Abstract
Cellular senescence induced by different stresses and telomere shortening appears to play an important role in the aging process. The products of the INK4a/ARF locus — p16INK4a and ARF — arrest cell proliferation at the senescence stage by exerting their effects on retinoblastoma protein– and p53-mediated responsive pathways. A study in this issue of the JCI provides experimental evidence of a specific upregulation of these cell cycle inhibitors in a variety of organs during mammalian aging.
According to a current hypothesis on aging, senescent cells accumulate in the organism, and this results in failure of organ homeostasis and function (1). Cellular senescence — characterized by the permanent arrest of cell proliferation — is detrimental to the regenerative capacity of organs during aging. However, the senescence checkpoint is also considered to be a major mechanism for suppressing tumors, protecting the organism from cancer during early life (2). A number of stimuli have been identified as inducing senescence, and these include telomere shortening (3), DNA damage (4), oxidative stress (5), sustained mitogen stimulation (6), and other cellular stresses. Senescence induced by telomere shortening has been called “replicative senescence” and is a result of DNA damage–like signals generated by dysfunctional telomeres (3, 4). In addition to telomere attrition, several other mechanisms can abruptly induce senescence independent of telomere length, termed “premature senescence,” including: overactivation of mitogenic pathways, such as Ras, Raf, or MEK, or overexpression of E2F or v-ets erythroblastosis virus E26 oncogene (Ets) transcription factors (5–9). The mechanisms leading to induction of premature senescence are less well understood compared with those leading to replicative senescence. However, the phenotypic characteristics and the molecular signals of premature senescence and replicative senescence appear to be very similar.
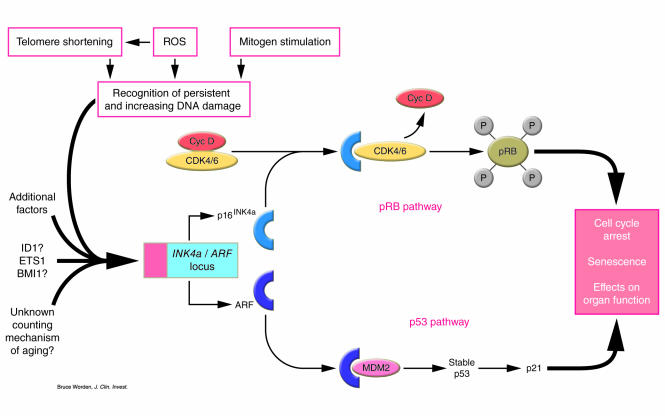
Under normal circumstances, cell cycle initiation and progression is cooperatively regulated by several classes of cyclin-dependent kinases (CDKs), whose activities are in turn regulated by CDK inhibitors (CDKIs). To allow cell cycle progression, retinoblastoma protein (pRB) is phosphorylated by a holoenzyme complex containing cyclin D and a cyclin-dependent kinase (CDK4 or CDK6) (10). The INK4 family of CDKIs comprises a number of small (“teenage”) proteins — p16INK4a, p14ARF (murine p19ARF), p15INK4b, and p18INK4c — all of which have a role in cell cycle regulation (10). In this commentary, p14ARF (murine p19ARF) will be referred to as ARF. Residing on chromosome 9p21 in humans and chromosome 4 (42.7) in mice, the INK4a/ARF locus encodes 2 different proteins, p16INK4a and ARF, via an alternative splicing mechanism. Lack of either protein predisposes the organism to the development of malignancy, though neither protein is required for normal growth and development. ARF and p16INK4a have long been recognized as mediators of senescence (6–8) (Figure 1). p16INK4a binds and induces an allosteric conformational change in CDK4/CDK6 that inhibits the binding of ATP and substantially reduces the formation of the CDK4/6–cyclin D interface, which leads to the disruption of the interaction with D-type cyclins. This antagonizes cyclin binding and activation of CDK, thereby maintaining pRB in its hypophosphorylated and growth-suppressive state and induces G1 cell cycle arrest (10). In contrast to p16INK4a, ARF has a major role in the pathway involving p53, a transcription factor that regulates several genes involved in cell cycle checkpoints, stress responses, DNA damage and repair, and apoptosis (Figure 1) (2). ARF binds the mouse double minute 2 (MDM2) protein and inhibits MDM2-mediated degradation of p53, which thus results in stabilization of p53. One of the important targets of p53 is the CDKI p21 Cip1/Waf1, which inhibits the activity of several cyclin-CDK complexes, thus arresting cells in both G1 and G2/M stages of the cell cycle (10).
