Abstract
Identifying and mimicking the signals that regulate stem cell self‐renewal, differentiation and maintenance in a petri dish is crucial to faithfully recapitulate stem cell behaviour in vitro. In this issue, Chacón‐Martínez et al (2017) describe novel culture conditions that allow the long‐term expansion and maintenance of functional murine hair follicle stem cells. This exciting discovery provides a faithful platform to study hair follicle stem cells in vitro and potentially perform drug screening for skin and hair follicle disorders.
Subject Categories: Development & Differentiation, Stem Cells, Systems & Computational Biology
Stem cells (SCs) are responsible for the maintenance of tissue homeostasis. They reside within specialized microenvironments, called niches. SC niches are composed of SCs themselves, neighbouring stromal, vascular and immune cells, as well as extracellular matrix, providing signals that regulate SCs' renewal, quiescence, proliferation and differentiation during homeostasis and repair (Blanpain & Fuchs, 2014). Therefore, in vitro models aimed at studying SC biology should incorporate niche cues to faithfully recapitulate the in vivo scenario. In this respect, Clevers and colleagues reported the successful generation of intestinal organoids by culturing intestinal SCs in matrigel together with the presence of a limited set of growth signals that are normally released by the intestinal SC niche (Sato et al, 2009). This approach has now been used to culture many tissues thought to be incapable to grow in vitro.
Hair follicle (HF) SCs, due to their high accessibility, their continual cycles of growth and degeneration and the different cell lineages they give rise to, represent an excellent model system to study all the essential characteristics of adult SC functions including quiescence, renewal, activation and differentiation (Blanpain & Fuchs, 2014). Pioneered by the work of Barrandon and Green, for the last several decades researchers had been studying HF and other types of epidermal SCs by culturing these cells on feeder layers (Barrandon & Green, 1987). HFSCs, although relatively quiescent in vivo, proliferate and renew relatively rapidly when cultured in vitro on feeder layers, giving rise to large undifferentiated colonies called holoclones. These cells can be expanded massively in vitro and transplanted back in vivo to generate a new epidermis (De Luca et al, 2006), which has saved thousands of lives of severely burnt patients. In addition, in the presence of dermal papillae (DP) cells, the mesenchymal cells that have HF‐inducing capacity, transplantation of cultured HFSCs can be induced to undergo HF differentiation even starting from a single HFSC (Blanpain et al, 2004). However, despite their capacity to differentiate into HF cells when co‐transplanted with DP cells, HFSCs cultured onto feeder layer in vitro rapidly lose many markers characteristic of their in vivo state, precluding studying in vitro the molecular mechanisms that regulate HF function. In this issue, Chacón‐Martínez et al (2017) uncover the culture conditions that allow the expansion and long‐term maintenance of HFSCs in the absence of heterologous cell types.
To define the culture conditions that recapitulate the in vivo HFSC niche and allow expansion and maintenance of HFSCs, the authors followed the approach developed by Sato and colleagues, namely to grow intestinal SC in vitro embedded in matrigel in the presence of defined morphogens normally expressed by the SC niche (Sato et al, 2009). Likewise, Chacón‐Martínez et al (2017) cultured epidermal cells embedded in matrigel in the presence of Rock inhibitor, which prevents anoikis resulting from the deprivation of cell–cell contact, and different growth factors expressed by HFSCs that regulate their growth. They monitored the maintenance of HFSC characteristics in culture by assessing the expression of CD34 (Fig 1), a marker specifically expressed by HFSC and rapidly lost in vitro using standard culture conditions (Blanpain et al, 2004). They found that matrigel or collagen 1 and Rock inhibitor together with FGF2 and VEGF‐A (Plikus & Chuong, 2014) in keratinocyte growth medium (referred to as 3C culture conditions thereafter) was sufficient to maintain and expand CD34+ HFSCs in culture (Fig 1). These cells were able to generate large colonies composed of tightly packed and undifferentiated cells, characteristic of the holoclones when cultured on feeders, and they can also form hair upon transplantation together with DP cells in vivo (Fig 1). These results demonstrate that the new culture conditions allows to maintain renewal and differentiation potential of HFSC in vitro. Remarkably, the authors show that the proportion of CD34+α6+ cells in their culture and the ability to generate de novo HF upon transplantation are conserved after 10 passages during 20 weeks and after freeze–thaw, showing the robustness of the 3C conditions.
Figure 1. Long‐term expansion and maintenance of murine hair follicle stem cells.
