Highlights
-
•
Vitamin D has a stimulatory effect on the mRNA expression of cathelicidin in vitro.
-
•
Anti-TB drugs abolish the vitamin D stimulatory effect of cathelicidin in vitro.
-
•
Subjects taking anti-TB drugs have no enhanced cathelicidin response to vitamin D.
-
•
Anti-TB drugs may alter the vitamin D stimulatory effect on anti-microbial peptides.
Keywords: Vitamin D, Tuberculosis, Cathelicidin, Antibiotics
Abstract
Tuberculosis (TB) is a major global health problem. Patients with TB have a high rate of vitamin D deficiency, both at diagnosis and during the course of treatment with anti-tuberculosis drugs. Although data on the efficacy of vitamin D supplementation on Mycobacterium tuberculosis (Mtb) clearance are uncertain from randomized controlled trials (RCTs), vitamin D enhances the expression of the anti-microbial peptide human cathelicidin (hCAP18) in cultured macrophages in vitro. One possible explanation for the mixed (primarily negative) results of RCTs examining vitamin D treatment in TB infection is that anti-TB drugs given to enrolled subjects may impact actions of vitamin D to enhance cathelicidin in macrophages. To address this hypothesis, human macrophage-like monocytic (THP-1) cells were treated with varying doses of first-line anti-tuberculosis drugs in the presence of the active form of vitamin D, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3). The expression of hCAP18 was determined by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). 1,25(OH)2D3 strongly induced expression of hCAP18 mRNA in THP-1 cells (fold-change from control). The combination of the standard 4-drug TB therapy (isoniazid, rifampicin, pyrazinamide and ethambutol) in the cultured THP-1 cells demonstrated a significant decrease in hCAP18 mRNA at the dosage of 10 µg/mL. In 31 subjects with newly diagnosed drug-sensitive TB randomized to either high-dose vitamin D3 (1.2 million IU over 8 weeks, n = 13) versus placebo (n = 18), there was no change from baseline to week 8 in hCAP18 mRNA levels in peripheral blood mononuclear cells or in plasma concentrations of LL-37, the protein product of hCAP18. These data suggest that first-line anti-TB drugs may alter the vitamin D-dependent increase in hCAP18 and LL-37 human macrophages.
Introduction
Tuberculosis (TB) remains a major worldwide health issue as the second most frequent cause of infection-related mortality [1], [2]. The World Health Organization (WHO) estimates that in 2013 there were ≈9 million new cases and ≈1.5 million deaths from TB disease [2]. The first line regimen of anti-TB drugs consists of a combination of isoniazid (INH), rifampicin, pyrazinamide and ethambutol [3], a combination with high success rates in various studies [4]. However, an epidemic of multi-drug resistant TB (MDR-TB) in which the infecting Mycobacterium tuberculosis (Mtb) organism is resistant to at least INH and rifampicin has emerged, including in the former Soviet Union Republics, such as Georgia [5].
Alternative and complementary therapies are needed in TB to improve clinical outcomes. Before the development of anti-TB drugs, both high-dose vitamin D and sunlight therapy had been used as a major treatment of TB [6], [7] although the pleiotropic effects of vitamin D on immune functions were not yet documented [8], [9], [10]. Several recent RCTs have re-examined the potential of vitamin D to help improve cure rates in patients with TB and MDR-TB [9], [11], [12], [13], [14], [15], [16], [17], [18], [19]. Many immune cells express the vitamin D receptor (VDR) including T-cells, B-cells, dendritic cells and macrophages [7], [8], [20], [21]. Both vitamin D and VDR influence the expression of specific endogenous antimicrobial peptides in the macrophage [21]. Vitamin D activation of the VDR is applicable to common infections such as influenza and TB. Further, the active vitamin D hormone can inhibit growth of Mtb in cultured human macrophages [22]. TB activates macrophages via the toll-like receptor (TLR) complex (TLR2/1) to stimulate the expression of VDR and 1α-hydroxylase (1α-OHase or CYP271B) [21]. In the presence of sufficient amounts of 25-hydroxyvitamin D3 (25(OH)D3) in the serum, it is converted to its active form, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) [21]. Binding of 1,25(OH)2D3 to the VDR induces transcription and translation of various genes, including the antimicrobial peptide cathelicidin (hCAP18) and its cleaved activated peptide LL-37, and β-defensin-4 (DEFB-4). Both hCAP18 and DEFB-4 stimulate the eradication of TB via the autolysosomal process [23]. Multiple in vitro experiments confirm that vitamin D up-regulates expression of hCAP18; however, several recent randomized control trials examining the efficacy of vitamin D in clearing active TB disease [9], [11], [12], [13], [14], [15], [16], [17], [18], [19] have been largely negative [9], [11], [12], [17], [18], [19]. One of the possible hypotheses for the discrepancy between the in vitro and in vivo data is that anti-TB drugs may interfere with vitamin D metabolism and thus the induction of hCAP18 by macrophages. Therefore, the objective of our study was to examine the impact of first line anti-TB drugs on vitamin D induced expression of hCAP18 in cultured human monocytes in vitro and in peripheral blood mononuclear cells in a randomized controlled clinical trial of high-dose vitamin D in adult patients with newly diagnosed TB disease.
