Abstract
Each year millions of Americans present to the emergency department (ED) for care after a motor vehicle collision (MVC), the great majority (>90%) are discharged to home after evaluation. Acute musculoskeletal pain is the norm in this population, and such patients are typically discharged to home with prescriptions for oral opioid analgesics or non-steroidal anti-inflammatory drugs (NSAIDs). The influence of acute pain management on subsequent pain outcomes in this common ED population is unknown. We evaluated the effect of opioid analgesics versus NSAIDs initiated from the ED on the presence of moderate to severe musculoskeletal pain and ongoing opioid use at 6 weeks in a large cohort of adult ED patients presenting to the ED after MVC (n=948). The effect of opioids versus NSAIDs was evaluated using an innovative quasi-experimental design method using propensity scores to account for covariate imbalances between the two treatment groups. No difference in risk for moderate to severe musculoskeletal pain at six weeks was observed between those discharged with opioid analgesics versus NSAIDS (Risk Difference = 7.2% (95% CI: -5.2,19.5%). However, at follow-up participants prescribed opioids were more likely than those prescribed NSAIDS to report use of prescription opioids medications at week 6 (Risk Difference = 17.5% (95% CI: 5.8,29.3%). These results suggest that analgesic choice at ED discharge does not influence the development of persistent moderate to severe musculoskeletal pain six weeks after an MVC, but may result in continued use of prescription opioids.
Keywords: Opioid analgesics, non-steroidal anti-inflammatory drugs, acute pain, persistent pain, chronic pain, motor-vehicle collision, causal inference, propensity matching
1. Introduction
Each year almost 4 million people are evaluated in US emergency departments (EDs) after experiencing a motor vehicle collision (MVC).[27] Greater than 90% of these individuals are discharged to home after ED evaluation.[11; 30] Acute musculoskeletal pain (most commonly in the neck or back) is the norm among those discharged from the ED,[3] and while the majority recover within the first few weeks, up to 40% transition to persistent post-traumatic musculoskeletal pain.[26] Persistent pain after MVC is a common and costly public health problem in industrialized countries.[5] Despite this, there is limited information about the influence of initial ED care, specifically analgesic medications, on persistent pain outcomes.
At the time of ED discharge, providers generally prescribe an opioid analgesic and/or a non-steroidal anti-inflammatory drug (NSAID).[16] Available data support the potential advantages of both options. Opioid analgesics may provide more potent acute analgesia than NSAIDs and may more effectively dampen peripheral and central nervous system neuroplastic changes mediating persistent pain and other adverse posttraumatic outcomes.[15; 19; 25; 36; 45] However, after an initial period of analgesia, opioids are also known to cause hyperalgesia.[20; 22; 24; 42] Consistent with these data, genetic epidemiologic studies suggest that endogenous opioids at the time of traumatic stressors such as MVC worsen pain outcomes.[1; 23] In addition, an early prescription for an opioid might also increase the risk of chronic opioid use[43] and chronic opioid use has been associated with dependence and abuse.[28] NSAIDs appear to have a lower risk of promoting hyperalgesia, but also have less acute pain efficacy and have not been shown to have any beneficial effects on longer term pain outcomes.[6] Finally, some individuals may respond better to opioid analgesics or NSAIDs, and this phenomenon may be dependent on factors such as age, medication history, and sex.[23]
Understanding the risks and benefits of analgesics prescribed for common ED conditions such as acute post-MVC pain is important to optimize pain reduction and improve trauma recovery. In this study, we applied innovative analytic methods to data from a large prospective cohort study of MVC patients to assess the effect of opioid versus NSAID prescription at the time of ED discharge on persistent pain outcomes. Our primary aim was to evaluate the average and individual-level effect of opioid versus NSAID analgesics on persistent moderate to severe musculoskeletal pain 6 weeks after an MVC. A secondary aim was to examine whether receipt of an opioid versus an NSAID was associated with ongoing prescription opioid use at 6 weeks. In addition, because of the potential effect of factors such age, prior opioid medication use, and sex on the effect of these analgesics, we explored whether the treatment effect of opioids versus NSAIDs was associated with these factors.
