Abstract
The therapeutic targeting of anaplastic lymphoma kinase (ALK) has been a burgeoning area of research since 2007 when ALK fusions were initially identified in patients with non-small cell lung cancer (NSCLC). The field has rapidly progressed through development of the first-generation ALK inhibitor, crizotinib, to an understanding of mechanisms of acquired resistance to crizotinib, and is currently witnessing an explosion in the development of next-generation ALK inhibitors such as certinib, alectinib, PF-06463922, AP26113, X-396, and TSR-011. As with most targeted therapies, acquired resistance appears to be an inevitable outcome. Current pre-clinical and clinical studies are focused on the development of rational therapeutic strategies, including novel ALK inhibitors as well as rational combination therapies to maximize disease control by delaying or overcoming acquired therapeutic resistance. This review summarizes the existing clinical data and ongoing research pertaining to the clinical application of ALK inhibitors in patients with NSCLC.
Keywords: Anaplastic lymphoma kinase, ALK, non-small cell lung cancer, lung adenocarcinoma, tyrosine kinase inhibitor, TKI, clinical trials, acquired resistance
Introduction
In 1994, the anaplastic lymphoma kinase (ALK) gene was first discovered as part of the characteristic t(2,5) found in patients with anaplastic large cell lymphoma, which results in a fusion between nucleophosmin (NPM) and ALK1. More then a decade later, analogous fusions involving ALK and the echinoderm microtubule associated protein like 4 (EML4) were discovered in patients with non-small cell lung cancer (NSCLC)2. The ALK gene encodes a receptor tyrosine kinase in the insulin receptor family with ill-defined but suspected ligands including midkine, heparin, and pleiotrophin3–5. The ALK receptor is thought to be activated by ligand-mediated dimerization and transphosphorylation4. The expression profile and normal function of ALK in humans is unknown, but in adult mice it is thought to assist in normal functioning of the frontal cortex and hippocampus6.
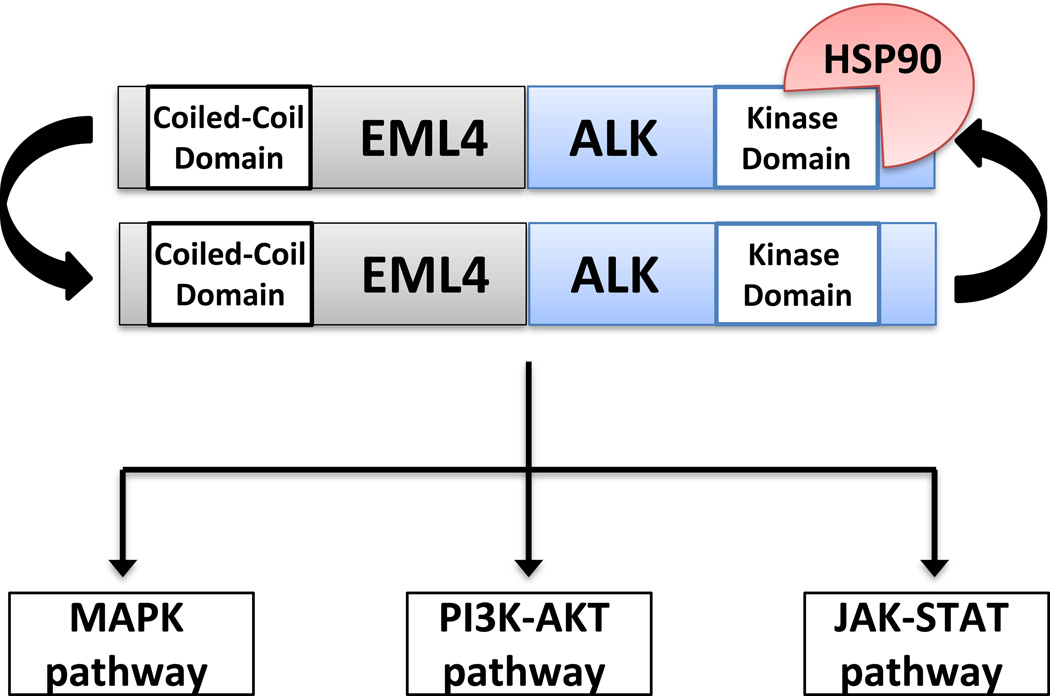
Amongst all patients with NSCLC, 3–7% harbor an ALK gene rearrangement in their tumor tissue. ALK rearrangements are more common in younger patients with little to no smoking history and adenocarcinoma histology7. In patients with NSCLC, many different ALK gene fusions have been identified, the most common of which is the EML4-ALK fusion. All ALK fusion proteins found in patients with NSCLC feature preservation of the ALK tyrosine kinase domain, utilization of a promoter from the N-terminal fusion partner gene, and exploitation of an oligomerization domain in the N-terminal fusion partner to achieve constitutive activation7 (Figure 1). ALK fusion proteins in lung cancer mediate downstream signaling through several pro-growth and anti-apoptotic pathways in the cell, including the mitogen activated protein kinase (MAPK) pathway, the phosphatidyl-inositol-3-kinase (PI3K)-AKT pathway, and the JAK-STAT pathway. ALK fusion proteins are also known to be clients of the heat shock protein-90 (HSP-90) chaperone which aids in stabilization of the fusion protein8.
