Abstract
Glomerular epithelial cells, podocytes, are insulin responsive and can develop insulin resistance. Here, we demonstrate that the small GTPase septin 7 forms a complex with nonmuscle myosin heavy chain IIA (NMHC-IIA; encoded by MYH9), a component of the nonmuscle myosin IIA (NM-IIA) hexameric complex. We observed that knockdown of NMHC-IIA decreases insulin-stimulated glucose uptake into podocytes. Both septin 7 and NM-IIA associate with SNAP23, a SNARE protein involved in GLUT4 storage vesicle (GSV) docking and fusion with the plasma membrane. We observed that insulin decreases the level of septin 7 and increases the activity of NM-IIA in the SNAP23 complex, as visualized by increased phosphorylation of myosin regulatory light chain. Also knockdown of septin 7 increases the activity of NM-IIA in the complex. The activity of NM-IIA is increased in diabetic rat glomeruli and cultured human podocytes exposed to macroalbuminuric sera from patients with type 1 diabetes. Collectively, the data suggest that the activity of NM-IIA in the SNAP23 complex plays a key role in insulin-stimulated glucose uptake into podocytes. Furthermore, we observed that septin 7 reduces the activity of NM-IIA in the SNAP23 complex and thereby hinders GSV docking and fusion with the plasma membrane.
Abbreviations: DN, diabetic nephropathy; GLUT4, glucose transporter 4; GSV, GLUT4 storage vesicle; NM-IIA, nonmuscle myosin IIA; NMHC-IIA, nonmuscle myosin heavy chain IIA; pp-RLC, phosphorylated myosin regulatory light chain; SNAP23, synaptosome-associated protein, 23 kDa; SNARE, N-ethylmaleimide–sensitive fusion protein attachment protein receptor; VAMP2, vesicle-associated membrane protein 2
Keywords: Septin 7, Nonmuscle myosin IIA, GLUT4 storage vesicle, SNARE, Glucose uptake, Podocytes
Graphical abstract
Highlights
-
•
Septin 7, nonmuscle myosin heavy chain IIA (NMHC-IIA) and SNAP23 form a complex.
-
•
Knockdown of septin 7 increases NM-IIA activity in the SNAP23 complex.
-
•
Insulin decreases septin 7 level and increases NM-IIA activity in the SNAP23 complex.
-
•
Septin 7 hinders GSV docking/fusion by reducing NM-IIA activity in the SNAP23 complex.
1. Introduction
Diabetic nephropathy (DN) is a serious complication of diabetes and the most common cause of end-stage renal disease [1]. Much effort has therefore been devoted to understanding the mechanisms that promote glomerular damage in DN. Risk factors for the development of DN include, for example, hyperglycemia, hypertension, dyslipidemia and genetic factors [2]. Also insulin resistance is a risk factor for DN [2], and has been reported to be associated with microalbuminuria in patients with type 1 as well as type 2 diabetes [3], [4].
At the cellular level the mechanisms leading to the development of insulin resistance include mutations in the insulin receptor itself [5] and impairments in the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway which mediates the uptake of glucose into cells [6]. Glucose transporter 4 (GLUT4) is the major insulin-inducible glucose transporter. Insulin activates the translocation of GLUT4 storage vesicles (GSVs) from the intracellular storage site to the plasma membrane [7]. GSV trafficking consists of several steps, and disturbances in this trafficking process may also cause insulin resistance. The trafficking involves actin and microtubule networks, the exocyst complex proteins that help to tether the GSVs with the plasma membrane, and the soluble N-ethylmaleimide–sensitive fusion protein attachment protein receptor (SNARE) complex that facilitates the tethering, docking and fusion of GSVs with the plasma membrane. The SNARE complex includes a vesicle-SNARE on GSVs, such as vesicle-associated membrane protein 2 (VAMP2), and target-SNAREs on the plasma membrane, such as syntaxin 4 and synaptosomal-associated protein, 23 kDa (SNAP23) [7].
Interestingly, kidney glomerular epithelial cells, or podocytes, are insulin sensitive and able to rapidly transport glucose using the glucose transporters GLUT4 and GLUT1 [8]. Insulin signaling is necessary for normal kidney function, as deletion of insulin receptor specifically in podocytes induces a disease state reminiscent of DN in a normoglycemic environment [9]. Furthermore, podocytes isolated from diabetic db/db mice are unable to respond to insulin indicating that podocytes can develop insulin resistance [10]. Nephrin, an essential structural protein of the glomerular filtration barrier, aids in GSV docking by interacting with the v-SNARE VAMP2 [11]. We showed previously that the small GTPase septin 7 associates with nephrin and VAMP2, and negatively regulates glucose uptake in podocytes [12]. Our data further revealed that knockdown of septin 7 increased the complex formation of VAMP2 with nephrin and syntaxin 4 [12], indicating that depletion of septin 7 enhances the final stages of GSV exocytosis.
In this study we set out to define whether septin 7 plays a specific, regulatory role in the docking of the GSVs with the plasma membrane as suggested by its association with nephrin [12]. We found that nonmuscle myosin heavy chain IIA (NMHC-IIA), which regulates the docking and fusion of GSVs in adipocytes [13], [14], [15], is a novel interaction partner of septin 7. Specifically, we observed that septin 7 reduces the activity of nonmuscle myosin IIA (NM-IIA) in the SNAP23-containing SNARE complex at the plasma membrane. Our study thus proposes a novel mechanism by which septin 7, by reducing the activity of NM-IIA in the SNAP23 complex, hinders GSV docking and fusion with the plasma membrane and reduces glucose uptake into podocytes.
2. Materials and methods
2.1. Cell culture, stable overexpression of nephrin in podocytes and preparation of cell lysates
Conditionally immortalized human podocytes (AB 8/13) were maintained in RPMI-1640 supplemented with 10% fetal calf serum (FCS) and 1% ITS (Sigma-Aldrich, St. Louis, MO) at 33 °C and shifted to 37 °C for two weeks for differentiation [16]. Rat nephrin was previously subcloned into pLNCX2 retroviral vector [17]. HEK293T cells were co-transfected with pLNCX2-nephrin and packaging pKAT2 vectors using Lipofectamine 2000 (Invitrogen, Camarillo, CA, USA). Virus-containing medium was filtered through a 0.45 µm filter and used to infect mouse podocytes. Podocytes overexpressing nephrin were selected by culturing the cells in medium supplemented with 2,5 mg/ml of geneticin (G418) (Gibco, Life Technologies, Carlsbad, CA, USA) for 12 days. Mouse podocytes and mouse podocytes stably overexpressing nephrin were maintained in DMEM containing 4.5 g/L glucose, 10% FCS, penicillin and streptomycin (Sigma-Aldrich), supplemented with 10 U/ml INF-γ (Sigma-Aldrich) at 33 °C. Where indicated, podocytes were starved and stimulated with 20 nM insulin (NovoNordisk, Bagsværd, Denmark). HEK293T cells were maintained in DMEM supplemented with 10% FCS, 1% ultraglutamin, 1% streptomycin and 1% penicillin at 37 °C. Cells were lysed in 1% Nonidet P-40 (NP-40), 20 mM HEPES, pH 7.5, 150 mM NaCl, in 50 mM HEPES, pH 7.6, 0.5% Triton X-100, 0.5% CHAPS or in 0.5% NP-40, 100 mM NaCl, 20 mM Tris-HCl, pH 8.0, 1 mM EDTA for co-immunoprecipitation as described [12]. All lysis buffers were supplemented with 50 mM NaF, 1 mM Na3VO4 and 1x Complete proteinase inhibitor cocktail (Roche, Basel, Switzerland).
2.2. Immunoprecipitation
Lysates were precleared with protein A-Sepharose (Invitrogen) or TrueBlot® anti-rabbit or anti-mouse Ig IP beads (eBiosciences, San Diego, CA, USA) and incubated with anti-septin 7, anti-SNAP23 or anti-nephrin antibodies and normal rabbit serum or rabbit/mouse IgGs (Zymed, South San Francisco, CA) as controls at 4 °C for 16 h. The immune complexes were bound to protein A-Sepharose or TrueBlot beads, washed with lysis buffer, stained with GelCode Blue (Pierce Chemical Co, Rockford, IL) or immunoblotted as described below.
2.3. Protein identification by LC-MS/MS
For mass spectrometry analysis the precipitated proteins were separated by SDS-PAGE and stained with GelCode Blue (Pierce Chemical Co). The >200 kDa band obtained in the septin 7 immunoprecipitation was excised from gel, in-gel digested with trypsin and the resulting peptides were analyzed by LC-MS/MS using an Ultimate 3000 nano-LC (Dionex, Sunnyvale, CA) and a QSTAR Elite hybrid quadrupole TOF-MS (Applied Biosystems/MDS Sciex, Life Technologies, Carlsbad, CA) with nano-ESI ionization as described previously [18]. The LC-MS/MS data was searched with in-house Mascot through ProteinPilot 2.0 interface against the SwissProt database using criteria: Human-specific taxonomy, trypsin digestion with one missed cleavage allowed, carbamidomethyl modification of cysteine as a fixed modification and oxidation of methionine as a variable modification.
2.4. Preparation of tissue lysates
Glomerular and tubular fractions were isolated from kidney cortices of male Sprague-Dawley and 40 weeks old, albuminuric obese or lean Zucker rats by graded sieving [19]. Zucker rats (Crl: ZUC-Leprfa) were purchased from Charles River Laboratories (Sulzfeld, Germany). Blood glucose, urinary albumin, and creatinine measurements have been described in [20]. The protocols were approved by the National Animal Experiment Board. Tissue lysates were prepared as above in NP-40 lysis buffer or in 50 mM HEPES, pH 7.6, 0.5% Triton X-100, 0.5% CHAPS.