2. Methods
2.1 Study Design and Setting
This investigation was a secondary analysis of data collected as part of a large, multi-center, prospective cohort study of adult patients who presented to an ED within 24 hours of an MVC and were discharged to home after evaluation. The primary aim of the parent study was to assess the association between epidemiologic and genetic characteristics and pain outcomes after an MVC. Data were collected at the ED visit via patient interview and self-report survey and 6 weeks, 6 months, and 1 year after the ED visit via internet-based self-report survey or telephone interview. Participants were enrolled at eight EDs in four states (Florida, Massachusetts, Michigan, and New York) between February 2009 and October 2011. The study was approved by the Institutional Review Board (IRB) at each of the study sites and all participants provided written informed consent. Further details of study methodology are described elsewhere.[26; 29]
2.2 Study Population
Patients aged 18 to 65 years old who presented to a participating ED within 24 hours of an MVC and were unlikely to require hospitalization were screened for study eligibility. Patients who were admitted to the hospital, had any fractures other than phalangeal fractures, had more than 4 lacerations requiring sutures or a single laceration more than 20 cm in length, or had intracranial or spinal injuries were excluded. Spinal injury was defined by the presence of a fracture, dislocation, or new neurologic deficit. Patients who were not alert and oriented also were excluded, as were pregnant patients, custodial prisoners, and patients unable to read and understand English. Enrollment was limited to non-Hispanic white subjects of European-American descent because the aims of the parent study included genetic analyses and studies involving genetic analyses can be biased by population stratification in a more diverse sample.[4]
2.3 Measures
2.3.1 Exposure Assessment
ED discharge medications were extracted from the electronic medical record (EMRs) of each study participant. Participants were categorized as having received an opioid analgesic at discharge if they received an opioid agonist medication prescription, such as hydrocodone or oxycodone. Participants who received a combination medication containing both an opioid and acetaminophen were also classified as having received an opioid. Participants were categorized as having received an NSAID if they received a non-specific cyclo-oxygenase enzyme inhibitor, such as ibuprofen or naproxen, but not aspirin. Participants who received both an NSAID and an opioid were excluded from the analyses.
2.3.2 Outcomes Assessment
The primary outcome was the presence of persistent self-reported moderate to severe pain 6 weeks after MVC. The six week time point was selected because it was the most proximate follow-up point to medication prescription, and because evidence suggests that chronic pain trajectories after MVC are generally established within 6-8 weeks after MVC.[17; 37; 39] Overall pain severity was assessed by asking individuals to rate their average overall pain intensity during the preceding week on a 0-10 numeric rating scale. Moderate to severe pain (>4 on this 0-10 numeric rating scale[21]) was chosen as the primary outcome because of its clinical relevance; its presence has been shown to be correlated with other patient-centered outcome measures, such as pain interference with function.[35] A secondary outcome of interest was the ongoing use of pain medication at week 6, because ongoing analgesic use is likely an important surrogate outcome in the measurement of pain[9] and because persistent opioid administration may increase the risk for problematic opioid use.
2.4 Statistical Analysis
2.4.1 Overview of Analyses
In a randomized controlled trial, the probability of receiving a given treatment is usually 50%. In an observational study like this, the probability that a physician will prescribe an opioid or an NSAID varies between patients, is dependent on many factors, and is likely confounded with the outcomes. To adjust for this confounding, we used multiple imputation propensity score methods with flexible parametric cubic splines along the propensity score (probability that a patient received an opioid). Using these methods, we estimated both average (group) effects and individual effect of opioid versus NSAIDs prescription on the likelihood of having moderate to severe musculoskeletal pain 6 weeks after an MVC.[12] In addition, we used innovative analytic techniques to estimate outcomes for individuals and groups had they received the other treatment option (opioid instead of NSAID, or vice versa).[7] By comparing these observed and estimated counterfactual (unobserved) outcomes, we determined both the overall average differential treatment effect of opioids versus NSAIDs and differential treatment effects according to individual characteristics.
As suggested by Rubin, we performed the statistical analysis in two stages.[32-34] The first stage comprised missing data imputation, propensity score estimation, and declaration of the procedures used for data analysis. All of these components were implemented without viewing the outcome data. The second stage involved imputation of missing counterfactual outcomes and estimation of treatment effects. All statistical analyses were conducted using Stata MP 13.1 (StataCorp, 2013. College Station, Texas) and R (R Foundation for Statistical Computing, Vienna, Austria).
2.4.2 Multiple Imputation of Missing Covariate Data
Multiple imputation of missing data was performed using chained equations; this method specifies the conditional models for all of the variables with missing values.[40; 44] To maintain objectivity of the analysis, covariates were imputed using only treatment indicators (opioid or NSAID) and other covariates. Imputation was performed using logistic regression for binary variables, ordinal logistic regression for ordinal variables, linear regression for continuous variables and predictive mean matching for semi-continuous variables. Twenty (m=20) complete imputed datasets were generated and used for calculation of the propensity score and further analyses.
2.4.3 Propensity Score Modeling
The conditional probability of receiving an opioid instead of an NSAID, given the observed covariates, was estimated using logistic regression in a stepwise iterative fashion.[18] In brief, initial covariate selection for the propensity score model was based on substantive knowledge about factors that might influence provider analgesic choice.[14; 38] Next, other covariates, as well as quadratic and interaction terms were selected based on likelihood ratio testing. The final propensity score model included 48 variables comprising socio-demographic data, baseline pain levels and body regions with pain, MVC characteristics, standardized assessments,[29] medical history (including previous opioid use), and study site. Individuals with propensity scores outside the region of common support (overlapping propensity scores between groups) were truncated. This truncation essentially excluded people whose probability of receiving a particular treatment approached 100%. A clinical analogy to this might be a patient who had a recent gastrointestinal bleed on NSAIDs, as this person will almost always be prescribed an opioid given the choice between an NSAID and an opioid. Since this was a comparative effectiveness study, we were interested in comparing participants who had some chance of receiving either treatment. Socio-demographic characteristics were compared between those included and excluded (truncated) from analyses.
