Abstract
Existing estimates of sociodemographic disparities in chronic pain in the United States are based on cross-sectional data, often treat pain as a binary construct, and rarely test for non-response or other types of bias. This study uses seven biennial waves of national data from the Health and Retirement Study (1998–2010; n = 19,776) to describe long-term pain disparities among older (age 51+) American adults. It also investigates whether pain severity, reporting heterogeneity, survey non-response, and/or mortality selection might bias estimates of social disparities in pain. In the process, the article clarifies whether two unexpected patterns observed cross-sectionally—plateauing of pain above age 60, and lower pain among racial/ethnic minorities—are genuine or artefactual. Findings show high prevalence of chronic pain: 27.3% at baseline, increasing to 36.6% thereafter. Multivariate latent growth curve models reveal extremely large disparities in pain by sex, education, and wealth, which manifest primarily as differences in intercept. Net of these variables, there is no racial/ethnic minority disadvantage in pain scores, and indeed a black advantage. Pain levels are predictive of subsequent death, even a decade in the future. No evidence of pain-related survey attrition is found, but surveys not accounting for pain severity and reporting heterogeneity are likely to underestimate socioeconomic disparities in pain. The lack of minority disadvantage (net of socioeconomic status) appears genuine. However, the age-related plateauing of pain observed cross-sectionally is not replicated longitudinally, and appears partially attributable to mortality selection, as well as to rising pain levels by birth cohort.
Keywords: Chronic pain, health disparities, HRS, mortality, mortality selection, persistent pain, reporting heterogeneity, socioeconomic disparities, socioeconomic status (SES)
INTRODUCTION
As awareness of chronic pain as a serious and costly public health problem grows [26, 34], an increasing number of studies provide estimates of pain’s prevalence and distribution in the general U.S. population [9, 29, 36, 37, 46, 47, 51, 54, 59, 61]. However, nearly all such studies rely on cross-sectional data and binary pain measures, and do not consider the potential role of measurement bias. The present study uses 12-year longitudinal data and directly tests for several types of bias to provide a more complete portrait of social disparities in chronic pain. In the process, the study clarifies whether findings from prior research are genuine or artefacts of measurement error.
While definitions and measures of chronic pain vary, sometimes quite markedly, existing studies agree that prevalence among U.S. adults is high: 11% to 47% [9, 46]. Moreover, four consistent patterns regarding sociodemographic disparities in chronic pain emerge: 1) Women experience higher rates of pain than do men [29, 36, 37, 47, 51, 54, 59, 61]. 2) Higher income individuals report pain less often than lower income individuals [9, 29, 36, 47, 51, 54]. 3) Likelihood of pain rises with age until approximately age 60, at which point it plateaus or decreases [9, 29, 36, 37, 46, 47, 54, 59]. 4) Non-Hispanic whites report pain more frequently than non-Hispanic blacks or Hispanics [29, 36, 37, 46, 47, 54, 59].
The first two of these patterns are unsurprising: voluminous clinical and experimental literature finds women to report more pain than men [25, 38], and disparities in health by income are widely documented [8, 44, 52]. The latter two patterns, however, are unexpected. Given that disease burden (including of pain-producing conditions such as osteoarthritis) increases with age, one might well expect a continuing increase in pain with age [20, 32]. Similarly, the observed minority advantage in pain is at odds with U.S. minorities’ (in particular African-Americans’) overall poorer health [15, 64, 65], and with minorities’ less comprehensive treatment for pain [1, 16, 49, 55].
Could these unexpected findings result from measurement bias? Both theoretical and empirical literature suggests that different social groups (men versus women, different racial/ethnic groups, etc.) may exhibit reporting heterogeneity, i.e., systematically different ways of rating pain [12, 17, 22, 28]. Non-response bias and mortality selection [63] are also potential sources of bias in any health survey.
This study uses seven waves of biennial data from a nationally-representative survey of older (age 51+) American adults to conduct two types of analyses. 1) First, it describes sociodemographic disparities in non-site-specific, chronic noncancer pain using longitudinal data. Do disparities widen with age (consistent with the cumulative disadvantage hypothesis) or narrow (consistent with the age-as-leveler hypothesis) [24]? Is the plateauing of pain after age 60, as observed cross-sectionally, replicated longitudinally? 2) Second, this study investigates four possible sources of measurement bias: pain severity, reporting heterogeneity, survey non-response, and mortality selection. Do these factors bias estimates of social disparities in pain, and if so, how? In particular, could the apparent minority advantage in pain be a consequence of reporting heterogeneity? Could the plateauing of pain after age 60 reflect mortality selection and/or non-response bias?
Overall, this study provides a fuller view of the national distribution of chronic pain, and identifies key sources of measurement bias relevant to pain disparities research.
METHODS
Data
This study is based on secondary analysis of seven consecutive biennial waves of the Health and Retirement Study (HRS; http://hrsonline.isr.umich.edu/index.php), a study sponsored by the National Institute of Aging (grant number NIA U01AG009740) and conducted by the University of Michigan. The HRS began in 1992, and by 1998 was expanded to be nationally representative of the non-institutionalized above-50 population of the United States. The 1998 response rate was 83.75% [58]. Respondents were non-institutionalized at baseline, but were followed if they moved to institutions in subsequent waves [30]. Surveys have been repeated every two years, with respondents interviewed by telephone or in-person depending on health and preferences. Follow-up response rates for the non-deceased have been high (85%-93%) [31], including among racial/ethnic minorities, a fact attributed to high quality training of interviewers and targeted follow-up strategies [50].
The present analyses start with the 1998 over-50 sample, and follow this group over seven waves. This closed cohort design was selected so that 12 years’ worth of data would be available for all respondents except in cases of non-response or death. Of baseline respondents (n = 20,007), 331 were excluded for missing all information about education or pain status, yielding an analytic sample of 19,776. The HRS oversamples African-Americans, Hispanics, and Floridians, and uses a different sampling frame for “oldest old” respondents [30]; sampling weights must thus be used to generate estimates representative of the population. Based on HRS-provided sampling weights, the analytic sample represents 67,338,111 Americans.
Measures
Pain
Since 1998, HRS biennial waves have used identical wording for pain-related questions. The initial question reads, “Are you often troubled with pain?” Respondents answering “yes” are here considered to be experiencing chronic pain. This wording has the advantage of not priming respondents to privilege continuous over episodic pain, or requiring respondents to be experiencing pain at the moment of the interview. Prior research finds that respondents are less than half as likely to report being “often troubled by pain” as to report experiencing “any pain in the last 30 days” [5], indicating that the HRS question is unlikely to capture fleeting or trivial experiences of pain.
Respondents answering “Yes” to the opening question are then asked, “How bad is the pain most of the time: mild, moderate or severe?”. Responses to this question were combined with the prior one to create a 4-category “pain status” variable for each survey wave: no pain (0), mild pain (1), moderate pain (2), and severe pain (3). Pain status was treated as a ratio variable in some analyses, as in prior studies [23]. Because this study focuses on chronic noncancer pain, pain status was set to missing when pain was likely due to cancer or cancer treatment, i.e., when respondents had received a new cancer diagnosis, received treatment for cancer, or reported that their cancer had gotten worse since the last study wave. This cancer exclusion affected between .67% and 3.38% of the sample depending on wave.
The final question in the series, “Does the pain make it difficult for you to do your usual activities such as household chores or work?”, serves as a measure of pain-related disability in the tests of reporting heterogeneity described below. Its mention of both household and out-of-house work makes it appropriate for a population of mixed employment and retirement statuses.
Item non-response for pain questions was very low, never exceeding 1.8%. Wave non-response (conditional on survivorship) ranged from 5.76% in 2000 to 14.37% in 2010.