Materials and methods
Cell preparation
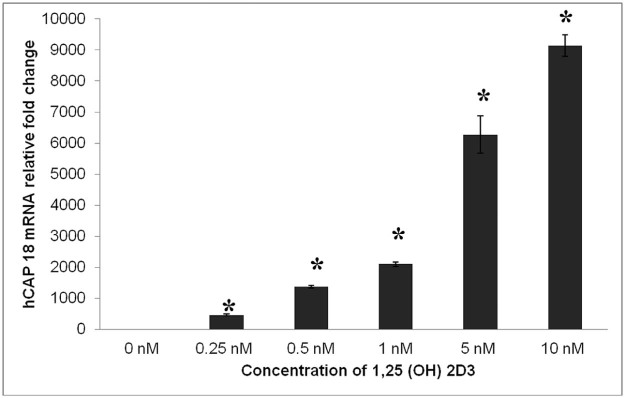
Human macrophage-like monocytic cells (THP-1) were obtained from American Type Culture Collection (ATCC; Manassas, VA) and grown in Roswell Park Memorial Institute medium (RPMI 1640) supplemented with 10% fetal bovine serum (FBS), 50 IU/mL penicillin and 50 µg/mL streptomycin. The concentration of THP-1 cells was adjusted to a concentration of 1 million cells per mL and then sub-cultured into 6-well tissue culture plates in the presence of 0.25, 0.50, 1.00, 5.00, and 10.00 nM of 1,25(OH)2D3 (Sigma Aldrich; St. Louis, MO). Cells were incubated for 12 hours to establish the optimal concentration of 1,25(OH)2D3 for induction of hCAP18 mRNA. The concentration of 5 nM of 1,25(OH)2D3 was selected for subsequent in vitro experiments.
The anti-TB drugs isoniazid, rifampicin, pyrazinamide and ethambutol were diluted in dimethyl sulfoxide (DMSO) and stored at −80 °C until use. The concentrations of each drug were examined over a range of doses known to inhibit Mtb growth from previously published studies [24], [25], [26], [27], [28], [29] at a final concentration of 1.0 µg/mL, 5.0 µg/mL and 10.0 µg/mL. THP-1 cells were then incubated in 37 °C, 5% CO2 for 6 hours, following which cell suspensions were centrifuged and supernatants removed. Harvested THP-1 cells were placed in RLT buffer (Qiagen; Hilden, Germany) and passed through QiaShredder columns (Qiagen; Hilden, Germany).
RNA extraction and quantitative real-time polymerase chain reaction
RNeasy mini kits (Qiagen; Hilden, Germany) were used to extract RNA according to the manufacturer's protocol. Extracted RNA was reverse transcribed to cDNA by using iscript cDNA (Bio-Rad Laboratories; Hercules, CA). Relative gene expression was determined by quantitative real-time polymerase chain reaction (RT-qPCR) using TaqMan® Universal PCR Master Mix (Life Technologies; Grand Island, NY). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a reference gene to calculate the fold change of gene expression using ΔΔCT method. Primers purchased from Sigma Aldrich were used for RT-qPCR reactions: human hCAP18; CTTCACCAGCCCGTCCTTC and CCAGGACGACACAGCAGTCA, GAPDH; CTTAGCACCCCTGGCCAAG and TGGTCATGAGTCCTTCCACG. TaqMan® MGB Probes 6000 pmol were purchased from Life Technologies: human LL-37; 6-FAM CAG AGG ATT GTG ACT TCA MGBNFQ, GAPDH; VIC-CAT CCA TGA CAA CTT TGG TA MGBNFQ.
Cellular viability measurement using the colorimetric MTT reduction assay
The tetrazolium salt dye is cleaved by live and metabolically active cells but not by dead cells; therefore, the MTT reduction assay was used to measure the viability of THP-1 cells treated with anti-TB drugs as described before [30]. Briefly, freshly grown THP-1 cells adjusted to 1 million cells/mL were treated with anti-TB drug doses ranging from 1 to 10 µg/mL and incubated overnight. Control cells treated with vehicle only were co-incubated under the same experimental conditions. Five mg of thiazolyl blue tetrazolium bromide was dissolved in 1 mL of PBS and 15 µL was added to 150 µL of treated THP-1 cells which were further incubated at 37 °C for one hour. Cells were centrifuged to remove extracellular dye then lysed in 150 µL of DMSO to dissolve the intracellular dye. The absorbance of reduced dye was measured at 591 nm wavelength using a Bio-Tek spectrophotometer.
Data from clinical studies
We obtained plasma and peripheral blood monocytes (PBMC) from pulmonary TB patients participating in a double-blind, randomized, placebo controlled clinical trial of high-dose vitamin D3 supplemented to newly diagnosed inpatients with pulmonary tuberculosis in Tbilisi, Republic of Georgia [9]. All patients received first-line 4 drug anti-tuberculosis therapy which included isoniazid, rifampicin, pyrazinamide and ethambutol plus either 50,000 IU of vitamin D3 or placebo orally three times weekly for 8 weeks then 50,000 IU of vitamin D3 or placebo every other week for the subsequent 8 weeks. The primary outcome of this study was the time to sputum culture conversion from positive to negative, during the 16-week study period, as defined by Martineau et al. [14]. All subjects provided informed consent for use of their blood samples for future sub-studies as outlined (clinicaltrials.gov NCT00918086). Blood for isolation of peripheral blood mononuclear cells (PBMCs) from subjects was collected at enrollment and at 8 weeks after treatment with high-dose vitamin D3 (1.2 million IU over 8 weeks, n = 13) or placebo (n = 18). Expression of hCAP18 mRNA from PBMC was determined by RT-qPCR. Plasma LL-37 (the protein product of hCAP18 mRNA) was also measured by enzyme-linked immunosorbent assay (ELISA, Hycult Biotech; Uden, The Netherlands) (n = 31).
