Summary
Background
Worldwide, ~35 million individuals are infected with HIV; ~25 million in sub-Saharan Africa (SSA). The WHO proposes using “treatment as prevention” (TasP) to eliminate HIV. Treatment suppresses viral load, decreasing the probability an individual transmits HIV. The elimination threshold is one new HIV infection per 1,000 individuals. Here, we test the hypothesis that TasP can substantially reduce epidemics and eliminate HIV. We estimate the impact of TasP, between 1996–2013, on the Danish HIV epidemic in Men-who-have-Sex-with-Men (MSM), an epidemic UNAIDS has identified as a priority for elimination.
Methods
We use a CD4-staged Bayesian back-calculation approach to estimate incidence, and the “hidden epidemic” (the number of HIV-infected undiagnosed MSM). We use data from an ongoing nationwide population-based study: the Danish HIV Cohort Study.
Findings
Incidence, and the hidden epidemic, decreased substantially after treatment was introduced in 1996. By 2013, incidence was close to the elimination threshold: 1·4 (median, 95% Bayesian Credible Interval (BCI): 0·4–2·1) new HIV infections per 1,000 MSM. There were only 617 (median, 95% BCI: 264–858) undiagnosed MSM. Decreasing incidence and increasing treatment coverage are highly correlated; a threshold effect is apparent.
Interpretation
Our study is the first to show that TasP can substantially reduce a country’s HIV epidemic, and bring it close to elimination. However, we have shown the effectiveness of TasP under optimal conditions: very high treatment coverage, and exceptionally high (98%) viral suppression rate. Unless these extremely challenging conditions can be met in SSA, the WHO’s global elimination strategy is unlikely to succeed.
Funding
NIAID/NIH
The World Health Organization (WHO)1 and UNAIDS2 have proposed using “treatment as prevention” (TasP) for the global elimination of HIV. They have set the elimination threshold at one new HIV infection per 1,000 individuals per year.3 To achieve their goal they propose diagnosing 90% of the ~35 million4 individuals currently infected with HIV, treating 90% of the diagnosed, and achieving viral suppression in 90% of treated individuals. In 2011 the landmark clinical trial, HPTN 052, demonstrated that treated individuals are less likely (than untreated individuals) to transmit HIV to their sex partners;5 i.e., treatment can act as prevention. The results showed that treating the HIV-infected partner in a discordant couple (i.e., a couple where only one partner is infected) was 96% effective in preventing HIV infection.5 More recently, the effect of treatment on reducing an individual’s risk of acquiring HIV infection has been demonstrated to occur in a rural community in KwaZulu-Natal in South Africa6 and in female sex workers in Kenya.7 However, it has not yet been determined whether TasP can be effective at the population-level and substantially reduce an HIV epidemic. The impact of TasP, on an epidemic, will depend upon: the number of HIV-infected individuals who are treated, the adherence levels of those individuals, and whether risk behavior changes. To have a major impact, TasP would need to substantially reduce the number of new HIV infections that occur each year; i.e., the incidence rate. Currently, four clinical trials8 are evaluating the effectiveness of TasP in decreasing incidence; the results will not be available for several years. Here, by using a historical dataset, we test the hypothesis that TasP can substantially reduce a country’s HIV epidemic and be an effective elimination tool for HIV.