Covariates
Demographic variables used in analyses include sex, age in 1998 (categorized as 51–59, 60–69, 70–79, and 80 or above), race/ethnicity (non-Hispanic white, non-Hispanic black or African-American, and Hispanic—henceforth “white”, “black” and “Hispanic” for brevity; the category “non-Hispanic other” was too small to be analyzed), highest level of education (less than high school, high school diploma, 4-year college degree, and graduate degree), 1998 household wealth quartiles (with mean values, in U.S. dollars, of $6,286, $75,032, $200,899, and $937,662), and a “survival status” variable indicating whether the respondent died before the end of the study period. Wealth was included rather than income based on evidence of non-linear relationships between income and overall socioeconomic standing when looking at populations of mixed retirement statuses [27]. Socioeconomic characteristics of the analytic sample are summarized in Table 1.
Table 1.
Sociodemographic Characteristics of Analytic Sample (n = 19,776; from the Health and Retirement Study, 1998)
| Proportion or Mean |
Standard Deviation |
N | |
|---|---|---|---|
| Sex | |||
| Female | 0.57 | 11,233 | |
| Male | 0.43 | 8,543 | |
| Age in 1998 | 66.98 | 10.17 | |
| Age Categories | |||
| 51–59 in 1998 | 0.28 | 5,513 | |
| 60–69 in 1998 | 0.34 | 6,801 | |
| 70–79 in 1998 | 0.24 | 4,809 | |
| 80 or above in 1998 | 0.13 | 2,653 | |
| Race/Ethnicity | |||
| White (non-Hispanic) | 0.77 | 15,132 | |
| Black (non-Hispanic) | 0.14 | 2,749 | |
| Hispanic | 0.08 | 1,591 | |
| Other (non-Hispanic) | 0.02 | 304 | |
| Education | |||
| Less than High School | 0.29 | 5,644 | |
| High School Degree | 0.54 | 10,765 | |
| 4-Year College Degree | 0.10 | 1,952 | |
| Graduate Degree | 0.07 | 1,415 | |
| Household income in 1998 | $48,902 | $97,678 | |
| Wealth in 1998 | $309,736 | $1,092,179 | |
| Survival Status (1998–2010) | |||
| Alive throughout Study Period | 0.64 | 12,671 | |
| Died during Study Period | 0.36 | 7,105 |
Analytic strategy
To summarize chronic pain’s prevalence and distribution, time series graphs of pain status were constructed for the full population and for specific sociodemographic groups. To assess multivariate associations, a probit-based latent growth curve model was run using the seven waves of pain data and all key covariates. Latent growth curve models depict the underlying latent trajectory of change in terms of an intercept and a slope, with variation by time-invariant covariates treated as deviations from this general intercept and slope [7]. The model was estimated under missing data theory using all available data [45]; findings using listwise deletion of respondents missing pain status were extremely similar.
The likely direction of bias due to reporting heterogeneity was estimated by comparing the association between reported levels of pain and of pain-related disability across groups. If one group is more stoical in pain reporting than another (for example, if its members call “moderate” what others would call “severe”), we would expect the stoical group to experience more disability for a given level of pain (i.e., we would expect their “moderate” pain to cause greater functional disruption than the other group’s “moderate” pain). Estimating such differences in reporting style could clarify whether studies likely under- or overestimate group differences in pain. Concretely, reporting heterogeneity was assessed via logistic regression of pain-related disability (yes/no) on the core sociodemographic variables plus self-reported pain severity. Because findings were similar across survey waves, results from a model pooling all seven waves of data are presented.
To clarify whether focusing on prevalence rather than severity might bias estimates of pain disparities, the distribution of any, mild, moderate, and severe pain by sociodemographic group was calculated, also using pooled data from all seven waves. To assess pain-related survey non-response (in the form of attrition bias), I conducted a logistic regression of wave nonresponse on categorical pain status at the prior survey wave, conditional on survivorship.
Mortality selection occurs if high-pain individuals are more likely to die and hence exit the population than low-pain individuals (regardless of whether this association is causal [57]). This leads to a reduced slope in the graph of pain by age, which reflects population compositional changes, i.e., it can occur even if mean pain increases steadily with age among survivors [63]. To test whether mortality selection contributes to the apparent plateauing of pain after age 60, I conducted a logistic regression of death by a given survey wave on categorical pain status at the prior wave. Mortality selection was also assessed by comparing mean pain across time for decedents, survivors, and the full sample, to examine whether decedents have higher average pain scores than survivors, and whether the full-sample slope is flatter than that for either survivors or decedents separately.
All presented graphs and analyses are sample weight-adjusted, except those aiming to identify quasi-causal relationships rather than to describe population parameters [66] (i.e., the tests of attrition bias, mortality selection, and reporting heterogeneity).
Stata MP/13.1 (StataCorp LP, College Station, TX) was used for all analyses except the latent growth curve model, which was implemented with Mplus version 7.31 (Muthén & Muthén, Los Angeles, CA). Code is available upon request.
RESULTS
Longitudinal disparities in pain: Bivariate results
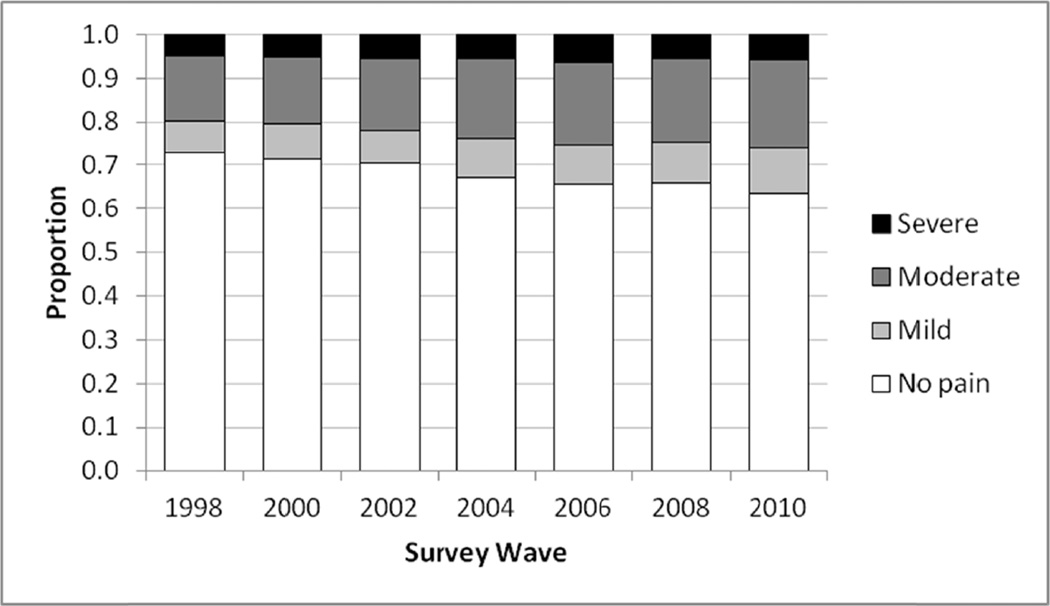
Figure 1 shows the proportion of respondents in each pain status category (no, mild, moderate, or severe pain) by survey wave. In 1998, 27.3% of respondents reported that they were often troubled with pain; by 2010 this had increased to 36.6%. The ratio of mild to moderate to severe pain remained fairly constant across the 7 waves, at approximately 3:6:2.
Fig. 1.
Chronic pain status by wave (n = 19,776; from the Health and Retirement Study, 1998–2010). Sample weight adjusted.