Statistical analysis
For the in vitro study, mean ± standard deviation and p-value from at least three independent experiments were analyzed with Student's t-test. For the data from the clinical study, comparisons between the vitamin D-treated and the placebo groups were analyzed with Chi square for categorical variables and Student's t-tests for continuous variables. Plasma LL-37 concentrations and PBMC hCAP18 mRNA at baseline and at week 8 were analyzed with two-way mixed model repeated measures analysis of variance (ANOVA). Analysis was performed by Microsoft Excel and IBM SPSS Statistics for Windows, version 23.0 (IBM Corp; Armonk, NY).
Results
Expression of hCAP18 mRNA in THP-1 cells
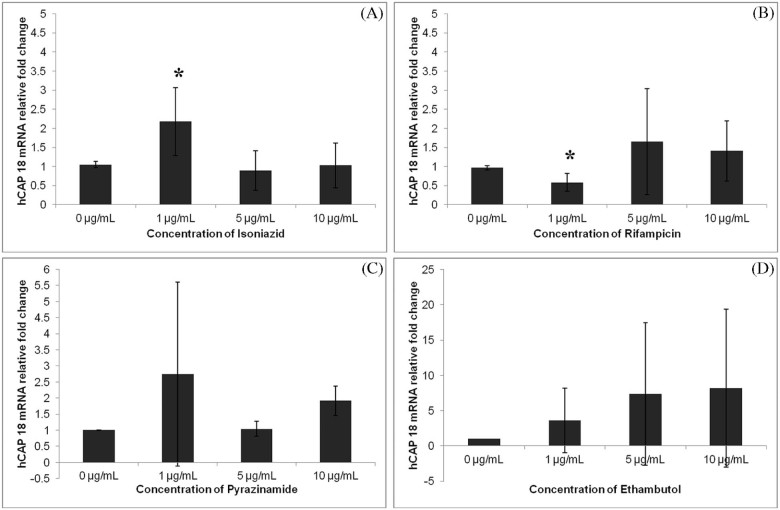
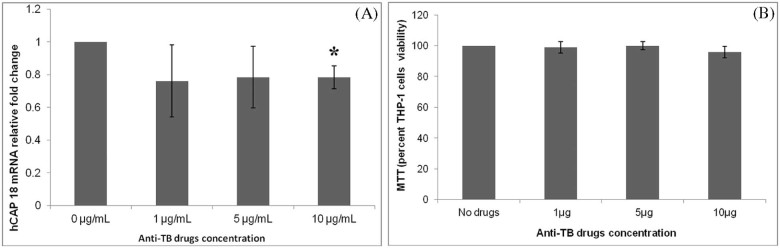
Addition of 1,25(OH)2D3 to cell culture media strongly induced expression of hCAP18 mRNA in THP-1 cells in a dose-dependent fashion (Fig. 1). INH at a concentration of 1.0 µg/mL significantly increased the expression of hCAP18 mRNA (Fig. 2A). In contrast, rifampicin at a concentration of 1.0 µg/mL significantly decreasedthe expression of hCAP18 (Fig. 2B). At higher concentrations of 5.0 and 10.0 µg/mL, both INH and rifampicin, respectively, did not alter hCAP18 mRNA expression cultured in the presence of 1,25(OH)2D3 (Fig. 2A and B). Neither pyrazinamide nor ethambutol at concentrations of 1.0, 5.0 and 10.0 µg/mL altered the expression of THP-1 cell hCAP18 mRNA cultured in the presence of 1,25(OH)2D3 (Fig. 2C and D). In contrast, cell culture with the combination of the 4 anti-TB drugs (INH, rifampicin, pyrazinamide and ethambutol) resulted in a significant decrease in the expression of hCAP18 mRNA at the dose of 10.0 µg/mL for each agent (Fig. 3A).
Figure 1.
Vitamin D induces expression of human cathelicidin in cultured THP-1 cells. THP-1 cells were cultured for 12 hours in the presence of increasing concentrations of 1,25(OH)2 D3 and harvested for RT-PCR for expression of human cathelicidin (hCAP18). The addition of 1,25(OH)2 D3 strongly induced the expression of hCAP18 mRNA relative to GAPDH in a dose dependent fashion in the cultured THP-1 cells (*p-value < 0.05).
Figure 2.
The impact of anti-tuberculosis drugs on induction of hCAP18 by vitamin D. The human monocytic cell line (THP-1) was cultured in the presence of 5 nM of 1,25(OH)2 D3 for 12 hours followed by 6 hours of exposure to increasing concentrations of anti-TB drugs including isoniazid (A), rifampicin (B), pyrazinamide (C) and ethambutol (D) (0, 1 µg/mL, 5 µg/mL, and 10 µg/mL). THP-1 cells were harvested and mRNA expression of hCAP18 was evaluated relative to GAPDH. 1 µg/mL of isoniazid significantly increased the expression of hCAP18 mRNA, but the 5 and 10 µg/mL had no effect (A). 1 µg/mL of rifampicin significantly decreased the expression of hCAP18 (B). Pyrazinamide and ethambutol at all of the concentrations evaluated did not significantly alter the expression of hCAP18 in the presence of 1,25(OH)2 D3 (C, D) (*p-value < 0.05).
Figure 3.
