Abstract
We recently showed that the anaplerotic enzyme pyruvate carboxylase (PC) is up-regulated in human breast cancer tissue and its expression is correlated with the late stages of breast cancer and tumor size [Phannasil et al., PloS One 10, e0129848, 2015]. In the current study we showed that PC enzyme activity is much higher in the highly invasive breast cancer cell line MDA-MB-231 than in less invasive breast cancer cell lines. We generated multiple stable PC knockdown cell lines from the MDA-MB-231 cell line and used mass spectrometry with 13C6-glucose and 13C5-glutamine to discern the pathways that use PC in support of cell growth. Cells with severe PC knockdown showed a marked reduction in viability and proliferation rates suggesting the perturbation of pathways that are involved in cancer invasiveness. Strong PC suppression lowered glucose incorporation into downstream metabolites of oxaloacetate, the product of the PC reaction, including malate, citrate and aspartate. Levels of pyruvate, lactate, the redox partner of pyruvate, and acetyl-CoA were also lower suggesting the impairment of mitochondrial pyruvate cycles. Serine, glycine and 5-carbon sugar levels and flux of glucose into fatty acids were decreased. ATP, ADP and NAD(H) levels were unchanged indicating that PC suppression did not significantly affect mitochondrial energy production. The data indicate that the major metabolic roles of PC in invasive breast cancer are primarily anaplerosis, pyruvate cycling and mitochondrial biosynthesis of precursors of cellular components required for breast cancer cell growth and replication.
Keywords: Gene knockout, Pyruvate carboxylase (PC), Breast cancer, Cell proliferation rate, Mitochondrial biosynthesis, Cancer biology, Anaplerosis, Mitochondrial metabolism
1. Introduction
Breast cancer is one of the most common malignancies in women, with an estimated one million cases diagnosed every year, and remains one of the cancer types that cause an extremely high mortality rate worldwide [1]. Similar to other cancers, regardless of tissue oxygen levels, breast cancer oxidizes glucose excessively via glycolysis known as the Warburg effect. Mitochondrial energy production by oxidative phosphorylation is essentially normal or occasionally slightly repressed in cancer cells [2]. This and other recent indirect evidence suggests that mitochondrial metabolism could be important in other ways, such as for supplying the substrates for biosynthesis of fatty acids, amino acids and nucleic acids as the structural and functional components of the rapidly dividing cells [2,3]. In this regard, “anaplerosis”, which is the net mitochondrial biosynthesis of certain citric acid cycle intermediates [4], could be extremely important for cancer cell proliferation and invasiveness. However, there is a paucity of data on the role of individual metabolic pathways that support cancer cell growth and survival, especially in breast cancer. Pyruvate carboxylation and glutaminolysis are the two major anaplerotic reactions that replenish citric acid cycle intermediates when they are depleted by their export from the mitochondria for biosynthetic reactions that take place mostly in the extramitochondrial compartment of the cell. A major anaplerotic reaction involves the carboxylation of pyruvate to oxaloacetate catalyzed by pyruvate carboxylase (PC) followed by oxaloacetate’s conversion to malate, citrate and aspartate and their export from mitochondria to the cytosol where they become precursors for the synthesis of carbohydrates, lipids and amino acids [5]. Glutaminolysis involves the conversion of glutamine to glutamate by glutaminase followed by oxidative deamination of glutamate to α-ketoglutarate catalyzed by glutamate dehydrogenase. α-Ketoglutarate is then converted to other citric acid cycle intermediates that can be exported from the mitochondria to the cytosol [6]. Different cancers use these anaplerotic reactions to various degrees [7–9].
We recently showed that PC is up-regulated in human breast cancer tissue and its expression levels correlated with the late stages of breast cancer and tumor size but not with the expression of estrogen receptor (ER), progesterone receptor (PR) and epidermal growth factor receptor 2 (HER2) in patients’ breast tissues [10]. In supporting these clinical data, we also found that the levels of PC and its mRNA were not correlated with expression of these three receptors in four independent breast cancer cell lines, namely MDA-MB-231 (ER−/PR−/HER2−), MDA-MB-435 (ER−/PR−/HER2+), MCF-7 (ER+/PR+/HER2−) and SKRB3 (ER−/ PR−/HER2+) [11] but highly correlated with their invasive phenotype i.e., highly expressed in highly metastasized cell lines (MDA-MB-231 > MDA-MB-435) but poorly expressed in low or non-metastasizing cell lines (SKRB3 and MCF-7) [10]. Ectopic expression of PC in MCF-7 cells increases their proliferation, motility and invasion abilities, suggesting a further link between PC and aggressive phenotype of breast cancer. Transient suppression of PC expression in MDA-MB-231 and MDA-MB-435 cell lines reduced their proliferation, migration and invasion in vitro indicating the essential role of PC in supporting breast cancer growth and invasiveness [10].
It remains unclear which biochemical pathways and metabolites are altered by PC knockdown and contribute to the slow proliferation and decreased motility phenotypes in breast cancer. In the current study we generated multiple stable PC knockdown cell lines from the MDA-MB-231 breast cancer cell line, a highly invasive cell line with a high PC enzyme activity. The cell lines with severe PC knockdown showed marked decreases in cell viability and proliferation rates. PC knockdown caused decreases in malate, citrate and pyruvate levels and glucose incorporation into these metabolites suggesting the inhibition of mitochondrial pyruvate cycling, decreases in aspartate and other amino acids, some nucleotides and their derivatives needed for cell structure, as well as decreased incorporation of glucose carbon into palmitate. ATP, ADP and pyridine nucleotide levels were not significantly affected demonstrating that PC knockdown does not inhibit mitochondrial energy production. The results demonstrate the important role of PC in anaplerosis and pyruvate cycling via mitochondrial biosynthesis for growth and survival of the MDA-MB-231 cells.
2. Materials and methods
2.1. Materials
Human breast cancer cell lines, MCF-7 and MDA-MB-231 were generous gifts from Dr. Wei Xu, University of Wisconsin-Madison. Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Corning) supplemented with 10% (v/v) fetal bovine serum (FBS), 100 units/ml penicillin and 100 µg/ml streptomycin, and maintained at 37 °C with 5% CO2.
2.2. Designation of Pcx shRNA constructs and generation of Pcx-knockdown MDA-MB-231 cell lines
Six different shRNAs targeted to human pyruvate carboxylase (hPcx) coding sequence (ACCESSION BC011617.2) were designed using “siRNA Wizard v 3.1” (http://www.invivogen.com/sirnawizard/design.php). The oligonucleotides corresponding to the shRNA sequences with BamHI or HindIII restriction sites overhang at their 5’-ends were synthesized by Eurofins (Fisher Scientific, USA), and their sequences are shown in Table 1. Double stranded oligonucleotide cassettes with BamHI and HindIII sites at 5’- and 3’-ends were generated upon annealing each pair of oligonucleotides, and subsequently ligated at the BamHI and HindIII sites of the modified pSilencer 2.1-U6 puro TOL2 vector (Ambion, USA) [16]. The constructs carrying six different Pcx shRNA cassettes in the pSilencer 2.1-U6 puromycin vector were sequenced to confirm the correct oligo nucleotide sequence. Similarly, a scrambled shRNA control from Ambion (Life Technologies) (5’-ACTACCGTTGTTATAGGTG-3’) was cloned into the same vector to serve as a control.
Table 1.
Oligonucleotides used to generate shRNA constructs for suppressing human pyruvate carboxylase (PC) expression.
| Sequence name | Sequence (5′ 3′) | Length (bp) |
|---|---|---|
| PC 179 (forward) | GATCCTCGGAGTATAAGCCCATCAAGAATGTGCTTTTCTTGATGGGCTTATACTCCTTTTTGGAAA | 66 |
| PC 179 (reverse) | AGCTTTTCCAAAAAGGAGTATAAGCCCATCAAGAAAAGCACATTCTTGATGGGCTTATACTCCGAG | 66 |
| PC 847 (forward) | GATCCTCGGAACATCCTGCACCTGTATGTGCTTTACAGGTGCAGGATGTTCCTTTTTGGAAA | 62 |
| PC 847 (reverse) | AGCTTTTCCAAAAAGGAACATCCTGCACCTGTATGTGCTTTACAGGTGCAGGATGTTCCGAG | 62 |
| PC 2054 (forward) | GATCCTCCGTGGTCTTCAAGTTCTGTTGTGCTTACAGAACTTGAAGACCACGTTTTTGGAAA | 62 |
| PC 2054 (reverse) | AGCTTTTCCAAAAACGTGGTCTTCAAGTTCTGTTGTGCTTACAGAACTTGAAGACCACGGAG | 62 |
| PC 2096 (forward) | GATCCTCGGATGTCTTCCGTGTGTTTGATGTGCTTTCAAACACACGGAAGACATCCTTTTTGGAAA | 66 |
| PC 2096 (reverse) | AGCTTTTCCAAAAAGGATGTCTTCCGTGTGTTTGATGTGCTTTCAAACACACGGAAGACATCCGAG | 66 |
| PC 2653 (forward) | GATCCTCGCAACTCGGACGTGTATGATGTGCTTTCATACACGTCCGAGTTGCTTTTTGGAAA | 62 |
| PC 2653 (reverse) | AGCTTTTCCAAAAAGCAACTCGGACGTGTATGAAAGCACATCATACACGTCCGAGTTGCGAG | 62 |
| PC 3436 (forward) | GATCCTCGGAAGGTGATAGACATCAAAGTGTGCTTCTTTGATGTCTATCACCTTCCTTTTTGGAAA | 66 |
| PC 3436 (reverse) | AGCTTTTCCAAAAAGGAAGGTGATAGACATCAAAGTGTGCTTCTTTGATGTCTATCACCTTCCGAG | 66 |
2.3. Generation of MDA-MB-231 PC knockdown cell lines
Approximately 1 × 106 MDA-MB-231 cells were plated in a 35-mm culture dish (6-well plate) containing DMEM supplemented with 100 units/ml penicillin and 100 µg/ml streptomycin, and grown at 37 °C with 5% CO2 overnight. The cells were then transfected with 2.0 µg of shRNA expression constructs along with 1.0 µg of pCMV-Tol2 vector using Lipofectamine2000 (Life Technologies, USA). After 24 h, the cells were selected in the complete medium containing 0.5 µg/ml puromycin (Invitrogen, USA) that was maintained throughout the selection. The MDA-MB-231 cell line stably transfected with a scrambled shRNA construct was similarly generated. Multiple puromycin resistant colonies were formed after 15 days of selection. Thus each “cell line” is actually a population of PC knockdown cells rather than a single clone. The selected cells were expanded and maintained in selection media at all times before subsequent biochemical analyses.
2.4. Quantitative real time reverse transcriptase polymerase chain reaction (QRT-PCR)
Total RNA was extracted from cells using RNeasy mini kit (Qiagen) following the manufacturer’s instructions. The 10 µl RT reaction contained 2 µg of total RNA and oligo(dT) primers (Ambion) at 85 °C for 3 min and chilled at 4 °C. Reverse transcription was initiated by adding 10 µl of mixture containing 2 µl 10x RT buffer, 0.5 mM dNTP mix, 2 units of RNase inhibitor and reverse transcriptase (Ambion), to the primed-RNA mixture and the reaction was incubated at 43 °C for 60 min, 92 °C for 10 min and held at 4 °C, respectively. The cDNA was stored at −20 °C until used. Quantitative real time PCR was performed using SYBR Premium Ex Taq (Takara) using MyiQ™ single-color real time PCR detection system (BioRad). The standard curve of PC cDNA was obtained from amplification plots of PC prepared from various dilutions of MDA-MB-231 cDNAs. The expression of PC mRNA was normalized to the glutamate dehydrogenase (GLUD) mRNA level and is shown as the relative gene expression. Fold change was calculated using the ΔQ method. The thermal profiles consisted of initial denaturation at 95 °C for 3 min followed by 40 cycles of denaturation at 95 °C for 10 s and annealing at 60 °C for 20 s and extension at 72 °C for 30 s.