2.5. Antibodies
Goat anti-septin 7 (N12) and rabbit anti-septin 7 (H120) IgGs were from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and rabbit anti-septin 7 (C) IgG was from Immuno-Biological Laboratories Co., Ltd. (Gumma, Japan). Rabbit anti-nonmuscle myosin IIA heavy chain IgG was from Biomedical Technologies, Inc. (Stoughton, MA, USA), and rabbit anti-phospho-myosin light chain 2 (Thr18/Ser19) IgG from Cell Signaling (Danvers, MA, USA). Rabbit anti-nephrin IgG (#1034) is described in [21], and mouse anti-nephrin IgG (5-1-6) in [22]. Guinea pig anti-nephrin IgG was from PROGEN Biotechnik (Heidelberg, Germany), and rabbit anti-SNAP23 and mouse anti-VAMP2 IgGs were from Synaptic System (Goettingen, Germany). Mouse anti-SNAP23, mouse anti-tubulin and mouse anti-actin IgGs were from Sigma-Aldrich.
2.6. Immunoblotting
Glomerular lysates of 40 weeks old six individual obese Zucker (fa/fa) and six individual lean Zucker (fa/+) rats were used for analyzing NMHC-IIA, septin 7 and pp-RLC expression levels. Differentiated human podocytes were exposed to 10% patient sera in FCS-free culture medium for 48 h. The serum samples were from 4 normoalbuminuric and 5 macroalbuminuric patients with type 1 diabetes from the Finnish Diabetic Nephropathy Study (FinnDiane; www.finndiane.fi) (Supplemental Table S1). Immunoblotting was performed as in [17] and blots were quantified using an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE, USA).
2.7. Immunoperoxidase staining
Human kidney samples were collected from surgical nephrectomies performed at Helsinki and Uusimaa Hospital district, and represented the non-malignant part of the kidney. Samples were fixed with 10% formalin, dehydrated, and embedded in paraffin. Immunoperoxidase staining was performed with a VectaStain Elite kit (Vector Laboratories, Burlingame, CA, USA). Sections were deparaffinized, antigen retrieval was performed by boiling in a microwave oven in 10 mM citric acid, pH 6.0 for 15 min, and endogenous peroxidase was inactivated by incubation in hydrogen peroxide in methanol for 30 min. Sections were blocked with CAS-block (Invitrogen) and incubated with primary antibodies diluted in ChemMate™ (DakoCytomation, Glostrup, Denmark) and with biotinylated secondary antibodies followed by incubation with ABC-reagent and AEC (Sigma-Aldrich) for colour development. Sections were counterstained with hematoxylin, mounted with Shandon Immu-Mount (Thermo Scientific, Waltham, MA, USA) and photographed using Nikon Eclipse 800 microscope. The use of human material was approved by the local Ethics Committee.
2.8. Immunoelectron microscopy
Adult rat kidney samples were fixed with 4% PFA in 0.1 M sodium phosphate buffer, pH 7.4 for 4 days at room temperature and infused with 2.3 M sucrose in PBS at 4 °C over night [23]. Ultrathin frozen sections were quenched with 50 mM NH4Cl in PBS for 10 min, blocked with 1% fish skin gelatin, 1% BSA in NH4Cl in PBS for 10 min, and incubated with rabbit anti-septin 7 (C) IgG followed by 10-nm gold-conjugated protein A (Department Cell Biology, Utrecht School of Medicine, the Netherlands), both incubations in a humidified chamber at room temperature for 60 min. Grids were stained in 2% neutral uranyl acetate for 10 min at room temperature, quickly rinsed and incubated in 2% methyl cellulose, 2% uranyl acetate (Sigma-Aldrich) for 15 min on ice. Samples were examined with JEM-1400 Transmission Electron Microscope (Jeol, Akishima, Tokyo, Japan) equipped with Olympus-SIS Morada CCD camera (Olympus Soft Imaging Solutions GmbH, Münster, Germany).
2.9. Indirect immunofluorescence
Surface labeling of nephrin was performed with nephrin IgG (5-1-6) as described in [19] using AlexaFluor 555 donkey anti-mouse IgG (Molecular Probes) as the secondary antibody. Nuclei were visualized with Hoechst 33342 (Sigma-Aldrich). Samples were examined with Leica SP8 confocal microscope (Wetzlar, Germany).
2.10. Duolink in situ
Interactions of SNAP23 with septin 7, NMHC-IIA, pp-RLC or VAMP2 were detected by the proximity ligation assay kit Duolink: PLA probe anti-rabbit plus, PLA probe anti-mouse minus (Olink Bioscience, Uppsala, Sweden). The samples were processed following the manufacturer's instructions and the fluorescence images were captured using Zeiss Axioplan2 microscope. Quantification of the detected PLA signals was performed using the Duolink Image Tool (Olink Bioscience). All samples were investigated with equal light intensity. Sensitivity of the software scan and blob threshold were set identically for all probes. The positive reaction dots from each interaction were counted automatically from at least 100 cells.
2.11. Silencing nonmuscle myosin IIA and septin 7 by siRNA
Mouse podocytes were transfected with 150 nmol ON-TARGET plus SMARTpool mouse nonmuscle myosin IIA heavy chain (L-040013-00-0005), siGENOME SMARTpool mouse septin 7 (M-042160-01-0005) or siCONTROL Non-Targeting Pool#2 (D-001206-14-05) siRNAs (Dharmacon, Lafayette, CO, USA) using Lipofectamine 2000 (Invitrogen). Cells were used for experiments after 48 h (NMHC-IIA siRNA) or 72 h (septin 7 siRNA).
2.12. 2-deoxy-D-glucose uptake assay
Glucose uptake of mouse podocytes was measured using 50 µmol/L (1 µCi/ml) 2-deoxy-D-[(1, 2-3H(N)]-glucose (PerkinElmer, Boston, MA) as described [12].
2.13. Statistical analysis
In all experiments, the differences between the groups were evaluated with the Student's t-test (Microsoft Excel). Sex frequencies were compared between cases and controls with the χ2 test. Results are presented as means±STDEV.
3. Results
3.1. Nonmuscle myosin heavy chain IIA interacts with septin 7 in podocytes
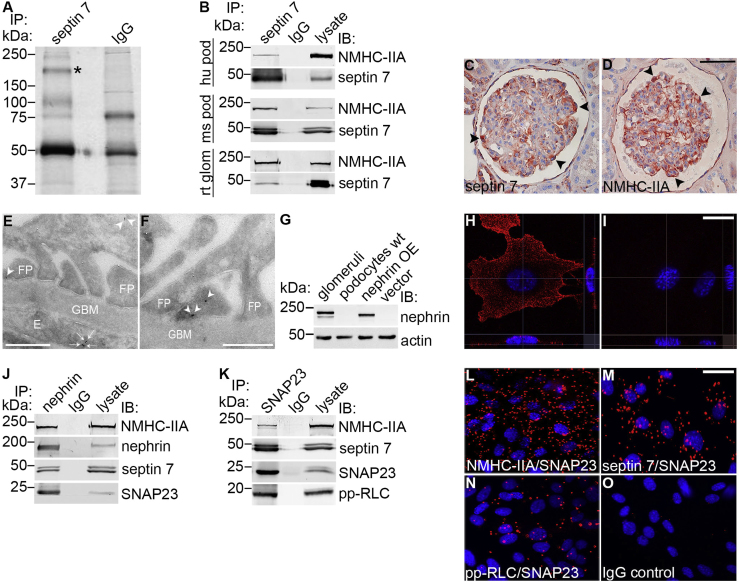
To define the role of septin 7 in podocytes and specifically in the regulation of GSV trafficking, we set out to identify novel interaction partners of septin 7. Co-immunoprecipitation analysis of human podocytes with anti-septin 7 antibodies identified a >200 kDa protein which associates with septin 7 (Fig. 1A). The corresponding band was excised from the gel, and the protein was identified by LC-MS/MS as NMHC-IIA. Two myosin heavy chains (MHCs) together with two pairs of light chains, essential light chains (ELCs) and regulatory light chains (RLCs) form the hexameric NM-II complex [24]. The NMHC-IIA isoform is encoded by the MYH9 gene [25] and it has previously been shown to be expressed in podocytes [26]. Co-immunoprecipitation and Western blotting confirmed that septin 7 and NMHC-IIA form a complex in cultured human and mouse podocytes in the basal state, and in isolated rat glomeruli (Fig. 1B). Immunoperoxidase staining indicated that septin 7 and NMHC-IIA are expressed in podocytes in human kidneys in vivo (Fig. 1C and D). Immunogold electron microscopy further confirmed that septin 7 is expressed in podocyte foot processes as well as in endothelial cells in glomeruli (Fig. 1E and F).
Fig. 1.
Nonmuscle myosin IIA is a novel interaction partner of septin 7 and it binds to the SNARE complex and nephrin (A) GelCode Blue-stained gel of immunoprecipitates obtained with septin 7 or rabbit IgGs from cultured human podocytes. The >200 kDa band (*) present only in the septin 7 precipitate was identified by mass spectrometry as nonmuscle myosin heavy chain IIA (NMHC-IIA). (B) Immunoblots showing NMHC-IIA in immunoprecipitates obtained with septin 7 IgG but not with rabbit IgG from human podocytes (hu pod), mouse podocytes (ms pod) or rat glomeruli (rt glom). Podocyte or glomerular lysates (30 µg) are included as controls. (C–D) Immunohistochemical staining shows that septin 7 (C) and NMHC-IIA (D) are expressed in podocytes in human glomeruli (arrowheads). (E–F) Immunogold electron microscopy of rat glomeruli shows septin 7 localization in podocyte foot processes (arrowheads) and endothelial cells (arrows). E, endothelial cell; FP, foot process; GBM, glomerular basement membrane. (G) In mouse podocytes stably expressing nephrin (nephrin OE), nephrin appears as an ∼180 kDa band that migrates slightly slower than the major band of endogenous nephrin in isolated rat glomeruli. Nephrin is not detected endogenously in podocytes (podocytes wt) or in podocytes infected with vector alone (vector). Actin is included as a loading control. (H–I) Surface labeling followed by confocal microscopy analysis confirms the localization of nephrin at the plasma membrane in podocytes overexpressing nephrin (H) whereas no signal is observed when the primary antibody is omitted (I). (J) Immunoblots showing NMHC-IIA, septin 7 and SNAP23 in immunoprecipitates obtained with nephrin IgG but not with rabbit IgG. Mouse podocyte lysate (30 µg) is included as a control. (K) Immunoblots showing NMHC-IIA, septin 7 and pp-RLC in immunoprecipitates obtained with SNAP23 IgG but not with rabbit IgG. Mouse podocyte lysate (30 µg) is included as a control. (L–O) Duolink proximity ligation assay shows interaction between SNAP23 and NMHC-IIA (L), septin 7 (M) and pp-RLC (N) in mouse podocytes. Each red spot represents an interaction detected by the kit. Scale bar, 50 µm (C, D), 0.5 µm (E–F), and 30 µm (H, I, L–O).
