Abstract
BP180 (type XVII collagen) is a transmembrane protein expressed in a variety of cell types. It is also the target of autoantibodies in cutaneous autoimmune disease including bullous pemphigoid and pemphigoid gestationis, a disease unique to pregnancy. The purpose of this study was to determine the prevalence and specificity of cutaneous autoantibodies in a cohort of pregnant women. De-identified sera were collected from pregnant women (n = 299) and from non-pregnant controls (n = 134). Sera were analyzed by ELISA for the presence of IgG and IgE autoantibodies directed against several cutaneous autoantigens. IgE antibodies against the NC16A domain of BP180 were detected in 7.7% of pregnant women, compared to 2.2% of healthy controls (p = 0.01). No increase in total or cutaneous autoantigen specific IgG was seen. Total serum IgE was within the normal range. Full-length BP180 was detected by western immunoblot in epidermal, keratinocyte, placental and cytotrophoblast (CTB) cell lysates. Furthermore, flow cytometry and indirect immunofluorescence confirmed the expression of BP180 on the surface of cultured CTBs. Finally, it was demonstrated that IgE antibodies in the pregnancy sera labeled not only cultured CTBs, but also the placental amnion and cutaneous basement membrane zone using indirect immunofluorescence. We conclude that some pregnant women develop antibodies specific for BP180, and that these autoantibodies are capable of binding both CTB and the placental amnion, potentially affecting placental function.
Keywords: IgE, Autoimmunity, Autoantibody, Pregnancy, Type XVII collagen
1. Introduction
During pregnancy the maternal immune system must balance the ability to tolerate fetal cells that express both maternal and paternal proteins with the ability to fight infections and prevent tumor growth. To maintain this balance, a relative shift from Th1 to Th2 immunity occurs. This shift causes an increase in the production of specific cytokines, IL-4, IL-5, IL-6, IL-10 and IL-13, which promote B cell expansion and antibody class switching. Total serum IgE and IL-4, a cytokine that stimulates class switching to IgE, are also elevated during pregnancy (Amoudruz et al., 2006; Persson et al., 2008). These immunologic changes have variable effects on the course and severity of autoimmune diseases depending on their etiology.
An autoimmune disease that develops under the unique conditions associated with pregnancy is pemphigoid gestationis (PG). PG is an autoantibody-mediated blistering skin disease that typically occurs during the second or third trimester of pregnancy and remits shortly after delivery. PG usually presents as urticarial plaques in the umbilical area that develop into tense, subepidermal blisters (Castro et al., 2006; Shornick et al., 1983). Twenty percent of infants born to women with PG develop transient blistering in the neonatal period due to transplacental passage of maternal autoantibodies (Shornick et al., 1983). These infants also tend to be small for gestational age (SGA), although increased mortality is not seen (Lawley et al., 1978; Ambros-Rudolf et al., 2006; Chi et al., 2009; Holmes and Black, 1984; Mascaro et al., 1995; Shornick and Black, 1992). Previous studies suggest the effects on the fetus are due to antibody accumulation in the placenta, resulting in placental insufficiency (Holmes and Black, 1984). However, the long-term outcomes of infants born to mothers with PG have not been sufficiently studied.
The target of the PG autoantibodies is BP180 (type XVII collagen) (Giudice et al., 1993), a cell attachment protein expressed in a variety of cells, including placental CTBs, epidermal keratinocytes and epithelial cells of the amniotic membrane (Fairley et al., 1995; Huilaja et al., 2008). Both IgG and IgE-class autoantibodies specific for BP180 have been observed in patients with PG (Chapman et al., 2007; Giudice et al., 1993). These IgG antibodies have been shown to induce dermal–epidermal separation in human skin cryosections, although studies evaluating pathogenicity of each isotype have not been conducted (Herrero-Gonzalez et al., 2006).
Interestingly, we have noted a higher incidence of IgE-class anti-BP180 autoantibodies in the sera of healthy pregnant women compared with non-pregnant controls (Fairley, unpublished results). Furthermore, a pathogenic role for IgE autoantibodies has been identified in a variety of autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis and autoimmune thyroiditis (Mikol et al., 2006; Valenta et al., 2009). Of particular interest, anti-SSA IgE antibodies were associated with fetal loss in lupus patients (Sekigawa et al., 2008). Similarly, anti-BP180 IgE autoantibodies are pathogenic in bullous pemphigoid (BP), an autoimmune disease with the same antigenic target as PG, causing the degranulation of mast cells, early urticarial plaque development, and recruitment of inflammatory cells to the skin (Fairley et al., 2007).
The goals of the current study were to determine the prevalence and specificity of cutaneous autoantibodies in pregnant women in order to understand better the role of these autoantibodies in PG and to determine the potential for placental binding of these autoantibodies in vivo. Our overall hypothesis is while only a small fraction of pregnant women who develop cutaneous autoantibodies will go on to develop PG, the presence of these autoantibodies might serve as an informative marker for other negative pregnancy outcomes such as SGA and prematurity.
