Abstract
In previous work, a T-helper epitope was mapped within the circumsporozoite protein of the murine malaria parasite Plasmodium yoelii. A 21-mer synthetic peptide corresponding to this epitope (amino acid positions 59-79; referred to as Py1) induced a specific T-cell proliferation in BALB/c and C57BL/6 mice and provided help for the production of antibodies to peptides from the repetitive region, (Gln-Gly-Pro-Gly-Ala-Pro)n, of the P. yoelii circumsporozoite protein when mice were immunized with the Py1 peptide conjugated to the repetitive peptide. Experiments were then designed to study the in vitro antiparasite efficacy of T cells elicited in vivo by peptide immunization. T-cell activity was evaluated on cultured hepatic stages of P. yoelii. Peptide immunizations led to the preferential activation of CD8+ T cells in BALB/c mice and of both CD4+ and CD8+ T cells in C57BL/6 mice. Parasite elimination was mediated directly by these cells and did not seem to be dependent on lymphokine secretion. These data suggest that peptide-primed CD4+ T cells as well as CD8+ T cells could be cytolytic for the hepatic phase of malaria parasites. The fact that the same peptide could activate different lymphocyte populations, depending on the strain of mouse, highlights the importance of a better understanding of the fine mechanisms behind the immune responses to synthetic peptides being tested for malaria vaccine development.
Full text
PDF
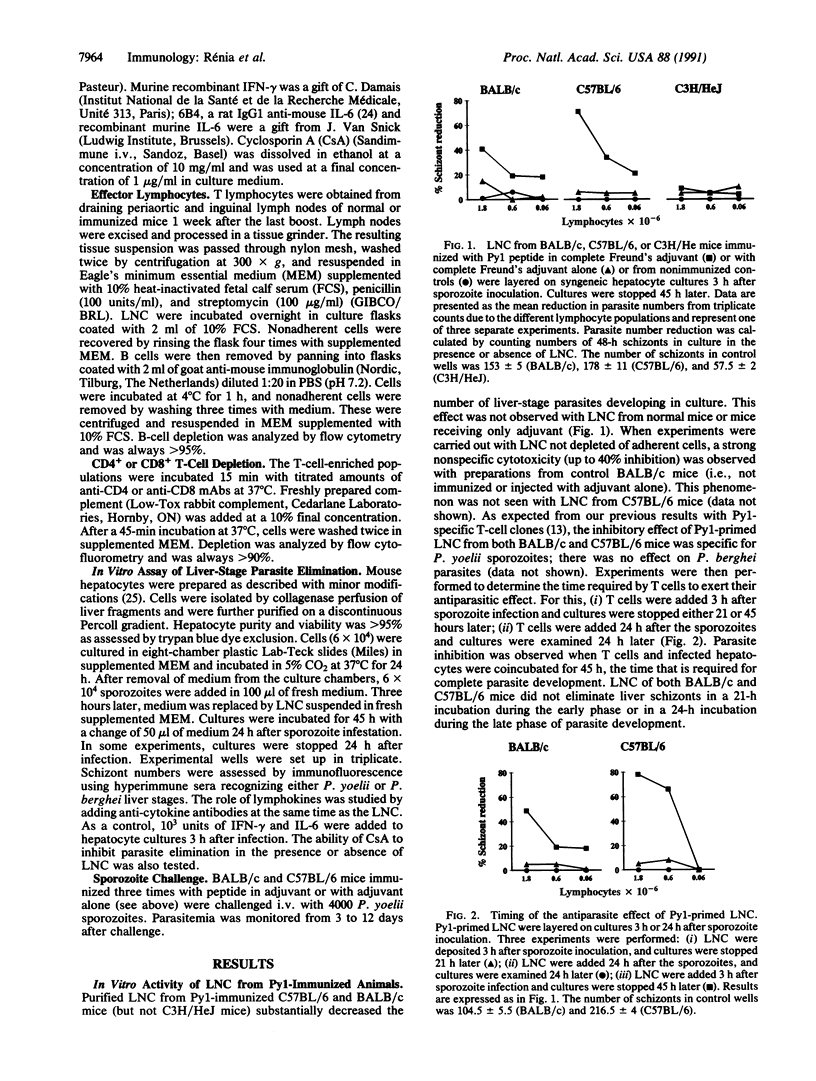
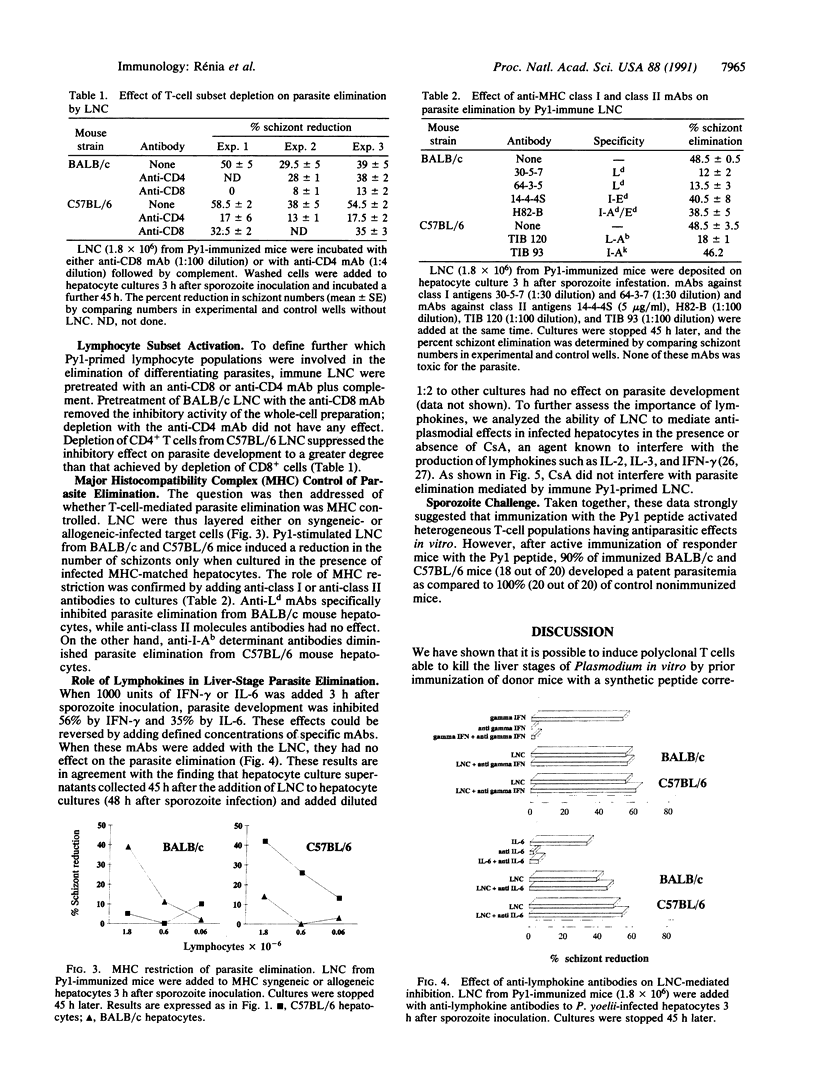


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharya A., Dorf M. E., Springer T. A. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981 Dec;127(6):2488–2495. [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Chen D. H., Tigelaar R. E., Weinbaum F. I. Immunity to sporozoite-induced malaria infeciton in mice. I. The effect of immunization of T and B cell-deficient mice. J Immunol. 1977 Apr;118(4):1322–1327. [PubMed] [Google Scholar]
- Del Giudice G., Grillot D., Rénia L., Müller I., Corradin G., Louis J. A., Mazier D., Lambert P. H. Peptide-primed CD4+ cells and malaria sporozoites. Immunol Lett. 1990 Aug;25(1-3):59–63. doi: 10.1016/0165-2478(90)90092-5. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Schofield L., Enea V., Schellekens H., van der Meide P., Collins W. E., Nussenzweig R. S., Nussenzweig V. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science. 1986 May 16;232(4752):881–884. doi: 10.1126/science.3085218. [DOI] [PubMed] [Google Scholar]