Impact of the 4 drug therapy (isoniazid, rifampicin, pyrazinamide and ethambutol) on vitamin D induced human cathelicidin (hCAP18) mRNA expression relative to GAPDH. The human monocytic cell line (THP-1) was cultured for 12 hours in the presence of 1,25(OH)2 D3 and all 4 anti-TB drugs were added at a concentration of 10 µg/mL for 6 hours. The THP-1 cells were harvested and mRNA was extracted for RT-PCR. The 4 drug combination demonstrated a significant decrease in the expression of hCAP18 mRNA at the highest concentration of 10 µg/mL (*p-value < 0.05) (A).
Further, the viability of THP-1 cells exposed to anti-TB drugs was assessed using the MTT assay as described [30]. The combination of the 4 anti-TB drugs treatment at 10.0 µg/mL of each drug did not affect the viability of THP-1 cells in presence of 1,25(OH)2D3, suggesting that the reduction in hCAP18 mRNA is caused by anti-TB drugs (Fig. 3B).
Expression of hCAP18 in PBMC and plasma LL-37 in subjects with pulmonary TB
The demographic information of the subjects participating in this sub-study of the larger double blind RCT [9] is presented in Table 1. There was no difference in the baseline demographic and clinical characteristics between the subjects receiving high-dose vitamin D3 and the subjects receiving placebo. No subject had MDR-TB and the mean plasma 25(OH)D concentration in each group was in the deficient range, similar to the plasma 25(OH)D concentrations in the full 199-subject RCT cohort [9]. All subjects received conventional dose regimens of INH, rifampicin, pyrazinamide and ethambutol, begun ≤7 days before the baseline blood sample and initiation of high-dose vitamin D3 or placebo.
Table 1.
Participant demographics
| Parameter | Placebo (n = 18) | Vitamin D (n = 13) | p-value |
|---|---|---|---|
| Age (yrs) (mean ± SD) | 34.1 ± 11.8 | 33.4 ± 10.9 | 0.862 |
| Gender (% male) | 61.1 | 69.2 | 0.641 |
| Employment status (% employed) | 50 | 53.8 | 0.833 |
| Yearly subject income (%) (<1000 Georgian lari) ($586) |
16.7 | 15.4 | 0.924 |
| Current smoker (%) | 27.8 | 46.2 | 0.291 |
| Body mass index (kg/m2) (mean ± SD) | 19.8 ± 2.4 | 21.4 ± 4.3 | 0.198 |
| Body mass index (kg/m2) (% BMI < 18.5) | 38.9 | 23.1 | 0.353 |
| Diabetes mellitus history (%) | 11.1 | 7.7 | 0.751 |
| Plasma 25(OH)D (ng/mL) (mean ± SD) | 10.4 ± 7.5 | 11.2 ± 6.1 | 0.733 |
| Isoniazid and rifampicin resistance (%) | 0 | 0 | 1.0 |
| TaqI genotype (%) | 0.425 | ||
| TT | 47.1 | 30.0 | |
| Tt | 29.4 | 40.0 | |
| tt | 23.5 | 30.0 |
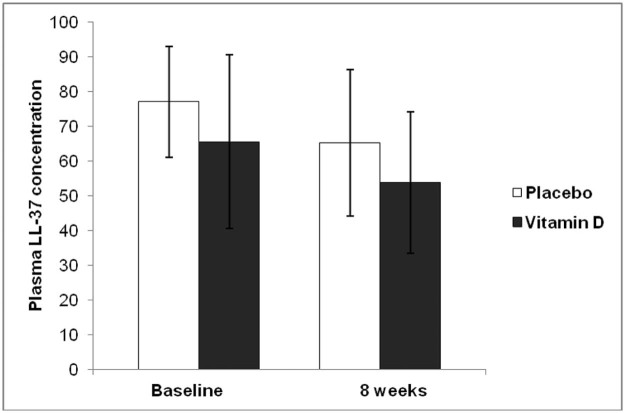
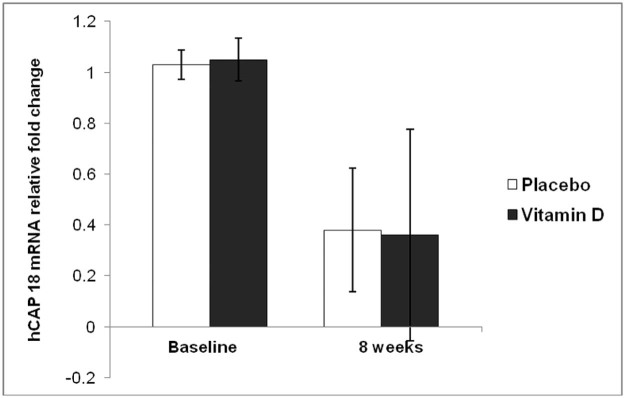
Plasma LL-37 concentrations did not differ between the two groups at baseline or after 8 weeks of anti-TB drug and vitamin D3 or placebo administration (P = 0.088) (Fig. 4). The expression of hCAP18 mRNA in PBMC was also similar between the two groups. The expression of hCAP18 mRNA in the PBMC from each group significantly decreased at 8 weeks compared to baseline (P < 0.001) but there was no significant difference between the vitamin D3 and placebo groups (P = 0.738) (Fig. 5).
Figure 4.
