Abstract
Acute kidney injury (AKI) is a significant independent risk factor for morbidity and mortality. In the last ten years a large number of publications have highlighted the limitations of traditional approaches and the inadequacies of conventional biomarkers to diagnose and monitor renal insufficiency in the acute setting. A great effort was directed not only to the discovery and validation of new biomarkers aimed to detect AKI more accurately but also to standardise the definition of AKI. Despite the advances in both areas, biomarkers have not yet entered into routine clinical practice and the definition of this syndrome has many areas of uncertainty. This review will discuss the controversies in diagnosis and the potential of novel biomarkers to improve the definition of the syndrome.
Introduction
Currently diagnostic approaches to AKI include careful history and thorough physical examination of the patient. Laboratory evaluation includes the measurement of serum creatinine (sCr), urea, and electrolytes. Urine analysis and microscopic examination as well as urinary chemistries may be helpful in determining the underlying cause of AKI. Imaging tests, especially ultrasound, are important components of the evaluation for patients with AKI.1,2 The use of an accurate definition of the syndrome that can be applied in clinical practice and in research is required in order for AKI to be diagnosed and staged early. Such definition might also enable differential diagnosis and help the physician in the selection of therapy. This ideal definition therefore should describe the syndrome and the course of a patient and correlate with the functional and structural changes of the kidneys during the course of the disease. It is obvious that such a definition would include measurement of biochemical markers. Although a number of biomarkers of functional change and cellular damage are under evaluation for early diagnosis, risk assessment and prognosis of AKI, the current definitions in use (which are consensus definitions), include only the measurement of sCr and urinary output (UO) and they are far from being ideal.3–5 Moreover the lack of a true gold standard in the diagnosis of AKI means that we don’t have any data on the sensitivity and specificity of a creatinine based definition of AKI.
These definitions may have helped clinicians to establish AKI diagnosis and staging, while urine examination can help in differential diagnosis. However after many years of clinical utilisation of these definitions, several limitations have been revealed. In the second part of our review on AKI we explore the limitations of these definitions and their impact on diagnosis and staging of the syndrome. We also explore the current status of established and emerging biomarkers and how they can be used to further refine the definition of AKI. We finally discuss the links of AKI with CKD.
Serum Creatinine and the Measurement of Kidney Function
GFR is considered the best indicator of overall kidney function and its assessment is an important clinical tool in the care of patients. Assessment of GFR can aid the clinician in measuring the degree of renal dysfunction, progression of established kidney disease, or both and for drug dosing.6,7 However, it does not give information on the cause of kidney disease, therefore it is important to interpret the GFR in the context of the clinical setting. GFR cannot be measured directly, but instead it can be assessed by the renal clearance of a marker that achieves stable plasma concentration, is inert, and is freely filtered by the glomeruli but not reabsorbed, secreted, or metabolised.6,8
Such an ideal endogenous marker does not exist. SCr has been used for many years as a marker of renal function in both AKI and CKD.3–5,9 Creatinine is formed from creatine in the muscles, has a molecular weight of 113 Da, and fulfils most of the requirements for a filtration marker. It is freely filtered at the glomerulus, it is not metabolised by the kidney, it is not bound to any protein and is non-toxic.10,11 It is completely cleared by renal excretion when renal function is normal. The proximal tubules secrete creatinine, which accounts for 10–20% of the excreted load, and results in overestimation of GFR when measured by creatinine clearance (CrCl).11,12 When GFR is reduced, the contribution of tubular creatinine secretion increases and may reach 50% of total CrCl, but it is highly variable among individuals.11 Tubular reabsorption is less important than secretion and appears later in the evolution of the CKD in patients with already significant alteration in urinary flow in some clinical settings such as decompensated heart failure and uncontrolled diabetes.11 Since there is little to no tubular reabsorption of creatinine, its renal clearance is often used to estimate GFR. Under stable kidney function sCr concentration can also reflect skeletal muscle mass if its non-muscle-mass-dependent variations (such as due to renal function or meat intake) can be accurately accounted for.13,14 In people with stable kidney function and UO, a 24 h urine creatinine (uCr) is usually a constant number based on skeletal muscle mass and any variation observed is due to changes in meat consumption.13,14 Given the fact that sCr co-varies closely with skeletal muscle mass, its utility in estimating GFR using equations such as MDRD or CKDEPI may not be appropriate when subjects exhibit weight variations during follow up as in the case of critically ill patients. Muscle loss might be misinterpreted as improvement of renal function.13,15 In a recent study Hoste et al. studied critically ill patients admitted to ICU with sCr levels within the normal range and found that 25% of these patients had CrCl <60 ml/min/1.73m2. UCr excretion was low in patients with low CrCl, suggesting a pronounced muscle loss and depressed production of creatinine.16
It is important to note that the isolated use of sCr concentration may not reflect the actual degree of kidney function of a certain patient. This is because multiple factors affect the concentration of sCr and because the inverse relationship between sCr and GFR is nonlinear, especially in patients with near-normal renal function. Measurement of CrCl can provide adequate clinical information under steady state conditions, but has two serious limitations: the difficulty to obtain accurate urine collections and the potential misinterpretation because of the large biologic variability of creatinine metabolism in various clinical settings, including the unpredictable level of creatinine secretion at different levels of GFR. At low GFR levels tubular secretion of creatinine occurs and therefore CrCl may overestimate GFR. Moreover the 24 h collection is not a useful time-frame for detecting AKI.17 Brief collections (2–8 h) have shown good correlation with 24 h collections and are better than sCr in detecting changes in GFR.18,19 Estimation of GFR by using creatinine-based mathematical equations is an alternative. These models are based on the inverse relationship of sCr with GFR, along with adjustment factors for measurable determinants of sCr concentration (e.g. age, sex, body size, race).20,21 More accurate methods of GFR measurement, such as the clearance of exogenous markers (i.e. inulin, iohexol or renally excreted isotopes), are expensive and usually not available for routine use. More novel serum measurements of analytes, such as cystatin C, are under investigation and are not yet fully validated in all clinical settings.22 Although determination of GFR with sCr measurement is adequate for the estimation of renal function in patients with stable CKD, it performs poorly in the setting of AKI for many reasons.
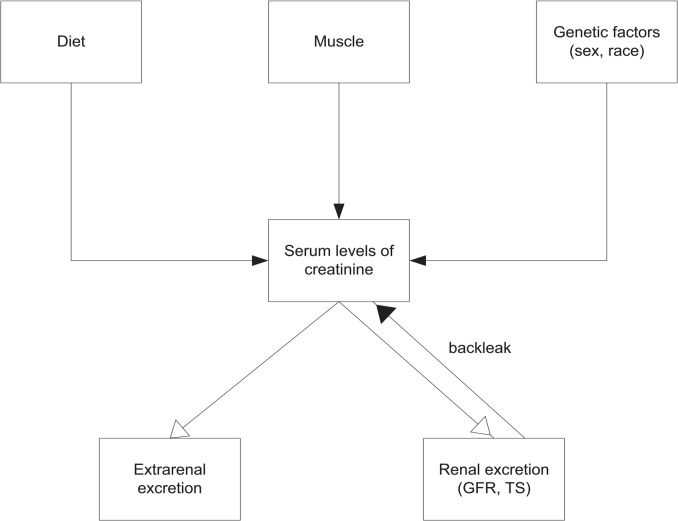
Evaluation of sCr changes present specific problems, which are related to its nature as a molecular marker and its kinetics. Creatinine is not an ideal molecular marker for the diagnosis of AKI. It is not specific for kidney injury, but rather a kidney function biomarker. There are many renal and non-renal factors that influence sCr levels in the blood and its secretion from the kidneys (Figure 1). Its production is proportional to muscle mass. While creatinine in muscle is produced at a constant rate its level in the blood can be elevated during catabolic states such as rhabdomyolysis. Age, sex, race, various drugs, diet, malnutrition, oedematous states, fluid overload, critical illness and sepsis can affect creatinine levels.
Figure 1.
Factors affecting serum creatinine levels
The sCr levels do not depend only on urinary clearance and on the rate of production but also on the volume of distribution. Aggressive fluid administration may dilute creatinine in the blood. Moran et al. demonstrated the effect of fluid accumulation on sCr concentrations and showed that increasing the total body water alters the volume of distribution of sCr, resulting in potential overestimation of the level of kidney function.23 More recently Macedo et al. in a study involving critically ill patients, showed that the dilution of sCr by fluid accumulation may lead to underestimation of the severity of AKI and increases the time required for AKI recognition.24 They suggested the use of an adjusted creatinine on the basis of fluid balance.24 More recently Pickering et al. proposed a model that combined volume and creatinine kinetics to assess changes in renal function. This model also takes into account fluid type, the rate of fluid infusion and urine output.25
Another drawback of sCr is that up to 50% of kidney function may be lost before any detectable rise of sCr, because of the large renal reserve. In addition, the half-life of creatinine is quite long and its blood serum variations over time are slow.26 The average biological half-life of sCr in healthy male adults between 20 and 39 years old is estimated to be 3.85 h. However this half-life is prolonged in patients with renal disease and becomes 77 h when renal function decreases to 5% of normal.27 SCr does not accurately show kidney function until steady state has been achieved. Chiou et al. also showed that the time required to reach a new steady-state sCr level after onset of renal failure is highly dependent upon the degree of renal insufficiency and it was estimated that it will take 1.1, 2.5, 6.7, and 13.4 days to reach 95% of the steady-state levels when the renal function drops to 50, 25, 10, and 5% of the normal capacity.27 When a non-steady state condition of renal function is involved, estimated GFR (eGFR) is of little utility in the diagnosis of AKI.15,28 SCr may take several days to reach a new steady state value after GFR has changed. Different forms of Renal Replacement Therapy (RRT) also affect the levels of sCr and its concentration during RRT may not reflect the actual level of renal function. Many factors interfere with laboratory assays and give false elevated (hyperglycaemia and hyperproteinaemia) or false reduced results (hyperbilirubinaemia and haemolysis)
The Relevance of Small Changes in sCr and the Impact of Biological and Analytical Variability
Current consensus definitions require small changes in sCr (26.5 µmol/L or 0.3 mg/dL or 50% baseline to peak) for diagnosis of AKI. These definitions do not take into account the magnitude of baseline creatinine value, the intra-individual biological variation of sCr and the numerous factors that interfere with its laboratory measurement (bias from IDMS reference method, analytical imprecision, interferences especially in Jaffé methods).
In the literature there are studies that demonstrate that even smaller changes (i.e. 8.8 µmol/L or 0.1 mg/dL or 1%–24%, baseline to peak) in patients, are independently associated with a 45% increase of ESRD or a two-fold risk for CKD.29–32 These associations of such small changes with adverse outcome in published studies may reveal the ability of confounders and not AKI to influence the development of CKD. Moreover there are questions beyond the association of small changes and outcome. The first question is how these minimal changes are defined. In most cases the definition is arbitrary and not uniform across all studies. In the study of Lassnigg et al. a minimal increase is considered any increase above 0 and below 44.2 µmol/L (0.5 mg/dL) and a significant increase is considered any increase above 44.2 µmol/L (0.5 mg/dL).30 In the study of Ishani et al. the magnitude of sCr increase was defined by the percent change from baseline to peak sCr levels after cardiac surgery and categorised as none (≤0%) or as class I, (1%–24%), II (25%–49%), III (50%–99%), or IV (≥100%).32 Another important question is whether these changes represent true changes in a patient’s health status or just random variation. The answer is not so straightforward. These studies do not take into account the variability in sCr measurement and its relevance to AKI. These small changes in sCr do not always reflect true changes in renal function as may be within the limits of the combined analytical and biological variation. It depends also on the baseline sCr of a specific patient.
It is possible to calculate objectively the change in sCr that represents a “true change in a patient’s health status”. This can be calculated by the reference change value: RCV = 21/2 * Z * (CVA2 + CVI2)1/2 where CVA is the analytical coefficient of variation, CVI = within subject biological variation and when Z = 1.96 then a change in any direction (2 tailed) to the RCV is “significant” at 95% probability.33 An optimal CVA is the half of the CVI. The CVI for sCr (taken from Ricos’ database) is 5.9% for healthy people and it is the median from 28 published studies.34,35 (See also supplemental data.)
Table 1 shows the RCVs that can be estimated for different baseline sCrs. The relatively small rises of sCr (26.5 µmol/L or 0.3 mg/dL) that is now incorporated into the definition of AKI have to be seen in this context and need re-evaluation. Looking at this table we can see that if we apply diagnostic criteria of any consensus definition that requires an absolute increase of sCr, we are going either to overestimate or underestimate the true clinical impact of sCr changes depending on the baseline sCr of a patient. If the patient has a baseline value of sCr of 221.0 µmol/L or 2.50 mg/dL, a rise of 35.3 µmol/L (0.40 mg/dL) can classify this as AKI while for this patient such a change is probably due to random variation. On the other hand a change in sCr of 17.6 µmol/L (0.20 mg/dL) in a patient with baseline sCr of 66.3 µmol/L (0.75 mg/dL), while being significant is overlooked by the definition. The issue of false positive and false negative rates in consensus definitions of AKI has been raised recently in some studies.36–38 The incorporation of the concept of RCV in laboratory reporting of consecutive measurements and in the definition of AKI has been suggested recently.38
Table 1.
