Abstract
Objective
Polypharmacy is common in hospitalized children in the United States and has been identified as a major risk factor for exposure to potential drug-drug interactions (PDDI). Little is known about the characteristics and prevalence of exposure of pediatric patients to polypharmacy and PDDI in pediatric intensive care units (PICUs).
Design
Retrospective cohort study using the Pediatric Information System database.
Setting
Forty-two freestanding children’s hospitals throughout the United States.
Patients
54,549 patients <18 years old cared for in PICUs in 2011. Patients in neonatal ICUs were not included.
Measurements and Main Results
PICU patients were on average exposed to 10 distinct drugs each hospital day and to 20 drugs cumulatively during their hospitalization. Seventy-five percent of patients were exposed to at ≥1 PDDI regardless of severity level, 6% to at ≥1 contraindicated PDDI, 69% to at ≥1 major PDDI, 57% to at ≥1 moderate PDDI, 19% to at ≥1 minor PDDI. PDDI exposures were significantly associated with specific diagnoses (p<0.001), presence of complex chronic conditions (p<0.001), increasing number of total distinct drugs used (p<0.001), increasing length of stay in PICU (p<0.001), and white race (p<0.001).
Conclusions
Many PICU patients are exposed to substantial polypharmacy and PDDI. Future research should identify the risk of adverse drug events (ADEs) following specific PDDI exposures, especially the risk of ADEs due to multiple PDDI exposures and determine the probability and magnitude of the actual harm (if any) for each specific PDDI, especially for multiple PDDI exposures.
Keywords: drug utilization, polypharmacy, drug-drug interaction, pediatrics, intensive care, pharmacoepidemiology
INTRODUCTION
Drugs are essential to effective care of patients in the pediatric intensive care units (PICUs). The 2002 Best Pharmaceuticals for Children Act (BPCA) set forth the goal of reducing pharmaceutical errors in the dispensing of drugs to hospitalized children (1). Both the BPCA and the Pediatric Research Equity Act also emphasized the need for pediatric drug studies (2). Translating these goals into the realm of pediatric intensive care for critically ill pediatric patients requires knowledge of both polypharmacy and drug-drug interactions (DDI).
While polypharmacy is known to be common among hospitalized adult patients (3, 4), we recently reported that polypharmacy is also highly prevalent among hospitalized children in the United States (5). Both PICU and adult ICU patients typically have multiple co-morbidities and are treated with many different drugs (6–8). In turn, patients treated with polypharmacy are exposed to multiple potential drug-drug interactions (PDDI) (8–11). Critically ill patients are at an even further increased risk for PDDI due not only to the complexity of the pharmacotherapy involved but also the physiologic dysfunction arising from critical illness (12, 13). Our recent work has found high rates of PDDI exposures in hospitalized children with complex chronic conditions (CCC) (14). PDDI may cause treatment failure or adverse drug events (ADEs), which are major cause of increased morbidity, mortality, and health care costs. ADEs rank as the 4th to 6th leading causes of death in inpatients (15). In the United States, for every dollar spent on medication in 2000, more than a dollar was estimated to have been spent on direct medical costs related to drug misadventures (16).
Despite the importance of pediatric polypharmacy and PDDI, and the likely associated increased risk of ADEs, research on these topics in the PICUs is limited. Information from such research will improve our understanding of medication usage patterns for critically ill pediatric patients, enable or enhance the design of safer systems of drug prescription ordering or subsequent drug monitoring (17, 18), and provide a framework for prioritizing future pharmacotherapeutic studies. Accordingly, we aimed in this large multicenter retrospective cohort study to: 1) quantify the rates of exposure to generic drugs; 2) describe patterns of polypharmacy among PICU patients; 3) quantify the rates of PDDI exposures in the PICU; and 4) asses patient demographic and clinical characteristics associated with PDDI exposures.
METHODS
Data sources
This study used the Pediatric Health Information System (PHIS) database (the Children’s Hospital Association (CHA), Kansas City, KS), a national administrative database containing resource utilization data from 43 freestanding general (rather than subspecialty) children’s hospitals representing most major U.S. metropolitan areas and approximately 70% of freestanding pediatric acute care hospital admissions in the United States (19). These participating hospitals are located in 25 US states and the District of Columbia. All these hospitals are affiliated with CHA, a national business alliance of children’s hospitals. Our study included all hospitalized children <18 years of age, who received care in PICUs, and were discharged from participating hospitals between 1 January 2011, and 31 December 2011. We excluded all patients in neonatal intensive care units (NICUs) because of differences in NICU patients compared to other pediatric patients regarding drug pharmacokinetics and medication exposure patterns (20–22).
The PHIS database includes patient demographics, diagnosis, and procedures as well as detailed pharmacy information, including ICD-9-CM (International Classification of Diseases, Ninth Revision, Clinical Modification) codes and CTC (Clinical Transaction Classification) code for each procedure, generic drug entity dispensed and clinical services for each day of hospital stay of each patient. Data quality are assured through a joint effort between CHA and participating hospitals, assuring that classified errors occur in less than 2% of a hospital’s data, which are de-identified before extraction and analysis (5, 19). One hospital without detailed pharmacy information was excluded. The institutional review board at The Children’s Hospital of Philadelphia deemed this study of de-identified data to not constitute human subjects research.
Data management
Drugs and therapeutic agents were recorded by CTC code and drug description. We converted CTC codes into National Drug Codes (NDC) using CTC-NDC crosswalk data provided by CHA. We implemented a standardized dictionary of 1227 generic drug entities. These generic drugs were grouped into 27 major categories using principally the American Hospital Formulary System Pharmacologic Therapeutic Classification hierarchy of drug and therapeutic agent classes and prevalent subclasses (5). CCC was defined as medical conditions that can be reasonably expected to last at least 12 months and that involved either several different organ systems or 1 organ system severely enough to require specialty pediatric care and probably some period of hospitalization in a tertiary care center. To identify whether an individual was diagnosed with a CCC, we used our previously published classification scheme base on ICD-9-CM codes (23).
