Abstract
The purpose of this study was to determine the effect of the diabetic phenotype on the mechanical properties of the native patellar tendon and its enthesis. Diabetes was induced via intraperitoneal injection of streptozotocin in Lewis rats. Control (n = 18) and diabetic animals(n = 20) were killed at 12 and 19 days for analysis. Statistical comparisons were performed using Student’s t-tests and a two-tailed Fisher test with significance set at p < 0.05. Pre- and post-injection intraperitoneal glucose tolerance tests demonstrated significant impairment of glycemic control in the diabetic compared to control animals (p = 0.001). Mean serum hemoglobin A1c levels at 19 days was 10.6 ± 2.7% and 6.0 ± 1.0% for the diabetic and control groups, respectively (p = 0.0001). Fifteen of sixteen diabetic animals demonstrated intrasubstance failure of the patellar tendon, while only 7 of 14 control specimens failed within the tendon substance. The Young’s modulus of the diabetic tendon was significantly lower than control specimens by 19 days post-induction (161 ± 10 N m−2 compared to 200 ± 46 N m−2, respectively) (p = 0.02). The metabolic condition of poorly controlled diabetes negatively affects the mechanical properties of the native patellar tendon. These altered structural properties may predispose diabetic patients to a greater risk of tendinopathy and/or traumatic rupture.
Keywords: diabetes, mechanical properties, patellar tendon, rat, streptozotocin
The impact of sustained hyperglycemia on the musculoskeletal system has only recently been examined. Clinical and experimental studies have revealed that the diabetic state is associated with impaired fracture healing,1–6 reduced skeletal mass,7 reduced bone mineral density,8 impaired collagen production,9,10 increased stiffness,11–15 increased risk of tendinopathy,16 increased presence of advanced glycation end products (AGE’s),17–20 abnormal cell morphology 21,22 as well as a greater risk of infection and complications following tendon repair.23,24 The effect of diabetes mellitus on native tendon tissue, however, has not been explored.
The primary function of the patellar tendon is to transmit forces from the patella to the tibia, producing joint motion. The tendon insertion (the enthesis) is a transitional zone where the tendon graduates to bone through a series of fibrocartilaginous layers. The enthesis allows a gradual change in mechanical properties from the flexible tendon to the rigid bone. Loss of tendon function due to tendinopathy, rupture or injury,16,23–28 will adversely affect joint function. Understanding the mechanical properties of tendons in diabetics will provide clinicians with critical information when counseling these patients on whether they are more predisposed to tendon degeneration and traumatic musculoskeletal injuries. We hypothesized that sustained hyperglycemia would adversely affect mechanical properties of native tendon compared to euglycemic controls. The purpose of this study was to determine the effect of the diabetic phenotype on the mechanical properties of the native patellar tendon and its enthesis. This hypothesis was tested using a controlled laboratory model of streptozotocin-induced diabetes in rats.
METHODS
Study Design
This study was approved by our Institutional Animal Care and Use Committee. Diabetes was induced via intraperitoneal (IP) injection of streptozotocin (STZ, 65 mg/kg; Sigma, St. Louis, MO), a selective toxin of pancreatic β-cells, in 20 male Lewis rats of identical age (weight: 250–300 g; Harlan, Indianapolis, IN). Induction of diabetes was confirmed with both pre- and post-STZ injection IP glucose tolerance tests (IPGTT). Eighteen control animals received an IP injection of citrate buffer solution only. Animals in both groups underwent rotator cuff surgery 5 days after injection to evaluate tendon-to-bone healing for a different study. The native tibia-patellar tendon-patella complex was harvested at 12 or 19 days post-induction from the right lower extremity. Our primary outcome measure was biomechanical analysis of the tendon and its enthesis while histological analysis was a secondary outcome.