Figure 1. Schematic representation of the EML4-ALK fusion protein.
The EML4-ALK fusion is formed via an abnormal rearrangement on chromosome 2 which juxtaposes part of the N-terminus of EML4 to a portion of the C-terminus of ALK. The N-terminus of EML4 contains a coiled-coiled domain, which mediates EML4-ALK dimerization. Dimerization has been shown to be critical for EML4-ALK activity (Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007:561–6). The ALK portion of the fusion contains the entire ALK tyrosine kinase domain. EML4-ALK is the most common ALK fusion described in lung cancer to date, however, other ALK fusions partners have also been described. EML4-ALK fusion proteins are clients of the heat shock protein-90 (HSP-90) chaperone, and they signal downstream through the mitogen activated protein kinase (MAPK) pathway, the phosphatidyl-inositol-3-kinase (PI3K)-AKT pathway, and the JAK-STAT pathway to mediate pleiotropic pro-growth and pro-survival effects.
Interestingly, aside from NSCLC, many other different tumor types – in both pediatric and adult malignancies - have been shown to harbor oncogenic ALK abnormalities. These include ALK amplification and ALK point mutations in patients with neuroblastoma, ALK point mutations in patients with anaplastic thyroid carcinoma, and ALK gene fusions in patients with anaplastic large cell lymphoma, diffuse large B cell lymphoma (DLBCL), inflammatory myofibroblastic tumor (IMT), and others9. For the specific details of known ALK point mutations and fusions we refer the reader to a recently published review7.
Initial therapeutic targeting
The therapeutic efficacy of ALK inhibition in patients with ALK-rearranged NSCLC was first demonstrated with crizotinib (PF-02341066) in the PROFILE series of clinical trials (Table 1). Crizotinib is an oral inhibitor of the tyrosine kinases ALK, mesenchymal-epithelial transition factor (MET), and c-ros oncogene 1 (ROS1)7. In a phase I clinical trial, crizotinib demonstrated a 60.8% objective response rate (ORR), a median progression free survival (PFS) of 9.7 months, and overall survival (OS) rates of 87.9% at six months and 74.8% at one year in patients with advanced ALK-rearranged NSCLC10. In this clinical trial, ALK rearrangements were identified with fluorescence in-situ hybridization (FISH), and the frequencies of specific ALK fusion gene types were not reported10. In a retrospective analysis using this phase I data comparing OS between 30 patients with ALK-rearranged NSCLC treated with crizotinib in the second or third line setting to 23 patients with ALK-rearranged NSCLC treated with standard, non-ALK inhibitor therapy in the second line setting, patients who received crizotinib had a significantly longer two-year OS, 55% vs 12% (p=0.004)11. The phase II clinical trial assessing crizotinib in patients with ALK-rearranged NSCLC who had undergone at least one prior line of therapy showed a similar ORR of 60% and a PFS of 8.1 months12.
Table 1.