2. Materials and methods
2.1. Sera
De-identified sera (n = 299), drawn from women at 16–20 weeks gestation, were obtained from the University of Iowa Hygienic Laboratory (Iowa City, IA). Control sera were collected from non-pregnant women (n = 134), in the same age distribution as pregnant women in the state of Iowa. Fifty-six of the women in the control group had previous pregnancies. Written consent was obtained from all control patients. This study was approved by the Institutional Review Board at the University of Iowa and was performed in adherence to the Declaration of Helsinki Principles.
2.2. ELISA
2.2.1. Total IgE and BP180-specific IgE
Total serum IgE level was measured by a human IgE capture ELISA (Bethyl Laboratories, Montgomery, TX), according to the manufacturer's standard protocol. In our hands, the minimum detectable level of IgE per sample is ~2 ng/ml. One IU of IgE = 2.4 ng/ml and the normal range of IgE is typically 0–90 IU/ml (0–216 ng/ml).
IgE-class autoantibodies against the primary antigenic region of BP180, termed NC16A, were measured by ELISA as previously described (Messingham et al., 2009). The minimum index value for a positive test was 18.92 U.
2.2.2. IgG specific for BP180, BP230, DSG1 and DSG3
IgG class antibodies against known cutaneous autoantigens BP180, BP230, DSG1 and DSG3 (Amagai, 1995; Giudice et al., 1993) were measured using commercially available ELISA kits (MBL, Nagoya, Japan), according to the manufacturer's standard protocols. All samples were tested for BP180-specific IgG with a minimum positive test 9 U/ml. Only the first 100 patient and control samples collected were screened for IgG autoantibodies to BP230, DGS1 and DSG3 with minimum positive tests of 9 U/ml, 14 U/ml and 9 U/ml, respectively.
2.3. Flow cytometry
Immortalized (HTR-8/SVneo) human CTBs (Graham et al., 1993) were routinely cultured in DMEM/10% FCS (GIBCO/Invitrogen, Carlsbad, CA). For comparison, primary cultures of normal human keratinocytes (KTC) were routinely cultured in keratinocyte serum free media (KSFM) supplemented with BPE, 0.2% (v/v); bovine insulin, 5 μg/ml; hydrocortisone, 0.18 μg/ml; bovine transferrin, 5 μg/ml; and human epidermal growth factor, 0.2 ng/ml (all from GIBCO/Invitrogen, Carlsbad, CA). All cells were maintained at less than 70% confluence on untreated tissue culture plastic at 37 °C, 5% CO2, 95% air. Cells were detached by incubation with 5 mM Na EDTA in PBS for 10–14 min at room temperature with occasional agitation, and a single cell suspension was produced by pipetting detached cells with an equal volume of KSFM. Cells were washed and resuspended in FACS buffer (PBS/10% FCS/0.002% NaN3).
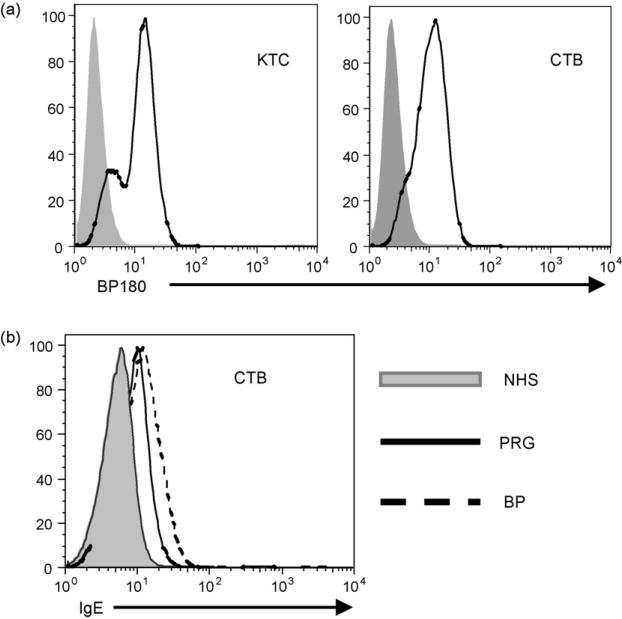
To examine surface expression of BP180 on CTB and KTC (positive control), cells were incubated (60 min, 4 °C) with a monoclonal antibody (HD18; 5 μg/ml) that recognizes the NC16A region of BP180 (Pohla-Gubo et al., 1995), or isotype control. After washing twice in FACS buffer, bound antibody was detected with a pre-determined optimal concentration of FITC-conjugated anti-mouse IgG (30 min, 4 °C Bethyl Laboratories, Montgomery, TX). Cells were washed and analyzed by flow cytometry. As shown in Fig. 1 KTC (MFI = 11.02 ± 0.13) and CTB (MFI = 10.98 ± 0.04) have similar levels of BP180 on their surface. Whether BP180-specific IgE, present in pregnancy sera, could bind CTB cells was determined through incubation (30 min, 4 °C) of cell suspensions with a 1:10 dilution of non-pregnant (NHS, negative) control sera, a serum obtained from a patient with BP or a pregnant patient (PRG), both known to have high levels of BP180-specific IgE by ELISA. After washing, bound IgE was detected with FITC-conjugated anti-human IgE (Bethyl Laboratories, Montgomery, TX). Immunostained cells (5 × 104) were analyzed by flow cytometry. Histograms are representative of three independent experiments.