- Franco A., Barnaba V., Natali P., Balsano C., Musca A., Balsano F. Expression of class I and class II major histocompatibility complex antigens on human hepatocytes. Hepatology. 1988 May-Jun;8(3):449–454. doi: 10.1002/hep.1840080302. [DOI] [PubMed] [Google Scholar]
- Franco A., Barnaba V., Ruberti G., Benvenuto R., Balsano C., Musca A. Liver-derived T cell clones in autoimmune chronic active hepatitis: accessory cell function of hepatocytes expressing class II major histocompatibility complex molecules. Clin Immunol Immunopathol. 1990 Mar;54(3):382–394. doi: 10.1016/0090-1229(90)90052-r. [DOI] [PubMed] [Google Scholar]
- Gajewski T. F., Joyce J., Fitch F. W. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989 Jul 1;143(1):15–22. [PubMed] [Google Scholar]
- Granelli-Piperno A., Inaba K., Steinman R. M. Stimulation of lymphokine release from T lymphoblasts. Requirement for mRNA synthesis and inhibition by cyclosporin A. J Exp Med. 1984 Dec 1;160(6):1792–1802. doi: 10.1084/jem.160.6.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillot D., Michel M., Müller I., Tougne C., Rènia L., Mazier D., Corradin G., Lambert P. H., Louis J. A., Del Guidice G. Immune responses to defined epitopes of the circumsporozoite protein of the murine malaria parasite, Plasmodium yoelii. Eur J Immunol. 1990 Jun;20(6):1215–1222. doi: 10.1002/eji.1830200604. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R. Phenotypic and functional alteration of CD4+ T cells after antigen stimulation. Resolution of two populations of memory T cells that both secrete interleukin 4. J Exp Med. 1989 Jun 1;169(6):2245–2250. doi: 10.1084/jem.169.6.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold K. C., Lancki D. W., Moldwin R. L., Fitch F. W. Immunosuppressive effects of cyclosporin A on cloned T cells. J Immunol. 1986 Feb 15;136(4):1315–1321. [PubMed] [Google Scholar]
- Hoffman S. L., Isenbarger D., Long G. W., Sedegah M., Szarfman A., Waters L., Hollingdale M. R., van der Meide P. H., Finbloom D. S., Ballou W. R. Sporozoite vaccine induces genetically restricted T cell elimination of malaria from hepatocytes. Science. 1989 Jun 2;244(4908):1078–1081. doi: 10.1126/science.2524877. [DOI] [PubMed] [Google Scholar]
- Kast W. M., Roux L., Curren J., Blom H. J., Voordouw A. C., Meloen R. H., Kolakofsky D., Melief C. J. Protection against lethal Sendai virus infection by in vivo priming of virus-specific cytotoxic T lymphocytes with a free synthetic peptide. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2283–2287. doi: 10.1073/pnas.88.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khusmith S., Charoenvit Y., Kumar S., Sedegah M., Beaudoin R. L., Hoffman S. L. Protection against malaria by vaccination with sporozoite surface protein 2 plus CS protein. Science. 1991 May 3;252(5006):715–718. doi: 10.1126/science.1827210. [DOI] [PubMed] [Google Scholar]