Induction of Diabetes
Rats were anesthetized with isoflurane and diabetes was induced by an IP injection of STZ. The STZ was dissolved in citrate buffer (pH 4.6) containing 75 mM citric acid, 150 mM NaOH, and 25 mM HCl. Four days after the injection, STZ-treated and control (untreated) rats were fasted for 6 h and subjected to an IPGTT. Blood glucose levels were measured at 0, 15, 30, 60, 90, and 120 min after an IP injection of a 50% dextrose solution in sterile saline at 1.5 g/kg body weight (Sigma). All rats maintained a sustained diabetic phenotype, defined by persistent fasting blood glucose levels equal or greater than 250 mg/dL. Blood glucose levels were measured every 3 days using a glucometer (True Result™, Fort Lauderdale, FL) in all rats to ensure maintenance of a euglycemic and hyperglycemic state in the control and diabetic groups, respectively.
Measurement of Glycosylated Hemoglobin
Immediately after the rats were killed, whole blood was collected into 3 ml EDTA tubes (BD, Franklin Lakes, NJ) via a single intracardiac puncture and immediately refrigerated. Blinded samples of blood were provided to an independent institution for analysis (Louisiana State University, Baton Rouge, LA). Serum HbA1c levels were measured to confirm a diabetic or euglycemic phenotype and to quantify the severity of sustained hyperglycemia.29–32 HbA1c was measured using standard affinity microchromatographic methodology (Helena Laboratories, Beaumont, TX).33
Histological Analysis
The right patella, patellar tendon, and tibia were carefully dissected free of all remaining soft tissues at the time of sacrifice (n = 4/group). The qualitative appearance (tendon swelling, thickening, and discoloration) of the native patellar tendon and its tubercle insertion were evaluated in a blinded-fashion by two individuals (AJF and AB). The tissue was fixed in 10% neutral buffered formalin at 4°C for 48 h, and then decalcified in formic acid (Immunocal, Tallman, NY) for 48 h and washed in phosphate-buffered saline solution. The samples were then dehydrated and embedded in paraffin following standard tissue processing techniques. Five micrometer thick, mid-sagittal sections of the specimen were mounted on silane-coated slides and were stained with hematoxylin and eosin, safranin-O, and picrosirius red. The patellar tendon and enthesis were qualitatively examined under light and polarized light microscopy at 40× to assess fibrocartilage and collagen organization (Eclipse E800; Nikon, Melville, NY).
Immunohistochemistry
Serial sections were treated with 3% H2O2 to quench endogenous peroxidase activity, and non-specific antibody binding was blocked with 5% goat serum. One percent bovine serum albumin/phosphate-buffered saline solution was used as a negative secondary reagent control. AGE staining of tissues was assessed using a monoclonal anti-AGE antibody (MP Biomedicals, Solon, OH) and was applied to sections for 60 min at 37°C. Bound antibodies were visualized using a goat avidin-biotin peroxidase system with diamino-benzidine (D.A.B., DakoCorp., Carpinteria, CA) as a substrate. Assessment of AGE deposition was performed at 100× by two independent observers (AB and AJF) who were not aware of the slide identification. The patellar tendon and enthesis were graded as 0, 1+, 2+, or 3+, based on the intensity of the staining. Distribution of staining was also documented.
Biomechanical Testing
Animals (n = 8/group) were killed at 12 or 19 days post-injection by CO2 inhalation. The right hind limb was disarticulated at the hip, placed in saline-soaked gauze and stored at −80°C until the time of biomechanical testing. On the day of testing, specimens were thawed overnight at 4°C and acclimated to room temperature. The patella-patellar tendon-tibia complex was carefully dissected under magnification in a blinded fashion with respect to group. The length of the tendon was viewed from the anterior surface from the distal pole of the patella to the tibial insertion. The length and cross-sectional area (width × thickness) of the tendon was calculated using measurements taken by a digital micrometer. The reproducibility of this technique was characterized by two individuals independently taking measurements in triplicate and averaging the dimensions and has been validated in previously published models.34,35 Each specimen was mounted on a custom-designed uniaxial system. The patella was secured in a screw grip using a cone-shaped wedge and the tibia was secured into a serrated vice grip that prevented slippage or fracture through the proximal tibial physis. A 45-N load cell attached to a linear bearing allowed uniaxial alignment of the tendon. The tibial jig was fixed to the linear stage and the specimen were pre-loaded to 0.5 N and then loaded to failure at a rate of 16.7 μm/s (1 mm/min). The preload and rate of loading is physiologically relevant and in concordance with previously validated experiments.34,35 A single operator performed all the biomechanical testing and the maximum load-to-failure and failure location (mode) were recorded. The linear region of the load-displacement curve was used to calculate the stiffness for each specimen. Young’s modulus was calculated as stress divided by strain.