Plasma concentrations of LL-37 (the active human cathelicidin protein) in subjects recently diagnosed with pulmonary TB receiving conventional 4-drug initial anti-TB therapy (INH, rifampicin, pyrazinamide and ethambutol) and receiving at total of 1.2 million units of vitamin D3 (dark bars, n = 13; 50,000 IU thrice weekly) or placebo (white bars, n = 18) over 8 weeks. There was no significant difference in the change of plasma LL-37 in between the two groups (P = 0.088).
Figure 5.
Expression of human cathelicidin (hCAP18) mRNA in peripheral blood mononuclear cells collected from subjects as outlined in Fig. 4. hCAP18 mRNA fell significantly in both groups from baseline to week 8 after entry (P < 0.001). There was no significant difference in the expression of hCAP18 mRNA relative to GAPDH between the two groups at either time point (P = 0.738).
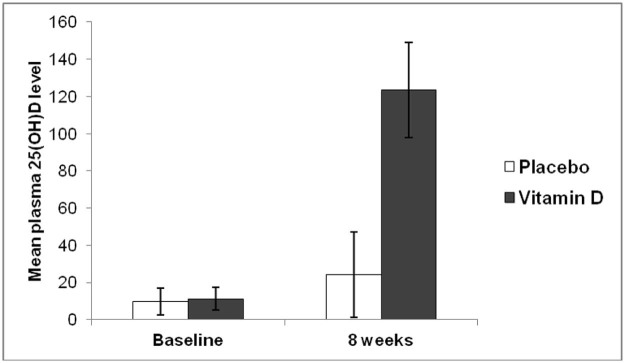
Mean plasma 25(OH)D concentration was significantly increased at 8 weeks compared to baseline in the vitamin D group (P < 0.001) whereas there was no significant change in 25(OH)D concentrations at 8 weeks in the placebo group (P = 0.07) (Fig. 6).
Figure 6.
Mean plasma 25(OH)D from subjects as outlined in Fig. 4. Mean plasma 25(OH)D concentration was significantly increased at 8 weeks compared to baseline in the vitamin D group (P < 0.001) whereas there was no significant change in 25(OH)D concentrations at 8 weeks in the placebo group (P = 0.07).
Discussion
Several studies have confirmed that vitamin D induces hCAP18 expression in cultured human macrophages [21], [24], [31], [32]. However many clinical studies have failed to show a positive effect of vitamin D on the treatment of tuberculosis infection [33]. One of the differences between the in vitro studies and the clinical studies is the potential confounding factor that all subjects receive standard anti-TB therapy in addition to the vitamin D or placebo, as it would be unethical to withhold standard anti-TB drug treatment. Our in vitro data confirm past published studies that show 1,25(OH)2D3 strongly induces the expression of hCAP18 mRNA in a human monocytic cell line THP-1. We also found that anti-TB drugs, when added to the culture medium of THP-1 cells, altered the action of vitamin D in terms of vitamin D induced hCAP18 mRNA expression. The major finding of our study was that when all 4 conventional first-line anti-TB drugs were added to culture media together (at 10.0 µg/mL each), the expression of hCAP18 mRNA by the cultured macrophages was significantly decreased in the presence of 1,25(OH)2D3.
These in vitro data may help to explain the lack of effect of high-dose vitamin D3 to enhance Mtb clearance drug-sensitive subjects, such as in our recent RCT in the country of Georgia. Our data from a subset of subjects from this RCT shown here demonstrate that high-dose oral vitamin D3 supplementation did not alter either hCAP18 mRNA expression in peripheral blood mononuclear cells or LL-37 protein expression in blood after 8 weeks. These negative findings may suggest that anti-TB drugs are impacting the action of vitamin D on induction of LL-37 in circulation and in PBMCs. In contrast, in a study of subjects with inflammatory bowel disease, vitamin D3 supplementation did increase blood levels of cathelicidin (LL-37) [34]. This would be consistent with our hypothesis that anti-TB drugs are impacting the action of vitamin D on induction of cathelicidin as patients with IBD are not on anti-TB drugs.
Several drugs may potentially impact the metabolism of vitamin D. Any drug that interferes with vitamin D absorption, metabolism and/or excretion process could potentially alter the action of vitamin D [35]. Several drugs are known to impact one of the two hydroxylation steps of vitamin D: (1) the hydroxylation of vitamin D to 25-hydroxyvitamin D (25(OH)D) via cytochrome P450 vitamin D 25-hydroxylase (25-OHase or CYP27A1) or (2) the hydroxylation of 25(OH)D to 1,25-dihydroxyvitamin D (1, 25(OH)2D) via the cytochrome P450 D-1-α-hydroxylase (1-OHase or CYP27B1) [36]. In a classic endocrine feedback loop, high blood concentrations of vitamin D also induces its own catabolism by increasing the expression of the 25-hydroxyvitamin D-24-hydroxylase (CYP24A1) which plays an important role in the catabolism of vitamin D [37]. Anti-convulsive medications such as phenytoin and phenobarbital can increase CYP24A1 expression, which results in the lowering of serum vitamin D [35].