Calculation of sCr RCV for different baseline values hypothesising optimal analytical variation (CVA=0.5CVI) and 95% significance level.*
| sCr at baseline (in mg/dL) | sCr at baseline (in μmol/L) | RCV(%) | Absolute changes needed to be significant (in mg/dL) | Absolute changes needed to be significant (in µmol/L) | Limit for a significant change (in mg/dL) | Limit for a significant change (in µmol/L) | KDIGO criterion in mg/dL | KDIGO criterion in µmol/L | % from baseline |
|---|---|---|---|---|---|---|---|---|---|
| 0.75 | 66.30 | 18.28 | 0.10 | 8.84 | 0.85 | 75.14 | 0.30 | 26.52 | 40.00 |
| 1.00 | 88.40 | 18.28 | 0.18 | 15.91 | 1.18 | 104.31 | 0.30 | 26.52 | 30.00 |
| 1.50 | 132.60 | 18.28 | 0.27 | 23.86 | 1.92 | 169.72 | 0.30 | 26.52 | 20.00 |
| 2.00 | 176.80 | 18.28 | 0.37 | 32.70 | 2.74 | 242.21 | 0.30 | 26.52 | 15.00 |
| 2.50 | 221.0 | 18.28 | 0.46 | 40.66 | 3.65 | 322.66 | 0.30 | 26.52 | 12.00 |
| 3.00 | 265.20 | 18.28 | 0.55 | 48.62 | 4.66 | 411.94 | 0.30 | 26.52 | 10.00 |
| 3.50 | 309.40 | 18.28 | 0.65 | 57.46 | 5.76 | 509.18 | 0.30 | 26.52 | 8.57 |
| 4.00 | 353.60 | 18.28 | 0.75 | 66.30 | 6.95 | 614.38 | 0.30 | 26.52 | 7.50 |
CVI is taken from current Ricos database and represents CVI in health. Calculation of RCV shows that different absolute values are needed for a significant change according to baseline. On the other hand the fixed 0.30 mg/dL (26.5 μmol/L) of the AKI definition gives false negative results when baseline sCr is < 2.00mg/dL (176.8 μmol/L) and false positive results when baseline sCr is > 2.00 mg/dL (176.8 μmol/L)
We believe that a modification in the definition of AKI might be appropriate and the issue of the fixed increase from baseline should be addressed. RCV calculates relative increases from baseline for each patient separately and takes into account the biological and analytical variation of sCr, and it is an objective measure of clinically significant changes in any biomarker. Finally “small creatinine changes and its relation to outcome” should be re-examined.
It is well known that biological variation (BV) is not the same in health and disease. Patients with chronic conditions (such as CKD, liver disease or diabetes) might exhibit higher BV than healthy subjects.39–44 Therefore the baseline renal function as well as chronic conditions should be taken into account (when these are known) and the appropriate BVI incorporated into calculations.44 The estimates of BV shown in Ricos’ database were derived from healthy subjects or stable CKD patients under highly standardised conditions, in the absence of factors that interfere with assay specificity.34,35 In acutely ill people BV is higher and therefore higher RCV values might be needed in order to determine that a change is clinically significant. Therefore the use of the BV derived from healthy people may underestimate the true RCV in patients with acute conditions (as those with AKI). However in such conditions it is difficult to estimate the true random variation. In consequence the use of healthy population RCVs might lead to labelling many hospitalised patients’ test results as having changed significantly. These changes could be called “false positives” since real RCV would be higher than in the healthy population.
On the other hand the RCV published in the literature uses the assumption that the values found in the studied subjects are forming a Gaussian distribution. This assumes truly random variation33,45 and also means that there is no correlation between successive results. This seems reasonable when we perform tests at medium to long-term intervals between samples. However, when we perform tests more frequently (on a daily basis), this serial correlation might exist. Estimates of within subject BV over short periods of time might be smaller than long-term estimates. This auto-correlation could make the CVi and therefore RCV smaller. Therefore the RCV that uses CVi from healthy people will lead to “false negative” changes. However these two effects tend to balance each other out and thus calculating RCV from healthy subjects is valid and widely applicable.33 The estimation of RCV has generated debate and discussion regarding the statistical approach that should be applied, especially when the analyte under investigation does not exhibit a normal distribution. For example in the case of sCr and plasma neutrophil gelatinase-associated lipocalin (NGAL), where these two analytes exhibit normal distribution the standard approach proposed by Fraser can be applied for the determination of RCV.46,47 On the other hand a non-parametric approach might be more appropriate for the determination of RCV in analytes that do not follow the normal distribution and are highly skewed like urinary NGAL.47 However the different approaches that have been proposed need careful validation.48–51
The Issues of Baseline Renal Function
The concept of AKI is based on “rapid worsening of kidney function from baseline levels”. The baseline sCr value is a measure of the patient’s pre-morbid kidney function and is necessary for two reasons: first to compare with the current value in order to define and stage AKI, and second to ascertain the extent of recovery of renal function after an AKI event, which is an important clinical endpoint.52 To apply the sCr component of the RIFLE, AKIN or KDIGO criteria in the diagnosis and classification of AKI the true baseline (pre-morbid levels) should be known. However very often this is not available or unknown. Methods of “looking back” to obtain a baseline value for the initial detection of AKI have been studied and suggested by various organisations. These methods can be summarised into three groups:
Those that require a measured sCr within seven days from the current value
Those that require a measured sCr between seven and 365 days before the current value
Those that back-calculate sCr using a formula
KDIGO suggests the use of the lowest creatinine during hospitalisation as the baseline value. KDIGO alsos allow the use of a “look-back” in laboratory results, in an otherwise stable patient, with no recent sCr and without (progressive) CKD. This “longer time frame” will detect more patients with less severe AKI, who may have a lower mortality. The KDIGO approach has the following reasoning:
Considers sCr,
Avoids use of sCr when values may be influenced by the illness prodrome, and
Considers previous values only as far as needed to earlier sCr values. The older the sCr result, the higher the chance that it will not reflect the true baseline function shortly before the onset of AKI.
The use of pre-admission values of sCr poses a great dilemma: how far back can a baseline value of sCr be retrieved and still be expected to be “valid” for the definition of AKI? In the general population, it is reasonable to assume that sCr will be stable over several months or even years, so that a sCr obtained six months or even one year previously would reasonably reflect the patient’s premorbid baseline.
The true problems arise when there is no pre-admission measured sCr. When there is no information on prior renal function of a patient the controversial use of estimated baseline sCr values has been advised by many organisations. Acute Dialysis Quality Initiative (ADQI) and KDIGO has recommended back-calculation of sCr from the MDRD formula, assuming an estimated GFR of 75mL/min/1.73m2.3,5 This method can be used when there is no evidence of CKD. In cases of known CKD a baseline sCr is usually available. Unfortunately in unidentified CKD cases when there is no baseline sCr value available, estimating the baseline sCr may mislabel a patient with AKI, when in reality the diagnosis is an unidentified CKD. This method has been used in many studies using RIFLE criteria to investigate the epidemiology of AKI.53–57 Although this method has recently been validated by Zavada et al.58 it also has the problem of underestimating the baseline sCr and therefore overestimating the GFR and several studies have shown that is prone to error.59
A different approach suggests the use of the minimum sCr during hospitalisation.60,61 The rationale for this is that it provides a reasonable estimate of baseline sCr among patients without AKI as well as among those with AKI who recover and may also be helpful in examining hospital-acquired AKI. In some cases, the lowest inpatient sCr may even more accurately reflect kidney function. However, in prolonged critical illness or sepsis, decreased creatinine generation may result in lower sCr values.62,63 Other studies have suggested that the presence of other confounders, such as fluid overload, may have an impact on AKI diagnosis.64
These limitations suggest that more sophisticated approaches may be needed to estimate baseline sCr.
Multiple imputation is a widely used approach that allows estimation of missing data in statistical analysis.65 Sew et al. using this approach showed that it can improve accuracy in estimating missing baseline sCr and reduce misclassification of AKI beyond currently proposed methods primarily by increasing specificity and positive predictive value among those with lower eGFR values.66 However the utility of this approach depends on the degree of missing data and the quality of the data that is available.
The choice of baseline sCr in clinical practice is critical not only for the diagnosis of AKI in a patient, but also for the staging of the severity of the case. Moreover misclassifications can have an impact on the choice of therapeutic approach (inappropriately aggressive in the case of a false positive AKI diagnosis or not intervening in the case of false negative diagnosis). In clinical studies, this has an effect on the prevalence of AKI in severity staging cases, in the mortality that is associated with AKI in the various stages.
Measuring UO in AKI. Is it Sensitive? Is it Specific? Is it Relevant?
UO is a rapid bedside test for kidney function. Reduced output is the oldest known biomarker for AKI. The theoretical advantages of UO over sCr include
The speed of the response. A rapid reduction of UO may be the earliest indication of decreased kidney function. For example, if GFR were to suddenly fall to zero, a rise in sCr would not be detectable for several hours. On the other hand, urine output would be affected immediately.
Low urine output is defined by a predefined cut-off value. There is no need to look for a baseline UO. In contrast sCr based definitions depend on a baseline sCr value which is often unknown and has to be estimated by processes that introduce significant errors.
Certain conditions (infections, sepsis, malnutrition) seriously affect creatinine production and make sCr use an unreliable surrogate marker of GFR.
It is well recognised that hydration status, use of diuretics and haemodynamic status influence UO in the absence of AKI. On the other hand it is also known that severe AKI can occur with normal UO. However the ADQI group has decided in the RIFLE consensus definition to use UO criteria to define and stage AKI.3 This criterion remained in the subsequent AKIN and KDIGO definitions.4,5 The accuracy and the usefulness of this criterion in clinical practice are not well verified. Very few studies have attempted to define an optimum UO and duration of urine collection for AKI diagnosis.67,68 Also in the consensus definitions the method of assessing oliguria is not specified. Whether the definition of oliguria should be made by the average flow over 6 h or from a persistent reduction over the 6 h is not clear. UO measurement has to be done manually and inputted into the hospital’s information system, which renders it to clerical errors. UO is also influenced by fluid balance, presence of hypotension and the use of diuretics and vasopressors. Moreover UO can be used as a biomarker only in those patients having a urine catheter in place. The difficulties in measuring, monitoring and recording accurately UO have resulted in the lack of a standardised approach to assessing changes in UO and identifying episodes of oliguria. Therefore it is not surprising that UO criteria for AKI diagnosis are often omitted from clinical studies.69
Although decrease of UO may be associated to a decline of GFR due to decrease of renal blood flow (RBF) or renal perfusion pressure, neurohormonal factors and functional changes may influence diuresis and natriuresis especially in critically ill patients. The decline of GFR and UO in response to a decrease of RBF is classically referred to as pre-renal azotaemia, which can evolve into structural damage if renal hypoperfusion persists. Into this context UO is used not only as a marker of AKI, but also to guide fluid resuscitation in critically ill patients. The mechanisms of diuresis regulation are discussed in a recent review. Elucidation of these mechanisms will allow us to interpret the UO in critically ill patients and the appropriate treatment to be initiated in case of changes in UO.70
The UO criteria for definition and staging AKI as well as their relation to outcomes (short-term and long-term mortality) have been assessed in a number of studies, where mostly critically ill patients are involved.67,68,71–74 However the number of studies is relatively small compared to those that use sCr criteria.
In relation to AKI diagnosis, the UO criterion consistently classifies more patients as “presenting with AKI” than the sCr criteria. Several studies report a higher incidence of AKI by UO compared with sCr implying a higher sensitivity of UO over sCr criterion.67 However the specificity of this criterion has not been defined yet. AKI patients are not stable patients and usually exhibit positive fluid balance, which alters the volume distribution (“dilute”) of sCr and results in overestimation of renal function and AKI diagnosis may be delayed by 48 to 72 h. In critically ill patients a combination of UO criteria with positive fluid balance (defined as >6.5 ml/kg) can diagnose patients prone to AKI earlier and more efficiently in the absence of sCr elevations. However this criterion should be applied after fluid volume correction.75 Although fluid resuscitation and optimisation of renal perfusion pressure is central to AKI prevention, excessive fluid volume correction may be harmful in some patients. Aggressive fluid resuscitation, although increasing renal blood flow, can be ineffective in restoring renal microvascular oxygenation since it increases haemodilution and not oxygen carriers in the circulation. When the diagnosis is based on the UO criterion alone it requires the exclusion of urinary tract obstructions that reduce UO or other easily reversible causes of reduced UO.