Potential drug-drug interactions
PDDIs were identified using DRUG-REAX® system (Thomson Micromedex®, Truven Health Analytics Inc., Greenwood Village, Co, USA). The software has been previously validated and described in detail (24, 25). The software classified drug interactions into four main levels of seriousness: contraindicated, major, moderate, minor. The seriousness category represents the seriousness of the PDDI if it were to occur. By definition, “contraindicated” drug pairs should not be used concurrently. “Major” interactions may be life threatening or require medical intervention to minimize or prevent ADEs. “Moderate” interactions may result in exacerbation of the patient’s condition or require an alteration in therapy. “Minor” interactions have limited clinical effect that may include an increase in the frequency or severity of the side effects but generally would not require a major alteration in therapy. The software also specified the level of the quality of scientific documentation of the PDDI as excellent, good, fair, poor, or unlikely; we did not consider PDDI in either the poor or unlikely categories. By definition, “excellent” indicates controlled studies have clearly established the existence of the drug interaction. “Good” indicates that documentation strongly suggests that a drug interaction exists, but well-controlled studies are lacking. “Fair” indicates that while the available documentation is scarce, pharmacological consideration may lead clinicians to suspect the existence of a potential drug interaction.
Statistical analysis
We first described the demographic and clinical characteristics of the patients in this cohort by calculating percentages. To describe the patterns of drug exposures, we calculated percentages of exposure to specific generic drugs by patient and by each day in PICU. We calculated patient-level percentiles of: (A) the number of exposures to distinct generic drugs on each day in PICU (reflecting concurrent exposure to medications that might interact); and (B) the cumulative number of exposures to distinct generic drugs on each successive day in PICU (reflecting the total exposure to different drugs that each pose some hazard of ADEs).
To describe the prevalence of PDDIs, we calculated percentages of specific PDDI by patient and by each day in PICU. We calculated patient-level percentiles of: (A) the number of exposures of distinct PDDI on each day in PICU (reflecting concurrent exposure to PDDIs; multiple PDDIs may increase the risk of additive ADEs); and (B) the cumulative number of exposures of distinct PDDI on each successive day in PICU (reflecting the total exposure to different PDDI that each may increase the risk of ADEs). We further computed the PDDI prevalence rate with 95% confidence intervals in each day up to 30 days in PICU stay and performed the trend analyses using Cochran-Armitage test.
To assess the association of patient’s demographic and clinical characteristic with PDDI exposures, we fitted multilevel logistic regression models including pertinent patient demographic and clinical characteristic variables, with a random intercept to account for the clustering of observations within hospitals. Variables in the multivariable regression included age in years, gender, race, geographical area, payers for the cost, diagnoses, number of CCCs, number of unique drugs used, and length of PICU stay in days. All data management and statistical analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
There were a total of 54,549 hospitalizations of children <18 years-old at the time of admission to the PICUs in 42 children’s hospitals throughout the United States in 2011. The median age was 3 years, 23.4% patients were infants <1 year old, 55.8% were males, and 51.7% were white. Respiratory system diseases and congenital anomalies were among the most common diagnoses. Nearly 70% of patients had at least one category of CCC present. The length of stay in ICU ranged from 1 to 768 days, with a median of 3 days. Half of the patients were insured by Medicaid (Supplemental Appendix 1).
A total of 1,008 unique generic drugs were administered. Table 1 lists the 12 most common medication exposures in rank order, stratified by 3 main age groups (infants, children, and adolescents, since patterns among patients within these age ranges were similar). Overall, the prevalence rate for the top 10 common administered drugs were acetaminophen (56.1% of patients), fentanyl (48.9%), midazolam (47.4%), ranitidine (46.1%), heparin (44.4%), morphine (43.8%), potassium chloride (34.1%), furosemide (33.8%), lidocaine (30.6%), and epinephrine (29.5%). A complete table of the prevalence rate with 95% confidence intervals (CIs) of all drugs used in the cohort, stratified by patient age, is provided in Supplemental Appendix 2.
Table 1.
Top dozen generic drug exposures by age group*
| Age (year) | Generic drug name | Patients, %(95% CI) |
|---|---|---|
| <1 | Acetaminophen | 57.90(56.78–59.03) |
| Fentanyl | 54.47(53.39–55.56) | |
| Heparin | 53.28(52.20–54.35) | |
| Ranitidine | 50.14(49.10–51.18) | |
| Midazolam | 48.93(47.90–49.96) | |
| Furosemide | 46.93(45.93–47.94) | |
| Morphine | 46.43(45.43–47.44) | |
| Potassium chloride | 44.61(43.63–45.60) | |
| Epinephrine | 41.65(40.69–42.60) | |
| Cefazolin | 31.85(31.01–32.68) | |
| Rocuronium | 29.86(29.05–30.66) | |
| Lidocaine | 28.28(27.50–29.06) | |
| 1–9 | Acetaminophen | 58.04(57.05–59.03) |
| Midazolam | 48.29(47.39–49.20) | |
| Fentanyl | 46.54(45.65–47.42) | |
| Ranitidine | 45.96(45.08–46.84) | |
| Morphine | 41.84(41.01–42.68) | |
| Heparin | 39.19(38.37–40.00) | |
| Albuterol | 34.31(33.55–35.08) | |
| Ondansetron | 32.90(32.16–33.65) | |
| Potassium chloride | 30.86(30.13–31.58) | |
| Lidocaine | 30.68(29.96–31.40) | |
| Furosemide | 29.68(28.97–30.39) | |
| Vancomycin | 28.52(27.82–29.21) | |
| 10–17 | Acetaminophen | 50.71(49.54–51.89) |
| Ondansetron | 47.01(45.88–48.14) | |
| Fentanyl | 45.96(44.84–47.08) | |
| Midazolam | 44.15(43.05–45.24) | |
| Morphine | 43.95(42.85–45.04) | |
| Heparin | 41.84(40.77–42.91) | |
| Ranitidine | 41.52(40.46–42.59) | |
| Lidocaine | 33.69(32.74–34.65) | |
| Propofol | 32.32(31.38–33.26) | |
| Vancomycin | 28.06(27.18–28.93) | |
| Cefazolin | 26.66(25.81–27.51) | |
| Potassium chloride | 26.49(25.64–27.34) |
Percentage of patients exposed to the named drug at any point during the ICU stay; rank ordering omits intravenous fluids, sterile water, dextrose water, heparin intravenous flushes, hyper alimentation, and glycerin suppository.