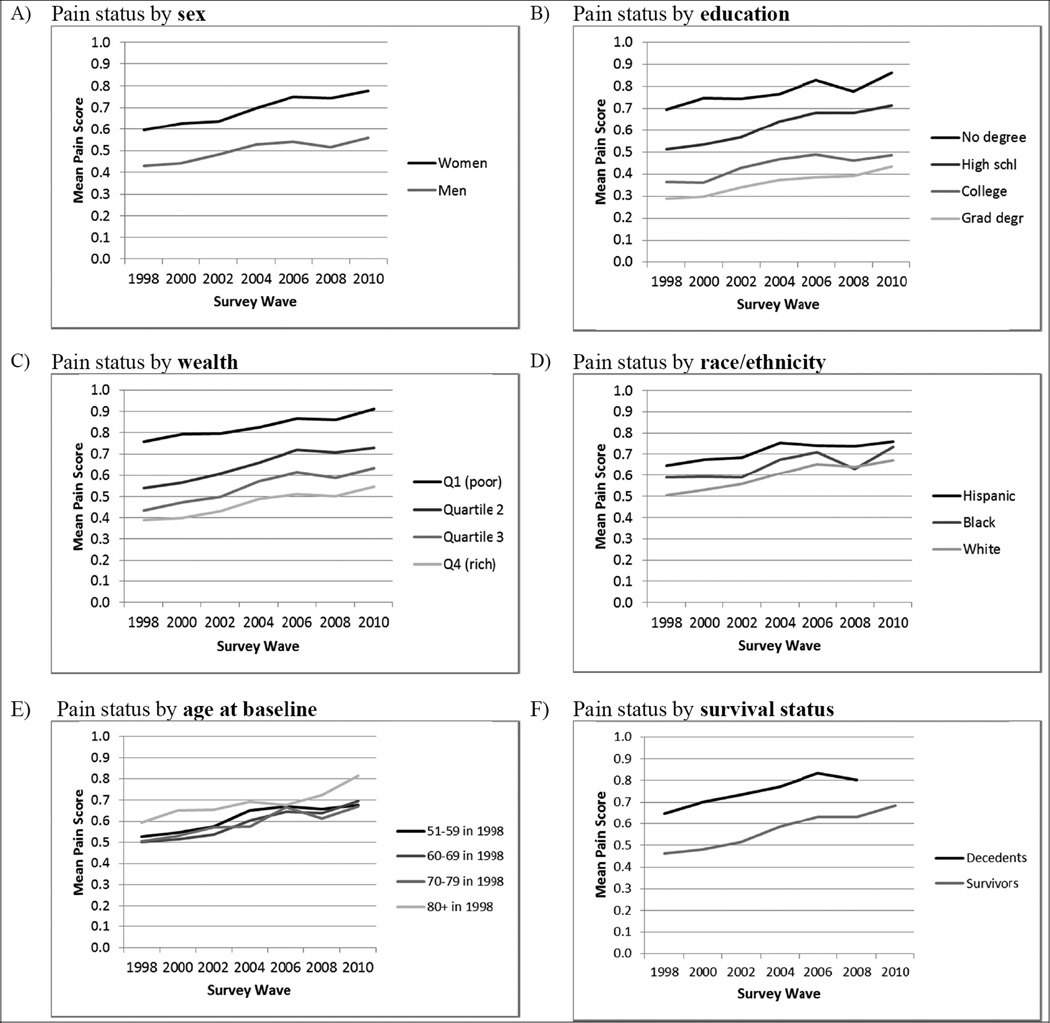
Figure 2 shows graphs of mean pain status over time by sex, education, wealth, race/ethnicity, age group, and survival status. All groups show an upward slope in mean pain, with slopes generally roughly parallel across groups. Intercepts, however, often differ dramatically across groups. As shown in panel A, women consistently report higher pain than men—on average, 38% higher. Panels B and C show large gradients by socioeconomic status, with pain scores monotonically lower with each categorical increase in education or wealth. On average, respondents with no high school degree have pain scores over twice as high as respondents with graduate degrees. Similarly, the least wealthy respondents have scores an average of 78% higher than the wealthiest. Mean pain scores by race/ethnicity (Figure 2 panel D) are relatively closely clustered together. While some racial/ethnic differences are significant in bivariate models, these patterns change in models with socioeconomic controls, as discussed below.
Fig. 2.
Mean pain status over time by sociodemographic characteristics (n = 19,776; from the Health and Retirement Study, 1998–2010). Means calculated by assigning scores: no pain = 0; mild pain = 1; moderate pain = 2; severe pain = 3. Sample weight adjusted. All differences by sex, education, wealth, and survival status (panels A, B, C, and F) are significant (P < 0.05, two-tailed) except between college and graduate degree, and, in 1998, between wealth quartiles 3 and 4. The only significant differences by race/ethnicity (panel D) are between Hispanics and whites, 1998–2006; and by baseline age (panel E) are between 80+ and younger groups, 1998 and 2000.
Mean pain scores for respondents in each baseline age group are shown in Figure 2, panel E. Mean pain rises with time in each age group, undermining the claim that pain plateaus or declines after age 60. At the same time, mean pain scores for most age groups are virtually indistinguishable throughout the 12-year period, with only respondents 80 or older showing noticeably higher pain scores in most waves. If a researcher were to use data from a single wave only, then mean pain scores would indeed appear to plateau with age, except for a possible rise after age 80.
This graph also indicates that pain is worsening by period, above and beyond worsening with age. For example, in 1998, respondents in their 60s had a mean pain score of .50. In 2008, respondents in their 60s (i.e., who had been in their 50s at baseline) had a significantly higher mean pain score of .66—a 32% increase. Findings are similar for respondents in their 70s, whose pain scores were 28% higher in 2008 than in 1998. (Respondents in their 80s had similar levels of pain in 1998 and 2008, however.)
Figure 2, panel F compares mean pain scores for respondents who died during the 12-year study period and those who survived throughout. The difference between the two groups is large and consistently significant, with decedents having, on average, 31% higher pain scores than survivors.
Longitudinal disparities in pain: Multivariate results
Results from a multivariate latent growth curve model of disparities in chronic pain over time are presented in Table 2. Goodness-of-fit measures suggest “extremely good” fit [40], with the RMSEA below .01 and the CFI and TLI very near one. The patterns observed in Figure 2 for sex, education, wealth, and survival status persist in multivariate context. As shown by substantively and statistically significant coefficients for the intercept (left half of Table 2), women have higher levels of pain than men (β = .199; P < .001), and both education and wealth are negatively associated with pain levels, with each increase in education or wealth associated with a lower intercept. Differences in intercept between those in the lowest and highest educational category (β = .415) or wealth category (β = .321) are quite large—larger than the difference between men and women. Respondents who died during the study also had a significantly higher intercept than those who survived (β = .283; P < .001), though also a lower slope (reflecting exit of high-pain individuals due to death).
Table 2.
