Abstract
DNA vaccination is a novel immunization strategy that has great potential for the development of vaccines and immune therapeutics. This strategy has been highly effective in mice, while less immunogenic in nonhuman primates and humans. Enhancing DNA vaccine potency remains a challenge. It is likely that APCs, and especially DCs, play a paramount role in the presentation of vaccine antigen to the immune system. A new study reports the synergistic recruitment, expansion, and activation of DCs in vivo in a mouse model through covaccination with plasmids encoding macrophage inflammatory protein-1α (MIP-1α), fms-like tyrosine kinase 3 ligand (Flt3L), and the DNA vaccine. Such cooperative strategies delivering vaccine in a single, simple platform result in improved cellular immunity in vivo, including enhanced tetramer responses and IFN-γ secretion by antigen-specific cells.
In theory, DNA vaccination combines the most desirable attributes of standard vaccine approaches in order to achieve functional enhancement of antigen-specific immune cells. Attenuated vaccine antigens are poorly taken up by and processed through the MHC class I pathway and thus primarily induce humoral immune responses and only weakly activate cytotoxic T cells. In contrast, live attenuated vaccines generate both cellular and humoral immune responses, and, potentially, lifelong immunity. However, safety concerns make development of new vaccines based on live, replicating vectors costly. Possible reversion and possible pathogenesis of the immunogen in immunocompromised individuals limit development of some live attenuated vaccines. The development of safer and more efficacious vaccine strategies that elicit both strong cellular and strong humoral immunity without viral replication is currently under intense investigation. One possible solution is the development of more effective DNA vaccines.
What are the mechanisms of immunity following DNA vaccination?
The efficiency of DNA vaccination depends on the interaction among genetic material, lymphocytes, and APCs. Following intramuscular injection, myocytes play the role of antigen factories. They primarily express MHC class I and under certain circumstances express MHC class II; however, they do not express the costimulatory molecules required for priming and activation of T cells and thus lack function as effective APCs (1). Alternatively, small numbers of bone marrow–derived DCs and other professional APCs that can be activated to express high levels of MHC, as well as costimulatory molecules, are present at the site of injection and become transfected with the injected DNA. Cross-priming may occur, in which CD8+ T cell responses are primed by exogenous class I–restricted peptides that are not expressed in, but rather are acquired by, local APCs (2, 3). Regardless of the underlying mechanism, it has become clear that in the context of DNA vaccination APCs are key inducers of immunity, as they are the pivotal mediators of immune responses between resident somatic cells and T cells in the lymph nodes. By trafficking antigen from the site of injection to the secondary lymphoid organs, APCs serve to efficiently present antigen to naive T cells.
Targeting DC maturation for improved DNA vaccine potency
Based on the initial reports of immune enhancement by delivery of plasmids encoding GM-CSF (4) or IL-12 (5) in combination with specific antigen-encoded plasmids, other molecules have been studied for manipulating the potency of DNA vaccines in mice. Molecules under investigation target Th1-type T cell expansion; DC/APC activation, expansion, or maturation; costimulation; and immune cell trafficking by chemokines (4, 6–13). Recently, some vaccine strategies have produced exciting results in nonhuman primates (14, 15).
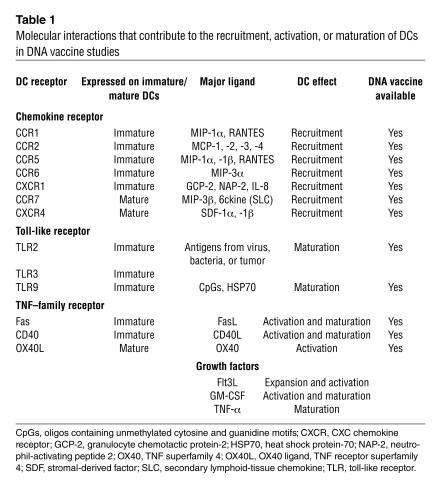
The ability of DCs to drive antigen-specific immunity is dependent on their degree of maturation. As shown in Table 1, a variety of molecules delivered as DNA vaccines can improve APC activation, expansion, or maturation following antigen uptake and processing in vivo. These include bacterially derived antigens, TNF-superfamily receptors, growth factors, inflammatory cytokines/chemokines, ligation of select cell surface receptors (CD40), and viral products. During their conversion from immature to mature cells, DCs undergo a number of phenotypic and functional changes, including increased MHC complex expression on their surface; increased surface expression of costimulatory molecules; morphological changes, including an increase in surface area, which allows DCs to interact with a greater number of T cells; secretion of chemokines and cytokines for attraction and expansion of T cells; protease production to convert molecules important in immune expansion to their active forms; and surface expression of adhesion molecules and chemokine receptors, allowing for increased interaction with other immune cells (Table 1). Through the use of combination immunomodulatory adjuvants that code for maturation, activation, or recruitment factors, it should be possible to further manipulate APCs in vivo to enhance DNA vaccine potency.