3.2. NMHC-IIA binds to nephrin and the SNARE complex
In adipocytes, NM-IIA has been shown to regulate glucose uptake by influencing the docking and fusion of GSVs as well as the activity of GLUT4 [13], [14], [15]. This suggests that septin 7 could, together with NMHC-IIA, regulate glucose uptake into podocytes by regulating the docking step. We next analyzed whether septin 7, nephrin, NM-IIA, and the plasma membrane SNARE proteins are part of the same macromolecular protein complex in the basal state. To mimic podocytes in vivo as closely as possible, we first produced a podocyte cell line stably expressing nephrin, as nephrin expression is typically lost from cultured podocytes during early passages. Nephrin was introduced into mouse podocytes by retroviral infection and cells stably expressing nephrin were obtained by antibiotic selection. Western blotting and surface labeling confirmed stable expression of nephrin in podocytes (Fig. 1G–I).
Immunoprecipitation analysis revealed that nephrin complex contains NMHC-IIA as well as septin 7 and SNAP23, a component of the t-SNARE complex at the plasma membrane (Fig. 1J). We further confirmed by co-immunoprecipitation with anti-SNAP23 antibodies that septin 7 and NMHC-IIA co-immunoprecipitate with SNAP23 (Fig. 1K). Further, as phosphorylation of the RLC regulates myosin motor activity [27] and GSV translocation and glucose uptake in adipocytes [28], we also studied the activity of NM-IIA in the SNAP23 complex by analyzing the presence of phosphorylated RLC (pp-RLC) in the complex. We found that NM-IIA, as visualized by the presence of pp-RLC in the precipitates, was active in the SNAP23 complex in podocytes (Fig. 1K).
To verify the interactions, we performed a Duolink proximity ligation assay (PLA) in which the interaction of the proteins can be visualized by immunofluorescence microscopy in the cells in situ. The method is based on application of specific primary antibodies raised in different species, followed by proximity probes (secondary antibodies coupled with oligonucleotides) and a connector oligonucleotide that produces a template for ligation and subsequent rolling circle PCR amplification. Each detected interaction gives rise to one rolling circle amplification product and consequently, quantification of the number of fluorescent spots observed reflects the level of interaction between the two proteins analyzed [29]. A previous study has confirmed that the method is suitable to analyze the SNARE complex formation [30]. Consistent with the co-immunoprecipitation data, Duolink showed that SNAP23 forms a complex with NMHC-IIA and septin 7 in mouse podocytes, and that NM-IIA is active in the complex as confirmed by the phosphorylation of the RLC (Fig. 1L–O).
3.3. NMHC-IIA reduces insulin-stimulated glucose uptake into podocytes
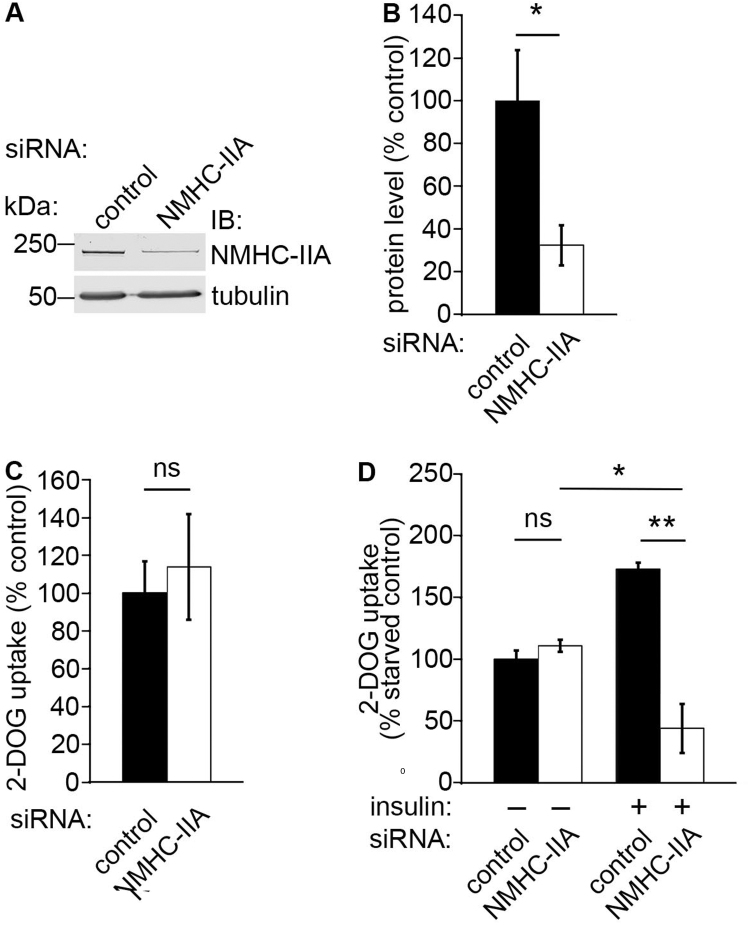
We next defined whether NM-IIA affects glucose uptake into podocytes by using previously characterized siRNAs specific for NMHC-IIA [15], [31] to reduce the expression level NMHC-IIA followed by glucose uptake assay. We observed that reduction of NMHC-IIA expression in nephrin-expressing podocytes by 68% (Fig. 2A and B) does not affect glucose uptake in basal (Fig. 2C) and starved states (Fig. 2D), but decreases insulin-stimulated glucose uptake by 56% compared to control siRNA-transfected, nonstimulated cells (set to 100%; Fig. 2D). In control siRNA-transfected cells insulin stimulation increases glucose uptake by 73% (Fig. 2D).
Fig. 2.
NMHC-IIA regulates insulin-stimulated glucose uptake in podocytes. (A) NMHC-IIA siRNA leads to 68% reduction of NMHC-IIA expression in mouse podocytes. Tubulin is included as a loading control. (B) Quantification of NMHC-IIA level in three replicate blots as in (A). (C) Depletion of NMHC-IIA does not affect glucose uptake activity of mouse podocytes compared to the control siRNA transfected cells (set to 100%) under basal conditions. (D) Knockdown of NMHC-IIA (NMHC-IIA siRNA, -insulin) does not affect glucose uptake activity of serum starved mouse podocytes. Glucose uptake activity of the control siRNA transfected and serum starved cells is set to 100% (control siRNA, -insulin). Insulin stimulation increases glucose uptake 73% in control siRNA transfected cells (control siRNA, +insulin), but decreases glucose uptake by 56% in myosin IIA siRNA transfected cells (NMHC-IIA siRNA, +insulin) compared to the control (control siRNA, -insulin). Bars show the mean and error bars STDEV of three independent experiments, Student's t-test. * p<0.05, ** p<0.01.
3.4. Downregulation of NMHC-IIA inhibits the association of VAMP2 with SNAP23
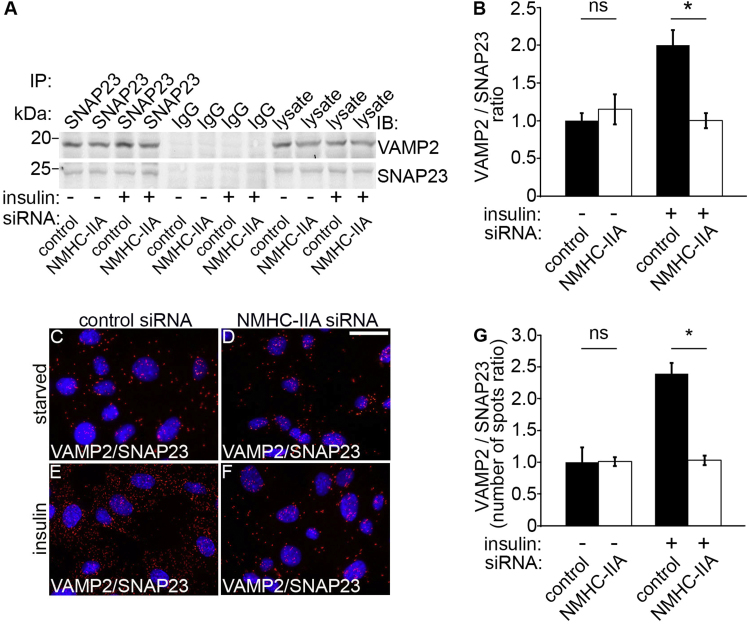
We further investigated the mechanism of attenuated insulin-stimulated glucose uptake in NMHC-IIA-depleted podocytes by studying the complex formation between the proteins on the GSVs and the plasma membrane. We speculated that in the absence of NMHC-IIA the complex formation between v-SNAREs and t-SNARES could be reduced. Indeed, we observed that in NMHC-IIA knockdown podocytes insulin stimulation fails to enhance complex formation between VAMP2 and SNAP23 compared to control siRNA-transfected, insulin-stimulated cells (Fig. 3A and B). Consistent with the co-immunoprecipitation data, Duolink showed decreased interaction of VAMP2 with SNAP23 in NMHC-IIA-depleted podocytes compared to the control siRNA-treated podocytes after insulin stimulation (Fig. 3C–G). This indicates that depletion of NMHC-IIA inhibits the insulin-induced complex formation between the v-SNARE VAMP2 and the t-SNARE SNAP23, and thereby reduces docking and fusion of the GSVs with the plasma membrane in podocytes, concomitant with reduced insulin-stimulated glucose uptake.
