tumors reminds that caution should be taken when using 111In-DOTA–Abegrin™ as a noninvasive imaging agent to directly quantify the integrin αvβ3 expression level in vivo. Aerts et al. recently found a disparity between the tumor uptake of 89Zr-labeled anti-EGFR antibody cetuximab and in vivo EGFR expression on tumors [38], which may be due to the complex microenvironment of tumors (e.g., inadequate vasculature and perfusion). In another report [39], the tumor uptake of 111In-labeled anti-HER2 antibody trastuzumab showed much stronger, nonlinear associations with HER2 density if it was corrected for nonspecific IgG localization (r2 = 0.99), but without the correction, the association between HER2 density and tumor uptake was poor (r2 = 0.22). Therefore, further study using 111In-labeled IgG or HSA as the nonspecific imaging agents to quantify the nonspecific uptake values in different tumor models may be helpful for quantitatively measuring the specific targeting values of 111In-DOTA–Abegrin™, which would provide more information on the receptor expression levels.
It also should be noted that the in vivo targeting property of 111In-DOTA–Abegrin™ in the present study may not truly reflect the clinical situation because Abegrin™ does not bind the murine integrin αvβ3 expressed on tumor new-bone vasculatures of mice. In human, Abegrin™ would bind both integrin αvβ3-positive tumor cells and the activated endothelial cells in tumor blood vessels. The high human integrin αvβ3 binding affinity and specificity of 111In-DOTA–Abegrin™ as demonstrated in this study guarantee that 111In-DOTA–Abegrin™ is promising for both integrin αvβ3-positive tumors imaging and tumor angiogenesis imaging in clinic.
In conclusion, 111In-labeled Abegrin™ exhibited high in vitro specificity and immunoreactivity against integrin αvβ3 on tumor cells. Due to the specific receptor targeting, the in vivo uptake of 111In-DOTA–Abegrin™ in integrin αvβ3-positive tumors was significantly higher than that in integrin αvβ3-negative tumors. The human integrin αvβ3-specific targeting of 111In-DOTA–Abegrin™ may be translated to clinic for noninvasive measurement of integrin αvβ3 in the tumors and also providing essential information in the evaluation of Abegrin™-based antiangiogenic therapy, as well as 90Y-Abegrin™-based radioimmunotherapy.
Acknowledgments
We thank Mr. Zhi Yang and Mr. Cunjing Jin for their excellent technical assistance with γ-imaging and biodistribution studies. This work is jointly supported by NSFC projects (30930030, 30870728, 30900373, and 20820102035), an 863 project (2007AA02Z467), and grants from the Ministry of Science and Technology of China (2009ZX09103-733, 2009ZX09301-010, and 2009ZX09103-746).
References
- 1.Eble JA, Haier J. Integrins in cancer treatment. Curr Cancer Drug Targets. 2006;6:89–105. doi: 10.2174/156800906776056518. [DOI] [PubMed] [Google Scholar]
- 2.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 3.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 4.Seftor RE, Seftor EA, Gehlsen KR, Stetler-Stevenson WG, Brown PD, Ruoslahti E, Hendrix MJ. Role of the alpha v beta 3 integrin in human melanoma cell invasion. Proc Natl Acad Sci U S A. 1992;89:1557–1561. doi: 10.1073/pnas.89.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eliceiri BP, Cheresh DA. The role of alphav integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest. 1999;103:1227–1230. doi: 10.1172/JCI6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar CC. Integrin alpha v beta 3 as a therapeutic target for blocking tumor-induced angiogenesis. Curr Drug Targets. 2003;4:123–131. doi: 10.2174/1389450033346830. [DOI] [PubMed] [Google Scholar]
- 7.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 8.Veeravagu A, Liu Z, Niu G, Chen K, Jia B, Cai W, Jin C, Hsu AR, Connolly AJ, Tse V, et al. Integrin alphavbeta3-targeted radioimmunotherapy of glioblastoma multiforme. Clin Cancer Res. 2008;14:7330–7339. doi: 10.1158/1078-0432.CCR-08-0797. [DOI] [PubMed] [Google Scholar]
