Abstract
Aging is associated with loss of functional potential of multiple tissue systems, and there has been significant interest in understanding how tissue specific cells contribute to this decline. DNA damage accumulation has been widely associated with aging in differentiated cell types. However, tissue specific stem cells were once thought to be a geno-protected population, as damage accrued in a stem cell population has the potential to be inherited by differentiated progeny as well as propagated within the stem cell compartment through self-renewal divisions. This review will discuss the evidence for DNA damage accumulation in the aged HSC compartment, potential drivers, and finally the consequences of the acquired damage.
Theories of aging encompassing damage accumulation
Aging is associated with significant loss in the ability of tissues to maintain homeostatic conditions, which is manifest by diminished capacity for maintenance and repair, often resulting in diminished numbers of functional cells. The loss of homeostasis manifests as deterioration of normal body processes, functional decline of the organism, and is associated with increased disease incidence. Theories stemming from the mid-1900’s have implicated DNA damage as playing a role in aging, when researchers correlated radiation exposure with accelerated aging phenotypes (1). Indeed, it has been shown that aging is associated with significant increases in DNA damage found in many different tissues of aged flies, mice, and humans (2–4). Perhaps this is not surprising in post-mitotic cells that compose brain, heart and skeletal muscle, given the constant barrage of genomic insults, both exogenous and endogenous, that a cell is exposed to on a daily basis (estimates of 10,000–100,000 lesions (5)). However, this damage accumulation is also seen in aged cells that are still proliferating (skin, intestines, liver, kidney, and hematopoietic cells) and capable of performing DNA damage repair. These proliferating cells have internal mechanisms to cope with the accumulation of damage, and can be prompted to undergo programmed cell death (apoptosis) or enter a state of irreversible growth arrest (senescence). These two mechanisms of handling DNA damage are thought to play a major role in tumor suppression, but may also contribute to aging phenotypes through cellular attrition due to apoptosis or tissue dysregulation caused by factors secreted by senescent cells (senescence-associated secretory phenotype or SASP)(6–8) with both contributing to loss of tissue homeostasis.
Organisms have mechanisms to manage cellular loss or dysregulation as they age. When systems are challenged, tissue specific stem cells have the capacity to mobilize and give rise to mature cells that repair and restore homeostasis. Adult stem cells are imbued with the capacity for self-renewal and the ability to generate all cells within the system, and thus they possess the ability to repair and sustain the their systems’ function throughout the life-span of the organism. However, studies repeatedly have shown that tissue specific stem cells lose their functional potential with age (9), and this is associated with a decrease in absolute stem cell numbers in tissues such as muscle(10) brain(11), and melanocytes (12, 13), suggesting that reductions in the total stem cell number restricts the regenerative potential of that tissue. Several studies have shown that there is also intrinsic loss of stem cell reconstitution potential on a per cell basis in aged stem cells, thereby contributing to loss of homeostasis and regenerative potential (14, 15). Within the hematopoietic stem cell (HSC) compartment, aging is associated with significant loss of reconstitution potential at an individual cell level as assessed in transplantation assays, but an expansion in cell number (14, 16–20). This phenomenon is also seen in aging epidermal stem cells (15). Further alterations of the aged HSC compartment involve changes in the clonal lineage potential (dominance of myeloid-biased HSCs)(14, 21–24), loss of cell polarity (25), and alterations in the epigenetic landscape (19, 20, 26). Given such changes in adult stem cells, there has also been significant interest in determining the susceptibility of these cells to DNA damage and whether accumulation of damage could contribute to any of these phenotypes.
The potential for stem cells to pass their genetic information to all their progeny during the process of differentiation, as well as their capacity to propagate damage within the stem cell compartment during self-renewal divisions, suggests that preserving genomic integrity of these cells would be critical for an organism’s survival. Indeed stem cells, including HSCs, exhibit many properties that suggest they have an enhanced capability to protect their genomes including high levels of expression of ABC transporters (27–30). Though expression of ABC transporters is not exclusive to stem cells (31), combinatorial expression of such transporters provides efflux potential conferring protection from exogenous insults. Moreover, HSCs are predominantly quiescent, and it is estimated that murine HSCs only undergo 5–20 cells divisions during a two-year lifespan (32–34), minimizing the introduction of replication-based errors. Furthermore, this reduced cycling minimizes the uncapping of telomeres during replication (35–37) and telomeres are further protected in stem cells by the expression of telomerase (36, 38, 39). HSCs also maintain low levels of reactive oxygen species (ROS) due to the combined effects of their low metabolic activity, their reliance on glycolytic metabolism, and their inherent hypoxic nature while within their specialized niche (40–45). However, substantial evidence suggests that even though HSCs have many protective features to minimize accumulation of DNA damage, they accrue damage as they age. In this review I will discuss various forms of damage in HSCs, potential drivers of damage, how such damage is accrued, and ultimately the functional consequences of accumulated DNA damage.