Fig. 3.
Knockdown of NMHC-IIA inhibits the interaction between VAMP2 and SNAP23. (A) Immunoblots showing VAMP2 in immunoprecipitates obtained with SNAP23 IgG but not with rabbit IgG from control or NMHC-IIA siRNA transfected mouse podocytes under starved (-insulin) or insulin-stimulated (+insulin) conditions. Control and NMHC-IIA siRNA-transfected podocyte lysates (30 µg) are included as controls. (B) Quantification of protein levels of three replicate blots as in (A) indicates that in insulin-stimulated mouse podocytes NMHC-IIA knockdown decreases complex formation between VAMP2 and SNAP23. (C–F) Duolink proximity ligation assay showing interaction between VAMP2 and SNAP23 in control siRNA (C, E) and NMHC-IIA siRNA (D, F) transfected cells. Each red spot represents an interaction detected by the kit. (G) Quantification of the spots in three replicate experiments as in (C–F) confirms the immunoprecipitation result showing that knockdown of NMHC-IIA fails to induce complex formation between VAMP2 and SNAP23. Scale bar, 30 µm. Bars show the mean and error bars STDEV of three independent experiments, Student's t-test. * p<0.05.
3.5. Insulin regulates the activity of NM-IIA bound to SNAP23
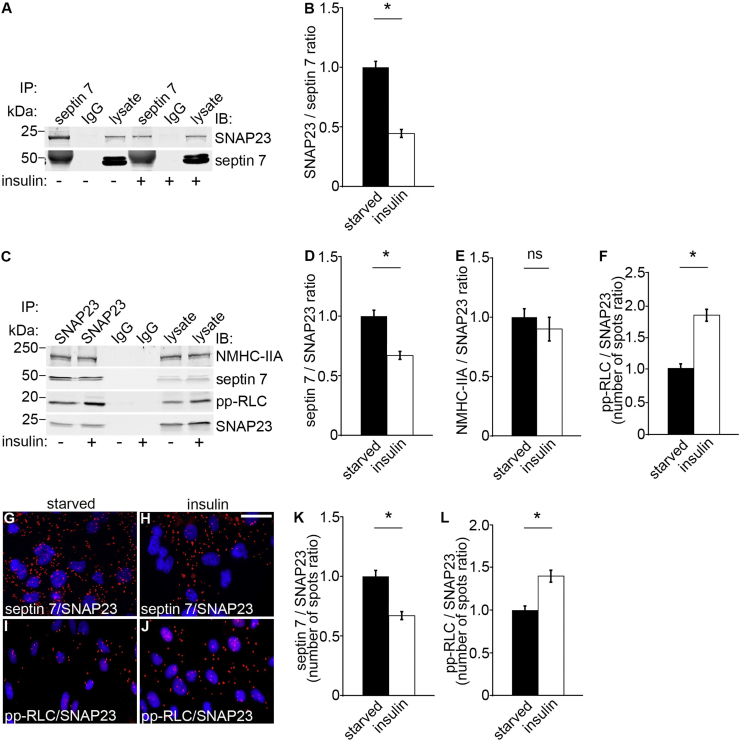
As knockdown of NMHC-IIA in podocytes reduces insulin-induced glucose uptake and as septin 7 and active NM-IIA bind to SNAP23, one could expect that insulin regulates the complex formation, or the activity of NM-IIA in complex with SNAP23. To investigate this, we performed co-immunoprecipitation and Duolink proximity ligation assays with and without insulin stimulation in podocytes. We found that insulin stimulation decreases the association of septin 7 with SNAP23 (Fig. 4A–D, G, H and K). However, insulin increases the activity of NM-IIA bound to SNAP23 as visualized by an increase of pp-RLC in the immunoprecipitate with SNAP23 and as also observed by the proximity ligation assay (Fig. 4C, F, I, J and L) without affecting the amount of NMHC-IIA in complex with SNAP23 (Fig. 4C and E). The data indicate an important regulatory role for the SNAP23 protein complex in glucose uptake into podocytes, insulin reducing the level of septin 7 in the complex with concomitant stimulation of the activity of NM-IIA in the complex.
Fig. 4.
Insulin regulates the activity of NM-IIA bound to SNAP23. (A) Immunoblots showing SNAP23 in immunoprecipitates obtained with septin 7 IgG but not with rabbit IgG from starved and insulin stimulated mouse podocytes. Lysates (30 µg) are included as controls. (B) Quantification of protein levels of three replicate blots as in (A) indicates that insulin decreases complex formation between septin 7 and SNAP23. (C) Immunoblots showing NMHC-IIA, septin 7 and pp-RLC in immunoprecipitates obtained with SNAP23 IgG but not with rabbit IgG from starved and insulin stimulated mouse podocytes. Lysates (30 µg) are included as controls. (D–F) Quantification of protein levels of three replicate blots as in (C). (G–J) Duolink proximity ligation assay showing interaction between septin 7 and SNAP23 (G, H) and between active NM-IIA (pp-RLC) and SNAP23 (I, J) in starved (G, I) and insulin stimulated (H, J) cells. Each red spot represents an interaction detected by the kit. (K–L) Quantification of the spots in three replicate experiments as in (G–J) showing that insulin decreases complex formation between septin 7 and SNAP23 (K) but increases the activity of NM-IIA, visualized by an increased amount of pp-RLC, in complex with SNAP23 (L). Scale bar, 30 µm. Bars show the mean and error bars STDEV of three independent experiments, Student's t-test. * p<0.05.
3.6. Septin 7 knockdown decreases the amount but increases the activity of NM-IIA in complex with SNAP23
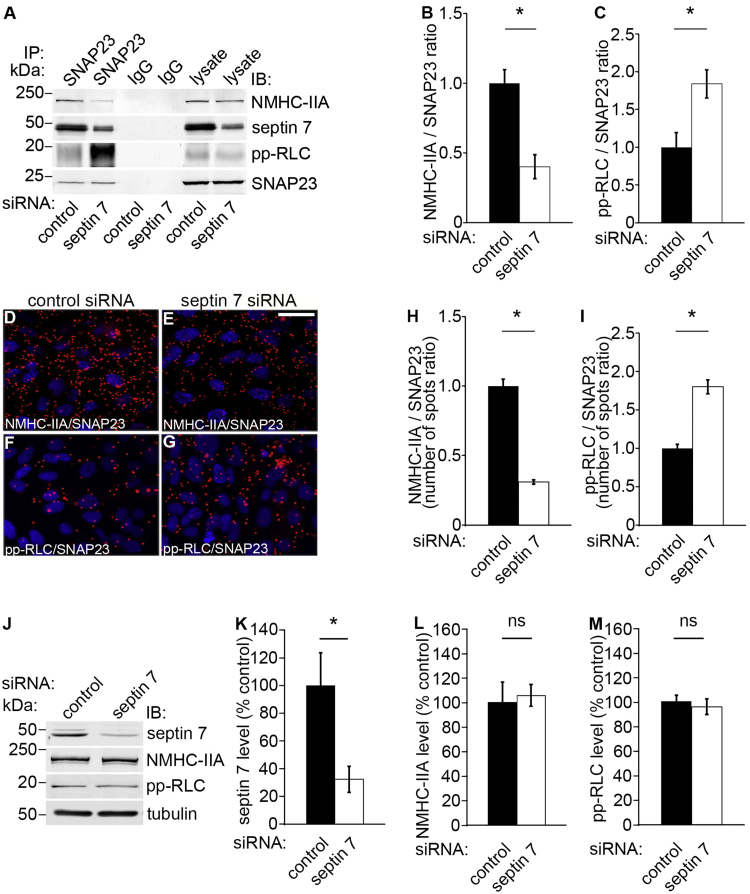
We next addressed whether septin 7 knockdown affects the activity of NM-IIA in the SNAP23 complex. To study this, we used septin 7-specific siRNAs [12] to knock down septin 7 in mouse podocytes followed by co-immunoprecipitation using an anti-SNAP23 antibody and immunoblotting for the complex components. We found that depletion of septin 7 decreases the interaction between SNAP23 and NMHC-IIA (Fig. 5A and B). However, depletion of septin 7 increases the activity of NM-IIA in complex with SNAP23 as visualized by increased level of pp-RLC in the SNAP23 immunoprecipitate (Fig. 5A and C). The interactions were further analyzed by the Duolink proximity ligation assay (Fig. 5D–I), which confirmed the data obtained by co-immunoprecipitation assays indicating that septin 7 enhances the complex formation between NM-IIA and SNAP23, and that suppression of septin 7 expression increases the activity of NM-IIA in complex with SNAP23. It is noteworthy that the increase in the activity of NM-IIA in complex with SNAP23 is not due to overall changes in the expression level or activity of NM-IIA, as Western blotting of whole cell lysates for NMHC-IIA and pp-RLC reveals no differences between septin 7 siRNA and control siRNA -transfected cells (Fig. 5J–M), as also shown previously [32].
Fig. 5.
Knockdown of septin 7 reduces the amount but increases the activity of NM-IIA in complex with SNAP23. (A) Immunoblots showing NMHC-IIA, septin 7 and pp-RLC in immunoprecipitates obtained with SNAP23 IgG but not with rabbit IgG from control or septin 7 siRNA transfected mouse podocytes. Control and septin 7 siRNA-transfected podocyte lysates (30 µg) are included as controls. (B–C) Quantification of protein levels of three replicate blots as in (A) indicates that septin 7 knockdown decreases complex formation between NMHC-IIA and SNAP23. However, septin 7 knockdown increases the activity of NM-IIA in complex with SNAP23 visualized by an increased level of pp-RLC in the precipitate. (D–G) Duolink proximity ligation assay shows interactions between NMHC-IIA and SNAP23 (D, E), and pp-RLC and SNAP23 (F, G) in control siRNA (D, F) and NMHC-IIA siRNA (E, G) transfected podocytes. Each red spot represents an interaction detected by the kit. (H–I) Quantification of the spots in three replicate experiments as in (D–G) confirms the immunoprecipitation result showing that knockdown of septin 7 decreases complex formation of NMHC-IIA with SNAP23, and increases the activity of NM-IIA in complex with SNAP23. (J) Immunoblots showing unchanged expression level of NMHC-IIA and pp-RLC in septin 7 depleted cells compared to the control siRNA-treated cells. (K-M) Quantification of protein levels of three replicate blots as in (J). Scale bar, 30 µm. Bars show the mean and error bars STDEV of three independent experiments, Student's t-test. * p<0.05.