Multivariate Latent Growth Curve Model for Pain Status over 12 Year Period (n = 19,776; from the Health and Retirement Study, 1998–2010)
| Intercept | Slope | |||
|---|---|---|---|---|
| β | SE | β | SE | |
| Sex (reference: Male) | ||||
| Female | 0.199*** | 0.016 | 0.001 | 0.004 |
| Education (reference: No degree) | ||||
| High school degree | −0.168*** | 0.024 | 0.016** | 0.006 |
| 4-year college degree | −0.309*** | 0.042 | 0.009 | 0.009 |
| Graduate degree | −0.415*** | 0.042 | 0.012 | 0.011 |
| Wealth (reference: Quartile 1) | ||||
| Quartile 2 | −0.218*** | 0.026 | 0.013* | 0.006 |
| Quartile 3 | −0.298*** | 0.024 | 0.013 | 0.007 |
| Quartile 4 (wealthiest) | −0.321*** | 0.025 | 0.003 | 0.007 |
| Race/Ethnicity (ref.: White non-Hisp.) | ||||
| Black (non-Hispanic) | −0.112*** | 0.029 | −0.002 | 0.007 |
| Hispanic | 0.002 | 0.033 | −0.015 | 0.008 |
| Age Categories (ref.: 51–59 in 1998) | ||||
| 61–69 in 1998 | −0.112*** | 0.023 | 0.016*** | 0.004 |
| 71–79 in 1998 | −0.203*** | 0.024 | 0.022*** | 0.005 |
| 80 or above in 1998 | −0.246*** | 0.037 | 0.029** | 0.009 |
| Survival Status (reference: Survived) | ||||
| Died during study period | 0.283*** | 0.023 | −0.017* | 0.007 |
| Constant | 0.000*** | 0.033*** | 0.008 | |
| Covariance of slope with intercept | −0.033*** | 0.002 | ||
| Sample size | 19,776 | |||
| Chi-square value (df=94) | 199.56*** | |||
| RMSEA | 0.008 | |||
| (and 90% CI) | (0.006–0.009) | |||
| CFI | 0.997 | |||
| TLI | 0.997 | |||
Notes:
= P < 0.05;
= P < 0.01;
= P < 0.001; two-tailed.
Estimation conducting using sample weights. Results are from a multivariate model simultaneously including all independent variables.
Abbreviations used: RMSEA (Root Mean Square Error of Approximation),CFI (Comparative Fit Index), and TLI (Tucker-Lewis Index). Intercepts for wealth quartile 3 and 4 are not significantly different from each other.
Not all patterns observed in Figure 2 persist in the multivariate model, however. Once education and wealth are controlled for, the previously observed pain disadvantage of Hispanics relative to whites disappears, as shown by the lack of significant differences in intercept or slope. Moreover, inclusion of socioeconomic controls shows African-Americans to have a significantly lower intercept than whites (β = −0.112; P < 0.001). In other words, the minority disadvantage in pain scores observed earlier is attributable to lower socioeconomic standing; once this is controlled for, the disadvantage disappears (for Hispanics) or reverses (for blacks).
Across successively older baseline age groups, the intercept for the latent curve decreases monotonically, i.e., net of other covariates, older cohorts have lower pain levels. This supports the earlier observation that pain levels appear to be increasing over time, disadvantaging younger birth cohorts. Nonetheless, the slope for all age groups is positive (since both the slope constant and the age-specific slope coefficients are positive), showing that within age categories pain increases with age, and accelerates at older ages.
With the noted exceptions, differences across groups in terms of slope were generally small and not statistically significant (Table 2, right half). In contrast, all intercept coefficients except one (for Hispanics) were statistically and substantively significant. Consistent with Figure 2, then, pain disparities across socioeconomic groups manifest primarily as differences in intercept.
Disparities in any pain versus in pain severity
Table 3 shows the percentage of respondents in each group who reported any pain and, conditional on reporting pain, who reported mild, moderate, and severe pain. (Data are pooled from all seven survey waves; individual waves show very similar percentages.) The table reveals that disparities in chronic pain by sex, education, wealth, and survival status are likely underestimated in studies relying on binary pain measures: not only are disadvantaged groups in these categories more likely to report any pain, but the pain is more likely to be severe. Thus, women are not only 28% more likely than men to report chronic pain (34.62% vs. 27.09%), they are also 37% more likely to report that their pain is severe (19.77% vs. 14.43%). Differences in pain severity are even more pronounced by education and wealth: e.g., 25.57% of pain sufferers without a high school degree term their pain “severe”, while only 9.79% of those with graduate degrees do. The difference across wealth quartiles is similar. Higher pain scores among respondents who died during the study period also reflect a combination of prevalence and severity: decedents were 18% more likely to report any pain, and, among those with pain, 48% more likely to deem the pain severe. (All differences noted are statistically significant.)
Table 3.
Percent Reporting Any Pain, Mild Pain, Moderate Pain, and Severe Pain, Based on Pooled Data from All Waves (n = 102,275; from the Health and Retirement Study, 1998–2010).
| Of Those Reporting Pain, % Reporting… |
||||
|---|---|---|---|---|
| % Who Report Any Pain (SE) |
Mild Pain (SE) |
Moderate Pain (SE) |
Severe Pain (SE) |
|
| Sex | ||||
| Female | 34.62 (0.50) | 24.03 (0.45) | 56.20 (0.50) | 19.77 (0.50) |
| Male | 27.09 (0.50) | 33.16 (0.63) | 52.41 (0.66) | 14.43 (0.51) |
| Education | ||||
| Less than high school | 37.79 (0.77) | 24.37 (0.64) | 50.06 (0.83) | 25.57 (0.70) |
| High school degree | 31.89 (0.47) | 26.29 (0.46) | 57.59 (0.59) | 16.12 (0.45) |
| 4-year college degree | 24.29 (0.89) | 33.80 (1.86) | 55.66 (1.66) | 10.54 (0.69) |
| Graduate degree | 21.08 (0.77) | 43.10 (1.64) | 47.11 (1.41) | 9.79 (1.23) |
| Wealth | ||||
| Quartile 1 (least wealthy) | 40.36 (0.86) | 23.19 (0.64) | 50.88 (0.73) | 25.93 (0.77) |
| Quartile 2 | 32.95 (0.59) | 26.26 (0.66) | 55.59 (0.91) | 18.15 (0.74) |
| Quartile 3 | 28.81 (0.62) | 29.34 (0.75) | 56.56 (0.88) | 14.09 (0.63) |
| Quartile 4 (wealthiest) | 25.53 (0.56) | 31.92 (1.01) | 56.65 (0.80) | 11.43 (0.57) |
| Race/Ethnicity | ||||
| White (non-Hispanic) | 30.98 (0.39) | 27.65 (0.47) | 56.34 (0.48) | 16.01 (0.38) |
| Black (non-Hispanic) | 31.78 (0.94) | 27.48 (0.85) | 44.75 (1.18) | 27.77 (1.58) |
| Hispanic | 35.74 (1.10) | 25.15 (0.99) | 51.59 (1.66) | 23.26 (1.66) |
| Age Categories | ||||
| 51–59 in 1998 | 32.62 (0.70) | 30.28 (0.69) | 52.82 (0.85) | 16.90 (0.57) |
| 61–69 in 1998 | 30.33 (0.50) | 25.80 (0.65) | 56.92 (0.63) | 17.27 (0.59) |
| 71–79 in 1998 | 29.73 (0.66) | 25.76 (0.64) | 55.05 (0.61) | 19.19 (0.63) |
| 80 or above in 1998 | 33.06 (0.82) | 22.86 (0.84) | 56.69 (1.03) | 20.44 (0.92) |
| Survival Status (1998–2010) | ||||
| Alive throughout study | 30.27 (0.41) | 28.96 (0.47) | 55.14 (0.54) | 15.90 (0.45) |
| Died during study period | 35.77 (0.63) | 22.64 (0.60) | 53.86 (0.51) | 23.50 (0.74) |
Notes: Sample weight adjusted; bivariate statistics.
Across racial/ethnic groups, the pattern in Table 3 is somewhat more complex, with Hispanics the most likely to report any pain, but blacks the most likely to report that their pain is severe. Indeed, combining information about pain prevalence and severity reveals that 8.8% of all blacks report severe pain, compared to 8.3% of all Hispanics. How one ranks blacks versus Hispanics in terms of pain burden thus depends on whether one prioritizes prevalence or severity. Whites have both the lowest prevalence and lowest level of severe pain among the three groups, with only 4.96% of whites experiencing severe pain. Differences across age categories were relatively small and usually not statistically significant.
