Abstract
We describe two methods for creating long (>1 kb) dsRNA molecules with specific, user-controlled overhangs for efficient hybridization and ligation. The two methods create double-stranded RNA (dsRNA) molecules with 5′ overhangs or with 3′ overhangs using T7 RNA polymerase (T7 RNAP) in transcription reactions of carefully designed PCR products. Primers utilized in the PCR reactions provide the template for the desired dsRNA overhangs. These methods provide complete control of the length and the sequence of the overhangs. This supplies a tool which is particularly lacking in dsRNA biochemistry given the absence of restriction endonucleases active on these substrates.
INTRODUCTION
The creation of specific overhangs (‘sticky ends’) for double-stranded DNA (dsDNA) by restriction endonuclease digestion and the resulting control of DNA ligation have revolutionized molecular biology over the past few decades, permitting such varied applications as the creation of specific plasmids and constructs with which to study protein–DNA interactions, the controlled modification of genes and many others. Similar control of double-stranded RNA (dsRNA) would greatly enhance the flexibility of working with this important molecule. This is especially important since the discovery of RNA interference has demonstrated that the role of long dsRNA molecules in the cellular life cycle is much more important than previously imagined (1,2).
One of the ingredients lacking in the dsRNA toolkit is sequence-specific dsRNA endonucleases (3) with which to create sticky ends on dsRNA molecules. Here, we demonstrate that this problem may be circumvented by protocols that produce dsRNA with user-controlled, specific overhangs. These protocols are flexible since they function on any DNA substrate and allow the user to determine the sequence of the overhangs incorporated. An important example of the utility of sticky ends for dsRNA is controlled, specific and efficient ligation of dsRNA molecules. This is not possible with blunt-ended dsRNA molecules due to the lack of hybridization. This makes it difficult to couple specific molecules to each other, results in a lack of control of the orientation of ligation partners with respect to each other, and decreases the ligation efficiency enormously due to the decreased time that the ligation partners spend in proximity. Effective creation of dsRNA with sticky ends will therefore facilitate studies of protein binding to dsRNA (4) by the creation of appropriate substrates, the implementation of DNA–RNA networks in biotechnology, and the inclusion of dsRNA in single molecule measurements (5–9). In addition, it will facilitate RNA labelling and the creation of long dsRNA molecules with overhangs tailored to RNA–protein interactions (e.g. Dicer, which prefers a 2-nt 3′ overhang).
MATERIALS AND METHODS
Oligonucleotide design
All PCR primers were purchased from Isogen Life Science (Maarssen, The Netherlands). We indicate the sequences of primers used to build overhangs into dsRNA molecules, using F to designate a forward primer and R to designate a reverse primer. Within the primers, the T7 promoter site is underlined, brackets indicate the part of the primer that is not complementary to the template DNA, and the future overhangs of the molecules are shown in italics.
- Use of the 3′-protocol to make 7.4 kb dsRNA with 4 nt overhangs. The DNA substrate used is Litmus 28i (New England Biolabs) with an insert from λ DNA. This substrate already contained two opposing T7 promoters separated by 7.4 kb, while the 3′-protocol in its most general form (Figure 1A) allows the user to incorporate the T7 promoters should they be absent on the substrate. The only consequence is that primers F1, R2 do not include the T7 promoters and anneal 3′ to the complements of the promoter sequences on the substrate.
- Main molecule, 7.4 kb long:
- F1 CTATGACCATGATTACGCCAAGC
- R1 [AGCT]GGCCTTGACTAGAGGGTACC
- F2 [GTAC]GGGCAGATCCACTCGTTATTCTC
- R2 CAAGGCGATTAAGTTGGGTAACG
- Use of the 5′-protocol to make 4.2 kb dsRNA with 4 nt overhangs. The DNA substrate used is recombinated pBAD vector.
- Main molecule, 4.2 kb long:
- F1 [TAATACGACTCACTATAGGTT]AAGATTAGCGGATCCTACCTGAC
- R1 GGTTAACCTCAACTTCCATTTCC
- F2 AAGATTAGCGGATCCTACCTGAC
- R2 [TAATACGACTCACTATAGGAA]GGTTAACCTCAACTTCCATTTCC
- Use of the 5′-protocol to make 0.4 and 4.2 kb dsRNA with 8 nt overhangs. The DNA substrate used is recombinated pBAD vector for the 4.2 kb dsRNA and bacteriophage λ DNA for the 0.4 kb fragments.