We focus on an epidemic UNAIDS has identified as a priority for elimination:2 the Danish HIV epidemic in Men-who-have-Sex-with-Men (MSM). In Denmark, as in other resource-rich countries, HIV epidemics are concentrated in risk-groups; the major risk group is MSM. To test our hypothesis we use a CD4-staged Bayesian back-calculation approach9 and analyze an exceptional longitudinal dataset (beginning in 1995) from the Danish HIV Cohort Study (DHCS), The study began as an open cohort study, but after a few years became a nationwide population study.10,11 The study, which is still referred to as the DHCS, is ongoing. The DHCS dataset includes diagnosis and treatment data from all MSM, who have been diagnosed with HIV over the past ~20 years. We use the diagnosis data to estimate the annual incidence rate in MSM in Denmark and the size of the “hidden epidemic” (i.e., the number of HIV-infected undiagnosed MSM) over the same time period. We then determine the strength of the statistical association between the annual incidence rate and treatment coverage between 1996 and 2013, using treatment data from the DHCS. Finally we discuss the significant implications of our results for the current global health policies proposed by the WHO and UNAIDS, and the control of the HIV pandemic.
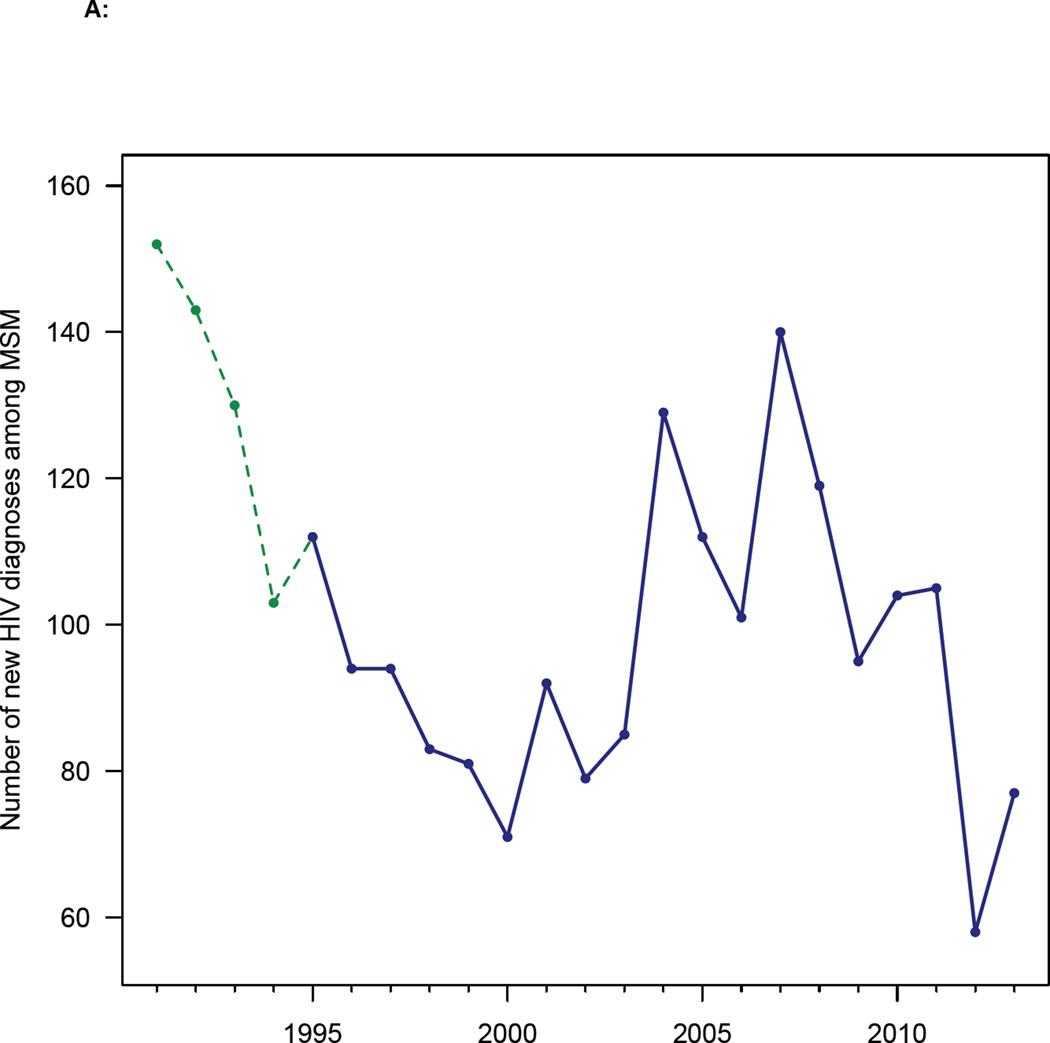
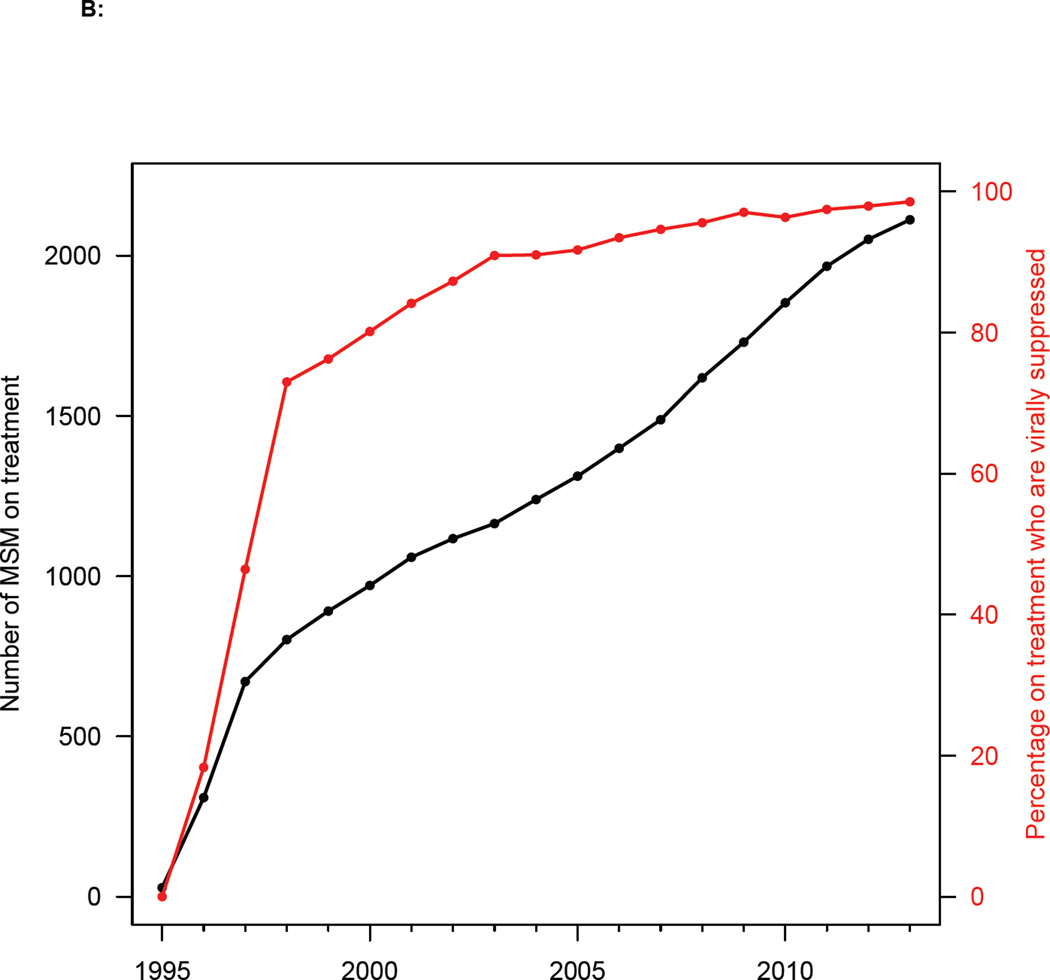
In Denmark the first AIDS case in MSM was diagnosed in 1982. HIV transmission among MSM was highest in the early/mid 1980’s12 and AIDS deaths peaked in the early 1990’s.13 Reporting HIV infections to a national surveillance system became mandatory in 1990; after 1995, the DHCS included all diagnosed individuals. Over the past 25 years the HIV diagnosis rate in MSM has varied considerably (figure 1A), as reflected in both the national surveillance data and the DHCS data. After the introduction of effective HIV therapies in Denmark in 1996, treatment coverage rapidly increased (figure 1B). The Danish treatment eligibility guidelines have changed over time. From 1996 to 2008, HIV-infected individuals were treated only after their CD4 cell count fell below 300 cells/mL. The treatment eligibility threshold was raised to 350 cells/mL in 2008. Beginning in 2011, all HIV-infected individuals (regardless of CD4 cell count) became eligible for immediate treatment. As coverage increased, so did the viral suppression rate (figure 1B), i.e., the percentage of patients who have a viral load of less than 200 copies/mL. The current viral suppression rate in Denmark, due to extremely high adherence, is 98%. These high adherence rates have resulted in negligible levels of transmitted drug resistance.14
Figure 1. Temporal trends in diagnosis and treatment rates.
(A) HIV diagnosis rates for all MSM diagnosed with HIV infection in Denmark between 1991 and 2013. Green dashed line shows national surveillance data and blue solid line shows Danish HIV Cohort Study (DHCS) data. (B) Uptake of treatment (black line), and increase in viral suppression rates (red line) after the introduction of combination therapies in 1996 (DHCS data). Data from all MSM treated in Denmark from 1995 to 2013.