Statistical Analysis
Statistical analysis was performed using SigmaStat (Systat Software Inc., Chicago, IL) with p < 0.05 defined as significant. Mean serum HbA1c levels, histological data, area under the curve (AUC), analysis of IPGTT, load-to-failure and stiffness were compared between control and diabetic groups using Student’s t-test. A two-tailed Fisher test was performed to compare the mode of failure between control and diabetic specimens. Results were reported as mean values ± standard error of the mean. The study was powered to detect significant differences in load-to-failure and stiffness between diabetic and control specimens.
RESULTS
Induction of Diabetes
Induction of diabetes with STZ was both effective and sustained. AUC analysis of IPGTT in both experimental and control groups provided a quantitative index of the severity of hyperglycemia. Mean AUC was significantly greater in the diabetic (21,510 ± 2,826) compared to control animals (9,826 ± 1,366) (p = 0.0001). Analysis of the AUC in experimental animals pre- and post-STZ injection demonstrated a significant reduction in glycemic control (10,278 ± 410 and 22,421 ± 3,162, respectively) (p = 0.001). Mean HbA1c level at 19 days was significantly greater in the diabetic group compared to the control group (10.6 ± 2.7% vs. 6.0 ± 1.0%, respectively) (p = 0.0001).
Gross and Histological Findings
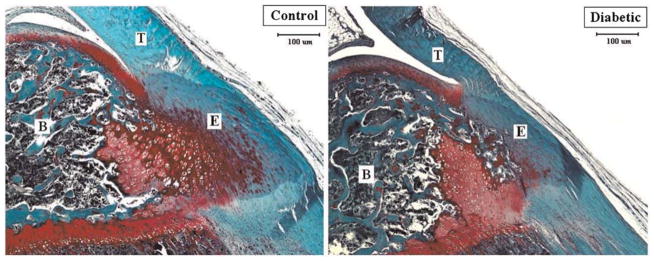
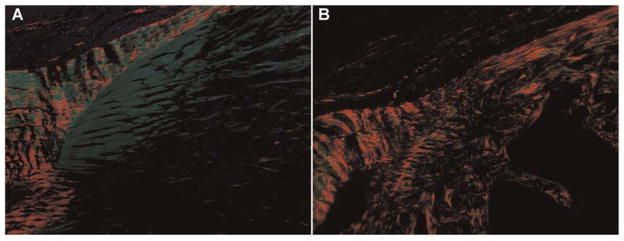
The patellar tendon of the diabetic animals appeared qualitatively different at sacrifice from those of control specimens. The tendon of the diabetic rats appeared fragile and demonstrated a yellowish discoloration compared to the more robust, healthy tissue observed in control animals. Mean length, width, and thickness of diabetic patellar tendons was 8.08 ± 0.14 mm, 2.52 ± 0.13 mm and 0.64 ± 0.08 mm, respectively, and 8.09 ± 0.29 mm, 2.37 ± 0.13 mm, 0.57 ± 0.05 mm in control tendons, respectively. There were no significant differences in length, width, or thickness between groups. The patellar tendons of diabetic animals demonstrated less fibrocartilage at the tibial tubercle enthesis compared to the control group at 19 days (Fig. 1). Examination of collagen birefringence revealed less organized collagen at the tubercle enthesis in the diabetic compared to control specimens at 19 days (Fig. 2). AGE’s were detected in minimal quantities within the tendon substance and enthesis in both groups. These observations were purely qualitative, however, as the study was underpowered to detect statistically significant differences in histomorphometric measurements.