DNA Damage in HSCs
Telomere attrition
Telomeres reside at the end of chromosomes and are made up of a serially repeated sequence of DNA (TTAGGG). These sequences are compacted and together with shelterin, prevent the ends of chromosomes from being recognized as damaged DNA and triggering a DNA damage response (46). Cell division in somatic cells can lead to telomere shortening due to the inability of DNA-polymerase to replicate telomere ends (47, 48). Critical shortening of telomeres due to proliferation can lead to a permanent cell cycle arrest, or senescence. These metabolically actively senescent cells have been shown to accrue during aging and have been implicated in aging phenotypes in many tissues (49). In cells required for life-long function, such as stem cells, telomere maintenance is critical. One potential mechanism that protects against telomere attrition in stem cells is their residence in G0 and limited numbers of cell division. Additionally, tissue specific stem cells maintain expression of telomerase, a reverse transcriptase that elongates telomeres after embryonic development. The importance of telomerase was demonstrated during stem cell challenges such as competitive or serial HSC transplants, as lack of telomerase expression leads to significant defects in functional potential (37, 50, 51). However, late generation telomerase deficient (G3) HSCs do not appear to be functionally impaired in young mice at steady state (50), but by one year of age, these HSCs lose serial reconstitution potential (18). The exhaustion of G3 mTerc−/− serial transplantation potential suggests that loss of telomere length may underlie this loss of function. However, maintenance of telomere length does not rescue the HSC potential in TERT overexpressing cells (52) suggesting that, at least in the murine system, telomere attrition is not the primary driving influence on age-associated loss of functional potential. Furthermore, during physiologic aging, telomere shortening does not appear to play a robust role in murine HSC aging phenotypes as there are nearly undetectable levels of shortening in the HSC compartment (19, 53). This may be attributed to the substantially long length of murine telomeres (5–10 times longer than human); however, studies in aged human hematopoietic stem and progenitor cells (HSPCs) also do not exhibit significant telomere loss during aging, thereby confirming this observation (54). It remains possible that even minor telomere loss may contribute to the altered potential of aging stem cells, as telomeres have been shown to also affect gene expression by modifying chromatin conformation (55). Comparatively, adult HSPCs contain shorter telomeres than those from highly cycling fetal liver and cord blood derived HSPCs (56). Studies of granulocytes, lymphocytes, and other bone marrow cells in aging individuals showed a robust decline in telomere length during adolescence (compared to length at birth). In these differentiated cell types this shortening also continues during physiologic aging, but is much less pronounced. This suggests that telomere attrition may occur in human hematopoietic progenitor cells, given the shortening was seen in all cells examined in the bone marrow (57). Recently, non-critical shortening of telomeres has been shown to affect the expression of genes several Mb away through a process of chromosomal looping (58). Thus, it is possible that even the moderate reduction in telomere length seen during aging may contribute to the altered function of aged HSCs.
DNA Strand Breaks
Mouse genetic models provided initial evidence suggesting that DNA repair is fundamental to adult HSC potential. Mice lacking key components required for nucleotide excision repair (NER), non-homologous end-joining (NHEJ), mismatch repair (MMR), and damage response all showed impairment in long-term reconstitution potential after transplant (18, 59–61). Without properly functioning DNA damage response and repair pathways, proliferation stresses (such as injury or transplant) could lead to increased levels of unrepaired DNA damage and loss of HSC function. Loss of reconstitution potential is a phenotype associated with HSC aging, but decreased DNA damage response and repair in mutant models may not completely represent what occurs during aging. To determine if changes in mutant DNA repair pathways contribute to physiologic aging, several groups have investigated if DNA damage accrues in HSCs with aging. Indeed, using multiple experimental paradigms, it is now well established that during physiologic aging, HSCs accumulate DNA damage (18, 62–65). In the murine system, purified HSCs (LSKCD34−Flk2− and LSKCD150+CD48−) from aged animals show elevated levels of gH2AX staining and increased alkaline comet tail moments (direct measurement of single and double strand breaks)(18, 62, 63, 65). Though gH2AX foci are not a direct measurement of DNA damage, the presence of phosphorylated gH2AX indicates a damage response (66). Similar to the elevated levels of DNA strand breaks seen in aged murine HSCs, there is also evidence of increased damage in human HSCs, as measured by increased gH2AX foci in aged human and transplanted CD34+CD38− cells (64, 67, 68).
Point mutations
Many adult tissue stem cells, including HSCs, largely reside in G0. Residence in a quiescent state is thought to minimize the introduction of replication-induced errors; however, increasing evidence indicates that point mutations accumulate in the primitive hematopoietic compartment during aging. While blasts from acute myeloid leukemia (AML) patients contain hundreds of somatic mutations (69, 70), these mutations could be traced back to mutations acquired in HSPCs (71–74). Mutations in epigenetic regulators were consistently over-represented in these studies, with several groups reporting the same mutational “hot spots” in genes such as DNMT3A, TET2, and IDH1/2. Interesting, these same mutations were found in the blood of aged, healthy individuals with non-malignant clonal hematopoiesis, implicating these mutations as drivers of clonal expansion (73, 75–77). Thus, it has been hypothesized that during normal aging, mutations in HSCs imbue them with a selective advantage leading to clonal hematopoiesis. This interpretation is supported by publications in murine models in which loss of Dnmt3a or Tet2 confers enhanced self-renewal potential to HSCs (77–81). These clones can then become pools for the stochastic acquisition of additional mutations that drive malignancy. With the ever-improving sequencing and analysis platforms, it is now established that these mutations driving clonal hematopoiesis, are present even in younger individuals (82) and are nearly ubiquitous in middle-aged healthy individuals (50–60 years old) who do not develop malignancies even over a follow-up period of 10 years (83). These middle-aged individuals have also acquired substantial numbers of other exonic, single nucleotide variants not previously annotated due to thresholds in detection levels in epigenetic regulators leading to accumulation of non-malignant clonal hematopoiesis (83). These data all support a model of increased point mutation burden in primitive HSCs, with aging. Notably, this increased mutation rate is not restricted to HSCs, as significant numbers of point mutations accrue in all organs with age, with similar mutation rates found in several different adult tissue specific stem cells (84).
Insertion / Deletions / Chromosomal abnormalities
Though fewer studies have addressed copy number variations, several groups have addressed structural variations that occur in human HSPCs during aging. Consistent with other forms of DNA damage, chromosomal anomalies such as insertions, deletions, duplications, and transpositions also increase with age. When these types of chromosomal lesions were assayed, they were present only at low frequencies from youth to middle-age (<50 years) but mosaicism was shown to rise significantly with age (68, 85–87).