Table 1.
Molecular interactions that contribute to the recruitment, activation, or maturation of DCs in DNA vaccine studies
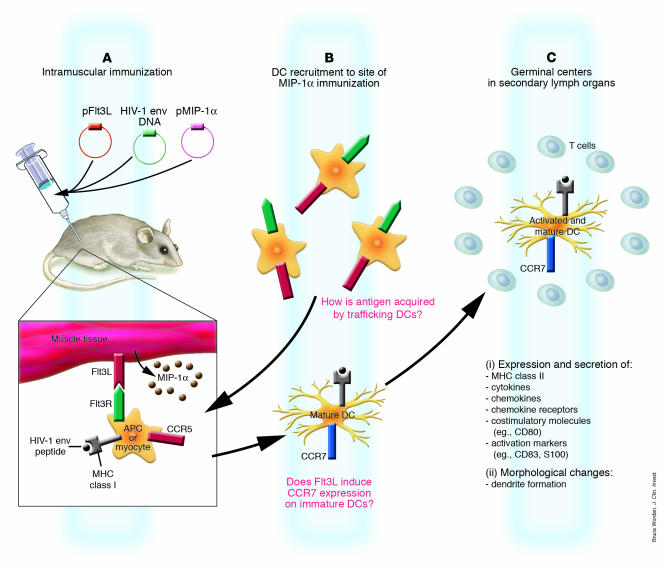
In this issue of the JCI, Sumida et al. (16) explore a combination of DC-specific chemotactic and growth factors, macrophage inflammatory protein-1α (MIP-1α) and fms-like tyrosine kinase 3 ligand (Flt3L), as part of a DNA vaccine cocktail. Previous DNA vaccine studies have shown that MIP-1α, which binds to CC chemokine receptor 5 (CCR5) on immature DCs, is able to recruit DCs to the site of inoculation in mice, resulting in enhanced cellular immune responses and increased antibody titers (17). In other studies, the use of maturation factors or DC growth factors such as Flt3L immunoadjuvants has been shown to expand DC numbers in both mice and humans (7, 18). However, the combination of MIP-1α and Flt3L tested by Sumida et al. (16) extends those early studies by demonstrating DC recruitment to the site of injection and migration of loaded APCs to the regional lymph nodes (16). Immunohistochemistry of injected muscle tissue shows that inoculation with plasmids encoding MIP-1α and Flt3L with HIV-1 envelope (env) DNA vaccine resulted in recruitment of DCs that express CD11b and the activation markers CD83 and S100, as well as the maturation markers MHC class II and CD80. In this model, DCs that traffic to the inoculation site in response to MIP-1α expression and contact Flt3L expressed on the muscle cells expand and mature in response to the growth factor Flt3L (Figure 1). Upregulation of CCR7 and loss of CCR5 on the mature DCs will cause them to traffic to the draining lymph node, where immune priming can occur in the germinal centers (Figure 1).
Figure 1.
Proposed schematic of chemokine-induced traffic and activation of DCs following DNA vaccination with plasmid-encoded Flt3L and MIP-1α. (A) Following intramuscular injection in mice of plasmid-encoded MIP-1α (pMIP-1α) and plasmid-encoded Flt3L (pFlt3L), in combination with HIV-1 env, muscle and resident APCs are transfected with plasmid, leading to protein production. MIP-1α protein is secreted, while Flt3L is expressed on muscle cells or on the surface of resident APCs. HIV-1 env is processed, and peptides are presented by MHC class I molecules; this peptide/MHC complex stimulates CD8+ T lymphocytes. Soluble protein released by transfected cells is taken up by APCs, and via the MHC Class II pathway, the peptide/MHC complex stimulates CD4+ T lymphocytes. Cross-priming occurs in which CD8+ T cell responses are primed by exogenous class I–restricted antigens that are not expressed in, but rather are acquired by, local APCs. (B) DCs that express CCR5 are recruited to the site of immunization in response to MIP-1α secretion. DCs bind Flt3L on the surface of local APCs or muscle cells, resulting in their expansion and maturation as they migrate to the draining lymph node. How antigen is acquired by the newly recruited DCs is an important question that remains poorly understood. These DCs acquire antigen, express CCR7, and are attracted by chemokines expressed in the draining lymph nodes. (C) The same DCs as in B traffic to the draining lymph nodes, where they prime naive T cells. Mature DCs express high levels of MHC class II, costimulatory molecules, activation markers, adhesion molecules, and chemokine receptors, secrete cytokines and chemokines, and form dendrites.