Figure 1.
Safranin-O staining of the native patellar tendon enthesis at the tibial tubercle in the control and diabetic group. At 19 days, the area of fibrocartilage was qualitatively less in the diabetic compared to the control group. B, bone; E, enthesis; T, tendon; (×40).
Figure 2.
Polarized light microscopy with picrosirius red staining revealed less organized collagen at the tubercle enthesis in the diabetic (B) compared to control (A) specimens at 19 days.
Biomechanical Findings
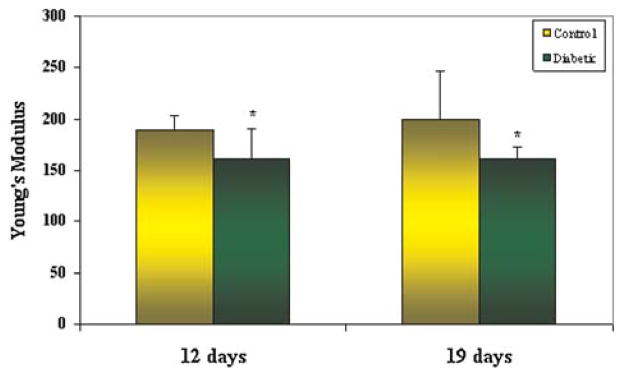
There was a significant difference in the mode of failure between the diabetic and control specimens. Fifteen of sixteen diabetic animals demonstrated intrasubstance failure of the patellar tendon during load-to-failure testing. In contrast, only 7 of 14 control specimens failed within the tendon substance, while the remaining specimens failed via avulsion of the tibial tubercle or fracture through the proximal tibial physis (p = 0.014). No significant differences in mean load-to-failure or stiffness were detected between diabetic and control animals at either time-point. The Young’s modulus of the diabetic patellar tendon was significantly lower than control specimens. At 19 days, the mean Young’s modulus of the diabetic patellar tendons was 162 ± 10 N m−2 compared to 200 ± 46 N m−2 in control tendons (p = 0.02). A significant difference in Young’s modulus of the tendon was also detected between diabetic (161 ± 30 N m−2) and control (189 ± 14 N m−2) groups at 12 days (p = 0.04) (Fig. 3).
Figure 3.
The mean Young’s modulus of the diabetic patellar tendon was significantly lower than control animals at both 12 days (p = 0.04) and 19 days (p = 0.02).
DISCUSSION
Diabetes mellitus is a complex disorder characterized by persistent hyperglycemia. The metabolic perturbations of diabetes result in detrimental changes to the musculoskeletal system,11–16,21,22,26–28,36–46 however the effect of diabetes on native tendon tissue has not been well defined.
The tensile strength of a tendon is highly dependent on the intra- and intermolecular cross-links, orientation, density, and length of collagen fibrils and fibers.27,47 Any disruption to the homeostatic environment will impact the micro-structural integrity of the tendon extra-cellular matrix (ECM). The patellar tendon has a parallel arrangement of collagen fibrils, providing the major resistance to mechanical loading. In this study, we found that the diabetic patellar tendons had a significantly reduced Young’s modulus and a predisposition for mid-substance failure compared to non-diabetic control specimens. Our results indicate that the diabetic phenotype alters the micro-structural properties of the tendons and their insertions that may predispose them to certain musculoskeletal injuries.
The results of our study suggest that diabetic patients may be uniquely susceptible to certain injury patterns. We found a predilection for mid-substance tendon rupture in the diabetic group, in contrast to the control specimens which often failed at the tibial enthesis. This finding is supported by the well-established increased prevalence of tendinopathy and tendon rupture in this patient population.11–17,23,25–28,36–45 Although the underlying pathological etiology of tendinopathy remains unclear, recent studies in the human population have reported that intrinsic factors such as mucoid and lipid accumulation may play a causative role in tendinopathy.46 Abnormalities in these intrinsic factors may result in altered tendon matrix metabolism, composition and organization and may have contributed to the tendon degeneration as seen in the diabetic group in this study. There may be a role for implementation of preventative exercise or rehabilitation programs in this patient population to help ameliorate the negative influences on musculoskeletal tissues. Furthermore, counseling to avoid certain activities that place diabetic patients at substantial risk for tendon injury or rupture may be appropriate. It is possible that hyperglycemia alters the mechanical properties of the enthesis and bone, which in-turn alters the relative location of load transmission and ultimate failure. Future studies to evaluate the effect of sustained hyperglycemia on bone and tendon microstructure in both animal and clinical contexts would provide further insight into these findings and their mechanism.