Methods
We used a CD4-staged Bayesian back-calculation approach9,15 to estimate the annual incidence rate in MSM in Denmark, and the size of the hidden epidemic. An HIV-infected individual’s CD4 cell count is a marker of time since infection: the lower the count, the longer (on average) the individual has been infected. Following the methodology of Sweeting and colleagues,9 the back-calculation model includes four CD4-stratified stages: stage one (≥ 500 cells/mL), stage two (350—500 cells/mL), stage three (200—350 cells/mL), and stage four (< 200 cells/mL). This classification is used in order to fit the model to the DHCS CD4-stratified diagnosis data.
We used data from the DHCS; this includes data on every MSM, diagnosed with HIV, since 1995. The DHCS uses the unique civil registration number assigned to all Danes, and immigrants, to identify individuals. This avoids replicate registrations and enables information (for each individual in the study) to be extracted from the Danish National Hospital Registry, the Danish Civil Registration System, and the Danish National Registry of Deaths. The Danish National Hospital Registry contains data on all hospital admission and discharge diagnoses since 1977. The Danish Civil Registration System contains data on immigration, emigration, and death. The Danish National Registry of Deaths documents causes of death. The DHCS dataset includes, for each HIV-infected individual: date of (and CD4 cell count at) diagnosis, complete history of treatment, and viral load and CD4 measurements over time.
We used 20,000 Markov Chain-Monte Carlo samples to simulate the back-calculation model, with an initial burn-in of 5,000 simulations. MATLAB was used for implementation. Following the methodology of Sweeting and colleagues,9 the annual incidence rate was modeled as a Poisson process. It was simulated using an autoregressive model on the log-scale to ensure it changed smoothly over time. During the simulations we updated the estimates for the incidence rate by using the DHCS diagnosis data and the likelihood function defined by Birrell and colleagues.15 Median values and Bayesian Credible Intervals (BCIs) for the estimates were calculated; they were then weighted using the likelihood function. Further methodological details are given in the Appendix.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Results
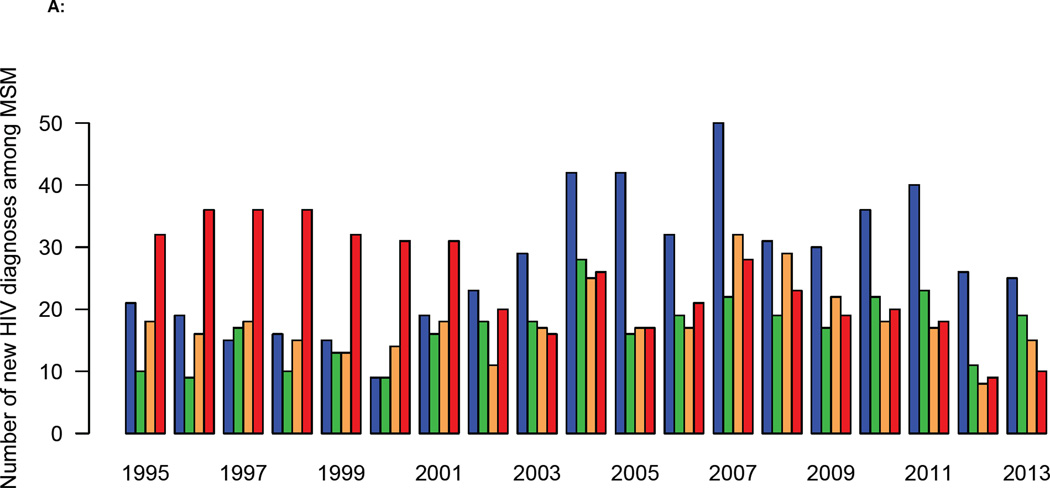
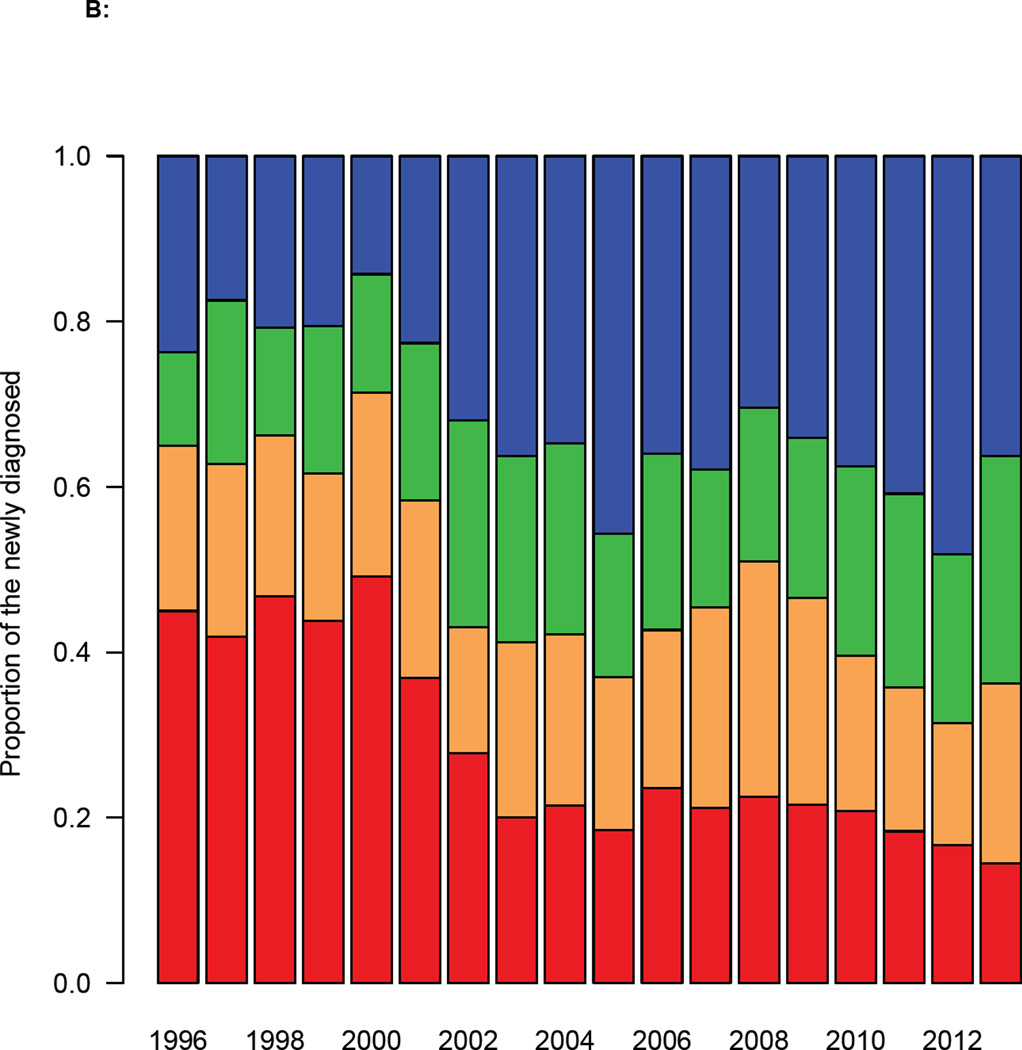
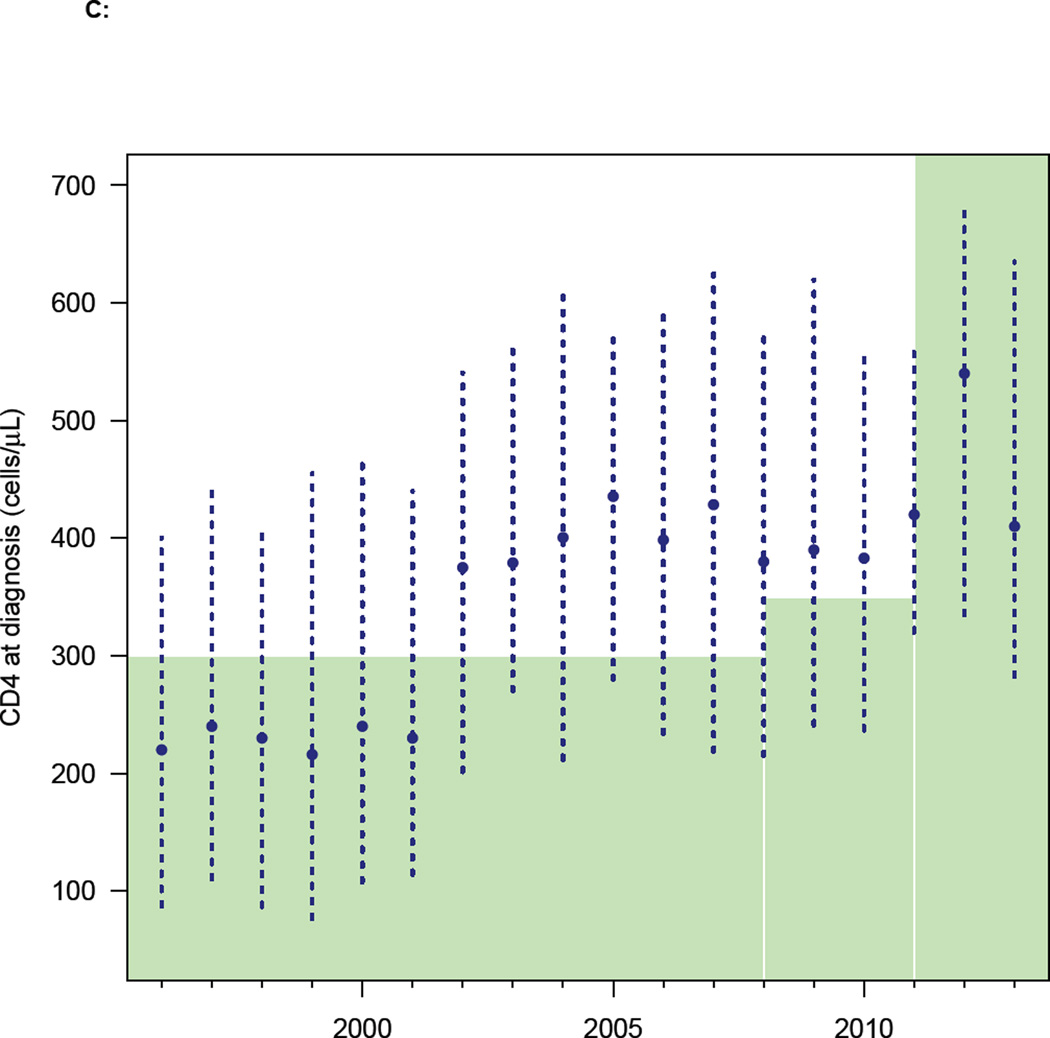
The DHCS diagnosis data, when stratified on the basis of the CD4 cell count at the time of diagnosis, show the HIV epidemic in MSM in Denmark has changed dramatically over the past two decades. From 2002 onwards the number of MSM with a high CD4 cell count at diagnosis has increased, and the number with a low count decreased (figure 2A). The distribution of CD4 cell counts at diagnosis reflects these temporal changes (figure 2B). In 2002 the median CD4 cell count at diagnosis increased fairly abruptly (figure 2C), indicating that the time between infection and diagnosis decreased. Notably, this occurred before the treatment eligibility threshold changed in 2008 (figure 2C).
Figure 2. Trends in diagnosis rates for all MSM diagnosed with HIV in Denmark between 1995 and 2013.