- 9.Shi J, Jia B, Liu Z, Yang Z, Yu Z, Chen K, Chen X, Liu S, Wang F. 99mTc-labeled bombesin(7–14)NH2 with favorable properties for SPECT imaging of colon cancer. Bioconjug Chem. 2008;19:1170–1178. doi: 10.1021/bc700471z. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Wang F, Chen X. Integrin alpha v beta 3-targeted cancer therapy. Drug Development Research. 2008;69:329–339. doi: 10.1002/ddr.20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beer AJ, Grosu AL, Carlsen J, Kolk A, Sarbia M, Stangier I, Watzlowik P, Wester HJ, Haubner R, Schwaiger M. [18F]galacto-RGD positron emission tomography for imaging of alphavbeta3 expression on the neovasculature in patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6610–6616. doi: 10.1158/1078-0432.CCR-07-0528. [DOI] [PubMed] [Google Scholar]
- 12.Beer AJ, Haubner R, Wolf I, Goebel M, Luderschmidt S, Niemeyer M, Grosu AL, Martinez MJ, Wester HJ, Weber WA, et al. PET-based human dosimetry of 18F–galacto-RGD, a new radiotracer for imaging alpha v beta3 expression. J Nucl Med. 2006;47:763–769. [PubMed] [Google Scholar]
- 13.Morrison MS, Ricketts SA, Barnett J, Cuthbertson A, Tessier J, Wedge SR. Use of a novel Arg-Gly-Asp radioligand, 18F–AH111585, to determine changes in tumor vascularity after antitumor therapy. J Nucl Med. 2009;50:116–122. doi: 10.2967/jnumed.108.056077. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Niu G, Shi J, Liu S, Wang F, Liu S, Chen X. (68)Ga-labeled cyclic RGD dimers with Gly(3) and PEG (4) linkers: promising agents for tumor integrin alpha (v)beta (3) PET imaging. Eur J Nucl Med Mol Imaging. 2009;36:947–957. doi: 10.1007/s00259-008-1045-1. [DOI] [PubMed] [Google Scholar]
- 15.Tucker GC. Integrins: molecular targets in cancer therapy. Curr Oncol Rep. 2006;8:96–103. doi: 10.1007/s11912-006-0043-3. [DOI] [PubMed] [Google Scholar]
- 16.Gutheil JC, Campbell TN, Pierce PR, Watkins JD, Huse WD, Bodkin DJ, Cheresh DA. Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin alphavbeta3. Clin Cancer Res. 2000;6:3056–3061. [PubMed] [Google Scholar]
- 17.Wu H, Beuerlein G, Nie Y, Smith H, Lee BA, Hensler M, Huse WD, Watkins JD. Stepwise in vitro affinity maturation of Vitaxin, an alphav beta3-specific humanized mAb. Proc Natl Acad Sci USA. 1998;95:6037. doi: 10.1073/pnas.95.11.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai W, Chen X. Anti-angiogenic cancer therapy based on integrin alphavbeta3 antagonism. Anticancer Agents Med Chem. 2006;6:407–428. doi: 10.2174/187152006778226530. [DOI] [PubMed] [Google Scholar]
- 19.Delbaldo C, Raymond E, Vera K, Hammershaimb L, Kaucic K, Lozahic S, Marty M, Faivre S. Phase I and pharmacokinetic study of etaracizumab (Abegrin), a humanized monoclonal antibody against alphavbeta3 integrin receptor, in patients with advanced solid tumors. Invest New Drugs. 2008;26:35–43. doi: 10.1007/s10637-007-9077-0. [DOI] [PubMed] [Google Scholar]
- 20.Posey JA, Khazaeli MB, DelGrosso A, Saleh MN, Lin CY, Huse W, LoBuglio AF. A pilot trial of Vitaxin, a humanized anti-vitronectin receptor (anti alpha v beta 3) antibody in patients with metastatic cancer. Cancer Biother Radiopharm. 2001;16:125–132. doi: 10.1089/108497801300189218. [DOI] [PubMed] [Google Scholar]
- 21.Cai W, Wu Y, Chen K, Cao Q, Tice DA, Chen X. In vitro and in vivo characterization of 64Cu-labeled Abegrin, a humanized monoclonal antibody against integrin alpha v beta 3. Cancer Res. 2006;66:9673–9681. doi: 10.1158/0008-5472.CAN-06-1480. [DOI] [PubMed] [Google Scholar]
- 22.Scheer MG, Stollman TH, Boerman OC, Verrijp K, Sweep FC, Leenders WP, Ruers TJ, Oyen WJ. Imaging liver metastases of colorectal cancer patients with radiolabelled bevacizumab: Lack of correlation with VEGF-A expression. Eur J Cancer. 2008;44:1835–1840. doi: 10.1016/j.ejca.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 23.Iagaru A, Gambhir SS, Goris ML. 90Y–ibritumomab therapy in refractory non-Hodgkin’s lymphoma: observations from 111In-ibritu-momab pretreatment imaging. J Nucl Med. 2008;49:1809–1812. doi: 10.2967/jnumed.108.052928. [DOI] [PubMed] [Google Scholar]