Published clinical trials of ALK inhibitors for patients with ALK-rearranged NSCLC
| ALK Inhibitor |
Reference | N | Phase | Objective Response Rate (%) |
Progression Free Survival (months) |
Distinguishing Toxicity (frequency, all grades) |
|---|---|---|---|---|---|---|
| Crizotinib | Camidge 2012 | 143 | I | 60.8 | 9.7 | Gastrointestinal (40–60%) |
| Crizotinib | Kim 2012 | 261 | II | 60 | 8.1 | Gastrointestinal (35–50%) |
| Crizotinib | Shaw 2013 | 347 | III | 65 | 7.7 | Gastrointestinal (45–60%) |
| Crizotinib | Solomon 2014 | 343 | III | 74 | 10.9 | Gastrointestinal (45–60%) |
| Ceritinib | Shaw 2014 | 114 | I | 58 | 7.0 | Gastrointestinal (65–80%) |
| Ceritinib | Mok 2015 | 140 | II | 54 | 5.7 | Gastrointestinal (60–80%) |
| Certinib | Felip 2015 | 124 | II | 79 | 11.1 | Gastrointestinal (65–80%) |
| Alectinib | Gadgeel 2014 | 44 | I | 55 | N/A | Myalgia (17%) |
| Alectinib | Seto 2013 and Ohe 2015 | 46 | I/II | 93.5 | >29 | Myalgia (13%) |
| Alectinib | Gandhi 2015 | 87 | II | 47.8 | N/A | Myopathy (8% grade 3–5) |
| Alectinib | Ou 2015 | 138 | II | 49.2 | N/A | Pulmonary (2–4% grade 3–5) |
| Brigatinib | Camidge 2015 | 79 | I/II | 72% | 14 | Gastrointestinal (35–45%) |
| X-396 | Horn 2014 | 17 | I | 59% | N/A | Rash (36%) |
| TSR-011 | Arkenau 2015 | 46 | I/II | 100% | N/A | QTc prolongation (22%) |
In a subsequent phase III, open label clinical trial involving 347 patients with locally advanced or metastatic ALK-rearranged NSCLC who had received one line of prior therapy with a platinum-based regimen, patients treated with crizotinib compared to standard cytotoxic chemotherapy (pemetrexed or docetaxel) had significantly better ORR and PFS, but no demonstrable OS benefit. In this clinical trial, the ORR was 65% in the crizotinib arm compared to 20% in the control arm (p<0.001), median PFS was 7.7 months in the crizotinib arm compared to 3.0 months in the control arm (p<0.001), and median OS was 20.3 months in the crizotinib arm compared to 22.8 months in the control arm (p=0.54). Importantly, the OS data was calculated on an intention-to-treat basis, and 64% of patients randomized to conventional chemotherapy in the second line setting went on to receive crizotinib. The frequencies of specific ALK fusion gene types were not reported in this clinical trial13. Similar results were obtained when crizotinib was compared to conventional cytotoxic chemotherapy with pemetrexed and either cisplatin or carboplatin in a phase III clinical trial involving 343 patients with treatment-naïve, ALK-rearranged, advanced NSCLC. In this cohort, the ORR was 74% in patients treated with crizotinib compared to 45% in patients treated with cytotoxic chemotherapy (p<0.001), and the median PFS was 10.9 months in patients treated with crizotinib compared to 7.0 months in patients treated with cytotoxic chemotherapy (p<0.001). One-year OS was not statistically different, as it was 84% in patients receiving crizotinib and 79% in patients receiving cytotoxic chemotherapy. Similar to the aforementioned second line phase III clinical trial, this first line therapy trial was assessed on an intention-to-treat basis, 70% of patients randomized to first-line cytotoxic chemotherapy eventually received crizotinib, and the frequencies of specific ALK fusion gene types were not reported14.
The time from the initial discovery of ALK alterations in patients with NSCLC to Food and Drug Administration (FDA) approval of crizotinib for the treatment of patients with ALK-rearranged NSCLC was four years. The impressive example of ALK-rearranged NSCLC serves as a paradigm for accelerated approval of molecularly targeted therapies in cancer care.