CI: confidence interval.
Eight-nine percent of patients had at least 1 day exposed to ≥5 distinct generic drugs, and 68.2% of patients had at least 1 day exposed to ≥10 distinct generic drugs. There were a total of 387,005 patient days, with 80.7% of patient days exposed to ≥ 5 distinct generic drugs, 60.5% of patient days exposed to ≥10 distinct generic drugs. Figure 1 displays daily and cumulative drug exposures up to 30 days (for patients who remained in PICU for that length of time). For example, on the first day in PICU, the median daily exposure of an infant patient was 7 distinct generic drugs, and this number rose to 9 by PICU stay day 2 through day 9, rose to 10 on day 10, then remained at 9 or 10 through day 30; the infant patient at the 90th percentile of daily drug exposures was exposed to 18 - 23 drugs in the PICU. The cumulative number of distinct generic drug exposures for each successive day in PICU for the median (and 90th percentile) rose on the first day in PICU from 7 (18) to 23 (53) by day 30. Similar patterns were observed for the other 2 age groups, but significantly increase in both daily and cumulative drug exposures.
Figure 1.
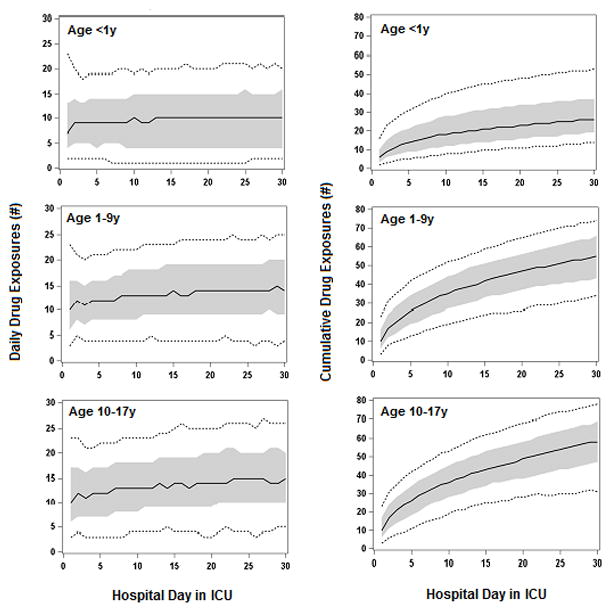
Number of daily and cumulative distinct drug and therapeutic agent exposures in PICU patients by age group. Shown are the levels of exposure to distinct drugs and therapeutic agents for each hospital day, from day 1 up to day 30 of hospitalization in ICU, for patients who remained in ICU for those lengths of stay. For each hospital day, we determined the level of exposure for patients at various percentiles of exposure; the plotted solid lines display the median, the plotted short dash lines display 10th and the 90th percentiles, and the shaded zone the interquartile range (IQR). Number of daily drug exposures is defined as the number of distinct drug and therapeutic agent that patients at each percentile were exposed to on that hospital day in ICU. Number of cumulative drug exposures is defined as the number of distinct drug and therapeutic agent that patients at each percentile were exposed to up to and including that hospital day in ICU.
A total of 1,332.168 PPDIs were identified in 41,034 patients. Among these PDDIs, 0.8% were contraindicated PDDI, 51.1% major PDDI, 42.9% moderate PDDI, and 5.3% minor PDDI. All these PDDIs were supported by excellent (7.2%), good (56.67%), and fair (36.2%) scientific evidence. There were 3,603 unique PPDI exposures in this cohort. Opioids was the most frequently implicated drug class in PDDI, and they were responsible for 29.7% of all PDDI exposures, following by neurologic drugs (23.6%), renal diuretics (18.8%), anti-infective agents (18.1%), cardiovascular agents(18.0%), analgesics and antipyretics (13.1%), psychotherapeutics (11.7%), gastrointestinal agents (10.5%), and blood and coagulation agents (10.4%). There were 543 unique generic drugs implicated in ≥1 PDDI exposure. The most common drugs involved in PDDI were midazolam (involved in 12.7% of all PDDIs), fentanyl (12.6%), morphine (11.2%), furosemide (9.9%), aspirin (8.1%), lorazepam (6.7%), phenobarbital (5.5%), heparin (5.5%), enalapril (4.3%), and spironolactone (4.2%). Seventy-five percent of patients had ≥1 PDDI regardless of severity level, 8.4% had ≥1 contraindicated PDDI, 68.8% had ≥1 major PDDI, 57% had ≥1 moderate PDDI, 19% had ≥1 minor PDDI. Table 2 lists the 10 most common PDDI exposures and their potential associated ADEs in rank order stratified by interaction severity level. A complete table of the prevalence rate with 95% CIs of all PDDIs stratified by age group is provided in Supplemental Appendix 3.
Table 2.