Mitochondrial DNA Damage
Loss of mitochondrial function is widely associated with aging processes, and there is evidence that the small mitochondrial genome (~16.2 kb in mouse and ~16.6 kb human) also accumulates various forms of DNA damage (point mutations and rearrangements) during aging (88). Several murine studies have used a mutator model expressing a proof-reading deficient variant of the mtDNA polymerase, POLG, and find that these mice exhibit premature aging phenotypes (89–91). However, Norddahl et al. have shown that in the hematopoietic system, mitochondrial mutations acquired by the mutator mice do not faithfully reproduce physiologic aging phenotypes in the bone marrow. Though the mutator mice have lineage skewing, anemia and loss of lymphoid cells, the HSC pool is not impacted and the lineage skewing is driven by loss of function in downstream progenitor cells (91). In human studies, primitive bone marrow cells (CD34+) from young and aged subjects showed no significant differences in the mtDNA mutational burden; however, both young and aged CD34+ had high levels of mtDNA damage (92). Similarly, in murine liver, high-density sequencing showed a lack of age-associated mtDNA mutations (90). This suggests that accumulation of mutations in the mitochondrial genome is not driven by aging, but rather that mtDNA mutations are driven by replications errors during early development when mtDNA is rapidly amplifying.
Drivers of DNA Damage
Cell Cycle
Quiescence
Since HSCs reside largely in a quiescent state, they have been thought to be protected from replication induced errors, but it has become clear recently that while in G0, HSCs also show attenuated DNA damage repair and response pathways (63). Thus, given that HSCs divide infrequently over the life span of an organism (33, 34, 93) but are subjected to daily genotoxic stress, it has been proposed that HSC quiescence may paradoxically promote age-associated DNA damage accumulation with aging. In fact, during quiescence, DNA damage repair is attenuated, but not absent, and HSCs preferentially utilize the NHEJ DNA repair mechanism (94). This error-prone pathway is available to cells in quiescence, but utilization of NHEJ is another mechanism by which HSCs can accrue DNA damage. Once the HSCs enter cycle, regardless of age, the cells robustly induce DNA damage response and repair of DNA strand breaks (62, 63). However, entry into the cell cycle that allows for the repair of structural damage, such as strand breaks, comes with the cost of potential loss of fidelity during DNA repair and introduction of new errors introduced during replication.
Replication: Introduction of Errors and Stalled Forks
The cell possesses many fail-safes to maintain DNA replication fidelity, however this process is not perfect. Replications errors, due to mismatches in the DNA, strand slippage, and template looping, can all introduce genomic mutations every time the cell undergoes division. Additionally, DNA lesions present before replication begins can lead to replication stress, including the stalling of replication forks. The stalling of replication forks can be resolved in several ways, including activation of the ATR pathway, firing of backup origins, lesion bypass, and fork collapse(95–98). MCM proteins form an important complex at origins of replication, and they also act to initiate backup origins in cells as a potential resolution of DNA replication stress. Reduction in the expression of these proteins causes cells to undergo division, but the progeny of these cell divisions progressively accumulate DNA lesions and chromosomal fragility (99). In aged HSCs, two MCM family members exhibit decreased protein expression, and the decrease in this complex may further contribute to the accumulation of DNA damage in aged HSCs in the murine system (100).
HSCs also robustly express Rad18, which allows redistribution of stalled DNA replication forks and promotes replicative bypass of DNA lesions, thereby leading to DNA damage tolerance. The high levels of Rad18 expression could also contribute to age-associated DNA damage in HSCs (101). The types of damage caused by lesion bypass or damage tolerance would largely lead to small deletions in the genome, but accumulation of small insertions or deletions in the aged HSC DNA has not yet been well characterized.
ROS
Reactive oxygen species (ROS) are known drivers of structural modifications of DNA. To mitigate ROS induced damage, HSCs reside in hypoxic niches (1–9% O2) (102) which act in concert with the overall low metabolic activity of HSCs, which largely use anaerobic glycolysis (40–45). HSCs also have low mitochondrial mass, which increases upon differentiation concomitant with an elevation in ROS levels (103). To further mitigate intracellular ROS levels, HSCs express FoxO family transcripts, indicating that HSCs possess several mechanisms to minimize ROS induced damage. Studies have shown that loss of FoxO family protein expression leads to significant functional defects associated with elevated ROS in the HSC compartment (104). Though expression of FoxO transcript levels do not significantly drop in aged HSCs, ROS levels are increased and increase even more after serial transplantation. These changes are also associated with loss of functional potential (105). This is similar to what is seen when HSCs are chronically stimulated, which results in proliferating HSCs exhibiting elevated levels of ROS damage, as measured by 8-oxo-2’-deoxyguanosine levels (65). Together, these data point to increased ROS levels contributing to age-associated DNA damage.
Epigenetic drivers
Though the amount of literature is modest, there is growing evidence supporting an interplay between epigenetic regulation and DNA damage. For DNA methylation, a deamination event can lead to the direct conversion of a mC to T. Given that CpG’s are highly methylated throughout the genome, it is perhaps not surprising that the most common mutation found in human DNA is the transition of C-T at CpG dinucleotides (106). Similarly, this is the most common single nucleotide change found in human blood during aging (69, 74) supporting an interplay between loss of epigenetic regulation and hematopoietic DNA damage accrual. Furthermore, alterations in epigenetic methylation landscapes are a hallmark of blood cancers and can effect initiation, development, and maintenance of disease. Though cancers are largely associated with global hypomethylation, critical tumor suppressor genes are often hypermethylated. These data support a complex relationship between DNA methylation, mutation acquisition, and gene regulation in which changes in DNA methylation can lead to point mutations (de-amination) as well as dysregulation of normally orchestrated transcription.
Histone modifications have also been shown to be critical for maintaining genomic stability, and loss of this regulation may also lead to genomic damage. During aging, there is a significant loss of histone methyltransferase SUV39H1 in HSCs leading to an overall decrease in H3K9me3 levels. H3K9me3 is a histone modification critical for heterochromatin maintenance and has been shown to protect DNA from double strand breaks (107). Thus, the age-associated decline of this histone methyltransferase could also contribute to the accrual of DNA damage due to genome instability (108). It is likely that as more information about epigenetic regulation in HSCs is revealed, more connections between histone modifications and age-associated DNA damage accrual will also be established.