Mechanisms of acquired resistance
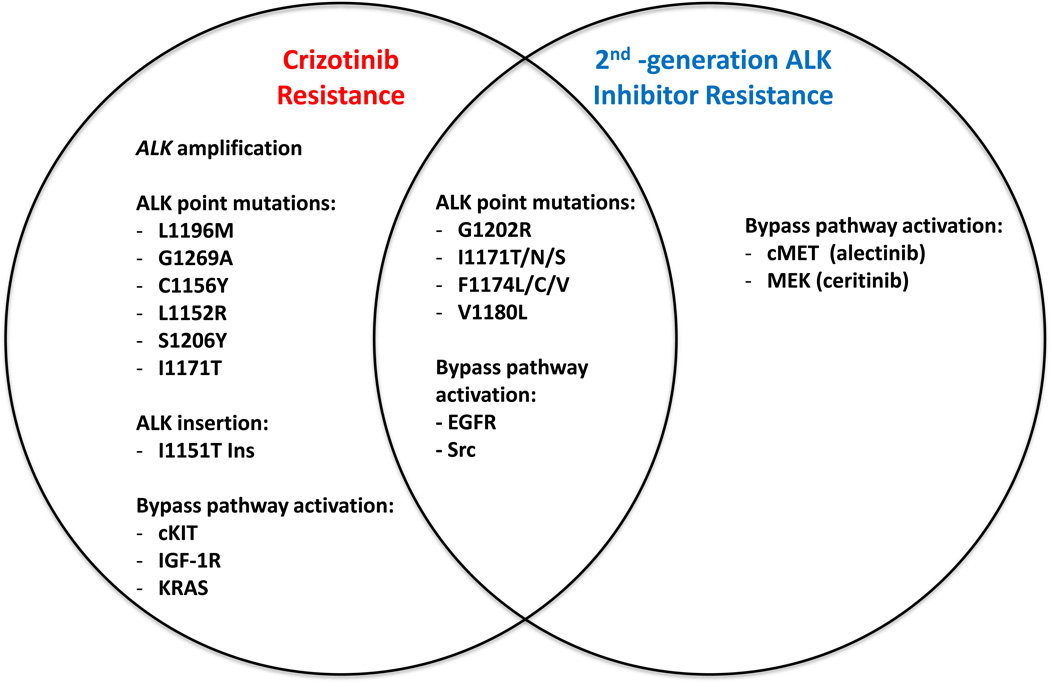
As occurs among patients with EGFR-mutant NSCLC treated with EGFR tyrosine kinase inhibitors (TKI’s), patients with ALK-rearranged NSCLC have been shown to acquire resistance to ALK inhibition with crizotinib by several mechanisms, broadly classified as target modification and ‘bypass’ signaling (Figure 2). Target modifications include ALK gene amplification, present in approximately 9% of tumors resistant to crizotinib15, as well as point mutations and insertion mutations in the tyrosine kinase domain of the EML4-ALK fusion protein7,16, which have been observed in approximately one third of patients with crizotinib-resistant tumors15. Unlike EGFR T790M ‘gatekeeper’ mutation, which is the predominant point mutation detected in approximately 50–60% of patients with acquired resistance to first- and second-generation EGFR TKIs, crizotinib resistance mutations appear to span the ALK kinase domain. The analogous gatekeeper mutation, L1196M, has been detected. Other mutations include L1152R, C1156Y, I1171T/N/S, F1174L/C/V, V1180L, G1202R, S1206Y, and G1269A. The clinical frequency of the various ALK kinase domain mutations which confer crizotinib resistance is actively being studied. In addition to modification of the ALK target, ‘bypass’ pathway signaling has also been shown to mediate crizotinib resistance in several pre-clinical and clinical studies. This ‘bypass’ signaling serves to provide an escape mechanism for the tumors to functionally circumvent the inhibited ALK fusion protein and signal to pro-growth downstream pathways through redundant pathways. For example, up-regulation of phosphorylated EGFR was detected in approximately 44% of patients at the time of crizotinib resistance17. Up-regulation of insulin-like growth factor receptor 1R (IGF-1R)18 and Src19 have also been detected at the time of crizotinib resistance. Finally, genomic amplification of cKIT has been detected in patients at the time of crizotinib resistance17.
Figure 2. Mechanisms of resistance to crizotinib and second-generation ALK inhibitors both share common strategies and exploit novel routes.
Specific ALK tyrosine kinase point mutations have been identified as imparting only resistance to crizotinib (L1196M) or enabling resistance to both crizotinib and second generation ALK inhibitors (G1202R). Similarly, specific bypass signaling pathways have been identified in conjunction with resistance to crizotinib (insulin-like growth factor 1 receptor, IGF-1R), resistance to second generation ALK inhibitors (mesenchymal-epithelial transition factor, MET), or resistance to both crizotinib and second generation ALK inhibitors (epidermal growth factor receptor, EGFR).
Toyokawa G, Seto T, Takenoyama M, et al. Crizotinib can overcome acquired resistance to CH5424802: is amplification of the MET gene a key factor? J Thorac Oncol 2014; 9: e27–8.
Crystal AS, Shaw AT, Sequist LV, et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science 2014; 346: 1480–6.