In relation to outcomes Cruz et al. found that sCr criteria were a better predictor of mortality than UO criteria and the combination of both sCr and UO criteria had the best predictive value for mortality.76 Haase et al. found that the UO criteria of the RIFLE or AKIN classification showed the lowest predictive value for in-hospital mortality. However, patients classified as RIFLE-F or AKIN-3 for the UO criteria compared with those with better diuresis had significantly longer lengths of stay in the ICU and the hospital and increased need for RRT initiation and in-hospital mortality.77
Prowle et al. showed that short duration of oliguria (<12 h) lacks clinical utility in AKI diagnosis (when AKI is defined using sCr criteria) with likelihood ratios (LR) ranging between 2.1 and 3.8.72 This means that the presence of this duration of oliguria in a patient one day does not increase the probability of AKI the next day (defined by sCr criterion) since a LR of >10 is considered necessary for a test to be considered clinically useful. On the other hand oliguria >12 h has increased clinical utility (LR=13.5). However using this cut-off resulted in an unacceptable low sensitivity (17%).
Ralib et al. tried to define an optimum UO and duration of collection for AKI diagnosis and to compare them with predefined clinical outcomes (death or need for dialysis).68 They found that oliguria defined by 0.5 ml/kg/h for 6 h was not predictive of survival whereas 0.3 ml/kg/h was. However they did not examine the combined effect of both sCr and UO for their cutoff value. In a more recent study Kellum et al. studied the combined effect of sCr and UO on survival, renal recovery and the risk for RRT.78 They found that patients meeting both sCr and UO criteria of KDIGO consensus definition have dramatically worse outcomes compared with patients who manifest AKI by one criterion. They also found that isolated oliguria is associated with decreased one year survival. Despite the differences in their results these studies emphasise the importance of measuring UO in AKI patients.
Can we Measure GFR in AKI?
Reduction in the GFR secondary to kidney injury is the hallmark of AKI and results in increased levels of both serum urea and creatinine. Unfortunately the rate of increase in sCr does not parallel the fall in GFR in a time frame that is clinically useful. Moreover since levels of sCr are determined by its production from muscle and by GFR, the use of sCr as an indicator of GFR is highly patient-specific and can be misleading. The formulas that use sCr to estimate GFR (i.e. MDRD, Cockcroft-Gault) were derived in subjects with CKD, and not with AKI.21,79 Furthermore the use of a single sCr measurement to estimate GFR relies on the assumption that the patient is in steady state with regard to creatinine production and excretion. This assumption is not valid for patients with AKI where changes in sCr are usually delayed and follow changes in GFR and the improvement in GFR often precedes the decline in sCr by days. Mathematical models have been proposed to predict GFR on the basis of sCr changes during AKI, but are not practical for clinical applications.23 Jellife et al. developed an equation to estimate GFR in the setting of non-steady kidney function.80 This equation has been validated recently.81
A rapid quantitative technique with clinical utility to measure GFR has not yet been developed.
Today there are no readily available methods for continuous monitoring of the GFR. Rabito et al. showed that such monitoring is feasible.82 It is likely to be introduced in the future once practical obstacles have been overcome. Short time urine collections (2–8 h) with a blood sample for CrCl determination are a useful method of relatively rapid kidney function assessment. This is the case in spite of oliguria or irregular UO in unstable AKI patients. The 4 h CrCl has been shown to detect AKI earlier than a plasma creatinine rise.18
The Issues of Differential Diagnosis (from Pre-renal Azotaemia to Pre-renal AKI to Transient AKI and to Functional Change) – the Role of Urinary Biochemistry, Derived Indices and Microscopy
This is an area with a lot of confusion.83–85 For many years the diagnosis and management of AKI was based on a concept where its aetiologies were divided into three categories: pre-renal, intrinsic and post-renal. The term “pre-renal” suggests that GFR is decreased as a consequence of renal hypo-perfusion in relation with events “outside” the kidney. The “pre-renal” term corresponds to various mechanisms that modify GFR but without primary damage of the kidney parenchyma, and that can be seen as adaptive responses of the kidney to external insults that are also reversible.84 However definitive diagnostic criteria are lacking. The current consensus definitions do not discriminate AKI by its aetiology.
This discrimination cannot be used to guide therapy because in cases like dehydration, rapid hydration is indicated, whereas in conditions with the same presentation like hepatorenal, nephrotic or cardiorenal syndrome hydration is prohibited. In cardiorenal syndrome it is the improvement of cardiac output and not the fluid administration that will improve renal function.86 Fluid responsiveness is not synonymous with “pre-renal” AKI. In a recent prospective study the investigators were able to classify the pre-renal versus intrinsic renal state based on fluid responsiveness in only 25% of cases.87
Although reversibility might imply structural integrity, there is actually little evidence that can confirm or exclude structural damage in pre-renal AKI. Its presence or absence cannot be excluded unless a biopsy is performed, which cannot be justified in such situations. Several cohort studies and the prospective EARLYARF study have identified that transient AKI was associated with adverse outcomes (need for dialysis and death) even when AKI is resolved within 24 h.88–91 A recent study that involved patients with septic AKI provides evidence on the concepts of intrinsic and pre-renal AKI.92 In this study, 77% of septic patients with AKI were found to have focal features of ATN on light microscopy, but only a small number of the renal tubules showed histopathological findings, and most of the microscopic fields appeared normal.92 These findings have led to the hypothesis that transient and persistent AKI in critically ill patients might share similar pathophysiological mechanisms and that the duration of AKI might reflect its severity rather than its mechanism.93 This hypothesis was supported by a large epidemiological study demonstrating a steady increase in hospital mortality associated with increased duration of AKI.88 Few studies to date have assessed the influence of AKI duration on the outcome of critically ill patients.94
Sometimes in the literature the term “transient-AKI” is used and as the name implies is defined by its duration of GFR decrease which is reflected by a transient increase in sCr or a transient decline in UO. Transient AKI is not separately recognised by current definitions. There is no consensus definition for “transient-AKI” but in most publications is referred to as short duration AKI that lasts between 24 and 72 h. Increases in sCr and decreases in UO should comply with AKI consensus definitions. These time points are arbitrary and create confusion since in some studies the investigators choose the 24 h to discriminate between transient and sustained AKI while in other 48 or 72 h. In practice the most common definition of transient AKI is “a reversal AKI in less than 72 h after fluid administration”.88,95–97 This definition might work in research studies but from a clinical perspective is not useful at all. In clinical practice where the aim is to triage patients as soon as possible waiting for this reversal in renal function is not acceptable since therapeutic and diagnostic interventions are delayed in those patients who require more than hydration. The emergence of novel biomarkers might enable us to classify AKI by functional change, kidney damage, or both and such discrimination will provide information that is interpretable, and will account for the dynamic nature of AKI.91,98 A recent meta-analysis showed that when biomarkers are positive even in the absence of apparent change in renal function, patients have worse hospital survival and even an increased need for dialysis compared to patients without AKI. The biomarkers may simply predict changes in function that would manifest later unless competing endpoints such as death or dialysis occur first.86,99
The terms “functional change” and “kidney damage” have been proposed recently to be used in preference to the terms ‘pre-, intra- and post-renal’ in order to narrow the differential diagnosis of AKI.86 A combination of history, clinical examination, renal imaging biopsy and measurement of biochemical markers is required to establish a causal diagnosis of AKI.
A number of urinary biochemistry tests and derived indices are traditionally used to aid clinicians in the early detection of AKI and to differentiate pre-renal azotaemia from intrinsic AKI. These tests are outlined in Table 2. Four review articles provide an excellent analysis of the recent literature.100–103
Table 2.
Traditional urinary tests used in AKI diagnosis and differentiation
| Test | Pre-renal azotaemia | Intrinsic AKI |
|---|---|---|
| Sediment | Normal or hyaline casts | Casts, tubular epithelial cells |
| Sp. gravity | High >1.020 | Low <1.020 |
| uNa | Low <20 mmol/L | High>40 mmol/L |
| FeNa | <1% | >1% |
| FeUr | <35% | >35% |
| Urine osmolality (mOsm/Kgr H2O) | High>500 | Near serum (<300) |
| uCr/sCr ratio | High>40 | Low<10 |
uNa=urinary Sodium, FeNa=fractional excretion of sodium, FeUr=fractional excretion of urea, uCr=urinary creatinine, sCr=serum creatinine
Fractional Excretion of Sodium (FeNa)
The FeNa is a measure of the extraction of sodium and water from the glomerular filtrate. It is the ratio of the sodium filtration rate to the overall GFR rate (estimated by the renal filtration of creatinine). A euvolaemic person with normal renal function and moderate salt intake in a steady state will have FeNa approximately 1%. When interpreting FeNa it is necessary to consider whether the patient has preexisting CKD as these patients might exhibit FeNa >1% in the absence of AKI depending on their GFR and daily dietary sodium intake.104,105 Traditionally FeNa has been used for the discrimination between pre-renal azotaemia and intrinsic AKI.106,107 In a case of pre-renal azotaemia the epithelial cells of proximal tubules reabsorb filtered sodium resulting in a very low concentration of sodium in urine (<20 mmol/L) and FeNa <1%, whereas in established AKI concentration of sodium in urine is higher than 40 mmol/L and the resulting FeNa is >1%. A low FeNa or low urine sodium reflects poor renal perfusion of any cause, not exclusively volume depletion. However there are many causes for a low FeNa despite AKI and for a high FeNa despite pre-renal AKI.. The use of diuretic agents, the presence of sepsis, myoglobinuria, acute glomerulonephritis, cirrhosis, congestive heart failure, and contrast induced nephropathy may seriously affect the performance of this test.96,108–113 A detailed list of the limitations of this test is presented in Perazella and Coca.102
Fractional Excretion of Urea (FeUr)
Its calculation is based on the same principle as FeNa except that since the reabsorption of urea occurs mainly at the proximal segment of the nephron, it is practically not affected by diuretic agents, which act distally to the proximal tubule. Therefore it should be more reliable than FeNa. However studies that evaluated the performance of FeUr in various clinical settings (including ICU patients) have produced discordant results.96,114–117 A low FeUr (Table 2) is usually indicative of pre-renal AKI, however recent studies indicate that ageing and sepsis may alter FeUr.108,118
Urine Microscopy
Examination of urinary sediment is one of the oldest tests used to evaluate AKI in clinical nephrology.107,119,120 An evaluation of the urine sediment is often considered as a complementary measure for the diagnosis and the severity of AKI since it provides additional information. Urine microscopy has many advantages: it is cheap, non-invasive and readily available. The presence and the type of casts in urine sediment can differentiate the aetiology of AKI.120,121 Visualisation of red cell casts is due to glomerulonephritis, whereas the presence of renal tubular epithelial cells and coarse granular or muddy brown casts as well as casts containing tubular epithelial cells is indicative of acute tubular necrosis. On the other hand, absent sediment or the presence of occasional hyaline casts is indicative of pre-renal azotaemia.120,122 An ischaemic or nephrotoxic insult causes tubular injury, which results in apoptosis or necrosis of the renal tubular epithelial cells.102 These are shed into the tubular lumen where they are excreted free or form casts which can be examined in fresh urine sediments. Since pre-renal azotaemia and AKI are not separate clinical entities but rather are a continuum, the presence of cells and casts would be expected to increase with the severity of the disease. It is logical to try to assess these findings quantitatively.
However, evidence that establishes the diagnostic value of urine microscopy has largely been lacking.
Two systematic reviews evaluated the usefulness of urinary microscopy and suggested it may have a limited role for the diagnosis, classification and prognosis of septic AKI.101,123 However recent data show that urine microscopy may have a complementary role for the discrimination of septic from non-septic AKI.113
Another systematic review evaluated the usefulness of urinary microscopy for the differentiation between pre-renal AKI and ATN and suggested that its clinical utility may be increased by the use of a simple urinary scoring system based on the number of renal tubular epithelial cells and casts.100 In a recent observational study the researchers used a simple urine sediment scoring system based on the presence and the quantity of granular casts and renal tubular epithelial cells, which was highly predictive for the final differential diagnosis of acute tubular necrosis from pre-renal azotaemia.124 However one major disadvantage, not only in this study but in general, is that there is no validated gold standard for the discrimination of pre-renal azotaemia and ATN except for kidney biopsy. In this study the clinical final diagnosis was the gold standard. Without a gold standard that would be able to diagnose a syndrome in a given patient, we cannot judge the performance of any biomarker objectively and its usefulness is likely to remain controversial. The same group evaluated the role of urine microscopy to predict the severity of AKI and adverse clinical outcomes during hospitalisation.125 Their results show that examination of urine sediment might have a role in determining the severity of AKI. Another more recent study compared the diagnostic value of urinalysis with NGAL for the early detection of AKI.126 Urinalysis was performed with the use of an automated dipstick and imaging system (IRIS) and only abnormal samples were selected for manual microscopy of the sediment. They found that NGAL exhibited only fair sensitivity (65%) and specificity (65%) in the detection of AKI. On the other hand urinalysis was more specific (91%), but its sensitivity was extremely low (22%). A different study provided evidence that there is low agreement between automated analysis systems and manual microscopic evaluation of urine sediment.127 Manual microscopy, if performed by an experienced clinical chemist or nephrologist, outperforms automated urinalysis systems mainly because the latter uses unspun urine. Centrifugation increases the probability of locating casts. On the other hand the major disadvantages of manual microscopy are the lack of methodological standardisation and the observer’s experience. The current information from published studies shows that the use of FeNa or FeUr may provide some insight into differential diagnosis when used in selected populations but has a small role in most clinical situations. On the other hand the clinical utility of urine microscopy is useful and its usefulness may be enhanced by using a scoring system based on the number of tubular epithelial cells and renal casts.