Consequences of DNA Damage
Loss / aberrant potential
While most age-associated DNA damage accrual in HSCs is in the form of strand breaks, the functional ramifications of this damage have yet to be fully characterized. In differentiation promoting media ex vivo, young and old HSCs are able to recognize damage and repair it to a state in which cell division can occur (63, 100). However, the fidelity of the repair of strand breaks or stalled forks is not known, and it appears that the progeny of some of these aged HSCs have functional defects such as clone collapse (63) or increased numbers of chromosomal abnormalities (100). Additionally, when aged HSCs are transplanted, they can reconstitute lethally irradiated recipient animals, suggesting that they can cycle and repair DNA. Another interpretation for these studies is that since in vivo only a single HSC would be required to reconstitute a lethally irradiated host animal, as few as one undamaged HSC could drive the reconstitution while the other damaged HSCs could undergo apoptosis. However, ex vivo very few aged HSCs underwent apoptosis to remove acquired DNA damage in differentiation-promoting culture conditions (63, 100), though ex vivo assays may not reflect HSC behavior in vivo. Indeed, other studies suggest that accumulated DNA damage leads to preferential differentiation of HSCs (109) or apoptosis due to the inability of HSCs to arrest in G1-S in vivo (62).
ROS appears to play a direct role in the functional decline in aging HSCs. Evidence supporting this model includes the strong correlation between increased ROS levels in human CD34+ bone marrow cells and decreased function of these cells in transplant experiments (110). ROS levels also are increased in HSCs from aged mice that diminished long-term reconstitution potential, and genomic rearrangements are more frequent in primitive cells with high levels of ROS (111). Additionally, the ability of HSCs to serially transplant was significantly improved in ATM deficient mice when they were treated with the ROS scavenging antioxidant NAC (112). The correlation between mitigating ROS and improved HSC function was further demonstrated when HSCs cultured under hypoxic conditions maintained more robust colony forming and reconstitution potential compared to those that cultured at ambient air oxygen levels (~21%) (113–115). More recently experiments by the Broxmeyer group have demonstrated that collection and ex vivo handling of HSCs under hypoxic conditions reduces the levels of ROS and significantly improves the functional potential of isolated bone marrow HSCs. By minimizing the mitochondrial activity of purified stem and progenitor cells, the progenitor compartment showed decreased differentiation potential in colony forming assays as well as increased long-term reconstitution potential upon transplantation as measured by donor chimerism in both primary and secondary transplants (115). The HSCs remaining under hypoxic conditions also showed reduced cycling compared to those exposed to higher oxygen levels, thereby suggesting that a hypoxic niche supports HSC function by minimizing levels of ROS, and increases in ROS levels leads to loss of reconstitution potential.
Clonal Hematopoiesis and Cancer
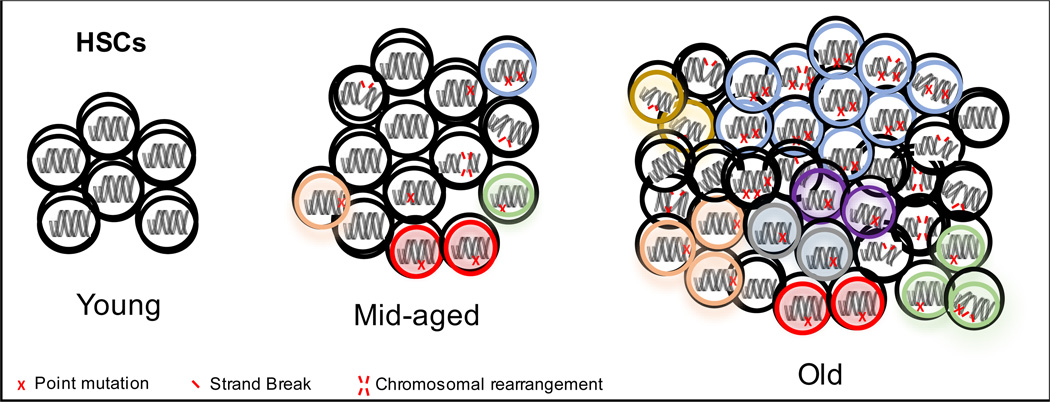
The increasing wealth of genome-wide sequencing data has demonstrated that point mutations accumulate in human hematopoietic cells with age. A subset of these mutations appears to confer a selective advantage to HSCs, allowing these cells to clonally expand (Fig 1). The clonal expansion of an HSC can be read out in the peripheral blood of aged individuals, as there is increased prevalence of mature hematopoietic cells carrying the same somatic mutations. Clonal hematopoiesis occurs in healthy individuals, and has been recently termed clonal hematopoiesis of indeterminate potential (CHIP)(116). While initial expansion of clones does not appear to be associated with significant disadvantages or effects on normal hematopoiesis (75, 83, 116), these driver mutations can predispose HSCs toward malignancies such as MDS, AML and aplastic anemia. While point mutations are acquired in HSCs during aging, it is not clear whether or not this is due to alterations in the fidelity of DNA damage repair in HSCs. The inappropriate repair of strand breaks, together with ROS-driven chromosomal rearrangements and altered epigenetic regulation of structure, can potentially contribute to age-associated changes in HSCs that can drive clonal hematopoiesis and can promote the development of cancer.
Figure 1.
Accumulation of various forms of DNA damage in the aged HSC compartment is present throughout aging of the HSC compartment, with certain combinations driving clonal expansion. The selection advantage conferred by mutations in these clones may also contribute to the progression towards hematopoietic malignancies.