- Main molecule, 4.2 kb long:
- F1 [TAATACGACTCACTATAGGATCGCC]AAGATTAGCGGATCCTACCTGAC
- R1 GGTTAACCTCAACTTCCATTTCC
- F2 AAGATTAGCGGATCCTACCTGAC
- R2 [TAATACGACTCACTATAGGGCTACC]GGTTAACCTCAACTTCCATTTCC
- Digoxigenin-labelled ends, 0.4 kb long:
- F1 [TAATACGACTCACTATAGGTAGCCC]GCTGTATAGTCAACTAACTCTTCTGTCG
- R1 GAGGCAAAGGCTACTCTTATTTCATCTTAC
- F2 GCTGTATAGTCAACTAACTCTTCTGTCG
- R2 [TAATACGACTCACTATAGG]GAGGCAAAGGCTACTCTTATTTCATCTTAC
- Biotin-labelled ends, 0.4 kb long:
- F1 [TAATACGACTCACTATAGGCGATCC]GCTGTATAGTCAACTAACTCTTCTGTCG
Primers R1, F2, R2 are identical to those used to generate dig-labelled fragments.
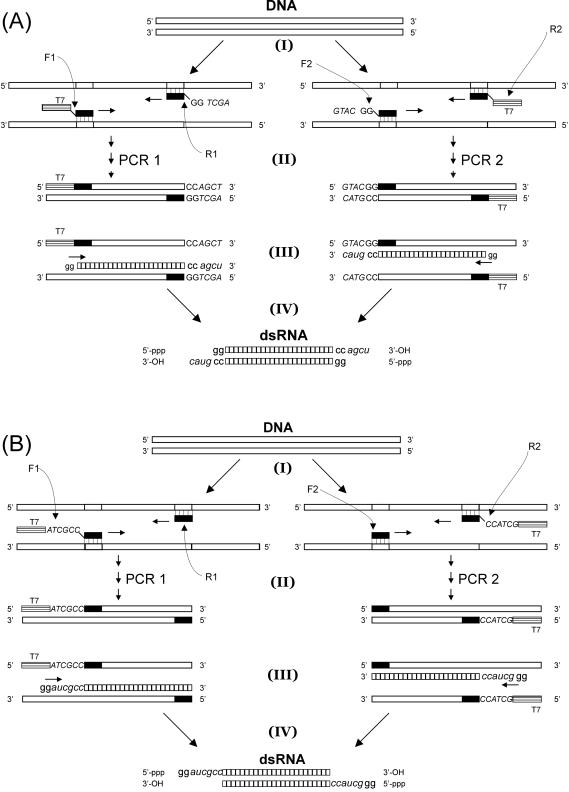
Figure 1.
Protocols for making long dsRNA with sticky ends (DNA sequences, uppercase; RNA sequences, lowercase). The sequence of the T7 promoter site is 5′-TAATACGACTCACTATAGG-3′. (A) Protocol for making long dsRNA with 3′ overhangs. Starting from a DNA template, two primer pairs are selected: each primer pair is used to build a T7 promoter site into one side of the PCR product and an overhang sequence (italicized) into the other side of the PCR product. The PCR products are subsequently combined in a transcription reaction using T7 RNAP. This results in the formation of a dsRNA molecule with distinct 3′ overhangs. (B) Protocol for making long dsRNA with 5′ overhangs. As before, two primer pairs are selected: each primer pair is used to incorporate a T7 promoter site directly followed by an overhang sequence (italicized) into a PCR product. The PCR products are subsequently combined in a transcription reaction withT7 RNAP. This results in the formation of a dsRNA molecule with two distinct 5′ overhangs.
The presence of overhangs in all three dsRNA molecules was tested by ligating them to each other. Transcription reactions from PCR templates synthesized using primers R1, F2 which included two 5′ end methyloxy nucleotides (10) were also successful. Such 5′ end methyloxy nucleotides limit transcription errors in the 3′ ends of the transcripts (see Discussion).
Parental PCRs
We used bacteriophage λ DNA (Promega), a recombinant pBAD vector (named pBADb10, kindly supplied by Valentin Rybenkov), and Litmus 28i plasmid (New England Biolabs) as DNA sources. Each 50 μl PCR reaction contained 5 U of Expand Long Template enzyme mix containing Taq and Tgo DNA polymerases (ELT, Roche Applied Science), ELT buffer 2 (1.75 mM MgCl2), 1.5 μM primers, 500 μM dNTPs and 1.5 ng template DNA. The samples were cycled in a Mastercycler with heated lid (Eppendorf) following a profile of gradually increasing extension times: one cycle 2 min at 94°C; ten cycles 10 s at 94°C, 30 s at 56°C, 5 min at 68°C; 20 cycles 15 s at 94°C, 30 s at 62°C, 5 min + 20 s/successive cycle at 68°C; one cycle 9 min at 68°C. The PCR products were purified using an UltraClean PCR Clean-up kit (MoBio Laboratories). They were then digested with mung bean nuclease (NewEngland Biolabs) at 30°C and purified again.