2.5. Cell viability assay
The numbers of viable cells were determined with the CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) kit (Promega). Four thousand cells of various stable PC knockdown MDA-MB-231 cell lines were plated into 96 well plates and grown in DMEM supplement with 10% (v/v) FBS, 100 units/ml penicillin and 100 µg/ml streptomycin, at 37 °C in a CO2 incubator overnight. Twenty microliters of CellTiter one solution reagent was added to the cells and incubated at 37 °C in CO2 incubator for 1 h. The amount of soluble formazan was measured immediately using a 96-well plate reader (Molecular Devices) at 490 nm. The absorbance is directly proportional to the number of viable cells in culture.
2.6. Cell proliferation
2 × 104 cells of the PC-knockdown MDA-MB-231 or the scrambled shRNA control cell line were plated into 35 mm2 dishes and cultured in complete DMEM supplemented with 1.0 µg/ml puromycin at 37 °C with 5% CO2. Cells were trypsinized and counted by staining with 0.4% (w/v) trypan blue (Gibco) at days 4, 5, 6 and 7.
2.7. PC enzyme activity
The cells were trypsinized off tissue culture plates with 0.05% trypsin and 0.5 mM EDTA. The cell pellet was washed twice with PBS and suspended in KMSH solution containing a protease inhibitor mixture (Pierce). PC enzyme activity was measured as previously described [12]. Ten microliters of the homogenate was incubated in a final volume of 50 µl of enzyme reaction mixture of 100 mMKCl,10mM MgCl2,2mM Na-ATP, 0.1% Triton X-100, 1 mM DTT, 1.6 mM acetyl CoA, 20 mM NaHCO3, 0.2 µCi [14C]NaHCO3, and 100 mM Tris-Cl buffer, pH 7.85 with or without 8 mM pyruvate at 37 °C for 30 min. The reaction was stopped by adding 50 µl 10% (v/v) trichloroacetic acid and after 10 min, 80 µl of the mixture was removed and added to a 20 ml scintillation vial that was left open for 2 h to allow evaporation of the unincorporated CO2. Then, 0.5 ml of water and 5 ml of Scintisafe scintillation mixture (catalog number SX21-5, Fisher Scientific) were added to the vial and the carbon fixed was measured by liquid scintillation spectrometry. Background radioactivity present in the absence of pyruvate was subtracted from the radioactivity in the presence of pyruvate to give the enzyme rate attributable to PC enzyme activity.
2.8. Measurement of pyruvate, malate and citrate from PC knockdown cells by alkali enhanced fluorescence
The cells were maintained in DMEM cell culture medium (contains 25 mM glucose and 4 mM glutamine) 10% (v/v) FBS 100 units/ml penicillin and 100 µg/ml streptomycin with 0.5 µg/ml puromycin on 150 mm. culture plates. The media was changed to RPMI 1640 cell culture medium (contains 2mM glutamine) modified to contain 5mM glucose the day before the experiment. On the day of the experiment the cells were washed twice with PBS and once with Krebs Ringer solution. Five milliliters of Krebs Ringer bicarbonate solution, pH 7.3, containing no glucose was added to the plates of cells, and the cells were incubated at 37 °C. After 10 min Krebs Ringer bicarbonate solution containing 10 mM glucose was added and the cells were incubated at 37 °C. After 35 min all liquid was quickly removed from the cells and the plates were put on ice. Then 0.75 ml of 6% PCA was added and the cells were scraped off the plates and transferred into the microtube. The mixture was homogenized and centrifuged to precipitate the protein and the supernatant fraction was neutralized with 30% KOH. The resulting precipitated potassium perchlorate was removed by centrifugation and the metabolite concentrations in the neutralized extract were measured by alkali enhanced fluorescence, as previously described [12].
2.9. Metabolites analysis by LC-MS and GC-MS
The cells were maintained in DMEM supplemented with 10% (v/v) FBS, 100 units/ml penicillin, 100 µg/ml streptomycin, and 0.5 µg/ml puromycin. Cells were plated at a density of 14 × 103 cells/cm2 in 6 cm culture dishes at 37 °C and 5% CO2 in a humidified atmosphere to 70% confluence over 5 days prior to experimentation. On the day of an experiment metabolism was stopped in four plates of each cell line and cells were harvested and saved for LC-MS analysis (time zero control). The medium was changed to DMEM without FBS modified to contain 10 mM 13C6-glucose plus 2 mM glutamine or 2 mM 13C5-glutamine plus 10 mM glucose. Four plates of each cell line were used for each condition. After incubation for 1 h, metabolism of cells was stopped and cells were harvested and analyzed with LC-MS as previously described [13,14].
2.10. Enzyme activities
Homogenates were prepared and activities of allenzymes except citrate synthase were measured as previously described [15]. The activity of citrate synthase was measured as described in reference [16].
2.11. Statistical analysis
Values of relative PC mRNA, PC enzyme activity, MTS cell proliferation and metabolite levels were expressed as mean ± standard error. The statistical analysis was confirmed with Student’s t-test.
3. Results
3.1. Characterization of the PC knockdown MDA-MB-231 cell lines
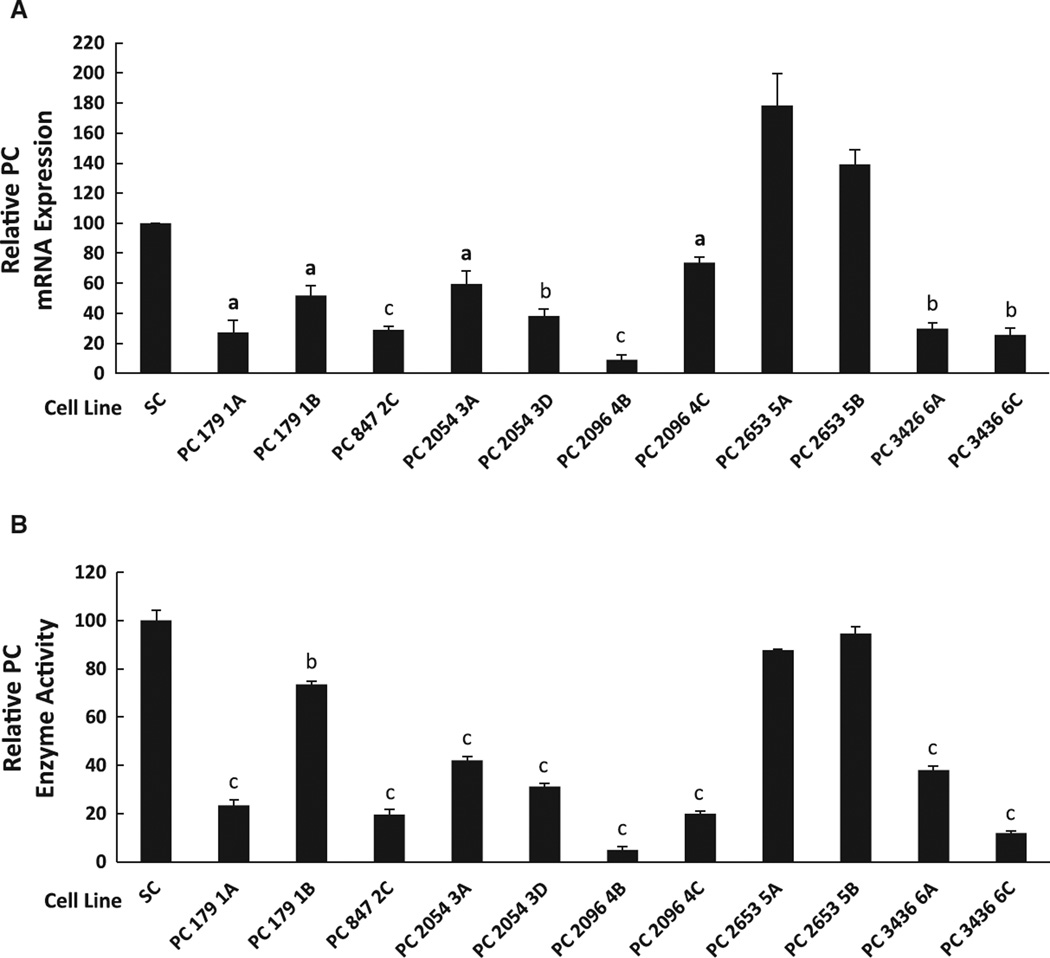
The PC enzyme activity of the MDA-MB-231 cell line, which is a highly invasive breast cancer cell line, was 10-fold higher than the PC enzyme activity of the less invasive breast cancer cell line MCF-7 (Table 2). Six shRNA constructs (PC179, PC847, PC2054, PC2096, PC2653 and PC3436) targeted to human PC mRNA were transfected to MDA-MB-231 cells. The stable cell lines were named according to the first nucleotide of the PC mRNA sequence targeted. Fig. 1A and B show that the degree of knockdown of PC mRNA correlated fairly well with the degree of knockdown of PC enzyme activity and PC protein in the various PC targeted cell lines. As we have previously observed, knockdown of PC enzyme activity and PC protein, although correlated well with the degree of mRNA knockdown was in general slightly less than the knockdown of PC mRNA [12]. Cell line PC 2096 4B possessed a PC mRNA level of approximately 10% compared to the scrambled shRNA control cell line, while cell lines PC 179 1A, PC 847 2C, PC 2054 3D, PC 3436 6A and PC 3436 6C contained PC mRNA levels of 20–40% that of the scrambled shRNA control cell line. Only modest reductions (50– 70%) of PC mRNA level were observed in cell lines PC 179 1B, PC 2054 3A and PC 2096 4C, while the level of PC mRNA was not decreased in cell lines PC 2653 5A and PC 2653 5B. PC enzyme activity of these cell lines was proportional to the levels of PC mRNA, with PC enzyme activity being lowest (5%) in the PC 2096 4B cell line (Fig. 1B). Cell lines PC 2096 4B, PC 179 1A, PC 847 2C, PC 2054 3D, PC 3436 6C and PC 2653 5B showed the highest, modest and lowest PC knockdown levels, respectively.
Table 2.
Pyruvate carboxylase (PC) enzyme activity is much higher in the invasive breast cancer cell line MDA-MB-231 than in the less invasive breast cancer cell line MCF-7. Results are means ± SE nmol CO2 fixed/min/mg cell protein of 4 replicate measurements.
| Cell line | PC enzyme activity (nmol CO2 fixed/min) |
|---|---|
| MDA-MB-231 | 13.2 ± 0.6 |
| MCF-7 | 1.2 ± 0.2 |
Fig. 1.
Decreased pyruvate carboxylase mRNA levels induced by gene silencing correlate with decreased PC enzyme activity in multiple cell lines derived from the breast cancer cell line MDA-MB 231.A, Relative pyruvate carboxylase (PC) mRNA expression in various PC knockdown cell lines (179 1A, 179 1B, 847 2C, 2054 3A, 2054 3D, 2096 4B, 2096 4C, 2653 5A, 2653 5B, 3426 6A, 34366C and scramble control (SC)). B, PC enzyme activity of PC knockdown cell lines relative to that of the scramble control which was arbitrarily set as 100%. ap < 0.05; bp < 0.01; cp < 0.001 vs scramble control.