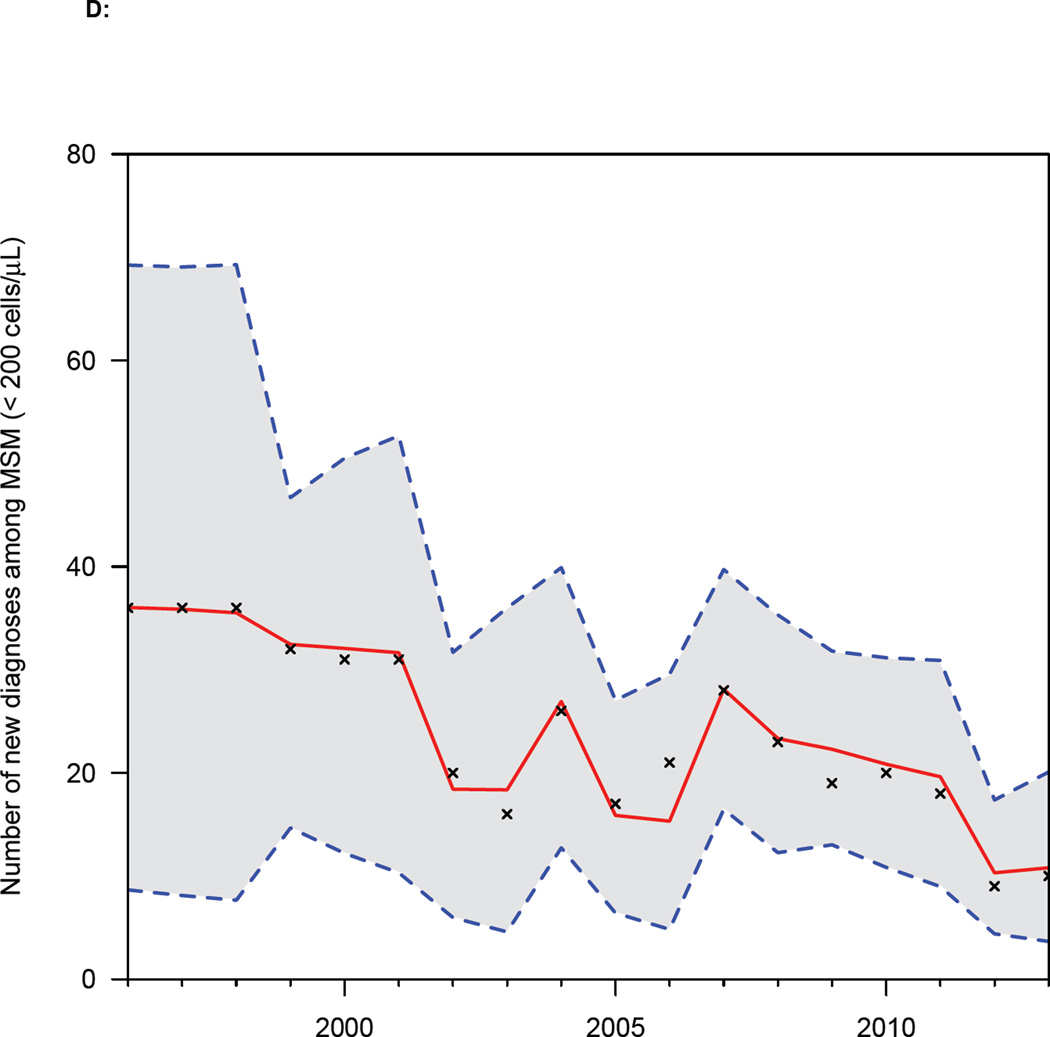
(A) Annual number of diagnoses (Danish HIV Cohort Study, DHCS, data) stratified by CD4 cell count (cells/µL): CD4 ≥ 500 (blue), 350 ≤ CD4 < 500 (green), 200 ≤ CD4 < 350 (orange), CD4 < 200 (red). (B) Distribution of CD4 cell counts (cells/µL) at diagnosis (DHCS data), color code as in figure 2A. (C) CD4 cell count (cells/µL) at diagnosis (DHCS data): blue dots (median values), dashed blue lines (Inter-quartile range). Shaded green area shows changes in the Danish HIV treatment guidelines, in terms of the CD4 cell count necessary for treatment initiation. (D) Goodness-of-fit to DHCS diagnosis data, for individuals with a CD4 cell count < 200 cells/µL at diagnosis: black crosses (DHCS data), red lines (estimates, median values), dashed blue lines (95% BCI).
The goodness-of-fit plots for the back-calculation model are shown in figure 2D and figure S1 in the Supplementary Material. The model’s estimates are an excellent fit to the DHCS diagnosis data: all data are contained within the 95% BCI of the estimates.
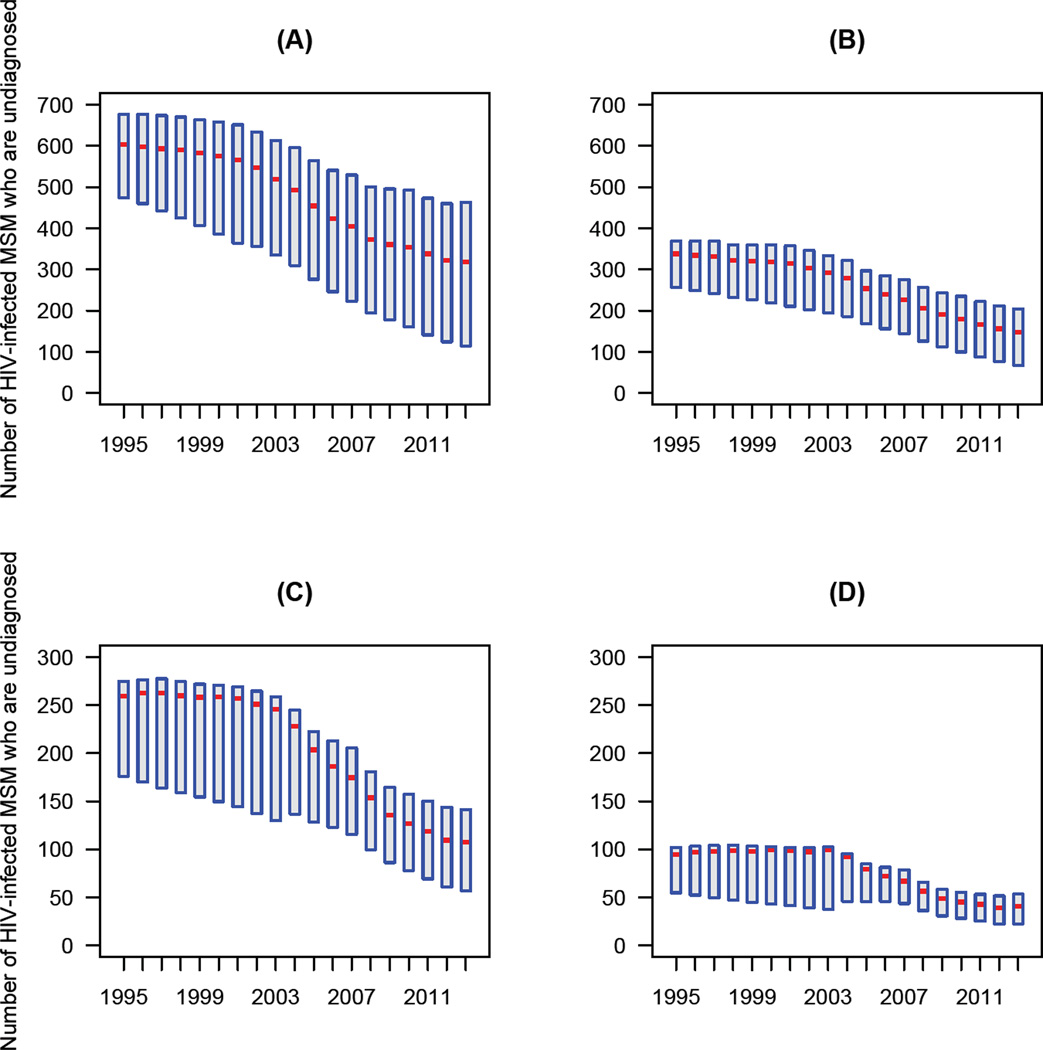
Our estimates of the number of HIV-infected undiagnosed MSM show the size of the hidden epidemic in Denmark has decreased substantially, in all four stages of infection, since treatment was introduced in 1996 (figure 3). The most likely values for the estimates and 95% BCIs (figure 3) were determined by the likelihood function. We estimate, that by 2013, only 21% (median, 95% BCI: 10%—27%) of all HIV-infected MSM in Denmark remained undiagnosed (figure S3 in the Supplementary Material). This percentage translates to only 617 (median, 95% BCI: 264—858) HIV-infected undiagnosed MSM in the entire country. The majority was in the two earliest stages of infection (figure 3A and 3B). Notably, there were only 107 (median, 95% BCI: 57—142) MSM in stage three (figure 3C), and 40 (median, 95% BCI: 22—53) in stage four (figure 3D).
Figure 3. Decrease in the size of the “hidden epidemic” after the introduction of combination therapies in 1996.
The hidden epidemic is shown in terms of the number of undiagnosed MSM stratified by CD4 count (cells/µL): (A) CD4 ≥ 500, (B) 350 ≤ CD4 < 500, (C) 200 ≤ CD4 < 350, and (D) CD4 < 200. Red lines show the most likely values (determined by the likelihood function), boxes show 95% BCIs.