Transcription reaction
Each 60 μl transcription reaction (HiScribe RNAi transcription kit, New England Biolabs) contained 0.5–1 μg of each PCR template, 300 U T7 RNAP, High Molecular Weight (HMW) Component Mix (0.67 mM Tris–HCl, pH 8.1, 0.05 mg/ml BSA, 3.33 units/ml inorganic pyrophosphatase, 400 units/ml pancreatic ribonuclease inhibitor and 1.67% glycerol), transcription buffer (40 mM Tris–HCl, pH 8.1, 19 mM MgCl2, 5 mM DTT, 1 mM spermidine), and NTPs (4 mM each). The samples were incubated for 3 h at 37°C.
Labelling dsRNA with biotin and digoxigenin
Incorporation of biotin-16-UTP and digoxigenin-11-UTP nucleotides into 0.4 kb dsRNA was executed by the addition of 1 molar unit modified UTP for every 2 molar units UTP in the transcription reactions.
Hybridization protocol
To improve the efficiency of strand annealing of the transcription products, the following step directly succeeded the 3 h transcription reaction: the reactions were heated to 65°C for at least 1 h in the presence of 30 mM EDTA and cooled down to room temperature by slowly decreasing the temperature (1.25°C/5 min) in a Mastercycler with heated lid (Eppendorf). Yields of up to 500 ng of 8 kb dsRNA and 2.5 μg of 0.4 kb dsRNA were obtained per 30 μl transcription reaction.
Purification
Following hybridization, the dsRNA transcription reactions were purified using an RNeasy MinElute Cleanup Kit (Qiagen). They were then treated with 5 U of RNase-free DNase I (Roche Applied Science) for 1 h at 37°C and repurified.
(De)phosphorylation
5′-triphosphates were replaced by 5′-monophosphates using a KinaseMax 5′ End-Labeling Kit (Ambion, Huntingdon, UK). Each 17 μl dephosphorylation reaction, containing 0.1 U calf intestine alkaline phosphatase (CIP), dephosphorylation buffer, and 0.1 (8 kb) to 10 (0.4 kb) pmol dsRNA was incubated for 1 h at 37°C. The reactions were purified by addition of 17 μl phosphatase removal reagent (PRR) and retrieval of the supernatant. Phosphorylation of the 5′ end was carried out in 35 μl reactions containing 0.57 mM ATP, kinase buffer, and 15 U polynucleotide kinase (PNK) and incubated for 1 h at 37°C. The reactions were purified using Qiagen RNeasy spin columns.
DNA ligation partners
Labelled 0.4 kb dsDNA fragments for ligation to 7.4 kb dsRNA with 3′ overhangs (Figure 1A) were synthesized in two PCR reactions using a pBADb10 substrate. One primer in each PCR reaction included a restriction site (for SacI or KpnI, respectively) 3 nt from the 5′ end. Biotin-16-dUTP and digoxigenin-11-dUTP nucleotides were incorporated during the PCR reaction by the addition of 1 molar unit modified dUTP for every 2 molar units dTTP. The PCR products were then digested using SacI and KpnI, respectively. Labelled 0.4 kb dsDNA fragments for ligation to 4.2 kb dsRNA with 5′ overhangs were made similarly except that the primers included the restriction site for BsmA1 as well as adjoining sequences to yield the correct overhangs upon digestion. The efficiency of restriction endonuclease digestion may decrease due to the presence of labelled nucleotides, but is typically not found to decrease below 50%.
Ligation
The dsRNA–dsDNA ligation reactions (total volume 20–100 μl) consisted of up to 100–500 ng 7.4 kb dsRNA, a 4-fold molar excess of both 0.4 kb dsDNAs, Quick Ligase buffer and 2 μl T4 DNA Quick Ligase (New England Biolabs), 20 U SacI and 20 U KpnI if necessary to prevent dsDNA–dsDNA ligation, and 10 μg BSA. These ligations were run at 16°C overnight and purified using Qiagen RNeasy spin columns. The dsRNA–dsRNA ligation reactions (total volume 20 μl) using T4 DNA ligase consisted of up to 100–500 ng 4.2 dsRNA, a 5-fold molar excess of both 0.4 kb dsRNAs, Quick Ligase buffer and 2 μl T4 DNA Quick Ligase (New England Biolabs), and 40 U of Protector RNase Inhibitor (Roche Applied Science, Germany). These ligations were incubated at 16°C overnight and purified using Qiagen RNeasy spin columns. The dsRNA–dsRNA ligation reactions (total volume 10 μl) using T4 RNA ligase 2 consisted of up to 100–500 ng 4.2 dsRNA, a 5-fold molar excess of both 0.4 kb dsRNAs, buffer (50 mM Tris-acetate pH 6.5, 40 mM NaCl, 5 mM DTT, 1 mM MgCl2), 1 mM ATP, 1 μl T4 RNA ligase 2 and 40 U of Protector RNase Inhibitor (Roche Applied Science, Germany).