It is currently unclear whether anti-TB drugs may impact vitamin D metabolism directly. Several studies conducted in patients infected with TB and taking the conventional 4 drug anti-TB regimen have suggested a very high prevalence of vitamin D deficiency in these populations [38], [39], [40], [41]. The association between TB and vitamin D deficiency suggests that several factors including the anti-TB therapy itself may have impacted the vitamin D status of the patients infected with TB. However, there is no clear mechanism by which anti-TB medications may lower circulating metabolites of vitamin D [42]. Relevant to our data, earlier studies have demonstrated that INH can either induce or inhibit the cytochrome P450 system which may result in the alterations of 25-OHase and 1-OHase [43], [44]. Furthermore, rifampicin can increase CYP3A4-dependent 25(OH)D3 metabolism in liver but not CYP24A1 [25], [45]. There are no available data on the impact of ethambutol and pyrazinamide on vitamin D metabolism or the effect of the 4-drug standard regimen studied in this report. In our study, hCAP18 mRNA in PBMCs fell significantly in both the vitamin D3-treated and the placebo cohorts from baseline to 8 weeks; LL-37 protein levels in plasma also declined over time, but not significantly. Whether this is due to the effect of the 4-drug regimen to clear Mtb and/or an impact on the anti-TB drug regimen on induction of hCAP18 mRNA and LL-37 in the circulation is unclear from our study. Moreover, there is no clear evidence that anti-TB drugs can have direct effects on cathelicidin independent of vitamin D in the literature.
While it is well established that vitamin D increases the expression of cathelicidin in vitro and in vivo in macrophages infected with TB, there may be other effects of vitamin D independent of cathelicidin and there may be other independent effects of TB medications. Salamon et al. [46] found that vitamin D may alter the growing environment of TB via PPAR by enhancing the antimicrobial effect that was induced by VDR. Tousif et al. [47] found that INH may decrease the function of CD4 T-cells via inducing apoptosis. These other potential vitamin D regulated pathways were worthy of further exploration.
Multiple RCTs have investigated the role of vitamin D as an adjunctive therapy in patients with active pulmonary TB. Most of clinical studies failed to show the improvement of treatment outcome. Our recent RCT of 199 pulmonary TB patients in Tbilisi, Georgia showed no difference in median sputum-culture conversion times between the high-dose vitamin D3 group and the placebo group [9]. Other negative clinical studies have other limitations as well, which include varying and insufficient doses of vitamin D [13], [14], small sample size, missing data [19] and absence of serum 25(OH)D concentrations [16]. The reasons why the results from the large RCTs have been negative are unclear despite very strong pre-clinical data that vitamin D triggers killing of Mtb via induction of LL-37 in vitro. However, one potential confounding factor in the investigation of vitamin D on clearance of Mtb in prospective clinical trials may be the use of anti-TB drugs by the patients enrolled in these trials. However, removing the potential confounding effect of anti-TB drugs may be challenging as it would be unethical to hold such effective drug therapy in these patients.
Our study has several strengths and limitations. One strength is that in vitro evaluation of 4 drugs commonly used in TB treatment has not been previously evaluated in terms of these drugs' effect on vitamin D induction of cathelicidin. We also linked our results to a recently conducted RCT of subjects with TB treated with the same 4 anti-TB drugs. Our study is limited in regard to the exact mechanism by which anti-TB impacts the action of vitamin D in macrophages and the lack of LL-37 protein concentration measures in either the cultured cells or in media. One possible explanation was the first-line anti-tuberculosis drugs might directly or indirectly interfere the enzymes in the vitamin D metabolism pathway such as CYP27A1 or CYP24A1 which may interfere with the activation of the vitamin D receptor in macrophages. Another limitation is the concentrations of the anti-TBs were based on the minimal inhibitory concentration (MIC) from published in vitro THP-1 cell culture and TB experiments in the literature [25], [26], [27], [28]. Also our THP-1 cells were not infected by Mtb. Although we cannot translate our in vitro findings to humans receiving anti-TB drugs, we do confirm that vitamin D induces hCAP18 in THP-1 cells in vitro and the impact of vitamin D to up-regulate hCAP18 mRNA and that hCAP18 induction by vitamin D is abolished when cultured in the presence of anti-TB drugs at 10 µg/mL. We speculate that the anti-TB medications given to subjects participating in our clinical RCT impacted the induction of LL-37 in blood or the mRNA expression of hCAP18 by the PBMCs from patients receiving high-dose vitamin D3.
In conclusion, our study is the first, to our knowledge, to demonstrate the effects of first-line anti-TB drugs on the induction of cathelicidin by vitamin D. We showed that 4 anti-TB drugs used in combination may alter the vitamin D-dependent increase in hCAP18 expression in cultured human macrophages. These findings suggest that further studies should be conducted to determine the mechanism of effect of anti-TB drugs on the action of vitamin D in macrophages.
Conflict of interest
The authors declare they have no conflicts of interest.
Acknowledgments
This work was supported by National Institutes of Health grants K24 DK096574 (to TRZ), D43TW007124 (to HMB), K23 AI103044 (to RRK), UL1 TR000454 (to the Atlanta Clinical and Translational Science Institute) and the Emory University Global Health Institute (to TRZ, VT, HMB).