3.2. Decreased cell proliferation rates in PC knockdown cell lines
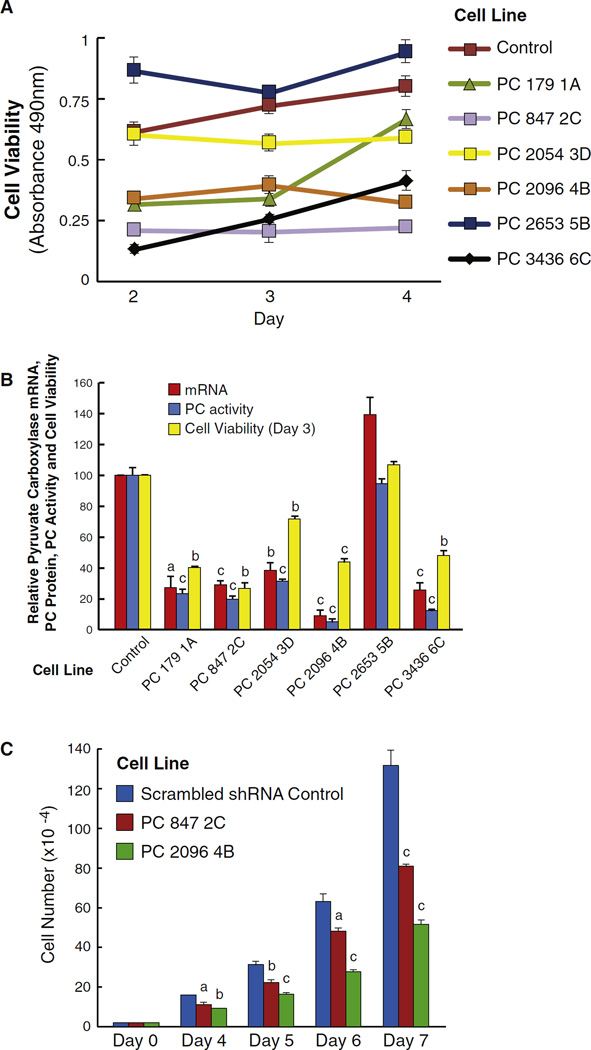
Cell lines with lower PC mRNA and enzyme activity showed lower cell viability (Fig. 2A and B). We selected two cell lines, PC 847 2C and PC 2096 4B, for further analysis of cell growth. Both knockdown cell lines showed decreased cell numbers at day 4, that became more obvious at days 5–7, with the PC 847 2C cell line showing a 35% lower cell count vs. the scrambled shRNA control cell line at day 7 and the PC 2096 B cell line showing a 65% lower cell count vs. the control cell line at day 7 (Fig. 2C).
Fig. 2.
Knockdown of pyruvate carboxylase (PC) mRNA and PC enzyme activity correlate with decreased breast cancer cell viability and growth. A, Viability of cells was measured with the MTS assay at days 2, 3 and 4. p > 0.001 vs the RNA scramble control of the four cell lines with the lowest viability. p < 0.05 for PC 2054 3D vs the control. B, PC mRNA, PC enzyme activity and viability of various knockdown cells at day 3. ap < 0.05; bp < 0.01, cp > 0.001 vs RNA scramble control. C. Decreased cell counts in the PC 2096 4B and PC 847 2C cell lines with knockdown of PC. ap < 0.05; bp < 0.01, cp > 0.001 vs RNA scramble control.
3.3. Metabolite analysis
Because most cancers use glucose and glutamine as the two main carbon sources for both energy production and biosynthesis [3], we used uniformly labeled 13C6-glucose or 13C5-glutamine and LC-MS or GC-MS to track the fluxes of these two substrates into the synthesis of various metabolites. This allowed us to distinguish which of the fluxes of the carbon sources might be more impaired in the PC knockdown cell lines PC 847 2C and PC 2096 4B compared to the shRNA scramble control MDA-MB-231 cell line. Prior to the experiment these three cell lines were maintained in DMEM cell culture medium for four or more days. DMEM is the standard cell culture medium used for maintaining the MDA-MB-231 cell line and it contains 25mM glucose and 4mM glutamine. For the LC-MS/MS analysis experiment, the cell lines were maintained for 1 h in DMEM medium modified to contain either 10 mM U-13C6 glucose plus 2 mM unlabeled glutamine or 2 mM U-13C5 glutamine plus 10 mM unlabeled glucose. For the experiment to look for a crossover point in the levels of the metabolites around the PC reaction, the cells were incubated in the presence of 5 mM glucose in RPMI 1640 cell culture medium because the high concentration of glucose in the DMEM medium would produce high levels of metabolites possibly obscuring a crossover point in the levels of the metabolites.
3.4. No decrease in glycolytic intermediates with PC knockdown
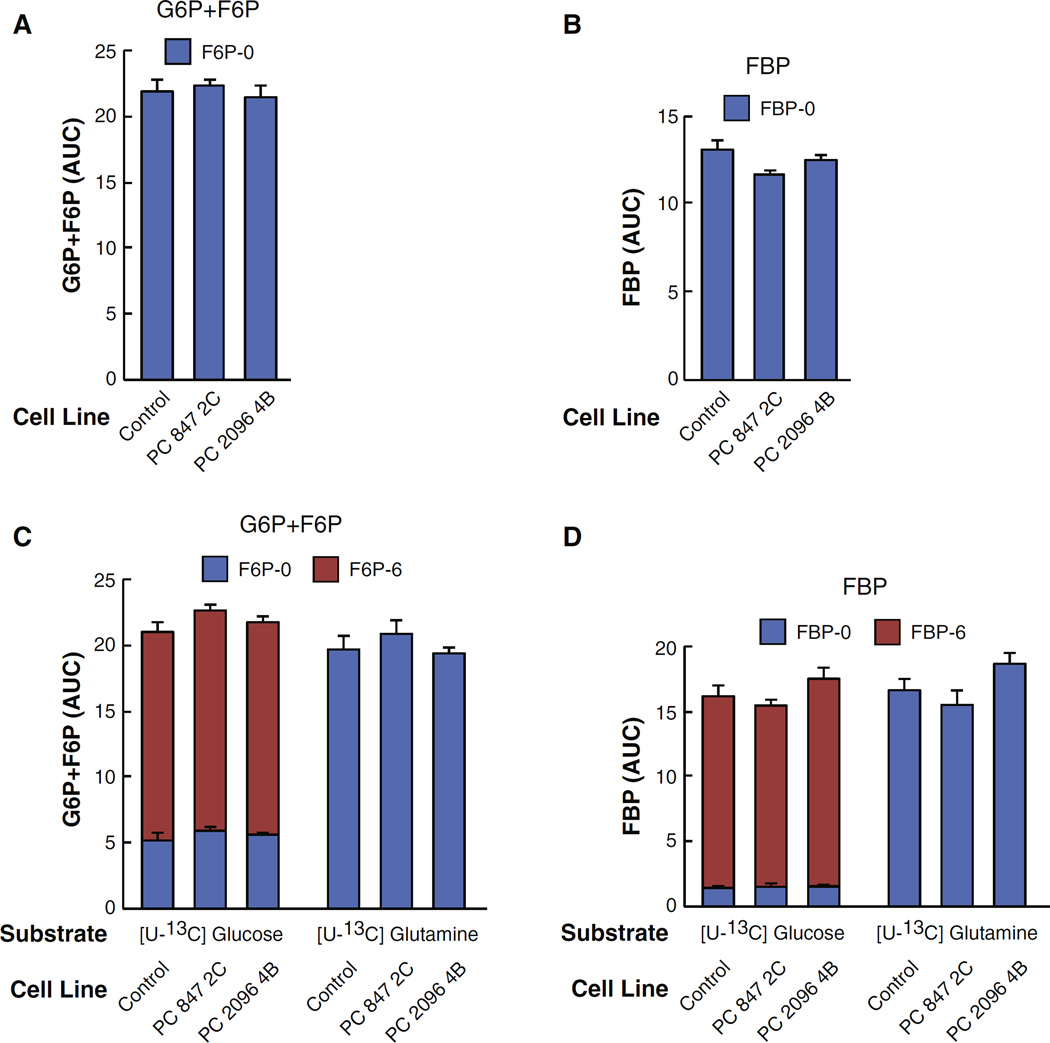
As expected, because PC is a mitochondrial enzyme its suppression did not affect the levels of glycolytic intermediates as glucose-6-phosphate, fructose-6-phosphate and fructose bisphosphate were not altered in each of these PC knockdown cell lines (Fig. 3A and B). Also as expected, glucose-6-phosphate and fructose-6-phosphate were labeled from U-13C6 glucose but not from U-13C5 glutamine (Fig. 3C and D).
Fig. 3.
Knockdown of pyruvate carboxylase (PC) expression does not alter the levels of glycolytic intermediates glucose-6-phosphate plus fructose-6-phosphate or fructose-biphosphate from U-13C6-glucose or U-13C5-glutamine in MDA-MB-231-derived cell lines. PC 847 2C and PC 2096 4B or a scramble shRNA control cell line were maintained in DMEM cell culture medium (contains 25 mM glucose and 4 mM glutamine) and 10% FBS for four or more days. Cells were then maintained in DMEM medium containing either 10 mM [U-13C6] glucose or 2 mM [U-13C5]glutamine for 1 h before metabolism was stopped and cells were analyzed by LC-MS/MS as described under Experimental Procedures. A, The levels of glucose-6-phosphate (G6P) plus fructose-6-phosphate (F6P) before the uniformly labeled glucose or glutamine was added (zero time point). B, The level of fructose-1,6-bisphosphate (FBP) before the uniformly labeled glucose or glutamine was added (zero time point). C and D, Upon adding the labeled glucose or glutamine to the culture media, the metabolites were extracted and analyzed by LC-MS/MS spectroscopy. Fractions of different isotopomers of U-13C glucose-6-phosphate plus U-13C fructose-6-phosphate or fructose-biphosphate are shown within the bars. AUC indicates “area under the curve”.
3.5. Suppression of PC lowers malate and citrate
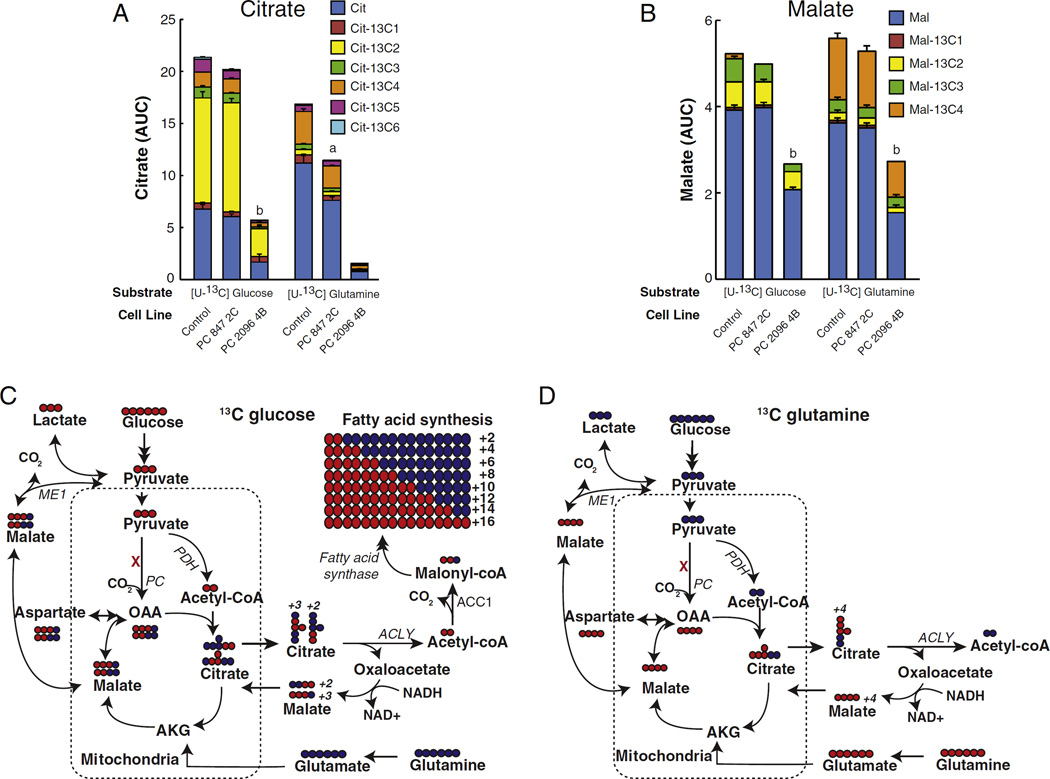
The immediate product of the PC reaction is oxaloacetate which is then directly converted to malate and citrate (Fig. 4). Oxaloacetate is very unstable and its concentration in most tissues is so low (about 5 µM) that it is impractical to accurately measure its concentration. Therefore, the levels of 13C incorporation into the immediate metabolites of oxaloacetate, which are citrate and malate, were measured. In the mass spectrometry experiments using either LC-MS/MS or GCMS, metabolite levels were measured in the cell lines immediately before (zero time control) and then 60 min after 10 mM [U-13C]glucose or 2 mM [U-13C]glutamine were added to the cells. Strong suppression of PC expression (cell line PC 2096 4B) markedly decreased the levels of both citrate and malate and decreased the incorporation of carbon from glucose and glutamine into citrate and malate (Fig. 5). Citrate was mainly +2 labeled with 13C6-glucose, suggesting that pyruvate de-hydrogenase supplied acetyl-CoA that was incorporated into citrate in the citrate synthase reaction. In contrast, malate was about equally +2 labeled and +3 labeled from 13C6-glucose. This indicates that the +3 labeled malate came from oxaloacetate formed in the PC reaction and the +2 labeled malate came from the citric acid cycle after the pyruvate dehydrogenase reaction produced +2 labeled acetyl-CoA that was incorporated into citrate that then became +2 labeled malate after flux through the citrate-pyruvate cycle or through the citric acid cycle. 13C5 labeled glutamine produced mostly +4 labeled citrate and malate that entered mitochondrial metabolism through α-ketoglutarate derived from glutamate in the glutamate dehydrogenase reaction. The decreased 13C incorporation into malate and citrate from glucose and glutamine in the PC 2096 4B cell line can be explained by PC knockdown inhibiting pyruvate flux through the citrate-pyruvate and the malate-pyruvate cycles.