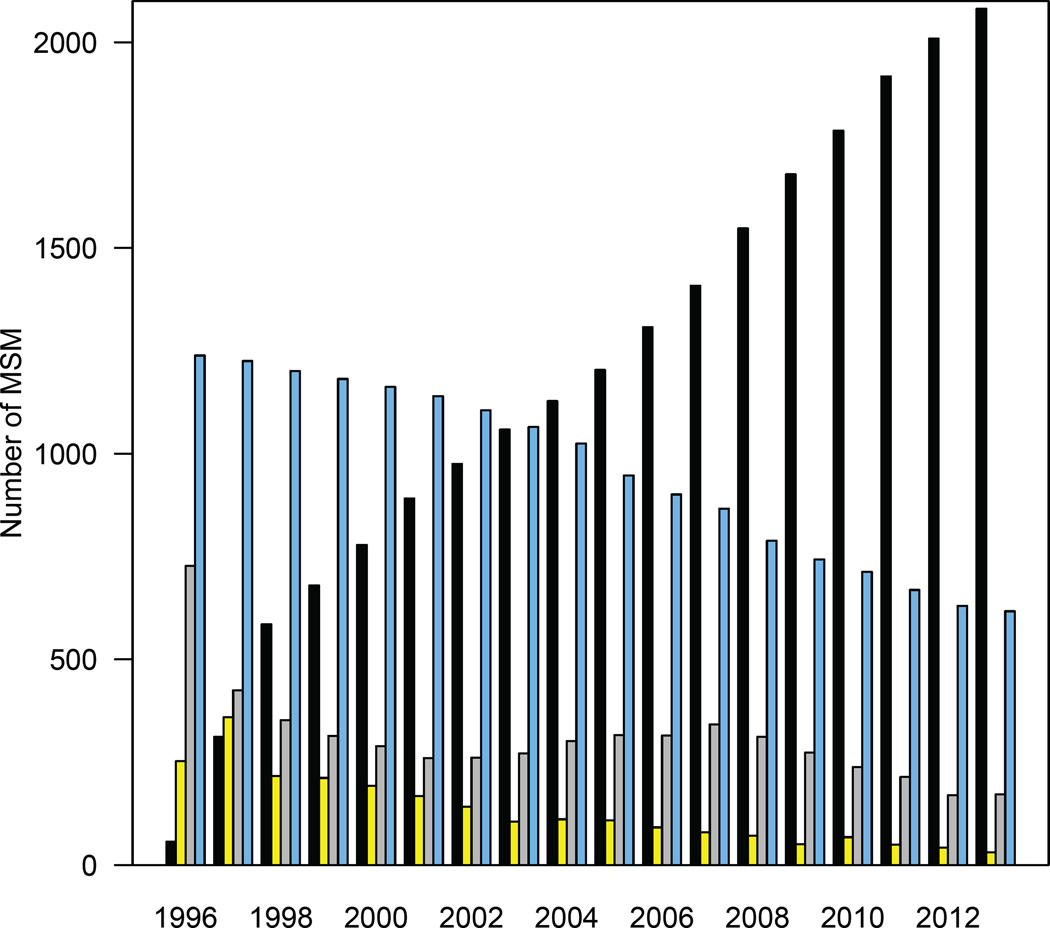
Our results (figure 4) show that the number of MSM in Denmark who are “potential transmitters” (PTs) of HIV has decreased substantially over the past ~20 years; this occurred as the number of MSM on treatment with completely suppressed virus increased. We define PTs as MSM who are: (i) undiagnosed, (ii) diagnosed but not on treatment, or (iii) on treatment with incomplete viral suppression. The number in the first group (i.e., the hidden epidemic) was estimated by the back-calculation; the number in groups two and three were calculated from the DHCS data. The total number of PTs has decreased by approximately two-thirds: from 2,218 (median, 95% BCI: 1,955—2,381) in 1996 to 819 (median, 95% BCI: 463—1,065) in 2013. In 2013, the vast majority (74%) of PTs were undiagnosed MSM (figure 4).
Figure 4. Temporal changes in the HIV epidemic in MSM in Denmark after the introduction of combination therapies in 1996.