3.7. Septin 7 overexpression increases the amount but decreases the activity of NM-IIA in complex with SNAP23
To confirm the septin 7 knockdown data, we investigated the effect of septin 7 overexpression on the activity of NM-IIA in complex with SNAP23. We first confirmed that septin 7 overexpression reduces glucose uptake as expected by performing 2-deoxy-D-glucose uptake assay on septin 7 overexpressing podocytes. Overexpression of septin 7 led to 1.6-fold upregulation of septin 7 (Supplemental Fig. S1A–E). 2-deoxy-D-glucose uptake assay indicated that glucose uptake is decreased by 20% in septin 7-overexpressing podocytes compared to empty vector-transfected cells (set to 100%) under basal conditions (Supplemental Fig. S1F). In serum-starved podocytes, overexpression of septin 7 did not affect glucose uptake compared to empty vector-transfected cells (set to 100%; Supplemental Fig. S1G). Insulin stimulation of podocytes increased glucose uptake in empty vector-transfected cells by 33% (Supplemental Fig. S1G), whereas the glucose uptake activity remained unchanged in septin 7-overexpressing podocytes (Supplemental Fig. S1G). These results confirm that septin 7 is a negative regulator of glucose uptake into podocytes as we showed previously [12].
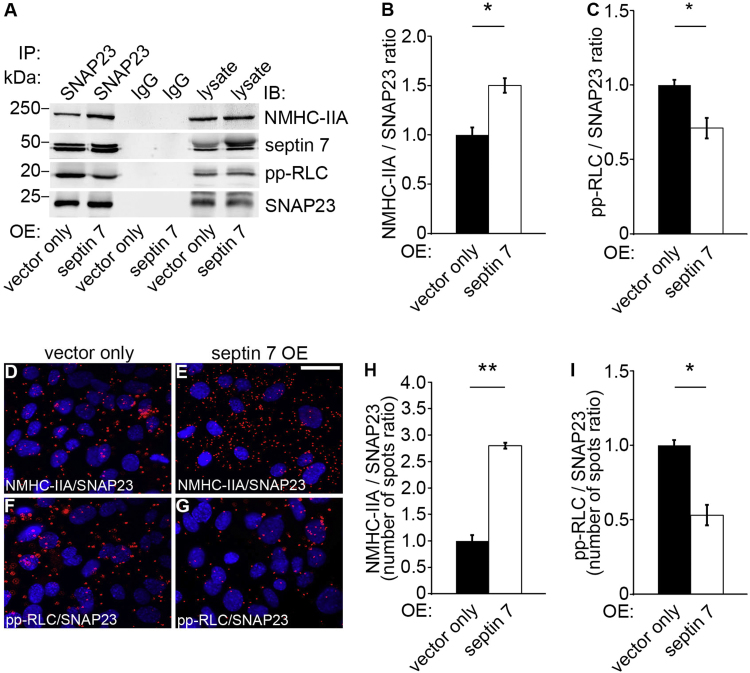
As expected, we found that septin 7 overexpression increases the complex formation between NMHC-IIA and SNAP23 (Fig. 6A–B), but decreases the activity of NM-IIA found in complex with SNAP23, as visualized by decreased level of pp-RLC in the complex (Fig. 6A and C). This was further validated by the Duolink proximity ligation assay (Fig. 6D–I). Collectively, the data on septin 7 knockdown and overexpression studies demonstrate that septin 7 enhances the complex formation between NM-IIA and SNAP23, but reduces the activity of NM-IIA in complex with SNAP23.
Fig. 6.
Septin 7 overexpression increases the amount but reduces the activity of NM-IIA in complex with SNAP23. (A) Immunoblots showing NMHC-IIA, septin 7 and pp-RLC in immunoprecipitates obtained with SNAP23 IgG but not with rabbit IgG from septin 7 or vector only transfected mouse podocytes. Lysates of podocytes overexpressing septin 7 or vector only (30 µg) are included as controls. (B–C) Quantitation of protein levels of three replicate blots as in (A) reveals that septin 7 overexpression increases complex formation of NMHC-IIA with SNAP23. However, septin overexpression decreases the activity of NM-IIA in complex with SNAP23. (D–G) Duolink proximity ligation assay shows interactions between NMHC-IIA and SNAP23 (D, E), and pp-RLC and SNAP23 (F, G) in vector only (D, F) and septin 7 (E, G) overexpressing podocytes. Each red spot represents an interaction detected by the kit. (H-I) Quantification of the spots in three replicate experiments as in (D–G) confirms the immunoprecipitation result showing that overexpression of septin 7 increases complex formation of NM-IIA with SNAP23, but decreases the activity of NM-IIA found in complex with SNAP23. Scale bar, 30 µm. Bars show the mean and error bars STDEV of three independent experiments, Student's t-test. * p<0.05, ** p<0.01.
3.8. NM-IIA is activated in the glomeruli of diabetic rats
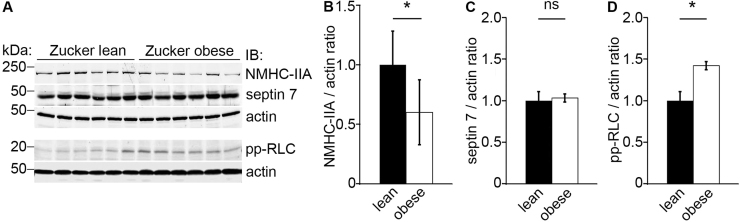
We next analyzed the expression and activity of NM-IIA in glomeruli in vivo using obese, albuminuric Zucker rats that are insulin resistant and slightly diabetic due to a mutation in the leptin receptor gene [33]. Quantitative immunoblot analysis of isolated glomeruli showed marked downregulation of NMHC-IIA in the obese (fa/fa) rats when compared to the lean controls (fa/+) (Fig. 7A–B) and no difference in the level of septin 7 (Fig. 7A and C), whereas the activity of NM-IIA, visualized by increased level of pp-RLC, was significantly increased in the obese rats (Fig. 7A and D).
Fig. 7.
NM-IIA is activated in glomeruli of diabetic rats. (A) Immunoblotting for NMHC-IIA, septin 7 and pp-RLC shows that NMHC-IIA is downregulated whereas the activity of NM-IIA is increased, visualized by an elevated level of pp-RLC, in glomeruli of obese Zucker rats compared to lean controls. The expression level of septin 7 does not change. Actin is included as a loading control (n=6 in each group). (B–D) Quantification of the levels of NMHC-IIA, septin 7 and pp-RLC in (A) normalized to actin. Bars show the mean and error bars STDEV of three independent experiments, Student's t-test. * p<0.05.
3.9. Macroalbuminuric sera from patients with type 1 diabetes induce activation of NM-IIA in cultured human podocytes
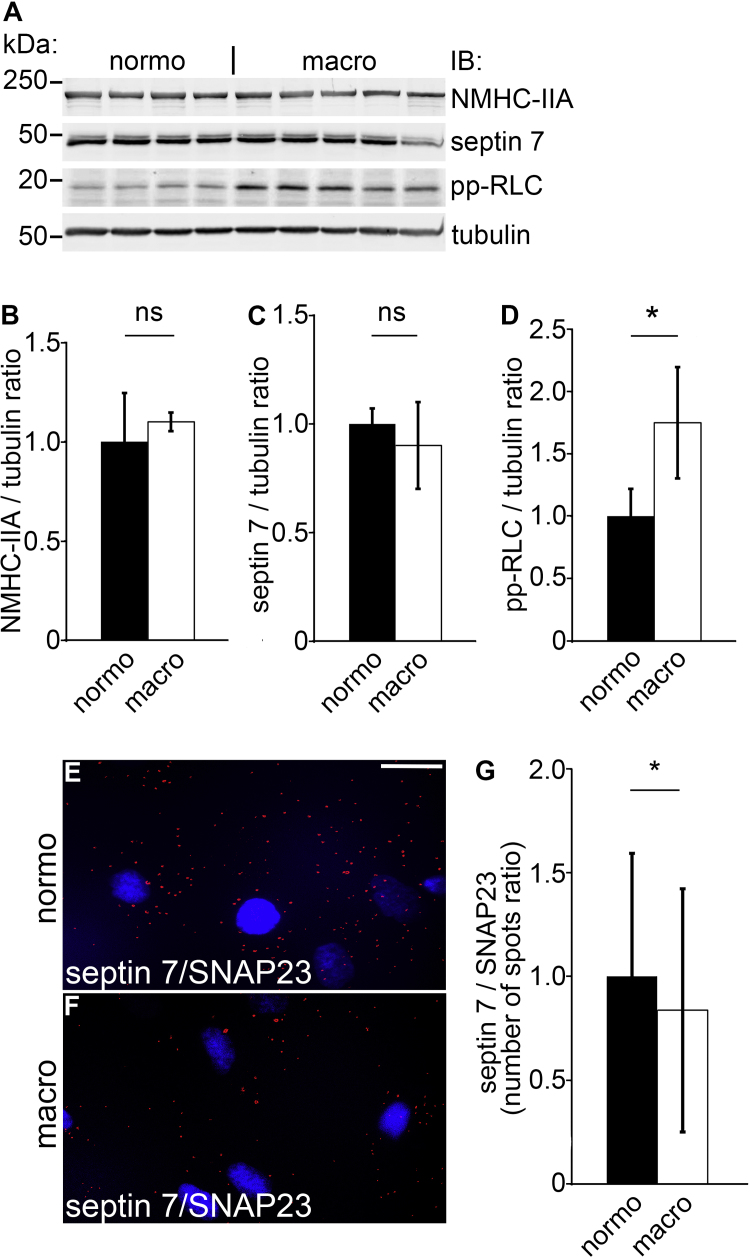
We also studied the expression of septin 7, and the expression and activity of NM-IIA in human podocytes treated with sera from male patients with type 1 diabetes presenting either normo- or macroalbuminuria (Fig. 8A–D). Macroalbuminuric patients were older and had longer duration of diabetes, as expected based on their diabetes status (Supplemental Table S1). The expression of NMHC-IIA and septin 7 did not differ between the groups (Fig. 8A–C). Interestingly, the activity of NM-IIA, visualized by increased level of pp-RLC, was increased by 75% in podocytes treated with sera from macroalbuminuric patients compared to podocytes treated with sera from normoalbuminuric patients (Fig. 8A and D). Furthermore, Duolink proximity ligation assay on human podocytes treated with sera form normo- and macroalbuminuric patients revealed, despite large variation, decreased interaction between septin 7 and SNAP23 after treatment with sera from patients presenting macroalbuminuria (Fig. 8E–G).