Reporting heterogeneity
Results of the logistic regression of pain-related disability on sociodemographic covariates (controlling for pain severity) are shown in Table 4. Odds ratios above 1 reflect relative stoicism in reporting pain, as they indicate that for a given level of reported pain, members of the group are more likely to experience pain-related disability (suggesting that the pain level itself may be understated). Odds ratios below 1 reflect greater expressiveness in reporting pain.
Table 4.
Multivariate Logistic Regression of Pain-Related Disability on Socioeconomic Covariates Controlling for Pain Severity, Based on Pooled Data from All Waves (n = 32,048; from the Health and Retirement Study, 1998–2010)
| Odds Ratio | SE | |
|---|---|---|
| Sex (reference: Male) | ||
| Female | 1.32*** | .03 |
| Education (reference: No degree) | ||
| High school degree | .82*** | .03 |
| 4-year college degree | .72*** | .04 |
| Graduate degree | .61*** | .04 |
| Wealth (reference: Quartile 1) | ||
| Quartile 2 | .87*** | .03 |
| Quartile 3 | .78*** | .03 |
| Quartile 4 (wealthiest) | .73*** | .03 |
| Race/Ethnicity (ref.: White non-Hisp.) | ||
| Black (non-Hispanic) | 1.01 | .04 |
| Hispanic | .75*** | .03 |
| Age Categories (ref.: 51–59 in 1998) | ||
| 61–69 in 1998 | .93* | .03 |
| 71–79 in 1998 | .94 | .03 |
| 80 or above in 1998 | .99 | .05 |
| Pain Severity (reference: Mild pain) | ||
| Moderate pain | 2.79*** | .08 |
| Severe pain | 8.55*** | .38 |
Notes:
= P < 0.05;
= P < 0.01;
= P < 0.001; two-tailed.
Results are from a multivariate model simultaneously including all independent variables.
By this interpretation, many socioeconomic disparities in pain reported earlier appear to be underestimates. Groups already reporting the most pain—women, the less educated, and the less wealthy—also appear to be the most stoical in reporting pain. If this were accounted for, disparities across groups would appear even larger.
Across racial/ethnic categories, no significant differences in reporting styles were found between blacks and whites, suggesting that the black advantage found in the multivariate model (Table 3) is genuine, i.e., not an artefact of reporting heterogeneity. However, Hispanics do appear significantly more expressive than whites, complicating interpretation of relative pain burden: Hispanics may, like blacks, have lower average pain scores than whites after all.
The foregoing interpretations assume that self-reported pain-related disability is free from, or at least less prone to, reporting heterogeneity than self-reported pain. If, however, both questions are subject to similar tendencies toward stoicism or expressiveness, then the present findings may be less illuminating than tautological. These findings are thus presented as tentative, not definitive, assessments of reporting heterogeneity.
Non-response bias and mortality selection
Results of tests of attrition bias can be briefly summarized: little to no evidence was found that pain predicts survey attrition. Odds of wave non-response were not significantly higher for those reporting pain (regardless of severity) in the prior wave than for those without pain, with the lone exception of higher non-response in 2000 among 1998 respondents with severe pain (OR = 1.29; P < .05).
Results of tests of mortality selection were also very consistent, but in the opposite direction: pain, specifically moderate and severe pain, strongly and consistently predicted death by the next wave (P < 0.01 in all cases). Compared to pain-free respondents and controlling for age in years, those with moderate pain had on average 0.72 the odds of surviving until the next survey wave (range across waves: .64–77), and those with severe pain had 0.50 the odds (range: .42–55). Those with mild pain had 0.87 the odds (range: .78–1.01), but this was not statistically significant.
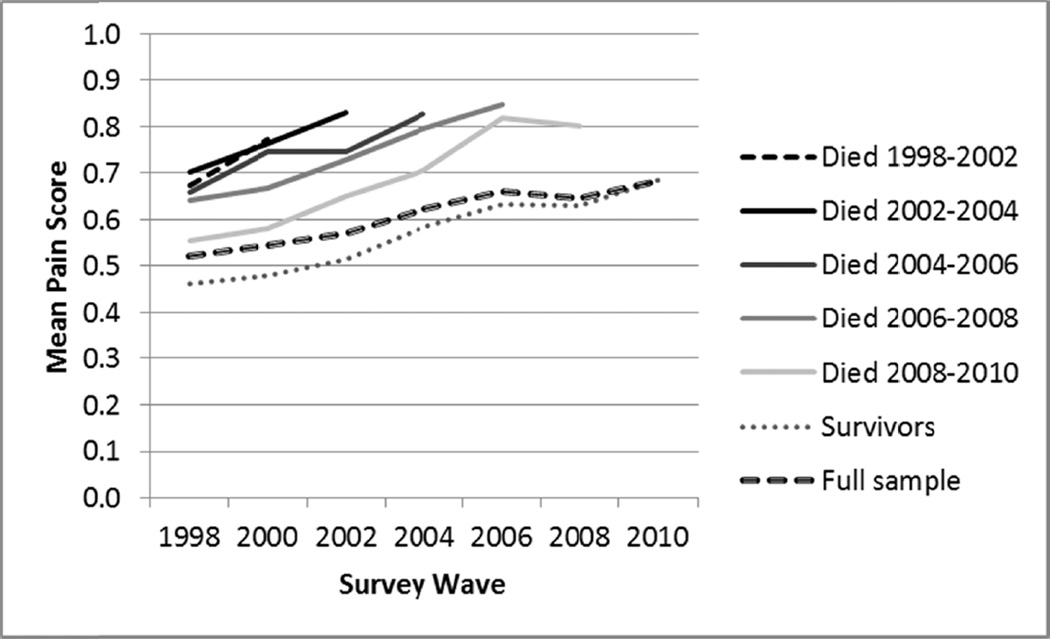
Figure 3 presents mean pain scores over time for decedents by period of death, for survivors, and for the full sample. As noted, decedents experience higher pain than survivors in the years before death. In addition, Figure 3 shows that the earlier in the study period respondents die, the higher their mean pain tends to be, both at baseline and in subsequent waves. (Similar patterns are observed when restricting the sample to specific age categories.) Higher pain among decedents cannot merely reflect acute pain in the final months of life, as higher pain at baseline predicts death even 10–12 years later. Pain appears to serve as a marker of long-term mortality risk.
Fig. 3.
Mean pain status over time by survival status (n = 19,776; from the Health and Retirement Study, 1998–2010). Means calculated by assigning scores: no pain = 0; mild pain = 1; moderate pain = 2; severe pain = 3. Sample weight adjusted. Because “Died 1998–2000” yielded only a single pain score (and thus was not easily observable on the graph), this category was merged with the subsequent one, yielding “Died 1998–2002.” Mean pain for those who died 1998–2000 was .73. All groups of decedents are statistically significantly different from survivors, in all waves (P < .02).
Figure 3 also shows that the slope for the full sample is less steep than the slope for survivors or for any group of decedents. This is a clear example of mortality selection: within all population subgroups, pain increases fairly steadily with time, but higher pain individuals are more likely to die and thus to exit the population. Such compositional changes lead the full population slope to appear more shallow than the slope for any individual subgroup.