Fig. 1.
Expression of BP180 and binding of IgE to the surface of cultured cytotrophoblasts. (a) Surface expression of BP180 on keratinocytes (KTC) and placental cytotrophoblasts (CTB) was evaluated using isotype control (shaded histogram) or a monoclonal antibody (HD18) that recognizes the NC16A region of BP180 followed by FITC-conjugated anti-mouse IgG. (b) CTB were incubated with serum obtained from a BP patient (BP; dashed line) or a pregnant patient (PRG; solid line)-both with high levels of BP180 IgE-or a healthy control (NHS; shaded histogram) and anti-human IgE-FITC and flow cytometry were used to detect bound antibody. Histograms are representative of at least 3 independent experiments.
2.4. Western blot
Cells, no more than 70% confluent in a T75 flask, were washed 2× in cold PBS and lysed by the addition 2 ml of ice-cold RIPA buffer (1% NP40, 0.5% sodium deoxycholate, 0.1% SDS in PBS, 1× EDTA-free protease inhibitor (Roche Diagnostics, Indianapolis, IN) + PMSF (10 mg/ml) and flasks were incubated for 5 min on ice. Cells were harvested, on ice, sonicated and the resulting lysates were centrifuged for 10 min at 10,000 × g, 4 °C to eliminate cell debris.
Tissue samples, were cut into small pieces and placed in ice-cold RIPA buffer + PMSF (10 mg/ml). Tissue was homogenized on ice, until no visible pieces remained. Lysates were centrifuged for 10 min at 10,000 × g, 4 °C, to eliminate cell debris. Total protein content of lysates and tissue samples was assayed using the BCA protein assay (Pierce, Rockford, IL) and samples were stored at −20 °C until use.
The presence of BP180 in cell or tissue lysates was determined by immunoblot using a polyclonal antibody (R136) against the cellular protein BP180 (Balding et al., 1997). Cell or tissue lysates (20 μg/well) were loaded on 7.5% SDS-PAGE and transferred onto nitrocellulose (100 V, 70 min). The membrane was incubated with blocking solution (5% BSA in 1 M tris-buffered saline containing 0.1% Tween-20 (TBST)) for 1 h at room temperature. R136 antibody was diluted 1:5000 in blocking solution and incubated with membrane overnight at 4 °C with gentle agitation. The membrane was washed 3 times with TBST and incubated with HRP-conjugated anti-rabbit IgG diluted 1:40,000 in blocking solution for 1 h at room temperature, washed 3 times, and developed using a standard chemiluminescence (Pierce, Rockford, IL).
2.5. Immunofluorescence
2.5.1. Immunostaining of CTBs
CTBs (~7000/well) were cultured overnight in 4 well Lab Tek chamberslides. The next day, media was aspirated and cells were washed with cold PBS and fixed in a 50%/50% (v/v) methanol:acetone solution for 10 min at RT. Cells were washed twice with cold PBS and primary antibodies diluted in PBS. Antibodies used included sera obtained from pregnant women with detectable levels of BP180-specific IgE by ELISA (PRG sera), a normal non-pregnant, non-autoimmune female (NHS; negative control) and a BP patient (BP sera; positive control). A BP180-specific monoclonal antibody (R136) or rabbit serum (R IgG) were used as additional controls. For CTBs, rabbit serum (1:500) and human serum (1:2) was incubated with cells for 30 min at RT. After extensive washing, secondary antibodies (anti-mouse IgG-Alexa 488 (1:800) or anti-human IgE-FITC (1:200) were added for 30 min at RT. Finally, slides were washed, nuclei were counterstained (DAPI) and cells were viewed using a Nikon epifluorescent microscope.
2.5.2. Indirect immunofluorescence
To assess the localization of binding within the placenta, fresh cryosections (6 μm) of human placenta or skin (control) were incubated with the human (1:20) or rabbit antibodies (1:500) described above, washed extensively in PBS, and bound antibodies were detected with anti-human IgE-FITC (1:200). Cell nuclei were counterstained (DAPI) and slides were viewed as above.
2.6. Statistics
Analysis of ELISA data revealed that data was not normally distributed so non-parametric analysis was employed. A Mann–Whitney Test was utilized to determine statistical significance and p values ≤0.05 were considered significant. Data was analyzed using GraphPad InStat 3 Statistical Software (GraphPad, San Diego, CA). Each data point represents the mean determination of duplicate samples.