Fig. 8.
Macroalbuminuric sera from human patients with type 1 diabetes activate NM-IIA in cultured human podocytes. (A) The activity of NM-IIA, visualized by increased level of pp-RLC, is increased by 75% in human podocytes treated with macroalbuminuric sera from patients with type 1 diabetes compared to treatment with normoalbuminuric sera. The expression levels of NMHC-II and septin 7 do not change. Tubulin is included as a loading control. (B–D) Quantification of the expression levels of NMHC-IIA, septin 7 and pp-RLC in (A) normalized to tubulin. (E–F) Duolink proximity ligation assay showing interaction between septin 7 and SNAP23 in podocytes treated with sera from patients with type 1 diabetes presenting either normo- or macroalbuminuria. Each red spot represents an interaction detected by the kit. (G) Quantification of the spots as in (E–F). Scale bar, 30 µm. Bars show the mean and error bars STDEV of two independent experiments, Student's t-test. * p<0.05.
4. Discussion
NMHC-IIA is expressed in glomerular podocytes and has been suggested to function as a major component of the actin-myosin contractile apparatus, which helps podocytes to respond to changes in the glomerular pressure in physiological conditions and contributes to foot process retraction in pathological conditions [26]. Here we present another function for NMHC-IIA as a regulator of GSV trafficking and glucose uptake in podocytes. We show that knockdown of NMHC-IIA reduces insulin-induced glucose uptake in podocytes. Furthermore, we found that the small GTPase septin 7 reduces the activity of NM-IIA in the SNAP23 complex proposing a new mechanism by which septin 7 and NM-IIA regulate glucose uptake into podocytes.
We previously found that septin 7 binds to the v-SNARE VAMP2 and reduces glucose uptake in HIRc cells (rat fibroblasts stably expressing human insulin receptor) and podocytes by apparently forming a barrier between the GSVs and the plasma membrane [12]. Here we show that septin 7 also binds to the t-SNARE SNAP23 suggesting that septin 7 could also be involved in the regulation of the docking process. We further observed that septin 7 forms a complex with NMHC-IIA, and that NMHC-IIA is necessary for insulin-stimulated glucose uptake into podocytes. The importance of NMHC-IIA in glucose uptake is supported by studies in adipocytes, in which pharmacological inhibition of the activity of NM-II or knockdown of NM-IIA reduced insulin-stimulated glucose uptake [13], [14], [15]. Our observations provide an insight how insulin-stimulated glucose uptake into podocytes is mechanistically regulated by septin 7 and NMHC-IIA. We found that insulin reduces the amount of septin 7 in the SNAP23 complex, and increases the activity of NM-IIA (an increase in pp-RLC) in the complex, thereby enhancing docking and fusion of GSVs with the plasma membrane and uptake of glucose into podocytes. Supporting our data, blocking the phosphorylation of RLC inhibits GSV translocation and glucose uptake in adipocytes [28].
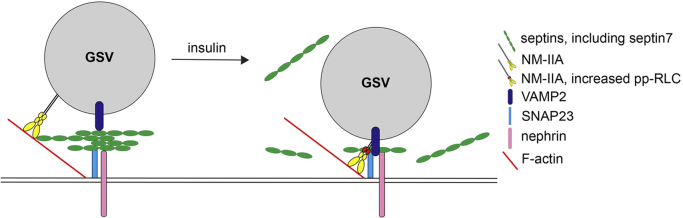
We found that in podocytes, knockdown of NMHC-IIA inhibits the insulin-stimulated interaction between SNAP23 and VAMP2, the t-SNARE and v-SNARE involved in GSV docking and fusion. Also in adipocytes knockdown of NMHC-IIA inhibits t-SNARE/v-SNARE (syntaxin 4/VAMP2) complex formation [15]. SNAP23 appears central for the insulin-stimulated regulatory actions of septin 7 and NM-IIA in glucose uptake in podocytes, as insulin stimulation reduces the interaction of septin 7 with SNAP23 and enhances the activity of NM-IIA in complex with SNAP23 (Fig. 9). Septin 7 knockdown, similarly as insulin stimulation, increases the activity of NM-IIA in the SNAP23 complex; yet we acknowledge that the signaling pathways activated by insulin stimulation and septin 7 knockdown may vary. Septin 7 overexpression has opposite effects further confirming the finding. These data together suggest that septin 7 plays a central role in GSV docking and fusion by regulating the activity of NM-IIA in the SNAP23 complex.
Fig. 9.
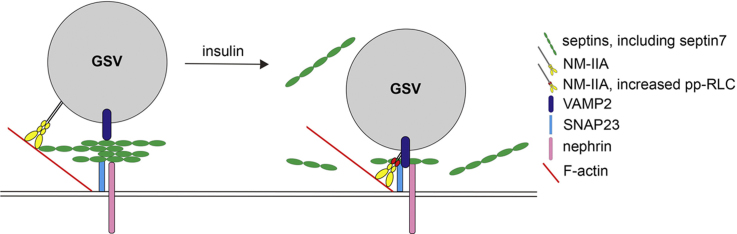
A cartoon showing the mechanism by which insulin stimulation regulates GSV docking and fusion in podocytes via septin 7 and NM-IIA. Septin 7, together with other septins in the complex, forms a barrier underneath the plasma membrane. This hinders the movement of GSVs and interaction of VAMP2 with SNAP23. Insulin stimulation triggers reduction of septins in complex with SNAP23 and increases activation of NM-IIA as revealed by an elevated level of phosphorylation of myosin RLC (pp-RLC). This enhances complex formation between VAMP2 and SNAP23 and thereby docking and fusion of the GSVs with the plasma membrane, leading to increased uptake of glucose into podocytes.
Previous data have revealed differential effects of various septins on the activity of myosins. Septin 9, which we previously found to be in complex with septin 7 in podocytes and downregulated upon septin 7 knockdown [12], has been shown to inhibit the actin-dependent ATPase activity of myosin V [34]. Septin 2, on the other hand, has been shown to scaffold NM-II and its kinases to activate NM-II by phosphorylation of the RLC [35]. These data, together with our results here, illustrate the complex relationship between the members of the septin and myosin families, which may cooperate in different manner in different cell types. Thus, further studies are required to determine the exact mechanisms by which septin 7 reduces the activity of NM-IIA in the SNAP23 complex in podocytes. One possible mechanism by which septin 7 may regulate the activity of NM-IIA in the SNAP23 complex is via recruitment of a phosphatase, the identity of which remains to be characterized. We can not rule out the possibility that NM-IIA regulates the final steps of GSV trafficking also via modulating actin dynamics, as the activity of NM-IIA is necessary for F-actin localization at the plasma membrane in adipocytes [36], and knockdown of NMHC-IIA leads to loss of actin stress fiber organization in podocytes [37]. Defective actin remodeling impairs the fusion of GSVs with the plasma membrane in adipocytes [38] and muscle cells [39], and glucose uptake depends on an intact actin cytoskeleton also in podocytes [8]. NM-IIA also regulates the intrinsic activity of GLUT4 at the plasma membrane after insulin stimulation [14], and also other proteins may, either positively or negatively, regulate GLUT4 activity [40], indicating the complexity of GLUT4 regulation.
Interestingly, we found that the activity of NM-IIA is increased in glomeruli of obese and albuminuric Zucker rats. Furthermore, we observed that exposure of cultured human podocytes to macroalbuminuric sera from patients with type 1 diabetes decreases the association between septin 7 and SNAP23 and increases the activation of NM-IIA compared to podocytes treated with normoalbuminuric sera. This indicates that some factors in the macroalbuminuric sera activate NM-IIA and may thereby enhance GSV docking and fusion and glucose uptake into podocytes. Further support for a role for NM-IIA and associated kinases in DN is provided in a study showing that the expression of myosin light chain kinase increases in the kidneys in streptozotocin-induced diabetes [41]. In addition, polymorphisms in MYH9, which encodes NMHC-IIA, are associated with DN in European Americans [42]. Whether increased glucose uptake by an increase in active NM-IIA in diabetes is protective or predisposes to DN awaits further studies. Excess glucose may activate several pathways that lead to podocyte injury [43]. The protective effect, on the other hand, could be expected based on data showing that insulin signaling, which may be coupled to increased glucose uptake, is essential for normal kidney function [9]. Furthermore, several clinical and experimental studies show that many, although not all, insulin sensitizers reduce albuminuria [44].