- 24.Pandit-Taskar N, O’Donoghue JA, Morris MJ, Wills EA, Schwartz LH, Gonen M, Scher HI, Larson SM, Divgi CR. Antibody mass escalation study in patients with castration-resistant prostate cancer using 111In-J591: lesion detectability and dosimetric projections for 90Y radioimmunotherapy. J Nucl Med. 2008;49:1066–1074. doi: 10.2967/jnumed.107.049502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behr TM, Behe M, Wormann B. Trastuzumab and breast cancer. N Engl J Med. 2001;345:995–996. doi: 10.1056/NEJM200109273451312. [DOI] [PubMed] [Google Scholar]
- 26.Banning A, Kipp A, Schmitmeier S, Lowinger M, Florian S, Krehl S, Thalmann S, Thierbach R, Steinberg P, Brigelius-Flohe R. Glutathione Peroxidase 2 Inhibits Cyclooxygenase-2-Mediated Migration and Invasion of HT-29 Adenocarcinoma Cells but Supports Their Growth as Tumors in Nude Mice. Cancer Res. 2008;68:9746–9753. doi: 10.1158/0008-5472.CAN-08-1321. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Yu Z, He W, Ma S, Sun L, Wang F. In-vitro internalization and in-vivo tumor uptake of anti-EGFR monoclonal antibody LA22 in A549 lung cancer cells and animal model. Cancer Biother Radiopharm. 2009;24:15–24. doi: 10.1089/cbr.2008.0537. [DOI] [PubMed] [Google Scholar]
- 28.Shao W, Zhao S, Liu Z, Zhang J, Ma S, Sato JD, Zhang P, Tong M, Han J, Wang Y, et al. Inhibition of human tumor xenograft growth in nude mice by a conjugate of monoclonal antibody LA22 to epidermal growth factor receptor with anti-tumor antibiotics mitomycin C. Biochem Biophys Res Commun. 2006;349:816–824. doi: 10.1016/j.bbrc.2006.08.114. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z, Yan Y, Chin FT, Wang F, Chen X. Dual Integrin and Gastrin-Releasing Peptide Receptor Targeted Tumor Imaging Using (18)F-labeled PEGylated RGD-Bombesin Heterodimer (18)F-FB-PEG (3)-Glu-RGD-BBN. J Med Chem. 2009;52:425–432. doi: 10.1021/jm801285t. [DOI] [PubMed] [Google Scholar]
- 30.He Q, Liu Z, Jia B, Li X, Shi J, Zhang J, Lan F, Yang Z, Liu Y, Shen L, et al. In vivo gamma imaging of the secondary tumors of transplanted human fetal striatum neural stem cells-derived primary tumor cells. Neuroreport. 2008;19:1009–1014. doi: 10.1097/WNR.0b013e328303c020. [DOI] [PubMed] [Google Scholar]
- 31.Maeda H, Fang J, Inutsuka T, Kitamoto Y. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. Int Immunopharmacol. 2003;3:319–328. doi: 10.1016/S1567-5769(02)00271-0. [DOI] [PubMed] [Google Scholar]
- 32.Boswell CA, Sun X, Niu W, Weisman GR, Wong EH, Rheingold AL, Anderson CJ. Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J Med Chem. 2004;47:1465–1474. doi: 10.1021/jm030383m. [DOI] [PubMed] [Google Scholar]
- 33.Liu Z, Li Z, Cao Q, Liu S, Wang F, Chen X. Small-Animal PET of Tumors with 64Cu-Labeled RGD-Bombesin Heterodimer. J Nucl Med. 2009;50:1168–1177. doi: 10.2967/jnumed.108.061739. [DOI] [PubMed] [Google Scholar]
- 34.Sprague JE, Peng Y, Sun X, Weisman GR, Wong EH, Achilefu S, Anderson CJ. Preparation and biological evaluation of copper-64-labeled tyr3-octreotate using a cross-bridged macrocyclic chelator. Clin Cancer Res. 2004;10:8674–8682. doi: 10.1158/1078-0432.CCR-04-1084. [DOI] [PubMed] [Google Scholar]
- 35.Prasanphanich AF, Nanda PK, Rold TL, Ma L, Lewis MR, Garrison JC, Hoffman TJ, Sieckman GL, Figueroa SD, Smith CJ. [64Cu-NOTA-8-Aoc-BBN(7–14)NH2] targeting vector for positron-emission tomography imaging of gastrin-releasing peptide receptor-expressing tissues. Proc Natl Acad Sci U S A. 2007;104:12462–12467. doi: 10.1073/pnas.0705347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gladson CL. Expression of integrin alpha v beta 3 in small blood vessels of glioblastoma tumors. J Neuropathol Exp Neurol. 1996;55:1143–1149. doi: 10.1097/00005072-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 38.Aerts HJ, Dubois L, Perk L, Vermaelen P, van Dongen GA, Wouters BG, Lambin P. Disparity between in vivo EGFR expression and 89Zr-labeled cetuximab uptake assessed with PET. J Nucl Med. 2009;50:123–131. doi: 10.2967/jnumed.108.054312. [DOI] [PubMed] [Google Scholar]
- 39.McLarty K, Cornelissen B, Scollard DA, Done SJ, Chun K, Reilly RM. Associations between the uptake of 111In-DTPA-trastuzumab, HER2 density and response to trastuzumab (Herceptin) in athymic mice bearing subcutaneous human tumour xenografts. Eur J Nucl Med Mol Imaging. 2009;36:81–93. doi: 10.1007/s00259-008-0923-x. [DOI] [PubMed] [Google Scholar]