3. Results
3.1. Pregnant women have an increased incidence of IgE-class autoantibodies to BP180
To determine the incidence and specificity of autoantibodies, sera from pregnant and non-pregnant women were screened by ELISA for IgG and IgE-class autoantibodies directed against BP180, BP230, desmoglein (DSG)1, and DSG3. Evaluation of sera from pregnant women (n = 299) did not reveal a difference in the level of BP180-specific IgG compared to controls (n = 134); however, 7.7% of pregnant women had BP180-specific IgE compared to 2.2% of healthy controls (Mann–Whitney Test, p = 0.01). Further evaluation revealed in increase in total serum IgE in the pregnant women (161.38 ng/ml ± SD 674.36) compared to healthy controls (69.80 ng/ml ± SD 140.76) that was not statistically significant (Mann–Whitney Test, p = 0.30). A subset of pregnant women (n = 100) and controls (n = 102) were also examined for IgG antibodies to BP230 and DSG1 and DSG3, but no reactivity was found. No differences were noted among the subset of control women that had experienced a previous pregnancy than those who were nulliparous.
3.2. BP180 is present in both skin and placenta
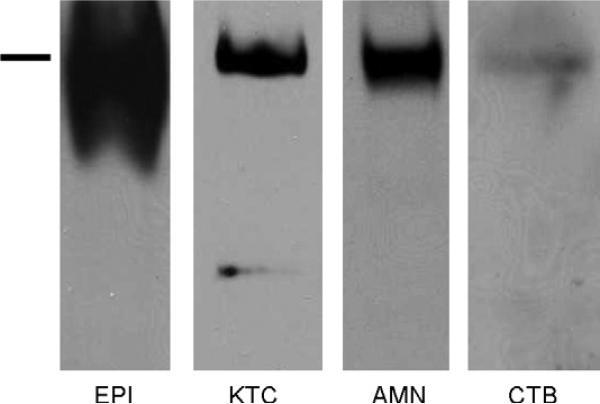
To determine if the same isoform of BP180 is expressed in both the placenta and skin, cell (KTC and CTB) and tissue (epidermis (EPI) and placental amnion (AMN) lysates were analyzed by immunoblot. The immunoblot reveals a band of varying intensity at the expected 180 kDa in all lysates tested, thus confirming the presence of full-length BP180 in these tissues (Fig. 2). An additional 120 kDa band was detectable in the KTC lysate represents the ectodomain of BP180, known to be shed in culture (Huilaja et al., 2008).
Fig. 2.
BP180 is present in both skin and placenta. BP180 protein was evaluated in primary human keratinocytes (KTC), a cytotrophoblast cell line (CTB), and fresh human epidermal (EPI) and amnionic (AMN) lysates by immunoblot using a polyclonal antibody (R136) directed against the C-terminus of the protein. The bar indicates 180 kDa, which corresponds to full-length BP180.
3.3. Serum containing BP180-specific IgE binds to placental CTBs expressing BP180
The ability of the BP180-specific maternal autoantibodies to bind to cytotrophoblasts, as would be encountered in the placenta, was evaluated using flow cytometry and indirect immunofluorescence. For the flow cytometric studies, we first confirmed the expression of BP180 on the surface of cultured CTB using cultured normal primary human keratinocytes as a positive control (Fig. 1a). Next, CTB and KTC were incubated with a serum obtained from a pregnant woman or a BP patient (both known to have BP180-specific IgE by ELISA), or a normal control serum. After washing the bound antibody was detected with anti-human IgE and flow cytometry (Fig. 1b). Incubation with either the pregnancy or BP sera resulted in similar levels of IgE bound to the surface of both cell types, suggesting that the maternal autoantibodies could bind to the placenta during pregnancy.
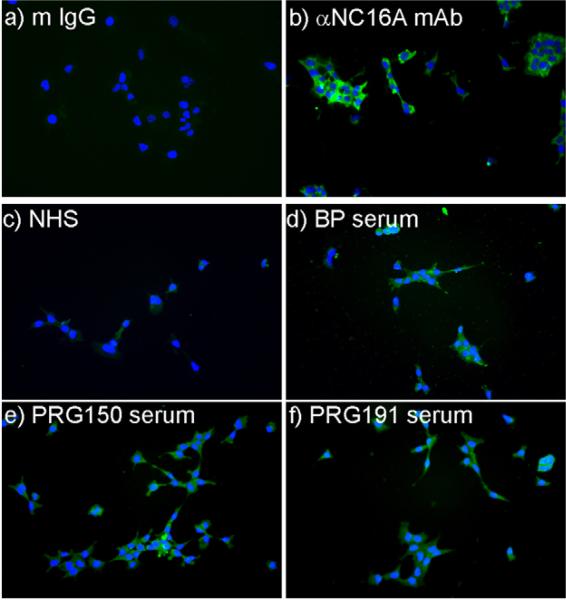
To confirm and extend the flow cytometry studies we evaluated the ability of maternal autoantibodies from a panel of 4 pregnancy sera, a BP sera (all known to have BP180-specfic IgE by ELISA) or NHS to bind CTBs using immunofluorescence. In all cases, autoantibody binding was detected with an IgE-specific secondary. A BP180-specific polyclonal antibody (R136) or rabbit serum (followed by anti-rabbit secondary) was also used as an additional control. The robust staining observed with R136 confirmed the surface expression of BP180 by cultured CTB (Fig. 3a and b). As expected, bound IgE was also detectable on the CTB when BP serum, but not NHS was used as the primary antibody (Fig. 3c and d). Binding of IgE to the CTB was also evident in 4/4 samples when BP180 ELISA positive pregnancy sera were used as the primary antibody (2 of 4 are shown in Fig. 3e and f).
