Abstract
Aim
Haemolysis has a major impact on patient safety as the need for a replacement specimen increases the risk of injury and infection, delays test results and extends the duration of hospital stays. Consistency of haemolysis detection and reporting can facilitate the generation of benchmark data used to develop quality practices to monitor and reduce this leading cause of pre-analytical laboratory error. This review aims to investigate current methods of haemolysis detection and reporting.
Method
Due to known heterogeneity and immaturity of the research field, a scoping search was conducted using PUBMED, Embase, Medline and CINAHL. Articles published between 2000 and 2014 that reported haemolysis rates in specimens from the general population were included.
Results
Of the 50 studies that met the inclusion criteria, 20 detected haemolysis using the Haemolysis Index (HI), 19 by visual inspection and 13 by undefined methods. There was large intra-study variation in the plasma free haemoglobin level used to establish haemolysis (HI: mean±SD 846±795 mg/L, range 150–3000 mg/L; Visual: 850±436 mg/L, 500–3000 mg/L). Sixteen studies reported the analyte of interest, with only three studies reporting a haemoglobin level at which the specimen would be rejected.
Conclusion
Despite haemolysis being a frequent and costly problem with a negative impact on patient care, there is poor consistency in haemolysis detection and reporting between studies. Improved consistency would facilitate the generation of benchmark data used to create quality practices to monitor and reduce this leading cause of pre-analytical laboratory error.
Introduction
Haemolysis refers to the breakdown of erythrocytes, commonly referred to as red blood cells, resulting in the release of haemoglobin into the surrounding fluid.1 While Carraro and Plebani reported a significant decrease in the number of errors observed in a clinical laboratory between 1996 and 2006, the proportion of pre-analytical errors remained relatively unchanged.2 As one of the leading causes of pre-analytical laboratory errors, reported to account for 40–70% of all specimen rejections, haemolysis constitutes an area of major importance for pathology laboratories.3,4 Although haemolysis may be caused by haemolytic anaemia, termed in vivo haemolysis, it is more commonly caused by incorrect procedures relating to the collection, handling, transportation or storage of the specimen, termed in vitro haemolysis.5
In vitro haemolysis is of particular concern when blood is collected for diagnostic testing as it has the potential to lead to incorrect measurements of some analytes, dilution effects, spectral interference and/or chemical elevations. Haemolysis may also lead to the release of proteases such as Cathepsin E which degrade troponin T and can lead to interference affecting antibody recognition in certain immunoassays.6 All of these factors can cause erroneous laboratory results.3 In these instances, a replacement specimen needs to be obtained to perform the requested tests, impacting available resources, delaying the availability of test results7 and potentially subjecting the patient to a repeat blood draw and increased risk of iatrogenic injury and infection.8 The need for a new specimen also contributes to delayed time to diagnosis and potentially longer episodes of hospital care and increases in laboratory costs.7 Therefore, as well as having a major bearing on the quality and efficiency of the laboratory process,9 haemolysis also has major implications for the quality and safety of patient care10,11 and hospital costs.12
Benchmark data are values of a given metric across a number of similar facilities, their critical characteristic being that the process for recording and calculating these values is adequately described so that comparable values can be recorded at other facilities and/or at other times. This standardisation in the measurement of the metric allows individual facilities to compare their performance to the benchmark and, where the comparison is unfavourable, consider the potential causes of the difference and strategies to improve their performance. Benchmark data of haemolysis prevalence in laboratory specimens could be used to develop safe and quality practices to reduce haemolysis and thereby potential errors in laboratory results. Such practices can contribute to an enhancement in the overall effectiveness of laboratory services and their contribution to safe and quality patient care.3
To enable the generation of accurate benchmark data, the sites generating the data must follow consistent procedures for haemolysis detection and reporting. While a consensus conference agreed that haemolysis should be defined as ‘any samples where one or more tests were not performed or one or more results were rejected or not reported due to haemolysis’,13 there is believed to be little consistency in the methods by which haemolysis is detected and reported between individual laboratories.3 This has led both Dolci and Panteghini14 and Lippi et al.15 to call for increased consistency in haemolysis detection. However, there is little evidence regarding the current level of consistency in haemolysis detection and reporting.
Traditionally, specimens were detected as being haemolysed by visual inspection, often on an arbitrary basis, a practice that is strongly discouraged today because of the unreliability and variability of results.4,16,17 Now, almost all biochemistry analytical platforms are equipped with hardware and software capable of detecting analytical interferences. One of these indicators is the HI, a quantitative value linked to the amount of free haemoglobin in the plasma or serum. Due to the ability to detect mild haemolysis and the potential for consistent detection methods across a range of laboratories, HI is now considered best practice for haemolysis detection.14,16,18 While it is known that the effect of haemolysis interference on test results depends on the analyte being tested,19 there is little information about the consistency of the methods by which haemolysis is detected and reported between individual laboratories. The aim of this narrative review was to investigate current methods of haemolysis detection and reporting as a step towards documenting levels of heterogeneity in current practice, and creating quality practices to monitor and reduce this leading cause of pre-analytical laboratory errors.