References
- 1.Palwatwichai A. Tuberculosis in Thailand. Respirology. 2001;6(1):65–70. doi: 10.1046/j.1440-1843.2001.00299.x. [DOI] [PubMed] [Google Scholar]
- 2.WHO . 2014. Global Tuberculosis Report 2014. WHO Press, World Health Organization, Geneva. ISBN 978 92 4 156480 9. [Google Scholar]
- 3.WHO . 2010. Treatment of Tuberculosis Guidelines. WHO Press, World Health Organization, Geneva. ISBN 10: 9241547839. [PubMed] [Google Scholar]
- 4.Mitchison D.A. Antimicrobial therapy of tuberculosis: justification for currently recommended treatment regimens. Semin Respir Crit Care Med. 2004;25(3):307–315. doi: 10.1055/s-2004-829503. [DOI] [PubMed] [Google Scholar]
- 5.Lew W., Pai M., Oxlade O., Martin D., Menzies D. Initial drug resistance and tuberculosis treatment outcomes: systematic review and meta-analysis. Ann Intern Med. 2008;149(2):123–134. doi: 10.7326/0003-4819-149-2-200807150-00008. [DOI] [PubMed] [Google Scholar]
- 6.Luong K., Nguyen L.T. Impact of vitamin D in the treatment of tuberculosis. Am J Med Sci. 2011;341(6):493–498. doi: 10.1097/MAJ.0b013e3182070f47. [DOI] [PubMed] [Google Scholar]
- 7.Battersby A.J., Kampmann B., Burl S. Vitamin D in early childhood and the effect on immunity to Mycobacterium tuberculosis. Clin Dev Immunol. 2012;2012:430972. doi: 10.1155/2012/430972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamshchikov A.V., Desai N.S., Blumberg H.M., Ziegler T.R., Tangpricha V. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr Pract. 2009;15(5):438–449. doi: 10.4158/EP09101.ORR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tukvadze N., Sanikidze E., Kipiani M., Hebbar G., Easley K.A., Shenvi N. High-dose vitamin D3 in adults with pulmonary tuberculosis: a double-blind randomized controlled trial. Am J Clin Nutr. 2015;102(5):1059–1069. doi: 10.3945/ajcn.115.113886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutaria N., Liu C.T., Chen T.C. Vitamin D status, receptor gene polymorphisms, and supplementation on tuberculosis: a systematic review of case-control studies and randomized controlled trials. J Clin Transl Endocrinol. 2014;1(4):151–160. doi: 10.1016/j.jcte.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nursyam E.W., Amin Z., Rumende C.M. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones. 2006;38(1):3–5. [PubMed] [Google Scholar]
- 12.Martineau A.R., Wilkinson R.J., Wilkinson K.A., Newton S.M., Kampmann B., Hall B.M. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176(2):208–213. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 13.Wejse C., Gomes V.F., Rabna P., Gustafson P., Aaby P., Lisse I.M. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009;179(9):843–850. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 14.Martineau A.R., Timms P.M., Bothamley G.H., Hanifa Y., Islam K., Claxton A.P. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011;377(9761):242–250. doi: 10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kota S.K., Jammula S., Kota S.K., Tripathy P.R., Panda S., Modi K.D. Effect of vitamin D supplementation in type 2 diabetes patients with pulmonary tuberculosis. Diabetes Metab Syndr. 2011;5(2):85–89. doi: 10.1016/j.dsx.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Ralph A.P., Waramori G., Pontororing G.J., Kenangalem E., Wiguna A., Tjitra E. L-arginine and vitamin D adjunctive therapies in pulmonary tuberculosis: a randomised, double-blind, placebo-controlled trial. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0070032. e70032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salahuddin N., Ali F., Hasan Z., Rao N., Aqeel M., Mahmood F. Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis. BMC Infect Dis. 2013;13:22. doi: 10.1186/1471-2334-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q., Ma A., Bygbjerg I.C., Han X., Liu Y., Zhao S. Rationale and design of a randomized controlled trial of the effect of retinol and vitamin D supplementation on treatment in active pulmonary tuberculosis patients with diabetes. BMC Infect Dis. 2013;13:104. doi: 10.1186/1471-2334-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daley P., Jagannathan V., John K.R., Sarojini J., Latha A., Vieth R. Adjunctive vitamin D for treatment of active tuberculosis in India: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2015;15(5):528–534. doi: 10.1016/S1473-3099(15)70053-8. [DOI] [PubMed] [Google Scholar]
- 20.Kamen D.L., Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med. 2010;88(5):441–450. doi: 10.1007/s00109-010-0590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu P.T., Stenger S., Li H., Wenzel L., Tan B.H., Krutzik S.R. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 22.White J.H. Vitamin D metabolism and signaling in the immune system. Rev Endocr Metab Disord. 2012;13(1):21–29. doi: 10.1007/s11154-011-9195-z. [DOI] [PubMed] [Google Scholar]