Both septin 7 and NMHC-IIA are in complex with nephrin (this study and [12]), an essential component of the interpodocyte cell adhesion structure called slit diaphragm [45]. Nephrin associates with the actin cytoskeleton [46] and serves as a modifying protein in the SNARE complex by associating with VAMP2 [9]. It is possible that in podocytes nephrin determines the specific membrane domain for NM-IIA and associated SNARE proteins to facilitate the docking and fusion of GSVs in the slit diaphragm region. As GLUT4 was previously found widely distributed in podocytes [8], other proteins than nephrin are apparently involved in the docking and fusion of GSVs in the basal and apical domains of the podocyte foot processes. The association of nephrin with NMHC-IIA suggests that nephrin could also play a role in syndromes associated with MYH9, so called MYH9-related disorders [47], [48], [49], as some patients with MYH9 mutations develop progressive proteinuric renal disease. However, the molecular mechanisms underlying the renal phenotype have thus far remained unclear. In mice, mutations in Myh9 mimicking the mutations identified in human MYH9-associated diseases or podocyte-specific deletion of Myh9 may lead to kidney dysfunction or predispose the mice to glomerulopathy depending on the genetic background and the type of the experimental injury [50], [51], [52]. Further studies analyzing the glucose uptake activity of the podocytes in these models will help to determine the role of NMHC-IIA in the insulin responsiveness of podocytes in vivo, and clarify whether this could be associated with the development of MYH9-associated glomerulopathy.
In addition to MYH9-related disorders, NMHC-IIA has also been associated with focal segmental glomerulosclerosis (FSGS) and end-stage renal disease [53]. Sekine et al. [54] suggested that mutations in NMHC-IIA could impair the structure and function of the slit diaphragm, which would result in proteinuria and the development of FSGS [54]. Our observation that NMHC-IIA and nephrin are found in the same multiprotein complex supports this. Further studies are required to define how the mutations in MYH9 might affect nephrin, its protein complexes, association with actin, and other functions, including the potential role of NM-IIA in regulating the insulin sensitivity of podocytes. This may provide novel clues to understanding the mechanisms that underlie disorders caused by mutations in MYH9.
In conclusion, we show here that NMHC-IIA is essential for insulin-stimulated glucose uptake into podocytes, and that SNAP23 and septin 7 are central regulators of the process. Interaction of NM-IIA with nephrin further suggests that NM-IIA may be involved in the regulation of the composition or function of the slit diaphragm.
Author contributions
AAW designed and performed the experiments, analyzed the data and wrote the paper. VD designed and performed the experiments. JT, TAN, CLF and EL performed the experiments. CF, ML and P-HG edited the paper, and SL wrote and edited the paper.
Competing interests
P-HG has received lecture honoraria from AstraZenega, Boehringer Ingelheim, Eli Lilly, Elo Water, Genzyme, MSD, Novartis, Novo Nordisk and Sanofi, and investigator-initiated grants from Eli Lilly and Roche. P-HG is an advisory Board Member of AbbVie, AstraZenega, Boehringer Ingelheim, Cebix, Eli Lilly, Janssen, Medscape, MSD, Novartis, Novo Nordisk and Sanofi.
Acknowledgments
This work was supported by the European Research Council (242820; SL), the Academy of Finland (131255, 218021, 255551, 277485; SL and 134379; P-HG), Helsinki Biomedical Graduate Program (AAW, VD), The Diabetes Research Foundation (AAW), The Kidney Foundation (AAW), Research Foundation of the University of Helsinki (VD), the Helsinki University Central Hospital Research Grant (JT), Folkhälsan Research Foundation (P-HG), Wilhelm and Else Stockmann Foundation (P-HG, ML, CLF) and the Novo Nordisk Foundation (NNF14SA0003 P-HG). We thank Dr. Moin Saleem (University of Bristol, Bristol Royal Hospital for Children, Bristol, UK) for kindly providing conditionally immortalized human podocyte cell line AB 8/13, Dr. Andrey S. Shaw (HHMI/Department of Pathology and Immunology, Washington University School of Medicine, St. Louis, MO, USA) for mouse podocyte cell line, Dr. Harry Holthöfer (Dublin City University, Ireland) and Dr. Hiroshi Kawachi (Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan) for anti-nephrin antibodies, and Dr. Koh-ichi Nagata (Aichi Human Service Center, Japan) for full-length septin 7 cDNA. Electron Microscopy Unit of the Institute of Biotechnology and Fang Zhao (University of Helsinki, Finland) are thanked for help with electron microscopy. Niina Ruoho, Anna Sandelin and Jaana Tuomikangas are thanked for skillful technical assistance.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.yexcr.2016.12.010.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Zimmet P., Alberti K.G., Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.Harjutsalo V., Groop P.H. Epidemiology and risk factors for diabetic kidney disease. Adv. Chronic Kidney Dis. 2014;21:260–266. doi: 10.1053/j.ackd.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Yip J., Mattock M.B., Morocutti A., Sethi M., Trevisan R., Viberti G. Insulin resistance in insulin-dependent diabetic patients with microalbuminuria. Lancet. 1993;342:883–887. doi: 10.1016/0140-6736(93)91943-g. [DOI] [PubMed] [Google Scholar]
- 4.Parvanova A.I., Trevisan R., Iliev I.P., Dimitrov B.D., Vedovato M., Tiengo A., Remuzzi G., Ruggenent P. Insulin resistance and microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes. 2006;55:1456–1462. doi: 10.2337/db05-1484. [DOI] [PubMed] [Google Scholar]
- 5.Accili D., Frapier C., Mosthaf L., McKeon C., Elbein S.C., Permutt M.A., Ramos E., Lander E., Ullrich A., Taylor S.I. A mutation in the insulin receptor gene that impairs transport of the receptor to the plasma membrane and causes insulin-resistant diabetes. EMBO J. 1989;8:2509–2517. doi: 10.1002/j.1460-2075.1989.tb08388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pessin J.E., Saltiel A.R. Signaling pathways in insulin action: molecular targets of insulin resistance. J. Clin. Invest. 2000;106:165–169. doi: 10.1172/JCI10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stöckli J., Fazakerley D.J., James D.E. GLUT4 exocytosis. Cell Sci. 2011;124:4147–4159. doi: 10.1242/jcs.097063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coward R.J., Welsh G.I., Yang J., Tasman C., Lennon R., Koziell A., Satchell S., Holman G.D., Kerjaschki D., Tavaré J.M. The human glomerular podocyte is a novel target for insulin action. Diabetes. 2005;54:3095–3102. doi: 10.2337/diabetes.54.11.3095. [DOI] [PubMed] [Google Scholar]
- 9.Welsh G.I., Hale L.J., Eremina V., Jeansson M., Maezawa Y., Lennon R., Pons D.A., Owen R.J., Satchell S.C., Miles M.J. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 2010;12:329–340. doi: 10.1016/j.cmet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tejada T., Catanuto P., Ijaz A., Santos J.V., Xia X., Sanchez P., Sanabria N., Lenz O., Elliot S.J., Fornoni A. Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int. 2008;73:1385–1393. doi: 10.1038/ki.2008.109. [DOI] [PubMed] [Google Scholar]
- 11.Coward R.J., Welsh G.I., Koziell A., Hussain S., Lennon R., Ni L., Tavaré J.M., Mathieson P.W., Saleem M.A. Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes. 2007;56:1127–1135. doi: 10.2337/db06-0693. [DOI] [PubMed] [Google Scholar]
- 12.Wasik A.A., Polianskyte-Prause Z., Dong M.Q., Shaw A.S., Yates J.R., 3rd, Farquhar M.G., Lehtonen S. Septin 7 forms a complex with CD2AP and nephrin and regulates glucose transporter trafficking. Mol. Biol. Cell. 2012;23:3370–3379. doi: 10.1091/mbc.E11-12-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steimle P.A., Fulcher F.K., Patel M. A novel role for myosin II in insulin stimulated glucose uptake in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2005;331:1560–1565. doi: 10.1016/j.bbrc.2005.04.082. [DOI] [PubMed] [Google Scholar]
- 14.Fulcher F.K., Smith B.T., Russ M., Patel Y.M. Dual role for myosin II in GLUT4-mediated glucose uptake in 3T3-L1 adipocytes. Exp. Cell Res. 2008;314:3264–3274. doi: 10.1016/j.yexcr.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung L.T.K., Hosaka T., Harada N., Jambaldorj B., Fukunaga K., Nishiwaki Y., Teshigawara K., Sakai T., Nakaya Y., Funaki M. Myosin IIA participates in docking of Glut4 storage vesicles with the plasma membrane in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2010;391:995–999. doi: 10.1016/j.bbrc.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Saleem M.A., O'Hare M.J., Reiser J., Coward R.J., Inward C.D., Farren T., Xing C.Y., Ni L., Mathieson P.W., Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 17.Lehtonen S., Lehtonen E., Kudlicka K., Holthofer H., Farquhar M.G. Nephrin forms a complex with adherens junction proteins and CASK in podocytes and in Madin-Darby canine kidney cells expressing nephrin. Am. J. Pathol. 2004;165:923–936. doi: 10.1016/S0002-9440(10)63354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Öhman T., Lietzén N., Välimäki E., Melchjorsen J., Matikainen S., Nyman T.A. Cytosolic RNA recognition pathway activates 14-3-3 protein mediated signaling and caspase-dependent disruption of cytokeratin network in human keratinocytes. J. Proteome Res. 2010;9:1549–1564. doi: 10.1021/pr901040u. [DOI] [PubMed] [Google Scholar]