Methods
Search Strategy
Due to the known heterogeneity in haemolysis detection and reporting, inconsistency in the detection of a haemolysed specimen and the relative immaturity of research in the field, a narrative review was deemed most appropriate to identify the current state of the evidence-base on this topic. As such, a scoping search was performed of PUBMED, Embase, Medline and CINAHL databases in January 2015 to identify studies that investigated pre-analytical errors, rejected specimens or in vitro haemolysis. Peer reviewed studies published between January 2000 and December 2014 were included if they reported on primary data from the general population, provided an overall rate of haemolysis, or provided sufficient information from which to calculate an overall rate, and provided at least an abstract in English. Articles that reported on specific patient conditions such as haemolysis rates for patients with diabetes were excluded from this review.20
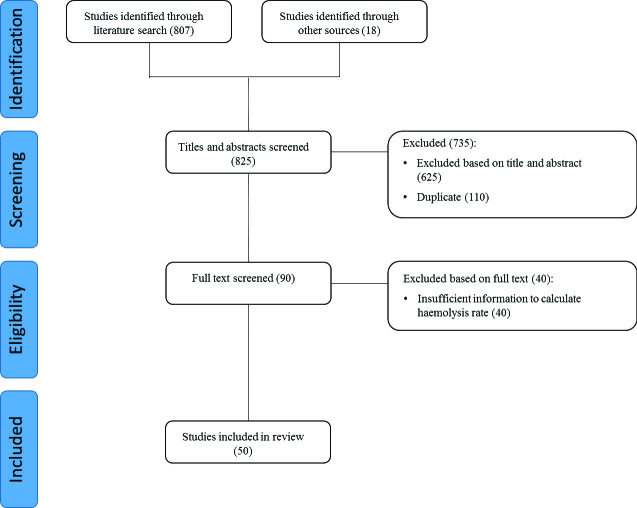
Broad search terms were used to maximise the identification of all studies that examined haemolysis rates. The search strategy used subject headings (e.g. MeSH where possible) and keywords. The following search terms were used individually and in combination, including both British and American English variants of spelling: h(a)emolysis, pre(−)analytical error(s), rate/frequency/prevalence, error(s), retrospective analysis/audit, rejection rate, h(a)emolysed/h(a)emolyzed, blood specimen/sample collection. Initially, all abstracts and titles were independently reviewed (EV, RL) and sorted based on the aforementioned predefined inclusion criteria. The full text of the studies that matched these criteria were then independently reviewed (EV, RL) and once again sorted based on the predefined inclusion criteria. Reference lists of studies that met the inclusion criteria were hand searched for relevant studies that were not returned by the initial search. The search protocol is summarised in the Figure.
Figure.
PRISMA flow diagram of search protocol.
Analysis
The following information was extracted from each study: the year and country in which the study was conducted; study design (including statistical methods); study setting; patient population (i.e. emergency, inpatient or outpatient); technique used to detect haemolysis (if stated); plasma free haemoglobin level used to detect haemolysis (if stated); plasma free haemoglobin level used to reject a specimen due to haemolysis (if stated); and the analyte of interest (if stated).
Studies were grouped based on haemolysis detection method, namely HI, visual inspection or undefined. Studies that used both the HI and visual inspection to detect haemolysiswere included in both categories.21,22 The mean plasma free haemoglobin level used to detect haemolysis was calculated for each group (±1 SD).
Results
Fifty studies published between 2000 and 2014 were included in this review. Twenty-three studies were conducted in Europe, 14 in North America, 12 in Asia and one in Australia. Thirty-one studies included emergency patients, 26 inpatients and 15 outpatients. One study included patients in both an ED and a labour/delivery ward and three studies did not state the patient population studied.
Detection of Haemolysis
Twenty studies detected haemolysis using the HI, 19 studies detected haemolysis visually and 13 studies did not state their method. Of the 20 studies that detected haemolysis using the HI, 10 different devices were used. The mean plasma free haemoglobin level used to detect haemolysis using the HI was 846 ± 795 mg/L, ranging from 150 mg/L to 3000 mg/L (Table 1). It was found that the Roche Modular (Roche, Basel, Switzerland) was the platform most commonly used to detect haemolysis across the studies. Large variation was found in the mean plasma free haemoglobin level used to detect haemolysis using both the same and different equipment, with studies using the Roche Modular platform using a free haemoglobin level ranging from 390 mg/L to 3000 mg/L, while studies that used other platforms used a free plasma haemoglobin level ranging from 150 mg/L to 2000 mg/L.
Table 1.
Characteristics of studies detecting haemolysis rate by the Haemolysis Index.