Figure 1.
Illustration showing possible mechanisms of p16INK4a and ARF induction and the role of these proteins in aging. The accumulation of persistent and increasing DNA damage in senescent cells in response to telomere shortening, DNA damage, inappropriate activation of signaling pathways, and production of ROS during aging results in transcriptional activation of the INK4a/ARF locus. Mitogen stimulation may amplify the signals of DNA damage. Alternative stochastic mechanisms of aging that lead to p16INK4a/ARF induction might also exist. Upregulation of p16INK4a and ARF activates pRB and p53 pathways, which in turn lead to cell cycle arrest and regenerative defects. In addition, p16INK4a/ARF upregulation might influence cellular functions in mitotically inactive organs during aging. Cyc D, cyclin D; BMI1, B lymphoma Mo-MLV insertion region; ID1, inhibitor of DNA binding 1; ETS1, v-ets erythroblastosis virus E26 oncogene homolog 1.
Understanding the role of p16INK4a and ARF during senescence has been complicated due to apparent differences between the mechanisms of senescence signalling induced by telomere dysfunction in mice and humans. In humans, telomere dysfunction induces senescence by activation of p53 and p16INK4a, whereas in mice only p53 is activated in response to telomere dysfunction (11). In addition, there might be differences in regulation of p16INK4a and ARF during replicative senescence and premature senescence. Whereas both genes are upregulated during premature senescence, only p16 but not ARF is upregulated during replicative senescence. During replicative senescence, upregulation of p16INK4a occurs shortly after the activation of p21, possibly to ensure maintenance of the senescence phenotype (12). Functional screens have shown that downregulation of ARF can rescue premature senescence (13). Understanding the regulation of p16INK4a and ARF in the different senescence pathways, the interaction between the pathways, and the functional role of these proteins during regeneration of organs and organismal aging are major questions for future research.
p16INK4a and ARF: molecular markers of aging?
In this issue of the JCI, Krishnamurthy et al. (14) attempted to identify molecular markers of aging in mice and rats by exploring the expression levels of several known cell cycle inhibitors. They show that the expression levels of both p16INK4a and ARF mRNA markedly increase with aging in most murine tissues, whereas there is no significant change in the expression levels of other related cell cycle inhibitors such as p15INK4b, p18INK4c, p21CIP, and p27KIP with aging. The authors substantiated their findings by demonstrating increased p16INK4a and ARF protein expression in some organ compartments. They found that in several organ compartments, the increase in the expression of p16INK4a and ARF was accompanied by an increased number of cells positive for senescence-associated β-galactosidase (SA-β-gal) — a biomarker of cellular senescence. While SA-β-gal has been used as a biomarker of senescence in vitro and in vivo, its lack of specificity has led to debate over the value of this method. However, although SA-β-gal is activated in response to several cellular stresses (15), it is still considered to be one of the best markers of senescence available today. It remains to be tested whether the accumulation of DNA-damage foci is a more specific marker for senescence. DNA damage foci accumulate in senescent cells with shortened telomeres; however, it is not yet clear whether they are present during premature senescence (16).
Krishnamurthy et al. show that the age-associated increase in expression of p16INK4a and ARF is attenuated in several tissues in response to caloric restriction (CR). CR is known to extend life span in a variety of species, including rats, mice, fish, flies, worms, and yeast. In addition, reduction of aging-associated pathology is evident in primates fed a calorie-restricted diet (17). The authors extended their studies further by exploring the role of p16INK4a and ARF in Igf1-null mice. There is experimental evidence that the lack of growth hormone–IGF (GH-IGF) signaling increases the lifespan in GH receptor (GHR) knockout (Ghr–/–) mice by approximately 25% (18). Krishnamurthy et al. did not see an impairment of p16INK4a or ARF expression in aging Ghr–/– mice, which suggests that GHR deficiency and CR extend the life span of organisms through distinct molecular mechanisms and that p16INK4a and ARF expression levels do not always correlate with lifespan.
Tangled regulation of p16INK4 and ARF during aging
At the level of transcription, the expression of p16INK4a is modulated by 3 principal regulators: ETS1, inhibitor of DNA binding 1 (ID1), and B lymphoma Mo-MLV insertion region (BMI1) (19, 20). The latter is also known to regulate ARF expression. Krishnamurthy et al. (14) found a strong correlation between the expression levels of p16INK4a and Ets-1, p16INK4 and ARF, but not between ARF and any of these 3 transcriptional regulators. These findings led to the speculation that an unknown coregulator(s) affects the expression of both p16INK4a and ARF with aging and that this coregulator(s) must be independent of Ets-1. In addition, the authors present what is believed to be the first in vivo evidence for a coordinated expression of p18INK4c and p19INK4d, possibly regulated by common or related transcriptional elements. With these studies as a basis, exploration of transcriptional control of the INK4a locus appears to be an interesting area of aging research (Figure 1).