Directions
While increasing lines of evidence support that DNA damage accumulates in the aged HSC compartment, many questions remain regarding the consequences of these genomic changes. As genomic sequencing technologies become more widespread, and methods to analyze these dense, high coverage datasets are constantly improving, we are beginning to understand the magnitude of point mutations that accrue during aging. While initial reports suggested a low frequency of clonal hematopoiesis in patients late in life, improvement in analysis and detection levels have demonstrated that clonal hematopoiesis is common, and many more mutations that have the potential to drive CHIP have been described than originally reported just a few years ago (83). Additionally, mutations driving clonal hematopoiesis have been identified in younger individuals than previously reported.
To begin to address how mutations drive clonal hematopoiesis and the development of hematologic malignancies, researchers can now employ site specific genome editing using CRISPR/Cas9 to recapitulate common mutations that are associated with CHIP as well as MDS and AML. These studies are just the initial steps to understand the contribution of DNA damage to HSC aging phenotypes as the field is still lacking experimentally tractable ways to assay HSCs ex vivo. Without the ability to maintain “stem-ness”, ex vivo analysis will continue to be limited and may not faithfully recapitulate HSC function in vivo. This limitation in understanding HSC function during aging is further exacerbated by our lack of knowledge regarding age-associated alterations in the HSC niche, which is still being characterized. In order to understand the consequences of DNA damage accrual (as well as other cell autonomous phenotypes) the field will also need to expand research beyond the mostly cell-intrinsic based assays using aged HSCs. This is especially critical for HSC biology since senescence and circulating factors appear to be major drivers of the loss in stem cell functional potential in other tissues; however, studies involving clearance of senescent cells (117) or restoration of circulating factors (118) do not appear to have significant effects on the hematopoietic system. Thus, it is clear that our understanding of how cells behave in the aged environment will be necessary to completely understand HSC aging. Ultimately, aging is a complex phenomenon, and the accumulation of DNA damage is one of many potential contributors to the loss of functional potential of HSCs. Characterizing the interplay among these diverse regulators of HSC function will undoubtedly be the continued focus of many investigators and will hopefully lead to discoveries that allow us to improve the function of tissue stem cells during aging.
Acknowledgments
Supported in part by K01 AG050813-01A1 and by the Intramural Research Program of the NIH, National institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Failla G. Considerations bearing on permissible accumulated radiation doses for occupational exposure; the aging process and cancerogenesis. Radiology. 1957;69(1):23–28. doi: 10.1148/69.1.23. discussion, 8–9. [DOI] [PubMed] [Google Scholar]
- 2.Dolle ME, Snyder WK, Dunson DB, Vijg J. Mutational fingerprints of aging. Nucleic acids research. 2002;30(2):545–549. doi: 10.1093/nar/30.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soares JP, Cortinhas A, Bento T, Leitao JC, Collins AR, Gaivao I, et al. Aging and DNA damage in humans: a meta-analysis study. Aging. 2014;6(6):432–439. doi: 10.18632/aging.100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moskalev AA, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, Yanai H, et al. The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing research reviews. 2013;12(2):661–684. doi: 10.1016/j.arr.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362(6422):709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 6.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, et al. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor. PLoS biology. 2008;6(12):2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509(7501):439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monti D, Grassilli E, Troiano L, Cossarizza A, Salvioli S, Barbieri D, et al. Senescence, Immortalization, and Apoptosis - an Intriguing Relationship. Annals of the New York Academy of Sciences. 1992;673:70–82. doi: 10.1111/j.1749-6632.1992.tb27438.x. [DOI] [PubMed] [Google Scholar]
- 9.Oh J, Lee YD, Wagers AJ. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nature medicine. 2014;20(8):870–880. doi: 10.1038/nm.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brack AS, Bildsoe H, Hughes SM. Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. Journal of cell science. 2005;118(20):4813–4821. doi: 10.1242/jcs.02602. [DOI] [PubMed] [Google Scholar]
- 11.Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE, et al. FoxO3 regulates neural stem cell homeostasis. Cell stem cell. 2009;5(5):527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inomata K, Aoto T, Binh NT, Okamoto N, Tanimura S, Wakayama T, et al. Genotoxic Stress Abrogates Renewal of Melanocyte Stem Cells by Triggering Their Differentiation. Cell. 2009;137(6):1088–1099. doi: 10.1016/j.cell.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307(5710):720–724. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- 14.Dykstra B, Olthof S, Schreuder J, Ritsema M, de Haan G. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. The Journal of experimental medicine. 2011;208(13):2691–2703. doi: 10.1084/jem.20111490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doles J, Keyes WM. Epidermal stem cells undergo age-associated changes. Aging. 2013;5(1):1–2. doi: 10.18632/aging.100530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(26):9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. The Journal of experimental medicine. 2000;192(9):1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447(7145):725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 19.Beerman I, Bock C, Garrison BS, Smith ZD, Gu H, Meissner A, et al. Proliferation-dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging. Cell stem cell. 2013;12(4):413–425. doi: 10.1016/j.stem.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS biology. 2007;5(8):e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Challen GA, Boles NC, Chambers SM, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell stem cell. 2010;6(3):265–278. doi: 10.1016/j.stem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beerman I, Bhattacharya D, Zandi S, Sigvardsson M, Weissman IL, Bryder D, et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(12):5465–5470. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111(12):5553–5561. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(50):20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Florian MC, Dorr K, Niebel A, Daria D, Schrezenmeier H, Rojewski M, et al. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell stem cell. 2012;10(5):520–530. doi: 10.1016/j.stem.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun D, Luo M, Jeong M, Rodriguez B, Xia Z, Hannah R, et al. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell stem cell. 2014;14(5):673–688. doi: 10.1016/j.stem.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nature medicine. 2001;7(9):1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 28.Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, et al. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. The Journal of biological chemistry. 2004;279(23):24218–24225. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- 29.Zhou S, Morris JJ, Barnes Y, Lan L, Schuetz JD, Sorrentino BP. Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(19):12339–12344. doi: 10.1073/pnas.192276999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin T, Islam O, Heese K. ABC transporters, neural stem cells and neurogenesis--a different perspective. Cell research. 2006;16(11):857–871. doi: 10.1038/sj.cr.7310107. [DOI] [PubMed] [Google Scholar]
- 31.Tang L, Bergevoet SM, Gilissen C, de Witte T, Jansen JH, van der Reijden BA, et al. Hematopoietic stem cells exhibit a specific ABC transporter gene expression profile clearly distinct from other stem cells. BMC Pharmacol. 2010;10:12. doi: 10.1186/1471-2210-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takizawa H, Regoes RR, Boddupalli CS, Bonhoeffer S, Manz MG. Dynamic variation in cycling of hematopoietic stem cells in steady state and inflammation. The Journal of experimental medicine. 2011;208(2):273–284. doi: 10.1084/jem.20101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foudi A, Hochedlinger K, Van Buren D, Schindler JW, Jaenisch R, Carey V, et al. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nature biotechnology. 2009;27(1):84–90. doi: 10.1038/nbt.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, et al. Hematopoietic Stem Cells Reversibly Switch from Dormancy to Self-Renewal during Homeostasis and Repair. Cell. 2008 doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 35.Flores I, Benetti R, Blasco MA. Telomerase regulation and stem cell behaviour. Current opinion in cell biology. 2006;18(3):254–260. doi: 10.1016/j.ceb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Morrison SJ, Prowse KR, Ho P, Weissman IL. Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity. 1996;5(3):207–216. doi: 10.1016/s1074-7613(00)80316-7. [DOI] [PubMed] [Google Scholar]
- 37.Allsopp RC, Morin GB, DePinho R, Harley CB, Weissman IL. Telomerase is required to slow telomere shortening and extend replicative lifespan of HSCs during serial transplantation. Blood. 2003;102(2):517–520. doi: 10.1182/blood-2002-07-2334. [DOI] [PubMed] [Google Scholar]
- 38.Broccoli D, Young JW, de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(20):9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiyama K, Hirai Y, Kyoizumi S, Akiyama M, Hiyama E, Piatyszek MA, et al. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J Immunol. 1995;155(8):3711–3715. [PubMed] [Google Scholar]
- 40.Shyh-Chang N, Daley GQ, Cantley LC. Stem cell metabolism in tissue development and aging. Development. 2013;140(12):2535–2547. doi: 10.1242/dev.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell stem cell. 2011;9(4):298–310. doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Nombela-Arrieta C, Pivarnik G, Winkel B, Canty KJ, Harley B, Mahoney JE, et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nature cell biology. 2013;15(8):1016. doi: 10.1038/ncb2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(13):5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kocabas F, Zheng J, Thet S, Copeland NG, Jenkins NA, DeBerardinis RJ, et al. Meis1 regulates the metabolic phenotype and oxidant defense of hematopoietic stem cells. Blood. 2012;120(25):4963–4972. doi: 10.1182/blood-2012-05-432260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell stem cell. 2010;7(3):391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 46.Sandell LL, Zakian VA. Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell. 1993;75(4):729–739. doi: 10.1016/0092-8674(93)90493-a. [DOI] [PubMed] [Google Scholar]
- 47.Olovnikov AM. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973;41(1):181–190. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- 48.Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51(6):887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- 49.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nature reviews Molecular cell biology. 2007;8(9):729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 50.Samper E, Fernandez P, Eguia R, Martin-Rivera L, Bernad A, Blasco MA, et al. Long-term repopulating ability of telomerase-deficient murine hematopoietic stem cells. Blood. 2002;99(8):2767–2775. doi: 10.1182/blood.v99.8.2767. [DOI] [PubMed] [Google Scholar]
- 51.Ju Z, Jiang H, Jaworski M, Rathinam C, Gompf A, Klein C, et al. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nature medicine. 2007;13(6):742–747. doi: 10.1038/nm1578. [DOI] [PubMed] [Google Scholar]
- 52.Allsopp RC, Morin GB, Horner JW, DePinho R, Harley CB, Weissman IL. Effect of TERT over-expression on the long-term transplantation capacity of hematopoietic stem cells. Nature medicine. 2003;9(4):369–371. doi: 10.1038/nm0403-369. [DOI] [PubMed] [Google Scholar]
- 53.Wahlestedt M, Norddahl GL, Sten G, Ugale A, Frisk MA, Mattsson R, et al. An epigenetic component of hematopoietic stem cell aging amenable to reprogramming into a young state. Blood. 2013;121(21):4257–4264. doi: 10.1182/blood-2012-11-469080. [DOI] [PubMed] [Google Scholar]
- 54.Wagner W, Bork S, Horn P, Krunic D, Walenda T, Diehlmann A, et al. Aging and replicative senescence have related effects on human stem and progenitor cells. PloS one. 2009;4(6):e5846. doi: 10.1371/journal.pone.0005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gottschling DE, Aparicio OM, Billington BL, Zakian VA. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990;63(4):751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- 56.Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(21):9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Werner B, Beier F, Hummel S, Balabanov S, Lassay L, Orlikowsky T, et al. Reconstructing the in vivo dynamics of hematopoietic stem cells from telomere length distributions. eLife. 2015;4 doi: 10.7554/eLife.08687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robin JD, Ludlow AT, Batten K, Magdinier F, Stadler G, Wagner KR, et al. Telomere position effect: regulation of gene expression with progressive telomere shortening over long distances. Genes & development. 2014;28(22):2464–2476. doi: 10.1101/gad.251041.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haneline LS, Gobbett TA, Ramani R, Carreau M, Buchwald M, Yoder MC, et al. Loss of FancC function results in decreased hematopoietic stem cell repopulating ability. Blood. 1999;94(1):1–8. [PubMed] [Google Scholar]