Danish HIV Cohort Study (DHCS) data (black) show the total number of MSM in Denmark on treatment with complete viral suppression (viral load < 200 copies/mL). Also shown are the numbers undiagnosed (blue; estimates, median values), diagnosed but not on treatment (grey; DHCS data), and on treatment with incomplete viral suppression (yellow; DHCS data).
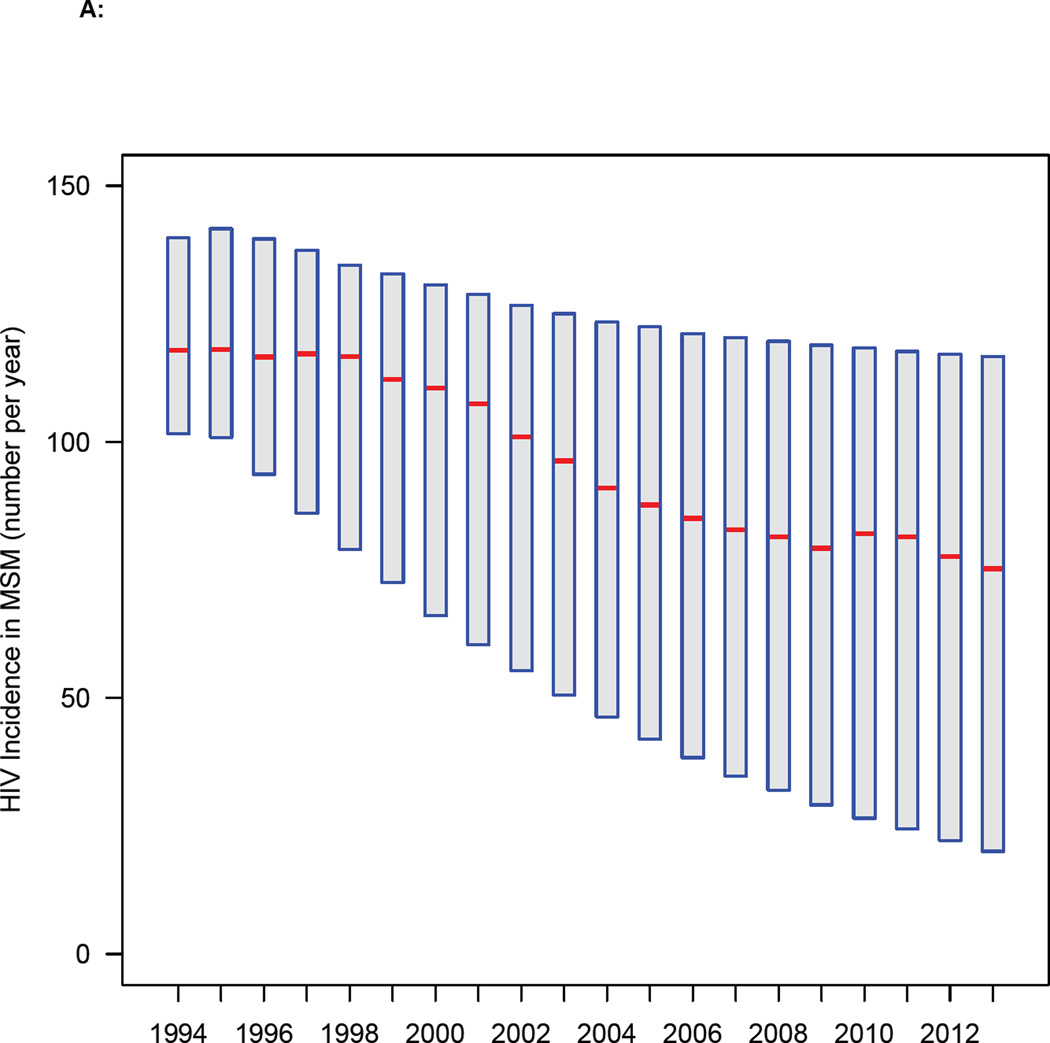
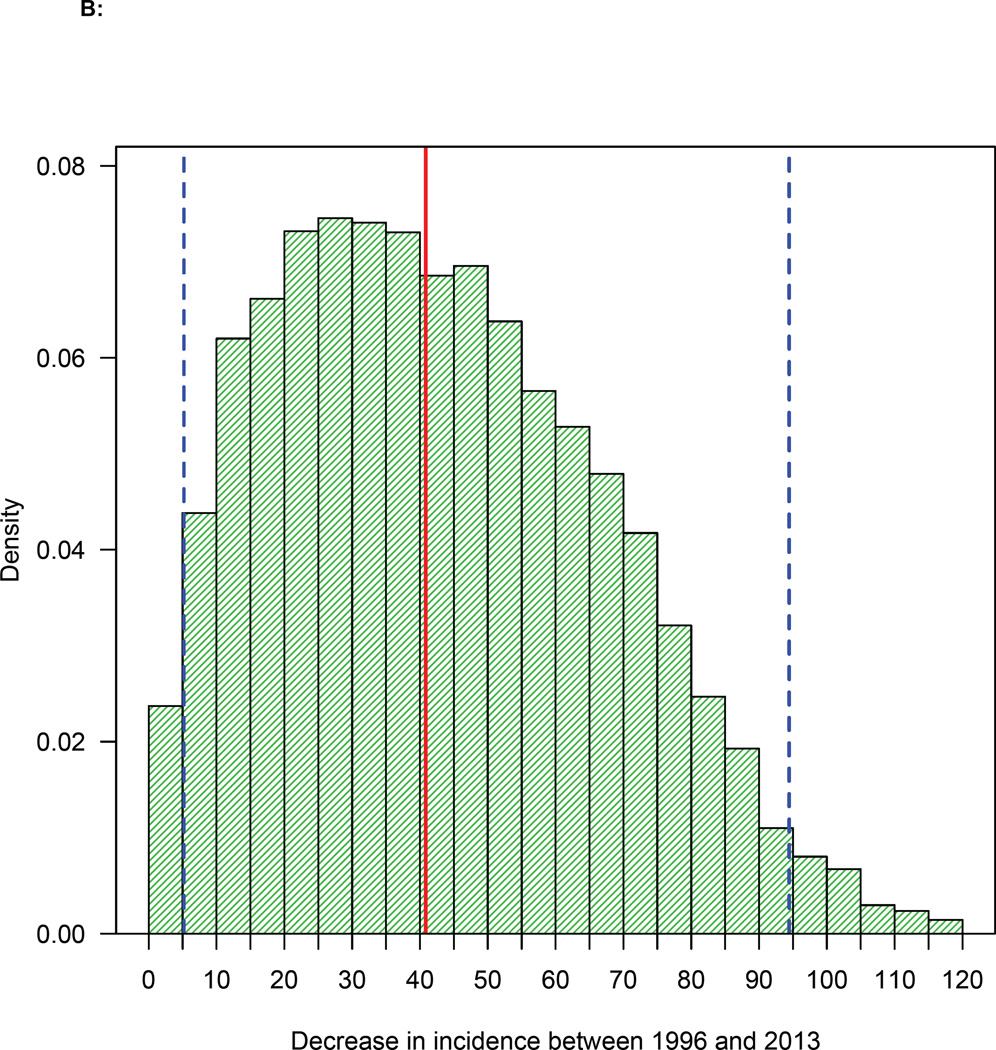
Our results (figure 5A) show that the annual HIV incidence rate in MSM in Denmark has been decreasing for almost two decades. The most likely values for the estimates and 95% BCIs (figure 5A) were determined by the likelihood function. The rate fell from 117 (median, 95% BCI: 94—140) new HIV infections per year in 1996 to a low of 75 (median, 95% BCI: 20—117) per year in 2013. Our results (figure 5B) provide strong supportive evidence that the annual incidence rate has decreased since the introduction of treatment in 1996: the 95% BCI does not include zero.
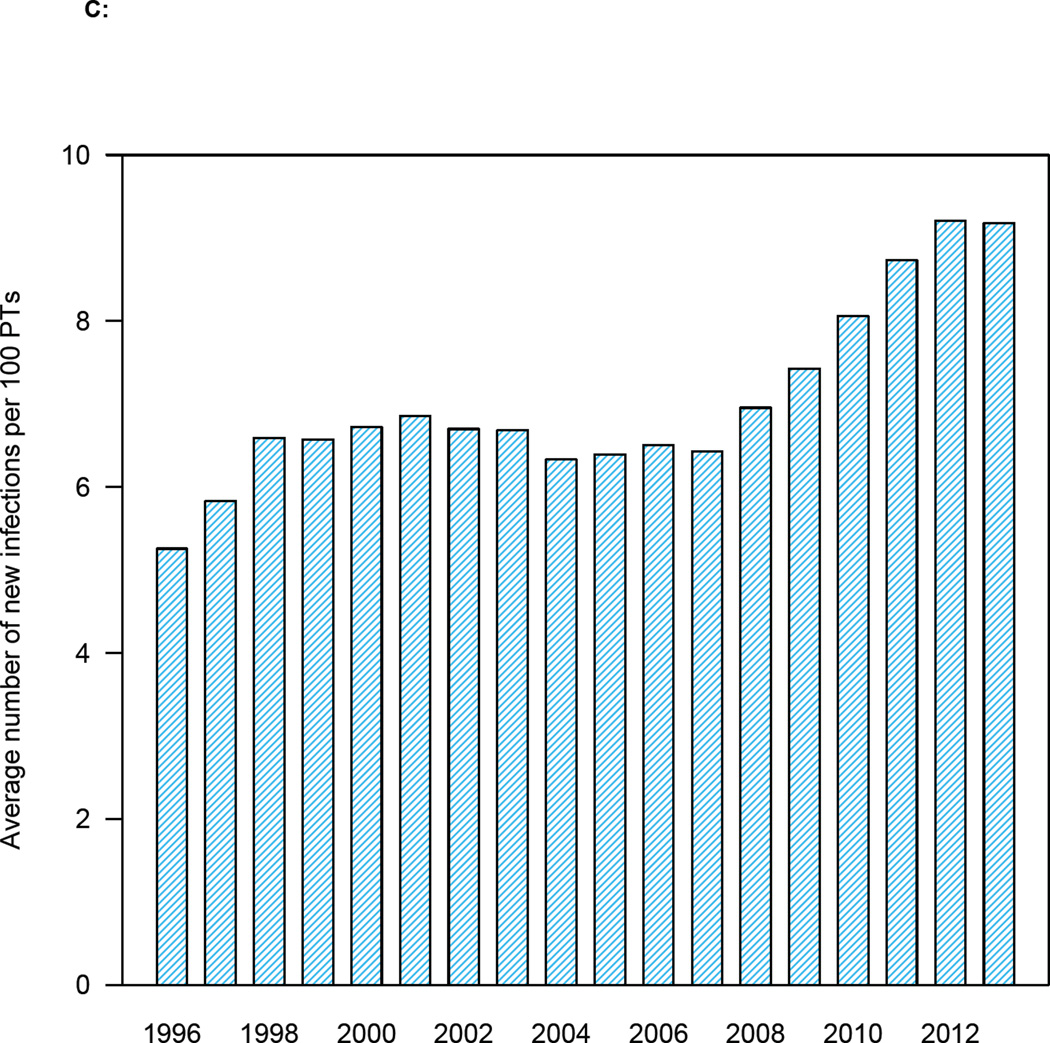
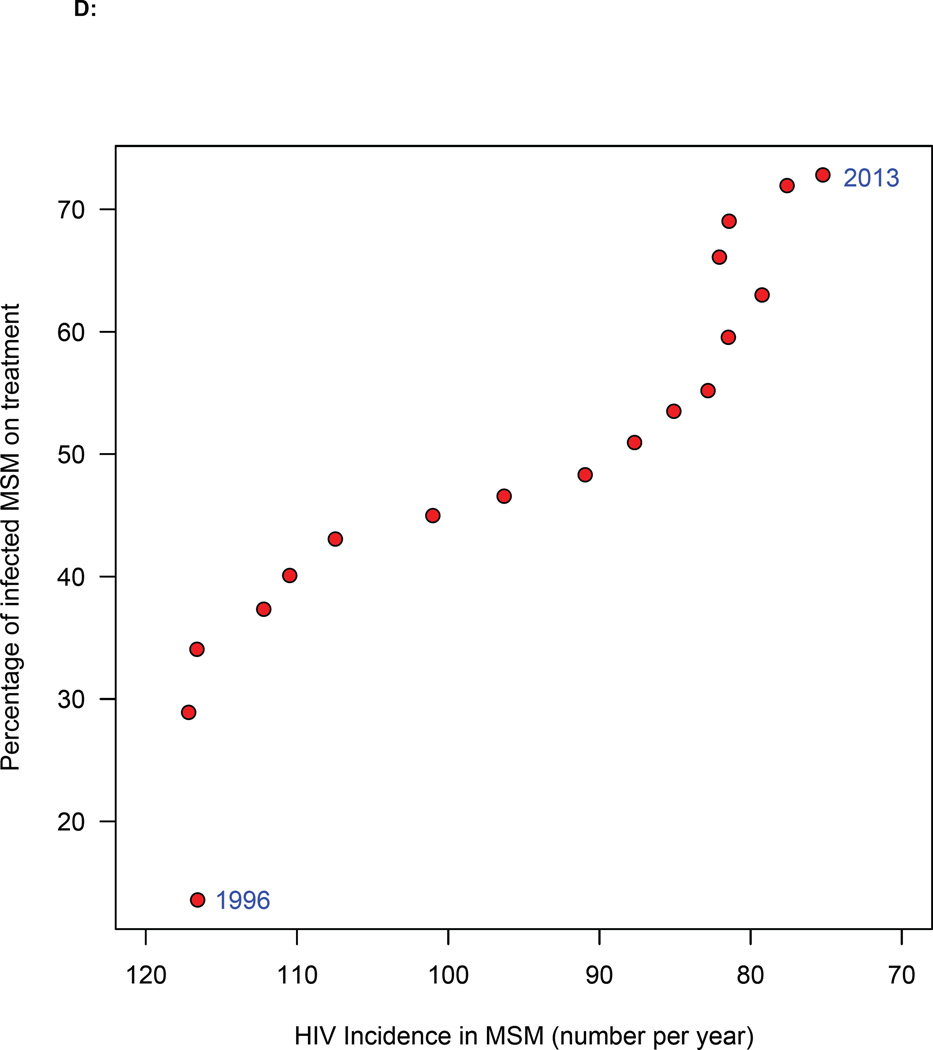
Figure 5. Temporal changes in treatment coverage and HIV incidence rates.