Fig. 3.
IgE antibodies in pregnancy and BP serum bind similarly to cytotrophoblasts. Placental cytotrophoblasts were grown overnight in chamber slides and stained with normal human serum ((c) NHS), BP serum (d) or sera obtained from pregnant women (e and f)—both with detectable levels of BP180-specific IgE by ELISA. A polyclonal rabbit serum specific for the NC16A region of BP180 ((b) αBP180), or unimmunized rabbit serum ((a) R serum) were included as controls. Bound antibodies were detected with anti-human IgE-FITC (c–f) or anti-rabbit IgG-Alexa 488 (a and b). Nuclei were counterstained with DAPI.
3.4. Antibody positive serum binds to the BMZ of the skin and placenta
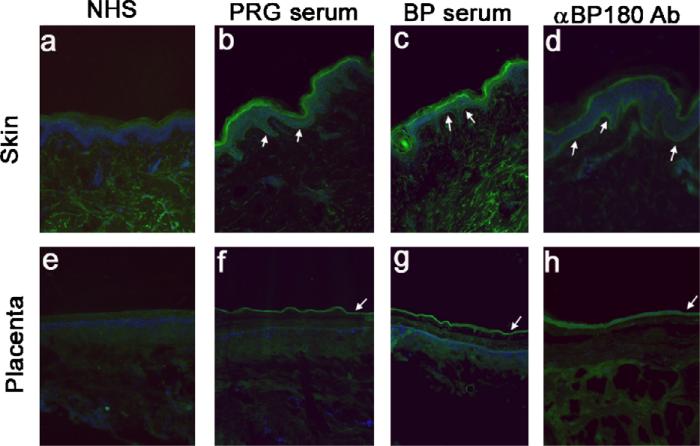
To determine if the maternal autoantibodies detected by ELISA (Table 1) were capable of binding to either intact skin or placenta, we performed indirect immunofluorescence. NHS was included as a negative control and BP serum and a BP180-specific monoclonal antibody (HD18) were used as positive controls. As expected, there was no staining in either tissue using normal human serum (Fig. 4a and e). Both the BP serum and the BP180-specific antibody result in linear staining at the BMZ of the skin (Fig. 4c and d) and at the amnion in the placental sections (Fig. 4g and h). Interestingly, the IgE in the serum from the pregnant patient bound the BMZ of the skin and amnion in the same pattern as the positive controls (BP serum and HD18). These studies confirm that the maternal IgE-class autoantibodies detectable in the sera of pregnant women, in the absence of clinical disease, react with proteins present in both the BMZ of the skin and placental amnion.
Table 1.
Pregnant women have increased levels of BP180-specfic IgE.a.
| Non-pregnant controls | Pregnant women | p-Valueb | |
|---|---|---|---|
| n = 134 | n = 299 | ||
| BP180-specific IgG | 12(9.0%) | 17(5.7%) | 0.21 |
| BP180-specific IgE | 3(2.2%) | 23(7.7%) | 0.01 |
| Total serum IgE | 69.8 ng/ml | 161.4 ng/ml | 0.30 |
Sera were screened by ELISA for BP180-specific IgE and IgG and total serum IgE.
p-Values were calculated using the Mann-Whitney U Test.
Fig. 4.
IgE antibodies in pregnancy serum bind to both skin and placenta. Cryo-sections of skin (a–d) and placenta (e–h) were incubated with serum from a non-pregnant control (a and e), a pregnant woman (BP180 IgE ELISA positive (b and f)), or a BP patient (known to contain IgE antibodies to BP180 (c and g)). As a positive control, skin and placenta were labeled with HD18, an anti-BP180 monoclonal antibody (d and h). Bound antibody was detected with either anti-human IgE or anti-murine IgG (HD18). There was no reactivity with the control human serum in either skin or placenta (a and e). Binding was observed in both tissues with IgE antibodies from a pregnant woman (b and f) and in both skin and placenta when probed with either the BP serum (c and g) or HD18 (d and h).