A second concern related to a potential therapeutic ceiling with crizotinib treatment for ALK-rearranged NSCLC is its central nervous system (CNS) penetration. While it has been noted that crizotinib’s cerebrospinal fluid (CSF) concentration is low compared to plasma (0.26% that of the plasma crizotinib concentration)20, the brain parenchymal concentrations of crizotinib are unknown. It has also been observed that in patients with ALK-rearranged NSCLC treated with crizotinib intracranial metastases are a common site of disease progression. However, it is not clear whether this represents primary or acquired resistance to crizotinib because the mechanisms underlying CNS progression in patients treated with crizotinib are unknown21. Targeting the CNS as a sanctuary site in patients with ALK-rearranged NSCLC is a key aspect of therapy with second-generation ALK inhibitors, and comparisons of the efficacy of these newer agents with crizotinib for CNS disease are ongoing.
Therapeutic strategies to overcome acquired resistance
Multiple second-generation ALK inhibitors are currently in clinical trials, both in the setting of patients with acquired resistance to crizotinib and as first-line therapies.
Ceritinib (LDK378) is a second-generation oral ALK inhibitor, which has shown efficacy when used to treat patients with locally advanced or metastatic ALK-rearranged NSCLC, including those whose tumors have acquired resistance to crizotinib. In a phase I clinical trial, among 114 patients with ALK-rearranged NSCLC who received at least 400 milligrams (mg) of ceritinib daily, the ORR was 58% and the PFS was 7.0 months. Among 86 patients who had previously been treated with crizotinib, the ORR remained 56%, indicating that ceritinib therapy was able to overcome acquired resistance to crizotinib in a substantial proportion of patients. Specifically, among seven patients in whom crizotinib resistance via ALK point mutations in the tyrosine kinase domain or ALK gene amplification were identified before treatment with ceritinib, six (86%) experienced an objective response22. In preliminary results from two larger clinical trials (ASCEND 2 and ASCEND 3; NCT01685060 and NCT01685138, respectively), among 140 patients previously treated with platinum based chemotherapy who had progressed through crizotinib, the ORR was 54% and the PFS was 5.7 months23. In 124 patients with ALK-rearranged NSCLC who were ALK inhibitor naïve, the ORR was 79% and the PFS was 11.1 months24.
Alectinib (CH5424802/RO5424802) is a second-generation oral ALK inhibitor that has also shown efficacy in patients with ALK-rearranged NSCLC. In a phase I clinical trial involving 44 patients with ALK-rearranged NSCLC who developed resistance to crizotinib or were intolerant of crizotinib, the ORR was 55%25. In a phase I/II clinical trial of alectinib in 46 patients with ALK-rearranged, advanced NSCLC with no prior ALK inhibitor therapy, the ORR was 93.5% and the PFS at the most recent update at the American Society of Clinical Oncology (ASCO) meeting in 2015 is expected to be greater than 29 months25,26. Furthermore, there has been suggestion that alectinib may have improved therapeutic efficacy for CNS metastases compared to crizotinib and ceritinib, as it achieves notably higher CSF levels and has induced CNS disease responses in tumors resistant to crizotinib and ceritinib27–29. There were also two phase II clinical trials presented at ASCO 2015, showing an ORR of 48–49% in patients with ALK-rearranged locally advanced or metastatic NSCLC who had progressed through crizotinib (NCT01801111 and NP28761)30–31.
PF-06463922 is an oral inhibitor of ALK and ROS1 that has been shown to have antitumor effects in both ALK-rearranged and ROS1-rearranged pre-clinical models32. PF-06463922 is currently in a phase I/II clinical trial, preliminary results of which were presented at ASCO 2015. Among 18 patients with ALK or ROS1-rearranged advanced NSCLC the ORR was 40%, and five of the six patients with documented responses to PF-06463922 had progressed through prior ALK TKI therapy (NCT01970865)33.
Brigatinib (AP26113) is an oral ALK inhibitor currently in a phase I/II clinical trial. At the most recent interval reporting of this trial at ASCO 2015, 79 patients with advanced, ALK-rearranged NSCLC had been treated with an ORR of 72% and median PFS of 14 months (NCT01449461)34.
X-396 is an oral ALK inhibitor also currently in a phase I/II clinical trial. At the most recent interval reporting of this trial in 2014, among 17 patients with ALK-rearranged NSCLC the ORR was 59%, and the ORR among the 13 patients previously treated with crizotinib was 54% (NCT01625234)35.
TSR-011 is an oral ALK and tropomyosin receptor kinase (TRK) inhibitor that is being evaluated in patients with ALK-rearranged tumors, including NSCLC, in a phase I trial. The first data was reported at ASCO 2015, and at an optimal dose regimen of 120mg per day, three of three patients achieved an objective response (NCT02048488)36.