| Author | Year | Country | Patient Type | Device | Haemolysis Level (mg/L free Hb) | Analyte |
|---|---|---|---|---|---|---|
| Davidson51 | 2014 | UK | IP, OP | Roche Modular | 390 | Iron, potassium |
| Fernandez et al.52 | 2014 | Spain | IP, OP | Various | Not given | ALT, AST, CK, cholesterol, phosphorus, ALP, iron, glucose, GGT, potassium, LDH, protein, triglycerides |
| Kara et al.35 | 2014 | Turkey | ED | Not given | Not given | ALT, amylase, AST, bilirubin, calcium, creatinine, glucose, LDH, potassium, sodium, urea nitrogen |
| Lippi et al.36 | 2014 | Italy | ED | Beckman Coulter | 500 | Not given |
| Lippi et al.57 | 2014 | Italy | ED | Beckman Coulter | 500 | Not given |
| Bolenius et al.23 | 2013 | Sweden | OP | Vitros 5,1 | 150 | ALP, amino transferases, iron, LDH, potassium |
| Dietrich17 | 2013 | USA | ED | Not given | 2000 | Not given |
| Wollowitz et al.24 | 2013 | USA | ED | Roche Modular | 1500 | Not given |
| Brunel et al.46 | 2012 | France | ED | Roche Modular | 700 | High sensitivity troponin T |
| Carraro et al.21 * | 2012 | Italy | IP | Dimension EXL | 500 | Not given |
| Berg et al.47 | 2011 | UK | ED, IP | Roche Modular | 700 | Potassium |
| BergerAchituv et al.29 | 2010 | Israel | Paed. IP | Hitachi 747 | 510 | Chloride, glucose, potassium, sodium, urea |
| Munnix et al.37 | 2010 | Netherlands | ED, OP | Roche Modular | 3000 | Not given |
| Ellis31 | 2009 | UK | IP | Abbott Aeroset | Not given (HI ≥0.6) | Not given |
| Romero et al.62 | 2009 | Spain | OP | Dimension RXL | Not given | Not given |
| Saleem et al.38 | 2009 | UK | IP | Roche Modular | 500 | Not given |
| Soderberg et al.66 | 2009 | Sweden | ED, OP | Vitros 5,1 | 150 | Not given |
| Dwyer et al.34 | 2006 | Australia | ED | Beckman LX20 | Not given (Grade 2) | Not given |
| Sodi et al.6 | 2006 | UK | Not given | Roche P-module | 744 | Cardiac troponin T |
| Cox et al.22 * | 2004 | USA | ED | Hitachi | Not given | Not given |
IP – Inpatient, OP – Outpatient, ED – Emergency, ALT - alanine aminotransferase, AST - aspartate aminotransferase, CK - creatine kinase, ALP - alkaline phosphatise, GGT - γ-glutamyltransferase, LDH - lactate dehydrogenase; *indicates a combination of the Haemolysis Index and visual inspection used to detect haemolysis.
Three studies also reported the levels of plasma free haemoglobin at which test results would be rejected when using the HI. Bölenius et al.23 reported a rejection level of 500 mg/L for lactate dehydrogenase (LDH) and 1000 mg/L for potassium (K), alkaline phosphatase (ALP) and the amino transferases alanine transaminase (ALT) and aspartate transaminase (AST); Wollowitz et al.24 reported a rejection level of 1500 mg/L for K; and Sodi et al.6 reported that 744 mg/L was the level for both haemolysis detection and result rejection for troponin T specimens.
When detecting haemolysis by visual inspection, only four studies reported the plasma free haemoglobin level equivalent to the colour change used to detect a haemolysed specimen. In these four studies, the mean plasma free haemoglobin level used to detect haemolysis by visual inspection was 850±436 mg/L (Table 2). Six studies reported that they detected haemolysis based on a mild or pink colour (Table 2). While Dugan et al.25 was the only study reporting an equivalent plasma free haemoglobin level at which a specimen would be rejected (2000 mg/L), Atay et al.26 and Grant27 both reported that specimens were rejected if they appeared dark red. Two studies that used visual inspection distinguished between specimens detected as haemolysed and specimens that displayed a sufficient level of haemolysis to be rejected. Grant reported that while 32% of specimens showed some level of haemolysis, defined as mild discolouration on visual inspection, in only 13% of cases was the discolouration judged sufficient for the specimen to be rejected.27 Carraro et al. reported that while they found a haemolysis rate of 1.8%, 64% of these specimens had only a small degree of haemolysis (a plasma free haemoglobin level of <50 mg/L), 31% had an intermediate level of haemolysis and 5% had a high level of haemolysis (a plasma free haemoglobin level of >300 mg/L).21
Table 2.
Characteristics of studies detecting haemolysis rate by visual inspection.
| Author | Year | Country | Patient Type | Haemolysis Level (mg/L free Hb) | Analyte |
|---|---|---|---|---|---|
| Atay et al.26 | 2014 | Turkey | IP, OP | Not given (Mild colour)* | Not given |
| Giménez-Marin et al.44 | 2014 | Spain | ED, IP, OP | Not given | Not given |
| Grecu et al.45 | 2014 | Romania | Ed, IP, OP | Not given | Not given |
| Carraro et al.21 * | 2012 | Italy | IP | 500 | Not given |
| Stauss et al.28 | 2012 | USA | ED | 1400 | Coagulation |
| Straszewski et al.55 | 2011 | USA | ED | Not given | Potassium |
| Chawla et al.60 | 2010 | India | IP, OP | Not given | Not given |
| Goswami et al.61 | 2010 | India | IP | Not given | Not given |
| Shah et al.39 | 2009 | USA | ED | Not given (Pink colour) | Type and Screen’ |
| Fang et al.40 | 2008 | Taiwan | ED, IP | Not given (Mild colour) | Not given |
| Lowe et al.30 | 2008 | USA | ED | 500 | Not given |
| Salvagno et al.67 | 2008 | Italy | ED, IP | Not given | Coagulation |
| Lippi et al.68 | 2006 | Italy | IP, OP | Not given | Not given |
| Dugan et al.25 | 2005 | USA | ED | 1000 | Not given |
| Cox et al.22 * | 2004 | USA | ED | Not given | Not given |
| Grant27 | 2003 | USA | ED | Not given (Pink colour) | Not given |
| Tanabe et al.41 | 2003 | USA | ED & Labour | Not given (Pink colour) | Not given |
| Burns et al.49 | 2002 | USA | ED, IP | Not given | Not given |
| Carraro et al.50 | 2000 | Italy | ED, IP | Not given (Pink colour) | Not given |
IP – Inpatient, OP – Outpatient, ED – Emergency; (*indicates a combination of the Haemolysis Index and visual inspection used to detect haemolysis.)
Notably, only 16 studies reported the analyte(s) of interest (Tables 1, 2 and 3). Of the nine studies that investigated a range of analytes, no studies reported different levels of haemolysis detection for different analytes. This made it impossible to stratify studies by analyte.