- 23.Hewison M. Vitamin D and the intracrinology of innate immunity. Mol Cell Endocrinol. 2010;321(2):103–111. doi: 10.1016/j.mce.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams J.S., Ren S., Liu P.T., Chun R.F., Lagishetty V., Gombart A.F. Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182(7):4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsson M.C., Lerm M., Angeby K., Nordvall M., Jureen P., Schon T. A luciferase-based assay for rapid assessment of drug activity against Mycobacterium tuberculosis including monitoring of macrophage viability. J Microbiol Methods. 2014;106:146–150. doi: 10.1016/j.mimet.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Verma R.K., Kaur J., Kumar K., Yadav A.B., Misra A. Intracellular time course, pharmacokinetics, and biodistribution of isoniazid and rifabutin following pulmonary delivery of inhalable microparticles to mice. Antimicrob Agents Chemother. 2008;52(9):3195–3201. doi: 10.1128/AAC.00153-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kos J., Nevin E., Soral M., Kushkevych I., Gonec T., Bobal P. Synthesis and antimycobacterial properties of ring-substituted 6-hydroxynaphthalene-2-carboxanilides. Bioorg Med Chem. 2015;23(9):2035–2043. doi: 10.1016/j.bmc.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 28.Stokes R.W., Doxsee D. The receptor-mediated uptake, survival, replication, and drug sensitivity of Mycobacterium tuberculosis within the macrophage-like cell line THP-1: a comparison with human monocyte-derived macrophages. Cell Immunol. 1999;197(1):1–9. doi: 10.1006/cimm.1999.1554. [DOI] [PubMed] [Google Scholar]
- 29.Sato E., Imafuku S., Ishii K., Itoh R., Chou B., Soejima T. Vitamin D-dependent cathelicidin inhibits Mycobacterium marinum infection in human monocytic cells. J Dermatol Sci. 2013;70(3):166–172. doi: 10.1016/j.jdermsci.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Zughaier S.M., Stauffer B.B., McCarty N.A. Inflammation and ER stress downregulate BDH2 expression and dysregulate intracellular iron in macrophages. J Immunol Res. 2014;2014:140728. doi: 10.1155/2014/140728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torres-Juarez F., Cardenas-Vargas A., Montoya-Rosales A., Gonzalez-Curiel I., Garcia-Hernandez M.H., Enciso-Moreno J.A. LL-37 immunomodulatory activity during mycobacterium tuberculosis infection in macrophages. Infect Immun. 2015;83(12):4495–4503. doi: 10.1128/IAI.00936-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuk J.M., Shin D.M., Lee H.M., Yang C.S., Jin H.S., Kim K.K. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6(3):231–243. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Kearns M.D., Alvarez J.A., Seidel N., Tangpricha V. Impact of vitamin D on infectious disease. Am J Med Sci. 2015;349(3):245–262. doi: 10.1097/MAJ.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raftery T., Martineau A.R., Greiller C.L., Ghosh S., McNamara D., Bennett K. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn's disease: results from a randomised double-blind placebo-controlled study. United European Gastroenterol J. 2015;3(3):294–302. doi: 10.1177/2050640615572176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robien K., Oppeneer S.J., Kelly J.A., Hamilton-Reeves J.M. Drug-vitamin D interactions: a systematic review of the literature. Nutr Clin Pract. 2013;28(2):194–208. doi: 10.1177/0884533612467824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christakos S., Ajibade D.V., Dhawan P., Fechner A.J., Mady L.J. Vitamin D metabolism. Endocrinol Metab Clin North Am. 2010;39(2):243–253. doi: 10.1016/j.ecl.2010.02.002. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holick M.F. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 38.Keflie T.S., Nolle N., Lambert C., Nohr D., Biesalski H.K. Vitamin D deficiencies among tuberculosis patients in Africa: a systematic review. Nutrition. 2015;31(10):1204–1212. doi: 10.1016/j.nut.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Desai N.S., Tukvadze N., Frediani J.K., Kipiani M., Sanikidze E., Nichols M.M. Effects of sunlight and diet on vitamin D status of pulmonary tuberculosis patients in Tbilisi, Georgia. Nutrition. 2012;28(4):362–366. doi: 10.1016/j.nut.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sloan D.J., Mwandumba H.C., Kamdolozi M., Shani D., Chisale B., Dutton J. Vitamin D deficiency in Malawian adults with pulmonary tuberculosis: risk factors and treatment outcomes. Int J Tuberc Lung Dis. 2015;19(8):904–911. doi: 10.5588/ijtld.15.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamshchikov A.V., Kurbatova E.V., Kumari M., Blumberg H.M., Ziegler T.R., Ray S.M. Vitamin D status and antimicrobial peptide cathelicidin (LL-37) concentrations in patients with active pulmonary tuberculosis. Am J Clin Nutr. 2010;92(3):603–611. doi: 10.3945/ajcn.2010.29411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Self T.H., Chrisman C.R., Baciewicz A.M., Bronze M.S. Isoniazid drug and food interactions. Am J Med Sci. 1999;317(5):304–311. doi: 10.1097/00000441-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Brodie M.J., Boobis A.R., Hillyard C.J., Abeyasekera G., MacIntyre I., Park B.K. Effect of isoniazid on vitamin D metabolism and hepatic monooxygenase activity. Clin Pharmacol Ther. 1981;30(3):363–367. doi: 10.1038/clpt.1981.173. [DOI] [PubMed] [Google Scholar]
- 44.Brodie M.J., Boobis A.R., Hillyard C.J., Abeyasekera G., Stevenson J.C., MacIntyre I. Effect of rifampicin and isoniazid on vitamin D metabolism. Clin Pharmacol Ther. 1982;32(4):525–530. doi: 10.1038/clpt.1982.197. [DOI] [PubMed] [Google Scholar]
- 45.Wang Z., Lin Y.S., Zheng X.E., Senn T., Hashizume T., Scian M. An inducible cytochrome P450 3A4-dependent vitamin D catabolic pathway. Mol Pharmacol. 2012;81(4):498–509. doi: 10.1124/mol.111.076356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salamon H., Bruiners N., Lakehal K., Shi L., Ravi J., Yamaguchi K.D. Cutting edge: vitamin D regulates lipid metabolism in Mycobacterium tuberculosis infection. J Immunol. 2014;193(1):30–34. doi: 10.4049/jimmunol.1400736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tousif S., Singh D.K., Ahmad S., Moodley P., Bhattacharyya M., Van Kaer L. Isoniazid induces apoptosis of activated CD4+ T cells: implications for post-therapy tuberculosis reactivation and reinfection. J Biol Chem. 2014;289(44):30190–30195. doi: 10.1074/jbc.C114.598946. [DOI] [PMC free article] [PubMed] [Google Scholar]