DISCUSSION
To heed recent calls to take chronic pain seriously as a public health problem [10, 19, 34], as well as to better understand and address U.S. health disparities [8, 11, 13], accurate estimation of pain’s prevalence, long-term patterns, and sociodemographic distribution is needed. This study uses 12-year longitudinal data to describe the national distribution of chronic noncancer pain among older (age 51+) American adults, and to investigate whether and how pain severity, reporting heterogeneity, survey non-response, and mortality selection might bias estimates of social disparities in pain. Like prior studies using national samples, this one finds chronic pain prevalence to be high: 27.3% in 1998, rising to 36.6% twelve years later. Several additional key points may be highlighted from the present findings.
First, disparities in chronic pain by sex and, especially, socioeconomic status (SES) are extremely large, in both bivariate and multivariate contexts. In contrast, while racial/ethnic minorities have slightly higher pain scores (and experience more severe pain) than whites in bivariate analyses, this disadvantage in pain scores disappears when controlling for socioeconomic status, and indeed a black advantage vis-à-vis whites emerges.
Next, studies that do not account for group differences in pain severity and/or in styles of rating pain (which includes most existing studies) are likely to underestimate pain disparities by sex and socioeconomic status. Women, the less educated, and the less wealthy experience not only more pain, but also more severe pain, as well as greater disability for a given reported level of pain. Tests of reporting heterogeneity show no difference between white and black pain rating styles, however, indicating that the aforementioned black advantage is likely genuine. (Hispanics show a more expressive rating style than other groups, consistent with prior studies [22, 56]; were this accounted for, Hispanics might also show a lower pain burden than whites in multivariate models.)
Next, chronic noncancer pain (in particular moderate or severe pain) strongly predicts death. Mortality selection thus leads to underestimation of the rise in pain with age when using cross-sectional data. The cross-sectional finding that pain plateaus or declines after age 60 [9, 29, 47, 59] is not replicated longitudinally. Instead, all age cohorts—including those above age 60—show steady increases in mean pain scores over time. Cross-sectional findings are biased by the higher death rates of high-pain individuals, leading to an apparent flattening of the pain curve.
Finally, the burden of pain appears to be increasing not only with age, but with time, i.e., younger birth cohorts faces higher pain levels than older ones (controlling for age). This phenomenon further contributes to the appearance of plateauing pain levels when using cross-sectional data. This finding corroborates recent research based on National Health Interview Survey data, which finds rising rates of chronic pain in the U.S. between 1997–1999 and 2011–2013 [14] (very close to the present study’s range of 1998–2010).
Comparing current findings with those of prior research more broadly, we may note that the main claims of cross-sectional pain disparities studies (summarized in the introduction) are supported here, except for the plateauing of pain with age. However, effect sizes from prior studies must be interpreted cautiously, as most studies do not incorporate information on pain severity or reporting heterogeneity, and thus likely underestimate disparities by sex and socioeconomic status. Previous longitudinal research on chronic conditions such as hypertension and diabetes suggests that cumulative disadvantage (health differentials widening with age) is likely to be observed when mortality selection is accounted for [24]. In the present study, however, differences in pain by sex, education, and wealth remain roughly parallel over time, even when controlling for mortality—a pattern consistent neither with the cumulative disadvantage hypothesis nor the age-as-leveler hypothesis. Chronic pain may follow a different long-term pattern than other common chronic conditions.
For health disparities researchers and pain epidemiologists, a key methodological implication of these findings is to attend to pain severity, reporting heterogeneity, and mortality selection as sources of measurement bias. (Techniques for overcoming reporting heterogeneity remain challenging to develop, however [28].) Non-response bias may be worth attending to as well, despite lack of evidence for it in this study. Not only did the present data permit testing only for attrition bias rather than general non-response bias, but even the findings regarding attrition could reflect a unique feature of the HRS: that respondents who become institutionalized continue to be interviewed. Many surveys exclude institutionalized respondents, and thus may lose high-pain individuals who move to nursing homes.
This study also underscores the importance of further research on mechanisms underlying socioeconomic disparities in pain. While sex differences in pain experiences have been “a topic of tremendous scientific interest” for the past two decades—yielding a broad set of biological and psychosocial explanations for such differences [25:447]—comprehensive explorations of mechanisms linking socioeconomic status to chronic pain at the population level have been much rarer [6]. This may be changing, however, with recent studies (especially European ones) exploring specific diseases [21], job characteristics [39], neighborhood effects [6], and mental health [41] as mediating factors. A U.S.-based article notes that low SES individuals are more likely to be perceived by healthcare providers as exaggerating pain, and to have bureaucratic difficulties contending with health insurance, if they have insurance at all [33]. (Clinicians may wish to take note of such findings, to ensure that their own clinical practices do not contribute to poor treatment of the socially disadvantaged.) Research clarifying the relative importance of these and additional factors, and testing for generalizability across countries, would help answer why large socioeconomic disparities in pain are observed, and how they might be effectively addressed.
Recent years have also seen growing scholarly interest in the association between chronic pain and death. Studies have tested whether or not specific pain conditions predict mortality [3, 4]), and whether the association between chronic pain and death is causal or spurious [2, 43, 60, 67]—a question still without definitive answer [57]. Further research would ideally clarify why chronic pain predicts death, and what can be done about it.
This study has several key limitations beyond those already discussed. First, the HRS’s measure of pain (“Are you often troubled by pain?”) does not match common definitions of chronic pain as pain lasting at least three months [35] or six months [62]. Nonetheless, estimates presented here align with those of several prior studies [29, 36, 37, 53]. For example, a study using 2010 NHIS data finds 27.6% of Americans 50 or older to experience “persistent pain” [37]—an estimate extremely close to the current baseline estimate of 27.3%. Moreover, the present pain measure has advantages over those of some other studies. Unlike in the National Center for Health Statistic’s 2006 report, there is no requirement that pain “persist for more than 24 hours” [47]—a criterion which could exclude people who experience pain regularly, even daily, but only in briefer bursts, e.g., while walking. Unlike in Tsang et al. (2008) [61], pain is not defined by specific conditions such as arthritis and headaches—which, if mild or well-managed, might not be considered chronic pain by the respondent [37]. Such differences in pain definition and measurement likely explain why this article’s prevalence estimates fall in the mid-range of recent estimates.
Additional limitations result from sample characteristics. Because the sample is restricted to Americans above age 50, generalization to the full U.S. population is not possible. Moreover, findings from the U.S. may not generalize to other countries, due to differences in health care systems, treatment regimens, etc. (One may note, for example, that Americans constitute only 4.6% of the world’s population, but consume “80% of the global opioid supply,” and even higher percentages of specific opioids [42]. Could such heavy use of opioid analgesics directly or indirectly contribute to the association between pain and death, as has been suggested [14]?)
A key message from this study for both researchers and policy makers is that addressing health and mortality disparities in the U.S. will have to involve addressing pain disparities. As shown, chronic pain is not only extremely common, but it is strongly patterned by socioeconomic class. It is also highly disabling [18], has been proposed as a cause of recent mortality increases among middle-aged Americans [14], and may well contribute to the U.S.’s poor standing among peer countries in rankings of life expectancy [48].
Especially when paired with evidence of rising pain prevalence, these are strong arguments for prioritizing chronic pain research and treatment. If pain is merely a marker of mortality risk, research should clarify and address the root causes of the association. If pain itself raises mortality risk, then pain is doubly injurious, depriving its sufferers not only of quality of life but of life itself. In either case, continued efforts to better understand pain at the population level—and especially to explain and address its high prevalence and dramatically unequal distribution—are needed.