Novel Biomarkers - Current Status
In everyday clinical practice, clinicians are facing several challenges with the interpretation of elevated sCr levels in a patient. These challenges include: differentiation between AKI and stable CKD, pre-renal and intrinsic AKI, estimation of the degree of renal dysfunction compared to baseline, estimation of the chance of renal recovery, the need for renal replacement therapy, and decision on the appropriate therapy.
Central to these challenges is the development of biomarkers that will improve the precision of the early diagnosis of AKI and facilitate effective therapeutic process. Moreover biomarkers may play a critical role in drug development process. The American Society of Nephrology have assigned the highest priority to the discovery and standardisation of new biomarkers.128 Since then, a significant number of studies have been published focusing on the detection and validation of new biomarkers in a variety of clinical settings and in different patient populations. New technology (genomics proteomics and metabolomics) triggered the discovery of a quite big number of potential biomarkers. Recently identified biomarkers of AKI, can be grouped into five categories:
Functional biomarkers (markers of glomerular filtration such as cystatin C)
Low molecular weight proteins, which are present in the systemic circulation and undergo glomerular filtration. Some of them, under normal situations, undergo complete tubular re-absorption (i.e. cystatin C, β2-microglobulin, α1-microglobulin, retinol binding protein) and therefore their presence in urine is indicative of tubular dysfunction
Enzymes that are released by tubular epithelial cells into urine after tubular cell injury and are direct markers of tubular damage (i.e. N-acetyl-beta-D-glucosaminidase (NAG), alpha-glutathione S-transferase (α-GST) piglutathione S-transferases (p-GST), γGT)
Inflammatory mediators released by renal cells or infiltrating inflammatory cells (IL-18)
Up-regulated proteins in response to cellular/tissue injury (NGAL, Kidney injury molecule 1 (KIM-1), L-type fatty acid-binding protein (L-FABP))
In the first phase of the discovery of these biomarkers, carefully selected cohorts of affected vs non-affected patients were used. This phase was followed by initial validation of these biomarkers in carefully selected and very homogenous populations. Under these conditions they exhibited excellent characteristics with areas under the curve in ROC analysis above 0.90. Subsequent validation using less homogenous populations came with mixed results for diagnosis, differentiation and prognosis of AKI. It is understandable that once the studies include patients of moderate and low risk and patients with comorbidities, the performance of these biomarkers will be reduced. Indeed many studies have yielded AUCs well below 0.75.
Although a number of criteria have been proposed in order to determine the clinical utility of these biomarkers,129,130 (outlined in Table 3), there are some questions that should be answered and concern their validation.
Is it rational to judge the performance of biomarkers that aim to diagnose structural tubular damage against functional markers that aim to measure GFR?
Is it appropriate to try to dichotomise this syndrome to pre-renal and intrinsic renal AKI when all evidence today shows that it is a continuum? Especially when no gold standard exists for the definition of pre-renal AKI, and definitive tubular damage can be proved only with biopsy which is rarely performed, and when it has been performed it showed that it was present in cases that were characterised as pre-renal and missing from cases that were characterised as intrinsic.
Are diagnosis, differentiation, and prognosis well served with the use of one biomarker or do we need to move to a multi marker approach.
Table 3.
Criteria to determine clinical usefulness of candidate biomarkers
|
Although these criteria (Table 3) seem logical to judge the performance of any candidate biomarker, their applicability depends on how definitive and robust they are.
Biomarkers for the Early Diagnosis of AKI
Many investigators have focused on the ability of novel biomarkers to diagnose AKI early, before sCr rises. Several reviews and metanalyses have focused on performance of these biomarkers.99,130–146 The performance of any candidate biomarker which will be used for the diagnosis of AKI, will be judged against sCr, since all current definitions of AKI use sCr changes. It has been discussed before that even minor imperfections in the diagnostic performance of a gold standard can result in significant misinterpretations of the diagnostic performance of a new biomarker.147,148 It is inevitable that the performance of novel biomarkers against these criteria will be extremely variable and their diagnostic values in clinical practice will be low, outside a well-defined research setting. A biomarker that is a “good performer” according to our gold standard may reflect the same inherent limitations of sCr whereas a “poor performer” may provide information completely different from sCr.147 This is true since the clinical condition (in this case AKI) that a novel biomarker is intended to identify is not synonymous with the clinical condition identified by the changes of sCr.148,149 We try to compare markers of tissue injury (which may range from low to extremely high) with markers that show loss of organ function (which is the outcome). Therefore we might have cases of AKI where sCr does not change (i.e. septic patients where creatinine is underproduced) and on the other hand we might also have cases where increases of sCr are observed without structural damage (i.e. hypovolaemia, diarrhoea etc.). Therefore it depends on how we define AKI and what we expect to diagnose. If we define AKI with sCr changes and we expect to find only those patients with structural damage, it is obvious that any biomarker aimed to identify tubular damage will give discrepant results when it is judged against our definition. In this case we should remove sCr from our diagnostic criteria and focus only on proof of structural damage. On the other hand if we appreciate AKI as a continuum, where functional changes and structural damage are intertwined, then we should find a way to incorporate some of the new biomarkers in our current definitions.
Siew et al. proposed a way worth exploring in interpreting data from clinical studies based on agreement and disagreement between sCr and biomarker data.147 McCullogh et al. reporting a statement from the 10th Acute Dialysis Quality Initiative Consensus Conference, focus on the use of both functional and injury biomarkers for the diagnosis of AKI.150,151 They propose that the interpretation of data from both functional and injury biomarkers could give complementary information (see Table 4)
Table 4.
Diagnosis of AKI using functional and injury biomarkers.
The current diagnostic criteria include only functional changes (lower line in table) The proposal of McCullough et al. enables the recognition of four subgroups according to AKI state. Patients without changes in sCr and who are negative for biomarker are considered as no-AKI. Patients with a biomarker only positive may represent a “subclinical-AKI” group in which loss of function is not present at the time of diagnosis but may develop several days after the diagnosis or may not develop at all. Loss of function without detectable injury represents a group of patients in whom a dynamic change in renal filtration of sCr is observed as in patients with dehydration. The last group of patients where both injury and loss of function are present represent the patients with worst prognosis. (adapted from ref 150)
| Biomarker positive | Biomarker negative | |
|---|---|---|
| No functional change | No functional changes or injury | Damage without functional change |
| Functional change | Loss of function without injury | Injury with loss of function |
Biomarkers for the Differentiation between Pre-renal and Intrinsic AKI
For differential diagnosis between pre-renal and intrinsic AKI there is no gold standard. Definitive diagnosis is always retrospective after a transient rise in sCr with recovery of function within 24 to 72 h. It is presumed that pre-renal AKI is not accompanied by structural tubular damage whereas intrinsic AKI does. However this can only be verified by biopsy which is not justified in most cases. A limited number of studies investigated the use of novel biomarkers to distinguish between pre-renal and intrinsic AKI. Two major problems exist with the definition of pre-renal AKI in these studies. First is the estimation of baseline sCr and second the duration of sCr increase. These are not uniform in all studies. Nejat et al. define pre-renal AKI using the AKIN criteria, that lasted between 24 and 48 h and combined with FeNA <1%.91 Only 51% of the patients had a sCr before admission to the ICU. For the rest, the lowest value of sCr during ICU stay served as baseline. In the study of Singer et al. the RIFLE criteria were used for the diagnosis.87 Patients were classified as “pre-renal AKI” when the increase in sCr concentration had been caused by factors that compromise renal perfusion, and creatinine rapidly improved to baseline with volume repletion or improvement in cardiac output within three days of directed therapy. However this criterion was not strict in the study as a number of patients with longer duration of normalisation of creatinine (seven days) were diagnosed as having pre-renal AKI. In this study the final diagnosis was made by two clinicians. As a result 38 patients out of 161 (23.6%) could not be classified using this approach. Finally, baseline sCr was determined by review of the previous 12 months of patients’ medical records, and if unavailable, it was assumed from the hospital course.
De Geus et al. used the RIFLE criteria to define AKI.152 Pre-renal AKI (named here transient AKI) was defined as AKI presenting only once and reaching normal sCr levels within 24 h and sustained AKI was defined as any AKI occurring and persisting for >24 h after admission. Baseline sCr was defined as the patient’s steady state level four weeks prior to ICU admission. If not available, the admission value was used as the baseline.
Nickolas et al. also used the RIFLE criteria to define AKI.153 Pre-renal AKI was defined as AKI persisting for only three days however when defining normal kidney function, different absolute increases were asked when baseline sCr was <88.4 µmol/L (1.0 mg/dL) (increase <17.6 µmol/L (0.2 mg/dL)) and when baseline was >88.4 µmol/L (1.0 mg/dL) (increase <26.5 µmol/L(0.3 mg/dL)). This dichotomisation seems arbitrary since they did not take into account biological and analytical variation of sCr. The second problem was the determination of baseline sCr. This was determined by review of the previous 12 months of records or, if unavailable, baseline sCr was assumed from the lowest recorded sCr level during the hospital course. Using this approach, only 63.5% of the patients had documented baseline sCr values at the time of presentation, a fact highlighting the difficulties of interpreting single measurements of sCr in triage.
It is obvious that when such discrepancies exist in the definition of pre-renal AKI and the estimation of baseline creatinine, novel biomarkers will always exhibit reduced performance. Singer et al. investigated if urinary NGAL can be used to differentiate between pre-renal and intrinsic AKI in 145 hospitalised patients with elevated sCr.87 Although urinary NGAL was found useful to stratify and classify patients with established AKI, its values showed considerable overlap among the pre-renal and unclassified patient groups. De Geus et al. evaluated urinary and plasma NGAL and cystatin C in 510 critically ill patients.152 They confirmed that urinary NGAL was the only biomarker to show significant differentiation between sustained and transient AKI on admission. But combination of different biomarkers or adding the results to a clinical prediction model led to marginal diagnostic improvement. Nickolas et al. evaluated five different biomarkers (NGAL, KIM-1, LFABPIL-18 and cystatin C) in 1635 patients who presented to the emergency department and were subsequently hospitalised for more than 24 h.153 Only urinary NGAL and urinary cystatin C were able to distinguish patients with pre-renal AKI from those with sustained AKI. Finally Nejat et al. measured urinary levels of NGAL, cystatin C, γGT, KIM-1 and IL-18 in 529 critically ill patients upon admission and at 24 and 48 h after admission to ICU.91 Urinary levels of cystatin C, IL-18 and KIM-1 could discriminate pre-renal AKI from intrinsic AKI and no-AKI whereas NGAL and γGT could not discriminate pre-renal AKI from those patients without AKI.
Use of Multiple Biomarkers
AKI is a complex syndrome with multiple diverse aetiologies, and often occurs in the setting of systemic diseases such as sepsis. Sepsis is also a syndrome that presents significant challenges for biomarker discovery. It is possible to enhance the ability to predict risk in such complex clinical situations by either measuring simultaneously multiple biomarkers or combining biomarkers with other risk estimates. Similar strategies have been tested in cardiovascular disease.154–156 This type of approach might be effective to define risk and prognosis of AKI.157 Combinations of AKI biomarkers have been described in several publications.157–159 More recently Arthur et al. studied the ability of 32 potential biomarkers to predict worsening of AKI or death in 95 patients with mild AKI after cardiac surgery.160 In this study the combination of a tubular damage marker (KIM-1) and an inflammatory mediator (IL-18) was the most predictive of death or advanced AKI. In another recent study that involved 93 high risk patients undergoing cardiopulmonary bypass, Prowle et al. showed that combinations that involved a tubular injury and a glomerular filtration biomarker best predicted AKI.161 These combinations displayed significantly better area under the curve (AUC-ROC) compared to the best single biomarker. However despite the statistical significance in ROC analysis, when they assessed their ability to divide patients into two groups of low and high risk, these combinations failed to improve classification of risk compared to the best single biomarkers.
Should we Normalise Urinary Biomarkers to Urinary Creatinine?