The remaining patients who were not truncated were classified into six strata based on quantiles of the propensity scores. We then assessed whether covariates were balanced between the NSAID and opioid groups within and across strata. Student's t-test for continuous variables and z-test for binary variables were used to assess differences within strata and balance of covariates across strata was assessed by testing the null hypothesis that block-adjusted average difference in average covariate values is equal to zero for each covariate. Imbalance of covariates was addressed by re-specification of the initial propensity score model and then repeating the procedures above until the distribution of covariates was similar between the opioid and NSAID groups.
2.4.4 Imputation of Missing Potential Outcomes
Because loss to follow-up occurred after the ED visit, it could not be adjusted for in our propensity score analysis.[31] However, loss to follow-up is an important factor when determining unobserved counterfactual outcomes. We therefore imputed loss to follow-up using a logistic regression model that included a cubic spline the propensity score, covariates, and an indicator value for opioids versus NSAIDS, and only imputed counterfactual primary outcome (presence or absence of moderate to severe pain at 6 weeks) and secondary outcome (ongoing pain medication use) within the strata of patients who were estimated to have followed up regardless of whether they received an opioid or an NSAID. The first model involved the presence of pain as a function of a cubic spline along with the propensity score and covariates. The second model involved the ongoing use of pain medication as a function of spline along the propensity score, covariates, and the presence of moderate to severe pain at 6 weeks. (The use of two separate models enabled us to account for possible associations between the primary and secondary outcomes.)
Each of the outcome imputations were performed (l=40) within each of the m=20 imputed datasets. All of the point estimates and standard errors were combined using the combination rules for two-stage imputation [13] and confidence intervals were obtained using the t-distribution. We examined the average difference between the observed and counterfactual outcomes between individuals to estimate the average treatment effect (risk difference) of opioids versus NSAIDs on the presence of moderate to severe pain at 6 weeks, as well as the ongoing use of pain medications. We further estimated the average risk difference in moderate to severe pain at week 6 among men and women in order to examine possible effect modification by sex. Lastly, we determined heterogeneity of treatment effect by reporting the proportion of participants who would have responded to: 1) opioids only, 2) NSAIDs only, 3) both treatments or 4) neither treatment. We further describe the age, gender, and history of opioid use prior to the MVC among these four possible types of treatment response.
3. Results
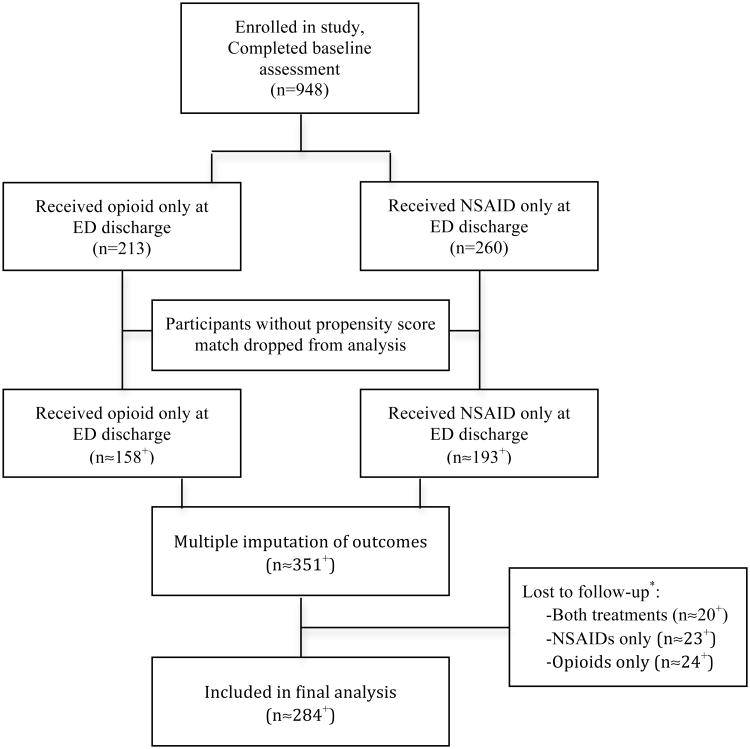
Figure 1 displays the flow of study participants from enrollment to inclusion in the final analyses. Slightly more than 60% of enrolled study participants were female, more than three-quarters had received formal education past high school, and more than half worked full time [26]. The median age of all study participants was 36 years (range 18 to 65). The vast majority (∼94%) had musculoskeletal strains only related to their MVC, the remainder also had minor associated injuries such as small lacerations or phalange fractures. The socio-demographic characteristics of participants who received either an opioid analgesic or an NSAID, before and after propensity score matching, are displayed in Table 1. Only educational status was significantly different between the opioid analgesic and NSAID groups in the unmatched sample (χ2=19.38, df=5, p<0.002).
Figure 1. Enrollment and analysis flow diagram.
+Numbers included in the analysis are approximate (≈) and equal to the mean of the number of participants included across each of the imputed datasets used in the analyses. *Loss to follow-up: Only individuals who would have followed up at 6 weeks regardless of whether they received an opioid or NSAID were included in the final analysis.