Prevalence of the first 10 most common PDDIs by interaction severity level
| Drug-Drug Combination | Potential ADE | Scientific Evidence | Number of Patients Exposed | %(95% CI) |
|---|---|---|---|---|
| Contraindicated | ||||
| Fluconazole + Ondansetron | An increased risk of QT interval prolongation. | Fair | 711 | 1.30(1.21–1.40) |
| Aspirin + Ketorolac | Enhanced gastrointestinal adverse effects (peptic ulcers, gastrointestinal bleeding and/or perforation). | Fair | 672 | 1.23(1.14–1.33) |
| Ibuprofen + Ketorolac | Enhanced gastrointestinal adverse effects (peptic ulcers, gastrointestinal bleeding and/or perforation). | Fair | 590 | 1.08(0.99–1.17) |
| Glycopyrrolate + Potassium chloride | Risk of gastrointestinal lesions. | Fair | 319 | 0.58(0.52–0.65) |
| Epinephrine + Linezolid | Increased hypertensive effects. | Fair | 275 | 0.50(0.44–0.56) |
| Atropine + Potassium chloride | Risk of gastrointestinal lesions. | Fair | 265 | 0.49(0.43–0.54) |
| Dopamine + Linezolid | Increased hypertensive effects. | Fair | 181 | 0.33(0.28–0.38) |
| Nitroprusside + Sildenafil | Potentiation of hypotensive effects. | Fair | 154 | 0.28(0.24–0.33) |
| Linezolid + Norepinephrine | Increased hypertensive effects. | Fair | 86 | 0.16(0.12–0.19) |
| Metoclopramide + Promethazine | Increased risk of extrapyramidal effects. | Fair | 58 | 0.11(0.08–0.13) |
| Major | ||||
| Fentanyl + Midazolam | Additive respiratory depression. | Good | 19,736 | 36.18(35.68–36.69) |
| Fentanyl + Morphine | Additive respiratory depression. | Good | 16,104 | 29.52(29.07–29.98) |
| Midazolam + Morphine | Additive respiratory depression. | Good | 14,896 | 27.31(26.87–27.75) |
| Fentanyl + Lorazepam | Additive respiratory depression. | Good | 8,304 | 15.22(14.94–15.55) |
| Lorazepam + Morphine | Additive respiratory depression. | Good | 7,853 | 14.40(14.08–14.71) |
| Lidocaine + Propofol | An increased hypnotic effect of propofol. | Good | 3,949 | 7.24(7.01–7.47) |
| Heparin + Ketorolac | Increased risk of gastrointestinal bleeding. | Fair | 3,316 | 6.08(5.87–6.29) |
| Bupivacaine + Propofol | An increased hypnotic effect of propofol. | Good | 3,267 | 5.99(5.78–6.19) |
| Alteplase + Heparin | An increased risk of bleeding. | Fair | 3,097 | 5.68(5.48–5.88) |
| Fentanyl + Hydromorphone | Additive respiratory depression. | Good | 3,054 | 5.60(5.40–5.80) |
| Moderate | ||||
| Midazolam + Ranitidine | Increased midazolam bioavailability. | Fair | 5,481 | 10.05(9.78–10.31) |
| Furosemide + Vecuronium | Increased or decreased neuromuscular blockade. | Good | 4,820 | 8.84(8.59–9.09) |
| Dexamethasone + Rocuronium | Decreased rocuronium effectiveness; prolonged muscle weakness and myopathy. | Fair | 4,076 | 7.47(7.24–7.70) |
| Furosemide + Ketorolac | Decreased diuretic and antihypertensive efficacy. | Good | 3,163 | 5.80(5.60–6.00) |
| Aspirin + Furosemide | Decreased diuretic and antihypertensive efficacy. | Good | 3,054 | 5.60(5.40–5.80) |
| Methylprednisolone + Rocuronium | Decreased rocuronium effectiveness; prolonged muscle weakness and myopathy. | Fair | 2,756 | 5.05(4.86–5.24) |
| Midazolam + Sevoflurane | Potentiation of anesthetic effects of sevoflurane. | Fair | 2,489 | 4.56(4.38–4.74) |
| Heparin + Vitamin A | Increased risk of bleeding. | Good | 2,483 | 4.55(4.37–4.73) |
| Dexamethasone + Vecuronium | Decreased vecuronium effectiveness; prolonged muscle weakness and myopathy. | Good | 2,457 | 4.50(4.33–4.68) |
| Magnesium + Rocuronium | An increased risk of rocuronium toxicity (neuromuscular block prolongation, respiratory depression, apnea). | Good | 2,444 | 4.48(4.30–4.66) |
| Minor | ||||
| Ampicillin + Gentamicin | Loss of aminoglycoside efficacy. | Good | 1,391 | 2.55(2.42–2.68) |
| Diazepam + Propofol | Diazepam toxicity (CNS depression). | Good | 1,319 | 2.42(2.29–2.55) |
| Aspirin + Ranitidine | Reduced salicylate plasma levels and decreased antiplatelet effect of aspirin. | Excellent | 1,001 | 1.84(1.72–1.95) |
| Gentamicin + Piperacillin | Loss of aminoglycoside efficacy. | Good | 891 | 1.63(1.53–1.74) |
| Copper + Zinc | Decreased zinc or copper absorption. | Fair | 763 | 1.40(1.30–1.50) |
| Fosphenytoin + Phenobarbital | Increased or decreased phenytoin levels. | Good | 733 | 1.34(1.25–1.44) |
| Piperacillin + Tobramycin | Loss of aminoglycoside efficacy. | Good | 654 | 1.20(1.11–1.29) |
| Furosemide + Hydralazine | An enhanced diuretic response to furosemide. | Good | 561 | 1.03(0.94–1.11) |
| Fosphenytoin + Ranitidine | Increased phenytoin concentrations. | Good | 519 | 0.95(0.87–1.03) |
| Furosemide + Succinylcholine chloride | Alterations of neuromuscular blockade. | Fair | 475 | 0.87(0.79–0.95) |
ADE, adverse drug events; CI, confidence interval.
As illustrated in Figure 2, the median daily PDDI exposure of an infant patient remained at 1 from the first day in the PICU through day 30 (for patients who remained in the PICU for that length of stay); the infant patient at the 90th percentile of daily PDDI exposure was 4 to 7 from day 1 to 30 in the PICU. The cumulative number of distinct PDDI exposures for each successive day in PICU for the median (and 90th percentile) infant patient rise on the first day in PICU from 1 (5) to 12 (29) by day 30. Similar patterns were observed for the other 2 age groups, but significantly increase in both daily and cumulative PDDI exposures. The daily PDDI exposure rates, for infant patients, was 62.8% on day 1 in PICU, 62.9% on day 7, 63.4% on day 15, 65.7% on day 30; for patients age 1–9 years, 70.1% on day 1, 77.4% on day 7, 81.4% on day 15, 83.3% on day 30; for patients age 10–17 years, 69.2% on day 1, 77.8% on day 7, 86.6% on day 15, 91.6% on day 30. For all 3 of these age groups, the increasing exposure rate over time was significant (p<0.0001).