4. Discussion
In this study, we report that a small but statistically significant proportion of pregnant women produce an IgE-class autoantibody response against BP180, a protein expressed in selected tissues, including skin, placenta and amnion. In addition, we determined that the IgE present in the pregnancy sera binds to the surface of keratinocytes and CTBs expressing BP180. Finally, we have shown that these antibodies are capable of binding both the skin and placental sections in areas of BP180 expression. Taken together these findings suggest that the maternal autoantibodies could have a direct effect on these cell types in vivo.
The hemidesmosomal protein BP180 functions in cell-matrix interactions and is the primary antigenic target in PG. There are two noteworthy points related to this issue. First, the IgE autoantibodies uncovered in this study recognize the same small antigenic domain of BP180 (NC16A) that is targeted by PG autoantibodies. Second, infants born to mothers with PG tend to be premature and small for gestational age (Lawley et al., 1978; Ambros-Rudolf et al., 2006; Holmes and Black, 1984; Merchaoui et al., 1992; Shornick and Black, 1992). It has been proposed that the effects on the fetus are due to the deposition of autoantibodies in the placenta, which leads to relative placental insufficiency. Because the pregnancy samples we obtained for this study were de-identified, we do not have data regarding pregnancy outcome in our IgE-positive subjects. Whether the IgE-class autoantibodies we have identified influence pregnancy outcome is a critical question and is the focus of an ongoing prospective study.
BP180 is expressed by the placenta and fetal membranes (Fairley et al., 1995; Huilaja et al., 2008). By the first trimester BP180 is expressed in both the syncytial and cytotrophoblastic cells of human placental villous epithelium. In addition, BP180 is expressed in the human amniotic membranes and its 120 kDa shed ectodomain can be detected in normal amniotic fluid (Fairley et al., 1995; Huilaja et al., 2008). In the current studies we have demonstrated that the anti-BP180 IgE produced by a subset of pregnant women without PG recognizes BP180 on the surface of cultured CTBs and can bind to placental tissue sections. The role of a placental antigen as the potential stimulus for autoantibody formation in PG, and now in a subset of normal pregnancies, has been a source of speculation, but the exact pathologic mechanism remains unknown.
During early gestation, CTBs invade the endometrium and myometrium and migrate in a retrograde fashion along the maternal spiral arteries in the uterus (Pijnenborg et al., 1983). From a position lining the maternal spiral arteries, the endovascular trophoblasts have direct contact with maternal blood and autoantibodies present in maternal blood could bind toBP180 on the surface of these cells. The interaction of maternal IgE autoantibodies with CTB would not be dependent on the expression of the high affinity IgE receptor (FcεRI), instead the interaction would be an antigen–antibody interaction. Moreover, antigen-specific maternal IgE has recently been demonstrated within the placenta (Joerink et al., 2009), raising the possibility that the IgE may cross the placental and potentially have more far ranging effects.
In another study (Sandberg et al., 2009), total IgE was increased during pregnancy in women with known allergic symptoms; however, antigen-specific IgE was not increased in these same women. An increase in total IgE production is not surprising in the Th2 milieu of pregnancy. In the current study, we also observed an increase in total IgE concentration; however, this was not significant due to the large amount of variation within the groups. In contrast, BP180-specific IgE was significantly elevated in the sera of pregnant women.
Given the rarity of PG (1 in 10,000–50,000 pregnancies (Holmes et al., 1982; Ambros-Rudolf et al., 2006)) compared to the relative frequency with which we saw anti-BP180 IgE in our pregnant population (7.7 in 100), it is highly unlikely that any of these antibody-positive subjects had PG. PG is associated with anti-BP180 autoantibodies of both the IgG and IgE classes (Chapman et al., 2007; Giudice et al., 1993), while in this study only IgE-class anti-BP180 autoantibodies were found to be elevated in the pregnancy sera, compared with non-pregnant controls. It is possible that skin disease does not develop in these antibody-positive pregnant women due to the absence of both classes of autoantibodies. However, a more careful comparison of the IgE autoantibodies in PG and “normal” pregnancy may reveal additional differences in fine specificity.
In conclusion, a small but statistically significant proportion of pregnant women produce an IgE-class autoantibody response against the skin and placental antigen BP180. These antibodies bind to the surface of keratinocytes and CTBs, suggesting that maternal autoantibodies could have a direct effect on these cell types in vivo. This development is particularly intriguing in light of recent studies that have linked polymorphisms in the IL-4 gene, a major regulator of IgE production, with an increased risk for SGA (Engel et al., 2005) and preterm birth (Annells et al., 2004). A critical question that remains for study is whether the production of IgE-class, anti-BP180 autoantibodies that do not lead to PG indicate a risk for negative outcomes, such as SGA or prematurity. We have recently initiated a prospective study designed to answer this important question.