Increasing evidence in the literature suggests that an accumulation of AGE’s (namely modifying collagen) may be responsible for the inferior biomechanical properties of connective tissues.9,18,31 While we did not detect significant AGE deposition within the tendon or enthesis, it is plausible that the normal, healthy turnover of other ECM molecules, growth factors and glycoaminoglycans (GAG’s) is perturbed by diabetes. GAG’s specifically are essential to tendon function and are responsible for cellular migration and differentiation and may also play a regulatory role in collagen fibril growth and in the three-dimensional arrangement of collagen fibrils.47 Furthermore, remodeling and homeostasis of the ECM is mediated primarily by matrix metalloproteinases (MMP) and their inhibitors, the tissue inhibitors of MMPs (TIMPs). Several studies have reported changes in MMP activity in diabetic tendinopathy.39–41,48 It is also plausible that the sustained hyperglycemia in our model produced an imbalance of MMP and TIMP activity, thus compromising the structural and functional integrity of the tendon. Future studies are warranted to determine if other ECM molecules, growth factors and/or GAG’s are up- or down-regulated with sustained hyperglycemia.
The limitations of this study are important to note. First, we evaluated animals for only 12 and 19 days. The short (acute) duration of the STZ-induced diabetic phenotype in these rats does not reflect the pathophysiologic condition in humans, which tends to occur over a period of years. Longer time-points may provide valuable information regarding the effect of chronic hyperglycemia on the tendon and enthesis. However, we were limited by deterioration in the general health of the animals in this STZ-induced diabetic model from pursuing later time-points. The loss of weight and increased lethargy displayed by the diabetic rats may influence the patellar tendons in a non-specific way such as reduced mechanical load. However, the finding of poor health using uncontrolled diabetic animals is not unique to our study.1,4,49 Second, our diabetic model creates a Type I insulin-dependent diabetic condition and presents a “worst case” scenario of glycemic control. The diabetic condition includes a broad spectrum of pathology, ranging from subtle insulin resistance to severe hyperglycemia, such that future studies using different models are necessary to elaborate upon the findings of our pilot study. Lastly, the animals in our study also underwent an acute rotator cuff repair at 5 days post-injection to evaluate tendon-bone healing for a different study. While both animal groups were subjected to the same treatment, it is possible that the post-operative stress condition affected the properties of the native patellar tendon.
SUMMARY
Sustained hyperglycemia resulted in a significantly reduced Young’s modulus of the patellar tendon compared to control specimens and resulted in an increased incidence of mid-substance tendon failure. Altered structural properties in musculoskeletal tissues may predispose diabetic patients to a greater of risk of tendinopathy and certain musculoskeletal injury patterns compared to euglycemic patients.