(A) Trend in incidence rate in MSM in Denmark since 1994: red lines represent the most likely values (determined by the likelihood function), boxes 95% BCIs. (B) Histogram of the estimated decrease in incidence between two time points (1996 and 2013): red line shows median, and dashed blue lines the 95% BCI. (C) Temporal relationship between the number of potential transmitters (PTs) and the average number of new HIV infections. (D) Relationship between increasing treatment coverage (Danish HIV Cohort Study, DHCS, data) and decreasing incidence: red dots show the most likely values (determined by the likelihood function) of the annual incidence rate between 1996 and 2013.
A recent study has determined that there are 50,000 MSM in Denmark.16 Using this value, we estimate that there were only 1·4 (median, 95% BCI: 0·4—2·1) new HIV infections in MSM per 1,000 MSM in Denmark in 2013. This is very close to the WHO elimination threshold.
The incidence rate (figure 5A) has not decreased in proportion to the reduction in the number of PTs (figure 4). Our results indicate that this may be due to an increase in risky sexual behaviors. We found there were ~5 new HIV infections (on average) for every 100 PTs in 1996 (figure 5C); by 2013, this had almost doubled (figure 5C).
Of greatest importance, we found a very strong correlation between increasing treatment coverage and decreasing incidence since 1996 (figure 5D). The most likely values for the incidence estimates were determined by the likelihood function. Whilst this statistical relationship does not demonstrate causality, it provides strong supportive evidence that TasP can reduce epidemics, and is biologically plausible. We found that the relationship between the effectiveness of TasP on decreasing incidence and treatment coverage is complex and nonlinear (Figure 5D). Notably, our results show there is a threshold effect: TasP did not have a significant effect on reducing the incidence rate until treatment coverage had reached ~35%.
Discussion
Our study is the first to show that TasP can substantially reduce a country’s HIV epidemic, and has the potential to be an effective elimination tool. Importantly, we have shown that a threshold effect exists; this implies that TasP is not effective in decreasing incidence unless the treatment coverage is moderately high. We have investigated the impact of TasP, over the past two decades, on an HIV epidemic that UNAIDS has identified as a priority for elimination: the Danish HIV epidemic in MSM. Notably, we have found that TasP has reduced the incidence rate close to the WHO elimination threshold. Treatment programs in Denmark have been, and are, outstanding: treatment coverage has been high for almost 20 years, and the viral suppression rate (due to high adherence) has been exceptional. TasP may only be successful in reducing epidemics and eliminating HIV, under these optimal treatment conditions. Under less optimal conditions, TasP (although effective at the individual-level5,6), may not be effective in reducing epidemics.
Taken together, our results suggest a conceptual framework for understanding how TasP could lead to the global elimination of HIV. They suggest that the introduction of treatment initiates a feedback cycle, but only after a threshold coverage level has been reached. As coverage and viral suppression rates increase, the number of PTs decreases; fewer PTs result in a reduction in the incidence rate. Decreasing incidence, coupled with increasing coverage, results in a further decline in the number of PTs. This, in turn, further reduces the incidence rate. If treatment coverage (and the viral suppression rate) is sufficiently high, the incidence rate could be reduced below the WHO elimination threshold.
Our estimates provide explicit targets for Denmark for the number of HIV-infected undiagnosed MSM that need to be found and provided with treatment, both for their clinical benefit17 and to eliminate the epidemic. We estimate that this hidden epidemic consists of only several hundred individuals. Therefore, it appears feasible, potentially through social media and mass testing campaigns, to find almost all of these individuals. Incidence in Denmark will decrease at a faster rate than currently if testing and diagnosis rates increase, risk behaviors decrease and/or effective interventions (e.g., pre-exposure prophylaxis)18 are introduced. Biomedical and behavioral interventions, targeting both HIV-infected and uninfected MSM, should be used. However incidence could rise if there is a substantial in-migration of HIV-infected MSM into Denmark, and/or risk behavior increases; temporary visitors could also, potentially, increase transmission. Past immigration of HIV-infected MSM may have increased incidence if sexual networks linked non-nationals with nationals. However, this appears unlikely as very few HIV-infected MSM have immigrated to Denmark: only 5% of the MSM in the DHCS are immigrants who were diagnosed with HIV within five years of arriving in Denmark. Recent data indicate that the number of HIV-infected immigrants may be increasing.
Our analysis has focused on the HIV epidemic in the major risk group, MSM. There are also individuals in Denmark who have become infected with HIV due to heterosexual transmission; ~25% of these are fairly recent immigrants, many from countries where HIV prevalence is high. This suggests they may have been infected before immigrating. If this is the case, using TasP in Denmark may not be very effective in reducing the number of HIV-infected heterosexuals. In many countries intravenous drug users (IDUs) are a key risk group for acquiring HIV. In Denmark there is almost no HIV transmission among IDUs as effective free needle exchange programs have been operating for decades.
Our results provide significant insights into the impact of increases in risk behavior on decreasing the effectiveness of TasP. We found that the incidence rate did not decrease to the extent expected, given the reduction in the number of PTs. Our results suggest this was due to an increase in risky sexual behaviors, which increased the HIV transmission rate. Notably, a recent study19 in Denmark has documented an increase in gonorrhea and syphilis rates in MSM indicating an increase in risky sex. However, even with an increase in risky behaviors, we found the incidence rate continued to decline; this occurred because the number of PTs continued to decrease. It is likely that TasP would have reduced incidence at a faster rate, and to a greater extent, if risk behavior had not increased. It will be essential to use other interventions with proven efficacy (e.g. pre-exposure prophylaxis)18 in combination with TasP, as well as to implement effective behavioral interventions that prevent risky behaviors increasing.
Theoretical studies20,21 (published soon after the introduction of effective HIV therapies in 1996) predicted that treatment would act as prevention, decrease incidence and could (if treatment coverage and viral suppression rates were very high) even eliminate HIV. Since then treatment has been shown to be effective in reducing an individual’s risk of acquiring HIV.5–7 However, prior to this study, TasP had not been shown to be effective in reducing epidemics. Previous studies have found that incidence rates have remained relatively constant, or increased15,22–25; notably, these studies were conducted in locations where viral suppression rates are lower than in Denmark. It is possible that TasP was effective in reducing incidence in some locations, but the effect was masked due to an increase in risky behavior. The early theoretical studies20,21 showed that only a relatively small increase in risky behavior could counter-balance the impact of TasP on reducing incidence.
Our results have implications for the effectiveness of TasP in reducing concentrated HIV epidemics in other resource-rich countries. Notably, there is universal access to healthcare in Denmark and HIV treatment is free. In addition, there are many easily accessible community walk-in clinics that provide HIV testing and linkage to care.26 Treatment programs in Denmark are outstanding. They have achieved high diagnosis rates (~80% of HIV-infected MSM have been diagnosed), high coverage (~92% of diagnosed MSM are on treatment), and an exceptionally high viral suppression rate (98%). In addition, patient retention in care is excellent: only ~2% of patients per year are lost to follow-up. Other resource-rich countries have high treatment coverage, but substantially lower viral suppression rates.27 In the United States, France, Australia, the United Kingdom and the Netherlands, HIV epidemics in MSM communities have begun to resurge.28,29 This comparison suggests, that for TasP to be an effective elimination tool, treatment programs in other countries will have to emulate those in Denmark.