The problems of reporting absolute concentrations of a urinary biomarker in random urine collections (single void) are easy to spot. Oliguria can cause an increase while polyuria a decrease in the concentration of a biomarker which has a constant production and excretion rate. In such situations normalisation to uCr takes into account urinary flow rate.
Urinary biomarkers (mainly albumin) are frequently reported as a normalised ratio to uCr concentration in order to control for variations in urine flow rate. This assumes that uCr excretion is constant across and within individuals. If the assumption is true then any changes in the ratio will reflect changes in the biomarker excretion.
In chronic conditions such as microalbuminuria in diabetes mellitus and proteinuria in nephrotic syndromes, it has been a standard practice for clinicians to normalise the urinary excretion of these biomarkers to uCr.162,163 It seems logical to normalise to uCr in these disease states, since the urinary excretion of any biomarker that is filtered via the glomerulus will be affected by the GFR and resultant urinary flow. In chronic states of reduced GFR, lack of normalisation may lead to false interpretation of low concentration of these biomarkers. In current clinical practice, spot urine collections have replaced timed collections assessments in many instances of biomarkers of chronic kidney diseases including assessments of microalbuminuria, proteinuria, and calciuria, and the results are reported normalised to uCr concentrations. However, although it has been assumed that normalisation of spot urinary biomarkers to uCr in an acute disease, such as AKI, can achieve the same goal, this has been challenged recently.164 In several clinical studies biomarkers of tubular injury are often normalised to uCr.97,165–170 This is based on the assumption that uCr excretion is constant, which is not correct when the renal function is changing rapidly. Before normalising we must take into account two parameters, the tubular secretion of creatinine and the variations in the creatinine excretion rate by the kidney when GFR is changing.11 Under non-steady-state conditions (such as AKI) the uCr excretion rate changes over time. Unless the biomarker behaves exactly like creatinine (that is filtered, secreted to some extent and not normally re-absorbed) the normalised level will be affected by differences in uCr excretion. This may lead to amplification of the “biomarker signal” and AKI overestimation.
In the literature, there are studies that prove there is a dynamic change in uCr excretion rate when the glomerular filtration changes, as in the case of AKI. Lower creatinine excretion rate therefore may amplify the signal of tubular injury biomarker and therefore will give us misleading information.
Moreover the generation rate of creatinine is reduced in acute illness and sepsis, extrarenal degradation of creatinine is substantial in patients with CKD, the rate of tubular excretion of creatinine is increasing as GFR decreases and there is also tubular back leak of creatinine to the systemic circulation in the case of ischaemic injury. It is difficult to calculate the contribution of each of these parameters to the urinary pool of creatinine and the added uncertainty to the calculated result.
On the other hand, in AKI the concentration of a biomarker might increase up to 1000-fold. When such increases are observed the normalisation to creatinine does not add to the interpretation of the results. This approach is useful in patients where the exact time of insult is known (i.e. surgical patients, patients receiving toxic medication, radio contrast agents etc.). In such populations, post-insult biomarker concentrations must be compared to baseline values for significant increases. Reference values are useful to compare the baseline value of such patients with a reference population. Therefore the same absolute units must be used for the reference population in order to facilitate direct comparisons.
It is known that low molecular weight proteins (NGAL, β-microglobulin, cystatin C) are freely filtered from the glomerulus and reabsorbed by the epithelial cells of the proximal tubules by the same receptor (megalin-cubulin). Albumin uses the same receptor for re-absorption and the presence of albumin in urine affects the levels of low molecular weight proteins in urine independently of AKI, since it has higher affinity for this receptor.171 Also higher levels of urinary NGAL can be observed in patients with CKD, which has been discussed as a sign of “on-going kidney damage”. Baseline levels can be useful to risk-stratify patients for post-operative AKI.
On the other hand in different populations where the time of insult is unknown and an urocatheter is in place (i.e. ICU patients), a more accurate approach could be the use of timed urine collections where we can estimate the actual excretion rate of NGAL in urine (i.e. ng/day).
The Interplay between Chronic and Acute Kidney Disease
AKI and CKD are closely associated. It is well established that both diseases share common risk factors as well as causes for the worsening and adverse outcomes, but the main question is whether one disease is a risk factor for developing the other.
There are three major questions that need to be answered in order to clarify the possible links between AKI and CKD.
Do patients with AKI progress to CKD/ESRD (is AKI a risk factor for CKD development?)
What happens to patients who already have CKD? (is AKI a risk factor for progression of pre-existing CKD?)
Is CKD per se a risk factor for AKI?
Recent epidemiologic studies have shown a complex interplay between these two clinical entities. A growing body of evidence supports a bidirectional relationship: AKI leads to CKD, and the presence of CKD increases the risk of AKI.
After an episode of AKI there are four potential outcomes172
Full recovery and return of renal function to baseline
Incomplete recovery of renal function resulting in CKD
Exacerbation of pre-existing CKD accelerating progression towards ESRD
Non-recovery of renal function leading to ESRD
Most cases of AKI are reversible within weeks of occurrence, with patients fully recovering and with their renal function returning to baseline. However there is now increasing concern that this is not necessarily the case. The short-term outcomes of AKI (length of hospitalisation and inpatient mortality) are well documented. However the long-term outcomes have been less elucidated and have only recently come into focus. It was the application of the consensus definitions that have enabled the categorisation of AKI cases according to severity and facilitated the analysis of milder degrees of AKI on long-term kidney function. One recent review and one meta-analysis have demonstrated a strong association between episodes of AKI and subsequent development and progression of CKD.173,174 It has been argued that most of the evidence we have today is from observational epidemiologic studies and not supported definitively by laboratory evidence. Moreover long-term prognosis after AKI varies depending on the cause and clinical setting.175 While these studies demonstrate a strong association between AKI and subsequent CKD, they are unable to establish AKI as a cause for CKD, especially when risk factors for the development of AKI overlap with those for progressive CKD.176,177 However controversy exists on the interpretation of current literature findings on this matter.176,178 The confounding factors we see in adults are missing in children thus the development of CKD subsequently to AKI could be readily more attributable to residual kidney injury due to AKI. In a recent meta-analysis Grenberg et al. found increased incidence rates of proteinuria, hypertension, GFR <60mL/min/1.73m2, ESRD and mortality.179 However there was considerable variation in the quality of these studies with substantial discrepancies in the definition of AKI and in the outcomes. Finally data from experimental studies using animal models of AKI have provided insight into biological mechanisms by which AKI contributes to subsequent development of CKD and support an aetiologic relationship.174,180
CKD is one of the strongest predictors of AKI. The term “acute-on-chronic kidney disease” is used when AKI occurs in the background of pre-existing CKD. The presence of CKD greatly increases the risk of developing renal injury. Epidemiological data is limited, as this entity has not been extensively investigated. Several studies suggest that the presence of underlying CKD is a risk factor for AKI and modifies the relationship between AKI and adverse outcomes.181–183 In a recent review it was shown that the presence of CKD was associated with doubling of mortality outcomes and a four-fold to five-fold increase in renal adverse outcomes.175
Although the relevance of small changes in sCr and their relation to adverse outcomes has been discussed in detail in a previous section of this article, there are more controversies concerning these relationships.
Cases of AKI may be Superimposed on Pre-existing and Previously Unrecognised CKD
If the status of kidney function before the acute event is not known then when these acute-on-chronic events resolve we cannot be sure if the patient has returned to its baseline or whether this event has left sequelae. On the other hand critical illness for example is associated with significant decreases in sCr through many potential mechanisms that persist to hospital discharge and can cause inaccurate assessment of renal function at discharge.63 Future AKI studies should report pre-AKI and post-AKI renal function consistently as factors that can modify AKI prognosis
The Fact that AKI Precedes CKD does not Necessarily Mean that it is the Cause of CKD
Risk factors of kidney injury that caused AKI to a patient may persist after the acute event and eventually lead to CKD. Age and pre-existing conditions such as diabetes, hypertension, cardiovascular, peripheral vascular, and liver disease as well as the presence of albuminuria are factors that also increase the risk for CKD. These conditions also make a person susceptible to AKI because they are more likely to be exposed to procedures (percutaneous coronary interventions, cardiopulmonary bypass, abdominal aortic aneurysm repair), nephrotoxic medications and acute illnesses (sepsis, acute myocardial infarction) that increase the risk of AKI.
Limitations in the Definition of AKI
Creatinine cannot easily distinguish between natural progression of CKD and acute-on chronic disease. The use of sCr levels to identify AKI has the limitation that it might not identify all AKI cases and also does not enable the distinction between changes in kidney function and structural kidney damage which may differ in their potential to cause CKD.
Is There a Need for Specific Biomarkers?
The potential pathologic mechanisms underlying the progression from AKI to CKD include glomerular hyperfiltration and hypertrophy, mitochondrial dysregulation, endothelial injury, promotion of tubule-interstitial inflammation/fibrosis cellular infiltration and paracrine actions of bioactive molecules and reduced capillary density. Endothelin-1, transforming growth factor β, endothelial hypoxia-inducible transcription factor, and galectin-3 appear to play important roles in these pathways and may be promising target molecules for future intervention studies. Optimal follow-up and management for patients surviving an AKI episode have no evidence base to-date. Research for novel biomarkers in this area is still in its infancy, however measuring a true post-recovery sCr, quantifying the degree of proteinuria and identifying risk factors for recurrent AKI or progression of CKD seem a safe approach for the moment.172
Conclusion
During the last ten years new biomarkers have emerged for the diagnosis of AKI. However these biomarkers have been validated against a gold standard which is a consensus definition based on sCr measurements. Although this definition has helped clinicians to define end stage AKI many elements of this definition are loosely defined and have been widely criticised. Despite its two revisions this definition needs significant changes in order that existing controversies can be addressed. A universal definition of AKI is important for its diagnosis and management. We need to find a way to incorporate in a definition of AKI, biomarkers that show direct injury together with biomarkers that currently are used for measurement of filtration function. This definition should evolve on the basis of evidence and not on the basis of opinion or consensus. The current experience with biomarkers of direct injury is still limited. Thus although our experience in assessing renal function is greater there is still room for improvement.
Supplemental Data
Carmen Ricos and the Analytical Quality Commission of the Spanish Society of Clinical Chemistry in 1999 presented an overview of estimates of BV for a large number of constituents.1 As a continuation of this work, estimates of CVI (within subject BV) and CVG (between subjects BV), identified by literature searches, and the associated analytical quality specifications for bias, imprecision and total error have been presented on the Westgard webpage,2 being updated every two years. This data has been used as the main source of estimates of BV by laboratory scientists. The impact of this information is and has been huge.
Supplemental References
Ricos C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, Jimenez CV, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest 1999;59:491–500.
Ricos C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, Jimenez CV, et al. Desirable specifications for total error, imprecision, and bias, derived from intra- and inter-individual biologic variation. The 2014 update. www.westgard.com/biodatabase1.htm (Accessed May 2016).
Footnotes
Competing Interests: None declared.