Figure 2.
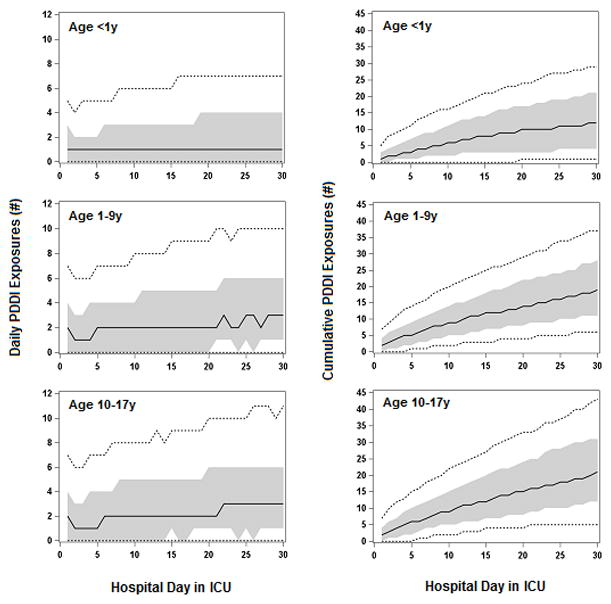
Number of daily and cumulative distinct PDDI exposures in PICU patients by age group. Shown are the levels of exposure to distinct PDDI for each hospital day, from day 1 up to day 30 of hospitalization in ICU, for patients who remained in ICU for those lengths of stay. For each hospital day, we determined the level of exposure for patients at various percentiles of exposure; the plotted solid lines display the median, the plotted short dash lines display 10th and the plotted 90th percentiles, and the shaded zone the interquartile range (IQR). Number of daily PDDI exposures is defined as the number of distinct PDDI that patients at each percentile were exposed to on that hospital day in ICU. Number of cumulative PDDI exposures is defined as the number of distinct PDDI that patients at each percentile were exposed to up to and including that hospital day in ICU.
The higher prevalence rate of PDDI (including least one PDDI regardless of severity level) was significantly associated with certain diagnoses (neoplasms, circulatory systems, congenital anomalies, nervous systems diseases), the presence of CCC, increasing number of distinct drug exposures, increasing length of stay in PICU, and white race (for all, p<0.001). Compared to those with <5 distinct drugs daily, those with 5–9 distinct drugs daily had 5 times higher likelihood of any PDDI exposure, and those with ≥10 distinct drugs daily had 37 times higher likelihood of any PDDI exposure. (Table 3).
Table 3.
Unadjusted and adjusted analysis of factors associated with PDDI
| Characteristics | Unadjusted OR (95%CI) | Adjusted OR(95%CI) |
|---|---|---|
| Age (years) | ||
| < 1 | 1.00 | 1.00 |
| 1–4 | 1.13(1.07––1.19) | 0.93(0.85–1.01) |
| 5–9 | 1.10(1.03–1.17) | 0.85(0.77–0.93) |
| 10–14 | 1.26(1.18–1.34) | 0.90(0.83–1.01) |
| 15–17 | 1.34(1.24–1.44) | 1.03(0.92–1.15) |
| Gender | ||
| Male | 1.00 | 1.00 |
| Female | 1.03(0.98–1.07) | 0.95(0.90–1.01) |
| Race | ||
| White | 1.00 | 1.00 |
| Hispanic | 0.77(0.72–0.82) | 0.86(0.79–0.95) |
| Black | 0.69(0.65–0.73) | 0.83(0.77–0.89) |
| Other | 0.75(0.70–0.80) | 0.79(0.72–0.87) |
| Region | ||
| Midwest | 1.00 | 1.00 |
| Northeast | 0.90(0.49–1.63) | 0.98(0.58–1.67) |
| South | 1.06(0.66–1.69) | 1.08(0.71–1.64) |
| West | 1.35(0.81–2.25) | 1.21(0.76–1.91) |
| Diagnosis categories | ||
| Respiratory system | 0.50(0.46–0.54) | 0.47(0.42–0.52) |
| Congenital anomalies | 5.39(4.86–5.98) | 2.29(1.99–2.63) |
| Injury and poisoning | 1.07(0.98–1.17) | 1.82(1.61–2.05) |
| Nervous system | 1.65(1.48–1.83) | 2.17(1.89–2.48) |
| Circulatory system | 2.17(1.92–2.45) | 2.45(2.12–2.90) |
| Neoplasms | 3.10(2.69–3.57) | 3.18(2.67–3.78) |
| Conditions in the perinatal period | 0.35(0.31–0.39) | 0.65(0.55–0.76) |
| Infectious diseases | 1.20(1.06–1.35) | 0.65(0.55–0.76) |
| Digestive system | 1.56(1.36–1.78) | 1.28(1.09–1.52) |
| Endocrine, nutritional & metabolic | 0.29(0.26–0.33) | 0.33(0.28–0.39) |
| All other diagnostic categories | 1.00 | 1.00 |
| Number of complex chronic condition | ||
| 0 | 1.00 | 1.00 |
| 1 | 4.52(4.29–4.76) | 1.77(1.65–1.90) |
| 2 | 5.88(5.51–6.27) | 1.77(1.62–1.93) |
| 3 | 6.55(6.02–7.11) | 1.64(1.47–1.83) |
| ≥4 | 9.33(8.35–10.43) | 1.90(1.65–2.18) |
| Average daily number of distinct medication exposure | ||
| 1–4 | 1.00 | 1.00 |
| 5–9 | 4.47(4.21–4.74) | 5.31(4.95–5.71) |
| ≥10 | 44.94(41.75–48.38) | 37.42(34.35–40.77) |
| Length of Stay in ICU (days) | ||
| 1 | 1.00 | 1.00 |
| 2–3 | 1.88(1.79–1.98) | 1.70(1.59–1.82) |
| 4–7 | 3.44(3.23–3.66) | 3.83(3.53–4.16) |
| 8–14 | 8.06(7.31–8.88) | 9.24(8.15–10.48) |
| 15–30 | 14.89(12.74–17.40) | 17.30(14.16–21.13) |
| >30 | 24.43(19.50–30.61) | 36.97(28.11–48.61) |
| Payers | ||
| Medicaid | 1.00 | 1.00 |
| Other Government Payers | 1.34(1.21–1.48) | 1.03(0.89–1.18) |
| Non Government Insurance | 1.12(1.06–1.17) | 0.96(0.90–1.03) |
| Other | 1.09(0.98–1.21) | 1.12(0.97–1.29) |
| Unknown | 1.26(0.96–1.64) | 1.04(0.74–1.45) |
OR, odds ratio; CI, confidence interval.