References
- 1.Macey LR, Kana SM, Jinushi S, et al. Defects of early fracture-healing in experimental diabetes. JBJS. 1989;71:722–733. [PubMed] [Google Scholar]
- 2.Loder RT. The influence of diabetes mellitus on the healing of closed fractures. Clin Orthop. 1988;232:210–216. [PubMed] [Google Scholar]
- 3.Cozen L. Does diabetes delay fracture healing? Clin Orthop. 1972;82:134–140. [PubMed] [Google Scholar]
- 4.Wray JB, Stunkle E. The effect of experimental diabetes on the breaking strength of the healing fracture in the rat. J Surg Res. 1965;5:479–481. [Google Scholar]
- 5.Herbsman H, Powers JC, Hirschman A, et al. Retardation of fracture healing in experimental diabetes. J Surg Res. 1968;8:424–431. doi: 10.1016/0022-4804(68)90058-9. [DOI] [PubMed] [Google Scholar]
- 6.Funk JR, Hale JE, Carmines D, et al. Biomechanical evaluation of early fracture healing in normal and diabetic rats. J Orthop Res. 2000;18:126–132. doi: 10.1002/jor.1100180118. [DOI] [PubMed] [Google Scholar]
- 7.Saha MT, Sievänen H, Salo MK, et al. Bone mass and structure in adolescents with type 1 diabetes compared to healthy peers. Osteoporos Int. 2009;20:1401–1406. doi: 10.1007/s00198-008-0810-0. [DOI] [PubMed] [Google Scholar]
- 8.Heilman K, Zilmer M, Zilkmer K, et al. Lower bone mineral density in children with type 1 diabetes is associated with poor glycemic control and higher serum ICAM-1 and urinary isoprostane levels. J Bone Miner Metab. 2009;27:598–604. doi: 10.1007/s00774-009-0076-4. [DOI] [PubMed] [Google Scholar]
- 9.Spanheimer RG. Correlation between decreased collagen production in diabetic animals and in cells exposed to diabetic serum: response to insulin. Matrix. 1992;12:101–107. doi: 10.1016/s0934-8832(11)80051-x. [DOI] [PubMed] [Google Scholar]
- 10.Leung MK, Folkes GA, Ramamurthy NS, et al. Diabetes stimulates procollagen degradation on rat tendon in vitro. Biochim Biophys Acta. 1986;880:147–152. doi: 10.1016/0304-4165(86)90074-7. [DOI] [PubMed] [Google Scholar]
- 11.Arkkila PE, Kantola IM, Viikari JS, et al. Shoulder capsulitis in type I and II diabetic patients: association with diabetic complications and related diseases. Ann Rheum Dis. 1996;55:907–914. doi: 10.1136/ard.55.12.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balci N, Balci MK, Tuzuner S. Shoulder adhesive capsulitis and shoulder range of motion in type II diabetes: association with diabetic complications. J Diab Comp. 1999;13:135–140. doi: 10.1016/s1056-8727(99)00037-9. [DOI] [PubMed] [Google Scholar]
- 13.Bridgman JF. Periarthritis of the shoulder and diabetes mellitus. Ann Rheum Dis. 1972;31:69–71. doi: 10.1136/ard.31.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao SR, Saltzman CL, Wilken J, et al. Increased passive ankle stiffness and reduced dorsiflexion range of motion in individuals with diabetes mellitus. Foot Ankle Int. 2006;27:617–622. doi: 10.1177/107110070602700809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trevino SG, Buford WL, Nakamura T, et al. Use of a torque-range-of-motion device for objective differentiation of diabetic from normal feet in adults. Foot Ankle Int. 2004;25:561–567. doi: 10.1177/107110070402500809. [DOI] [PubMed] [Google Scholar]
- 16.Batista F, Nery C, Pinzur M, et al. Achilles tendinopathy in diabetes mellitus. Foot Ankle Int. 2008;29:498–501. doi: 10.3113/FAI-2008-0498. [DOI] [PubMed] [Google Scholar]
- 17.James VJ, Delbridge L, McLennan SV, et al. Use of X-ray diffraction in study of human diabetic and aging collagen. Diabetes. 1991;40:391–394. doi: 10.2337/diab.40.3.391. [DOI] [PubMed] [Google Scholar]