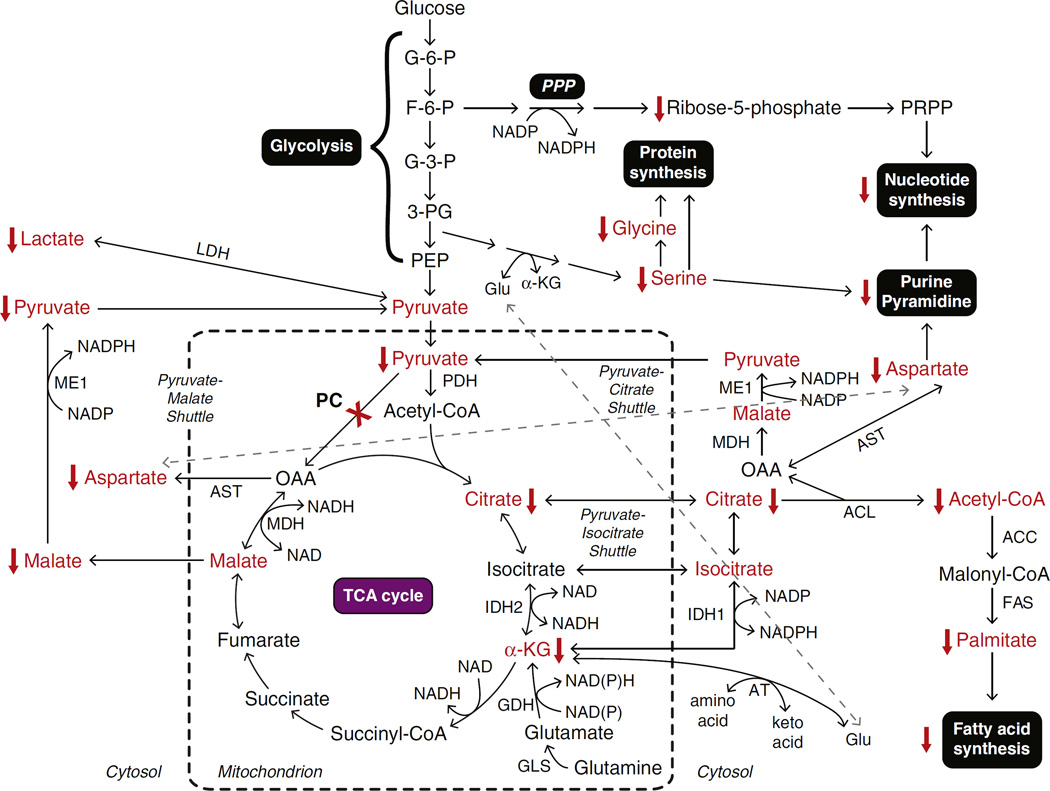
Fig. 4.
Schematic summary of metabolic pathways disturbance caused by suppression of pyruvate carboxylase in MDA-MB-231-derived cell lines. The abbreviations used are: ACC, acetyl-CoA carboxylase; α-KG, α-ketoglutarate; AT, aminotransferase; ACL, ATP-citrate lyase; AST, aspartate aminotransferase; F-6-P, fructose-6-phosphate; FAS, fatty acid synthase; G-3-P, glyceraldehyde-3-phosphate; G-6-P, glucose-6-phosphate; GDH, glutamate dehydrogenase; GLS, glutaminase; Glu, glutamate; IDH1, isocitrate dehydrogenase 1; IDH2, isocitrate dehydrogenase 2; LDH, lactate dehydrogenase; MDH, malate dehydrogenase; ME, cytosolic malic enzyme; 3-PG, 3-bisphosphoglycerate; PC, pyruvate carboxylase; PDH, pyruvate dehydrogenase; PEP, phosphoenolpyruvate; PPP, pentose phosphate pathway; PRPP, phosphoribosylpyrophosphate. Metabolites that are altered in the knockdown cell lines are shown in red.
Fig. 5.
Decreased labeled citrate and malate from U-13C6-glucose or U-13C5-glutamine in pyruvate carboxylase (PC) knockdown cell lines derived from the MDA-MB-231 breast cancer cell line. PC847 2C and PC 20964B cell linesor the scramble control cell line were maintained in the presence of U-13C6 glucose or U-13C5 glutamine for 1 h in the same experiment described in Fig. 3. Fractions of different isotopomers of 13C citrate (A) and 13C malate (B) in the PC knockdown cell lines and scramble control cell line are shown within the same bars. ap <0.01, bp < 0.001 vs scramble control. Metabolic pathways showing incorporation and distribution of labeled carbon from 13C6-glucose (C) or 13C5-glutamine (D) to various downstream metabolites. Labeled glucose and glutamine are indicated in red while unlabeled glucose and glutamine are indicated in blue. Abbreviations: ACC1, acetyl-CoA carboxylase1; α-KG, α-ketoglutarate; ACLY, ATP-citrate lyase; PC, pyruvate carboxylase; PDH, pyruvate dehydrogenase.
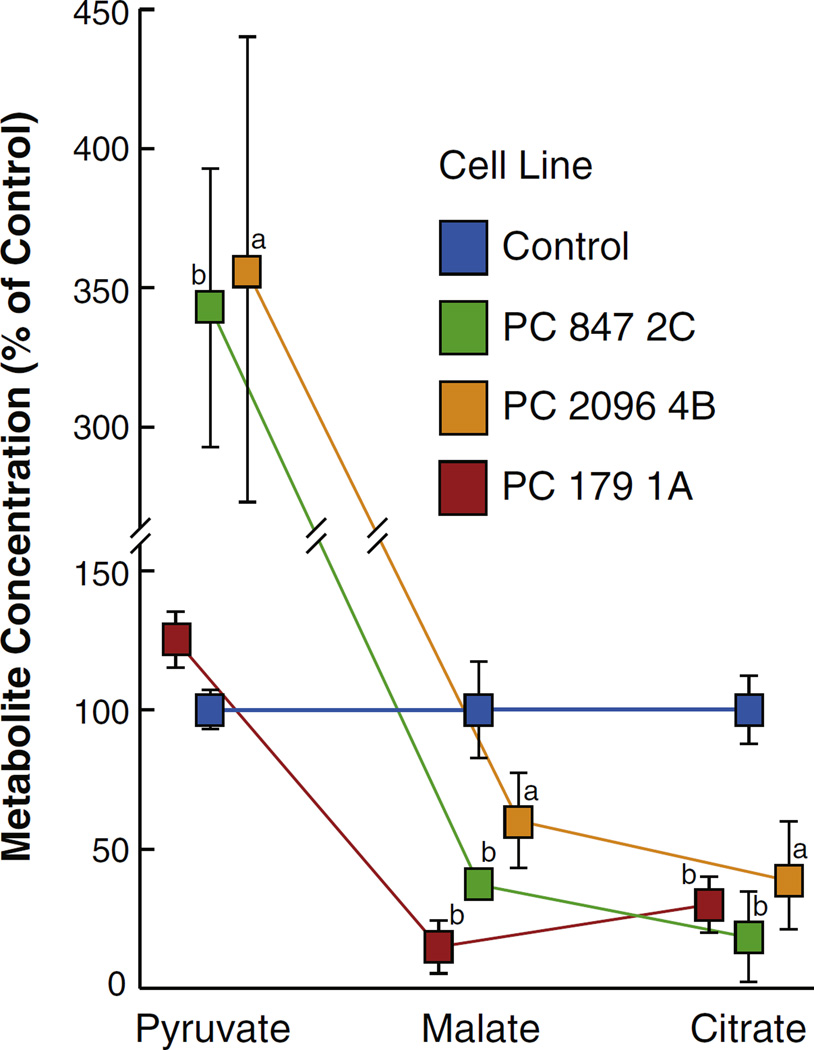
To confirm the mass spectrometry measurements the levels of malate and citrate were measured by alkali-enhanced fluorescence in cell lines maintained in RPMI 1640 tissue culture medium (usually contains 11.1 mM glucose and 2mM glutamine) modified to contain a physiologically normal concentration of glucose (5 mM) (and still 2 mM glutamine) for one day followed by a brief starvation period in the presence of no fuel and then a 35 min incubation period in Krebs Ringer bicarbonate buffer solution containing 10 mM glucose. Similarly to the mass spectrometry measurements of malate and citrate in the cell lines maintained in DMEM cell culture medium the cell lines with knocked down PC maintained in the modified RPMI 1640 medium followed by the Krebs Ringer solution, the PC knockdown cell lines PC 847 2C, PC 2096 4B and PC 179 1A, showed decreased levels of malate and citrate compared to the control cell line containing a scrambled shRNA (Fig. 6).
Fig. 6.
An increase in pyruvate and decreases in malate and citrate in pyruvate carboxylase (PC) knockdown MDA-MB-231 cells show a cross over point at the PC consistent with inhibition of the PC reaction. PC 847 2C, PC 2096 4B and PC179 1A cell lines and the scramble shRNA control cell line were maintained in RPMI 1640 medium containing 5 mM glucose for 22 h and then in Krebs Ringer bicarbonate solution containing 10 mM glucose for 35 min before the concentrations of metabolites were measured as described under Experimental Procedures. The concentrations of each metabolite in the three PC knockdown cell lines are shown relative to those of the scramble shRNA control cell line were measured by alkali enhanced fluorescence. ap < 0.05, bp < 0.01 vs scramble control.