Table 1. Socio-demographic characteristics of participants discharged from the emergency department with a prescription for an opioid (with or without acetaminophen) vs an NSAID prescription only, before and after multiple imputation of covariates and propensity score matching.
| Unmatched Sample | Matched Sample* | |||
|---|---|---|---|---|
| Opioids (n=213) | NSAIDs (n=260) | Opioids (n≈158+) | NSAIDs (n≈193+) | |
| Median age, years | 33.0 (31.1 – 34.9) | 31.5 (29.6 – 33.4) | 36.3 (34.3 – 38.3) | 34.5 (32.6 – 36.3) |
| Gender (% female) | 59.2 (51.8 – 66.2) | 53.6 (48.0 - 59.1) | 58.5 (50.9 – 66.1) | 58.2 (51.1 – 65.3) |
| Education completed | ||||
| Some high school | 10.1 (6.4 – 15.4) | 2.0 (1.0 – 4.3) | 5.9 (2.0 – 9.8) | 2.6 (0.4 – 4.8) |
| High school | 20.1 (14.8 – 26.7) | 19.9 (15.8 – 24.8) | 28.3 (21.2 – 35.4) | 26.5 (20.2 – 32.8) |
| Some college | 45.3 (35.3 – 57.4) | 37.2 (30.2 – 45.7) | 33.5 (26.1 – 40.9) | 29.0 (22.5 – 35.5) |
| College graduate | 24.5 (16.4 – 32.5) | 40.7 (32.8 – 49.2) | 18.9 (12.8 – 25.0) | 29.5 (23.0 – 36.0) |
| Partner status | ||||
| Married | 42.3 (35.2 – 49.8) | 37.0 (31.7 – 42.6) | 46.7 (38.9 – 54.5) | 35.2 (28.5 – 41.9) |
| Living with partner | 15.3 (10.7 – 21.4) | 15.4 (10.3 – 20.5) | 12.3 (7.0 – 17.6) | 15.4 (10.3 – 20.5) |
| Serious relationship | 10.2 (6.5 – 15.6) | 18.0 (14.0 – 22.8) | 13.6 (8.3 – 18.9) | 21.1 (15.4 – 26.8) |
| Single | 32.2 (25.7 – 39.4) | 30.7 (26.7 – 36.1) | 27.3 (20.2 – 34.4) | 28.3 (22.0 – 34.6) |
| Employment status | ||||
| Employed | 79.8 (74.8 – 84.4) | 83.6 (76.7 – 90.9) | 64.0 (56.6 – 71.4) | 61.1 (54.2 – 68.0) |
| Disabled | 0.6 (0.1 – 3.8) | 2.6 (1.4 – 5.1) | 0.7 (0.0 – 2.1) | 2.1 (0.1 – 4.1) |
| Student | 22.3 (16.8 – 29.1) | 16.3 (12.6 – 20.9) | 19.9 (13.6 – 26.2) | 18.1 (12.6 – 23.6) |
| Unemployed | 11.7 (7.8 – 17.3) | 9.1 (6.3 – 12.9) | 10.8 (5.9 – 15.7) | 7.8 (4.1 – 11.5) |
Data are displayed as proportions (%) and 95% confidence intervals unless otherwise specificed.
Point estimates average across subclasses. NSAIDS = nonsteroidal anti-inflammatory drugs.
Numbers included in the analysis are approximate (≈) and equal to the mean of the number of participants included across each of the imputed datasets
Of the 948 enrolled in the initial cohort study, 6 week follow-up data were obtained in 91% of participants. Participants who were lost to follow-up in the initial cohort tended to be male, younger, and of lower socio-economic status.[26] Of the 859 cohort participants with complete follow-up data, 25% were discharged with an opioid analgesic (with or without acetaminophen) and 30% were discharged with an NSAID alone. Among those prescribed an opioid analgesic at ED discharge (n=213), 84% received hydrocodone and the rest received oxycodone. Among those participants prescribed only an NSAID (n=260), 90% received ibuprofen. The most frequently prescribed dose of hydrocodone and oxycodone was 5mg (range 5 – 10mg); the most frequently prescribed dose of ibuprofen was 800mg (range 400 – 800mg). Participants in both medication groups received a median duration of 3 days of analgesic medications. The median pain score among participants who received opioid was 7 (IQR 5 – 8); among those who received an NSAID, the median pain score was 5 (IQR 4 – 7).
In unadjusted analyses, 49% of all participants receiving NSAIDS and 56% of all participants receiving opioids reported moderate to severe musculoskeletal pain at 6 weeks post ED MVC visit. After propensity score methods were applied, there was no difference in risk of persistent moderate to severe musculoskeletal pain when participants were discharged from the ED with an opioid analgesic compared to an NSAID (Risk Difference = 7.2% (95% CI: -5.2, 19.5%). When stratified by sex, there was no statistically significant difference in the risk for persistent pain in women (Risk Difference = 11.1% (95% CI: -3.5 – 25.5%) or men (Risk Difference = 2.2% (95% CI: -14.4 – 18.8%). However, participants prescribed opioid analgesics were more likely than those who received NSAIDs to report using prescription opioids at the 6-week follow-up (Risk Difference = 17.5% (95% CI: 5.8, 29.3%).