What is the functional role and the mechanism of induction of p16INK4a and ARF during aging?
The study by Krishnamurthy et al. (14) appears to be of general interest, since the development of reliable biomarkers of aging will certainly be immensely useful in various areas of medicine. It is tempting to speculate that p16INK4a and/or ARF are not only good candidate biomarkers of aging, but that they may in fact have a functional role in the aging process. p16INK4a and ARF have both been linked to the induction of cell cycle arrest in response to DNA damage (21, 22). This finding seems of particular interest, since there are several connections among the different senescence stimuli that point to DNA damage as a major factor inducing senescence: (a) telomere shortening induces senescence by activation of the DNA-damage response (16); (b) ROS increase the rate of telomere shortening and induces multiple forms of DNA damage (23, 24); and (c) mitogen stimulation cooperates with telomere shortening to induce a DNA-damage response (25). It has recently been shown that DNA damage accumulates in senescent cells and aging murine tissues (26). Thus the DNA-damage hypothesis of aging could give a plausible explanation for the upregulation of p16INK4a and ARF during aging. Alternatively p16INK4a and ARF upregulation during aging might be regulated by an as-yet-unknown counting mechanism that differs from accumulation of DNA damage or telomere shortening (Figure 1). Understanding the signaling pathways involved in p16INK4a and ARF upregulation in organs and tissues could eventually point to new therapeutic targets to improve regenerative capacity during aging. Importantly, the experimental evidence for a functional role of p16INK4a and ARF in the aging process has yet to be established. Studies focused on the tumor suppressor p53 have revealed diverse results regarding the role of this key component of the senescence pathway during aging. In mouse models, the deletion of p53 has rescued the adverse effect of telomere dysfunction on organ homeostasis (27), whereas transgenic expression of a mutant p53 with increased protein stability induced premature aging (28), which indicates that p53 is indeed a mediator of aging phenotypes. However, inserting an extra copy of wild-type p53 in the mouse genome resulted in increased p53 activity in response to DNA damage and improved tumor suppression but did not enhance aging phenotypes (29). In this model, the extremely long telomeres in laboratory inbred mouse strains compared with house mice or humans might explain the lack of premature aging. To date there are no reports of improved organ regeneration in aging knockout mice lacking p16Ink4a or ARF or both genes. In addition, it is not known whether transgenic mice with increased expression levels of 1 of these proteins would develop premature aging phenotypes. However, there is indirect evidence from studies in proliferation associated SNF-2–like gene–knockout (PASG-knockout) mice, which have a defect in DNA-methylation. These mice show a severe premature aging phenotype accompanied by decreased expression of Bmi1 (a negative regulator of both p16INK4a and ARF) and increased levels of p16INK4a (30). It is interesting that Krishnamurthy et al. (14) observed an upregulation of p16INK4a and ARF in a wide spectrum of different organs and tissues in aging mice. Many of these tissues are mitotically inactive; thus, it appears to be important to analyze the impact of p16INK4a and ARF on cellular functions other than cell proliferation to evaluate their role in aging (Figure 1). In addition, these findings suggest that there might be a secreted factor leading to upregulation of p16INK4a and ARF in a variety of organs during aging.
The constant increase in maximum lifespan of human beings over recent centuries is certainly one of the biggest achievements of humankind. The identification and functional analysis of aging-associated molecular changes appears to be a promising area of research and should help us continue to make gains in this area.
Footnotes
See the related article beginning on page 1299.