DISCUSSION
This study, based on data from 54,549 admissions to PICU in 42 children’s hospitals in the United States, provides a broad overview of PICU patient drug utilization. While there is no consensus in defining polypharmacy, the majority of studies have applied 5 or more drugs as threshold for polypharmacy (4, 26, 27), and “excessive polypharmacy” has been defined as the concurrent use of 10 or more in the adult population (4, 28). Our study indicates that 89% of the PICU patients had at least 1 day exposed to 5 or more distinct generic drugs, while 68.2% of patients had at least 1 day exposed to 10 or more distinct generic drugs. The total numbers of drug exposures over the course of the entire PICU stay equaled 20 drugs for the typical patient admitted to PICU (with a median length of PICU stay of 3 days).
We identified a total of 543 unique generic drugs in this cohort that could have potential interacted with other drugs administered concurrently. With 75.2% of all PICU patients exposed to at least one PDDI; we identified a total of 1,332,168 PDDI exposures, comprising 3,603 distinct PDDIs. Among these PDDIs, 70% were classified as of either major or contraindicated severity and almost 64% were supported by good scientific evidence.
We recently reported that 49% of all hospitalized pediatric patient were exposed at least 1 PDDI (14), but to date, no study has specifically evaluated the prevalence of PDDI exposure in the PICUs. The overall prevalence of PDDI exposure in adult ICUs has been reported to range from 44.3% and 87.9% (29–31). The variation among these various studies likely arose from differences in study design, patient populations, and the software or algorithms used to identify PDDI. The high prevalence of exposure to PDDI found in our study may be due to several factors, including the nature of the underlying medical conditions (such as cancer, cardiac defects, congenital anomalies, or central nervous system disorders), long stays in the PICU, and high levels of polypharmacy exposure, all of which are known risk factors for PDDI exposure. Our previous studies showed that patients with CCC take more medications and use more drugs with potential for interactions, such as opioids (14, 32). Similar to other studies conducted in adults (9, 10), increased numbers of total daily medications was associated with a greater potential for DDI in our population.
The fact that a large fraction of PPDIs were due to opioids and benzodiazepines warrants discussion. Similar to other reports conducted in adults ICU (29), the most common PDDI exposure observed in our cohort was the combination of an opioid (fentanyl) and a benzodiazepine (midazolam), and overall opioids were involved in 30% of all PDDIs. Hospitalized children are often treated with opioids and benzodiazepines, especially in PICUs (5, 32, 33), and analgesia-sedation in the ICU provides comfort, anxiety relief, and facilitates synchronizing the patient and the ventilator to optimize oxygenation and ventilation (34–37). Accordingly, due to the common use of opioids and benzodiazepines, clinicians are likely quite aware of the potential interactions among these drugs, and manage patients in a manner to ensure safe and effective analgesia-sedation. At the same time, recent concerns about the long-term effects of opioids, benzodiazepines, and other sedation medications on the developing brain encourage us to avoid unnecessary polypharmacy and to seek to reduce exposures to PDDI and appropriate monitoring for ADEs in sedation protocols (34, 37). More generally, the most common PDDI exposures may ultimately not be the ones that pose the greatest risk for child wellbeing, since clinicians may be familiar and prepared to manage the drug interactions; instead, the less common PDDI, arising from drugs that are likewise used less commonly, may pose the larger health risk.
This study has both strengths and weaknesses that warrant consideration. Regarding strengths, our retrospective cohort design, based on the large multicenter PHIS database, enabled us to describe the extent of polypharmacy and the frequency and pattern of PDDI exposures in PICU patients throughout the United States. Previous estimates of drug utilization in PICU are based on data from either a single hospital or a few centers, and these estimates do not provide information about the patterns of polypharmacy and PDDI exposures during the course of hospitalization. Regarding weaknesses, first, while the PDDIs were identified by the validated DRUG-REAX® system, these were all potential DDIs, and we do not know what fraction resulted in clinically significant ADEs, the risk of which varies based on individual genetic makeup, clinical condition, and other factors. Second, although the DRUG-REAX® system has proven to be highly accurate (24), there are disagreements among drug interaction compendia including the DRUG-REAX® system in identifying and ranking the severity of PDDIs in adult population (24, 25), no such reports in pediatric setting. We may miss some critical PDDIs and some severity ratings may be not accurate. Third, since we did not have data regarding precise timing of drug administration, all drugs given in a 24 hour period were regarded as concurrent exposure. This would result in overestimating PDDIs for certain drug combinations; For example, the interaction between nitroprusside and sildenafil would not be a DDI if nitroprusside is given and stopped with adequate time prior to administering sildenafil given nitroprusside’s very short half-life. Fourth and relatedly, since the PHIS database had no results of laboratory or imaging tests, we could not evaluate the clinical significance of PDDI exposure in this study.