Acknowledgements
We would like to acknowledge Dr. George Giudice for his insight and thoughtful comments and Amber Onoh and Danial Samar for technical assistance. We also thankfully acknowledge the Congenital and Inherited Disorders Advisory Committee of the Iowa Development of Public Health and the University Hygienic Laboratory for their help obtaining the pregnancy serum. This work was supported by a Merit Review Award from the Department of Veterans Affairs (JAF), The Doris Duke Charitable Foundation (MHN), The Institute for Clinical, Translational Research at the University of Iowa (MHN), and the Herzog Endowment to the University of Iowa Department of Dermatology.
References
- Amagai M. Adhesion molecules. I: Keratinocyte-keratinocyte interactions; cadherins and pemphigus. J. Invest. Dermatol. 1995;104:146–152. doi: 10.1111/1523-1747.ep12613668. [DOI] [PubMed] [Google Scholar]
- Ambros-Rudolf CM, Müllegger RR, Vaughan-Jones SA, Kerl H, Black MM. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J. Am. Acad. Dermatol. 2006;54(3):395–404. doi: 10.1016/j.jaad.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Amoudruz P, Minang JT, Sundstrom Y, Nilsson C, Lilja G, Troye-Blomberg M, et al. Pregnancy, but not the allergic status, influences spontaneous and induced interleukin-1beta (IL-1beta), IL-6, IL-10 and IL-12 responses. Immunology. 2006;119:18–26. doi: 10.1111/j.1365-2567.2006.02400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annells MF, Hart PH, Mullighan CG, Heatley SL, Robinson JS, Bardy P, et al. Interleukins-1, -4, -6, -10, tumor necrosis factor, transforming growth factor-beta, FAS, and mannose-binding protein C gene polymorphisms in Australian women: risk of preterm birth. Am. J. Obstet. Gynecol. 2004;191:2056–2067. doi: 10.1016/j.ajog.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Balding SD, Diaz LA, Giudice GJ. A recombinant form of the human BP180 ectodomain forms a collagen-like homotrimeric complex. Biochemistry. 1997;36:8821–8830. doi: 10.1021/bi970675n. [DOI] [PubMed] [Google Scholar]
- Castro LA, Lundell RB, Krause PK, Gibson LE. Clinical experience in pemphigoid gestationis: report of 10 cases. J. Am. Acad. Dermatol. 2006;55:823–828. doi: 10.1016/j.jaad.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Chapman MA, Olague-Marchan M, Yancey KB, Giudice GJ, Fairley JA. IgE class autoantibodies against BP180 in herpes gestationis and cicatricial pemphigoid. J. Invest. Dermatol. 2007;127:S15. [Google Scholar]
- Chi CC, Wang SH, Charles-Holmes R, Ambros-Rudolph C, Powell J, Jenkins R, et al. Pemphigoid gestationis: early onset and blister formation are associated with adverse pregnancy outcomes. Br. J. Dermatol. 2009 doi: 10.1111/j.1365-2133.2009.09086.x. [DOI] [PubMed] [Google Scholar]
- Engel SA, Olshan AF, Savitz DA, Thorp J, Erichsen HC, Chanock SJ. Risk of small-for-gestational age is associated with common anti-inflammatory cytokine polymorphisms. Epidemiology. 2005;16:478–486. doi: 10.1097/01.ede.0000164535.36412.6b. [DOI] [PubMed] [Google Scholar]
- Fairley JA, Burnett CT, Fu CL, Larson DL, Fleming MG, Giudice GJ. A pathogenic role for IgE in autoimmunity: bullous pemphigoid IgE reproduces the early phase of lesion development in human skin grafted to nu/nu mice. J. Invest. Dermatol. 2007;127:2605–2611. doi: 10.1038/sj.jid.5700958. [DOI] [PubMed] [Google Scholar]
- Fairley JA, Heintz PW, Neuburg M, Diaz LA, Giudice GJ. Expression pattern of the bullous pemphigoid-180 antigen in normal and neoplastic epithelia. Br. J. Dermatol. 1995;133:385–391. doi: 10.1111/j.1365-2133.1995.tb02665.x. [DOI] [PubMed] [Google Scholar]
- Giudice GJ, Emery DJ, Zelickson BD, Anhalt GJ, Liu Z, Diaz LA. Bullous pemphigoid and herpes gestationis autoantibodies recognize a common non-collagenous site on the BP180 ectodomain. J. Immunol. 1993;151:5742–5750. [PubMed] [Google Scholar]
- Graham CH, Hawley TS, Hawley RG, Macdougall JR, Kerbel RS, Khoo N, et al. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp. Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- Herrero-Gonzalez JE, Brauns O, Egner R, Ronspeck W, Mascaro JM, Jr., Jonkman MF, et al. Immunoadsorption against two distinct epitopes on human type XVII collagen abolishes dermal-epidermal separation induced in vitro by autoantibodies from pemphigoid gestationis patients. Eur. J. Immunol. 2006;36:1039–1048. doi: 10.1002/eji.200535349. [DOI] [PubMed] [Google Scholar]