Acknowledgments
This article uses data from the Health and Retirement Study (HRS), which is sponsored by the NIA (grant number NIA U01AG009740) and is conducted by the University of Michigan. Early stages of this research were supported by core grants to the Center for Demography of Health and Aging (NIA P30 AG017266) and the Center for Demography and Ecology and (NICHD R24 HD047873) at the University of Wisconsin–Madison. The author thanks Joan Fujimura, Robert M. Hauser, Pamela Herd, and Cameron Macdonald for helpful comments on an early version of this manuscript.
Footnotes
The author has no conflicts of interest to declare.
REFERENCES
- 1.Anderson KO, Green CR, Payne R. Racial and ethnic disparities in pain: Causes and consequences of unequal care. J Pain. 2009;10(12):1187–1204. doi: 10.1016/j.jpain.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Andrews JS, Cenzer IS, Yelin E, Covinsky KE. Pain as a risk factor for disability or death. J Am Geriatr Soc. 2013;61(4):583–589. doi: 10.1111/jgs.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asberg AN, Stovner L, Zwart JA, Winsvold BS, Heuch I, Hagen K. Chronic musculoskeletal complaints as a predictor of mortality. The HUNT study. Pain. 2016 doi: 10.1097/j.pain.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 4.Asberg AN, Stovner LJ, Zwart JA, Winsvold BS, Heuch I, Hagen K. Migraine as a predictor of mortality: The HUNT study. Cephalalgia. 2016;36(4):351–357. doi: 10.1177/0333102415593090. [DOI] [PubMed] [Google Scholar]
- 5.Banks J, Kapteyn A, Smith JP, van Soest A. Work disability is a pain in the ****, especially in England, the Netherlands, and the United States. In: Cutler DM, Wise DA, editors. Health at older ages: The causes and consequences of declining disability among the elderly. Chicago: University of Chicago Press; 2009. pp. 251–293. [Google Scholar]
- 6.Blyth F. The demography of chronic pain: An overview. In: Croft P, Blyth FM, van der Windt D, editors. Chronic pain epidemiology: From aetiology to public health. Oxford: Oxford University Press; 2010. pp. 19–27. [Google Scholar]
- 7.Bollen KA, Curran PJ. Latent curve models: A structural equation perspective. Hoboken, NJ: John Wiley and Sons, Inc; 2006. [Google Scholar]
- 8.Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: What the patterns tell us. Am J Public Health. 2010;100:S186–S196. doi: 10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown A. Chronic pain rates shoot up until Americans reach late 50s; low-income and obese americans more likely to have chronic pain. 2012 Gallup.Com http://www.Gallup.Com/poll/154169/chronic-pain-rates-shoot-until-americans-reach-late-50s.Aspx.Brown.
- 10.Brown M, Crowe A, Cousins S. Educating patients and caregivers about pain management: What clinicians need to know. In: Moore RJ, editor. Handbook of pain and palliative care. New York: Springer; 2013. pp. 53–67. [Google Scholar]
- 11.Burgard SA, Chen PV. Challenges of health measurement in studies of health disparities. Soc Sci Med. 2014;106:143–150. doi: 10.1016/j.socscimed.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bzostek S, Sastry N, Goldman N, Pebley A, Duffy D. Using vignettes to rethink Latino-white disparities in self-rated health. Soc Sci Med. 2016;149:46–65. doi: 10.1016/j.socscimed.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell LC, Robinson K, Meghani SH, Vallerand A, Schatman M, Sonty N. Challenges and opportunities in pain management disparities research: Implications for clinical practice, advocacy, and policy. J Pain. 2012;13(7):611–619. doi: 10.1016/j.jpain.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Case A, Deaton A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc Natl Acad Sci U S A. 2015;112(49):15078–15083. doi: 10.1073/pnas.1518393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow EA, Foster H, Gonzalez V, McIver L. The disparate impact of diabetes on racial/ethnic minority populations. Clin Diabetes. 2012;30(3):130–133. [Google Scholar]
- 16.Cintron A, Morrison RS. Pain and ethnicity in the United States: A systematic review. J Palliat Med. 2006;9(6):1454–1473. doi: 10.1089/jpm.2006.9.1454. [DOI] [PubMed] [Google Scholar]
- 17.Courtenay WH. Constructions of masculinity and their influence on men’s well-being: A theory of gender and health. Soc Sci Med. 2000;50(10):1385–1401. doi: 10.1016/s0277-9536(99)00390-1. [DOI] [PubMed] [Google Scholar]
- 18.Covinsky KE, Lindquist K, Dunlop DD, Yelin E. Pain, functional limitations, and aging. J Am Geriatr Soc. 2009;57(9):1556–1561. doi: 10.1111/j.1532-5415.2009.02388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Croft P, Blyth FM, van der Windt D. Chronic pain as a topic for epidemiology and public health. In: Croft P, Blyth FM, van der Windt D, editors. Chronic pain epidemiology: From aetiology to public health. Oxford: Oxford University Press; 2010. pp. 3–8. [Google Scholar]
- 20.Dionne CE, Dunn KM, Croft PR. Does back pain prevalence really decrease with increasing age? A systematic review. Age Ageing. 2006;35(3):229–234. doi: 10.1093/ageing/afj055. [DOI] [PubMed] [Google Scholar]
- 21.Dorner TE, Muckenhuber J, Stronegger WJ, Ràsky É, Gustorff B, Freidl W. The impact of socio-economic status on pain and the perception of disability due to pain. European Journal of Pain. 2011;15(1):103–109. doi: 10.1016/j.ejpain.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Dowd JB, Todd M. Does self-reported health bias the measurement of health inequalities in U.S. adults? Evidence using anchoring vignettes from the Health and Retirement Study. J Gerontol B Psychol Sci Soc Sci. 2011;66B(4):478–489. doi: 10.1093/geronb/gbr050. [DOI] [PubMed] [Google Scholar]
- 23.Dunn KM, Jordan K, Croft PR. Characterizing the course of low back pain: A latent class analysis. Am J Epidemiol. 2006;163(8):754–761. doi: 10.1093/aje/kwj100. [DOI] [PubMed] [Google Scholar]
- 24.Dupre ME. Educational differences in age-related patterns of disease: Reconsidering the cumulative disadvantage and age-as-leveler hypotheses. J Health Soc Behav. 2007;48(1):1–15. doi: 10.1177/002214650704800101. [DOI] [PubMed] [Google Scholar]
- 25.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley Iii JL. Sex, gender, and pain: A review of recent clinical and experimental findings. J Pain. 2009;10(5):447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Grol-Prokopczyk H, Freese J, Hauser RM. Using anchoring vignettes to assess group differences in general self-rated health. J Health Soc Behav. 2011;52(2):246–261. doi: 10.1177/0022146510396713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grol-Prokopczyk H, Verdes-Tennant E, McEniry M, Ispány M. Promises and pitfalls of anchoring vignettes in health survey research. Demography. 2015;52(5):1703–1728. doi: 10.1007/s13524-015-0422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardt J, Jacobsen C, Goldberg J, Nickel R, Buchwald D. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9(7):803–812. doi: 10.1111/j.1526-4637.2008.00425.x. [DOI] [PubMed] [Google Scholar]
- 30.Health and Retirement Study. Sample evolution: 1992–1998. 2008 http://hrsonline.isr.umich.edu/sitedocs/surveydesign.pdf.
- 31.Health and Retirement Study. Sample sizes and response rates. 2011 http://hrsonline.isr.umich.edu/sitedocs/sampleresponse.pdf.