- 18.Reddy GK, Stehno-Bittel L, Enwemeka CS. Glycation-induced matrix stability in the rat Achilles tendon. Arch Biochem Biophys. 2002;399:174–180. doi: 10.1006/abbi.2001.2747. [DOI] [PubMed] [Google Scholar]
- 19.Sell DR, Monnier VM. End-stage renal disease and diabetes catalyze the formation of a pentose-derived crosslink from aging human collagen. J Clin Invest. 1990;85:380–384. doi: 10.1172/JCI114449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Degroot J, Verzijl N, Budde M, et al. Accumulation of advanced glycation end products decreases collagen turnover by bovine chondrocytes. Exp Cell Res. 2001;266:303–310. doi: 10.1006/excr.2001.5224. [DOI] [PubMed] [Google Scholar]
- 21.Grant WP, Sullivan R, Sonenshine DE, et al. Electron microscopic investigation of the effect of diabetes mellitus on the Achilles tendon. J Foot Ankle Surg. 1997;36:272–278. doi: 10.1016/s1067-2516(97)80072-5. [DOI] [PubMed] [Google Scholar]
- 22.Odetti P, Aragno I, Rolandi R, et al. Scanning force microscopy reveals structural alterations in diabetic rat collagen fibrils: role of protein glycation. Diab Metab Res Rev. 2000;16:74–81. doi: 10.1002/(sici)1520-7560(200003/04)16:2<74::aid-dmrr80>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Chen AL, Shapiro JA, Ahn AK, et al. Rotator cuff repair in patients with type 1 diabetes mellitus. J Shoulder Elbow Surg. 2003;12:416–421. doi: 10.1016/s1058-2746(03)00172-1. [DOI] [PubMed] [Google Scholar]
- 24.Saxena A, Maffulli N, Nguyen A, et al. Wound complications from surgeries pertaining to the Achilles tendon. J Am Podiatr Med Assoc. 2008;98:95–101. [PubMed] [Google Scholar]
- 25.Ramirez LC, Raskin P. Diabetic foot tendinopathy: abnormalities in the flexor plantar tendons in patients with diabetes mellitus. J Diabetes Compl. 1998;12:337–339. doi: 10.1016/s1056-8727(98)00024-5. [DOI] [PubMed] [Google Scholar]
- 26.Cook JL, Khan KM, Kiss ZS, et al. Patellar tendinopathy in junior basketball players: a controlled clinical and ultrsonographic study of 268 patellar tendons in players aged 14–18 years. Scand J Med Sci Sports. 2000;10:216–220. doi: 10.1034/j.1600-0838.2000.010004216.x. [DOI] [PubMed] [Google Scholar]
- 27.Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendons. Clin Sports Med. 2003;22:675–692. doi: 10.1016/s0278-5919(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 28.Maffulli N, Testa V, Capasso G, et al. Surgery for chronic Achilles tendinopathy yields worse results in nonathletic patients. Clin J Sport Med. 2006;16:123–128. doi: 10.1097/00042752-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Rahbar S, Blumenfeld O, Ranney HM. Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem Biophys Res Commun. 1969;36:838–843. doi: 10.1016/0006-291x(69)90685-8. [DOI] [PubMed] [Google Scholar]
- 30.Gabbay KH, Hasty K, Breslow JL, et al. Glycosylated hemoglobins and long-term blood glucose control in diabetes mellitus. J Clin Endocrinol Metab. 1977;44:859–864. doi: 10.1210/jcem-44-5-859. [DOI] [PubMed] [Google Scholar]
- 31.Bunn HF, Shapiro R, McManus M, et al. Structural heterogeneity of human hemoglobin A due to nonenzymatic glycosylation. J Biol Chem. 1979;254:3892–3898. [PubMed] [Google Scholar]
- 32.Koenig RJ, Peterson CM, Kilo C, et al. Hemoglobin A1c as an indicator of the degree of glucose intolerance in diabetes. Diabetes. 1976;25:20–232. doi: 10.2337/diab.25.3.230. [DOI] [PubMed] [Google Scholar]
- 33.Yue DK, McLennan S, Church DB, et al. The measurement of glycosylated haemoglobin in man and animals by aminophenylboronic acid affinity chromatography. Diabetes. 1982;31:701–705. doi: 10.2337/diab.31.8.701. [DOI] [PubMed] [Google Scholar]
- 34.Gulotta LV, Kovacevic D, Ehteshami JR, et al. Application of bone-marrow derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37:2126–2133. doi: 10.1177/0363546509339582. [DOI] [PubMed] [Google Scholar]