- Holmes RC, Black MM, Dann J, James DC, Bhogal B. A comparative study of toxic erythema of pregnancy and herpes gestationis. Br. J. Dermatol. 1982;106:499–510. doi: 10.1111/j.1365-2133.1982.tb04551.x. [DOI] [PubMed] [Google Scholar]
- Holmes RC, Black MM. The fetal prognosis in pemphigoid gestationis (herpes gestationis). Br. J. Dermatol. 1984;110:67–72. doi: 10.1111/j.1365-2133.1984.tb07314.x. [DOI] [PubMed] [Google Scholar]
- Huilaja L, Hurskainen T, Autio-Harmainen H, Hofmann SC, Sormunen R, Rasanen J, et al. Pemphigoid gestationis autoantigen, trans-membrane collagen XVII, promotes the migration of cytotrophoblastic cells of placenta and is a structural component of fetal membranes. Matrix Biol. 2008;27:190–200. doi: 10.1016/j.matbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Joerink M, Rindsjo E, Stenius F, Alm J, Lilja G, Gronlund H, et al. Evidence for allergen-specific IgE of maternal origin in human placenta. Allergy. 2009 doi: 10.1111/j.1398-9995.2009.01941.x. [DOI] [PubMed] [Google Scholar]
- Lawley TJ, Stingl G, Katz SI. Fetal and maternal risk factors in herpes gestationis. Arch. Dermatol. 1978;114:552–555. [PubMed] [Google Scholar]
- Mascaro JM, Jr., Lecha M, Mascaro JM. Fetal morbidity in herpes gestationis. Arch. Dermatol. 1995;131:1209–1210. doi: 10.1001/archderm.1995.01690220117027. [DOI] [PubMed] [Google Scholar]
- Merchaoui J, Makhlouf T, Sfar R, Mokthar I, Fazaa B, Kamoun MR. Obstetrical prognosis of gestational pemphigoid. Study of a series of 13 cases and review of the literature. J. Gynecol. Obstet. Biol. Reprod. (Paris) 1992;21:963–967. [PubMed] [Google Scholar]
- Messingham KA, Noe MH, Chapman MA, Giudice GJ, Fairley JA. A novel ELISA reveals high frequencies of BP180-specific IgE production in bullous pemphigoid. J. Immunol. Methods. 2009 doi: 10.1016/j.jim.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikol DD, Ditlow C, Usatin D, Biswas P, Kalbfleisch J, Milner A, et al. Serum IgE reactive against small myelin protein-derived peptides is increased in multiple sclerosis patients. J. Neuroimmunol. 2006;180:40–49. doi: 10.1016/j.jneuroim.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Persson M, Ekerfelt C, Ernerudh J, Matthiesen L, Jenmalm M, Jonsson Y, et al. Increased circulating paternal antigen-specific IFN-gamma-and IL-4-secreting cells during pregnancy in allergic and non-allergic women. J. Reprod. Immunol. 2008;79:70–78. doi: 10.1016/j.jri.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983;4:397–413. doi: 10.1016/s0143-4004(83)80043-5. [DOI] [PubMed] [Google Scholar]
- Pohla-Gubo G, Lazarova Z, Giudice GJ, Liebert M, Grassegger A, Hint-ner H, et al. Diminished expression of the extracellular domain of bullous pemphigoid antigen 2 (BPAG2) in the epidermal basement membrane of patients with generalized atrophic benign epidermolysis bullosa. Exp. Dermatol. 1995;4:199–206. doi: 10.1111/j.1600-0625.1995.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Sandberg M, Frykman A, Jonsson Y, Persson M, Ernerudh J, Berg G, et al. Total and allergen-specific IgE levels during and after pregnancy in relation to maternal allergy. J. Reprod. Immunol. 2009 doi: 10.1016/j.jri.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Sekigawa I, Kaneda K, Kaneko H, Takasaki Y, Takamori K, Ogawa H. Detection of serum IgE class anti-SSA antibodies in mothers with foetal loss. Rheumatol. Int. 2008;28:623–626. doi: 10.1007/s00296-007-0494-3. [DOI] [PubMed] [Google Scholar]
- Shornick JK, Bangert JL, Freeman RG, Gilliam JN. Herpes gestationis: clinical and histologic features of twenty-eight cases. J. Am. Acad. Dermatol. 1983;8:214–224. doi: 10.1016/s0190-9622(83)70027-7. [DOI] [PubMed] [Google Scholar]
- Shornick JK, Black MM. Fetal risks in herpes gestationis. J. Am. Acad. Dermatol. 1992;26:63–68. doi: 10.1016/0190-9622(92)70008-4. [DOI] [PubMed] [Google Scholar]
- Valenta R, Mittermann I, Werfel T, Garn H, Renz H. Linking allergy to autoimmune disease. Trends Immunol. 2009;30:109–116. doi: 10.1016/j.it.2008.12.004. [DOI] [PubMed] [Google Scholar]