References
- 1.Kellum JA, Lameire N, KDIGO AKI Guideline Work Group Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahman M, Shad F, Smith MC. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician. 2012;86:631–9. [PubMed] [Google Scholar]
- 3.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2(Suppl):1–138. [Google Scholar]
- 6.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–83. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis. 2014;63:820–34. doi: 10.1053/j.ajkd.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens LA, Levey AS. Measurement of kidney function. Med Clin North Am. 2005;89:457–73. doi: 10.1016/j.mcna.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 10.Hosten AO. BUN and Creatinine. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. [PubMed] [Google Scholar]
- 11.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–53. [PubMed] [Google Scholar]
- 12.Shemesh O, Golbetz H, Kriss JP, Myers BD. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28:830–8. doi: 10.1038/ki.1985.205. [DOI] [PubMed] [Google Scholar]
- 13.Patel SS, Molnar MZ, Tayek JA, Ix JH, Noori N, Benner D, et al. Serum creatinine as a marker of muscle mass in chronic kidney disease: results of a cross-sectional study and review of literature. J Cachexia Sarcopenia Muscle. 2013;4:19–29. doi: 10.1007/s13539-012-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S. Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr. 1983;37:478–94. doi: 10.1093/ajcn/37.3.478. [DOI] [PubMed] [Google Scholar]
- 15.Bragadottir G, Redfors B, Ricksten SE. Assessing glomerular filtration rate (GFR) in critically ill patients with acute kidney injury—true GFR versus urinary creatinine clearance and estimating equations. Crit Care. 2013;17:R108. doi: 10.1186/cc12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoste EA, Damen J, Vanholder RC, Lameire NH, Delanghe JR, Van den Hauwe K, et al. Assessment of renal function in recently admitted critically ill patients with normal serum creatinine. Nephrol Dial Transplant. 2005;20:747–53. doi: 10.1093/ndt/gfh707. [DOI] [PubMed] [Google Scholar]
- 17.Endre ZH, Pickering JW, Walker RJ. Clearance and beyond: the complementary roles of GFR measurement and injury biomarkers in acute kidney injury (AKI) Am J Physiol Renal Physiol. 2011;301:F697–707. doi: 10.1152/ajprenal.00448.2010. [DOI] [PubMed] [Google Scholar]
- 18.Pickering JW, Frampton CM, Walker RJ, Shaw GM, Endre ZH. Four hour creatinine clearance is better than plasma creatinine for monitoring renal function in critically ill patients. Crit Care. 2012;16:R107. doi: 10.1186/cc11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrera-Gutiérrez ME, Seller-Pérez G, Banderas-Bravo E, Muñoz-Bono J, Lebrón-Gallardo M, Fernandez-Ortega JF. Replacement of 24-h creatinine clearance by 2-h creatinine clearance in intensive care unit patients: a single-center study. Intensive Care Med. 2007;33:1900–6. doi: 10.1007/s00134-007-0745-5. [DOI] [PubMed] [Google Scholar]
- 20.Florkowski CM, Chew-Harris JS. Methods of estimating GFR - different equations including CKD-EPI. Clin Biochem Rev. 2011;32:75–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 22.Chew JS, Saleem M, Florkowski CM, George PM. Cystatin C—a paradigm of evidence based laboratory medicine. Clin Biochem Rev. 2008;29:47–62. [PMC free article] [PubMed] [Google Scholar]
- 23.Moran SM, Myers BD. Course of acute renal failure studied by a model of creatinine kinetics. Kidney Int. 1985;27:928–37. doi: 10.1038/ki.1985.101. [DOI] [PubMed] [Google Scholar]
- 24.Macedo E, Bouchard J, Soroko SH, Chertow GM, Himmelfarb J, Ikizler TA, et al. Program to Improve Care in Acute Renal Disease Study Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care. 2010;14:R82. doi: 10.1186/cc9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pickering JW, Ralib AM, Endre ZH. Combining creatinine and volume kinetics identifies missed cases of acute kidney injury following cardiac arrest. Crit Care. 2013;17:R7. doi: 10.1186/cc11931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ronco C. Kidney attack: overdiagnosis of acute kidney injury or comprehensive definition of acute kidney syndromes? Blood Purif. 2013;36:65–8. doi: 10.1159/000354768. [DOI] [PubMed] [Google Scholar]
- 27.Chiou WL, Hsu FH. Pharmacokinetics of creatinine in man and its implications in the monitoring of renal function and in dosage regimen modifications in patients with renal insufficiency. J Clin Pharmacol. 1975;15:427–34. doi: 10.1002/j.1552-4604.1975.tb02364.x. [DOI] [PubMed] [Google Scholar]
- 28.Molitoris BA. Measuring glomerular filtration rate in the intensive care unit: no substitutes please. Crit Care. 2013;17:181. doi: 10.1186/cc12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liotta M, Olsson D, Sartipy U, Holzmann MJ. Minimal changes in postoperative creatinine values and early and late mortality and cardiovascular events after coronary artery bypass grafting. Am J Cardiol. 2014;113:70–5. doi: 10.1016/j.amjcard.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 30.Lassnigg A, Schmid ER, Hiesmayr M, Falk C, Druml W, Bauer P, et al. Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: do we have to revise current definitions of acute renal failure? Crit Care Med. 2008;36:1129–37. doi: 10.1097/CCM.0b013e318169181a. [DOI] [PubMed] [Google Scholar]
- 31.Newsome BB, Warnock DG, McClellan WM, Herzog CA, Kiefe CI, Eggers PW, et al. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med. 2008;168:609–16. doi: 10.1001/archinte.168.6.609. [DOI] [PubMed] [Google Scholar]
- 32.Ishani A, Nelson D, Clothier B, Schult T, Nugent S, Greer N, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171:226–33. doi: 10.1001/archinternmed.2010.514. [DOI] [PubMed] [Google Scholar]
- 33.Fraser CG. Biological Variation: From Principles to Practice. Washington DC: AACC press; 2001. [Google Scholar]
- 34.Minchinela J, Ricós C, Perich C, Fernández-Calle P, Alvarez V, Domenech M, et al. Biological variation database, and quality specifications for imprecision, bias and total error (desirable and minimum). The 2012 update. http://www.westgard.com/biodatabase-2012-update.htm (Accessed 12 October 2016)
- 35.Ricós C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, Jimenez CV, et al. Desirable specifications for total error, imprecision, and bias, derived from intra- and inter-individual biologic variation. The 2014 update. http://www.westgard.com/biodatabase1.htm (Accessed 12 October 2016)
- 36.Lin J, Fernandez H, Shashaty MG, Negoianu D, Testani JM, Berns JS, et al. False-positive rate of AKI using consensus creatinine-based criteria. Clin J Am Soc Nephrol. 2015;10:1723–31. doi: 10.2215/CJN.02430315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas ME, Blaine C, Dawnay A, Devonald MA, Ftouh S, Laing C, et al. The definition of acute kidney injury and its use in practice. Kidney Int. 2015;87:62–73. doi: 10.1038/ki.2014.328. [DOI] [PubMed] [Google Scholar]
- 38.Garner AE, Lewington AJ, Barth JH. Detection of patients with acute kidney injury by the clinical laboratory using rises in serum creatinine: comparison of proposed definitions and a laboratory delta check. Ann Clin Biochem. 2012;49:59–62. doi: 10.1258/acb.2011.011125. [DOI] [PubMed] [Google Scholar]
- 39.Fraser CG, Williams P. Short-term biological variation of plasma analytes in renal disease. Clin Chem. 1983;29:508–10. [PubMed] [Google Scholar]
- 40.Hölzel WG. Intra-individual variation of some analytes in serum of patients with insulin-dependent diabetes mellitus. Clin Chem. 1987;33:57–61. [PubMed] [Google Scholar]
- 41.Hölzel WG. Intra-individual variation of some analytes in serum of patients with chronic renal failure. Clin Chem. 1987;33:670–3. [PubMed] [Google Scholar]
- 42.Hölzel WG. Intra-individual variation of analytes in serum from patients with chronic liver diseases. Clin Chem. 1987;33:1133–6. [PubMed] [Google Scholar]
- 43.Carter JL, Parker CT, Stevens PE, Eaglestone G, Knight S, Farmer CK, et al. Biological Variation of Plasma and Urinary Markers of Acute Kidney Injury in Patients with Chronic Kidney Disease. Clin Chem. 2016;62:876–83. doi: 10.1373/clinchem.2015.250993. [DOI] [PubMed] [Google Scholar]
- 44.Ricós C, Iglesias N, García-Lario JV, Simón M, Cava F, Hernández A, et al. Within-subject biological variation in disease: collated data and clinical consequences. Ann Clin Biochem. 2007;44:343–52. doi: 10.1258/000456307780945633. [DOI] [PubMed] [Google Scholar]
- 45.Fraser CG, Harris EK. Generation and application of data on biological variation in clinical chemistry. Crit Rev Clin Lab Sci. 1989;27:409–37. doi: 10.3109/10408368909106595. [DOI] [PubMed] [Google Scholar]
- 46.Ceriotti F, Boyd JC, Klein G, Henny J, Queraltó J, Kairisto V, et al. IFCC Committee on Reference Intervals and Decision Limits (C-RIDL) Reference intervals for serum creatinine concentrations: assessment of available data for global application. Clin Chem. 2008;54:559–66. doi: 10.1373/clinchem.2007.099648. [DOI] [PubMed] [Google Scholar]
- 47.Makris K, Stefani D, Makri E, Panagou I, Lagiou M, Sarli A, et al. Evaluation of a particle enhanced turbidimetric assay for the measurement of neutrophil gelatinase-associated lipocalin in plasma and urine on Architect-8000: Analytical performance and establishment of reference values. Clin Biochem. 2015;48:1291–7. doi: 10.1016/j.clinbiochem.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Fokkema MR, Herrmann Z, Muskiet FA, Moecks J. Reference change values for brain natriuretic peptides revisited. Clin Chem. 2006;52:1602–3. doi: 10.1373/clinchem.2006.069369. [DOI] [PubMed] [Google Scholar]
- 49.Braga F, Ferraro S, Ieva F, Paganoni A, Panteghini M. A new robust statistical model for interpretation of differences in serial test results from an individual. Clin Chem Lab Med. 2015;53:815–22. doi: 10.1515/cclm-2014-0893. [DOI] [PubMed] [Google Scholar]
- 50.Lund F, Petersen PH, Fraser CG, Sölétormos G. Calculation of limits for significant unidirectional changes in two or more serial results of a biomarker based on a computer simulation model. Ann Clin Biochem. 2015;52:237–44. doi: 10.1177/0004563214534636. [DOI] [PubMed] [Google Scholar]
- 51.Lund F, Petersen PH, Fraser CG, Sölétormos G. Calculation of limits for significant bidirectional changes in two or more serial results of a biomarker based on a computer simulation model. Ann Clin Biochem. 2015;52:434–40. doi: 10.1177/0004563214555163. [DOI] [PubMed] [Google Scholar]
- 52.Ricci Z, Cruz DN, Ronco C. Classification and staging of acute kidney injury: beyond the RIFLE and AKIN criteria. Nat Rev Nephrol. 2011;7:201–8. doi: 10.1038/nrneph.2011.14. [DOI] [PubMed] [Google Scholar]
- 53.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34:1913–7. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- 54.Bagshaw SM, George C, Dinu I, Bellomo R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1203–10. doi: 10.1093/ndt/gfm744. [DOI] [PubMed] [Google Scholar]
- 55.Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007;35:1837–43. doi: 10.1097/01.CCM.0000277041.13090.0A. quiz 1852. [DOI] [PubMed] [Google Scholar]
- 56.Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bell M, Liljestam E, Granath F, Fryckstedt J, Ekbom A, Martling CR. Optimal follow-up time after continuous renal replacement therapy in actual renal failure patients stratified with the RIFLE criteria. Nephrol Dial Transplant. 2005;20:354–60. doi: 10.1093/ndt/gfh581. [DOI] [PubMed] [Google Scholar]
- 58.Závada J, Hoste E, Cartin-Ceba R, Calzavacca P, Gajic O, Clermont G, et al. AKI6 investigators A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol Dial Transplant. 2010;25:3911–8. doi: 10.1093/ndt/gfp766. [DOI] [PubMed] [Google Scholar]
- 59.Pickering JW, Endre ZH. Back-calculating baseline creatinine with MDRD misclassifies acute kidney injury in the intensive care unit. Clin J Am Soc Nephrol. 2010;5:1165–73. doi: 10.2215/CJN.08531109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hsu CY, McCulloch CE, Fan D, Ordoñez JD, Chertow GM, Go AS. Community-based incidence of acute renal failure. Kidney Int. 2007;72:208–12. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Broce JC, Price LL, Liangos O, Uhlig K, Jaber BL. Hospital-acquired acute kidney injury: an analysis of nadir-to-peak serum creatinine increments stratified by baseline estimated GFR. Clin J Am Soc Nephrol. 2011;6:1556–65. doi: 10.2215/CJN.08470910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doi K, Yuen PS, Eisner C, Hu X, Leelahavanichkul A, Schnermann J, et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009;20:1217–21. doi: 10.1681/ASN.2008060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prowle JR, Kolic I, Purdell-Lewis J, Taylor R, Pearse RM, Kirwan CJ. Serum creatinine changes associated with critical illness and detection of persistent renal dysfunction after AKI. Clin J Am Soc Nephrol. 2014;9:1015–23. doi: 10.2215/CJN.11141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu KD, Thompson BT, Ancukiewicz M, Steingrub JS, Douglas IS, Matthay MA, et al. National Institutes of Health National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network Acute kidney injury in patients with acute lung injury: impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit Care Med. 2011;39:2665–71. doi: 10.1097/CCM.0b013e318228234b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Little RJA, Rubin DB. Statistical Analysis with Missing Data. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 66.Siew ED, Peterson JF, Eden SK, Moons KG, Ikizler TA, Matheny ME. Use of multiple imputation method to improve estimation of missing baseline serum creatinine in acute kidney injury research. Clin J Am Soc Nephrol. 2013;8:10–8. doi: 10.2215/CJN.00200112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Macedo E, Malhotra R, Claure-Del Granado R, Fedullo P, Mehta RL. Defining urine output criterion for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2011;26:509–15. doi: 10.1093/ndt/gfq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Md Ralib A, Pickering JW, Shaw GM, Endre ZH. The urine output definition of acute kidney injury is too liberal. Crit Care. 2013;17:R112. doi: 10.1186/cc12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int. 2008;73:538–46. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 70.Legrand M, Payen D. Understanding urine output in critically ill patients. Ann Intensive Care. 2011;1:13. doi: 10.1186/2110-5820-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Macedo E, Malhotra R, Bouchard J, Wynn SK, Mehta RL. Oliguria is an early predictor of higher mortality in critically ill patients. Kidney Int. 2011;80:760–7. doi: 10.1038/ki.2011.150. [DOI] [PubMed] [Google Scholar]