Table 3.
Characteristics of studies detecting haemolysis rate by undefined means.
| Author | Year | Country | Patient Type | Analyte |
|---|---|---|---|---|
| OrtellsAbuye et al.32 | 2014 | Spain | IP | Not given |
| Sinici Lay et al.58 | 2014 | Turkey | IP, OP | Amylase, AST, calcium, cholesterol, creatinine, CK, glucose, potassium, sodium, urea |
| Tóth et al.69 | 2014 | Hungary | Not given | Not given |
| Upreti et al.59 | 2013 | India | IP, OP | Not given |
| Bhat et al.53 | 2012 | India | IP | Not given |
| Ashakiran et al.54 | 2011 | India | Not given | Not given |
| Ong et al.33 | 2009 | Singapore | ED | Electrolyte, urea |
| Alsina et al.48 | 2008 | Spain | ED, IP, OP | Not given |
| Ong et al.42 | 2008 | Singapore | ED | Electrolyte, urea |
| Pretlow et al.56 | 2008 | USA | ED, IP | Not given |
| Stark et al.70 | 2007 | USA | ED, IP, OP | Not given |
| Fernandes et al.43 | 2004 | Canada | ED | Haemoglobin, potassium |
| Bonini et al.10 | 2002 | Italy | IP, OP | Not given |
IP – Inpatient, OP – Outpatient, ED – Emergency, AST - aspartate aminotransferase, CK - creatine kinase
Study Designs, Specimens and Settings
Thirty-nine (78%) of the 50 studies were retrospective or observational cohort studies. Of the remaining studies, three (6%) were comparative,28–30 three (6%) were randomised crossover studies,22,31,32 two (4%) were crossover studies23,33 and two (4%) were randomised controlled trials.34,35 Three (6%) of the studies reported mean haemolysis rates calculated from fewer than 100 specimens29,35,36 and 16 (32%) studies reported mean haemolysis rates calculated from fewer than 1000 specimens.25,27–30,32,33,35–43 Eighteen (36%) studies provided no information about the study setting,6,10,17,23,27,29,35,37,39,41,42,44–50 with a further seven (14%) studies providing only limited data about the study setting, such as providing only the hospital name.40,51–56 Seventeen studies (36%) employed no statistical analysis.10,17,37,39,46,48,50,51,53,54,56–62
Discussion
This review identified inconsistency in haemolysis detection and reporting. While there was consistency in the mean plasma free haemoglobin levels used to detect haemolysis using either the HI or visual inspection, with a mean haemoglobin level of 846 mg/L for HI and 850 mg/L for visual, there was large variation in the mean plasma free haemoglobin levels used to detect haemolysis within each technique. This variation was evident even when studies were using the same equipment.
When considering inconsistency in haemolysis detection it is important to note that the effect of haemolysis interference on test results depends on the analyte being tested, with specimens that are regarded as being haemolysed for a certain analyte, such as K, potentially demonstrating a level of haemolysis that does not impact upon other analytes, such as ALT.19 The fact that only 16 of the studies included in this review stated the analyte of interest meant that it was not possible to stratify the mean plasma free haemoglobin levels used to detect haemolysis by this factor. However, the mean plasma free haemoglobin used to detect haemolysis using both the HI and visual inspection is within 100 mg/L of the value of 750 mg/L at which Koseoglu et al.19 found a statistically significant variation in ALP, amylase, AST, bilirubin, cholesterol, creatine kinase, glucose, LDH, phosphorus, K and triglycerides due to haemolysis, and within 250 mg/L of the value of 600 mg/L at which Lippi et al.63 found clinically meaningful variations in AST, chloride, LDH, K and sodium (Na) due to haemolysis. Therefore, this presents the possibility that a standard plasma free haemoglobin level, such as the level of 750 mg/L identified by Koseoglu et al.,19 could be used to determine haemolysis for a range of common laboratory tests.
While this article agrees with both Dolci and Panteghini14 and Lippi et al.15 who have called for greater consistency of haemolysis detection, it must also be considered that site-specific variation, particularly pertaining to the equipment available at each laboratory and the patient mix at each facility, may currently make this difficult to achieve. This is exacerbated by the fact that different manufacturers report HI in different ways, with some systems reporting HI as plasma free haemoglobin equivalents in mg/dL while others report it in g/L. Further, the methods and algorithms used to determine the HI are manufacturer- and, frequently, instrument-specific and are generally not in the public domain. While most of the devices used in the articles included in this review are multi-wavelength spectrophotometric measures, the actual wavelengths and coefficients used to determine the HI are proprietary and not freely available. It must also be considered that the HI is only an estimate of plasma free haemoglobin. Despite these drawbacks, some photometric methods for plasma free haemoglobin have been shown to be safer, easier and more accurate than chemical methods,64 with satisfactory consistency of HI observed amongst different analytical platforms.15,52 Therefore, while not a panacea, the adaption of technical standards, quality practices and benchmark data generated to account for the nuances of specific facilities, or groups of facilities, may be achievable at this time.
One concerning outcome of this review is that, despite haemolysis rates being a primary outcome measure in all of the studies, 26% of the studies did not state the method of haemolysis detection. While it can be assumed that haemolysis was detected using either the HI or visual inspection, such information is essential for establishing future benchmark data which can be used to develop safe and quality practices to reduce haemolysis rates. A number of other factors such as patient location (e.g. emergency department, inpatient ward), qualifications and skills of specimen collection staff, day-of-week and time-of-day of the collection, patient demographics and phlebotomy methods may also affect haemolysis rates. While further study is required to explore the effect of these factors on haemolysis rates, these factors must be considered in tandem with the methods used to detect and report haemolysis in future studies that aim to generate benchmark data of haemolysis.