Gel electrophoresis
All gel electrophoresis analyses shown in the figures were conducted on a 0.75% agarose gel with an electric field of 4 V/cm.
RESULTS
DsRNA molecules with 3′ overhangs can be made using the following protocol (Figure 1A). Two PCR products are generated in separate reactions PCR 1 and PCR 2, both containing a single promoter sequence for T7 RNA polymerase (T7 RNAP). Each PCR product hence forms a template for the transcription of a single strand of RNA. In PCR 1, the forward primer (F1) incorporates the T7 promoter sequence, while the reverse primer (R1) consists of (from 5′ to 3′) an overhang sequence (AGCT in Figure 1A), two guanine residues and a sequence complementary to the DNA substrate. In PCR 2, the forward primer (F2) consists of (from 5′ to 3′) an overhang sequence (GTAC in Figure 1A), two guanine residues and a sequence complementary to the DNA substrate; the reverse primer (R2) is used to build in the T7 promoter sequence. In our example, the two overhang sequences in PCR 1 and PCR 2 contain an identical number of nucleotides, but this need not generally apply. In a single reaction, both PCR products are then transcribed into RNA by T7 RNAP, starting with the two guanines at the 3′ end of the promoter sequence which are a prerequisite for efficient transcription (11). This yields transcripts which are entirely complementary save at the 3′ ends. Subsequent hybridization of these strands yields a dsRNA molecule with two distinct 3′ overhangs. Note that the two guanine residues incorporated into primers F2, R1 are transcribed into cytosines which hybridize with the two 5′-guanines synthesized by T7 RNAP on the opposite strand (Figure 1A).
DsRNA molecules with 5′ overhangs can be made by choosing primer configurations such that the promoter site and the overhang sequence are part of a single primer (Figure 1B). In PCR 1, the forward primer (F1) consists of (from 5′ to 3′) the T7 promoter sequence, an overhang sequence (ATCGCC in Figure 1B), and a sequence complementary to the DNA substrate. The reverse primer (R1) anneals entirely to the DNA substrate. In PCR 2, the forward primer (F2) anneals entirely to the DNA substrate, while the reverse primer (R2) consists of (from 5′ to 3′) the T7 promoter sequence, an overhang sequence (GCTACC in Figure 1B), and a sequence complementary to the DNA substrate. The single-stranded RNA transcripts of the two PCR products are complementary except at the 5′ ends, where they contain (from 5′ to 3′) two guanines followed by the overhang sequences. Subsequent hybridization of these strands yields a dsRNA molecule with two distinct 5′ overhangs, 5′-GGAUCGCC-3′ and 5′-GGGCUACC-3′.
In both protocols, the specific choice of overhangs is determined by the user. Thus, the protocols permit the creation of sticky ends that are palindromic (so that they can be ligated to dsDNA fragments digested by standard restriction endonucleases) as well as the creation of sticky ends that are not palindromic. The latter can present an advantage as it prevents unwanted creation of dimers. Of course, one can also consider combining the two protocols to create dsRNA molecules with a 5′ overhang on one side and a 3′ overhang on the other side.
We now demonstrate the synthesis and ligation of dsRNA molecules using these protocols. We synthesize three long dsRNA molecules using these protocols and demonstrate the successful incorporation of sticky ends by ligating them to both dsDNA and dsRNA fragments. For an application in single-molecule biophysics, we seek to create a long (4 kb or larger) main dsRNA molecule ligated at each end to short 0.4 kb fragments that are multiply labelled with biotin and digoxigenin, respectively. This will therefore constitute our example reaction, of which the potential products are the main molecule plus a biotin fragment, the main molecule plus a digoxigenin fragment and the main molecule plus both labelled fragments. The latter three-body reaction is less efficient but can be observed in ligation reactions with T4 RNA ligase 2 [Rnl2, gift of Stewart Shuman (12)] and by using single-molecule techniques, as we explain below.