- Lal A. A., de la Cruz V. F., Welsh J. A., Charoenvit Y., Maloy W. L., McCutchan T. F. Structure of the gene encoding the circumsporozoite protein of Plasmodium yoelii. A rodent model for examining antimalarial sporozoite vaccines. J Biol Chem. 1987 Mar 5;262(7):2937–2940. [PubMed] [Google Scholar]
- Lie W. R., Myers N. B., Gorka J., Rubocki R. J., Connolly J. M., Hansen T. H. Peptide ligand-induced conformation and surface expression of the Ld class I MHC molecule. Nature. 1990 Mar 29;344(6265):439–441. doi: 10.1038/344439a0. [DOI] [PubMed] [Google Scholar]
- Mazier D., Beaudoin R. L., Mellouk S., Druilhe P., Texier B., Trosper J., Miltgen F., Landau I., Paul C., Brandicourt O. Complete development of hepatic stages of Plasmodium falciparum in vitro. Science. 1985 Jan 25;227(4685):440–442. doi: 10.1126/science.3880923. [DOI] [PubMed] [Google Scholar]
- Mazier D., Rénia L., Nussler A., Pied S., Marussig M., Goma J., Grillot D., Miltgen F., Drapier J. C., Corradin G. Hepatic phase of malaria is the target of cellular mechanisms induced by the previous and the subsequent stages. A crucial role for liver nonparenchymal cells. Immunol Lett. 1990 Aug;25(1-3):65–70. doi: 10.1016/0165-2478(90)90093-6. [DOI] [PubMed] [Google Scholar]
- Mellouk S., Maheshwari R. K., Rhodes-Feuillette A., Beaudoin R. L., Berbiguier N., Matile H., Miltgen F., Landau I., Pied S., Chigot J. P. Inhibitory activity of interferons and interleukin 1 on the development of Plasmodium falciparum in human hepatocyte cultures. J Immunol. 1987 Dec 15;139(12):4192–4195. [PubMed] [Google Scholar]
- Momburg F., Koch N., Möller P., Moldenhauer G., Butcher G. W., Hämmerling G. J. Differential expression of Ia and Ia-associated invariant chain in mouse tissues after in vivo treatment with IFN-gamma. J Immunol. 1986 Feb 1;136(3):940–948. [PubMed] [Google Scholar]
- Natali P. G., Bigotti A., Nicotra M. R., Viora M., Manfredi D., Ferrone S. Distribution of human Class I (HLA-A,B,C) histocompatibility antigens in normal and malignant tissues of nonlymphoid origin. Cancer Res. 1984 Oct;44(10):4679–4687. [PubMed] [Google Scholar]
- Nussler A., Pied S., Goma J., Rénia L., Miltgen F., Grau G. E., Mazier D. TNF inhibits malaria hepatic stages in vitro via synthesis of IL-6. Int Immunol. 1991 Apr;3(4):317–321. doi: 10.1093/intimm/3.4.317. [DOI] [PubMed] [Google Scholar]