While generation of useful benchmark data of haemolysis rates relies on well documented and reproducible methods for the detection of haemolysis, creation of benchmarks and frameworks that can be used to monitor and reduce rates of haemolysis also rely on the scientific rigour of studies in this area. This review found that 32% of studies reported haemolysis rates calculated from fewer than 1000 specimens and that 36% of studies employed no statistical analysis. Such low sample sizes and lack of statistical analysis mean that caution must be applied when interpreting the results from these individual studies and using the data for benchmarking. Furthermore, half of the studies included in this narrative review provided limited or no contextual data relating to the study setting. Such information is essential to determine the generalisability of the results.
While this is not a systematic review, the search strategy was broad and included a search for ‘grey literature’ (research output produced by organisations outside of commercial or academic publishing and distribution channels) from a variety of sources.65 As outlined in the methods section, such a search strategy was used due to the known heterogeneity in haemolysis detection and reporting, inconsistency in the detection of a haemolysed specimen and the relative immaturity of the field, while minimising the risk of publication bias.
Conclusion
While haemolysis continues to pose a significant problem for clinical laboratories, this narrative review demonstrates that there is inconsistency in the way haemolysis is detected and reported in individual studies, indicating a potential variation in practice between individual laboratories. There was found to be large interstudy variability in both the method and plasma free haemoglobin level used to detect haemolysis. Furthermore, few studies reported either the analyte of concern or plasma free haemoglobin level at which a specimen would be rejected, with large variation in study design. To improve the consistency of haemolysis detection and reporting, we recommend that laboratories strive to: a preferance for analysers that conduct a spectrophotometric assessment of the plasma free haemoglobin to detect haemolysis; provide a clear statement of the units of measure (mg/L preferred) and the analyte of interest; and perform an analyte-specific assessment for determination of significance and provide a clear statement of the plasma free haemoglobin level at which a specimen would be rejected. A future move to improve the consistency of haemolysis detection and reporting between laboratories will facilitate the generation of reliable benchmark data of haemolysis rates. This benchmark data could be used to support the design and testing of quality practices that monitor and reduce haemolysis, improving the safety and efficiency of laboratory processes and patient care.
Acknowledgments
This project was funded by the Royal College of Pathologists Australasia Quality Assurance Program (RCPAQAP) and a National Health and Medical Research Council (NHMRC) Program Grant.
Footnotes
Competing Interests: None declared.
References
- 1.Franco RS. The measurement and importance of red cell survival. Am J Hematol. 2009;84:109–14. doi: 10.1002/ajh.21298. [DOI] [PubMed] [Google Scholar]
- 2.Carraro P, Plebani M. Errors in a stat laboratory: types and frequencies 10 years later. Clin Chem. 2007;53:1338–42. doi: 10.1373/clinchem.2007.088344. [DOI] [PubMed] [Google Scholar]
- 3.Lippi G, Blanckaert N, Bonini P, Green S, Kitchen S, Palicka V, et al. Haemolysis: an overview of the leading cause of unsuitable specimens in clinical laboratories. Clin Chem Lab Med. 2008;46:764–72. doi: 10.1515/CCLM.2008.170. [DOI] [PubMed] [Google Scholar]
- 4.Šimundić A-M, Topic E, Nikolac N, Lippi G. Hemolysis detection and management of hemolyzed specimens. Biochem Med. 2010;20:154–9. [Google Scholar]
- 5.Lippi G, Plebani M, Di Somma S, Cervellin G. Hemolyzed specimens: a major challenge for emergency departments and clinical laboratories. Crit Rev Clin Lab Sci. 2011;48:143–53. doi: 10.3109/10408363.2011.600228. [DOI] [PubMed] [Google Scholar]
- 6.Sodi R, Darn SM, Davison AS, Stott A, Shenkin A. Mechanism of interference by haemolysis in the cardiac troponin T immunoassay. Ann Clin Biochem. 2006;43:49–56. doi: 10.1258/000456306775141687. [DOI] [PubMed] [Google Scholar]
- 7.Green SF. The cost of poor blood specimen quality and errors in preanalytical processes. Clin Biochem. 2013;46:1175–9. doi: 10.1016/j.clinbiochem.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Kim JY, Kamis IK, Singh B, Batra S, Dixon RH, Dighe AS. Implementation of computerized add-on testing for hospitalized patients in a large academic medical center. Clin Chem Lab Med. 2011;49:845–50. doi: 10.1515/CCLM.2011.140. [DOI] [PubMed] [Google Scholar]