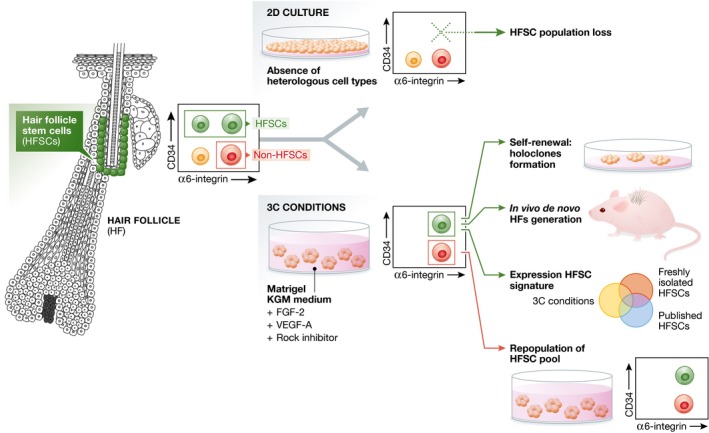
Hair follicle stem cells from the bulge region are characterized by high expression of CD34. Culture of epidermal cell mixtures in 2D conditions in the absence of heterologous cell types leads to an exhaustion of the HFSC pool. Culture of epidermal cell mixtures in 3C conditions leads to the generation of cultures that contain HFSCs and non‐HFSCs (mainly progenitors). 3C conditions allow the expansion of functional HFSCs that generate holoclones upon co‐culturing with dermal cells, generate de novo HFs upon transplantation to mice and share high similarity to FACS‐isolated HFSCs and published HFSC signatures. Moreover, the non‐HFSC population within the 3C culture conditions is able to give rise to the HFSC population.
Using RNA‐seq and RT–PCR analysis, the authors defined the genes preferentially expressed by CD34‐expressing cells (α6+CD34+ vs. α6+CD34−) in 3C conditions in vitro and compared them to CD34+ HFSC in vivo and previously reported HFSC signatures (Morris et al, 2004; Tumbar et al, 2004). Remarkably, their cultured cells present a transcriptional profile that shares important similarity with freshly FACS‐isolated HFSC and published HFSC signatures (Fig 1), whereas their α6+CD34− progeny resembles more closely the hair germ HFSC progeny in vivo (Greco et al, 2009). These findings highlight that this new culture condition preserves the preferential expression of the HFSC signature found in vivo, without preventing their normal lineage commitment.
The authors then analysed the dynamics and relative ratio of CD34+ vs. CD34− cells in the new culture condition. They found that the proportion of cells expressing CD34 rapidly decreased after plating and reached a plateau of 50% of CD34+. EdU incorporation demonstrates that CD34+ cells divide faster than CD34− cells, while their rate of apoptosis was similar, suggesting that CD34+ cells must continuously differentiate into CD34− cells to maintain a constant ratio. They used differential equations to model the constant proportion of CD34+/CD34− cells in which they allow CD34+ and CD34− bidirectional interconversion. To test this prediction, they isolated CD34− cells and cultured them in the 3C conditions. In good accordance with the prediction of their model, CD34− cells give rise to CD34+ cells and also reach the equilibrium after several days (Fig 1). Different ratio of CD34+ and CD34− cells co‐cultured in 3C conditions revealed that they reach 50/50 equilibrium at the same rate independently of the initial proportion of CD34+ cells in the culture. These findings highlight that the culture system developed by Chacón‐Martínez et al (2017) not only contains the cues required to expand CD34+ HFSCs in vitro but is also able to recapitulate the plasticity reported in vivo leading to the repopulation of the HFSC by non‐HFSCs following their depletion (Ito et al, 2004). In addition, this new method could be used as a platform to study the effect of activation/inhibition of signalling pathways controlling HFSC functions. The authors showed that alterations in the Shh and BMP signalling pathways change the proportion of CD34+ cells as predicted by their effects on HFSC functions in vivo.
The study of Chacón‐Martínez et al (2017) describes the culture conditions that mimic the in vivo HFSC niche, allowing to maintain and expand HFSCs in vitro in a state that faithfully recapitulates the identity and behaviour observed in HFSC in their native state. The model implemented has the potential to become a valuable platform to discover novel mechanisms controlling HFSC functions and potentially other epithelial SCs and can in addition be utilized for drug screening. It would be of great interest to determine whether this approach could be applicable to human HFSCs.
See also: CA Chacón‐Martínez et al (January 2017)
References
- Barrandon Y, Green H (1987) Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA 84: 2302–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E (2004) Self‐renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118: 635–648 [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E (2014) Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science 344: 1242281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacón‐Martínez CA, Klose M, Niemann C, Glauche I, Wickström SA (2017) Hair follicle stem cell cultures reveal self‐organizing plasticity of stem cells and their progeny. EMBO J 36: 151–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Pellegrini G, Green H (2006) Regeneration of squamous epithelia from stem cells of cultured grafts. Regen Med 1: 45–57 [DOI] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz‐Racelis J, Fuchs E (2009) A two‐step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 4: 155–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Kizawa K, Hamada K, Cotsarelis G (2004) Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation 72: 548–557 [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G (2004) Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 22: 411–417 [DOI] [PubMed] [Google Scholar]
- Plikus MV, Chuong CM (2014) Macroenvironmental regulation of hair cycling and collective regenerative behavior. Cold Spring Harb Perspect Med 4: a015198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H (2009) Single Lgr5 stem cells build crypt‐villus structures in vitro without a mesenchymal niche. Nature 459: 262–265 [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E (2004) Defining the epithelial stem cell niche in skin. Science 303: 359–363 [DOI] [PMC free article] [PubMed] [Google Scholar]