As described in the above methods section, we used innovative analytic techniques[7] to predict treatment success (no moderate or severe pain six weeks after MVC) for each individual for each treatment. Using the results of these analyses, we classified individuals into one of four predicted treatment success categories i.e. treatment success with: 1) opioids alone, 2) NSAIDs alone, 3) either treatment, or 4) neither treatment (Table 2). Treatment effects according to participant characteristics are displayed in Table 2. No treatment response was predicted in ∼3/10 individuals, with responses to either treatment or NSAIDs alone predicted in ∼1/4 individuals, and response to opioids alone predicted in 1/5 individuals. There was no significant difference in age, sex or prior opioid use across the 4 different types of treatment response.
Table 2. Proportion of individuals in four different categories of treatment response* along with selected characteristics.
| Response to opioids only (n=54) | Response to NSAIDs only (n=71) | Response to both (n=68) | No treatment response (n= 92) | |
|---|---|---|---|---|
| Total proportion | 19.4 (12.5 – 26.3) | 26.6 (18.9 – 34.2) | 24.2 (16.7 – 31.6) | 29.8 (22.1 – 37.6) |
| Median age, years | 27.6 (22.9 – 32.3) | 32.8 (27.2 – 38.4) | 30.2 (24.0 – 36.4) | 35.8 (29.2 – 42.3) |
| Gender (% female) | 50.0 (34.4 – 65.6) | 59.6 (46.3 – 72.9) | 60.3 (46.4 – 74.2) | 53.5 (41.2 – 65.7) |
| Chronic opioid use | 5.4 (0.0 – 12.3) | 1.3 (0.0 – 4.1) | 2.9 (0.0 – 7.4) | 1.8 (0.0 – 5.2) |
Lack of moderate to severe pain at week 6 was considered treatment response. Variables are displayed as the proportion (%) of participants with 95% confidence intervals unless otherwise indicated. Participants with missing outcomes at 6 weeks were omitted from the analysis.
4. Discussion
The results from this investigation suggest that the initial ED prescription for an NSAID versus opioid medication at ED discharge following an MVC-related visit does not influence the development of persistent moderate to severe musculoskeletal pain six weeks after MVC. Although patients in our study who were discharged with NSAIDS, on average seemed to be experiencing less pain than those released with opioids (RD= -7.2%), this result was not significant.
In addition, participants prescribed opioid analgesics were nearly 20% more likely to report prescription opioid medication use at 6-week follow-up. It is possible that individuals who were experiencing greater levels of pain continued to take opioids, or this observation may also reflect the properties of opioid medications to induce dependence, tolerance, or hyperalgesia. While the reason for the finding is unknown, it is worth further examination given the recent stance by the Centers for Disease Control (CDC) to limit opioid prescribing for chronic pain.[8]
Study results also suggest that there is individual variability in treatment response. Approximately 1 in 5 individuals would not have persistent moderate or severe pain six weeks after MVC if they were prescribed an opioid, but they would if they had moderate to severe pain had they been prescribed an NSAID. Conversely, 1 in 4 patients would have only experienced such treatment success with an NSAID. These results add to growing data that opioids are effective in some individuals, but in others opioids may increase the risk of persistent pain development and/or persistent opioid use.
These data further underscore the importance of individualized medicine – determining which treatment is right for which individual and under what conditions. In this context, it is important to note that this study did not address other adverse effects of opioids (ex. nausea, constipation, overdose) or NSAIDs (ex. hypertension, dyspepsia, or gastrointestinal bleed), only continued opioid use. Future work should focus on the utility of a clinical decision tool to aid prescribing based on the likelihood of both treatment response, as well as unwanted sequelae (continued opioid use, adverse effects). Such a tool could optimize pain outcomes and reduce unnecessary medication use. Based on this study, one can infer that approximately 19% of people will only improve with an opioid (when given the choice between an opioid and an NSAID), but 25% of the study cohort received an opioid. If only the people who were going to get better on an opioid, actually received an opioid, opioid prescribing would be reduced based on this estimate. Moreover, if patients receive the treatment that they will respond to best (whether it be an opioid or an NSAID), the proportion of participants experiencing improvements in pain would be increased. Notably, there are some individuals will not respond to either an opioid or an NSAID. This emphasizes the importance of finding alternatives to our usual arsenal of pain medications.
In these preliminary analyses, we did not detect any significant differences in age, gender or history of opioid use to explain the variation in individual treatment response. This suggests that other mechanisms, clinical factors or even genetics, might determine whether an individual responds to treatment. It is worthwhile to note that the point estimate for the increased risk of persistent pain among women prescribed an opioid was 11% (vs. only 2% for men). This could represent a clinically meaningful difference and a larger study primarily aimed to examine gender difference might demonstrate statistical significance. Women have been shown to be at greater risk for chronic pain and chronic widespread pain development compared to men for some painful conditions.[41] Subsequent research should explore whether sex differences in chronic pain risk are mediated by opioid use.