- 9.Georgiou A, Williamson M, Westbrook JI, Ray S. The impact of computerised physician order entry systems on pathology services: a systematic review. Int J Med Inform. 2007;76:514–29. doi: 10.1016/j.ijmedinf.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Bonini P, Plebani M, Ceriotti F, Rubboli F. Errors in laboratory medicine. Clin Chem. 2002;48:691–8. [PubMed] [Google Scholar]
- 11.Lippi G, Guidi GC, Mattiuzzi C, Plebani M. Preanalytical variability: the dark side of the moon in laboratory testing. Clin Chem Lab Med. 2006;44:358–65. doi: 10.1515/CCLM.2006.073. [DOI] [PubMed] [Google Scholar]
- 12.Foley M, Kifaieh N, Mallon WK. Financial impact of emergency department crowding. West J Emerg Med. 2011;12:192–7. [PMC free article] [PubMed] [Google Scholar]
- 13.Plebani M, Astion ML, Barth JH, Chen W, de Oliveira Galoro CA, Escuer MI, et al. Harmonization of quality indicators in laboratory medicine. A preliminary consensus. Clin Chem Lab Med. 2014;52:951–8. doi: 10.1515/cclm-2014-0142. [DOI] [PubMed] [Google Scholar]
- 14.Dolci A, Panteghini M. Harmonization of automated hemolysis index assessment and use: Is it possible? Clin Chim Acta. 2014;432:38–43. doi: 10.1016/j.cca.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Lippi G, Luca Salvagno G, Blanckaert N, Giavarina D, Green S, Kitchen S, et al. Multicenter evaluation of the hemolysis index in automated clinical chemistry systems. Clin Chem Lab Med. 2009;47:934–9. doi: 10.1515/CCLM.2009.218. [DOI] [PubMed] [Google Scholar]
- 16.Šimundić A-M, Nikolac N, Ivankovic V, Ferenec-Ruzic D, Magdic B, Kvaternik M, et al. Comparison of visual vs. automated detection of lipemic, icteric and hemolyzed specimens: can we rely on a human eye? Clin Chem Lab Med. 2009;47:1361–5. doi: 10.1515/CCLM.2009.306. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich H. One poke or two: can intravenous catheters provide an acceptable blood sample? A data set presentation, review of previous data sets, and discussion. J Emerg Nurs. 2014;40:575–8. doi: 10.1016/j.jen.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Lippi G, Cervellin G, Favaloro EJ, Plebani M. In Vitro and In Vivo Hemolysis - An Unresolved Dispute in Laboratory Medicine. Germany: De Gruyter; 2012. [Google Scholar]
- 19.Koseoglu M, Hur A, Atay A, Cuhadar S. Effects of hemolysis interferences on routine biochemistry parameters. Biochem Med (Zagreb) 2011;21:79–85. doi: 10.11613/bm.2011.015. [DOI] [PubMed] [Google Scholar]
- 20.Errico MK, Iovane B, Bernardini A, Gliati D, Scarabello C, Fainardi V, et al. Haemolysis during diabetic ketoacidosis treatment in two girls with incomplete glucose-6-phosphate dehydrogenase deficiency. Acta Biomed. 2009;80:69–72. [PubMed] [Google Scholar]
- 21.Carraro P, Zago T, Plebani M. Exploring the initial steps of the testing process: frequency and nature of prepreanalytic errors. Clin Chem. 2012;58:638–42. doi: 10.1373/clinchem.2011.175711. [DOI] [PubMed] [Google Scholar]
- 22.Cox SR, Dages JH, Jarjoura D, Hazelett S. Blood samples drawn from IV catheters have less hemolysis when 5-mL (vs 10-mL) collection tubes are used. J Emerg Nurs. 2004;30:529–33. doi: 10.1016/j.jen.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Bölenius K, Söderberg J, Hultdin J, Lindkvist M, Brulin C, Grankvist K. Minor improvement of venous blood specimen collection practices in primary health care after a large-scale educational intervention. Clin Chem Lab Med. 2013;51:303–10. doi: 10.1515/cclm-2012-0159. [DOI] [PubMed] [Google Scholar]
- 24.Wollowitz A, Bijur PE, Esses D, John Gallagher E. Use of butterfly needles to draw blood is independently associated with marked reduction in hemolysis compared to intravenous catheter. Acad Emerg Med. 2013;20:1151–5. doi: 10.1111/acem.12245. [DOI] [PubMed] [Google Scholar]
- 25.Dugan L, Leech L, Speroni KG, Corriher J. Factors affecting hemolysis rates in blood samples drawn from newly placed IV sites in the emergency department. J Emerg Nurs. 2005;31:338–45. doi: 10.1016/j.jen.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Atay A, Demir L, Cuhadar S, Saglam G, Unal H, Aksun S, et al. Clinical biochemistry laboratory rejection rates due to various types of preanalytical errors. Biochem Med (Zagreb) 2014;24:376–82. doi: 10.11613/BM.2014.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant MS. The effect of blood drawing techniques and equipment on the hemolysis of ED laboratory blood samples. J Emerg Nurs. 2003;29:116–21. doi: 10.1067/men.2003.66. [DOI] [PubMed] [Google Scholar]
- 28.Stauss M, Sherman B, Pugh L, Parone D, Looby-Rodriguez K, Bell A, et al. Hemolysis of coagulation specimens: a comparative study of intravenous draw methods. J Emerg Nurs. 2012;38:15–21. doi: 10.1016/j.jen.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Berger-Achituv S, Budde-Schwartzman B, Ellis MH, Shenkman Z, Erez I. Blood sampling through peripheral venous catheters is reliable for selected basic analytes in children. Pediatrics. 2010;126:e179–86. doi: 10.1542/peds.2009-2920. [DOI] [PubMed] [Google Scholar]