Despite these limitations, the depth and breadth of the findings from this study can inform and guide the design of future studies. At a practical level, our estimates of the proportion of patients exposed to drugs and to PDDIs graded by severity level provides information that can assist with selecting specific drugs or PDDIs to study and estimating the likely size of the exposed population. At a more theoretical level, while our study identified only 2-drug interactions (which is the reported standard in other DDI studies); our findings regarding polypharmacy inform us that ≥3 drug combinations are very common. A greater number of drugs used concurrently may have a potentially multiplicative increase in the number of ADEs, especially for specific drug classes such as sedation (38) or QT-prolonging medications (39). Similarly, exposures to multiple PDDIs, as we found occurring in our cohort, could result in compounded effects of ADEs. Finally, although we identified 543 unique generic drugs that had PDDI with other drugs, and 3,603 specific PDDIs in this cohort, we believe (but cannot prove) that most of these PDDIs are well-understood, well-managed, and present a small risk of harm, whereas a few of the less common PDDIs may be quite harmful. We must identify the truly dangerous “needles amidst the PDDI haystack” in order to develop decision support tools not just to detect ADEs (40) but also to assist clinicians in minimizing exposing patients to harmful PDDI while improving outcomes (18, 41).
CONCLUSIONS
A large portion of PICU patients are exposed to substantial polypharmacy, and many patients are exposed to multiple PDDIs. Future research should investigate whether the potential benefits of additional concurrent medication are offset by potential harms of PDDI and quantify the realized risk of ADEs following specific PDDI exposures, especially the risk of ADEs due to multiple PDDI exposures.
Supplementary Material
Demographic and clinical characteristics of patients and prevalence of PDDI in PICU
Complete List of All Distinct Generic Drug Exposures in Study Population
Complete List of All Distinct PDDIs Occurring in Study Population
Acknowledgments
The authors thank Truven Health Analytics and Brian Cohan, RPH, for their assistance in performing the en masse identification of PDDIs using the DRUG-REAX® system.
Funding/Support: This study was supported by the Agency for Healthcare Quality and Research, Comparative Effectiveness and Safety of Hospital-Based Pediatric Palliative Care (1R01HS018425).
Footnotes
Financial Disclosures: The authors declare that they have no relevant financial relationships to disclose.
Conflict of Interest: The authors have no conflicts of interest to disclose.
Role of the Sponsor: The funding organization had no role in the conduct of the study, including the collection, analysis, and preparation of the data or the drafting, editing, review, or approval of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
References
- 1.US Department of Health and Human Services. [Accessed September 12, 2014];Best Pharmaceuticals for Children Act Literature Reviews and Assessments. Available at: http://www.bpca.nichd.nih.gov/resources/reviews/index.cfm.
- 2.Kweder S. [Accessed September 12, 2014];Programs Affecting Safety and Innovation in Pediatric Therapies. Available at: http://www.fda.gov/NewsEvents/Testimony/ucm153848.htm.
- 3.Mizokami F1, Koide Y, Noro T, Furuta K. Polypharmacy with common diseases in hospitalized elderly patients. Am J Geriatr Pharmacother. 2012;10:123–128. doi: 10.1016/j.amjopharm.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Petersson G, Hovstadius B. Factors leading to excessive polypharmacy. Clin Geriatr Med. 2012;28:159–172. doi: 10.1016/j.cger.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Feudtner C, Dai D, Hexem KR, et al. Prevalence of polypharmacy exposure among hospitalized children in the United States. Arch Pediatr Adolesc Med. 2012;166:6–16. doi: 10.1001/archpediatrics.2011.161. [DOI] [PubMed] [Google Scholar]
- 6.Edwards JD1, Houtrow AJ, Vasilevskis EE, et al. Chronic conditions among children admitted to U.S. pediatric intensive care units: their prevalence and impact on risk for mortality and prolonged length of stay. Crit Care Med. 2010;40:2196–2203. doi: 10.1097/CCM.0b013e31824e68cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonnell C1, Hum S, Frndova H, Parshuram CS. Pharmacotherapy in pediatric critical illness: a prospective observational study. Paediatr Drugs. 2009;11:323–331. doi: 10.2165/11310670-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Papadopoulos J, Smithburger PL. Common drug interactions leading to adverse drug events in the intensive care unit: Management and pharmacokinetic considerations. Crit Care Med. 2010;38:S126–S135. doi: 10.1097/CCM.0b013e3181de0acf. [DOI] [PubMed] [Google Scholar]
- 9.Hanlon JT, Pieper CF, Hajjar ER, et al. Incidence and predictors of all and preventable adverse drug reactions in frail elderly persons after hospital stay. J Gerontol A Biol Sci Med Sci. 2006;61:511–516. doi: 10.1093/gerona/61.5.511. [DOI] [PubMed] [Google Scholar]
- 10.Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30:911–918. doi: 10.2165/00002018-200730100-00009. [DOI] [PubMed] [Google Scholar]
- 11.Nobili A, Garattini S, Mannucci PM. Multiple diseases and polypharmacy in the elderly: Challenges for the internist of third millennium. J Co-morb. 2011;1:28–44. doi: 10.15256/joc.2011.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mouly S, Meune C, Bergmann JF. Mini-series: I. Basic science. Uncertainty and inaccuracy of predicting CYP-mediated in vivo drug interactions in the ICU from vitro models: focus on CYP3A4. Intensive Care Med. 2009;35:417–429. doi: 10.1007/s00134-008-1384-1. [DOI] [PubMed] [Google Scholar]
- 13.Askari M, Eslami S, Louws M, et al. Frequency and nature of drug-drug interactions in the intensive care unit. Pharmacoepidemiol Drug Saf. 2013;22:430–437. doi: 10.1002/pds.3415. [DOI] [PubMed] [Google Scholar]
- 14.Feinstein J, Dai D, Zhong W, et al. Potential Drug-Drug Interactions in Infant, Child, and Adolescent Patients in Children’s Hospitals. Pediatrics. 2015;135:e99–e108. doi: 10.1542/peds.2014-2015. [DOI] [PubMed] [Google Scholar]