Our study has significant implications for the current global health policies proposed by the WHO and UNAIDS, and for the control of the HIV pandemic. The vast majority (~25 million) of HIV-infected individuals live in resource-constrained countries in sub-Saharan Africa (SSA) where treatment coverage is currently moderate to low. HIV epidemics in sub-Saharan African countries are very different from those in resource-rich countries. They are generalized throughout the population, rather than concentrated in risk-groups. Furthermore, incidence rates are significantly higher: e.g., ~100 new HIV infections per 1,000 per year.30 It is essential to increase treatment coverage in SSA, as this will provide substantial clinical benefits for millions of HIV-infected individuals.17 However, for TasP to be effective in reducing HIV epidemics in SSA, treatment programs will need to be more successful than many of the current programs in resource-rich countries. Unless this occurs, the WHO’s global elimination strategy is unlikely to succeed.
Supplementary Material
Acknowledgments
J.T.O., D.R., L.P., and S.B acknowledge the financial support of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants R01 AI116493, R56 AI041935 and R21 AI114478). J.G. and N.O. acknowledge support from Preben og Anna Simonsens Fond, NOVO Nordisk Foundation and the Danish AIDS Foundation. The Danish HIV Cohort Study includes patients from the Departments of Infectious Diseases at Copenhagen University Hospitals, Rigshospitalet (J. Gerstoft, N. Obel) and Hvidovre (G. Kronborg), Odense University Hospital (C. Pedersen), Aarhus University Hospitals, Skejby (C.S. Larsen) and Aalborg (G. Pedersen), Herning Hospital (R. Mohey), Hillerød Hospital (L. Nielsen), Roskilde Hospital (L. Weise) and Kolding Hospital (J. Jensen). We are grateful to Bradley Wagner, Katie Sharp, Nicholas Jewell and Nelson Freimer for discussions throughout the course of this research.
Footnotes
Contributors
J.T.O., D.R., L.P., J.G., N.O., and S.B. designed the project, interpreted the results, and jointly wrote the manuscript. J.T.O and D.R. performed all statistical analysis and coding of the back-calculation model. L.P. provided additional technical support. J.G. and N.O. provided access to the DHCS data as well as detailed information about Denmark’s HIV epidemic.
Conflicts of interests
J.T.O., D.R., L.P., J.G., N.O., and S.B. declare that they have no conflicts of interest.
Ethics Approval
No approval from an ethics committee was necessary as we conducted an analysis of a dataset that does not include identifiers.
References
- 1.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 2.UNAIDS. Strategy for 2016—2021: fast-tracking to zero. Geneva: UNAIDS; 2015. [Google Scholar]
- 3.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 4.UNAIDS. The GAP report. Geneva: UNAIDS; 2014. [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339(6122):966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClelland RS, Richardson BA, Cherutich P, et al. A 15-year study of the impact of community antiretroviral therapy coverage on HIV incidence in Kenyan female sex workers. AIDS. 2015;29(17):2279–2286. doi: 10.1097/QAD.0000000000000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwuji CC, McGrath N, de Oliveira T, et al. The art of HIV elimination: past and present science. J AIDS Clin Res. 2015;6(11) doi: 10.4172/2155-6113.1000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweeting MJ, De Angelis D, Aalen OO. Bayesian back-calculation using a multi-state model with application to HIV. Stat Med. 2005;24(24):3991–4007. doi: 10.1002/sim.2432. [DOI] [PubMed] [Google Scholar]
- 10.Omland LH, Ahlstrom MG, Obel N. Cohort profile update: the Danish HIV cohort study (DHCS) Int J Epidemiol. 2014;43(6):1769—9e. doi: 10.1093/ije/dyu153. [DOI] [PubMed] [Google Scholar]
- 11.Obel N, Engsig FN, Rasmussen LD, Larsen MV, Omland LH, Sorensen HT. Cohort profile: the Danish HIV cohort study. Int J Epidemiol. 2009;38(5):1202–1206. doi: 10.1093/ije/dyn192. [DOI] [PubMed] [Google Scholar]
- 12.Amundsen EJ, Aalen OO, Stigum H, et al. Back-calculation based on HIV and AIDS registers in Denmark, Norway and Sweden 1977—95 among homosexual men: estimation of absolute rates, incidence rates and prevalence of HIV. J Epidemiol Biostat. 2000;5(4):233–243. [PubMed] [Google Scholar]
- 13.HIV/AIDS-surveillance, quarterly report 30 June 1999. Copenhagen: Statens Serum Institut; 2000. [Google Scholar]
- 14.Audelin AM, Lohse N, Obel N, Gerstoft J, Jorgensen LB. The incidence rate of HIV type-1 drug resistance in patients on antiretroviral therapy: a nationwide population-based Danish cohort study 1999—2005. Antivir Ther. 2009;14(7):995–1000. doi: 10.3851/IMP1412. [DOI] [PubMed] [Google Scholar]
- 15.Birrell PJ, Gill ON, Delpech VC, et al. HIV incidence in men who have sex with men in England and Wales 2001—10: a nationwide population study. Lancet Infect Dis. 2013;13(4):313–318. doi: 10.1016/S1473-3099(12)70341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcus U, Hickson F, Weatherburn P, Schmidt AJ. Estimating the size of the MSM populations for 38 European countries by calculating the survey-surveillance discrepancies (SSD) between self-reported new HIV diagnoses from the European MSM internet survey (EMIS) and surveillance-reported HIV diagnoses among MSM in 2009. BMC Public Health. 2013;13:919. doi: 10.1186/1471-2458-13-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lundgren JD, Babiker AG, Gordin F, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowan SA, Gerstoft J, Haff J, Christiansen AH, Nielsen J, Obel N. Stable incidence of HIV diagnoses among Danish MSM despite increased engagement in unsafe sex. J Acquir Immune Defic Syndr. 2012;61(1):106–111. doi: 10.1097/QAI.0b013e31825af890. [DOI] [PubMed] [Google Scholar]
- 20.Blower SM, Gershengorn HB, Grant RM. A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science. 2000;287(5453):650–654. doi: 10.1126/science.287.5453.650. [DOI] [PubMed] [Google Scholar]
- 21.Velasco-Hernandez JX, Gershengorn HB, Blower SM. Could widespread use of combination antiretroviral therapy eradicate HIV epidemics? Lancet Infect Dis. 2002;2(8):487–493. doi: 10.1016/s1473-3099(02)00346-8. [DOI] [PubMed] [Google Scholar]
- 22.van Sighem A, Nakagawa F, De Angelis D, et al. Estimating HIV incidence, time to diagnosis, and the undiagnosed HIV epidemic using routine surveillance data. Epidemiology. 2015;26(5):653–660. doi: 10.1097/EDE.0000000000000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS ONE. 2010;5(6):e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Q, Boulos D, Yan P, et al. Estimates of the number of prevalent and incident human immunodeficiency virus (HIV) infections in Canada, 2008. Can J Public Health. 2010;101(6):486–490. doi: 10.1007/BF03403969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montaner JS, Lima VD, Harrigan PR, et al. Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: the "HIV Treatment as Prevention" experience in a Canadian setting. PLoS ONE. 2014;9(2):e87872. doi: 10.1371/journal.pone.0087872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qvist T, Cowan SA, Graugaard C, Helleberg M. High linkage to care in a community-based rapid HIV testing and counseling project among men who have sex with men in Copenhagen. Sex Transm Dis. 2014;41(3):209–214. doi: 10.1097/OLQ.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 27.European Centre for Disease Prevention and Control. Thematic report: HIV continuum of care. Stockholm: ECDC; 2015. [Google Scholar]
- 28.Phillips AN, Cambiano V, Nakagawa F, et al. Increased HIV incidence in men who have sex with men despite high levels of ART-induced viral suppression: analysis of an extensively documented epidemic. PLoS ONE. 2013;8(2):e55312. doi: 10.1371/journal.pone.0055312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan PS, Hamouda O, Delpech V, et al. Reemergence of the HIV epidemic among men who have sex with men in North America, Western Europe, and Australia, 1996—2005. Ann Epidemiol. 2009;19(6):423–431. doi: 10.1016/j.annepidem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.