- 30.Lowe G, Stike R, Pollack M, Bosley J, OBrien P, Hake A, et al. Nursing blood specimen collection techniques and hemolysis rates in an emergency department: analysis of venipuncture versus intravenous catheter collection techniques. J Emerg Nurs. 2008;34:26–32. doi: 10.1016/j.jen.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Ellis G. An episode of increased hemolysis due to a defective pneumatic air tube delivery system. Clin Biochem. 2009;42:1265–9. doi: 10.1016/j.clinbiochem.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Ortells-Abuye N, Busquets-Puigdevall T, Díaz-Bergara M, Paguina-Marcos M, Sánchez-Pérez I. A cross-sectional study to compare two blood collection methods: direct venous puncture and peripheral venous catheter. BMJ Open. 2014;4:e004250. doi: 10.1136/bmjopen-2013-004250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ong ME, Chan YH, Lim CS. Reducing blood sample hemolysis at a tertiary hospital emergency department. Am J Med. 2009;122:1054.e1–6. doi: 10.1016/j.amjmed.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 34.Dwyer DG, Fry M, Somerville A, Holdgate A. Randomized, single blinded control trial comparing haemolysis rate between two cannula aspiration techniques. Emerg Med Australas. 2006;18:484–8. doi: 10.1111/j.1742-6723.2006.00895.x. [DOI] [PubMed] [Google Scholar]
- 35.Kara H, Bayir A, Ak A, Degirmenci S, Akinci M, Agacayak A, et al. Hemolysis associated with pneumatic tube system transport for blood samples. Pak J Med Sci. 2014;30:50–8. doi: 10.12669/pjms.301.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lippi G, Avanzini P, Aloe R, Cervellin G. Blood collection from intravenous lines: is one drawing site better than others? Lab Med. 2014;45:172–5. doi: 10.1309/lm2xcv5sqml1ontm. [DOI] [PubMed] [Google Scholar]
- 37.Munnix IC, Schellart M, Gorissen C, Kleinveld HA. Factors reducing hemolysis rates in blood samples from the emergency department. Clin Chem Lab Med. 2011;49:157–8. doi: 10.1515/CCLM.2011.012. [DOI] [PubMed] [Google Scholar]
- 38.Saleem S, Mani V, Chadwick MA, Creanor S, Ayling RM. A prospective study of causes of haemolysis during venepuncture: tourniquet time should be kept to a minimum. Ann Clin Biochem. 2009;46:244–6. doi: 10.1258/acb.2009.008228. [DOI] [PubMed] [Google Scholar]
- 39.Shah KG, Idrovo JP, Nicastro J, McMullen HF, Molmenti EP, Coppa G. A retrospective analysis of the incidence of hemolysis in type and screen specimens from trauma patients. Int J Angiol. 2009;18:182–3. doi: 10.1055/s-0031-1278350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang L, Fang S-H, Chung Y-H, Chien S-T. Collecting factors related to the haemolysis of blood specimens. J Clin Nurs. 2008;17:2343–51. doi: 10.1111/j.1365-2702.2006.02057.x. [DOI] [PubMed] [Google Scholar]
- 41.Tanabe P, Kyriacou DN, Garland F. Factors affecting the risk of blood bank specimen hemolysis. Acad Emerg Med. 2003;10:897–900. doi: 10.1111/j.1553-2712.2003.tb00637.x. [DOI] [PubMed] [Google Scholar]
- 42.Ong ME, Chan YH, Lim CS. Observational study to determine factors associated with blood sample haemolysis in the emergency department. Ann Acad Med Singapore. 2008;37:745–8. [PubMed] [Google Scholar]
- 43.Fernandes CM, Worster A, Hill S, McCallum C, Eva K. Root cause analysis of laboratory turnaround times for patients in the emergency department. CJEM. 2004;6:116–22. doi: 10.1017/s1481803500009088. [DOI] [PubMed] [Google Scholar]
- 44.Giménez-Marín A, Rivas-Ruiz F, Pérez-Hidalgo MDELM, Molina-Mendoza P. Pre-analytical errors management in the clinical laboratory: a five-year study. Biochem Med (Zagreb) 2014;24:248–57. doi: 10.11613/BM.2014.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grecu DS, Vlad DC, Dumitrascu V. Quality indicators in the preanalytical phase of testing in a stat laboratory. Lab Med. 2014;45:74–81. doi: 10.1309/lm9zy92ybzrfpfqy. [DOI] [PubMed] [Google Scholar]
- 46.Brunel V, Larson T, Peschanski N, Cauliez B. Evaluation of haemolysis in emergency department samples requesting high sensitivity troponin T measurement. Ann Clin Biochem. 2012;49:509–10. doi: 10.1258/acb.2012.012032. [DOI] [PubMed] [Google Scholar]
- 47.Berg JE, Ahee P, Berg JD. Variation in phlebotomy techniques in emergency medicine and the incidence of haemolysed samples. Ann Clin Biochem. 2011;48:562–5. doi: 10.1258/acb.2011.011099. [DOI] [PubMed] [Google Scholar]
- 48.Alsina MJ, Alvarez V, Barba N, Bullich S, Cortés M, Escoda I, et al. Preanalytical quality control program -an overview of results (20012005 summary) Clin Chem Lab Med. 2008;46:849–54. doi: 10.1515/CCLM.2008.168. [DOI] [PubMed] [Google Scholar]
- 49.Burns ER, Yoshikawa N. Hemolysis in serum samples drawn by emergency department personnel versus laboratory phlebotomists. Lab Med. 2002;33:378–80. [Google Scholar]
- 50.Carraro P, Servidio G, Plebani M. Hemolyzed specimens: a reason for rejection or a clinical challenge? Clin Chem. 2000;46:306–7. [PubMed] [Google Scholar]