- 15.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 16.Malone DC, Abarca J, Hansten PD, et al. Identification of serious drug-drug interactions: results of the partnership to prevent drug-drug interactions. Am J Pharm Assoc. 2004;44:142–151. doi: 10.1331/154434504773062591. [DOI] [PubMed] [Google Scholar]
- 17.Aagaard L, Christensen A, Hansen EH. Information about adverse drug reactions reported in children: a qualitative review of empirical studies. Br J Clin Pharmacol. 2010;74:481–491. doi: 10.1111/j.1365-2125.2010.03682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harper MB, Longhurst CA, McGuire TL, et al. Core Drug-drug interaction alerts for inclusion in pediatric electronic health records with computerized prescriber order entry. J Patient Saf. 2014;10:59–63. doi: 10.1097/PTS.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 19.Fisher BT, Lindenauer PK, Feudtner C. In-Hospital Databases. In: Strom B, Kimmel S, Hennessy S, editors. Pharmacoepidemiology. 5. Hoboken, NJ: Wiley-Blackwell; 2012. pp. 244–258. [Google Scholar]
- 20.Hsieh EM, Hornik CP, Clark RH, et al. Medication Use in the Neonatal Intensive Care Unit. Am J Perinatol. 2014;31:811–821. doi: 10.1055/s-0033-1361933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alcon J, McNamara PJ. Pharmacokinetics in the newborn. Adv Drug Deliv Rev. 2003;55:667–686. doi: 10.1016/s0169-409x(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 22.Kearns GL1, Abdel-Rahman SM, Alander SW, et al. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 23.Feudtner C, Feinstein J, Zhong W, Hall M, Dai D. Pediatric Complex Chronic Conditions Classification System Version 2: Updated for ICD-10 and Complex Medical Technology Dependence and Transplantation. BMC Pediatrics. 2014;14:199. doi: 10.1186/1471-2431-14-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrons R. Evaluation of personal digital assistant software for drug interactions. Am J Health-Syst Pharm. 2004;61:380–385. doi: 10.1093/ajhp/61.4.380. [DOI] [PubMed] [Google Scholar]
- 25.Vonbach P, Dubied A, Krahenbuhl S, Beer JH. Evaluation of frequently used drug interaction screening programs. Pharm World Sci. 2008;30:367–374. doi: 10.1007/s11096-008-9191-x. [DOI] [PubMed] [Google Scholar]
- 26.Viktil KK, Blix HS, Moger TA, Reikvam A. Polypharmacy as commonly defined is an indicator of limited value in the assessment of drug-related problems. Br J Clin Pharmacol. 2007;63:187–195. doi: 10.1111/j.1365-2125.2006.02744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin interventions in aging. 2008;3:383–389. doi: 10.2147/cia.s2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haider SI, Johnell K, Weitoft GR, et al. The influence of educational level on polypharmacy and inappropriate drug use: a register-based study of more than 600,000 older people. J Am Geriatr Soc. 2009;57:62–69. doi: 10.1111/j.1532-5415.2008.02040.x. [DOI] [PubMed] [Google Scholar]
- 29.Reis AM, Cassiani Prevalence of potential drug interactions in patients in an intensive care unit of a university hospital in Brazil. Clinics. 2011;66:9–15. doi: 10.1590/S1807-59322011000100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smithburger PL, Kane-Gill SL, Seybert AL. Drug-drug interactions in the medical intensive care unit: an assessment of frequency, severity and the medications involved. Int J Pharm Pract. 2012;20:402–408. doi: 10.1111/j.2042-7174.2012.00221.x. [DOI] [PubMed] [Google Scholar]
- 31.Uijtendaal EV, van Harssel LL, Hugenholtz GW, et al. Analysis of potential drug-drug interactions in medical intensive care unit patients. Pharmacotherapy. 2014;34:213–219. doi: 10.1002/phar.1395. [DOI] [PubMed] [Google Scholar]
- 32.Womer J, Zhong W, Kraemer FW, et al. Variation of opioid use in pediatric inpatients across hospitals in the United States. J Pain Symptom Manage. 2014;48:908–914. doi: 10.1016/j.jpainsymman.2013.12.241. [DOI] [PubMed] [Google Scholar]
- 33.Zuppa AF, Adamson PC, Mondick JT, et al. Drug utilization in the pediatric intensive care unit: Monitoring prescribing trends and establishing prioritization of pharmacotherapeutic evaluation of critically ill children. J Clin Pharmacol. 2005;45:1305–1312. doi: 10.1177/0091270005280966. [DOI] [PubMed] [Google Scholar]
- 34.Devlin JW. Pharmacology of over sedation in mechanically ventilated adults. Curr Opin Crit Care. 2008;14:403–407. doi: 10.1097/MCC.0b013e32830280b3. [DOI] [PubMed] [Google Scholar]
- 35.Devlin JW, Roberts RJ. Pharmacology of commonly used analgesia and sedatives in the ICU: benzodiazepines, propofol, and opioids. Crit Care Clin. 2009;25:431–449. doi: 10.1016/j.ccc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Barr J, Fraser GL, Puntillo K, et al. Clinical guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–360. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 37.Schweickert WD, Kress JP. Strategies to optimize analgesia and sedation. Crit Care. 2008;12(Suppl 3):S6. doi: 10.1186/cc6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neutel I, Skurtveit S, Berg C. Polypharmacy of potentially addictive medication in the older persons – quantifying usage. Pharmacoepidemiol Drug Saf. 2012;22:199–206. doi: 10.1002/pds.2214. [DOI] [PubMed] [Google Scholar]
- 39.Tay KY, Ewald MB, Bourgeois FT. Use of QT-prolonging medications in US emergency departments, 1995–2009. Pharmacoepidemiol Drug Saf. 2013;23:9–17. doi: 10.1002/pds.3455. [DOI] [PubMed] [Google Scholar]
- 40.Takata GS, Mason W, Taketomo C, et al. Development, Testing, and Findings of a Pediatric-Focused Trigger Tool to Identify Medication-Related Harm in US Children’s Hospitals. Pediatrics. 2008;121:e927–e935. doi: 10.1542/peds.2007-1779. [DOI] [PubMed] [Google Scholar]
- 41.Walsh KE, Landrigan CP, Adams WG, et al. Effect of computer order entry on prevention of serious medication errors in hospitalized children. Pediatrics. 2008;121:e421–e427. doi: 10.1542/peds.2007-0220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demographic and clinical characteristics of patients and prevalence of PDDI in PICU
Complete List of All Distinct Generic Drug Exposures in Study Population
Complete List of All Distinct PDDIs Occurring in Study Population