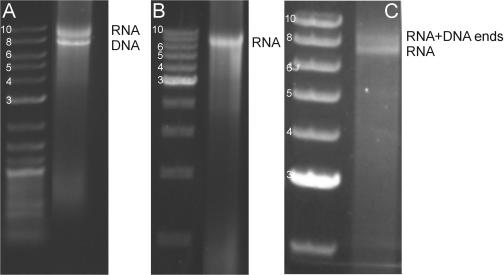
First, we demonstrate the creation of dsRNA molecules with 3′ overhangs and their ligation to dsDNA fragments. The primers were chosen as described above to yield 4 nt overhangs 5′-GUAC-3′ and 5′-AGCU-3′ (Figure 1A), which are complementary to DNA overhangs created by the restriction endonucleases KpnI and SacI. Following formation of the 7.4 kb PCR products, a transcription reaction was run. Gel electrophoresis following this reaction (Figure 2A) showed two bands: the dsDNA PCR products (denoted DNA) and a second, bright band which migrated more slowly than the dsDNA PCR products (denoted RNA). Several controls indicated that this retarded band consisted of dsRNA: (i) transcription reactions run using only a single PCR product did not display this band; (ii) addition of RNase-free DNase I to the transcription reaction product removed the band corresponding to the dsDNA PCR products, but not the retarded band (Figure 2B); (iii) addition of a mixture of RNases (which digests ssRNA) to the transcription reaction product did not remove the retarded band; (iv) addition of RNase III (which digests dsRNA) to the transcription reaction product did remove the retarded band. Therefore, we concluded that this retarded band corresponded to dsRNA. Next, using T4 DNA ligase, we ligated this 7.4 kb dsRNA molecule to 0.4 kb labelled dsDNA fragments that had been digested with KpnI and SacI. In order to limit dsDNA–dsDNA ligation (probably since the efficiency of dsDNA–dsDNA ligation greatly exceeds the efficiency of dsDNA–dsRNA ligation when using T4 DNA ligase [Paul Walsh, NEB, personal communication; (13)], we included the restriction endonucleases KpnI and SacI in the reaction. This greatly improved the final yield of the dsDNA–dsRNA construct (Figure 2C, band denoted RNA + DNA ends), presumably since the restriction endonucleases cleave ligated dsDNA dimers but do not cleave dsDNA fragments ligated to dsRNA. This joining of dsDNA fragments to dsRNA molecules demonstrates the successful incorporation of sticky ends with the correct sequences into dsRNA.
Figure 2.
Gel electrophoresis demonstrating the creation of dsRNA overhangs using the 3′ overhang protocol through the ligation to dsDNA fragments. In all gels, the left lane shows a dsDNA ladder with molecule lengths indicated in kb. (A) The direct outcome of the transcription reaction on two 7.4 kb PCR templates: the more slowly migrating band only appears following transcription. (B) The transcription reaction after treatment with DNase I: the PCR products have disappeared, leaving a single band which must be dsRNA. (C) Test of the incorporation of overhangs through the ligation of the 7.4 kb dsRNA product to two distinct 0.4 kb dsDNA fragments (labelled with biotin and digoxigenin, respectively) by T4 DNA ligase. The dsDNA ends have been digested with KpnI and SacI. A new band appears that migrates more slowly on gel, corresponding to molecules that have been ligated using the designed 3′ overhangs.
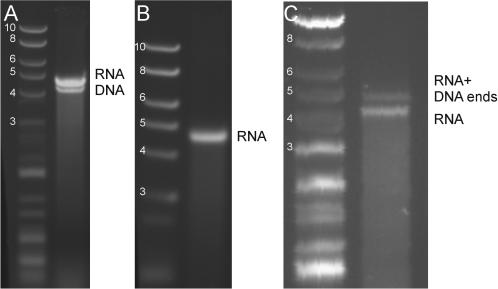
Next, we synthesize dsRNA molecules with overhangs using the 5′-protocol (Figure 1B). Using two 4.2 kb PCR templates which encoded for overhangs 5′-GGTT-3′ and 5′-GGAA-3′, a transcription reaction was run as previously, yielding a more slowly moving band (denoted dsRNA) above the PCR products (Figure 3A). As before, the transcription product was then digested with RNase-free DNase I which removed the faster band. This confirmed that the retarded band in the transcription reaction was dsRNA (Figure 3B). T7 RNAP synthesizes a ribonucleotide polymer with one NTP at the 5′ end. For optimal ligation, this NTP can be converted into an NMP. The 4.2 kb dsRNA fragment was therefore treated with CIP and T4 polynucleotide kinase to obtain monophosphates at its 5′-termini prior to ligation (this was not found to be efficient in the 3′-protocol, see below). The ligation partners for the 4.2 kb dsRNA molecules were two labelled dsDNA molecules which had been digested with BsmA1 to yield overhangs 5′-CCAA-3′ and 5′-CCTT-3′, respectively. These overhangs are complementary to those incorporated into the 4.2 kb dsRNA molecule. Given these non-palindromic overhangs, the dsDNA molecules could not ligate to each other, but they could ligate to the RNA overhangs. Following ligation (Figure 3C), an additional band appeared in the gel (denoted RNA + DNA ends) which migrated more slowly than the 4.2 kb dsRNA fragment, as expected. This demonstrated that the 5′-protocol could also be used to successfully incorporate overhangs into dsRNA molecules.