- 51.Davidson DF. A survey of some pre-analytical errors identified from the Biochemistry Department of a Scottish hospital. Scott Med J. 2014;59:91–4. doi: 10.1177/0036933014529056. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez P, Llopis MA, Perich C, Alsina MJ, Alvarez V, Biosca C, et al. Harmonization in hemolysis detection and prevention. A working group of the Catalonian Health Institute (ICS) experience. Clin Chem Lab Med. 2014;52:1557–68. doi: 10.1515/cclm-2013-0935. [DOI] [PubMed] [Google Scholar]
- 53.Bhat V, Tiwari M, Chavan P, Kelkar R. Analysis of laboratory sample rejections in the pre-analytical stage at an oncology center. Clin Chim Acta. 2012;413:1203–6. doi: 10.1016/j.cca.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 54.Ashakiran S, Sumati ME, Murthy NK. A study of pre-analytical variables in clinical biochemistry laboratory. Clin Biochem. 2011;44:944–5. doi: 10.1016/j.clinbiochem.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Straszewski SM, Sanchez L, McGillicuddy D, Boyd K, Dufresne J, Joyce N, et al. Use of separate venipunctures for IV access and laboratory studies decreases hemolysis rates. Intern Emerg Med. 2011;6:357–9. doi: 10.1007/s11739-011-0568-9. [DOI] [PubMed] [Google Scholar]
- 56.Pretlow L, Gandy T, Leibach EK, Russell B, Kraj B. A quality improvement cycle: hemolyzed specimens in the emergency department. Clin Lab Sci. 2008;21:219–24. [PubMed] [Google Scholar]
- 57.Lippi G, Bonelli P, Cervellin G. Prevalence and cost of hemolyzed samples in a large urban emergency department. Int J Lab Hematol. 2014;36:e24–6. doi: 10.1111/ijlh.12135. [DOI] [PubMed] [Google Scholar]
- 58.Sinici Lay I, Pınar A, Akbıyık F. Classification of reasons for rejection of biological specimens based on prepreanalytical processes to identify quality indicators at a university hospital clinical laboratory in Turkey. Clin Biochem. 2014;47:1002–5. doi: 10.1016/j.clinbiochem.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 59.Upreti S, Upreti S, Bansal R, Jeelani N, Bharat V. Types and frequency of preanalytical errors in haematology lab. J Clin Diagn Res. 2013;7:2491–3. doi: 10.7860/JCDR/2013/6399.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chawla R, Goswami B, Tayal D, Mallika V. Identification of the types of preanalytical errors in the clinical chemistry laboratory: 1-Year study at G.B. Pant Hospital. Lab Med. 2010;41:89–92. [Google Scholar]
- 61.Goswami B, Singh B, Chawla R, Mallika V. Evaluation of errors in a clinical laboratory: a one-year experience. Clin Chem Lab Med. 2010;48:63–6. doi: 10.1515/CCLM.2010.006. [DOI] [PubMed] [Google Scholar]
- 62.Romero A, Cobos A, López-León A, Ortega G, Muñoz M. Preanalytical mistakes in samples from primary care patients. Clin Chem Lab Med. 2009;47:1549–52. doi: 10.1515/CCLM.2009.338. [DOI] [PubMed] [Google Scholar]
- 63.Lippi G, Salvagno GL, Montagnana M, Brocco G, Guidi GC. Influence of hemolysis on routine clinical chemistry testing. Clin Chem Lab Med. 2006;44:311–6. doi: 10.1515/CCLM.2006.054. [DOI] [PubMed] [Google Scholar]
- 64.Malinauskas RA. Plasma hemoglobin measurement techniques for the in vitro evaluation of blood damage caused by medical devices. Artif Organs. 1997;21:1255–67. doi: 10.1111/j.1525-1594.1997.tb00486.x. [DOI] [PubMed] [Google Scholar]
- 65.Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, et al. Dissemination and publication of research findings: an updated review of related biases. Health Technol Assess. 2010;14:iii, ix–xi, 1–193. doi: 10.3310/hta14080. [DOI] [PubMed] [Google Scholar]
- 66.Söderberg J, Jonsson PA, Wallin O, Grankvist K, Hultdin J. Haemolysis indexan estimate of preanalytical quality in primary health care. Clin Chem Lab Med. 2009;47:940–4. doi: 10.1515/CCLM.2009.227. [DOI] [PubMed] [Google Scholar]
- 67.Salvagno GL, Lippi G, Bassi A, Poli G, Guidi GC. Prevalence and type of pre-analytical problems for inpatients samples in coagulation laboratory. J Eval Clin Pract. 2008;14:351–3. doi: 10.1111/j.1365-2753.2007.00875.x. [DOI] [PubMed] [Google Scholar]
- 68.Lippi G, Bassi A, Brocco G, Montagnana M, Salvagno GL, Guidi GC. Preanalytic error tracking in a laboratory medicine department: results of a 1-year experience. Clin Chem. 2006;52:1442–3. doi: 10.1373/clinchem.2006.069534. [DOI] [PubMed] [Google Scholar]
- 69.Tóth J, Lenkey Á, V Oláh A, Köteles J, Kissné Sziráki V, Kerényi A, et al. [Pneumatic tube system for transport of laboratory samples: preanalytical aspects]. (English synopsis provided by Tóth, J. via personal communication: September 2014) Orv Hetil. 2014;155:1113–20. doi: 10.1556/OH.2014.29895. [DOI] [PubMed] [Google Scholar]
- 70.Stark A, Jones BA, Chapman D, Well K, Krajenta R, Meier FA, et al. Clinical laboratory specimen rejectionassociation with the site of patient care and patients characteristics: findings from a single health care organization. Arch Pathol Lab Med. 2007;131:588–92. doi: 10.5858/2007-131-588-CLSRWT. [DOI] [PubMed] [Google Scholar]