Resistance to second-generation agents
Resistance to second-generation ALK inhibitors is an active area of investigation. Mechanisms of resistance to crizotinib and second-generation ALK inhibitors show overlap as well as novel mechanisms outlined in Figure 2. Similar to crizotinib resistance mechanisms but with less well-defined frequencies, resistance to second-generation ALK inhibitors has been observed to occur through ALK point mutations and alternative signaling pathways including EGFR, Src, MAP2K1, MEK, and MET activation37–38.
Sequential ALK inhibition and combination strategies
One of the most pressing questions facing the application of ALK inhibitors in patients with ALK-rearranged NSCLC is the optimal sequential or combination therapy regimen to utilize in order to maximize disease control and survival. In an early assessment of sequential ALK inhibitor therapy, a retrospective analysis has shown that among patients treated sequentially with crizotinib followed by ceritinib after progression on crizotinib the median combined PFS is 17.4 months and OS is 49.4 months29. In this analysis, there was no difference in PFS with certinib therapy based on the presence or absence of a known crizotinib-resistance-mediating ALK mutation29. Prospective trials evaluating the sequence of investigational ALK TKIs versus crizotinib are being planned.
To maximize disease control and patient survival, heterogeneous resistance mechanisms, such as the simultaneous existence of ALK point mutations and alternative pathway activation, must be addressed. Acknowledging the need to simultaneously inhibit multiple oncogenic checkpoints in the same patient, several combination strategies including vertical pathway inhibition, horizontal pathway inhibition, heat shock protein-90 (HSP-90) inhibition, ALK inhibition combined with immunotherapy, and ALK inhibition combined with cytotoxic chemotherapy have been proposed. Vertical pathway inhibition refers to inhibiting multiple components within the same oncogenic pathway, such as combined MEK and ALK inhibition. Horizontal inhibition, in contrast, refers to inhibiting multiple different oncogenic pathways simultaneously, such as combining ALK inhibition with Src, IGF-1R, EGFR, or cKIT inhibition.
The combination of ALK inhibition with immunotherapy is an active area of investigation, stimulated in part by an analogous finding that EGFR-mutant NSCLC utilizes activation of the programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) pathway to evade destruction by a patient’s immune system39. This treatment strategy is being assessed through a phase I clinical trial of combination ceritinib and the PD-1 inhibitor nivolumab (NCT02393625).
Finally, based on data suggesting that patients with ALK-rearranged NSCLC are sensitive to pemetrexed-based cytotoxic chemotherapy40, there is an ongoing clinical trial comparing the combination of pemetrexed and crizotinib to pemetrexed alone in patients with ALK-rearranged NSCLC that has progressed through crizotinib monotherapy (NCT02134912).
Conclusions
ALK fusions in lung cancer serve as the paradigm for the rapid and successful translation of a new biomarker from initial discovery to full clinical integration and widespread use of ALK directed targeted therapies for patients whose tumors harbor this alteration. There are several important questions to pursue moving forward in this field: (1) With the on-going clinical development of several new ALK TKIs, how can we systematically study and define the optimal sequence of ALK directed therapies in this cohort of patients? (2) All of the studies to date have examined the efficacy of ALK TKIs in patients with metastatic disease. Will ALK TKIs be efficacious in the adjuvant setting? This question is being addressed in the ALCHEMIST-ALK clinical trial which will assess the efficacy of crizotinib vs. placebo in patients with ALK-rearranged lung cancer following standard of care adjuvant therapy41. (3) Will the specific ALK gene fusion dictate clinical responses? At present, ALK fusions are detected primarily by FISH, which only detects an ALK gene rearrangement and cannot detect the actual fusion variant. However, as next generation sequencing based technologies move to the forefront of clinical diagnostics, the field will be able to assess how the various different ALK fusion partners and fusion variants effect responses. (4) Finally, as the field of ALK inhibition in patients with NSCLC advances, there is a pressing need to determine the generalizability of findings in NSCLC to other tumor types where ALK point mutations, amplification, and rearrangements have been identified. As an example, crizotinib has shown clinical activity in patients with ALK-rearranged anaplastic large cell lymphoma and inflammatory myofibroblastic tumors (IMT), and in a minority of patients with neuroblastoma42–43. To address this question, one arm of the NCI Molecular Analysis for Therapy Choice Program (NCI-MATCH) is testing crizotinib in patients with ALK-rearranged malignancies besides NSCLC7.