- Nussler A., Pied S., Pontet M., Miltgen F., Renia L., Gentilini M., Mazier D. Inflammatory status and preerythrocytic stages of malaria: role of the C-reactive protein. Exp Parasitol. 1991 Jan;72(1):1–7. doi: 10.1016/0014-4894(91)90114-c. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Pied S., Nussler A., Pontent M., Miltgen F., Matile H., Lambert P. H., Mazier D. C-reactive protein protects against preerythrocytic stages of malaria. Infect Immun. 1989 Jan;57(1):278–282. doi: 10.1128/iai.57.1.278-282.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pied S., Rénia L., Nüssler A., Miltgen F., Mazier D. Inhibitory activity of IL-6 on malaria hepatic stages. Parasite Immunol. 1991 Mar;13(2):211–217. doi: 10.1111/j.1365-3024.1991.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Pierres M., Goridis C., Golstein P. Inhibition of murine T cell-mediated cytolysis and T cell proliferation by a rat monoclonal antibody immunoprecipitating two lymphoid cell surface polypeptides of 94 000 and 180 000 molecular weight. Eur J Immunol. 1982 Jan;12(1):60–69. doi: 10.1002/eji.1830120112. [DOI] [PubMed] [Google Scholar]
- Poo W. J., Conrad L., Janeway C. A., Jr Receptor-directed focusing of lymphokine release by helper T cells. Nature. 1988 Mar 24;332(6162):378–380. doi: 10.1038/332378a0. [DOI] [PubMed] [Google Scholar]
- Potocnjak P., Yoshida N., Nussenzweig R. S., Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J Exp Med. 1980 Jun 1;151(6):1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero P., Maryanski J. L., Corradin G., Nussenzweig R. S., Nussenzweig V., Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989 Sep 28;341(6240):323–326. doi: 10.1038/341323a0. [DOI] [PubMed] [Google Scholar]
- Schofield L., Ferreira A., Altszuler R., Nussenzweig V., Nussenzweig R. S. Interferon-gamma inhibits the intrahepatocytic development of malaria parasites in vitro. J Immunol. 1987 Sep 15;139(6):2020–2025. [PubMed] [Google Scholar]
- Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- Schulz M., Zinkernagel R. M., Hengartner H. Peptide-induced antiviral protection by cytotoxic T cells. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):991–993. doi: 10.1073/pnas.88.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroishi T., Evans G. A., Appella E., Ozato K. Role of a disulfide bridge in the immune function of major histocompatibility class I antigen as studied by in vitro mutagenesis. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7544–7548. doi: 10.1073/pnas.81.23.7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoskiewicz M. J., Colvin R. B., Schneeberger E. E., Russell P. S. Widespread and selective induction of major histocompatibility complex-determined antigens in vivo by gamma interferon. J Exp Med. 1985 Nov 1;162(5):1645–1664. doi: 10.1084/jem.162.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitalny G. L., Havell E. A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984 May 1;159(5):1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitalny G. L., Verhave J. P., Meuwissen J. H., Nussenzweig R. S. Plasmodium berghei: T cell dependence of sporozoite-induced immunity in rodents. Exp Parasitol. 1977 Jun;42(1):73–81. doi: 10.1016/0014-4894(77)90063-7. [DOI] [PubMed] [Google Scholar]
- Sypek J. P., Wyler D. J. Susceptibility of lymphokine-resistant Leishmania to cell contact-mediated macrophage activation. J Infect Dis. 1988 Aug;158(2):392–397. doi: 10.1093/infdis/158.2.392. [DOI] [PubMed] [Google Scholar]
- Vink A., Coulie P. G., Wauters P., Nordan R. P., Van Snick J. B cell growth and differentiation activity of interleukin-HP1 and related murine plasmacytoma growth factors. Synergy with interleukin 1. Eur J Immunol. 1988 Apr;18(4):607–612. doi: 10.1002/eji.1830180418. [DOI] [PubMed] [Google Scholar]
- Walker C. M., Moody D. J., Stites D. P., Levy J. A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986 Dec 19;234(4783):1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- Weiss W. R., Mellouk S., Houghten R. A., Sedegah M., Kumar S., Good M. F., Berzofsky J. A., Miller L. H., Hoffman S. L. Cytotoxic T cells recognize a peptide from the circumsporozoite protein on malaria-infected hepatocytes. J Exp Med. 1990 Mar 1;171(3):763–773. doi: 10.1084/jem.171.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss W. R., Sedegah M., Beaudoin R. L., Miller L. H., Good M. F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci U S A. 1988 Jan;85(2):573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala F., Tam J. P., Barr P. J., Romero P. J., Ley V., Nussenzweig R. S., Nussenzweig V. Synthetic peptide vaccine confers protection against murine malaria. J Exp Med. 1987 Nov 1;166(5):1591–1596. doi: 10.1084/jem.166.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz V. F., Lal A. A., McCutchan T. F. Variation among circumsporozoite protein genes from rodent malarias. Mol Biochem Parasitol. 1988 Feb;28(1):31–38. doi: 10.1016/0166-6851(88)90176-4. [DOI] [PubMed] [Google Scholar]