Figure 3.
Gel electrophoresis demonstrating the creation of dsRNA overhangs using the 5′ overhang protocol through the ligation to dsDNA fragments. In all gels, the left lane shows a DNA ladder with molecule lengths indicated in kb. (A) Result of the transcript reaction of a 4.2 kb dsRNA molecule with 5′ overhangs complementary to those of 0.4 kb dsDNA molecules: the more slowly migrating band only appears following transcription. (B) The transcription reaction following treatment with DNase I. The single remaining band must be dsRNA. (C) Ligation by T4 DNA ligase of the 4.2 kb dsRNA molecule with 5′ overhangs to 0.4 kb dsDNA ends tagged with biotin and digoxigenin. The dsDNA molecules were digested with BsmA1 to yield overhangs complementary to those incorporated into the 4.2 kb dsRNA molecule. The appearance of a new more slowly migrating band indicates the presence of molecules that have been ligated using the designed 5′ overhangs.
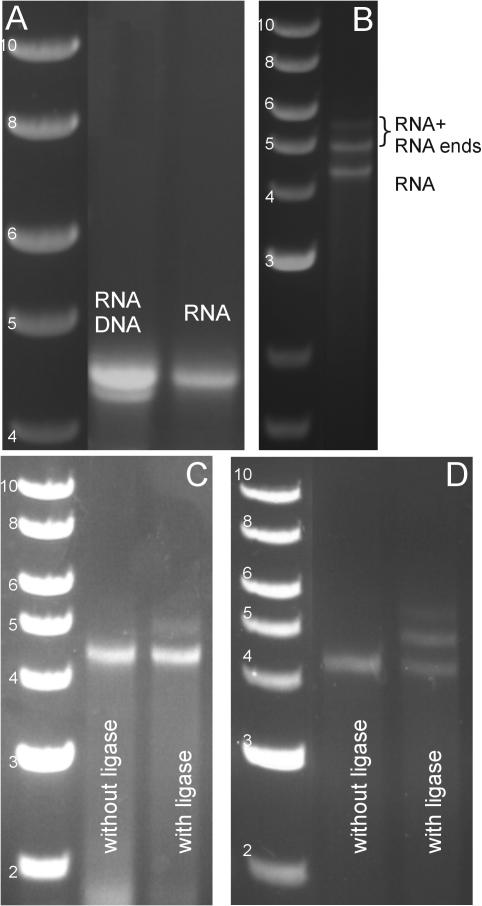
Finally, we demonstrate that dsRNA molecules can be joined to other dsRNA molecules using the 5′-protocol (Figure 1B). For this, we construct three dsRNA molecules with 5′ overhangs (one 4.2 kb molecule with 8 nt overhangs 5′-GGAUCGCC-3′ and 5′-GGGCUACC-3′ and two 0.4 kb labelled fragments with overhangs 5′-GGUAGCCC-3′ and 5′-GGCGAUCC-3′, respectively). The transcription reaction and the control with DNase I are shown for the 4.2 kb dsRNA (Figure 4A). All three dsRNA molecules were subsequently treated with CIP and kinase. In the case of dsRNA–dsRNA ligation, this step is essential since its omission results in the complete absence of 5′-monophosphates in all ligation partners, and hence in the absence of any ligation product. Following this treatment, the ligation reaction was carried out, resulting in additional bands corresponding to ligation to one or both labelled fragments (Figure 4B, denoted RNA + RNA ends). This demonstrated that the 5′-protocol can be used to join to dsRNA molecules to each other. However, since the longer length of the overhangs incorporated into these molecules (8 nt compared to 4 nt utilized in the previous ligations) implied that hybridization products could be stably formed at room temperature, we applied an additional control to distinguish hybridization from ligation. An identical reaction to the one described in Figure 4B was carried out, but in the absence of T4 DNA ligase. Gel electrophoresis of the reaction product at room temperature showed a pattern similar to Figure 4B, which indicates that there is good hybridization between the molecules. Next, the products of both reactions were heated to 55°C (a temperature well above the melting temperature Tm ≈ 28°C of the overhangs) for 15 min, rapidly chilled on ice and run on gel again. In the reaction without ligase (Figure 4C, left lane), only the band corresponding to the 4.2 kb dsRNA molecule remained, whereas in the reaction with ligase (Figure 4C, right lane), both the 4.2 kb dsRNA band and a longer band remained. The fact that a longer band remained in the presence of heating demonstrated ligation of the phosphodiester backbone. A similar experiment using Rnl2, which is a ligase specific to dsRNA, confirmed this result and demonstrated an increased ligation efficiency (Figure 4D).
Figure 4.