Acknowledgments
WI would like to thank the Vanderbilt Internal Medicine Residency Program for support and research time to complete this manuscript. CML was supported in part by the National Institutes of Health (NIH) and National Cancer Institute (NCI) R01CA121210 and P01CA129243. CML was additionally supported by a Damon Runyon Clinical Investigator Award and a LUNGevity Career Development Award.
CML has served as a consultant for Pfizer, Novartis, Sequenom, and Genoptix and has been an invited speaker for Abbott and Qiagen. CML has received research funds from Astra Zeneca and Novartis.
Footnotes
Conflict of Interest: WI has no conflicts of interest to report.
References
- 1.Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 2.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 3.Stoica GE, Kuo A, Aigner A, et al. Identification of anaplastic lymphoma kinase as a receptor for the growth factor pleiotrophin. J Biol Chem. 2001:16772–16779. doi: 10.1074/jbc.M010660200. [DOI] [PubMed] [Google Scholar]
- 4.Murray PB, Lax I, Reshetnyak A, et al. Heparin is an activating ligand of the orphan receptor tyrosine kinase ALK. Sci Signal. 2015;8:ra6. doi: 10.1126/scisignal.2005916. [DOI] [PubMed] [Google Scholar]
- 5.Stoica GE, Kuo A, Powers C, et al. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J Biol Chem. 2002:35990–35998. doi: 10.1074/jbc.M205749200. [DOI] [PubMed] [Google Scholar]
- 6.Bilsland JG, Wheeldon A, Mead A, et al. Behavioral and neurochemical alterations in mice deficient in anaplastic lymphoma kinase suggest therapeutic potential for psychiatric indications. Neuropsychopharmacology. 2008:685–700. doi: 10.1038/sj.npp.1301446. [DOI] [PubMed] [Google Scholar]
- 7.Katayama R, Lovly CM, Shaw AT. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: a paradigm for precision cancer medicine. Clin Cancer Res. 2015:2227–2235. doi: 10.1158/1078-0432.CCR-14-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sang J, Acquaviva J, Friedland JC, et al. Targeted inhibition of the molecular chaperone Hsp90 overcomes ALK inhibitor resistance in non-small cell lung cancer. Cancer Discov. 2013;3:430–443. doi: 10.1158/2159-8290.CD-12-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer. 2013:685–700. doi: 10.1038/nrc3580. [DOI] [PubMed] [Google Scholar]
- 10.Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011–1019. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011:1004–1012. doi: 10.1016/S1470-2045(11)70232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim D, Ahn M, Yang P, et al. Updated results of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC) Ann Onc. 2012;23(suppl 9) [Google Scholar]
- 13.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 14.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 15.Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res. 2013:4273–4281. doi: 10.1158/1078-0432.CCR-13-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363:1734–1739. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 17.Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med. 2012;8(4(120)):120. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovly CM, McDonald NT, Chen H, et al. Rationale for co-targeting IGF-1R and ALK in ALK fusion-positive lung cancer. Nat Med. 2014;20(9):1027–1034. doi: 10.1038/nm.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crystal AS, Shaw AT, Sequist LV, et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science. 2014;346(6216):1480–1486. doi: 10.1126/science.1254721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011:e443–e445. doi: 10.1200/JCO.2010.34.1313. [DOI] [PubMed] [Google Scholar]
- 21.Costa DB, Shaw AT, Ou SHI, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small cell lung cancer and brain metastases. J Clin Oncol. 33(17):1881–1888. doi: 10.1200/JCO.2014.59.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370:1189–1197. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mok T, Spigel D, Felip E, et al. ASCEND 2: A single arm, open-label, multicenter phase II study of ceritinib in adult patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC) previously treated with chemotherapy and crizotinib (CRZ) JCO. 2015;33(Suppl) abstract 8059. [Google Scholar]
- 24.Felip E, Orlov S, Park K, et al. ASCEND 3: A single arm, open-label, multicenter phase II study of ceritinib in ALKi-naïve adult patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC) JCO. 2015;33(Suppl) abstract 8060. [Google Scholar]