4.1 Limitations
Several limitations should be considered when interpreting the study results. As with any observational study where the assignment to treatment is not randomized, the possibility of unmeasured confounding exists and could bias the study results. In addition, this investigation was an analysis of observational data, participants were aware of their “treatment assignment” and their expectations of pain and pain relief could also impact their reported pain scores. However, it is unlikely that expectations based on recollection of medication prescription at the time of ED discharge affected pain reporting at six weeks.
In addition, the study findings are only generalizable to the population of patients included in the analysis. In particular, the analysis only included participants who received an opioid or an NSAID at discharge – participants who received nothing or something other than these analgesics could have been systematically different than those included in the study. However, we did not identify any differences in sociodemographic characteristics (as listed in Table 1) between those included and not included in the analyses. In addition, the prospective cohort study from which these data were drawn included only white participants of self-reported European ancestry. It is possible that a more diverse or different racial and ethnic group could yield different findings.
This study only addressed the comparative effectiveness of opioid analgesics versus NSAIDs; we did not make a comparison to no analgesics or other types of analgesics. Comparative effectiveness assessments of two treatments is also intended to reflect clinical practice, and most ED patients are either prescribed or recommended to use some type of analgesic medication after presenting to the ED after an MVC.[2] As such, a comparison to no analgesics is likely not feasible, and in a randomized controlled trial it might be considered unethical to discharge people without analgesics. We also did not assess whether or not the combination of opioid analgesics and NSAIDs was superior to either type of medication alone. However, a recent randomized controlled trial among acute low back patients demonstrated no difference in pain and functional outcomes when oxycodone was added to the NSAID naproxen.[10] In addition, data were not available to examine adverse effects other than persistent pain and/or continued opioid use in this analysis, and this could possibly influence analgesic treatment choice. Lastly, our investigation was an intention-to-treat analysis based on prescription provided at discharge. As such, it reflects only the initial treatment choice by the provider and does not reflect adherence or efficacy and is also subject to confounding after the point of treatment assignment, as in an RCT. In addition, we did not examine the impact of different prescription regimens (strength, frequency, or “as needed” dosing) as there was not sufficient heterogeneity to examine these factors.
4.2 Conclusion
Based upon the results of this investigation, opioid analgesics prescribed to post-MVC ED patients use was not associated with greater improvements in pain outcomes than NSAIDS at 6 weeks after the ED visit. Accordingly, there is insufficient evidence to support recommending an opioid analgesic over an NSAID for all post-MVC ED patients. Of concern, individuals prescribed an opioid analgesic were more likely to report ongoing use of this medication at 6 weeks. There is likely variation in individual response to treatment and this phenomenon should be further explored in randomized controlled trials. Additional research is needed to determine the optimal intervention to prevent persistent or chronic pain following an MVC.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number R01AR056328. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supported by NIAMS R01AR056328 and AHRQ 5K12HS022998.
Footnotes
Scientific Meeting Presentation: American Pain Society Meeting 2015, Palm Springs, CA; Society for Academic Emergency Medicine Annual Meeting 2015, San Diego, CA; International Conference on Health Policy Statistics 2015, Providence, RI
Conflicts of Interest: The authors have no conflicts of interest to report.
References
- 1.Ballina LE, Ulirsch JC, Soward AC, Rossi C, Rotolo S, Linnstaedt SD, Heafner T, Foley KA, Batts J, Collette R, Holbrook D, Zelman S, McLean SA. mu-Opioid Receptor Gene A118G Polymorphism Predicts Pain Recovery After Sexual Assault. The journal of pain : official journal of the American Pain Society. 2013;14(2):165–171. doi: 10.1016/j.jpain.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Berger ML, Mamdani M, Atkins D, Johnson ML. Good research practices for comparative effectiveness research: defining, reporting and interpreting nonrandomized studies of treatment effects using secondary data sources: the ISPOR Good Research Practices for Retrospective Database Analysis Task Force Report--Part I. Value Health. 2009;12(8):1044–1052. doi: 10.1111/j.1524-4733.2009.00600.x. [DOI] [PubMed] [Google Scholar]
- 3.Bortsov AV, Platts-Mills TF, Peak DA, Jones JS, Swor RA, Domeier RM, Lee DC, Rathlev NK, Hendry PL, Fillingim RB, McLean SA. Pain distribution and predictors of widespread pain in the immediate aftermath of motor vehicle collision. Eur J Pain. 2013;17(8):1243–1251. doi: 10.1002/j.1532-2149.2013.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardon LR, Palmer LJ. Population stratification and spurious allelic association. Lancet. 2003;361(9357):598–604. doi: 10.1016/S0140-6736(03)12520-2. [DOI] [PubMed] [Google Scholar]