- 72.Prowle JR, Liu YL, Licari E, Bagshaw SM, Egi M, Haase M, et al. Oliguria as predictive biomarker of acute kidney injury in critically ill patients. Crit Care. 2011;15:R172. doi: 10.1186/cc10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vanmassenhove J, Glorieux G, Hoste E, Dhondt A, Vanholder R, Van Biesen W. Urinary output and fractional excretion of sodium and urea as indicators of transient versus intrinsic acute kidney injury during early sepsis. Crit Care. 2013;17:R234. doi: 10.1186/cc13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leedahl DD, Frazee EN, Schramm GE, Dierkhising RA, Bergstralh EJ, Chawla LS, et al. Derivation of urine output thresholds that identify a very high risk of AKI in patients with septic shock. Clin J Am Soc Nephrol. 2014;9:1168–74. doi: 10.2215/CJN.09360913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chau K, Schisler T, Er L, Jaswal D, Cheung C, Israel A, et al. Fluid balance, change in serum creatinine and urine output as markers of acute kidney injury post cardiac surgery: an observational study. Can J Kidney Health Dis. 2014;1:19. doi: 10.1186/s40697-014-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cruz DN, Bolgan I, Perazella MA, Bonello M, de Cal M, Corradi V, et al. North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI) Investigators North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI): targeting the problem with the RIFLE Criteria. Clin J Am Soc Nephrol. 2007;2:418–25. doi: 10.2215/CJN.03361006. [DOI] [PubMed] [Google Scholar]
- 77.Haase M, Bellomo R, Matalanis G, Calzavacca P, Dragun D, Haase-Fielitz A. A comparison of the RIFLE and Acute Kidney Injury Network classifications for cardiac surgery-associated acute kidney injury: a prospective cohort study. J Thorac Cardiovasc Surg. 2009;138:1370–6. doi: 10.1016/j.jtcvs.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 78.Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G. Classifying AKI by Urine Output versus Serum Creatinine Level. J Am Soc Nephrol. 2015;26:2231–8. doi: 10.1681/ASN.2014070724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 80.Jelliffe R. Estimation of creatinine clearance in patients with unstable renal function, without a urine specimen. Am J Nephrol. 2002;22:320–4. doi: 10.1159/000065221. [DOI] [PubMed] [Google Scholar]
- 81.Bouchard J, Macedo E, Soroko S, Chertow GM, Himmelfarb J, Ikizler TA, et al. Program to Improve Care in Acute Renal Disease Comparison of methods for estimating glomerular filtration rate in critically ill patients with acute kidney injury. Nephrol Dial Transplant. 2010;25:102–7. doi: 10.1093/ndt/gfp392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rabito CA, Panico F, Rubin R, Tolkoff-Rubin N, Teplick R. Noninvasive, real-time monitoring of renal function during critical care. J Am Soc Nephrol. 1994;4:1421–8. doi: 10.1681/ASN.V471421. [DOI] [PubMed] [Google Scholar]
- 83.Parikh CR, Coca SG. Acute kidney injury: defining prerenal azotemia in clinical practice and research. Nat Rev Nephrol. 2010;6:641–2. doi: 10.1038/nrneph.2010.128. [DOI] [PubMed] [Google Scholar]
- 84.Bellomo R, Bagshaw S, Langenberg C, Ronco C. Prerenal azotemia: a flawed paradigm in critically ill septic patients? Contrib Nephrol. 2007;156:1–9. doi: 10.1159/000102008. [DOI] [PubMed] [Google Scholar]
- 85.Uchino S. The meaning of transient azotemia. Contrib Nephrol. 2010;165:337–44. doi: 10.1159/000313775. [DOI] [PubMed] [Google Scholar]
- 86.Endre ZH, Kellum JA, Di Somma S, Doi K, Goldstein SL, Koyner JL, et al. Differential diagnosis of AKI in clinical practice by functional and damage biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol. 2013;182:30–44. doi: 10.1159/000349964. [DOI] [PubMed] [Google Scholar]
- 87.Singer E, Elger A, Elitok S, Kettritz R, Nickolas TL, Barasch J, et al. Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int. 2011;80:405–14. doi: 10.1038/ki.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Uchino S, Bellomo R, Bagshaw SM, Goldsmith D. Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant. 2010;25:1833–9. doi: 10.1093/ndt/gfp624. [DOI] [PubMed] [Google Scholar]
- 89.Brown JR, Kramer RS, Coca SG, Parikh CR. Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg. 2010;90:1142–8. doi: 10.1016/j.athoracsur.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Coca SG, King JT, Jr, Rosenthal RA, Perkal MF, Parikh CR. The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int. 2010;78:926–33. doi: 10.1038/ki.2010.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nejat M, Pickering JW, Devarajan P, Bonventre JV, Edelstein CL, Walker RJ, et al. Some biomarkers of acute kidney injury are increased in pre-renal acute injury. Kidney Int. 2012;81:1254–62. doi: 10.1038/ki.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013;187:509–17. doi: 10.1164/rccm.201211-1983OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schneider AG, Bellomo R. Urinalysis and pre-renal acute kidney injury: time to move on. Crit Care. 2013;17:141. doi: 10.1186/cc12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perinel S, Vincent F, Lautrette A, Dellamonica J, Mariat C, Zeni F, et al. Transient and persistent acute kidney injury and the risk of hospital mortality in critically ill patients: results of a multicenter cohort study. Crit Care Med. 2015;43:e269–75. doi: 10.1097/CCM.0000000000001077. [DOI] [PubMed] [Google Scholar]
- 95.Macedo E, Mehta RL. Prerenal failure: from old concepts to new paradigms. Curr Opin Crit Care. 2009;15:467–73. doi: 10.1097/MCC.0b013e328332f6e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pépin MN, Bouchard J, Legault L, Ethier J. Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis. 2007;50:566–73. doi: 10.1053/j.ajkd.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 97.Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–9. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Doi K, Katagiri D, Negishi K, Hasegawa S, Hamasaki Y, Fujita T, et al. Mild elevation of urinary biomarkers in prerenal acute kidney injury. Kidney Int. 2012;82:1114–20. doi: 10.1038/ki.2012.266. [DOI] [PubMed] [Google Scholar]
- 99.Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–61. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kanbay M, Kasapoglu B, Perazella MA. Acute tubular necrosis and pre-renal acute kidney injury: utility of urine microscopy in their evaluation- a systematic review. Int Urol Nephrol. 2010;42:425–33. doi: 10.1007/s11255-009-9673-3. [DOI] [PubMed] [Google Scholar]
- 101.Bagshaw SM, Langenberg C, Bellomo R. Urinary biochemistry and microscopy in septic acute renal failure: a systematic review. Am J Kidney Dis. 2006;48:695–705. doi: 10.1053/j.ajkd.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 102.Perazella MA, Coca SG. Traditional urinary biomarkers in the assessment of hospital-acquired AKI. Clin J Am Soc Nephrol. 2012;7:167–74. doi: 10.2215/CJN.09490911. [DOI] [PubMed] [Google Scholar]
- 103.Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB. Toward the optimal clinical use of the fraction excretion of solutes in oliguric azotemia. Ren Fail. 2010;32:1245–54. doi: 10.3109/0886022X.2010.517353. [DOI] [PubMed] [Google Scholar]
- 104.Sodium homeostasis in chronic renal disease. Kidney Int. 1982;21:886–97. doi: 10.1038/ki.1982.116. [DOI] [PubMed] [Google Scholar]
- 105.Gotfried J, Wiesen J, Raina R, Nally JV., Jr Finding the cause of acute kidney injury: which index of fractional excretion is better? Cleve Clin J Med. 2012;79:121–6. doi: 10.3949/ccjm.79a.11030. [DOI] [PubMed] [Google Scholar]
- 106.Espinel CH. The FENa test. Use in the differential diagnosis of acute renal failure. JAMA. 1976;236:579–81. doi: 10.1001/jama.236.6.579. [DOI] [PubMed] [Google Scholar]
- 107.Miller TR, Anderson RJ, Linas SL, Henrich WL, Berns AS, Gabow PA, et al. Urinary diagnostic indices in acute renal failure: a prospective study. Ann Intern Med. 1978;89:47–50. doi: 10.7326/0003-4819-89-1-47. [DOI] [PubMed] [Google Scholar]
- 108.Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB. The comparative benefits of the fractional excretion of urea and sodium in various azotemic oliguric states. Nephron Clin Pract. 2010;114:c145–50. doi: 10.1159/000254387. [DOI] [PubMed] [Google Scholar]
- 109.Vaz AJ. Low fractional excretion of urine sodium in acute renal failure due to sepsis. Arch Intern Med. 1983;143:738–9. [PubMed] [Google Scholar]
- 110.Corwin HL, Schreiber MJ, Fang LS. Low fractional excretion of sodium. Occurrence with hemoglobinuricand myoglobinuric-induced acute renal failure. Arch Intern Med. 1984;144:981–2. doi: 10.1001/archinte.144.5.981. [DOI] [PubMed] [Google Scholar]
- 111.Fang LS, Sirota RA, Ebert TH, Lichtenstein NS. Low fractional excretion of sodium with contrast media-induced acute renal failure. Arch Intern Med. 1980;140:531–3. [PubMed] [Google Scholar]
- 112.Zarich S, Fang LS, Diamond JR. Fractional excretion of sodium. Exceptions to its diagnostic value. Arch Intern Med. 1985;145:108–12. [PubMed] [Google Scholar]
- 113.Bagshaw SM, Bennett M, Devarajan P, Bellomo R. Urine biochemistry in septic and non-septic acute kidney injury: a prospective observational study. J Crit Care. 2013;28:371–8. doi: 10.1016/j.jcrc.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Darmon M, Vincent F, Dellamonica J, Schortgen F, Gonzalez F, Das V, et al. Diagnostic performance of fractional excretion of urea in the evaluation of critically ill patients with acute kidney injury: a multicenter cohort study. Crit Care. 2011;15:R178. doi: 10.1186/cc10327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pons B, Lautrette A, Oziel J, Dellamonica J, Vermesch R, Ezingeard E, et al. Diagnostic accuracy of early urinary index changes in differentiating transient from persistent acute kidney injury in critically ill patients: multicenter cohort study. Crit Care. 2013;17:R56. doi: 10.1186/cc12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Carvounis CP, Nisar S, Guro-Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int. 2002;62:2223–9. doi: 10.1046/j.1523-1755.2002.00683.x. [DOI] [PubMed] [Google Scholar]
- 117.Kaplan AA, Kohn OF. Fractional excretion of urea as a guide to renal dysfunction. Am J Nephrol. 1992;12:49–54. doi: 10.1159/000168417. [DOI] [PubMed] [Google Scholar]
- 118.Musch W, Verfaillie L, Decaux G. Age-related increase in plasma urea level and decrease in fractional urea excretion: clinical application in the syndrome of inappropriate secretion of antidiuretic hormone. Clin J Am Soc Nephrol. 2006;1:909–14. doi: 10.2215/CJN.00320106. [DOI] [PubMed] [Google Scholar]
- 119.Espinel CH, Gregory AW. Differential diagnosis of acute renal failure. Clin Nephrol. 1980;13:73–7. [PubMed] [Google Scholar]
- 120.Esson ML, Schrier RW. Diagnosis and treatment of acute tubular necrosis. Ann Intern Med. 2002;137:744–52. doi: 10.7326/0003-4819-137-9-200211050-00010. [DOI] [PubMed] [Google Scholar]
- 121.Bagshaw SM, Gibney RT. Acute kidney injury: clinical value of urine microscopy in acute kidney injury. Nat Rev Nephrol. 2009;5:185–6. doi: 10.1038/nrneph.2009.23. [DOI] [PubMed] [Google Scholar]
- 122.Perazella MA, Parikh CR. How can urine microscopy influence the differential diagnosis of AKI? Clin J Am Soc Nephrol. 2009;4:691–3. doi: 10.2215/CJN.06691208. [DOI] [PubMed] [Google Scholar]
- 123.Bagshaw SM, Langenberg C, Wan L, May CN, Bellomo R. A systematic review of urinary findings in experimental septic acute renal failure. Crit Care Med. 2007;35:1592–8. doi: 10.1097/01.CCM.0000266684.17500.2F. [DOI] [PubMed] [Google Scholar]