3.6. Effects of suppression of PC on levelsof pyruvate, lactate and acetyl-CoA
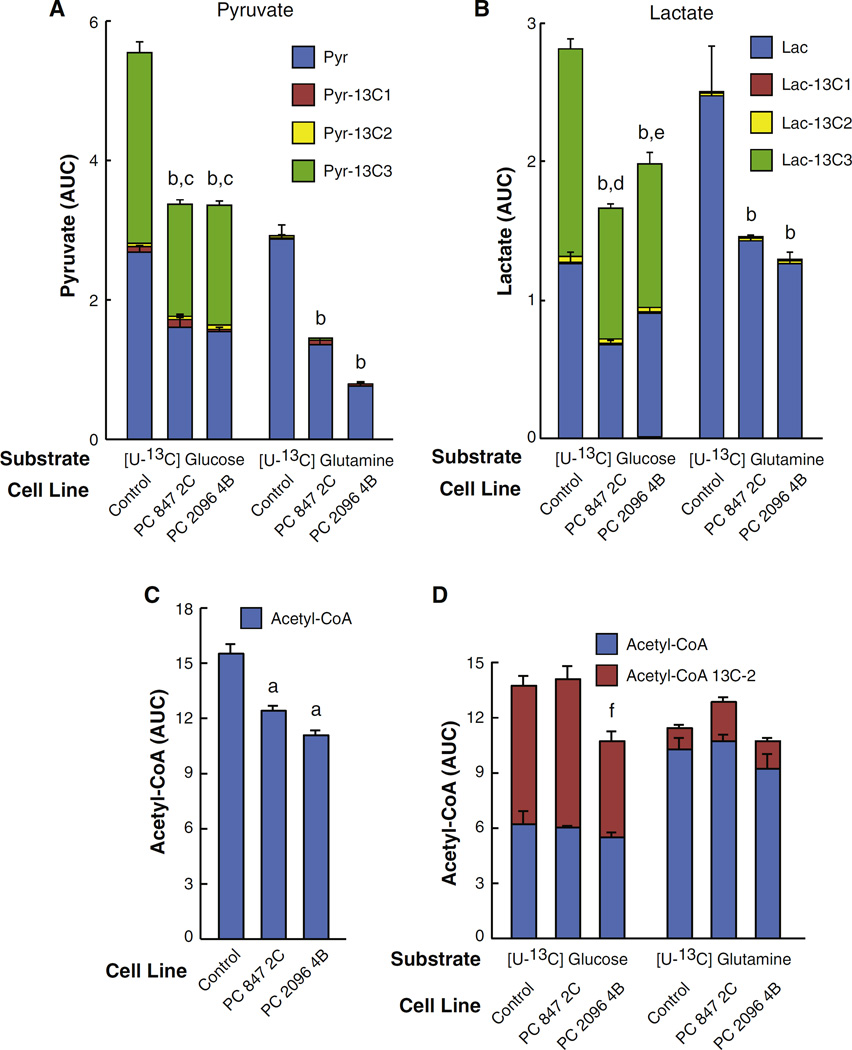
The mass spectrometry measurements showed that pyruvate was mostly labeled from glucose rather than from glutamine (Fig. 7A). Suppression of PC caused a marked reduction in the level of glucose-derived pyruvate in the mass spectrometry experiments in which the cells were cultured in DMEM for four or more days prior to the experiment (see Fig. 7A). Since pyruvate is the substrate for the PC reaction, it might be expected that suppression of PC expression would result in the accumulation of pyruvate, which is the substrate of PC, as we previously observed in pancreatic beta cells with knocked down PC [12]. The lower levels of pyruvate seen in the PC knockdown cell lines were likely due to decreased malate and citrate cycling to pyruvate because the levels of these two metabolites were markedly decreased by PC knockdown (Fig. 5). As shown in Fig. 4, malate can exit mitochondria and be converted back to pyruvate by malic enzyme in the cytosol. Pyruvate can then re-enter mitochondria and be reconverted to oxaloacetate by PC (the pyruvate-malate shuttle) [17,18]. Alternatively in the pyruvate-citrate shuttle, citrate can exit mitochondria and be converted to oxaloacetate and acetyl-CoA by ATP-citrate lyase [18,19]. Oxaloacetate can then be converted to malate by cytosolic malate dehydrogenase. Malic enzyme can then convert malate to pyruvate the same as in the pyruvate-malate cycle. The malate-pyruvate and citrate-pyruvate cycles are very active in pancreatic beta cells where PC protein and enzyme activity are high and support these cycles [17,18]. The low pyruvate also explains the low level of lactate (Fig. 7B), which is the redox partner of pyruvate, in these cells. Because glycolysis was not inhibited (Fig. 3), the decreased 13C6-glucose incorporation into pyruvate and lac-tate in the two PC knockdown cell lines (Fig. 7A and B) can only be explained by a decreased activity of pyruvate cycling due to decreased PC enzyme activity.
Fig. 7.
Decreased labeled pyruvate, lactate and acetyl-CoA from U-13C6-glucose or U-13C5-glutamine in pyruvate carboxylase (PC) knockdown cell lines derived from the MDA-MB 231 breast cancer cell line. PC 847 2C and PC 2096 4B cell lines or scramble control cell line were maintained in the presence of U-13C6 glucose or U-13C5 glutamine for 1 h in the same experiment described in Fig. 3. Fractions of different isotopomers of 13C-pyruvate (A), 13C–lactate (B) or 13C-acetyl-CoA (D) in the PC knockdown cell lines and the scramble control cell line are shown within the same bars. C, The levels of acetyl-CoA in cells before labeled glucose or glutamine was added to the culture media. ap < 0.01; bp < 0.001 total metabolite vs scramble control same substrate; cp < 0.01 Pyr-13C3 vs scramble control; dp < 0.001 or ep < 0.01 Lac-13C3 vs scramble control; fp < 0.05 acetyl-CoA-13C2 vs scramble control.
The concentration of glucose in the cell culture medium can have a strong influence on the concentration of pyruvate in the cells. As mentioned above, for LC-MS or GC-MS analysis the cells were maintained for four or more days in DMEM cell culture medium right up to the time of the experiment when the cells were maintained in DMEM medium modified to contain 10 mM glucose and 2 mM glutamine for 60 min. DMEM contains a high concentration of glucose (25 mM glucose) and glutamine (4 mM) which are much higher concentrations of these fuels than in the RPMI 1640 cell culture medium that was modified to contain 5 mM glucose (and contains 2 mM glutamine) that the cells were maintained in for 24 h before the cells were incubated in Krebs Ringer bicarbonate solution for the experiments in which malate, citrate and pyruvate were measured by alkali enhanced fluorescence. Because the concentration of glucose was so high in the DMEM medium, it was expected that this could cause a higher level of pyruvate in both the control and PC knockdown cell lines making it difficult to see a crossover point with an expected even higher level of pyruvate in the PC knockdown cell lines. Therefore the incubation conditions of the experiment shown in Fig. 6 were made identical to those of a previous experiment with pancreatic beta cells in which the cell lines were incubated in the presence of the physiological concentration of glucose and glucose-starved for a short time period enabling us to observe an increase in pyruvate after glucose (10 mM) was added to the cells for 30 min [12]. Similarly to experiments in which pure beta cells with knocked down PC were maintained at a physiological concentration of glucose prior to a 35 min incubation with 10 mM glucose, an increase in pyruvate was observed along with the decreases in malate and citrate in all three PC knockdown cell lines shown in Fig. 6. This crossover point with high pyruvate and low malate and low citrate [12] is consistent with a block at the PC reaction [17,18]. The levels of malate and citrate were much lower in cell line PC 179 1A than in the other two PC knockdown cell lines shown in Fig. 6, and the level of pyruvate was increased less than in the other two cell lines in this cell line (only 24% higher than in the control cell line, as compared to 350% higher in the other two PC knockdown cell lines (Fig. 6)). Cell line PC 179 1A also showed a 50% lower level of malic enzyme (as later shown in Fig. 12) that, in addition to the extremely low levels of the substrates malate and citrate could contribute to decreased pyruvate cycling.
Fig. 12.
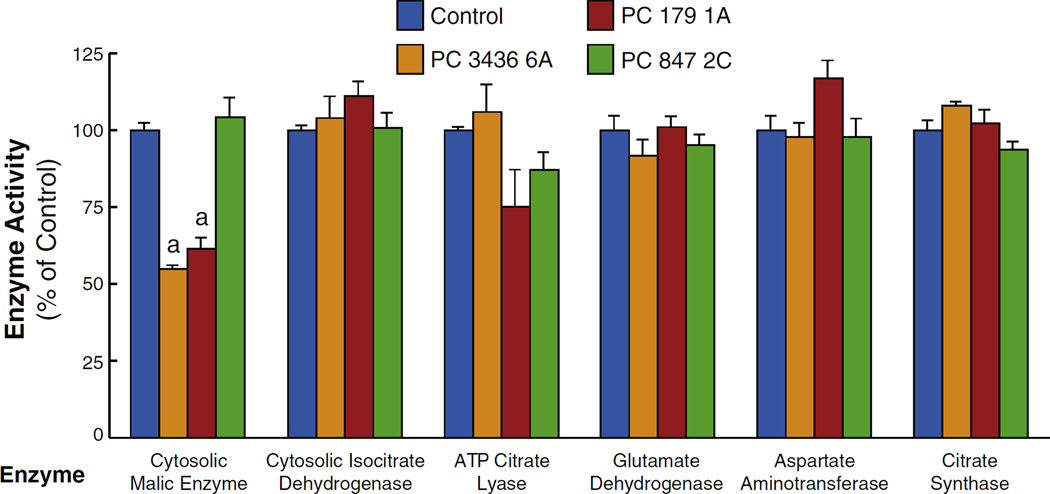
Malic enzyme is the only enzyme out of several anaplerotic/cataplerotic pyruvate cycling enzymes that is decreased in breast cancer cell lines with severely knocked down pyruvate carboxylase (PC). Cell lines PC 3436 6A and PC 179 1A possess severely lower levels of pyruvate carboxylase enzyme activity and cell line and PC 847 2C possesses moderately decreased PC activity compared to the scrambled shRNA control cell line. These cell lines show low cell proliferation rates compared to the control cell line (Figs. 1 and 2). The lower level malic enzyme in two cell lines with severely knocked down PC is consistent with lower pyruvate cycling and lower malate, citrate and pyruvate levels (Figs. 5–8) in these cell lines (means ± SE, ap < 0.01 vs scramble control). Only malic enzyme showed decreased activity vs the control cell line.
The decreased levels of acetyl-CoA (Fig. 7C) and the decreased labeling of acetyl-CoA from glucose (Fig. 7D) in the PC 2096 4B cell line are also likely due to the decreased citrate levels. Most of the +2 labeled acetyl-CoA would be expected to come from the pyruvate dehydrogenase reaction. However, there was slightly decreased +2 labeling of acetyl-CoA from glucose and from glutamine in the PC 2096 4B cell line (Fig. 7D) and this can only be explained by the decreased PC enzyme activity inhibiting flux of citrate through the citrate-pyruvate cycle. The lower concentrations of total cellular acetyl-CoA (Fig. 7C) and lower 13C incorporation into acetyl-CoA (Fig. 7D) in the PC knockdown cells are consistent with a lower cytosolic level of acetyl-CoA resulting from decreased formation of mitochondrial citrate and its export to the cytosol via the pathway that uses ATP citrate lyase shown in Fig. 4.