- 35.Bedi A, Kovacevic D, Hettrich C, et al. The effect of matrix metalloproteinase inhibition on tendon-bone healing in a rotator cuff repair model. J Shoulder Elbow Surg. 2010;19:384–391. doi: 10.1016/j.jse.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Chbinou N, Frenette J. Insulin-dependent diabetes impairs the inflammatory response and delays angiogenesis following Achilles tendon injury. Am J Physiol Regul Comp Physiol. 2004;286:R952–R957. doi: 10.1152/ajpregu.00536.2003. [DOI] [PubMed] [Google Scholar]
- 37.Darby IA, Bisucc T, Hewitson TD, et al. Apoptosis is increased in a model of diabetes-impaired wound healing in genetically diabetic mice. Int J Biochem Cell Biol. 1997;29:191–200. doi: 10.1016/s1357-2725(96)00131-8. [DOI] [PubMed] [Google Scholar]
- 38.Loots M, Lamme EN, Mekkes JR, et al. Cultured fibroblasts from chronic diabetic wounds on the lower extremity (non-insulin-dependent diabetes mellitus) show disturbed proliferation. Arch Dermatol Res. 1999;291:93–99. doi: 10.1007/s004030050389. [DOI] [PubMed] [Google Scholar]
- 39.Maffulli N, Sharma P, Luscombe KL. Achilles tendinopathy: aetiology and management. J R Soc Med. 2004;97:472–476. doi: 10.1258/jrsm.97.10.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magra M, Maffulli N. Matrix metalloproteases: a role in overuse tendinopathies. Br J Sports Med. 2005;39:789–791. doi: 10.1136/bjsm.2005.017855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woo SLY, Ritter MA, Amiel D, et al. The biomechanical and biochemical properties of swine tendons — long term effects of exercise on the digital extensors. Connect Tissue Res. 1980;7:177–183. doi: 10.3109/03008208009152109. [DOI] [PubMed] [Google Scholar]
- 42.Simonsen EB, Klitgaard H, Bojsen-Moller F. The influence of strength training, swim training and ageing on the Achilles tendon and M. soleus of the rat. J Sports Sci. 1995;13:291–295. doi: 10.1080/02640419508732242. [DOI] [PubMed] [Google Scholar]
- 43.Viidik S. Tensile strength properties of Achilles tendon systems in trained and untrained rabbits. Acta Orthop Scand. 1969;40:261–272. doi: 10.3109/17453676908989506. [DOI] [PubMed] [Google Scholar]
- 44.Andreassen TT, Seyer-Hansen K, Bailey AJ. Thermal stability, mechanical properties and reducible cross-links of rat tail tendon in experimental diabetes. Biochim Biophys. 1981;677:313–317. doi: 10.1016/0304-4165(81)90101-x. [DOI] [PubMed] [Google Scholar]
- 45.Altinel L, Cagri Kose K, Degirmenci B, et al. The midterm effect of diabetes mellitus on quadriceps and patellar tendons in patients with knee arthrosis: a comparative radiologic study. J Diabetes Complications. 2007;21:392–396. doi: 10.1016/j.jdiacomp.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Gaida JE, Ashe MC, Bass SL, et al. Is adiposity an under-recognized risk factor for tendinopathy? A systemic review. Arthritis Care Res. 2009;61:840–849. doi: 10.1002/art.24518. [DOI] [PubMed] [Google Scholar]
- 47.Scott JE. Proteoglycan–fibrillar collagen interactions. Biochem J. 1988;252:313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corps AN, Jones GC, Harrall RL, et al. The regulation of aggrecanase ADAMTS-4 expression in human Achilles tendon and tendon-derived cells. Matrix Biol. 2008;27:393–401. doi: 10.1016/j.matbio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yue DK, Swanson B, McLennan S, et al. Abnormalities of granulation tissue and collagen formation in experimental diabetes, uraemia and malnutrition. Diabetic Med. 1986;3:221–225. doi: 10.1111/j.1464-5491.1986.tb00748.x. [DOI] [PubMed] [Google Scholar]