Gel electrophoresis demonstrating the creation of dsRNA overhangs using the 5′ overhang protocol through the ligation to dsRNA fragments. In all gels, the left lane shows a DNA ladder with molecule lengths indicated in kb. (A) Left lane: results of a transcription reaction on two separate 4.2 kb PCR products: the more slowly migrating band only appears following transcription. Right lane: the same reaction following treatment with DNase I. The band corresponding to the PCR products has been eliminated by DNase digestion, leaving a 4.2 kb dsRNA molecule. (B) The 4.2 kb dsRNA molecule ligated by T4 DNA ligase to 0.4 kb dsRNA fragments, all with 5′ overhangs. Two additional, more slowly migrating bands have appeared, corresponding to ligation products. (C) Controls applied to distinguish hybridization from ligation using T4 DNA ligase. We carried out two separate reactions, one reaction in the absence of ligase, and another in the presence of ligase. Both cases result in a gel pattern similar to (B) prior to heating. However, following heating of the reaction in the absence of ligase (left lane), only the band corresponding to the 4.2 kb dsRNA molecule remained, whereas following heating of the reaction in the presence of ligase (right lane), both the 4.2 kb dsRNA band and a longer band remained. This demonstrated that ligase repaired the phosphodiester backbone. We estimate that approximately 10% of the 4.2 kb dsRNA molecules were ligated to at least one of the shorter fragments. (D) The same experiment as in (C), but using Rnl2. The reaction was incubated for 30 min, heated to 55°C (well above the Tm ≈ 28°C for the 8 nt overhangs) for 15 min, rapidly chilled on ice, and run on gel. Left lane: in the absence of Rnl2; right lane: in the presence of Rnl2. Again, the fact that longer bands remain in the right lane following heating indicates the presence of ligation products. The use of Rnl2 appears to increase the ligation efficiency to ≥50%.
For all three reactions described above, the products were successfully tethered in a single-molecule apparatus and measured to have correct lengths (J. A. Abels, F. Moreno-Herrero, T. van der Heijden, C. Dekker, and N. H. Dekker, submitted). Such tethering of molecules is only possible in the presence of biotin- and digoxigenin-labelled extremities. Furthermore, both gel electrophoresis and single-molecule analysis of ligation reactions with blunt-ended dsRNA molecules yielded null results (data not shown). This demonstrates the utility of the RNA sticky ends generated using the above protocols.
DISCUSSION
We now compare the two protocols to create dsRNA molecules with sticky ends. While both provide the user with great flexibility by allowing the creation of dsRNA molecules with sticky overhangs from any DNA substrate, the 3′-protocol is the more versatile of the two techniques because the entire sequence of the overhang can be set by the user (Figure 1A). This is not strictly true for the 5′-protocol if high efficiency transcription is required, because T7 RNAP is most efficient when the last two nucleotides of the promoter sequence are guanines (11). The two corresponding 5′-ribonucleotides will form part of the 5′ overhang sequence; nevertheless, the remainder of the overhang sequence can be freely chosen (Figure 1B). The choice of protocol may also depend on whether one aims to join dsRNA molecules through hybridization or through ligation. Since dephosphorylation/phosphorylation (an important consideration with dsRNA–dsRNA ligation) is significantly more efficient on 5′ overhangs than on 3′ overhangs (14), ligation may proceed more efficiently using the 5′-protocol. Alternatively, the 3′-protocol can be adapted by including strategies for increasing the efficiency of dephosphorylation and phosphorylation of 3′ overhangs [such as temporary heating and rapid chilling (14)] or by allowing the two single-stranded RNA molecules generated during transcription to hybridize only following incorporation of monophosphates.
Next, we discuss the fidelity of T7 RNAP. In certain cases, T7 RNAP does not faithfully generate the 5′ end or the 3′ end of its transcript (10,15,16). Such errors would generate sticky ends which deviate from the intended overhang sequences, which, though it need not prevent successful joining of dsRNA molecules by hybridization of long sticky ends, might prohibit ligation of dsRNA molecules. However, the fidelity of T7 RNAP can be maximized in several ways: by preventing cases where the first five nucleotides transcribed by T7 RNAP are all guanines (thought to cause errors in the 5′ end of the transcript due to slippage of the RNA polymerase) and by utilizing the technique developed by Kao et al. (10) in which the PCR primers include several 5′ end methyloxy nucleotides (which prevents errors in the 3′ end of the transcript due to the addition of nontemplated nucleotides).
Finally, we discuss the efficiency with which these protocols yield dsRNA with the correct overhangs. This efficiency can be estimated from the ligation reactions (it is necessarily a lower bound since the ligation efficiency may not attain 100%). The experiments with Rnl2 demonstrate a ligation efficiency ≥50% (Figure 4D, right lane) using the 5′-protocol, indicating very successful synthesis of dsRNA with the correct overhangs. We have further investigated whether the presence of labeled nucleotides in or near the overhangs (Figure 1B) affects this figure. For the labelling conditions described in the Materials and Methods, however, we have determined that this is not the case.