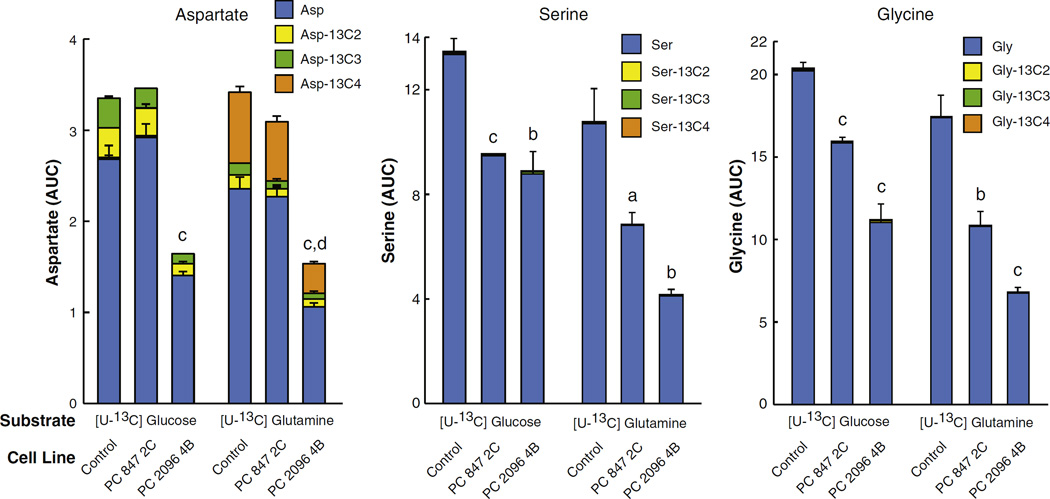
3.7. Suppression of PC lowers aspartate, glycine and serine
Aspartate was labeled from both glucose and glutamine, albeit slightly more from glutamine, indicating that both nutrients contribute to aspartate synthesis (Fig. 8). Aspartate is produced directly from oxaloacetate catalyzed by aspartate aminotransferase. Suppression of PC expression should lower oxaloacetate, resulting in a lowered level of aspartate. As expected, the decrease of +2 and +3 aspartate from glucose and +4 labeled aspartate from glutamine in the PC 2096 4B cell line are consistent with a depleted oxaloacetate level caused by PC suppression in the case of labeling from glucose and decreased activity of the citrate-pyruvate cycle in the case of labeling from glutamine (Fig. 8). Both PC 847 2C and PC 2096 4B knockdown cell lines showed decreased levels of serine and glycine (Fig. 8). The negligible or absent incorporation of 13C from glucose and glutamine into both amino acids is due to the short incubation time of the cells in the presence of the labeled glucose or glutamine and indicates that the levels of these two amino acids was low before two cells were incubated in the presence of the labeled fuels.
Fig. 8.
Decreased labeled aspartate, glycine and serine from U-13C6-glucose or U-13C5-glutamine in pyruvate carboxylase (PC) knockdown cell lines derived from MDA-MB 231 breast cancer cell line. PC 847 2C and PC 2096 4B cell lines or the shRNA scramble control cell line were maintained in the presence of U-13C6-glucose or U-13C5-glutamine for 1 h in the same experiment described in Fig. 3. Fractions of different isotopomers of 13C aspartate, 13C-glycine and 13C-serine between the PC knockdown cell lines and scramble control cell lines are shown within the same bars. ap < 0.05, bp < 0.01 and cp < 0.001 total metabolite level vs scramble control. dp < 0.001 vs Asp-13C4.
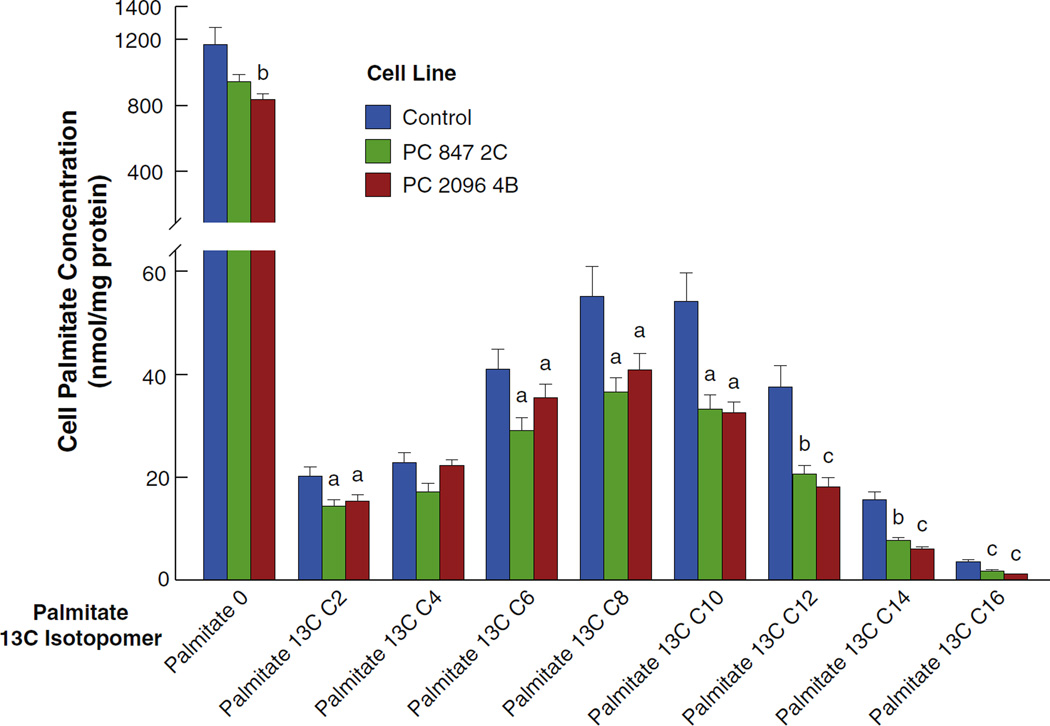
3.8. Decreased 13C-glucose incorporation into palmitate in PC knockdown cell lines
Fatty acids act as a sink for carbon flowing from mitochondrial citrate and then through cytosolic acetyl-CoA and malonyl-CoA (Fig. 4). Thus, the 13C incorporation from glucose into palmitate is an indication of the rate of flux of glucose through biosynthetic pathways. Cell lines were incubated in the presence of the 13C-labeled glucose for 18 h. Fig. 9 shows that suppression of PC decreased the incorporation of glucose carbon into palmitate. This is consistent with PC suppression inhibiting the mitochondrial synthesis of citrate and consequently the export of citrate to the cytosol thus lowering the supply of cytosolic acetyl-CoA and malonyl-CoA needed for fatty acid synthesis as depicted in Fig. 4.
Fig. 9.
Decreased U-13C6-glucose incorporation into palmitate in pyruvate carboxylase (PC) knockdown cell lines derived from the MDA-MB-231 breast cancer cell line. PC 847 2C and PC 2096 4B cell lines or the shRNA scramble control cell line were maintained in DMEM for 24 h or longer without labeled glucose of glutamine (the same as the zero time control shown in Fig. 3A and B). Cells were then maintained for 18 h in DMEM modified to contain 10 mM [U-13C6]glucose and 13C incorporation into palmitate was measured by LC-MS/MS. ap < 0.05, bp < 0.01 and cp < 0.001 vs scramble control.
3.9. PC knockdown lowers various metabolites including nucleotides
Table 3 and the heat map in Fig. 10 show several selected metabolites that were altered in the strong knockdown cell line PC 2096 4B or both PC knockdown cell lines. In addition to the metabolites discussed above moderate or strong suppression of PC resulted in the decreased levels of α-ketoglutarate, ADP-glucose, GDP-fucose and GDP-mannose while decreases in the levels of ribose-5-phosphate, CTP, hypoxanthine, UDP and GDP were observed only in the strong PC suppression cell line PC 2096 4B (Fig. 10 and Table 3).
Table 3.
Selected metabolites that are altered in pyruvate carboxylase (PC) knockdown MDA-MB-231 cell lines.
| Metabolite | KD cell line PC 847 2C |
KD cell line PC 2096 4B |
||
|---|---|---|---|---|
| Fold change | P-value | Fold change | P-value | |
| Ribose-5-phosphate | 0.98 | NS | 0.84 | P < 0.05 |
| α-ketoglutarate | 0.70 | P < 0.05 | 0.72 | P < 0.05 |
| ADP-glucose | 0.85 | P < 0.05 | 0.80 | P < 0.01 |
| CTP | 0.9 | NS | 0.70 | P < 0.01 |
| Hypoxanthine | 1.01 | NS | 0.84 | P < 0.05 |
| UDP | 0.78 | NS | 0.70 | P < 0.05 |
| GDP | 0.85 | NS | 0.76 | P < 0.05 |
| GDP-fucose | 0.84 | P < 0.05 | 0.76 | P < 0.005 |
| GDP-mannose | 0.84 | P < 0.05 | 0.45 | P < 0.005 |
Fig. 10.
Heat map showing global lowering of metabolites in pyruvate carboxylase (PC) knockdown MDA-MB 231 breast cancer cell lines. Metabolites were measured by LC-MS in the same experiments shown in Fig. 3 at the zero time point, i.e. before a 13C labeled substrate was added. Metabolite levels are coded by m/z. The asterisk indicates different from the scramble control cell line with p < 0.05.
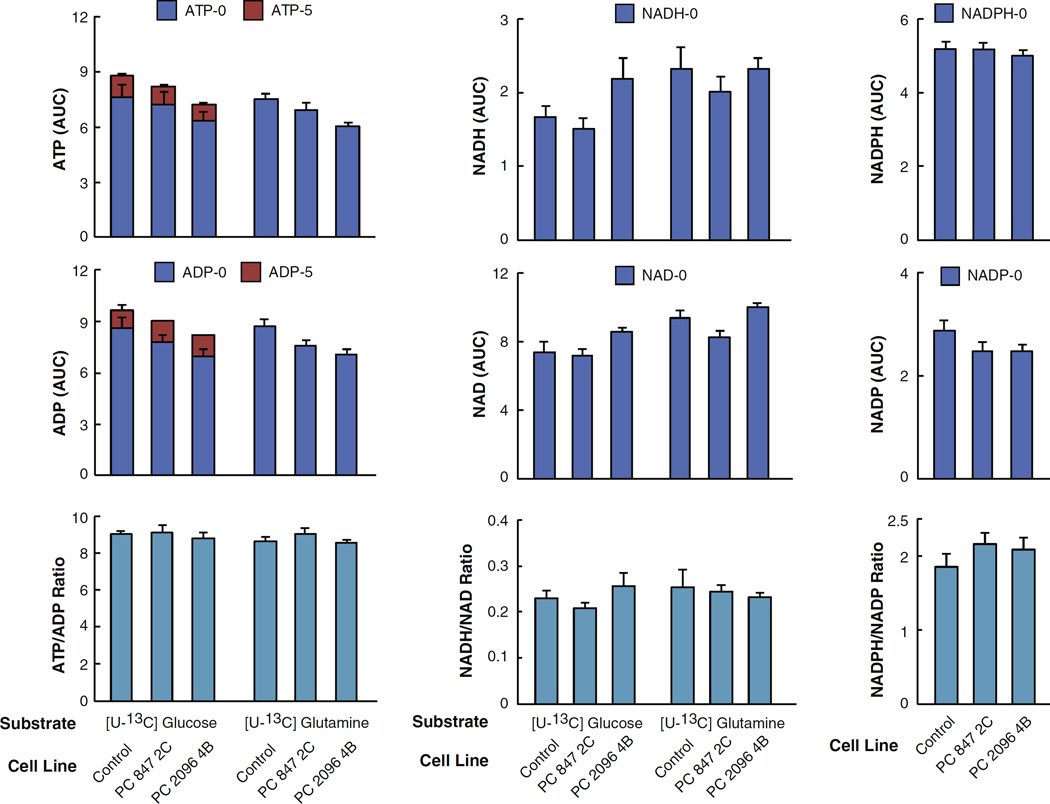
Suppression of PC caused no or a very slight and insignificant reduction of unlabeled and labeled ATP and ADP from glucose (Fig. 11, left panel) such that the ATP/ADP ratio was unaltered by PC knockdown. This indicates that the energy charge of the cell was not impaired by PC knockdown. The slightly decreased levels of ADP and ATP in the PC 2096 4B cell line may be linked to the lowered levels of ribose-5-phosphate (see Table 3 and Fig. 10), which supplies the ribose moiety of these nucleotides and/or decreased aspartate (Fig. 8) which contributes to nitrogen-donation in purine ring synthesis. The levels of NADH and NAD+ including NADH/NAD+ ratios were not different between the knockdown cells and the scramble control (Fig. 11) also indicating that the energy charge of the cell was not affected by PC not down.
Fig 11.