Nonstandard abbreviations used: CDK, cyclin-dependent kinase; CDKI, CDK inhibitor; CR, caloric restriction; GH, growth hormone; GHR, GH receptor; MDM2, mouse double minute 2; pRB, retinoblastoma protein; SA-β-gal, senescence-associated β-galactosidase.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Kim S, Kaminker P, Campisi J. Telomeres, aging and cancer: in search of a happy ending. Oncogene. 2002;21:503–511. doi: 10.1038/sj.onc.1205077. [DOI] [PubMed] [Google Scholar]
- 2.Sharpless NE, DePinho RA. p53: good cop/bad cop. Cell. 2002;110:9–12. doi: 10.1016/s0092-8674(02)00818-8. [DOI] [PubMed] [Google Scholar]
- 3.Campisi J. The biology of replicative senescence. Eur. J. Cancer. 1997;33:703–709. doi: 10.1016/S0959-8049(96)00058-5. [DOI] [PubMed] [Google Scholar]
- 4.Reaper PM, di Fagagna F, Jackson SP. Activation of the DNA damage response by telomere attrition: a passage to cellular senescence. Cell Cycle. 2004;3:543–546. [PubMed] [Google Scholar]
- 5.Chen QM, et al. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem. J. 1998;332:43–50. doi: 10.1042/bj3320043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin AW, et al. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 1998;12:3008–3019. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huschtscha LI, Reddel RR. p16(INK4a) and the control of cellular proliferative life span. Carcinogenesis. 1999;20:921–926. doi: 10.1093/carcin/20.6.921. [DOI] [PubMed] [Google Scholar]
- 8.Ferbeyre G, et al. Oncogenic ras and p53 cooperate to induce cellular senescence. Mol. Cell. Biol. 2002;22:3497–3508. doi: 10.1128/MCB.22.10.3497-3508.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowland BD, et al. E2F transcriptional repressor complexes are critical downstream targets of p19(ARF)/p53-induced proliferative arrest. Cancer Cell. 2002;2:55–65. doi: 10.1016/s1535-6108(02)00085-5. [DOI] [PubMed] [Google Scholar]
- 10.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 11.Smogorzewska A, de Lange T. Different telomere damage signaling pathways in human and mouse cells. EMBO J. 2002;21:4338–4348. doi: 10.1093/emboj/cdf433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stein GH, Drullinger LF, Soulard A, Dulic V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol. Cell. Biol. 1999;19:2109–2117. doi: 10.1128/mcb.19.3.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shvarts A, et al. A senescence rescue screen identifies BCL6 as an inhibitor of anti-proliferative p19(ARF)-p53 signaling. Genes Dev. 2002;16:681–686. doi: 10.1101/gad.929302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnamurthy J, et al. Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 2004;114:1299–1307. doi:10.1172/JCI200422475. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Severino J, Allen RG, Balin S, Balin A, Cristofalo VJ. Is beta-galactosidase staining a marker of senescence in vitro and in vivo? Exp. Cell Res. 2000;225:162–171. doi: 10.1006/excr.2000.4875. [DOI] [PubMed] [Google Scholar]
- 16.d’Adda di Fagagna F, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 17.Lane MA, et al. Caloric restriction in primates. Ann. N. Y. Acad. Sci. 2001;928:287–295. doi: 10.1111/j.1749-6632.2001.tb05658.x. [DOI] [PubMed] [Google Scholar]
- 18.Bartke A, et al. Insulin-like growth factor 1 (IGF-1) and aging: controversies and new insights. Biogerontology. 2003;4:1–8. doi: 10.1023/a:1022448532248. [DOI] [PubMed] [Google Scholar]
- 19.Ohtani N, et al. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature. 2001;409:1067–1070. doi: 10.1038/35059131. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro GI, Edwards CD, Ewen ME, Rollins BJ. p16INK4A participates in a G1 arrest checkpoint in response to DNA damage. Mol. Cell. Biol. 1998;18:378–387. doi: 10.1128/mcb.18.1.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honda R, Yasuda H. Association of p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J. 1999;18:22–27. doi: 10.1093/emboj/18.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez RD, et al. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev. 2001;15:398–403. doi: 10.1101/gad.859201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, Trimarchi JR, Smith PJ, Keefe DL. Mitochondrial dysfunction leads to telomere attrition and genomic instability. Aging Cell. 2002;1:40–46. doi: 10.1046/j.1474-9728.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 25.Satyanarayana A, et al. Mitogen stimulation cooperates with telomere shortening to activate DNA damage responses and senescence signaling. Mol. Cell. Biol. 2004;24:5459–5474. doi: 10.1128/MCB.24.12.5459-5474.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sedelnikova OA, et al. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat. Cell. Biol. 2004;6:168–170. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- 27.Chin L, et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97:527–538. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- 28.Tyner SD, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Cao I, et al. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 2002;21:6225–6235. doi: 10.1093/emboj/cdf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun LQ, et al. Growth retardation and premature aging phenotypes in mice with disruption of the SNF2-like gene, PASG. Genes Dev. 2004;18:1035–1046. doi: 10.1101/gad.1176104. [DOI] [PMC free article] [PubMed] [Google Scholar]