- 5.Carroll LJ, Holm LW, Hogg-Johnson S, Cote P, Cassidy JD, Haldeman S, Nordin M, Hurwitz EL, Carragee EJ, van der Velde G, Peloso PM, Guzman J. Course and prognostic factors for neck pain in whiplash-associated disorders (WAD): results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976) 2008;33(4 Suppl):S83–92. doi: 10.1097/BRS.0b013e3181643eb8. [DOI] [PubMed] [Google Scholar]
- 6.Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane database of systematic reviews (Online) 2013;7:CD008307. doi: 10.1002/14651858.CD008307.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dore DD, Swaminathan S, Gutman R, Trivedi AN, Mor V. Different analyses estimate different parameters of the effect of erythropoietin stimulating agents on survival in end stage renal disease: a comparison of payment policy analysis, instrumental variables, and multiple imputation of potential outcomes. Journal of clinical epidemiology. 2013;66(8 Suppl):S42–50. doi: 10.1016/j.jclinepi.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA : the journal of the American Medical Association. 2016;315(15):1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Immpact. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1-2):9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Friedman BW, Dym AA, Davitt M, Holden L, Solorzano C, Esses D, Bijur PE, Gallagher EJ. Naproxen With Cyclobenzaprine, Oxycodone/Acetaminophen, or Placebo for Treating Acute Low Back Pain: A Randomized Clinical Trial. JAMA : the journal of the American Medical Association. 2015;314(15):1572–1580. doi: 10.1001/jama.2015.13043. [DOI] [PubMed] [Google Scholar]
- 11.Gopinath B, Jagnoor J, Harris IA, Nicholas M, Maher CG, Casey P, Blyth F, Sindhusake D, Cameron ID. Comparison of health outcomes between hospitalised and non-hospitalised persons with minor injuries sustained in a road traffic crash in Australia: a prospective cohort study. BMJ Open. 2015;5(9):e009303. doi: 10.1136/bmjopen-2015-009303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutman R, Rubin DB. Estimation of causal effects of binary treatments in unconfounded studies. Stat Med. 2015;34(26):3381–3398. doi: 10.1002/sim.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harel O. Inferences on missing information under multiple imputation and two-stage multiple imputation. Statistical Methodology. 2007;4(1):75–89. [Google Scholar]
- 14.Heins JK, Heins A, Grammas M, Costello M, Huang K, Mishra S. Disparities in analgesia and opioid prescribing practices for patients with musculoskeletal pain in the emergency department. Journal of emergency nursing: JEN : official publication of the Emergency Department Nurses Association. 2006;32(3):219–224. doi: 10.1016/j.jen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL. Morphine use after combat injury in Iraq and post-traumatic stress disorder. N Engl J Med. 2010;362(2):110–117. doi: 10.1056/NEJMoa0903326. [DOI] [PubMed] [Google Scholar]
- 16.Hoppe JA, Houghland J, Yaron M, Heard K. Prescription history of emergency department patients prescribed opioids. The western journal of emergency medicine. 2013;14(3):247–252. doi: 10.5811/westjem.2012.2.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu J, Bortsov AV, Ballina LE, Orrey DC, Swor RA, Peak DA, Jones JS, Rathlev N, Lee DC, Domeier R, Hendry P, McLean SA. Chronic Widespread Pain after Motor Vehicle Collision Typically Occurs via Immediate Development and Non-Recovery: Results of an Emergency Department-Based Cohort Study. Pain. 2015 doi: 10.1097/j.pain.0000000000000388. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imbens GW, Rubin DB. Causal Inference in Statistics, Social, and Biomedical Sciences. Cambridge University Press; 2015. [Google Scholar]
- 19.Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26(12):696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Joly V, Richebe P, Guignard B, Fletcher D, Maurette P, Sessler DI, Chauvin M. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology. 2005;103(1):147–155. doi: 10.1097/00000542-200507000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Krebs EE, Carey TS, Weinberger M. Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med. 2007;22(10):1453–1458. doi: 10.1007/s11606-007-0321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Roy C, Laboureyras E, Gavello-Baudy S, Chateauraynaud J, Laulin JP, Simonnet G. Endogenous opioids released during non-nociceptive environmental stress induce latent pain sensitization Via a NMDA-dependent process. The journal of pain : official journal of the American Pain Society. 2011;12(10):1069–1079. doi: 10.1016/j.jpain.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Linnstaedt SD, Hu J, Bortsov AV, Soward AC, Swor R, Jones J, Lee D, Peak D, Domeier R, Rathlev N, Hendry P, McLean SA. mu-Opioid Receptor Gene A118 G Variants and Persistent Pain Symptoms Among Men and Women Experiencing Motor Vehicle Collision. J Pain. 2015;16(7):637–644. doi: 10.1016/j.jpain.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLean SA. The scientific journey to predicting and preventing postoperative pain: recalling Dr. Wall's stories along the way. Anesthesiology. 2013;119(6):1244–1246. doi: 10.1097/ALN.0000000000000014. [DOI] [PubMed] [Google Scholar]