In the current study (16), vaccine-elicited immune responses were not detectably augmented by plasmid-encoded Flt3L or plasmid-encoded MIP-1α alone. In contrast, tetramer responses and IFN-γ responses were enhanced in mice that received the DNA vaccine along with both adjuvant plasmids. This finding is in contrast to earlier studies in which vaccination with MIP-1α expanded both humoral and cellular immune responses (17) and vaccination with Flt3L also exhibited enhancement of immunogenicity (18). These differences may reflect the properties of different plasmid-based vectors, properties unique to the antigens involved, different forms of the adjuvants, or other, unknown biases.
Furthermore, improved protection against challenge with recombinant HIV env and vaccinia virus in mice was achieved following vaccination with plasmid-encoded MIP-1α, Flt3L, and HIV-1 env DNA vaccine (16). Prior studies have also shown enhanced immunogenicity and protection against lethal challenge with herpes simplex virus-2 in mouse model systems using chemokines that bind CCR5 as adjuvants. For example, RANTES coimmunization significantly enhanced CD8+ and CD4+ T cell responses and resulted in enhanced survival in a lethal mucosal herpes simplex virus-2 challenge model (19). However, in the same system, immunization with MIP-1α alone was not effective, which suggests that not all CCR5 ligands are similar and that Flt3L, as reported here (16), complements MIP-1α activity.
Overall, these studies support a model where the number of DCs present at the site of inoculation during antigen expression is a possible rate-limiting factor for DNA vaccine effectiveness. In this context, coimmunization with a plasmid chemokine for attracting DCs to the site of injection appears important. For example, in other DNA vaccine studies, the use of apoptotic molecules as plasmid adjuvants results in the death of antigen-bearing cells, leading to an increase in antigen acquisition by APCs, including DCs (20, 21).
Important issues that should be investigated include elucidation of how the antigenic plasmid is trafficked to APCs following immunization, the efficacy of different adjuvant combinations, the most relevant timing of adjuvant delivery, and validation using other antigenic plasmids in additional model systems. The question remains of exactly how and in what context DCs that migrate to the site of injection acquire plasma-delivered antigen. For future development of this strategy, validation needs to be carried out in primate models. Many vaccine studies have shown that mice and primates may not respond similarly to the same adjuvants. For example, GM-CSF was shown to be a superior adjuvant for DNA vaccines in mice but was a poor DNA adjuvant in nonhuman primates (15). Such validation studies are an important step since the immune response induced in nonhuman primates appears to be more representative of the responses ultimately observed in humans. Sumida et al. (16) provide additional evidence that simple DNA vaccine formulations can manipulate complex host immunity. A more detailed study of these and other combination strategies aimed at improved APC function will likely represent an important area of DNA vaccine research. Such combination strategies may ultimately allow this novel approach to achieve improved potency in clinical evaluation.
Footnotes
See the related article beginning on page 1334.