- 60.Reese JS, Liu L, Gerson SL. Repopulating defect of mismatch repair-deficient hematopoietic stem cells. Blood. 2003;102(5):1626–1633. doi: 10.1182/blood-2002-10-3035. [DOI] [PubMed] [Google Scholar]
- 61.Carreau M, Gan OI, Liu L, Doedens M, Dick JE, Buchwald M. Hematopoietic compartment of Fanconi anemia group C null mice contains fewer lineage-negative CD34+ primitive hematopoietic cells and shows reduced reconstruction ability. Experimental hematology. 1999;27(11):1667–1674. doi: 10.1016/s0301-472x(99)00102-2. [DOI] [PubMed] [Google Scholar]
- 62.Moehrle BM, Nattamai K, Brown A, Florian MC, Ryan M, Vogel M, et al. Stem Cell-Specific Mechanisms Ensure Genomic Fidelity within HSCs and upon Aging of HSCs. Cell reports. 2015;13(11):2412–2424. doi: 10.1016/j.celrep.2015.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beerman I, Seita J, Inlay MA, Weissman IL, Rossi DJ. Quiescent Hematopoietic Stem Cells Accumulate DNA Damage during Aging that Is Repaired upon Entry into Cell Cycle. Cell stem cell. 2014 doi: 10.1016/j.stem.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rube CE, Fricke A, Widmann TA, Furst T, Madry H, Pfreundschuh M, et al. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PloS one. 2011;6(3):e17487. doi: 10.1371/journal.pone.0017487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walter D, Lier A, Geiselhart A, Thalheimer FB, Huntscha S, Sobotta MC, et al. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature. 2015;520(7548):549–552. doi: 10.1038/nature14131. [DOI] [PubMed] [Google Scholar]
- 66.Turinetto V, Giachino C. Multiple facets of histone variant H2AX: a DNA double-strand-break marker with several biological functions. Nucleic acids research. 2015;43(5):2489–2498. doi: 10.1093/nar/gkv061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yahata T, Takanashi T, Muguruma Y, Ibrahim AA, Matsuzawa H, Uno T, et al. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood. 2011;118(11):2941–2950. doi: 10.1182/blood-2011-01-330050. [DOI] [PubMed] [Google Scholar]
- 68.Jacobs KB, Yeager M, Zhou W, Wacholder S, Wang Z, Rodriguez-Santiago B, et al. Detectable clonal mosaicism and its relationship to aging and cancer. Nature genetics. 2012;44(6):651–658. doi: 10.1038/ng.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ley TJ, Mardis ER, Ding L, Fulton B, McLellan MD, Chen K, et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature. 2008;456(7218):66–72. doi: 10.1038/nature07485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. The New England journal of medicine. 2009;361(11):1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jan M, Snyder TM, Corces-Zimmerman MR, Vyas P, Weissman IL, Quake SR, et al. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Science translational medicine. 2012;4(149):149ra18. doi: 10.1126/scitranslmed.3004315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506(7488):328–333. doi: 10.1038/nature13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nature medicine. 2014;20(12):1472–1478. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150(2):264–278. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. The New England journal of medicine. 2014;371(26):2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Busque L, Patel JP, Figueroa ME, Vasanthakumar A, Provost S, Hamilou Z, et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nature genetics. 2012;44(11):1179–1181. doi: 10.1038/ng.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Genovese G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. The New England journal of medicine. 2014;371(26):2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Challen GA, Sun D, Mayle A, Jeong M, Luo M, Rodriguez B, et al. Dnmt3a and Dnmt3b Have Overlapping and Distinct Functions in Hematopoietic Stem Cells. Cell stem cell. 2014 doi: 10.1016/j.stem.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ko M, Bandukwala HS, An J, Lamperti ED, Thompson EC, Hastie R, et al. Ten-Eleven-Translocation 2 (TET2) negatively regulates homeostasis and differentiation of hematopoietic stem cells in mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(35):14566–14571. doi: 10.1073/pnas.1112317108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moran-Crusio K, Reavie L, Shih A, Abdel-Wahab O, Ndiaye-Lobry D, Lobry C, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer cell. 2011;20(1):11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Challen GA, Sun D, Jeong M, Luo M, Jelinek J, Berg JS, et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nature genetics. 2012;44(1):23–31. doi: 10.1038/ng.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McKerrell T, Park N, Moreno T, Grove CS, Ponstingl H, Stephens J, et al. Leukemia-associated somatic mutations drive distinct patterns of age-related clonal hemopoiesis. Cell reports. 2015;10(8):1239–1245. doi: 10.1016/j.celrep.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Young AL, Challen GA, Birmann BM, Druley TE. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nature communications. 2016;7:12484. doi: 10.1038/ncomms12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blokzijl F, de Ligt J, Jager M, Sasselli V, Roerink S, Sasaki N, et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature. 2016;538(7624):260–264. doi: 10.1038/nature19768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Laurie CC, Laurie CA, Rice K, Doheny KF, Zelnick LR, McHugh CP, et al. Detectable clonal mosaicism from birth to old age and its relationship to cancer. Nature genetics. 2012;44(6):642–650. doi: 10.1038/ng.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Machiela MJ, Zhou W, Caporaso N, Dean M, Gapstur SM, Goldin L, et al. Mosaic 13q14 deletions in peripheral leukocytes of non-hematologic cancer cases and healthy controls. Journal of human genetics. 2016;61(5):411–418. doi: 10.1038/jhg.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Forsberg LA, Rasi C, Razzaghian HR, Pakalapati G, Waite L, Thilbeault KS, et al. Age-related somatic structural changes in the nuclear genome of human blood cells. American journal of human genetics. 2012;90(2):217–228. doi: 10.1016/j.ajhg.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Larsson NG. Somatic mitochondrial DNA mutations in mammalian aging. Annu Rev Biochem. 2010;79:683–706. doi: 10.1146/annurev-biochem-060408-093701. [DOI] [PubMed] [Google Scholar]