Normal ATP and ADP concentrations, ATP/ADP ratio, NAD(P)H and NAD(P) concentrations and NAD(P)H/NAD(P) ratios in pyruvate carboxylase (PC) knockdown cell lines derived from the MDA-MB-231 breast cancer cell line supplied with U-13C6-glucose or U-13C5-glutamine. PC 847 2C and PC 2096 4B cell lines or the shRNA scramble control cell line were maintained in the presence of U-13C6 glucose or U-13C5 glutamine for 1 h in the same experiment described in Fig. 3. Fractions of different isotopomers of 13C-ATP and 13C-ADP and levels of the pyridine nucleotides NAD(P), NAD(P)H (which showed no incorporation of 13C) between the PC knockdown cell lines and scramble control group are shown within the same bars. ap < 0.01 vs total metabolite same substrate control.
3.10. Enzyme levels in PC knockdown cell lines
We measured the activities of several mitochondrial and cytosolic enzymes that catalyze reactions of anaplerosis/cataplerosis including pyruvate cycling or might influence the levels of the metabolites measured in our study. As shown in Fig. 4, these included cytosolic malic enzyme, NADP-isocitrate dehydrogenase, ATP-citrate lyase, glutamate dehydrogenase, aspartate aminotransferase and citrate synthase. The activity of only one enzyme in the PC knockdown cells was significantly different from that of the scrambled shRNA control cell line. This was malic enzyme and in two of the three cell lines with very low PC malic enzyme activity was about 50% lower than that of the control cell line (Fig. 12). Malic enzyme activity was not lower in the PC cell line with moderate knockdown of PC, PC 847 2C (Fig. 12). The shRNA nucleotide sequences used to target the PC mRNA were not similar to any of the nucleotide sequence of cytosolic malic enzyme mRNA. Therefore, the lower malic enzyme activities were probably a response to the effects of lower PC enzyme activity. The 50% lower activity of malic enzyme in the cell lines with severe knockdown of PC might contribute to the lower pyruvate levels seen in these cell lines (Fig. 5) due to lower pyruvate cycling from malate and citrate. The enzyme activities were measured in the cell lines after they had been frozen for several months and then re-plated. Unfortunately the cell line PC 2096 4B with one of the lowest levels of PC and that was used for mass spectrometry studies would not grow after having been stored frozen so that the enzyme activities could not be measured in this cell line.
4. Discussion
4.1. The role of PC in cancer cell proliferation
Pyruvate carboxylation is an important anaplerotic reaction that replenishes citric acid cycle intermediates when they intermediates are removed from mitochondria by their export to the cytosol (cataplerosis). Anaplerosis and cataplerosis are necessary for gluconeogenesis in liver and kidney, lipogenesis in liver and adipose tissue, glutamate synthesis in astrocytes and glucose-induced insulin secretion in pancreatic beta cells [5,18–21]. We and others have shown that PC mRNA and PC protein are up-regulated in many cancers [7–10].
In the current study we generated multiple stable PC knockdown MDA-MB-231-derived cell lines by shRNA with various levels of PC knockdown and investigated the biochemical changes associated with the defects in growth and motility phenotypic defects of the PC knockdown cells. The various degrees of decreases in PC mRNA and PC enzyme activity were correlated with the decreased cell proliferation rates among the cell lines (Fig. 2). This is a confirmation of the relationship between PC enzyme levels and proliferation rates in breast cancer cells. This retarded proliferation phenotype of the PC knockdown MDA-MB-231 cell lines was also similar to that of the transient knockdown of PC in the MDA-MB-231 cell line that we reported previously [10]. A dose-dependent suppression effect on metabolism and inhibition of insulin secretion were also observed in the rat insulinoma cell line INS-1 832/13 with different degrees of PC suppression [12]. A similar disturbance of common metabolites was observed in both moderate and strong PC suppression MDA-MB-231-derived cells as shown in Table 3 and Figs. 5 and 6.
4.2. Knockdown of PC inhibits anaplerosis
The central metabolic pathway affected by the suppression of PC appears to lie within the anaplerosis. The product of the PC reaction is oxaloacetate, which immediately condenses with acetyl-CoA to produce citrate or is converted to malate in the mitochondrial malate dehydrogenase reaction. Suppression of PC resulted in the depletions of malate and citrate levels as expected (Figs. 5 and 6). Suppression of PC decreased the levels of total citrate and malate and 13C incorporation into their isotopomers from 13C6-labeled glucose and 13C5-labeled glutamine (Fig. 5). This indicates that PC knockdown inhibits anaplerosis from both pyruvate carboxylation and glutaminolysis.
4.3. PC knockdown lowers pyruvate cycling
In contrast to suppression of PC expression in pancreatic beta cells, in which the knockdown cells showed a metabolic crossover point with increased levels of pyruvate and low levels of malate and citrate [12], suppression of PC in MDA-MB-231 cell lines showed lowered levels of pyruvate and lactate when cells were maintained in the presence of a high concentration of glucose (25 mM) (in DMEM cell culture medium) prior to the experiment (Fig. 7). Similar to experiments with pancreatic beta cells [12], when the PC knockdown cells were maintained in a cell culture medium containing a lower and physiologic concentration of glucose (5 mM) before glucose was added as in the experiments with beta cells, pyruvate was increased and malate and citrate were decreased indicating a crossover point and a block at the PC reaction (Fig. 6). The changes in the levels of pyruvate, lactate, malate and citrate raise the possibility of certain pathway(s) in which these three metabolites are connected. In pancreatic beta cells, there is a cycling of pyruvate into mitochondria known as the pyruvate-malate shuttle [17,18], as well as in the pyruvate-citrate cycle [18,19]. In the pyruvate-malate shuttle PC converts pyruvate to oxaloacetate followed by the conversion of oxaloacetate to malate by mitochondrial malate dehydrogenase, enabling malate to exit mitochondria. In the cytosol malate is in turn converted back to pyruvate by cytosolic malic enzyme, allowing pyruvate to re-enter mitochondria for carboxylation again by PC (Fig. 4). In the citrate-pyruvate shuttle citrate is exported from the mitochondria and cleaved to oxaloacetate in the ATP citrate lyase reaction. The oxaloacetate is then reduced to malate catalyzed by cytosolic malate dehydrogenase. The resulting malate then participates in the pyruvate-malate shuttle as shown in Fig. 4. These cycles provide cytosolic NADPH, a coupling factor, required for glucose-induced insulin secretion in pancreatic beta cells [17,21] and lipid synthesis in many different tissues. In addition, the acetyl-CoA derived from the ATP citrate lyase reaction can be converted to malonyl-CoA and utilized for lipid synthesis in the cytosol. The higher levels of PC mRNA and PC protein [10] and PC enzyme activity (Table 2) in the highly metastatic MDA-MB-231 cell line than in the low metastatic MCF-7 cell line suggest that similar to pyruvate cycling operative in pancreatic beta cells, pyruvate cycling is important for cell invasiveness or metastasis in breast cancer. In the PC knockdown MDA-MB-231 cells, the depleted levels of malate and citrate caused by the chronic suppression of PC may slow down the overall pyruvate cycling rate, resulting in the low level of pyruvate (Fig. 7).
Interestingly, the level of cytosolic malic enzyme was 50% lower in two of the three cell lines with severe knockdown of PC studied. The shRNA nucleotide sequence used to target the PC mRNA was not similar to any of the nucleotide sequence of the malic enzyme mRNA indicating the lower level of the malic enzyme was not due to an off target effect of PC targeting. Therefore, the lower malic enzyme level was probably a down-regulatory response of the cell to a lower level of the substrate malate for reasons that are not exactly clear. In any case, the 50% lower level of malic enzyme is probably enough lower to contribute to decreased conversion of malate to pyruvate and thus a decrease in pyruvate cycling.
4.4. Generalized effects of PC knockdown on metabolite precursors needed for cell structure and energy production
Since citrate and malate are the two major citric acid cycle intermediates that are capable of exiting mitochondria for the biosynthesis of lipids, nucleic acids and certain amino acids, the reduction in the cellular levels of nucleotides (Table 3 and Fig. 11), aspartate, glycine, serine (Fig. 8) and lower glucose carbon incorporation into palmitate (Fig. 9) are consistent with the anaplerotic/cataplerotic role of PC in growth of breast cancer cells.
Transamination of oxaloacetate with glutamate catalyzed by aspartate aminotransferase would produce aspartate. Therefore, it is likely that the lowered level of oxaloacetate caused by suppression of PC may be responsible for the low level of aspartate (Fig. 8). A lowered level of aspartate was also reported in the PC knockdown non-small cell lung cancer (NSCLC), which showed a marked decrease in the cellular aspartate level [9]. In renal adenocarcinoma and paraganglioma cancers harboring loss of function mutations of succinate dehydrogenase, PC was found essential to support cancer proliferation [22,23]. Suppression of PC slows down proliferation of these cancers, concomitant with reduced cellular levels of aspartate. Supplementation of the knockdown cancer cells with aspartate rescued this slow growth phenotype. As aspartate is the structural component of several biomolecules including the backbone of purine and pyrimidine rings in nucleic acids, the decreased levels of nucleotides (Fig. 10 and Table 3) and as-partate observed in the PC knockdown MDA-MB-231 cells can potentially slow the synthesis of these nucleotides, contributing to the low rates of cell proliferation. As the ribose-5-phosphate is also the backbone of nucleotides, the lowered level of total ribose-5-phosphate in the knockdown cells as shown in Table 3 and may also contribute to the decreased levels of some nucleotides and their derivatives i.e. hypoxanthine, ADP-glucose, UDP, GDP, GDP-mannose and GDP-fucose. Similar to our study, the PC knockdown non-small cell lung cancer (NSCLC) also showed reduction of glucose- and glutamine- derived CTP and UTP levels [9].
The marked reduction of serine and glycine levels in the PC knockdown cells may underlie the retarded growth phenotype of the knockdown cells because serine contributes to various biosynthetic pathways including protein synthesis, phospholipids and nucleotides which are in high demand during tumorigenesis [24].
The perturbation of serine biosynthesis may occur during the conversion of 3-phosphoglycerate to serine. The conversion of 3-phosphoglycerate to serine is mediated through three sequential reactions (see Fig. 4). The first reaction is the conversion of 3-phosphoglycerate to 3-phosphohydroxypyruvate by phosphoglycerate dehydro genase followed by further conversion to 3-phosphoserine by phosphoserine aminotransferase before the final conversion to serine by protein serine phosphatase. Then phosphoserine aminotransferase catalyzes the transfer of the amino group from glutamate to 3-phosphohydroxypyruvate. Because glutamate is produced from α-ketoglutarate via a transamination reaction, the lowered level of α-ketoglutarate in the PC-knockdown MDA-MB-231 cells (Fig. 10) may in turn lower the rate of glutamate formation which consequently affects the transamination reaction catalyzed by phosphoserine aminotransferase. Interestingly, up-regulation of serine and glycine biosynthesis caused by amplification of phosphoglycerate dehydrogenase gene copy number was also reported to contribute to oncogenesis in melanoma and breast cancer [24,25]. Suppression of phosphoglycerate dehydrogenase expression in several breast cancer cell lines including MDA-MB-231 cells, lowered serine biosynthesis concomitant with a decreased cell proliferation rate, indicating the crucial role of serine in supporting breast cancer growth. Suppression of phosphoglycerate dehydrogenase expression in invasive breast cancer cells also reduces the level of α-ketoglutarate which is produced from the transamination of glutamate [26]. These findings mirror the PC knockdown MDA-MB-231 cells that showed lowered levels of α-ketoglutarate and serine in the current study.
Lowered levels of total cellular acetyl-CoA in the PC knockdown cell lines can most likely be attributed to decreased export of citrate from mitochondria causing decreased citrate available for conversation to oxaloacetate and acetyl-CoA catalyzed by ATP citrate lyase in the cytosol. The latter route for acetyl-CoA regeneration also forms part of alternate route of pyruvate cycling: the well-known pyruvate/citrate cycle [4,18, 19] (Fig. 4).