In conclusion, the protocols which we have demonstrated provide a much needed tool lacking in the molecular biology of dsRNA with which to prepare dsRNA molecules with sticky ends. We expect that this can be used in various settings such as the creation of dsRNA molecules for specific dsRNA–protein interactions and the efficient labelling of dsRNA molecules, and that, in general, it greatly enhances the flexibility of working with this molecule.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Stewart Shuman for a gift of T4 RNA ligase 2. We are also grateful to Dennis Kainov, René Ketting, Daniël Koster and Paul Walsh for useful discussions, and to Maarten de Smit and Stewart Shuman for a critical reading of the manuscript. This work is part of the research program of the ‘Stichting voor Fundamenteel Onderzoek der Materie (FOM)’, which is financially supported by the ‘Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO)’.
REFERENCES
- 1.Fire A., Xu,S., Montgomery,M.K., Kostas,S.A., Driver,S.E. and Mello,C.C. (1998) Potent and specific genetic interference by double stranded RNA in Caenorhabditis elegans. Nature, 391, 806–811. [DOI] [PubMed] [Google Scholar]
- 2.Timmons L. and Fire,A. (1998) Specific interference by ingested dsRNA. Nature, 395, 854–857. [DOI] [PubMed] [Google Scholar]
- 3.Lapham J. and Crothers,D. (1996) RNase H cleavage for processing of in vitro transcribed RNA for NMR studies and RNA ligation. RNA, 2, 289–296. [PMC free article] [PubMed] [Google Scholar]
- 4.Ryter J.M. and Schultz,S.C. (1998) Molecular basis of double-stranded RNA–protein interactions: structure of a dsRNA-binding domain complexed with dsRNA. EMBO J., 17, 7505–7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henn A., Medalia,O., Shi,S.-P., Steinberg,M., Franceschi,F. and Sagi,I. (2001) Visualization of unwinding activity of duplex RNA by DbpA, a DEAD box helicase, at single-molecule resolution by atomic force microscopy. Proc. Natl Acad. Sci. USA, 98, 5007–5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagerman P.J. (1997) Flexibility of RNA. Annu. Rev. Biophys. Biomol. Struct., 26, 139–156. [DOI] [PubMed] [Google Scholar]
- 7.Bonin M., Zhu,R., Klaue,Y., Oberstrass,J., Oesterschulze,E. and Nellen,W. (2002) Analysis of RNA flexibility by scanning force spectroscopy. Nucleic Acids Res., 30, e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dekker N.H., Rybenkov,V.V., Duguet,M., Crisona,N.J., Cozzarelli,N.R., Bensimon,D. and Croquette,V. (2002) The mechanism of type IA topoisomerases. Proc. Natl Acad. Sci. USA, 99, 12126–12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liphardt J., Onoa,B., Smith,S.B., Tinoco,I.,Jr. and Bustamante,C. (2001) Reversible unfolding of single RNA molecules by mechanical force. Science, 292, 733–737. [DOI] [PubMed] [Google Scholar]
- 10.Kao C., Zheng,M. and Rüdisser,S. (1999) A simple and efficient method to reduce nontemplated nucleotide addition at the 3′ terminus of RNAs transcribed by T7 RNA polymerase. RNA, 5, 1268–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milligan J.F., Groebe,D.R., Witherell,G.W. and Uhlenbeck,O.C. (1987) Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res., 15, 8783–8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nandakumar J., Ho,C.K., Lima,C.D. and Shuman,S. (2004) RNA substrate specificity and structure-guided mutational analysis of bacteriophage T4 RNA ligase 2. J. Biol. Chem., 279, 31337–31347. [DOI] [PubMed] [Google Scholar]
- 13.Kleppe K., van de Sande,J.H. and Khorana,H.G. (1970) Polynucleotide ligase-catalyzed joining of deoxyribo-oligonucleotides on ribopolynucleotide templates and of ribo-oligonucleotides on deoxyribopolynucleotide templates. Proc. Natl Acad. Sci. USA, 67, 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sambrook J. and Russell,D.W. (2001) Molecular Cloning: A Laboratory Manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 15.Lyakhov D.L., He,B., Zhang,X., Studier,F.W., Dunn,J.J. and McAllister,W.T. (1998) Pausing and termination by bacteriophage T7 RNA polymerase. J. Mol. Biol., 280, 201–213. [DOI] [PubMed] [Google Scholar]
- 16.Pleiss J.A., Derrick,M.L. and Uhlenbeck,O.C. (1998) T7 RNA polymerase produces 5′ end heterogeneity during transcription from certain templates. RNA, 4, 1313–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]