Nonstandard abbreviations used: CCR, CC chemokine receptor; env, envelope; Flt3L, fms-like tyrosine kinase 3 ligand; MIP-1α, macrophage inflammatory protein-1α.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Hohlfeld R, Engel AG. The immuno-biology of muscle. Immunol. Today. 1994;15:269–274. doi: 10.1016/0167-5699(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 2.Heath WR, Carbone FR. Cross-presentation in viral immunity and self-tolerance. Nat. Rev. Immunol. 2001;1:126–134. doi: 10.1038/35100512. [DOI] [PubMed] [Google Scholar]
- 3.Ulmer JB, Otten GR. Priming of CTL responses by DNA vaccines: direct transfection of antigen presenting cells versus cross-priming. Dev. Biol. (Basel). 2000;104:9–14. [PubMed] [Google Scholar]
- 4.Xiang Z, Ertl HC. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995;2:129–135. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 5.Kim JJ, et al. In vivo engineering of a cellular immune response by coadministration of IL-12 expression vector with a DNA immunogen. J. Immunol. 1997;158:816–826. [PubMed] [Google Scholar]
- 6.Gurunathan S, Wu CY, Freidag BL, Seder RA. DNA vaccines: a key for inducing long-term cellular immunity. Curr. Opin. Immunol. 2000;12:442–447. doi: 10.1016/s0952-7915(00)00118-7. [DOI] [PubMed] [Google Scholar]
- 7.Chan K, et al. The roles of MHC class II, CD40, and B7 costimulation in CTL induction by plasmid DNA. J. Immunol. 2001;166:3061–3066. doi: 10.4049/jimmunol.166.5.3061. [DOI] [PubMed] [Google Scholar]
- 8.Shedlock DJ, Weiner DB. DNA vaccination: antigen presentation and the induction of immunity. J. Leukoc. Biol. 2000;68:793–806. [PubMed] [Google Scholar]
- 9.Kim JJ, Yang JS, Dentchev T, Dang K, Weiner DB. Chemokine gene adjuvants can modulate immune responses induced by DNA vaccines. J. Interferon Cytokine Res. 2000;20:487–498. doi: 10.1089/10799900050023906. [DOI] [PubMed] [Google Scholar]
- 10.Pasquini S, et al. Cytokines and costimulatory molecules as genetic adjuvants. Immunol. Cell Biol. 1997;75:397–401. doi: 10.1038/icb.1997.62. [DOI] [PubMed] [Google Scholar]
- 11.Biragyn A, et al. DNA vaccines encoding human immunodeficiency virus-1 glycoprotein 120 fusions with proinflammatory chemoattractants induce systemic and mucosal immune responses. Blood. 2002;100:1153–1159. doi: 10.1182/blood-2002-01-0086. [DOI] [PubMed] [Google Scholar]
- 12.Spies B, et al. Vaccination with plasmid DNA activates dendritic cells via Toll-like receptor 9 (TLR9) but functions in TLR9-deficient mice. J. Immunol. 2003;171:5908–5912. doi: 10.4049/jimmunol.171.11.5908. [DOI] [PubMed] [Google Scholar]
- 13.Wan T, et al. Novel heat shock protein Hsp70L1 activates dendritic cells and acts as a Th1 polarizing adjuvant. Blood. 2004;103:1747–1754. doi: 10.1182/blood-2003-08-2828. [DOI] [PubMed] [Google Scholar]
- 14.Barouch DH, et al. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 15.Calarota SA, Weiner DB. Enhancement of human immunodeficiency virus type 1-DNA vaccine potency through incorporation of T-helper 1 molecular adjuvants. Immunol. Rev. 2004;199:84–99. doi: 10.1111/j.0105-2896.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- 16.Sumida SM, et al. Recruitment and expansion of dendritic cells in vivo potentiate the immunogenicity of plasmid DNA vaccines. J. Clin. Invest. 2004;114:1334–1342. doi:10.1172/JCI200422608. doi: 10.1172/JCI22608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKay PF, et al. Recruitment of different subsets of antigen-presenting cells selectively modulates DNA vaccine-elicited CD4+ and CD8+ T lymphocyte responses. Eur. J. Immunol. 2004;34:1011–1020. doi: 10.1002/eji.200324840. [DOI] [PubMed] [Google Scholar]
- 18.Westermann J, et al. Flt-3 ligand as adjuvant for DNA vaccination augments immune responses but does not skew TH1/TH2 polarization. Gene Ther. 2004;11:1048–1056. doi: 10.1038/sj.gt.3302261. [DOI] [PubMed] [Google Scholar]
- 19.Sin J, Kim JJ, Pachuk C, Satishchandran C, Weiner DB. DNA vaccines encoding interleukin-8 and RANTES enhance antigen-specific Th1-type CD4(+) T-cell-mediated protective immunity against herpes simplex virus type 2 in vivo. J. Virol. 2000;74:11173–11180. doi: 10.1128/jvi.74.23.11173-11180.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chattergoon MA, et al. Targeted antigen delivery to antigen-presenting cells including dendritic cells by engineered Fas-mediated apoptosis. Nat. Biotechnol. 2000;18:974–979. doi: 10.1038/79470. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki S, Amara RR, Oran AE, Smith JM, Robinson HL. Apoptosis-mediated enhancement of DNA-raised immune responses by mutant caspases. Nat. Biotechnol. 2001;19:543–547. doi: 10.1038/89289. [DOI] [PubMed] [Google Scholar]