- 19.Heikkilä E., Ristola M., Havana M., Jones N., Holthöfer H., Lehtonen S. trans-interaction of nephrin and Neph1/Neph3 induces cell adhesion that associates with decreased tyrosine phosphorylation of nephrin. Biochem. J. 2011;435:619–628. doi: 10.1042/BJ20101599. [DOI] [PubMed] [Google Scholar]
- 20.Hyvönen M.E., Saurus P., Wasik A., Heikkilä E., Havana M., Trokovic R., Saleem M., Holthöfer H., Lehtonen S. Lipid phosphatase SHIP2 downregulates insulin signalling in podocytes. Mol. Cell Endocrinol. 2010;328:70–79. doi: 10.1016/j.mce.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Ahola H., Heikkilä E., Aström E., Inagaki M., Izawa I., Pavenstädt H., Kerjaschki D., Holthöfer H. A novel protein, densin, expressed by glomerular podocytes. J. Am. Soc. Nephrol. 2003;14:1731–1737. doi: 10.1097/01.asn.0000075553.33781.9f. [DOI] [PubMed] [Google Scholar]
- 22.Topham P.S., Kawachi H., Haydar S.A., Chugh S., Addona T.A., Charron K.B., Holzman L.B., Shia M., Shimizu F., Salant D.J. Nephritogenic mAb 5-1-6 is directed at the extracellular domain of rat nephrin. J. Clin. Invest. 1999;104:1559–1566. doi: 10.1172/JCI7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tokuyasu K.T. Use of poly(vinylpyrrolidone) and poly(vinyl alcohol) for cryoultramicrotomy. Histochem J. 1989;21:163–171. doi: 10.1007/BF01007491. [DOI] [PubMed] [Google Scholar]
- 24.Sellers J.R. Myosins: a diverse superfamily. Biochim. Biophys. Acta. 2010;17:3–22. doi: 10.1016/s0167-4889(00)00005-7. [DOI] [PubMed] [Google Scholar]
- 25.Simons M., Wang M., McBride O.W., Kawamoto S., Yamakawa K., Gdula D., Adelstein R.S., Weir L. Human nonmuscle myosin heavy chains are encoded by two genes located on different chromosomes. Circ. Res. 1991;69:530–539. doi: 10.1161/01.res.69.2.530. [DOI] [PubMed] [Google Scholar]
- 26.Arrondel C., Vodovar N., Knebelmann B., Grünfeld J.P., Gubler M.C., Antignac C., Heidet L. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J. Am. Soc. Nephrol. 2002;13:65–74. doi: 10.1681/ASN.V13165. [DOI] [PubMed] [Google Scholar]
- 27.Li X.D., Saito J., Ikebe R., Mabuchi K., Ikebe M. The interaction between the regulatory light chain domains on two heads is critical for regulation of smooth muscle myosin. Biochemistry. 2000;39:2254–2260. doi: 10.1021/bi9924617. [DOI] [PubMed] [Google Scholar]
- 28.Choi Y.O., Ryu H.J., Kim H.R., Song Y.S., Kim C., Lee W., Choe H., Leem C.H., Jang Y.J. Implication of phosphorylation of the myosin II regulatory light chain in insulin-stimulated GLUT4 translocation in 3T3-F442A adipocytes. Exp. Mol. Med. 2006;30:180–189. doi: 10.1038/emm.2006.22. [DOI] [PubMed] [Google Scholar]
- 29.Weibrecht I., Leuchowius K.J., Clausson C.M., Conze T., Jarvius M., Howell W.M., Kamali-Moghaddam M., Söderberg O. Proximity ligation assays: a recent addition to the proteomics toolbox. Expert Rev. Proteom. 2010;7:401–409. doi: 10.1586/epr.10.10. [DOI] [PubMed] [Google Scholar]
- 30.Kioumourtzoglou D., Gould G.W., Bryant N.J. Insulin stimulates syntaxin4 SNARE complex assembly via a novel regulatory mechanism. Mol. Cell Biol. 2014;34:1271–1279. doi: 10.1128/MCB.01203-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woody S., Stall R., Ramos J., Patel Y.M. Regulation of myosin light chain kinase during insulin-stimulated glucose uptake in 3T3-L1 adipocytes. PLoS One. 2013;8:e77248. doi: 10.1371/journal.pone.0077248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tooley A.J., Gilden J., Jacobelli J., Beemiller P., Trimble W.S., Kinoshita M., Krummel M.F. Amoeboid T lymphocytes require the septin cytoskeleton for cortical integrity and persistent motility. Nat. Cell Biol. 2009;11:17–26. doi: 10.1038/ncb1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chua S.S., Jr, Chung W.K., Wu-Peng X.S., Zhang Y., Liu S.M., Tartaglia L., Leibel R.L. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;27:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- 34.Smith C., Dolat L., Angelis D., Forgacs E., Spiliotis E.T., Galkin V.E. Septin 9 Exhibits Polymorphic Binding to F-Actin and Inhibits Myosin and Cofilin Activity. J. Mol. Biol. 2015;427:3273–3284. doi: 10.1016/j.jmb.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joo E., Surka M.C., Trimble W.S. Mammalian SEPT2 is required for scaffolding nonmuscle myosin II and its kinases. Dev. Cell. 2007;13:677–690. doi: 10.1016/j.devcel.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Stall R., Ramos J., Kent Fulcher F., Patel Y.M. Regulation of myosin IIA and filamentous actin during insulin-stimulated glucose uptake in 3T3-L1 adipocytes. Exp. Cell Res. 2014;322:81–88. doi: 10.1016/j.yexcr.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hays T., Ma'ayan A., Clark N.R., Tan C.M., Teixeira A., Teixeira A., Choi J.W., Burdis N., Jung S.Y., Bajaj A.O. Proteomics Analysis of the Non-Muscle Myosin Heavy Chain IIa-Enriched Actin-Myosin Complex Reveals Multiple Functions within the Podocyte. PLoS One. 2014;9:100660. doi: 10.1371/journal.pone.0100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanzaki M., Pessin J.E. Insulin-stimulated GLUT4 translocation in adipocytes is dependent upon cortical actin remodeling. J. Biol. Chem. 2001;276:42436–42444. doi: 10.1074/jbc.M108297200. [DOI] [PubMed] [Google Scholar]
- 39.Tong P., Khayat Z.A., Huang C., Patel N., Ueyama A., Klip A. Insulin-induced cortical actin remodeling promotes GLUT4 insertion at muscle cell membrane ruffles. J. Clin. Invest. 2001;108:371–381. doi: 10.1172/JCI12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furtado M.L., Poon V., Klip A. GLUT4 activation: thoughts on possible mechanisms. Acta Physiol. Scand. 2003;178:287–296. doi: 10.1046/j.1365-201X.2003.01160.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhu H., Zhang X., Zuo L., Zhou Q., Gui S., Wei W., Wang Y. Expression of myosin light chain kinase in kidney of streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2006;7:510–518. [Google Scholar]
- 42.Cooke J.N., Bostrom M.A., Hicks P.J., Ng M.C., Hellwege J.N., Comeau M.E., Divers J., Langefeld C.D., Freedman B.I., Bowden D.W. Polymorphisms in MYH9 are associated with diabetic nephropathy in European Americans. Nephrol. Dial. Transplant. 2012;27:1505–1511. doi: 10.1093/ndt/gfr522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewko B., Stepinski J. Hyperglycemia and mechanical stress: targeting the renal podocyte. J. Cell Physiol. 2009;221:288–295. doi: 10.1002/jcp.21856. [DOI] [PubMed] [Google Scholar]
- 44.Jauregui A., Mintz D.H., Mundel P., Fornoni A. Role of altered insulin signaling pathways in the pathogenesis of podocyte malfunction and microalbuminuria. Curr. Opin. Nephrol. Hypertens. 2009;18:539–545. doi: 10.1097/MNH.0b013e32832f7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kestilä M., Lenkkeri U., Männikkö M., Lamerdin J., McCready P., Putaala H., Ruotsalainen V., Morita T., Nissinen M., Herva R. Positionally cloned gene for a novel glomerular protein-nephrin-is mutated in congenital nephrotic syndrome. Mol. Cell. 1998;4:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 46.Yuan H., Takeuchi E., Salant D.J. Podocyte slit-diaphragm protein nephrin is linked to the actin cytoskeleton. Am. J. Physiol. Ren. Physiol. 2002;282:585–591. doi: 10.1152/ajprenal.00290.2001. [DOI] [PubMed] [Google Scholar]
- 47.Epstein C.J., Sahud M.A., Piel C.F., Goodman J.R., Bernfield M.R., Kushner J.H., Ablin A.R. Hereditary macrothrombocytopathia, nephritis and deafness. Am. J. Med. 1972;52:299–310. doi: 10.1016/0002-9343(72)90017-4. [DOI] [PubMed] [Google Scholar]
- 48.Peterson L.C., Rao K.V., Crosson J.T., White J.G. Fechtner syndrome-a variant of Alport's syndrome with leukocyte inclusions and macrothrombocytopenia. Blood. 1985;65:397–406. [PubMed] [Google Scholar]
- 49.Greinacher A., Nieuwenhuis H.K., White J.G. Sebastian platelet syndrome: a new variant of hereditary macrothrombocytopenia with leukocyte inclusions. Blut. 1990;61:282–288. doi: 10.1007/BF01732878. [DOI] [PubMed] [Google Scholar]
- 50.Johnstone D.B., Zhang J., George B., Léon C., Gachet C., Wong H., Parekh R., Holzman L.B. Podocyte-specific deletion of Myh9 encoding nonmuscle myosin heavy chain 2A predisposes mice to glomerulopathy. Mol. Cell Biol. 2011;31:2162–2170. doi: 10.1128/MCB.05234-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y., Conti M.A., Malide D., Dong F., Wang A., Shmist Y.A., Liu C., Zerfas P., Daniels M.P., Chan C.C. Mouse models of MYH9-related disease: mutations in nonmuscle myosin II-A. Blood. 2012;5:238–250. doi: 10.1182/blood-2011-06-358853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnstone D.B., Ikizler O., Zhang J., Holzman L.B. Background strain and the differential susceptibility of podocyte-specific deletion of Myh9 on murine models of experimental glomerulosclerosis and HIV nephropathy. PLoS One. 2013;8:67839. doi: 10.1371/journal.pone.0067839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kopp J.B., Smith M.W., Nelson G.W., Johnson R.C., Freedman B.I., Bowden D.W., Oleksyk T., McKenzie L.M., Kajiyama H., Ahuja T.S. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat. Genet. 2008;40:1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sekine T., Konno M., Sasaki S., Moritani S., Miura T., Wong W.S., Nishio H., Nishiguchi T., Ohuchi M.Y., Tsuchiya S. Patients with Epstein-Fechtner syndromes owing to MYH9 R702 mutations develop progressive proteinuric renal disease. Kidney Int. 2010;78:207–214. doi: 10.1038/ki.2010.21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material