- 89.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429(6990):417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 90.Ameur A, Stewart JB, Freyer C, Hagstrom E, Ingman M, Larsson NG, et al. Ultra-deep sequencing of mouse mitochondrial DNA: mutational patterns and their origins. PLoS genetics. 2011;7(3):e1002028. doi: 10.1371/journal.pgen.1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Norddahl GL, Pronk CJ, Wahlestedt M, Sten G, Nygren JM, Ugale A, et al. Accumulating mitochondrial DNA mutations drive premature hematopoietic aging phenotypes distinct from physiological stem cell aging. Cell stem cell. 2011;8(5):499–510. doi: 10.1016/j.stem.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 92.Yao YG, Kajigaya S, Feng X, Samsel L, McCoy JP, Jr, Torelli G, et al. Accumulation of mtDNA variations in human single CD34+ cells from maternally related individuals: effects of aging and family genetic background. Stem cell research. 2013;10(3):361–370. doi: 10.1016/j.scr.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shepherd BE, Guttorp P, Lansdorp PM, Abkowitz JL. Estimating human hematopoietic stem cell kinetics using granulocyte telomere lengths. Experimental hematology. 2004;32(11):1040–1050. doi: 10.1016/j.exphem.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 94.Mohrin M, Bourke E, Alexander D, Warr MR, Barry-Holson K, Le Beau MM, et al. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell stem cell. 2010;7(2):174–185. doi: 10.1016/j.stem.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McGlynn P, Lloyd RG. Recombinational repair and restart of damaged replication forks. Nat Rev Mol Cell Bio. 2002;3(11):859–870. doi: 10.1038/nrm951. [DOI] [PubMed] [Google Scholar]
- 96.Lehmann AR, Fuchs RP. Gaps and forks in DNA replication: Rediscovering old models. DNA repair. 2006;5(12):1495–1498. doi: 10.1016/j.dnarep.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 97.Sale JE, Lehmann AR, Woodgate R. Y-family DNA polymerases and their role in tolerance of cellular DNA damage. Nat Rev Mol Cell Bio. 2012;13(3):141–152. doi: 10.1038/nrm3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang J, Liu SO, Bellani M, Ling C, Wang YS, Wang WD, et al. Fanconi anemia protein FANCM promotes replication traverse of DNA interstrand crosslinks. Faseb Journal. 2014;28(1) [Google Scholar]
- 99.Ibarra A, Schwob E, Mendez J. Excess MCM proteins protect human cells from replicative stress by licensing backup origins of replication. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(26):8956–8961. doi: 10.1073/pnas.0803978105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Flach J, Bakker ST, Mohrin M, Conroy PC, Pietras EM, Reynaud D, et al. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature. 2014;512(7513):198–202. doi: 10.1038/nature13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang Y, Poe JC, Yang L, Fedoriw A, Desai S, Magnuson T, et al. Rad18 confers hematopoietic progenitor cell DNA damage tolerance independently of the Fanconi Anemia pathway in vivo. Nucleic acids research. 2016;44(9):4174–4188. doi: 10.1093/nar/gkw072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell stem cell. 2010;7(2):150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 103.Rehman J. Empowering self-renewal and differentiation: the role of mitochondria in stem cells. J Mol Med (Berl) 2010;88(10):981–986. doi: 10.1007/s00109-010-0678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128(2):325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 105.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nature medicine. 2006;12(4):446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 106.Bestor TH, Coxon A. Cytosine methylation: the pros and cons of DNA methylation. Current biology : CB. 1993;3(6):384–386. doi: 10.1016/0960-9822(93)90209-7. [DOI] [PubMed] [Google Scholar]
- 107.Burman B, Zhang ZZ, Pegoraro G, Lieb JD, Misteli T. Histone modifications predispose genome regions to breakage and translocation. Genes & development. 2015;29(13):1393–1402. doi: 10.1101/gad.262170.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Djeghloul D, Kuranda K, Kuzniak I, Barbieri D, Naguibneva I, Choisy C, et al. Age-Associated Decrease of the Histone Methyltransferase SUV39H1 in HSC Perturbs Heterochromatin and B Lymphoid Differentiation. Stem cell reports. 2016;6(6):970–984. doi: 10.1016/j.stemcr.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang J, Sun Q, Morita Y, Jiang H, Gross A, Lechel A, et al. A differentiation checkpoint limits hematopoietic stem cell self-renewal in response to DNA damage. Cell. 2012;148(5):1001–1014. doi: 10.1016/j.cell.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 110.Kong Y, Song Y, Hu Y, Shi MM, Wang YT, Wang Y, et al. Increased reactive oxygen species and exhaustion of quiescent CD34-positive bone marrow cells may contribute to poor graft function after allotransplants. Oncotarget. 2016 doi: 10.18632/oncotarget.8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Richardson C, Yan S, Vestal CG. Oxidative stress, bone marrow failure, and genome instability in hematopoietic stem cells. Int J Mol Sci. 2015;16(2):2366–2385. doi: 10.3390/ijms16022366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Porto ML, Rodrigues BP, Menezes TN, Ceschim SL, Casarini DE, Gava AL, et al. Reactive oxygen species contribute to dysfunction of bone marrow hematopoietic stem cells in aged C57BL/6 J mice. J Biomed Sci. 2015;22:97. doi: 10.1186/s12929-015-0201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood. 1993;82(7):2031–2037. [PubMed] [Google Scholar]
- 114.Koller MR, Bender JG, Miller WM, Papoutsakis ET. Reduced oxygen tension increases hematopoiesis in long-term culture of human stem and progenitor cells from cord blood and bone marrow. Experimental hematology. 1992;20(2):264–270. [PubMed] [Google Scholar]
- 115.Mantel CR, O'Leary HA, Chitteti BR, Huang X, Cooper S, Hangoc G, et al. Enhancing Hematopoietic Stem Cell Transplantation Efficacy by Mitigating Oxygen Shock. Cell. 2015;161(7):1553–1565. doi: 10.1016/j.cell.2015.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126(1):9–16. doi: 10.1182/blood-2015-03-631747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530(7589):184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344(6184):649–652. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]