In addition to the lowered levels of certain mitochondrial metabolites, pyruvate cycling and nucleotide synthesis, we also found that suppression of PC causes a reduction of glucose incorporation into palmitate (Fig. 9), suggesting that inhibition of anaplerosis caused by PC knockdown results in lower fatty acid synthesis which restricts membrane biogenesis of the newly dividing cells.
Suppression of PC only slightly and insignificantly lowered the concentrations of ATP and ADP without affecting the ATP/ADP ratio and NAD(P)(H) concentrations (Fig. 11) indicating suppression of PC did not seriously affect the cell energy charge. The decreases in other nucleotides and nucleotide derivatives (Table 3 and Fig. 11) can be attributed to decreased nucleotide biosynthesis via the reduced level of ribose-5-phosphate which is a backbone nucleotide derivatives and decreased aspartate which is a precursor for purine and pyrimidine synthesis.
Since we studied the MDA-MB-231 cell line which is triple negative for estrogen receptor (ER), progesterone receptor (PR) and epidermal growth factor receptor 2 (HER2)in our study, one might argue whether overexpression of PC is a specific characteristic of breast cancer cell lines. Using various other breast cancer cell lines expressing these three receptors differently, we found no correlation between the expression of these receptors and PC expression. For example, the two most invasive breast cancer cell lines, MDA-MB-231 and MDA-MB-435, which are ER−/PR−/HER2− and ER−/PR2−/HER2+, respectively, express PC much higher than MCF-7 and SKBR3 cell lines, which are ER+/PR+/HER2− and ER−/PR−/HER2+, respectively (10). Furthermore, unlike MDA-MB-231, MCF-10A, a non-invasive cell line that is also negative for those three receptors was found to possess an extremely low level of PC enzyme activity (data not shown). Most importantly, our clinicopathological investigation of breast cancer patients has also shown that the level of PC expression was not correlated with the status of these receptors in breast cancer tissues of patients but rather shows a positive correlation with tumor size and stages (10). Thus the level of expression of PC appears to be independent of the status of ER, PR and HER2 receptor expression.
5. Conclusion
Fig. 4 summarizes the disturbance of various metabolic pathways resulting in the growth retarded phenotype of PC knockdown MDA-MB-231 cells. In conclusion, suppression of PC expression in MDA-MB-231 breast cancer cells results in the lowered levels of citrate, malate and α-ketoglutarate. The depleted levels of these metabolites perturb mitochondrial cataplerosis for the synthesis of serine, glycine, aspartate and fatty acids which are used as the building blocks for synthesis of proteins, lipids and nucleotides. The lowered levels of citrate and malate also impair pyruvate cycling between mitochondria and cytosol. This global perturbation of biosynthesis contributes to the retarded growth phenotype of the PC knockdown MDA-MB-231 cells. The findings that suppression of PC inhibits cell proliferation in glioblastoma [7], NSCLC [9], renal carcinoma, paraganglioma [22,23] and, in our study, breast cancer (Fig. 2), highlights the crucial role of PC in cancer cell growth and suggests PC may be an attractive drug target of cancer treatment.
Supplementary Material
Acknowledgments
The excellent technical support of Mindy A. Kendrick and Scott W. Stoker is greatly appreciated.
Funding
This work was supported by grant BRG5780007 from the Thailand Research Fund and Mahidol University to S.J. and a gift to M.J.M. from the Nowlin Family Trust of the In Faith Community Foundation. P.P. was supported by the RGJ-PhD scholarship (PHD/0412/2552) from the Thailand Research Fund. K.R. was supported by the Science Achievement Scholarship of Thailand. C.F.B. was supported by the Michigan Regional Comprehensive Metabolomics Resource Core Graft U24 (NIH Grant Number DK097153).
Abbreviations
- PC
pyruvate carboxylase
- MS
mass spectrometry
Footnotes
Author contribution
Phatchariya Phannasil, Israr Ansari, Mahmoud El Azzouny, Khanti Rattanapornsompong, Sarawut Jitrapakdee and Michael MacDonald designed the experiments and interpreted data. Phatchariya Phannasil, Israr Ansari, Mahmoud El Azzouny, Melissa Longacre (Melissa Longacre designed parts of experiments) and Khanti Rattanapornsompong performed the experiments. Phatchariya Phannasil, Sarawut Jitrapakdee and Michael MacDonald wrote the manuscript. Israr Ansari and Mahmoud El Azzouny contributed to the writing of the manuscript. Sarawut Jitrapakdee, Michael MacDonald and Charles Burant contributed reagents, materials and analysis tools. All authors analyzed the results and approved the final version of the manuscript. Michael MacDonald and Sarawut Jitrapakdee contributed equally to this work.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Transparency document
The Transparency document associated with this article can be found, in online version.
References
- 1.Welch HG, Gorski DH, Albertsen PC. Trends in metastatic breast and prostate cancer - lessons in cancer dynamics. N. Engl. J. Med. 2015;373:1685–1687. doi: 10.1056/NEJMp1510443. [DOI] [PubMed] [Google Scholar]
- 2.Lee SY, Jeon HM, Ju MK, Kim CH, Yoon G, Han SI, Park HG, Kang HS. Wnt/ Snail signaling regulates cytochrome C oxidase and glucose metabolism. Cancer Res. 2012;72:3607–3617. doi: 10.1158/0008-5472.CAN-12-0006. [DOI] [PubMed] [Google Scholar]
- 3.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem. 2002;277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 5.Jitrapakdee S, Maurice MSt, Rayment I, Cleland WW, Wallace JC, Attwood PV. Structure, mechanism and regulation of pyruvate carboxylase. Biochem. J. 2008;413:369–387. doi: 10.1042/BJ20080709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreadith RA, Lehninger AL. The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD(P)+-dependent malic enzyme. J. Biol. Chem. 1984;259:6215–6221. [PubMed] [Google Scholar]
- 7.Cheng T, Sudderth J, Yang C, Mullen AR, Jin ES, Matés JM, DeBerardinis RJ. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc. Natl. Acad. Sci. U. S. A. 2011;108:8674–8679. doi: 10.1073/pnas.1016627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan TW, Lane AN, Higashi RM, Farag MA, Gao H, Bousamra M, II, Miller DM. Altered regulation of metabolic pathways in human lung cancer discerned by (13) C stable isotope-resolved metabolomics (SIRM) Mol. Cancer. 2009;8:41. doi: 10.1186/1476-4598-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sellers K, Fox MP, Bousamra M, II, Slone SP, Higashi RM, Miller DM, Lane AN. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J. Clin. Invest. 2015;125:687–698. doi: 10.1172/JCI72873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phannasil P, Thuwajit C, Warnnissorn M, Wallace JC, MacDonald MJ, Jitrapakdee S. Pyruvate carboxylase is up-regulated in breast cancer and essential to support growth and invasion of MDA-MB-231 cells. PLoS One. 2015;10:e0129848. doi: 10.1371/journal.pone.0129848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subik K, Lee J-F, Baxter L, Strzepek T, Costello D, Crowley P, Xing L, Hung M-C, Bonfiglio T, Hicks DG, Tang P. The expression patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by immunohistochemical analysis in breast cancer cell lines. Breast Cancer (Auckl.) 2010;4:35–41. [PMC free article] [PubMed] [Google Scholar]
- 12.Hasan NM, Longacre MJ, Stoker SW, Boonsaen T, Jitrapakdee S, Kendrick MA, MacDonald MJ. Impaired anaplerosis and insulin secretion in insulinoma cells caused by small interfering RNA-mediated suppression of pyruvate carboxylase. J. Biol. Chem. 2008;283:28048–28059. doi: 10.1074/jbc.M804170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorenz MA, Burant CF, Kennedy RT. Reducing time and increasing sensitivity in sample preparation for adherent mammalian cell metabolomics. Anal. Chem. 2011;83:3406–3414. doi: 10.1021/ac103313x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorenz MA, El Azzouny MA, Kennedy RT, Burant CF. Metabolome response to glucose in the β-cell line INS-1 832/13. J. Biol. Chem. 2013;288:10923–10935. doi: 10.1074/jbc.M112.414961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDonald MJ, Longacre MJ, Stoker SW, Kendrick MA, Thonpho A, Brown LJ, Hasan NM, Jitrapakdee S, Fukao T, Hanson MS, Fernandez LA, Odorico J. Differences between human and rodent pancreatic islets: low pyruvate carboxylase, ATP citrate lyase and pyruvate carboxylation; high glucose-stimulated acetoacetate in human pancreatic islets. J. Biol. Chem. 2011;286:18383–18396. doi: 10.1074/jbc.M111.241182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srere PA. In: Citrate Synthase in Methods in Enzymology, Citric Acid Cycle. Lowenstein JM, editor. XIII. New York, NY: Academic Press, Inc; 1969. [Google Scholar]
- 17.MacDonald MJ. Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets. Further implications of cytosolic NADPH in insulin secretion. J. Biol. Chem. 1995;270:20051–20058. [PubMed] [Google Scholar]
- 18.MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2005;288:E1–E15. doi: 10.1152/ajpendo.00218.2004. [DOI] [PubMed] [Google Scholar]
- 19.Farfari S, Schulz V, Corkey B, Prentki M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes. 2000;49:718–726. doi: 10.2337/diabetes.49.5.718. [DOI] [PubMed] [Google Scholar]
- 20.Jitrapakdee S, Slawik M, Medina-Gomez G, Campbell M, Wallace JC, Sethi JK, O’Rahilly S, Vidal-Puig AJ. The peroxisome proliferator-activated receptor-γ regulates murine pyruvate carboxylase gene expression in vivo and in vitro . J. Biol. Chem. 2005;280:27466–27476. doi: 10.1074/jbc.M503836200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jitrapakdee S, Wutthisathapornchai A, Wallace JC, MacDonald MJ. Regulation of insulin secretion: role of mitochondrial signaling. Diabetologia. 2010;53:1019–1032. doi: 10.1007/s00125-010-1685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardaci S, Zheng L, MacKay G, Van Den Broek NJ, MacKenzie ED, Nixon C, Stevenson D, Tumanov S, Bulusu V, Kamphorst JJ, Vazquez A, Fleming S, Schiavi F, Kalna G, Blyth K, Strathdee D, Gottlieb E. Pyruvate carboxylation enables growth of SDH-deficient cells by supporting aspartate biosynthesis. Nat. Cell Biol. 2015;17:1317–1326. doi: 10.1038/ncb3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lussey-Lepoutre C, Hollinshead KE, Ludwig C, Menara M, Morin A, Castro-Vega LJ, Parker SJ, Janin M, Martinelli C, Ottolenghi C, Metallo C, Gimenez-Roqueplo AP, Favier J, Tennant DA. Loss of succinate dehydrogenase activity results in dependency on pyruvate carboxylation for cellular anabolism. Nat. Commun. 2015;6:8784. doi: 10.1038/ncomms9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, Sethumadhavan S, Woo HK, Jang HG, Jha AK, Chen WW, Barrett FG, Stransky N, Tsun ZY, Cowley GS, Barretina J, Kalaany NY, Hsu PP, Ottina K, Chan AM, Yuan B, Garraway LA, Root DE, Mino-Kenudson M, Brachtel EF, Driggers EM, Sabatini DM. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Locasale JW, Grassian AR, Melman T, Lyssiotis CA, Mattaini KR, Bass AJ, Heffron G, Metallo CM, Muranen T, Sharfi H, Sasaki AT, Anastasiou D, Mullarky E, Vokes NI, Sasaki M, Beroukhim R, Stephanopoulos G, Ligon AH, Meyerson M, Richardson AL, Chin L, Wagner G, Asara JM, Brugge JS, Cantley LC, Vander Heiden MG. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat. Genet. 2011;43:869–874. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.