- 32.Helme RD, Gibson SJ. Pain in older people. In: Crombie IK, Croft PR, Linton SJ, LeResche L, Von Korff M, editors. Epidemiology of pain. Seattle: IASP Press; 1999. pp. 103–112. [Google Scholar]
- 33.Ikezi S, Campbell N, Frost EAM. Impact of socioeconomic, ethnic, cultural, and sociobehavioral differences on management of chronic pain syndromes: Can we manage pain better and without bias? Topics in Pain Management. 2015;31(2):1–7. [Google Scholar]
- 34.Institute of Medicine, Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington, D.C: National Academies Press; 2011. [PubMed] [Google Scholar]
- 35.International Association for the Study of Pain. Classification of chronic pain: Descriptions of chronic pain syndromes and definitions of pain terms. Pain. 1986;3(Suppl.):1–226. [PubMed] [Google Scholar]
- 36.Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: Results of an internet-based survey. J Pain. 2010;11(11):1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy J, Roll JM, Schraudner T, Murphy S, McPherson S. Prevalence of persistent pain in the U.S. adult population: New data from the 2010 National Health Interview Survey. J Pain. 2014;15(10):979–984. doi: 10.1016/j.jpain.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Kroenke K, Spitzer RL. Gender differences in the reporting of physical and somatoform symptoms. Psychosom Med. 1998;60(2):150–155. doi: 10.1097/00006842-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Lacey RJ, Belcher J, Croft PR. Does life course socio-economic position influence chronic disabling pain in older adults? A general population study. The European Journal of Public Health. 2013;23(4):534–540. doi: 10.1093/eurpub/cks056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacCallum RC. Power analysis and determination of sample size for covariance structure modeling. Psychol Methods. 1996;1(2):130–149. [Google Scholar]
- 41.Macfarlane GJ, Norrie G, Atherton K, Power C, Jones GT. The influence of socioeconomic status on the reporting of regional and widespread musculoskeletal pain: Results from the 1958 British Birth Cohort Study. Ann Rheum Dis. 2009;68(10):1591–1595. doi: 10.1136/ard.2008.093088. [DOI] [PubMed] [Google Scholar]
- 42.Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: A ten-year perspective. Pain Physician. 2010;13(5):401–435. [PubMed] [Google Scholar]
- 43.McBeth J, Symmons DP, Silman AJ, Allison T, Webb R, Brammah T, Macfarlane GJ. Musculoskeletal pain is associated with a long-term increased risk of cancer and cardiovascular-related mortality. Rheumatology (Oxford) 2009;48(1):74–77. doi: 10.1093/rheumatology/ken424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mili F, Helmick CG, Zack MM. Prevalence of arthritis: Analysis of data from the US Behavioral Risk Factor Surveillance System, 1996–99. J Rheumatol. 2002;29(9):1981–1988. [PubMed] [Google Scholar]
- 45.Muthén LK, Muthén BO. Mplus user’s guide. 6th. Los Angeles, CA: Muthén & Muthén; 2010. [Google Scholar]
- 46.Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain. 2015;16(8):769–780. doi: 10.1016/j.jpain.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Center for Health Statistics. Health, United States, 2006, with chartbook on trends in the health of Americans. Hyattsville, MD: National Center for Health Statistics; 2006. [PubMed] [Google Scholar]
- 48.National Research Council. International differences in mortality at older ages: Dimensions and sources. In: Crimmins EM, Preston SH, Cohen B, editors. Panel on Understanding Divergent Trends in Longevity in High-Income Countries. Committee on Population, Division of Behavioral and Social Sciences and Education. Washington, D.C: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 49.Nguyen M, Ugarte C, Fuller I, Haas G, Portenoy RK. Access to care for chronic pain: Racial and ethnic differences. J Pain. 2005;6(5):301–314. doi: 10.1016/j.jpain.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Ofstedal MB, Weir DR. Recruitment and retention of minority participants in the Health and Retirement Study. Gerontologist. 2011;51(Suppl 1):S8–S20. doi: 10.1093/geront/gnq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Portenoy RK, Ugarte C, Fuller I, Haas G. Population-based survey of pain in the United States: Differences among white, African American, and Hispanic subjects. J Pain. 2004;5(6):317–328. doi: 10.1016/j.jpain.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Rabi DM, Edwards AL, Southern DA, Svenson LW, Sargious PM, Norton P, Larsen ET, Ghali WA. Association of socio-economic status with diabetes prevalence and utilization of diabetes care services. BMC Health Serv Res. 2006;6:124–124. doi: 10.1186/1472-6963-6-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reyes-Gibby CC, Aday LA, Todd KH, Cleeland CS, Anderson KO. Pain in aging community-dwelling adults in the United States: Non-Hispanic whites, non-Hispanic blacks, and Hispanics. J Pain. 2007;8(1):75–84. doi: 10.1016/j.jpain.2006.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riskowski JL. Associations of socioeconomic position and pain prevalence in the United States: Findings from the National Health and Nutrition Examination Survey. Pain Med. 2014;15(9):1508–1521. doi: 10.1111/pme.12528. [DOI] [PubMed] [Google Scholar]
- 55.Shavers VL, Bakos A, Sheppard VB. Race, ethnicity, and pain among the U.S adult population. J Health Care Poor Underserved. 2010;21(1):177–220. doi: 10.1353/hpu.0.0255. [DOI] [PubMed] [Google Scholar]
- 56.Shetterly SM, Baxter J, Mason LD, Hamman RF. Self-rated health among Hispanic vs non-Hispanic white adults: The San Luis valley health and aging study. Am J Public Health. 1996;86(12):1798–1801. doi: 10.2105/ajph.86.12.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith D, Wilkie R, Uthman O, Jordan JL, McBeth J. Chronic pain and mortality: A systematic review. PLoS One. 2014;9(6):e99048. doi: 10.1371/journal.pone.0099048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JWR, Weir DR. Cohort profile: The Health and Retirement Study (hrs) Int J Epidemiol. 2014 doi: 10.1093/ije/dyu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sternbach RA. Survey of pain in the United States: The Nuprin pain report. Clin J Pain. 1986;2(1):49–53. doi: 10.1016/0304-3959(86)90224-1. [DOI] [PubMed] [Google Scholar]
- 60.Torrance N, Elliott AM, Lee AJ, Smith BH. Severe chronic pain is associated with increased 10 year mortality: A cohort record linkage study. Eur J Pain. 2010;14(4):380–386. doi: 10.1016/j.ejpain.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 61.Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, Borges GLG, Bromet EJ, de Girolamo G, de Graaf R, Gureje O, Lepine J-P, Haro JM, Levinson D, Oakley Browne MA, Posada-Villa J, Seedat S, Watanabe M. Common chronic pain conditions in developed and developing countries: Gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9(10):883–891. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 62.U.S. Department of Health and Human Services. National pain strategy: A comprehensive population health-level strategy for pain. 2016 [Google Scholar]
- 63.Vaupel JW, Yashin AI. Heterogeneity’s ruses: Some surprising effects of selection on population dynamics. Am Stat. 1985;39(3):176–185. [PubMed] [Google Scholar]
- 64.Williams DR. Race, SES, and health: The added effects of racism and discrimination. In: Brown P, editor. Perspectives in medical sociology. 4th. Long Grove, IL: Waveland Press, Inc; 2008. pp. 24–40. [Google Scholar]
- 65.Williams DR. Miles to go before we sleep: Racial inequities in health. J Health Soc Behav. 2012;53(3):279–295. doi: 10.1177/0022146512455804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winship C, Radbill L. Sampling weights and regression analysis. Sociol Method Res. 1994;23(2):230–257. [Google Scholar]
- 67.Zhu K, Devine A, Dick IM, Prince RL. Association of back pain frequency with mortality, coronary heart events, mobility, and quality of life in elderly women. Spine (Phila Pa 1976) 2007;32(18):2012–2018. doi: 10.1097/BRS.0b013e318133fb82. [DOI] [PubMed] [Google Scholar]