- 25.Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1–2 study. Lancet Oncol. 2013;14:590–598. doi: 10.1016/S1470-2045(13)70142-6. [DOI] [PubMed] [Google Scholar]
- 26.Ohe Y, Nishio M, Kiura K, Seto T, et al. A phase I/II study with a CNS-penetrant, selective ALK inhibitor alectinib in ALK-rearranged non-small cell lung cancer (ALK+ NSCLC) patients (pts): updates on progression free survival (PFS) and safety results from AF-001JP. JCO. 2015;33(Suppl) abstract 8061. [Google Scholar]
- 27.Ajimizu H, Kim YH, Mishima M. Rapid response of brain metastases to alectinib in a patient with non-small-cell lung cancer resistant to crizotinib. Med Oncol. 2015;32:477. doi: 10.1007/s12032-014-0477-7. [DOI] [PubMed] [Google Scholar]
- 28.Kodama T, Hasegawa M, Takanashi K, Sakurai Y, Kondoh O, Sakamoto H. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol. 2014;74:1023–1028. doi: 10.1007/s00280-014-2578-6. [DOI] [PubMed] [Google Scholar]
- 29.Gainor JF, Tan DS, De Pas T, et al. Progression-Free and Overall Survival in ALK-Positive NSCLC Patients Treated with Sequential Crizotinib and Ceritinib. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-14-3009. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ou SHI, Ahn JS, Petris LD, et al. Efficacy and safety of the ALK inhibitor alectinib in ALK+ non-small cell lung cancer (NSCLC) patients who have failed prior crizotinib: an open-label, single arm, global phase 2 study (NP28673) JCO. 2015;33(Suppl) abstract 8008. [Google Scholar]
- 31.Ghandi L, Shaw A, Gadgeel SM, et al. A phase II, open-label, multicenter study of the ALK inhibitor alectinib in an ALK+ non-small cell lung cancer (NSCLC) U.S./Canadian population who had progressed on crizotinib (NP28761) JCO. 2015;33 Abstract 8019. [Google Scholar]
- 32.Zou HY, Li Q, Engstrom LD, et al. PF-06463922 is a potent and selective next-generation ROS1/ALK inhibitor capable of blocking crizotinib-resistant ROS1 mutations. Proc Natl Acad Sci. 2015;112(11):3493–3498. doi: 10.1073/pnas.1420785112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaw AT, Bauer MT, Felip E, et al. Clinical activity and safety of PF-06463922 from a dose escalation study in patients with advanced ALK+ or ROS+ NSCLC. JCO. 2015;33(Suppl) abstract 8018. [Google Scholar]
- 34.Camidge DR, Bazhenova L, Salgia R, et al. Safety and efficacy of brigatinib (AP26113) in advanced malignancies, including ALK+ non-small cell lung cancer (NSCLC) JCO. 2015 May;33(15) Abstract 8062. [Google Scholar]
- 35.Horn L, Infante JR, Blumenshcein G, et al. A phase I trial of X-396, a novel ALK inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2014;32(5s) doi: 10.1200/JCO.2013.52.3993. (suppl; abstr 8030^) [DOI] [PubMed] [Google Scholar]
- 36.Arkenau HT, Sachdev JC, Mita MM, et al. Phase (Ph) 1/2a study of TSR-011, a potent inhibitor of ALK and TRK, in advanced solid tumors including crizotinib-resistant non-small cell lung cancer. J Clin Oncol. 2015;33(suppl) abstr 8063. [Google Scholar]
- 37.Katayama R, Friboulet L, Koike S, et al. Two novel ALK mutations mediate acquired resistance to the next-generation ALK inhibitor alectinib. Clin Cancer Res. 2014:5686–5696. doi: 10.1158/1078-0432.CCR-14-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanimoto A, Yamada T, Nanjo S, et al. Receptor ligand-triggered resistance to alectinib and its circumvention by Hsp90 inhibition in EML4-ALK lung cancer cells. Oncotarget. 2014:4920–4928. doi: 10.18632/oncotarget.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Camidge DR, Kono SA, Lu X, et al. Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J Thorac Oncol. 2011;6(4):774–780. doi: 10.1097/JTO.0b013e31820cf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerber DE, Oxnard GR, Mandrekar SJ, et al. ALCHEMIST: A clinical trial platform to bring genomic discovery and molecularly targeted therapies to early-stage lung cancer. J Clin Oncol. 2015;33(suppl) doi: 10.1002/cpt.91. abstr TPS7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butrynski JE, D'Adamo DR, Hornick JL, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363:1727–1733. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosse YP, Lim MS, Voss SD, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children's Oncology Group phase 1 consortium study. Lancet Oncol. 2013;14:472–480. doi: 10.1016/S1470-2045(13)70095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]