- 124.Perazella MA, Coca SG, Kanbay M, Brewster UC, Parikh CR. Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2008;3:1615–9. doi: 10.2215/CJN.02860608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Perazella MA, Coca SG, Hall IE, Iyanam U, Koraishy M, Parikh CR. Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2010;5:402–8. doi: 10.2215/CJN.06960909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schinstock CA, Semret MH, Wagner SJ, Borland TM, Bryant SC, Kashani KB, et al. Urinalysis is more specific and urinary neutrophil gelatinase-associated lipocalin is more sensitive for early detection of acute kidney injury. Nephrol Dial Transplant. 2013;28:1175–85. doi: 10.1093/ndt/gfs127. [DOI] [PubMed] [Google Scholar]
- 127.Sharda N, Bakhtar O, Thajudeen B, Meister E, Szerlip H. Manual urine microscopy versus automated urine analyzer microscopy in patients with acute kidney injury. Lab Med. 2014;45:e152–5. doi: 10.1309/LMVJK6W4KQL1ZHKS. [DOI] [PubMed] [Google Scholar]
- 128.American Society of Nephrology Renal Research Report. J Am Soc Nephrol. 2005;16:1886–903. doi: 10.1681/ASN.2005030285. [DOI] [PubMed] [Google Scholar]
- 129.Nguyen MT, Devarajan P. Biomarkers for the early detection of acute kidney injury. Pediatr Nephrol. 2008;23:2151–7. doi: 10.1007/s00467-007-0470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ostermann M, Philips BJ, Forni LG. Clinical review: Biomarkers of acute kidney injury: where are we now? Crit Care. 2012;16:233. doi: 10.1186/cc11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ho J, Tangri N, Komenda P, Kaushal A, Sood M, Brar R, et al. Urinary, Plasma, and Serum Biomarkers’ Utility for Predicting Acute Kidney Injury Associated With Cardiac Surgery in Adults: A Meta-analysis. Am J Kidney Dis. 2015;66:993–1005. doi: 10.1053/j.ajkd.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 132.Haase-Fielitz A, Haase M, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Ann Clin Biochem. 2014;51:335–51. doi: 10.1177/0004563214521795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu S, Che M, Xue S, Xie B, Zhu M, Lu R, et al. Urinary L-FABP and its combination with urinary NGAL in early diagnosis of acute kidney injury after cardiac surgery in adult patients. Biomarkers. 2013;18:95–101. doi: 10.3109/1354750X.2012.740687. [DOI] [PubMed] [Google Scholar]
- 134.Susantitaphong P, Siribamrungwong M, Doi K, Noiri E, Terrin N, Jaber BL. Performance of urinary liver-type fatty acid-binding protein in acute kidney injury: a meta-analysis. Am J Kidney Dis. 2013;61:430–9. doi: 10.1053/j.ajkd.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang Z, Lu B, Sheng X, Jin N. Cystatin C in prediction of acute kidney injury: a systemic review and meta-analysis. Am J Kidney Dis. 2011;58:356–65. doi: 10.1053/j.ajkd.2011.02.389. [DOI] [PubMed] [Google Scholar]
- 136.Waanders F, Navis G, van Goor H. Urinary tubular biomarkers of kidney damage: potential value in clinical practice. Am J Kidney Dis. 2010;55:813–6. doi: 10.1053/j.ajkd.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 137.Waanders F, van Timmeren MM, Stegeman CA, Bakker SJ, van Goor H. Kidney injury molecule-1 in renal disease. J Pathol. 2010;220:7–16. doi: 10.1002/path.2642. [DOI] [PubMed] [Google Scholar]
- 138.Koyner JL, Vaidya VS, Bennett MR, Ma Q, Worcester E, Akhter SA, et al. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol. 2010;5:2154–65. doi: 10.2215/CJN.00740110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–93. doi: 10.1146/annurev.pharmtox.48.113006.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vaidya VS, Waikar SS, Ferguson MA, Collings FB, Sunderland K, Gioules C, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. 2008;1:200–8. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.de Geus HR, Betjes MG, Bakker J. Biomarkers for the prediction of acute kidney injury: a narrative review on current status and future challenges. Clin Kidney J. 2012;5:102–8. doi: 10.1093/ckj/sfs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vanmassenhove J, Vanholder R, Nagler E, Van Biesen W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrol Dial Transplant. 2013;28:254–73. doi: 10.1093/ndt/gfs380. [DOI] [PubMed] [Google Scholar]
- 143.Tsigou E, Psallida V, Demponeras C, Boutzouka E, Baltopoulos G. Role of new biomarkers: functional and structural damage. Crit Care Res Pract. 2013;2013:361078. doi: 10.1155/2013/361078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Makris K, Rizos D, Kafkas N, Haliassos A. Neurophil gelatinase-associated lipocalin as a new biomarker in laboratory medicine. Clin Chem Lab Med. 2012;50:1519–32. doi: 10.1515/cclm-2012-0227. [DOI] [PubMed] [Google Scholar]
- 145.Makris K, Kafkas N. Neutrophil gelatinase-associated lipocalin in acute kidney injury. Adv Clin Chem. 2012;58:141–91. doi: 10.1016/b978-0-12-394383-5.00012-6. [DOI] [PubMed] [Google Scholar]
- 146.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A, NGAL Meta-analysis Investigator Group Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–24. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 147.Siew ED, Ware LB, Ikizler TA. Biological markers of acute kidney injury. J Am Soc Nephrol. 2011;22:810–20. doi: 10.1681/ASN.2010080796. [DOI] [PubMed] [Google Scholar]
- 148.Waikar SS, Betensky RA, Emerson SC, Bonventre JV. Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol. 2012;23:13–21. doi: 10.1681/ASN.2010111124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Waikar SS, Betensky RA, Bonventre JV. Creatinine as the gold standard for kidney injury biomarker studies? Nephrol Dial Transplant. 2009;24:3263–5. doi: 10.1093/ndt/gfp428. [DOI] [PubMed] [Google Scholar]
- 150.McCullough PA, Shaw AD, Haase M, Bouchard J, Waikar SS, Siew ED, et al. Diagnosis of acute kidney injury using functional and injury biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol. 2013;182:13–29. doi: 10.1159/000349963. [DOI] [PubMed] [Google Scholar]
- 151.McCullough PA, Bouchard J, Waikar SS, Siew ED, Endre ZH, Goldstein SL, et al. Implementation of novel biomarkers in the diagnosis, prognosis, and management of acute kidney injury: executive summary from the tenth consensus conference of the Acute Dialysis Quality Initiative (ADQI) Contrib Nephrol. 2013;182:5–12. doi: 10.1159/000349962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.de Geus HR, Woo JG, Wang Y, Devarajan P, Betjes MG, le Noble JL, et al. Urinary Neutrophil Gelatinase-Associated Lipocalin Measured on Admission to the Intensive Care Unit Accurately Discriminates between Sustained and Transient Acute Kidney Injury in Adult Critically Ill Patients. Nephron Extra. 2011;1:9–23. doi: 10.1159/000330428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nickolas TL, Schmidt-Ott KM, Canetta P, Forster C, Singer E, Sise M, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012;59:246–55. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Koenig W. Cardiovascular biomarkers: added value with an integrated approach? Circulation. 2007;116:3–5. doi: 10.1161/CIRCULATIONAHA.107.707984. [DOI] [PubMed] [Google Scholar]
- 155.Morrow DA, Braunwald E. Future of biomarkers in acute coronary syndromes: moving toward a multimarker strategy. Circulation. 2003;108:250–2. doi: 10.1161/01.CIR.0000078080.37974.D2. [DOI] [PubMed] [Google Scholar]
- 156.Morrow DA. New insight into clinical risk scores for patients with acute coronary syndromes. Am Heart J. 2003;146:754–6. doi: 10.1016/S0002-8703(03)00460-5. [DOI] [PubMed] [Google Scholar]
- 157.Katagiri D, Doi K, Honda K, Negishi K, Fujita T, Hisagi M, et al. Combination of two urinary biomarkers predicts acute kidney injury after adult cardiac surgery. Ann Thorac Surg. 2012;93:577–83. doi: 10.1016/j.athoracsur.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 158.Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73:863–9. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2009;4:873–82. doi: 10.2215/CJN.04810908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Arthur JM, Hill EG, Alge JL, Lewis EC, Neely BA, Janech MG, et al. SAKInet Investigators Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int. 2014;85:431–8. doi: 10.1038/ki.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Prowle JR, Calzavacca P, Licari E, Ligabo EV, Echeverri JE, Bagshaw SM, et al. Combination of biomarkers for diagnosis of acute kidney injury after cardiopulmonary bypass. Ren Fail. 2015;37:408–16. doi: 10.3109/0886022X.2014.1001303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Goldstein SL. Urinary kidney injury biomarkers and urine creatinine normalization: a false premise or not? Kidney Int. 2010;78:433–5. doi: 10.1038/ki.2010.200. [DOI] [PubMed] [Google Scholar]
- 163.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 164.Waikar SS, Sabbisetti VS, Bonventre JV. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010;78:486–94. doi: 10.1038/ki.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, et al. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care. 2007;11:R84. doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wagener G, Minhaz M, Mattis FA, Kim M, Emond JC, Lee HT. Urinary neutrophil gelatinase-associated lipocalin as a marker of acute kidney injury after orthotopic liver transplantation. Nephrol Dial Transplant. 2011;26:1717–23. doi: 10.1093/ndt/gfq770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kaufeld JK, Gwinner W, Scheffner I, Haller HG, Schiffer M. Urinary NGAL ratio is not a sensitive biomarker for monitoring acute tubular injury in kidney transplant patients: NGAL and ATI in renal transplant patients. J Transplant. 2012;2012:563404. doi: 10.1155/2012/563404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, et al. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant. 2006;6:1639–45. doi: 10.1111/j.1600-6143.2006.01352.x. [DOI] [PubMed] [Google Scholar]
- 169.Herget-Rosenthal S, Poppen D, Hüsing J, Marggraf G, Pietruck F, Jakob HG, et al. Prognostic value of tubular proteinuria and enzymuria in nonoliguric acute tubular necrosis. Clin Chem. 2004;50:552–8. doi: 10.1373/clinchem.2003.027763. [DOI] [PubMed] [Google Scholar]
- 170.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 171.Nejat M, Hill JV, Pickering JW, Edelstein CL, Devarajan P, Endre ZH. Albuminuria increases cystatin C excretion: implications for urinary biomarkers. Nephrol Dial Transplant. 2012;27(Suppl 3):iii96–103. doi: 10.1093/ndt/gfr222. [DOI] [PubMed] [Google Scholar]
- 172.Varrier M, Forni LG, Ostermann M. Long-term sequelae from acute kidney injury: potential mechanisms for the observed poor renal outcomes. Crit Care. 2015;19:102. doi: 10.1186/s13054-015-0805-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–8. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516–24. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 175.Sawhney S, Mitchell M, Marks A, Fluck N, Black C. Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. BMJ Open. 2015;5:e006497. doi: 10.1136/bmjopen-2014-006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rifkin DE, Coca SG, Kalantar-Zadeh K. Does AKI truly lead to CKD? J Am Soc Nephrol. 2012;23:979–84. doi: 10.1681/ASN.2011121185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Belayev LY, Palevsky PM. The link between acute kidney injury and chronic kidney disease. Curr Opin Nephrol Hypertens. 2014;23:149–54. doi: 10.1097/01.mnh.0000441051.36783.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hsu CY. Yes, AKI truly leads to CKD. J Am Soc Nephrol. 2012;23:967–9. doi: 10.1681/ASN.2012030222. [DOI] [PubMed] [Google Scholar]
- 179.Greenberg JH, Coca S, Parikh CR. Long-term risk of chronic kidney disease and mortality in children after acute kidney injury: a systematic review. BMC Nephrol. 2014;15:184. doi: 10.1186/1471-2369-15-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol. 2010;298:F1078–94. doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Singh P, Rifkin DE, Blantz RC. Chronic kidney disease: an inherent risk factor for acute kidney injury? Clin J Am Soc Nephrol. 2010;5:1690–5. doi: 10.2215/CJN.00830110. [DOI] [PubMed] [Google Scholar]
- 182.Hsu CY, Ordoñez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74:101–7. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lafrance JP, Djurdjev O, Levin A. Incidence and outcomes of acute kidney injury in a referred chronic kidney disease cohort. Nephrol Dial Transplant. 2010;25:2203–9. doi: 10.1093/ndt/gfq011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Carmen Ricos and the Analytical Quality Commission of the Spanish Society of Clinical Chemistry in 1999 presented an overview of estimates of BV for a large number of constituents.1 As a continuation of this work, estimates of CVI (within subject BV) and CVG (between subjects BV), identified by literature searches, and the associated analytical quality specifications for bias, imprecision and total error have been presented on the Westgard webpage,2 being updated every two years. This data has been used as the main source of estimates of BV by laboratory scientists. The impact of this information is and has been huge.
Supplemental References
Ricos C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, Jimenez CV, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest 1999;59:491–500.
Ricos C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, Jimenez CV, et al. Desirable specifications for total error, imprecision, and bias, derived from intra- and inter-individual biologic variation. The 2014 update. www.westgard.com/biodatabase1.htm (Accessed May 2016).