- 25.McLean SA, Clauw DJ, Abelson JL, Liberzon I. The development of persistent pain and psychological morbidity after motor vehicle collision: integrating the potential role of stress response systems into a biopsychosocial model. Psychosomatic Medicine. 2005;67(5):783–790. doi: 10.1097/01.psy.0000181276.49204.bb. [DOI] [PubMed] [Google Scholar]
- 26.McLean SA, Ulirsch JC, Slade GD, Soward AC, Swor RA, Peak DA, Jones JS, Rathlev NK, Lee DC, Domeier RM, Hendry PL, Bortsov AV, Bair E. Incidence and predictors of neck and widespread pain after motor vehicle collision among US litigants and nonlitigants. Pain. 2014;155(2):309–321. doi: 10.1016/j.pain.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niska R, Bhuiya F, Xu J. National Hospital Ambulatory Medical Care Survey: 2007 emergency department summary. National health statistics reports. 2010;(26):1–31. [PubMed] [Google Scholar]
- 28.Palmer RE, Carrell DS, Cronkite D, Saunders K, Gross DE, Masters E, Donevan S, Hylan TR, Von Kroff M. The prevalence of problem opioid use in patients receiving chronic opioid therapy: computer-assisted review of electronic health record clinical notes. Pain. 2015;156(7):1208–1214. doi: 10.1097/j.pain.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 29.Platts-Mills TF, Ballina L, Bortsov AV, Soward A, Swor RA, Jones JS, Lee DC, Peak DA, Domeier RM, Rathlev NK, Hendry PL, McLean SA. Using emergency department-based inception cohorts to determine genetic characteristics associated with long term patient outcomes after motor vehicle collision: methodology of the CRASH study. BMC Emerg Med. 2011;11:14. doi: 10.1186/1471-227X-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Platts-Mills TF, Hunold KM, Esserman DA, Sloane PD, McLean SA. Motor vehicle collision-related emergency department visits by older adults in the United States. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2012;19(7):821–827. doi: 10.1111/j.1553-2712.2012.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenbaum PR. The consquences of adjustment for a concomitant variable that has been affected by the treatment. Journal of the Royal Statistical Society Series A (General) 1984:656–666. [Google Scholar]
- 32.Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2(3-4):169–188. [Google Scholar]
- 33.Rubin DB. The design versus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Statistics in medicine. 2007;26(1):20–36. doi: 10.1002/sim.2739. [DOI] [PubMed] [Google Scholar]
- 34.Rubin DB. For objective causal inference, design trumps analysis. The Annals of Applied Statistics. 2008:808–840. [Google Scholar]
- 35.Sadosky AB, Taylor-Stokes G, Lobosco S, Pike J, Ross E. Relationship between self-reported low-back pain severity and other patient-reported outcomes: results from an observational study. Journal of spinal disorders & techniques. 2013;26(1):8–14. doi: 10.1097/BSD.0b013e3182296c15. [DOI] [PubMed] [Google Scholar]
- 36.Sheridan RL, Stoddard FJ, Kazis LE, Lee A, Li NC, Kagan RJ, Palmieri TL, Meyer WJ, 3rd, Nicolai M, Stubbs TK, Chan G, Hinson MI, Herndon DN, Tompkins RG Multi-Center Benchmarking S. Long-term posttraumatic stress symptoms vary inversely with early opiate dosing in children recovering from serious burns: effects durable at 4 years. J Trauma Acute Care Surg. 2014;76(3):828–832. doi: 10.1097/TA.0b013e3182ab111c. [DOI] [PubMed] [Google Scholar]
- 37.Sterling M, Hendrikz J, Kenardy J. Similar factors predict disability and posttraumatic stress disorder trajectories after whiplash injury. Pain. 2011 doi: 10.1016/j.pain.2011.01.056. [DOI] [PubMed] [Google Scholar]
- 38.Terrell KM, Hui SL, Castelluccio P, Kroenke K, McGrath RB, Miller DK. Analgesic prescribing for patients who are discharged from an emergency department. Pain medicine (Malden, Mass) 2010;11(7):1072–1077. doi: 10.1111/j.1526-4637.2010.00884.x. [DOI] [PubMed] [Google Scholar]
- 39.Ulirsch JC, Weaver MA, Bortsov AV, Soward AC, Swor RA, Peak DA, Jones JS, Rathlev NK, Lee DC, Domeier RM, Hendry PL, McLean SA. No man is an island: Living in a disadvantaged neighborhood influences chronic pain development after motor vehicle collision. Pain. 2014;155(10):2116–2123. doi: 10.1016/j.pain.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Statistical methods in medical research. 2007;16(3):219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 41.Viniol A, Jegan N, Brugger M, Leonhardt C, Barth J, Baum E, Becker A, Strauch K. Even Worse - Risk Factors and Protective Factors for Transition from Chronic Localized Low Back Pain to Chronic Widespread Pain in General Practice: A Cohort Study. Spine (Phila Pa 1976) 2015;40(15):E890–899. doi: 10.1097/BRS.0000000000000980. [DOI] [PubMed] [Google Scholar]
- 42.Wall PD. On the relation of injury to pain. The John J. Bonica lecture. Pain. 1979;6(3):253–264. doi: 10.1016/0304-3959(79)90047-2. [DOI] [PubMed] [Google Scholar]
- 43.Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine (Phila Pa 1976) 2007;32(19):2127–2132. doi: 10.1097/BRS.0b013e318145a731. [DOI] [PubMed] [Google Scholar]
- 44.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 45.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288(5472):1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]